Abstract
Background
Celiac disease (CD) is common but under-diagnosed in the United States (US). A proposed quality guideline recommends that ≥4 specimens be submitted during duodenal biopsy. Adherence to this recommendation in clinical practice is unknown.
Objectives
To measure the number of specimens submitted during duodenal biopsy among patients throughout the U.S., and to determine the incremental diagnostic yield of adherence to the recommended number of specimens.
Design
Retrospective cohort study.
Methods
132,352 patients without known CD underwent duodenal biopsy submitted to a pathology laboratory operating in 43 states in the U.S. We used multivariate logistic regression to identify factors associated with submitting ≥4 specimens. We also compared prevalence of newly diagnosed CD in biopsies with ≥4 specimens to <4 specimens.
Results
Of the 132,352 patients who underwent biopsy (67% female, mean age 52.9), ≥4 specimens were submitted in 45,995 (35%). A modest increase in the proportion of biopsies with ≥4 specimens occurred after this guideline was proposed in 2006 (OR for 2009 vs. 2006 1.51 95%CI 1.22–1.88), but the rate of adherence in 2009 remained low at 37%. Among patients in whom the indication was malabsorption/suspected CD (n=3,261), adherence to this standard was only 39.5%. The probability of a new diagnosis of CD was increased when ≥4 specimens were submitted (1.8% vs. 0.7%, p<0.0001).
Limitations
Retrospective analysis lacking clinical follow-up. Guideline publication occurred during the study period, possibly influencing clinical practice and confounding results.
Conclusions
Although this proposed standard remains a subject of debate, adherence to submitting ≥4 specimens is low in the U.S. Adherence yields a diagnosis rate of 1.8%, a small absolute increase, but a doubling of the diagnosis rate of CD. Efforts to increase adherence are warranted.
Keywords: Celiac disease, quality indicators, pathology, endoscopy
INTRODUCTION
Celiac disease (CD) is an autoimmune disease that is triggered by the ingestion of gluten in genetically predisposed individuals.1 The prevalence of CD in the United States is 0.8%,2 but the vast majority of patients are not diagnosed, 3 even though the disease is associated with an increased risk of malignancy and mortality that are both reduced after diagnosis and treatment with a gluten-free diet. 4–11
Deficiencies in quality related to endoscopic evaluation may contribute to the low rates of diagnosis of CD in the United States. A multicenter endoscopy database study found that the majority of patients undergoing upper gastrointestinal endoscopy for such indications as anemia, iron deficiency, and weight loss did not have a duodenal biopsy done during the procedure.12
As the histopathologic features of CD are patchy, guidelines recommend that 4–6 biopsy specimens of the small bowel be submitted during upper endoscopy when CD is under consideration. 1, 13 These proposed quality guidelines have been borne out by studies of patients with known CD in which the sensitivity of duodenal biopsy was shown to decline when fewer than four specimens were examined. 14–15 The degree to which endoscopists adhere to such recommendations in clinical practice, and the diagnostic yield of adherence to this standard, have not been studied.
Using a large national pathology database spanning the first four years during which these recommendations appeared (2006 through 2009), we assessed adherence to these proposed guidelines. To determine the diagnostic yield of the recommendation to submit ≥4 specimens, we investigated the association between adherence to this standard and the proportion of patients with the finding of a new diagnosis of CD. We also aimed to identify patient and procedure-related factors associated with the submission of ≥4 specimens. In so doing, this study elucidates how a guideline plays out in clinical practice, both in terms of adherence to the recommendation, as well as the incremental yield of adherence.
METHODS
Study setting
The gastrointestinal pathology division of Caris Life Sciences (Irving, TX) is a specialized pathology laboratory that receives specimens from outpatient gastrointestinal endoscopy centers in 43 states throughout the United States, as well as the District of Columbia and Puerto Rico. Caris Life Sciencesmaintains a database of all patients who had endoscopic procedures in which a specimen was submitted to the laboratory. Patients and providers were de-identified in the preparation of the database for this analysis. For each specimen, the following is available: gender and age of the patient; procedure year, location, and provider; a summary of the clinical history; the endoscopic impressions; and histopathologic findings. For a subset of procedures, more detailed information on the indication for the examination and endoscopic findings are exported from the endoscopy report and are retrievable via free-text search.
Histopathologic criteria
In this laboratory, biopsies are interpreted by a group of gastrointestinal pathologists who share a common approach to biopsy evaluation and use a pre-determined approach to specimen handling, diagnostic criteria, and terminology.
