Abstract
NOXO1 (Nox Organizer 1) is a homolog of the NAPDH oxidase protein p47phox. NADPH oxidases transfer electrons from NADPH to molecular oxygen, generating the superoxide anion. NOXO1 contains an N-terminal PX (phox homology) domain and is one of several PX domain-containing proteins found in the cytosolic subunits of the NADPH oxidase complex. These PX domains bind to membrane lipids and target the protein to membranes, recruiting other cytosolic components to the membrane bound components and aiding formation of a active enzyme complex. This recruitment represents a level of regulation of these oxidases. Here we report the backbone assignments of NOXO1β PX.
Keywords: PX domain, NOXO1, NADPH oxidase, NMR backbone assignments
Biological context
The NADPH oxidases are multiprotein enzyme complexes that generate reactive oxygen species (ROS). ROS generated by these complexes are involved in numerous biological processes including host immune response, cell growth and differentiation, signaling and cell death (reviewed in Krause and Bedard 2007). These complexes include both membrane-bound and cytosolic components. Active complex requires assembly of the cytosolic components with the membrane-bound components at the plasma membrane.
Several of the cytosolic proteins in the NADPH complexes contain a Phox homology (PX) domain. PX domains bind to phosphorylated lipids, most notably the phosphatidylinositol phosphates (Sato et al. 2001). One of these PX domain-containing cytosolic proteins is the NADPH oxidase organizer protein NOXO1. NOXO1 is a 376 residue protein that acts as an organizer within the NADPH oxidase complex to recruite the activator protein, NOXA1, to the membrane-bound catalytic subunit (Banfi et al. 2003, Geiszt et al. 2003 and Takeya et al. 2003). This colocalization represents a major regulatory element of ROS generation. One additional feature of NOXO1 is the presence of four splice variants termed α, β, γ and δ, with NOXO1β being the predominant isoform (Cheng and Lambeth 2005). These splice variants result in insertions and/or deletions within the PX domain. Here, we report the backbone 1H, 13C and 15N assignments for the NOXO1β PX domain (residues 1–144) and discuss additional methods used to obtain the chemical shift assignments.
Experimental methods
NOXO1β PX Expression and Purification
The sequence for human NOXO1β PX (1–144) was cloned into pGEX-6P-1 (GE Biosciences) and transformed into the E. coli strain BL21(DE3)Codon + RIPL (Stratagene). Cultures were grown at 32°C in minimal media containing 1 g/l 15NH4Cl and 2 g/l 13C6 glucose (2H713C6 glucose for 2H samples) until OD600 reached 0.6 and induced with 0.5 mM IPTG at 22°C overnight (1H media) or for 72 h (2H media). Stable isotopes were purchased from Isotec/ Sigma and Cambridge Isotope Laboratories. The harvested cell pellet was resuspended in lysis buffer (20 mM Tris, 20 mm NaCl, 2 mM DTT, 0.1% (v/v) Tween-20, pH 8.0) and lysed on ice by sonication (8 cycles, 4 min/cycle, level 8, 30% duty cycle, 1/2″ probe, Branson Sonifier 250). The lysate was incubated with 10 μl Benzonase (EMD) at room temperature for one hour to digest bacterial DNA. Lysates were clarified by centrifugation for 45 min at 30,000 × g at 4°C. Clarified lysate containing GST-NOXO1β PX was run over SP Sepharose resin equilibrated in SP-A buffer (50 mM NaPi, 100 mM NaCl, 2 mM DTT, pH 7.4). The fusion protein was eluted with a salt gradient (SP-B, 50 mM NaPi, 1.5 M NaCl, 2 mM DTT, pH 7.4) and eluted at ∼800 mM NaCl. The combination of Benzonase treatment and use of SP Sepharose, rather than GSH Sepharose, for initial capture of the fusion protein eliminated nucleic acid contamination of the product.
The pooled SP Sepharose peak was loaded onto GSH-Sepharose FF resin (GE Biosciences) equilibrated in GSH-A (50 mM NaPi, 800 mM NaCl, 2 mM DTT, pH 7.4). GST-NOXO1β PX was eluted with 15 mM reduced glutathione in GSH-A. The eluted GSH-Sepharose peak was dialyzed against 2l SP-A overnight at 4°C in the presence of 80 μg PreScission Protease (GE Biosciences) to cleave the GST. The digested fusion protein was loaded onto Source 15S resin (GE Biosciences) equilibrated in SP-A. Cleaved NOXO1β PX was eluted with a salt gradient (SP-B), with the protein eluting at ∼400 mM NaCl. The pooled NOXO1β PX was treated with Pefabloc PSC (0.1 mg/ml final concentration) for 30 min at 4°C. Final yield of purified NOXO1β PX was ∼3 mg/l culture.
