Abstract
Background
It is unclear if the higher burden from colorectal cancer among blacks is due to an increased biological susceptibility.
Objective
To determine whether non-Hispanic blacks (blacks) have a higher risk of adenoma recurrence than non-Hispanic whites (whites) after removal of colorectal adenomas.
Design
Secondary analysis of the Polyp Prevention Trial (PPT) data
Setting
United States.
Patients
1,668 self-identified whites and 153 blacks who completed the 4-year trial. Of these, 688 whites and 55 blacks enrolled in a post-trial passive PPT Continued Follow-up Study (PPT-CFS) and underwent another colonoscopy.
Main outcome measurements
Recurrence and location of adenoma and advanced adenoma by race-ethnicity during the PPT and cumulative recurrence over a mean follow-up of 8.3 years (range 4.9–12.4 years) among PPT-CFS enrollees.
Results
Blacks had a similar risk of any adenoma recurrence (39.2% versus 39.4%; incidence risk ratio (RR) =0.98; 95%CI: 0.80-1.20) and advanced adenoma (8.5% versus 6.4%; RR=1.18; 95%CI: 0.68-2.05) as whites at the end of the PPT. Recurrence risk did not differ by colon subsite.
Among PPT-CFS enrollees, the cumulative recurrence rate was similar for blacks and whites for any adenoma (67.3% versus 67.0%; RR=1.01; 95%CI: 0.84-1.21) and advanced adenoma (14.5% versus 16.9%; RR=1.03; 95%CI: 0.60-1.79).
Limitation
There were few blacks in the long-term follow-up study
Conclusions
Adenoma and advanced adenoma recurrence did not differ by race. Our study does not support more frequent surveillance colonoscopies for blacks with a personal history of adenoma as an intervention to reduce colorectal cancer disparity.
Keywords: Adenomatous polyps, race-ethnicity, colonoscopy, health disparity
INTRODUCTION
Blacks have the highest incidence of and mortality from colorectal cancer (CRC) in the United States. 1-3 Studies have reported that blacks develop CRC at earlier ages with more right sided cancers when compared to whites. 4, 5 They also present at more advanced stages of disease even among insured patients when CRC screening was a covered benefit. 6 These findings suggest that blacks may have a higher biological susceptibility to CRC. However, there is evidence that black-white differences in CRC risk may be related to differences in access to, or use of, healthcare services. 7-11 Furthermore, similar CRC survival was reported for blacks and whites in the U.S. Veterans Affairs Health Care System where access to healthcare resources is equivalent12 and also among participants in a randomized clinical trial of adjuvant chemotherapy. 13 These studies suggest a less important role of biology as the main factor for CRC disparity by race-ethnicity.
In spite of the uncertainties of the cause of CRC disparity by race, some organizations recommend screening for blacks to start at age 40 14 or 45 years 15, 16 while others recommend average risk screening at age 50 years regardless of race-ethnicity. 17-19 These race-based differences in CRC screening recommendations create confusion for healthcare providers in the delivery of screening services to their patients and complicate the measurement of the quality of services provided. For instance, Goodwin et al. 20 recently reported that blacks were more likely to undergo early repeat screening after a negative colonoscopy among Medicare population, an overuse of limited healthcare resources. It is unclear if this overuse is related to the suggestion that blacks were more likely to receive substandard care 21-23 and perhaps, more likely to have missed lesions at colonoscopy.
To date, most studies evaluating white-black differences in CRC risk have focused on differences in neoplasia prevalence 1, 2, 4-7 but another measure of biological susceptibility is the risk of recurrence after polypectomy. Using data from the Polyp Prevention Trial (PPT), we assessed whether blacks were more likely to have adenomas that were missed at baseline colonoscopy. We also evaluated the risk of adenoma and advanced adenoma recurrence over 4 years, and the cumulative recurrence risk over a maximal follow-up period of 12 years.
METHODS
Study population
The design and results of the PPT have been published. 24-26 In summary, the PPT was a 4-year multicenter, randomized, controlled trial that evaluated the effect of a low-fat, high-fiber, fruit and vegetable diet on the risk of colorectal adenoma recurrence. A total of 2,079 participants who were at least 35 years old and had one or more histologically confirmed adenomatous polyps removed within 6 months were randomized between June 1991 and January 1994. The clinical trial was approved by the Institutional Review Boards of the National Cancer Institute (NCI), and each of the eight participating clinical centers. All participants gave written informed consent. The trial was completed in 1998. After the trial ended, 1,297 subjects participated in a passive “Continued Follow-up Study” (PPT-CFS), which was intended to ascertain the long-term effect of the dietary intervention during the trial on subsequent adenoma recurrence. The extended follow-up began in 1995 and ended in 2004. The current study was a secondary analysis of de-identified data from the PPT and PPT-CFS. Final approval for the current study was obtained from the NCI on July 11, 2012.
Exposure and outcome assessment
At baseline, characteristics of the participants including self identified race-ethnicity and health-related lifestyle was obtained through interviews. Per design and protocol of the PPT, dietary counseling took place in the clinical centers while colonoscopic examinations were performed by community gastroenterologists, and hence reflected the performance of colonoscopy in community-based practice. The participants underwent a clearing colonoscopy approximately one year after randomization (T1) to remove any lesion which may have been missed at qualifying colonoscopy (T0). They were followed for four years after randomization, and had an end-of-trial (T4) colonoscopy to ascertain adenoma recurrence. Any adenoma found during an incidental colonoscopy after the T1 examination (i.e. outside PPT protocol) was considered to be recurrent. For the PPT-CFS participants, the post-trial colonoscopies were performed per routine care as determined by the participants’ care providers. The reports of the first colonoscopy performed after the trial were included in this analysis. The colonoscopy reports provided information on size, number, and location of polyps. The histology and degree of dysplasia during the main PPT trial were confirmed by two central gastrointestinal trial pathologists but the histopathology determination during PPT-CFS were by the community pathologists where the colonoscopies took place. We defined an advanced adenoma as an adenoma with size ≥1 cm in diameter, or villous histology or high grade dysplasia or invasive cancer.
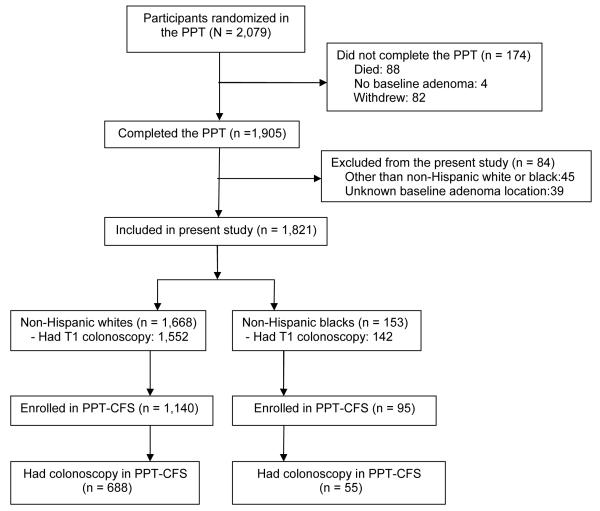
A total of 1,905 (91.6%) participants completed the trial by undergoing the end of trial (T4) colonoscopy and 774 (59.7%) of PPT-CFS enrollees (n = 1,297) had a colonoscopy during the extended follow-up period. The dietary intervention did not affect adenoma recurrence during the PPT 26 or during the PPT-CFS. 27 Our analytic cohort for this study comprises 1,668 non-Hispanic white (whites) and 153 non-Hispanic black (blacks) participants who completed the trial and had complete information on the location of their baseline adenoma and 688 white and 55 black enrollees in the PPT-CFS who had at least one colonoscopy subsequent to the trial (Figure 1). We excluded all Hispanics (whites and blacks) and other races (Asians and American Indians) from this analysis.
Figure 1. Diagram of flow of participants through the study.