Data Collection
NMR spectra were acquired at 293 K on a Bruker Avance DRX-600 spectrometer equipped with a triple resonance Z-gradient cryoprobe. For NMR experiments, NOXO1β PX was dialyzed into 100 mM NaPi, 100 mM NaCl, 0.1 mM EDTA, pH 6.9. 2H5 glycerol was added to 5% (v/v) after samples were concentrated. NOXO1β PX was concentrated to ∼500 μM for all NMR experiments. Standard pulse sequences were used to acquire 2D HSQC and 3D HNCA, HNCO, HN(CA)CB, HN(CO)CA, HN(CA)CO and HN(COCA)CB data. NMR data were processed using NMRPipe (Delaglio et al. 1995) and analyzed using NMRView (Johnson et al. 1994).
Assignments and data deposition
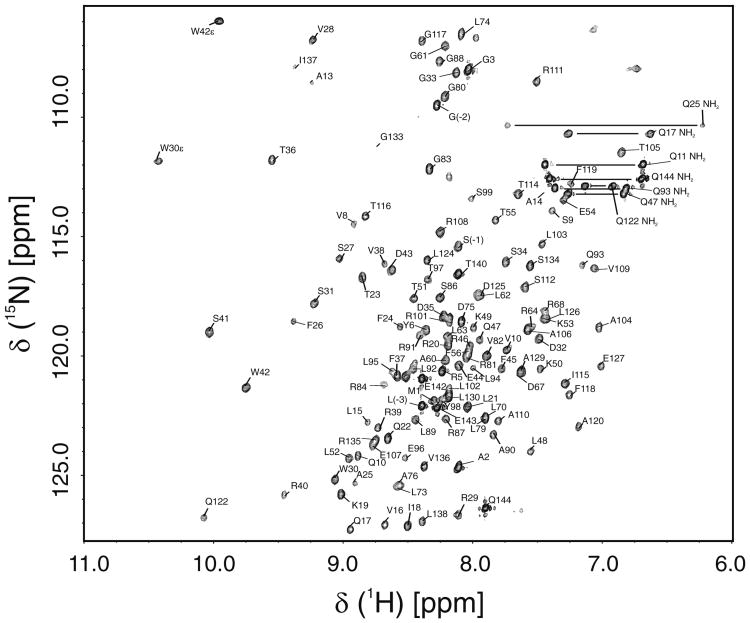
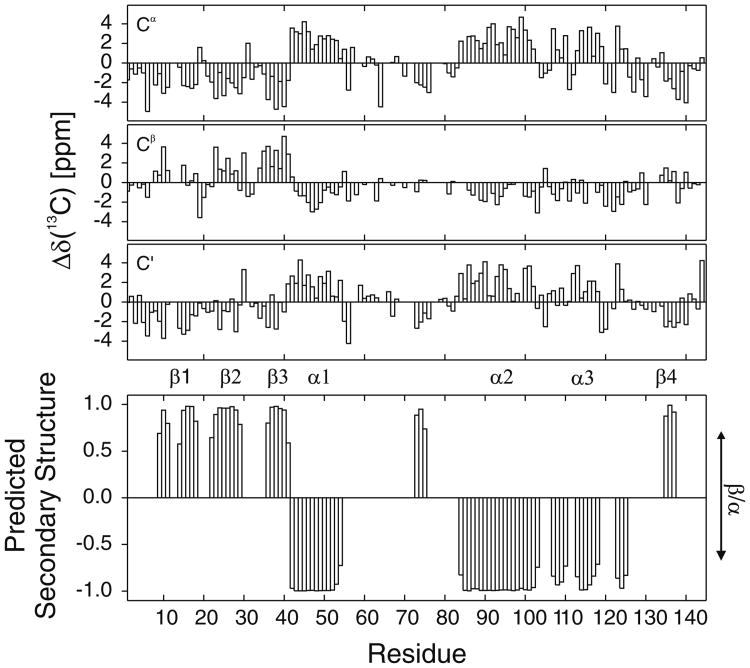
Backbone assignments for NOXO1β PX have been made for 125 of 131 non-proline residues (Fig. 1). We have assigned 95% of HN/N, 89% of Cα, 82% of Cβ and 90% of C′ atoms, 7/7 Gln sidechain NH2 resonances 2/2 Trp indole NH resonances. 13C chemical shift deviations from consensus CSI values (Wishart and Sykes 1994) and secondary structure predicted by TALOS+ (Shen et al. 2009) are shown in Fig. 2. Predicted secondary structure of NOXO1β PX is consistent with observed secondary structure in other solved PX domains. Backbone assignments for NOXO1β PX have been deposited in the BMRB with accession code 16749.
Fig. 1.
Assigned 15N-1H HSQC of NOXO1β PX. The spectrum was obtained with ∼ 500 μM NOXO1β PX in 100 mM NaPi, 100 mM NaCl, 0.1 mM EDTA, 5% (v/v) 2H5 glycerol, pH 6.9
Fig. 2.