PPT = Polyp Prevention Trial
PPT-CFS = Polyp Prevention Trial and Continued Follow-up Study
Statistical analyses
We compared the baseline demographic, lifestyle and baseline adenoma characteristics of PPT participants by race-ethnicity (whites versus blacks). We also compared the characteristics of PPT-CFS participants to non-participants by race-ethnicity. We used the Wilcoxon rank-sum (Mann-Whitney) test to compare age as a continuous variable and used the chi-squared test to compare categorical variables. We analyzed the rate of missed adenoma (defined by PPT protocol as adenoma found at T1 colonoscopy) by race-ethnicity as a quality measure because of the suggestion in the literature that substandard care received by blacks is a substantial contributor to health disparities by race-ethnicity. 21-23 We used Poisson regression models with robust standard error estimation to compare the risk of missed adenoma (T1) versus no missed adenoma by race-ethnicity. We also compared the risk of adenoma recurrence versus no adenoma recurrence at the end of the PPT (T4). We defined adenoma recurrence as adenoma found subsequent to the T1 clearing colonoscopy. For the 127 participants who did not undergo T1 clearing colonoscopy, any adenoma at colonoscopy after 2 years from the baseline examination was regarded as recurrent per PPT protocol. Assuming an adenoma recurrence rate of 35% among whites, our study has 80% power to detect a 33% increased risk of recurrence among blacks. We calculated incidence risk ratios (RR) and 95% confidence intervals (CI). We used multinomial logistic regression models to evaluate adenoma recurrence by colon subsite. We categorized adenoma recurrence by colon subsite location as: no recurrence; distal only recurrence; at least one proximal recurrence, and we calculated relative risk ratio (RRR) and 95% CI. Advanced adenoma recurrence by colon subsite was categorized in a similar fashion. Because of the suggestion of earlier age of CRC among blacks, 11 we performed an exploratory analysis among participants who were younger than 50 years at baseline. We also performed a sensitivity analysis in which we added adenomas removed at T1 clearing colonoscopy to the T4 colonoscopy (as recurrent lesions) and repeated our analyses. Finally, we compared the cumulative adenoma recurrence (T1 plus T4 plus PPT-CFS colonoscopy findings) by race-ethnicity among PPT-CFS participants. Assuming a cumulative adenoma recurrence rate of 60% among whites, our study has 88% power to detect a 33% increased risk of cumulative adenoma recurrence among blacks. We included age, sex, randomization assignment, body mass index, highest education attained (as a surrogate for socioeconomic status), use of non steroidal anti-inflammatory drugs, smoking, family history of CRC in our final multivariable models. For the end-of-PPT analysis, we had information on whether the cecum was reached during colonoscopy and on the adequacy of bowel preparation. These were also added to the multivariable models. We used Stata ® statistical software version 11.2 (College Station, Texas) for our analyses. All reported P-values correspond to two-sided tests.
RESULTS
Baseline characteristics in the PPT
A total of 1,668 (91.6%) whites and 153 (8.4%) blacks had information on the location of their baseline adenoma and completed the 4-year trial. Table 1 shows selected baseline characteristics of study participants by race-ethnicity. When compared with whites, blacks had less formal education and were more likely to be obese, but there were no differences in age or sex. Black participants in the trial had a higher prevalence of advanced adenoma at baseline (44.4% versus 37.0%; P=0.07). Of note, there was no difference by race-ethnicity in the number of people who failed to complete the PPT (8.3% whites versus 9.9% blacks, P=0.47).
Table 1.
| PPT | PPT-CFS | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Characteristics | Non- Hispanic whites |
Non- Hispanic blacks |
P value |
Non- Hispanic whites |
Non- Hispanic blacks |
P value |
|
| ||||||
| (n=1,668) | (n=153) | (n=688) | (n=55) | |||
| Mean age, years (SD) | 61.3 (9.9) | 60.8 (9.5) | 0.53 | 59.9 (8.8) | 58.7 (8.8) | 0.29 |
|
| ||||||
| Randomization assignment, n (%) | ||||||
| Control | 843 (50.5) | 66 (43.1) | 0.08 | 341 (49.6) | 23 (41.8) | 0.27 |
| Intervention | 825 (49.5) | 87 (56.9) | 347 (50.4) | 32 (58.2) | ||
| Sex, n (%) | ||||||
| Female | 600 (36.0) | 51 (33.3) | 0.52 | 235 (34.2) | 16 (29.1) | 0.45 |
| Male | 1,068 (64.0) | 102 (66.7) | 453 (65.8) | 39 (70.9) | ||
| Education, n (%) ‡ | ||||||
| High school or less | 396 (23.8) | 54 (35.3) | 0.002 | 132 (19.2) | 16 (29.1) | 0.077 |
| More than high school | 1,271 (76.2) | 99 (64.7) | 556 (80.8) | 39 (70.9) | ||
| Body mass index, kg/m2, n (%) § | ||||||
| < 25 | 448 (26.9) | 28 (18.3) | 0.006 | 172 (25.0) | 10 (18.2) | |
| 25-29 | 791 (47.4) | 69 (45.1) | 346 (50.3) | 23 (41.8) | 0.043 | |
| ≥ 30 | 429 (25.7) | 56 (36.6) | 170 (24.7) | 22 (40.0) | ||
| Smoking, n (%) | ||||||
| Never | 658 (39.5) | 48 (31.4) | 0.055 | 283 (41.1) | 16 (29.1) | |
| Former | 797 (47.8) | 77 (50.3) | 333 (48.4) | 31 (56.4) | 0.194 | |
| Current | 213 (12.8) | 28 (18.3) | 72 (10.5) | 8 (14.6) | ||
|
Positive first degree
family history of colon cancer, n (%) |
457 (27.4) | 34 (22.2) | 0.167 | 225 (32.7) | 16 (29.1) | 0.58 |
|
Non-steroidal anti-
inflammatory drugs at least once/month, n (%) |
573 (34.4) | 45 (29.4) | 0.22 | 227 (33.0) | 22 (40.0) | 0.29 |
| Baseline adenoma, n (%) | ||||||
| ≥3 synchronous | 252 (15.1) | 16 (10.5) | 0.12 | 92 (13.4) | 5 (9.1) | 0.36 |
| Adenomas | ||||||
| Advanced adenoma∥ | 617 (37.0) | 68 (44.4) | 0.069 | 269 (39.1) | 20 (36.4) | 0.69 |
| Baseline adenoma location, n (%) | ||||||
| Distal only | 927 (55.6) | 74 (48.4) | 387 (56.3) | 23 (41.8) | ||
| Proximal only | 447 (26.8) | 50 (32.7) | 0.198 | 188 (27.3) | 22 (40.0) | 0.086 |
| Synchronous proximal and distal adenomas |
294 (17.6) | 29 (19.0) | 113 (16.4) | 10 (18.2) | ||
PPT = Polyp prevention Trial
PPT-CFS = Polyp Prevention Trial and the Continued Follow-Up Study
Missing education status for 1 white participant
Highest body mass index in PPT = 38.8 kg/m2
Advanced adenoma = size ≥1 cm in diameter, or villous histology or high grade dysplasia or invasive cancer.
Missed adenoma and advanced adenoma
Of 1,821 participants in the current study, 1,694 (93.0%) underwent the clearing (T1) colonoscopy designed per PPT protocol to remove missed adenoma approximately 1 year after randomization. There was no difference in the receipt of T1 colonoscopy by race-ethnicity (1,552 (93.1%) whites versus 142 (92.8%) blacks; P=0.91). Blacks and whites had similar risks of missed adenoma and advanced adenoma (Table 2).
Table 2.
The risk of missed adenoma and missed advanced adenoma at T1 colonoscopy by race-ethnicity
| Race-ethnicity | No missed adenoma, n (%) |
Any missed adenoma, n (%) |
Missed adenoma versus no missed adenoma RR (95% CI) |
Missed advanced adenoma, n (%) |
Missed advanced adenoma versus no missed adenoma RR (95% CI) |
|---|---|---|---|---|---|
| White (n = 1,552) | 1,010 (65.1) |
542 (34.9) | Reference | 93 (6.0) | Reference |
| Black (n = 142) | 94 (66.2) | 48 (33.8) | 0.97 (0.77-1.22) | 8 (5.6) | 0.97 (0.49-1.91) |
Adjusted for age, sex, randomization assignment, education, body mass index, use of non-steroidal anti-inflammatory drugs, smoking and family history of colorectal cancer
Adenoma and advanced adenoma recurrence in the PPT (short term)
At the end-of-trial (T4) colonoscopy, cecal intubation was documented for 151 (98.7%) blacks versus 1,591 (95.4%) whites (P=0.054) and bowel preparation was documented as adequate for 153 (100%) blacks versus 1,619 (97.1%) whites (P=0.032). However, information on the degree of adequacy of the bowel preparation such as good or excellent bowel preparation was not available. Adenoma recurrence occurred among 717 (39.4%) participants and 120 (6.6%) participants had advanced adenoma recurrence. Overall, the recurrence rates were similar by race-ethnicity (Table 3). A total of 60 (39.2%) blacks and 657 (39.4%) whites had adenoma recurrence (RR=0.98; 95%CI: 0.80-1.20) whereas 13 (8.5%) blacks and 107 (6.4%) whites had advanced adenoma recurrence (RR=1.18; 95%CI: 0.68-2.05). There was no difference in the recurrence of adenoma and advanced adenoma by colon subsite (Table 3).
Table 3.