Δδ (13C) for a Cα, b Cβ, and c C′ from CSI consensus values, referenced to DSS. d TALOS + predicted secondary structure of NOXO1β PX. Residue number is indicated along the x-axis and the secondary structure is indicated along the y-axis with a value of +1 for β-sheet and −1 for α-helix. The height of the bars indicate the probability of the residue being either a β-sheet (+1 maximum) or α-helix (−1 maximum). Major PX domain structural elements are labeled
Although NOXO1β PX is a small domain (∼16 kDa MW), perdeuteration of the protein was necessary to obtain backbone carbon assignments. With the 1H/15N/13C-labeled protein, only 152 total Cα peaks and 15 total Cβ peaks were observed in HNCA and HNCACB spectra, respectively. In contrast, the 2H/15N/13C NOXO1β PX yielded 253 Cα peaks and 182 Cβ peaks in HNCA and HNCACB spectra, sufficient to allow resonance assignment. We hypothesize that the poor magnetization transfer observed in the protonated protein was a result of a longer τc due to the addition of glycerol to the sample buffer and the need for running experiments at a cooler temperature (293 K).
While cryogenic probes are able to yield a marked increase in signal-to-noise relative to standard probes, they are sensitive to electrically conductive samples and yield reduced S/N in high-salt buffers. For NOXO1β PX solubility and stability, it was necessary for the sample buffer to contain high levels of both phosphate and salt. Sodium phosphate and sodium chloride were chosen over their potassium counterparts due to a slightly lower conductivity (Kelly et al. 2002). Voehler et al. (2006) reported that a smaller diameter NMR tube is a viable avenue for increasing S/N of high conductivity samples in a cryogenic probe, and we have used a 4 mm Shigemi tube in these experiments in order to decrease requisite 1H power and to increase S/N. Specifically, ∼ 180 μl of the protein sample was put into a 4 mm Shigemi tube which was placed inside a 5 mm flat bottomed tube containing D2O. Use of D2O in the outer tube allowed spectrometer lock without HN/DN exchange dilution effects to the protein sample. Although the sample volume within the detect coil decreases by ∼ 30%, the S/N of data acquired in a 4 mm tube is higher than that for a sample of the same concentration in a 5 mm tube. Utilizing a smaller tube also decreased the 1H 90° pulse from 15 μs to 10.2 μs.
Acknowledgments
This work is funded by NIH R01AI064609 to D.A.H. and R01AI022564 to L.C.M. The authors would like to thank Tom L. Leto (NIH) for the NOXO1 plasmid.
References
- Banfi B, Clark RA, Steger K, Krause K-H. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem. 2003;278:3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Cheng G, Lambeth JD. Alternative mRNA splice forms of NOXO1: differential tissue expression and regulation of Nox1 and Nox3. Gene. 2005;356:118–126. doi: 10.1016/j.gene.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- Geiszt M, Lekstrom K, Witta J, Leto TL. Proteins homologous to p47phox and p67phox support superoxide production by NAD(P)H oxidase 1 in colon epithelial cells. J Biol Chem. 2003;278:20006–20012. doi: 10.1074/jbc.M301289200. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Blevins RA. NMR view: a computer program for the visualization and analysis of NMR data. J Biomol NMR. 1994;4:603–614. doi: 10.1007/BF00404272. [DOI] [PubMed] [Google Scholar]
- Kelly AE, Ou HD, Withers R, DÖtsch V. Low-conductivity buffers for high-sensitivity NMR measurements. J Am Chem Soc. 2002;124:12013–12019. doi: 10.1021/ja026121b. [DOI] [PubMed] [Google Scholar]
- Sato TK, Overduin M, Emr SD. Location, location, location: membrane targeting directed by PX domains. Science. 2001;294:1881–1885. doi: 10.1126/science.1065763. [DOI] [PubMed] [Google Scholar]
- Shen Y, Delaglio F, Cornilescu G, Bax A. TALOS + : a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR. 2009;44:213–223. doi: 10.1007/s10858-009-9333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya R, Ueno N, Kami K, Taura M, Kohjima M, Izaki T, Nunoi H, Sumimoto H. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J Biol Chem. 2003;278:25234–25246. doi: 10.1074/jbc.M212856200. [DOI] [PubMed] [Google Scholar]
- Voehler MV, Collier G, Young JK, Stone MP, Germann MW. Performance of cryogenic probes as a function of ionic strength and sample tube geometry. J Magn Reson. 2006;183:102–109. doi: 10.1016/j.jmr.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart DS, Sykes BD. The 13C chemical-shift index: a simple method for the identification of protein secondary structure using 13C chemical-shift data. J Biomol NMR. 1994;4:171–180. doi: 10.1007/BF00175245. [DOI] [PubMed] [Google Scholar]