Risk of adenoma and advanced adenoma recurrence by race-ethnicity in the 4-year Polyp Prevention Trial
| Characteristics | Non-Hispanic Whites (n = 1,668) |
Non-Hispanic Blacks (n = 153) |
|---|---|---|
| No adenoma recurrence, n (%) | 1,011 (60.6) | 93 (60.8) |
| Any adenoma recurrence, n (%) | 657 (39.4) | 60 (39.2) |
| Any adenoma recurrence versus no recurrence, RR (95% CI) |
Reference | 0.98 (0.80-1.20) |
| Any advanced adenoma recurrence, n (%) | 107 (6.4) | 13 (8.5) |
| Any advanced adenoma recurrence versus no recurrence, RR (95% CI) |
Reference | 1.18 (0.68-2.05) |
| Distal only adenoma recurrence, n (%) | 205 (12.3) | 15 (9.8) |
| Distal only adenoma recurrence versus no recurrence, RRR (95% CI) |
Reference | 0.78 (0.44-1.38) |
| Any proximal adenoma recurrence, n (%) | 452 (27.1) | 45 (29.4) |
| Any proximal adenoma recurrence versus no recurrence, RRR (95% CI) |
Reference | 1.05 (0.71-1.54) |
| Distal only advanced adenoma recurrence, n (%) | 42 (2.5) | 5 (3.3) |
| Distal only advanced adenoma recurrence versus no recurrence, RRR (95% CI) |
Reference | 1.14 (0.43-3.01) |
| Any proximal advanced adenoma recurrence, n (%) | 64 (3.8) | 8 (5.2) |
| Any proximal advanced adenoma recurrence versus no recurrence, RRR (95% CI) |
Reference | 1.31 (0.59-2.91) |
Adjusted for age, sex, randomization assignment, education, body mass index, use of non-steroidal anti-inflammatory drugs, smoking, family history of colorectal cancer, cecum reached during colonoscopy and adequacy of bowel preparation.
Adenoma recurrence among participants younger than 50 years old at baseline
No previous study has evaluated adenoma recurrence risk by race-ethnicity among those younger than 50 years of age. Therefore, we conducted an exploratory analysis in this regard. There were a total of 230 participants: 213 whites and 17 blacks. There was no age difference between the two groups (mean age of whites = 44.1 years versus mean age of blacks = 44.3 years; P=0.98). In this subset, we observed a lower adenoma recurrence among blacks (1 black (5.9%) versus 50 whites (23.5%); P=0.093), but there was no significant difference in the risk of recurrence by race-ethnicity in the multivariable model (RR=0.24; 95% CI: 0.04 – 1.60).
Sensitivity analysis
Although adenomas found at T1 colonoscopies were regarded as missed lesions in the PPT, there is also a possibility that these lesions may be new. Therefore, we performed a sensitivity analysis and added adenomas found at T1 to those identified at T4 and repeated our analysis. This did not change the results by race-ethnicity. The adenoma recurrence rate was 56.2% among blacks and 54.4% among whites (RR=1.03; 95%CI: 0.89-1.19) and advanced adenoma recurrence rate was 12.4% among blacks and 10.5% among whites (RR=1.16; 95%CI: 0.77-1.76).
Cumulative adenoma and advanced adenoma recurrence in the PPT-CFS (long term)
There was no difference in participation in PPT-CFS by race-ethnicity. A total of 95 (62.1%) black and 1,140 (68.4%) white PPT completers agreed to participate in the PPT-CFS (P=0.113). Of the PPT-CFS enrollees, there was no difference in the receipt of post-trial colonoscopy by race-ethnicity as 55 (36.0%) blacks and 688 (41.3%) whites had another colonoscopy during the follow-up period (P=0.64). The comparison of the baseline characteristics of these PPT-CFS participants by race-ethnicity was similar to the race-ethnicity comparison of the entire cohort included in this analysis except that the difference in the highest education attained, our marker for socioeconomic status, was no longer significantly different (Table 1).
Including the 4-year trial period, the cumulative mean follow-up period among the PPT-CFS participants was 8.3 years (range 4.9 to 12.4 years). The total mean follow-up period was similar among blacks and whites (8.5 years among blacks versus 8.3 years among whites; P=0.50). A total of 37 (67.3%) blacks and 461 (67.0%) whites had at least 1 adenoma recurrence during the entire follow-up period. There was no difference in the cumulative adenoma and advanced adenoma recurrence by race-ethnicity (Table 4). The sample size was too small for a meaningful analysis of recurrence by colon subsite.
Table 4.
Cumulative risk of adenoma and advanced adenoma recurrence by race-ethnicity in PPT and PPT-CFS over a mean follow-up of 8.3 years (range 4.9 to 12.4 years)
| Race- ethnicity |
No adenoma recurrence, n (%) |
Adenoma recurrence, n (%) |
Adenoma versus no adenoma recurrence RR (95% CI) |
Advanced adenoma recurrence, n (%) |
Advanced adenoma recurrence versus no adenoma recurrence RR (95% CI) |
|---|---|---|---|---|---|
| White (n = 688) |
227 (33.0) | 461 (67.0) | Reference | 116 (16.9) | Reference |
| Black (n = 55) |
18 (32.7) | 37 (67.3) | 1.01 (0.84-1.21) | 8 (14.5) | 1.03 (0.60-1.79) |
Adjusted for age, sex, randomization assignment, education, body mass index, use of non-steroidal anti-inflammatory drugs, smoking and family history of colorectal cancer
DISCUSSION
We compared the risk of adenoma and advanced adenoma recurrence among white and black PPT participants in the short-term (4 years) and in the PPT-CFS participants in the long-term (maximal follow-up of 12 years) and we did not find any racial difference in the risk of recurrence. It is noteworthy that the uptake of surveillance colonoscopy was comparable between blacks and whites. Furthermore, there was no difference in adenoma detection within a year of index colonoscopy by race-ethnicity. Our study suggests that the observed disparity in CRC by race may not be due to increased susceptibility to carcinogenesis among blacks, at least in the early phase of neoplastic disease. Our findings do not provide evidence to support more frequent surveillance for blacks with a personal history of adenoma as an intervention to reduce CRC disparity. The implication of our findings is that blacks may not need a different postpolypectomy surveillance colonoscopy recommendation from whites.
Three domains of CRC disparities have been identified: healthcare access which includes availability of health insurance, accessible facilities and providers; healthcare utilization, which entails the use of healthcare resources when it is available; and biological differences. 28 We have previously evaluated 2 domains (healthcare utilization and biology) within the same cohort of subjects in a screening population. 29 In that study, we used data from the recently concluded Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO) and evaluated CRC disparity by race-ethnicity by examining the rate of diagnostic colonoscopy following an abnormal screening flexible sigmoidoscopy among 57,561 white and 3,011 black participants. There was a comparable rate of finding polyps or masses during flexible sigmoidoscopy screening among whites and blacks (23.9% versus 25.5%, P=0.26) at screening, but blacks were less likely to undergo diagnostic colonoscopy (relative risk (RR) = 0.88; 95%CI: 0.83-0.93) within a year of the abnormal screening. However, when compared with whites, blacks had comparable yield of adenoma (RR=1.01; 95%CI: 0.92-1.11), advanced adenoma (RR=1.11; 95%CI: 0.94-1.30) and CRC (RR=1.58; 95%CI: 0.80-3.12) among those who underwent diagnostic colonoscopy. That study suggested that healthcare utilization may be playing more of a role in the observed CRC disparity by race. Taken together, the aforementioned study and the current study provide some evidence that blacks may not be more susceptible to colorectal carcinogenesis than whites in terms of prevalence and recurrence of precursor lesions of CRC.
We are only aware of 2 studies that have compared the risk of adenoma recurrence between blacks and whites. Using data from the adenoma pooling project, Martinez et al. 30 reported similar risk of adenoma recurrence among blacks and whites over a median of 47.2 months of follow-up, (odds ratio (OR) = 1.12; 95%CI: 0.92–1.37) for non-advanced and (OR=1.08; 95%CI: 0.79–1.47) for advanced adenoma, but the authors did not report on the risk of recurrence by colon subsite. Using retrospective data collection, Penn et al. 31 reported that race was not associated with the prevalence of polyps at baseline (OR=1.04; 95%CI: 0.85-1.28). However, among 57 patients (7 blacks) with baseline adenoma who had a follow-up colonoscopy after a median of 3.6 years later, race was not associated with polyp recurrence, (hazard ratio (HR) = 1.89; 95%CI: 0.68-5.24). This study was limited by its retrospective design, lack of histopathological diagnosis for all patients with the use of polyps as a proxy for neoplasia, small sample size for the postpolypectomy cohort (with only 7 blacks) and short follow-up interval. In our observational study using data from a completed randomized trial, we did not find any difference in the risk and location of adenoma and advanced adenoma recurrence among blacks and whites despite a higher prevalence of advanced adenoma among blacks at baseline. There was no difference in recurrence risk over a maximal follow-up period of 12 years.
There are many notable strengths of our study. Our study population is from a large randomized controlled trial with participants recruited from geographically dispersed areas, dedicated central pathologists with expertise in gastrointestinal tumors examined the adenomas and all patients had planned colonoscopic assessment for recurrence after an adequate follow-up period of 4 years. Furthermore, we were able to assess recurrence among a subset of the study population over a maximum follow-up of 12 years.
Our study also has some limitations. The participants in the PPT were self-selected and may be healthier than comparable members of the general population. We have a relatively small number of blacks, particularly in the long-term follow-up study; therefore, our study power was limited to detect a 33% increased risk of adenoma recurrence among blacks. Finally, we did not have any information about serrated lesions since it was not a well known phenomenon at the time the trial was conducted. However, we are not aware of any conclusive evidence that blacks are more likely than whites to have sessile serrated adenoma that could potentially advance to CRC through an alternate pathway.
In conclusion, we did not find an increased risk of adenoma or advanced adenoma recurrence among blacks as compared to whites. Our study does not support more frequent surveillance for blacks with a personal history of adenoma as an intervention to reduce CRC disparity. Rather, our study suggests that when the uptake and quality of colonoscopy performed are equal, blacks may not be at an increased risk of CRC to warrant different screening guideline recommendations. However, further studies are needed to elucidate the underlying factors for the increased burden of CRC among blacks and possibly identify a subset of blacks at increased biological susceptibility to CRC.
Acknowledgments
Grant support: Dr Laiyemo is supported by the National Cancer Institute’s new faculty recruitment supplement to the Comprehensive Minority Institution/Cancer Center Partnership between Howard University Cancer Center and Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University (5U54CA091431-09 S1).
Dr. Doubeni is supported by a mentored career development award (5K01CA127118). Dr. Platz is supported by NIH grant P30 CA006973.
Financial disclosure: The study was funded by the Division of Cancer Prevention and the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health.
Footnotes
Conflict of interest: No conflicts of interest exist
Disclosure: An abstract from this study was presented at the Digestive Diseases Week in San Diego in May 2012. (Gastroenterology 2012; 142 (5):S408).
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012 Jan-Feb;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations) National Cancer Institute; Bethesda, MD: [Accessed on May 25, 2012]. 2012. http://seer.cancer.gov/csr/1975_2009_pops09/, based on November 2011 SEER data submission, posted to the SEER web site. at http://seer.cancer.gov/statfacts/html/colorect.html. [Google Scholar]
- 3.Chien C, Morimoto LM, Tom J, Li CI. Differences in colorectal carcinoma stage and survival by race and ethnicity. Cancer. 2005;104(3):629–39. doi: 10.1002/cncr.21204. [DOI] [PubMed] [Google Scholar]
- 4.Fairley TL, Cardinez CJ, Martin J, et al. Colorectal cancer in U.S. adults younger than 50 years of age, 1998-2001. Cancer. 2006;107(5 Suppl):1153–61. doi: 10.1002/cncr.22012. [DOI] [PubMed] [Google Scholar]
- 5.Shavers VL. Racial/ethnic variation in the anatomic subsite location of in situ and invasive cancers of the colon. J Natl Med Assoc. 2007;99(7):733–48. [PMC free article] [PubMed] [Google Scholar]
- 6.Doubeni CA, Field TS, Buist DS, et al. Racial differences in tumor stage and survival for colorectal cancer in an insured population. Cancer. 2007;109(3):612–20. doi: 10.1002/cncr.22437. [DOI] [PubMed] [Google Scholar]
- 7.Siegel RL, Jemal A, Thun MJ, Hao Y, Ward EM. Trends in the incidence of colorectal cancer in relation to county-level poverty among blacks and whites. J Natl Med Assoc. 2008;100(12):1441–4. doi: 10.1016/s0027-9684(15)31544-3. [DOI] [PubMed] [Google Scholar]
- 8.Kinsey T, Jemal A, Liff J, Ward E, Thun M. Secular trends in mortality from common cancers in the United States by educational attainment, 1993-2001. J Natl Cancer Inst. 2008;100(14):1003–12. doi: 10.1093/jnci/djn207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma S, O’Keefe SJ. Environmental influences on the high mortality from colorectal cancer in African Americans. Postgrad Med J. 2007;83(983):583–9. doi: 10.1136/pgmj.2007.058958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le H, Ziogas A, Lipkin SM, Zell JA. Effects of socioeconomic status and treatment disparities in colorectal cancer survival. Cancer Epidemiol Biomarkers Prev. 2008;17(8):1950–62. doi: 10.1158/1055-9965.EPI-07-2774. [DOI] [PubMed] [Google Scholar]
- 11.Jerant AF, Fenton JJ, Franks P. Determinants of racial/ethnic colorectal cancer screening disparities. Arch Intern Med. 2008;168(12):1317–24. doi: 10.1001/archinte.168.12.1317. [DOI] [PubMed] [Google Scholar]
- 12.Dominitz JA, Samsa GP, Landsman P, Provenzale D. Race, treatment, and survival among colorectal carcinoma patients in an equal-access medical system. Cancer. 1998;82(12):2312–20. doi: 10.1002/(sici)1097-0142(19980615)82:12<2312::aid-cncr3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 13.McCollum AD, Catalano PJ, Haller DG, et al. Outcomes and toxicity in African Americans and Caucasian patients in a randomized adjuvant chemotherapy trial for colon cancer. J Natl Cancer Inst. 2002;94:1160–1167. doi: 10.1093/jnci/94.15.1160. [DOI] [PubMed] [Google Scholar]
- 14.Qaseem A, Denberg TD, Hopkins RH, Jr, et al. Clinical Guidelines Committee of the American College of Physicians Screening for colorectal cancer: a guidance statement from the American College of Physicians. Ann Intern Med. 2012;156(5):378–86. doi: 10.7326/0003-4819-156-5-201203060-00010. [DOI] [PubMed] [Google Scholar]
- 15.Cash BD, Banerjee S, Anderson MA, et al. Ethnic issues in endoscopy. Gastrointest Endosc. 2010;71(7):1108–12. doi: 10.1016/j.gie.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM, American College of Gastroenterology American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104(3):739–50. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Preventive Services Task Force Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 18.Levin B, Lieberman DA, McFarland B, et al. American Cancer Society Colorectal Cancer Advisory Group. US Multi-Society Task Force. American College of Radiology Colon Cancer Committee Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58(3):130–60. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 19.Gupta S, Shah J, Balasubramanian BA. Strategies for reducing colorectal cancer among blacks. Arch Intern Med. 2012;172(2):182–4. doi: 10.1001/archinternmed.2011.594. [DOI] [PubMed] [Google Scholar]
- 20.Goodwin JS, Singh A, Reddy N, Riall TS, Kuo YF. Overuse of screening colonoscopy in the Medicare population. Arch Intern Med. 2011;171(15):1335–43. doi: 10.1001/archinternmed.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onega T, Duell EJ, Shi X, Demidenko E, Goodman DC. Race versus place of service in mortality among medicare beneficiaries with cancer. Cancer. 2010;116(11):2698–706. doi: 10.1002/cncr.25097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341(16):1198–205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 23.Steyerberg EW, Earle CC, Neville BA, Weeks JC. Racial differences in surgical evaluation, treatment, and outcome of locoregional esophageal cancer: a population-based analysis of elderly patients. J Clin Oncol. 2005;23(3):510–7. doi: 10.1200/JCO.2005.05.169. [DOI] [PubMed] [Google Scholar]
- 24.Schatzkin A, Lanza E, Freedman LS, et al. The polyp prevention trial I: rationale, design, recruitment, and baseline participant characteristics. Cancer Epidemiol Biomarkers Prev. 1996;5(5):375–83. [PubMed] [Google Scholar]
- 25.Lanza E, Schatzkin A, Ballard-Barbash R, et al. The polyp prevention trial II: dietary intervention program and participant baseline dietary characteristics. Cancer Epidemiol Biomarkers Prev. 1996;5(5):385–92. [PubMed] [Google Scholar]; Cancer Epidemiol Biomarkers Prev. 1996;5(7):584. Erratum in: [Google Scholar]
- 26.Schatzkin A, Lanza E, Corle D, et al. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. Polyp Prevention Trial Study Group. N Engl J Med. 2000;342(16):1149–55. doi: 10.1056/NEJM200004203421601. [DOI] [PubMed] [Google Scholar]
- 27.Lanza E, Yu B, Murphy G, et al. The Polyp Prevention Trial Continued Follow-up Study: No Effect of a Low-Fat, High-Fiber, High-Fruit, and -Vegetable Diet on Adenoma Recurrence Eight Years after Randomization. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1745–1752. doi: 10.1158/1055-9965.EPI-07-0127. [DOI] [PubMed] [Google Scholar]
- 28.Laiyemo AO. In search of a perfect solution to ensure that “no colon is left behind”. Dig Dis Sci. 2012;57(2):263–5. doi: 10.1007/s10620-011-2010-6. [DOI] [PubMed] [Google Scholar]
- 29.Laiyemo AO, Doubeni C, Pinsky PF, et al. Race and colorectal cancer disparities: health-care utilization vs different cancer susceptibilities. J Natl Cancer Inst. 2010;102(8):538–46. doi: 10.1093/jnci/djq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martínez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009;136(3):832–41. doi: 10.1053/j.gastro.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penn E, Garrow D, Romagnuolo J. Influence of Race and Sex on Prevalence and Recurrence of Colon Polyps. Arch Intern Med. 2010;170(13):1127–1132. doi: 10.1001/archinternmed.2010.152. [DOI] [PubMed] [Google Scholar]