Pathologic abnormalities of the duodenum in this laboratory are grouped in accordance with the classification developed by Marsh and Oberhuber. 16–17 As in a previous analysis of yield of duodenal biopsy according to indication using a subset of this data, 18 the following classification of outcomes was used: normal duodenal mucosa; duodenal intraepithelial lymphocytosis, as defined as >25 intraepithelial lymphocytes per 100 enterocytes, with or without crypt hypertrophy (equivalent to Marsh I or II lesions); blunted villi (Marsh IIIA); or flat villi (Marsh IIIB/C). Other recorded pathologic abnormalities include gastric metaplasia of the duodenal mucosa regardless of the presence of Helicobacter Pylori (“peptic duodenopathy” or “peptic duodenitis”)19 and mild intraepithelial lymphocytosis (as indicated by the presence of intraepithelial lymphocytes not meeting the threshold for Marsh I).
Case identification
We queried the database for all small intestinal specimens retrieved during upper endoscopy on individuals ≥18 years during the four year period spanning from January 1, 2006 to December 31, 2009. This query included any specimen labeled with the term “duodenum,” “duodenal,” “small bowel” or “small intestine” and excluded any specimen that contained the word “aspirate” or “aspiration” so as to exclude fluid analysis from the dataset.
For individuals who underwent more than one examination during this period we included only the first chronological examination. As the primary aim was to assess biopsy practices among patients without known CD, we excluded any patient with a known history of CD as described in the clinical indication field. To determine the number of duodenal biopsy specimens for each biopsy set we used a free text search of the pathologist’s description of each sample. When present, specimens from the duodenal bulb (identified either in the endoscopist’s report or the histopathologic interpretation) were included in the total number of specimens submitted. Cases in which the number of specimens submitted was not quantified (either not stated or characterized as “multiple”) were excluded.
Data analysis
We used the chi square test to assess the association between adherence to the recommendation of submitting ≥4 submitted specimens and the proportion of patients with pathological findings consistent with CD. As this dataset did not contain information on serological findings or follow-up clinical information, we defined a priori having a result of either blunted villi (Marsh IIIA) or flat villi (Marsh IIIB/C) as meeting the pathological definition of CD. For assessing the relationship between ordinal categories such as year or number of specimens and the pathologic diagnosis of CD, we used the Cochran-Armitage test for trend.
Given the possibility that gross endoscopic findings may be associated with both the number of specimens submitted and the probability of CD, we investigated the relationship between adherence to ≥4 specimens and CD while stratifying by gross endoscopic findings. We used the Breslow-Day test for homogeneity of odds ratios so as to assess whether gross appearance modifies this association.
Generalized estimating equation (GEE) multivariate logistic regression was used to determine factors associated with the submission of ≥4 specimens, adjusted for clustering by individual provider. We postulated that adherence to this practice was correlated with individual providers. Using GEE in this multivariate analysis takes such clustering into account when estimating the variance of hypothesized associations.
We used SAS version 9.1 (Cary, NC) for all statistical calculations. All p values presented are 2-sided. The Institutional Review Board of Columbia University Medical Center evaluated this study protocol and designated it as “Non-Human Subject Research” involving de-identified records.
RESULTS
A total of 150,155 procedures involving a duodenal/small bowel biopsy were submitted for histopathologic evaluation during the four-year period. Of these 150,155 procedures, 17,803 patients met at least one of the exclusion criteria: known CD at the start of the procedure (n=1,841), repeated procedures (n=9,531), and biopsies in which the number of specimens was not noted (n=7,871). The remaining sample size for the analysis was 132,352.
Characteristics of the 132,352 patients included in the analysis are enumerated in Table 1. It can be seen that 67% of the patients were female, and the mean (±SD) age was 52.9 (±16.7) years. Gross abnormalities such as scalloping and decreased folds accounted for less than 2% of all gross descriptions.
Table 1.
Characteristics of patients undergoing duodenal biopsy, not known to have celiac disease (n=132,352).
| Characteristic | Number of patients (%) |
|---|---|
|
| |
| Gender* | |
| Male | 44,256 (33.5) |
| Female | 88,016 (66.5) |
| Age (mean±SD) | 52.9 (±16.7) |
| Year of procedure | |
| 2006 | 20,209 (15.3) |
| 2007 | 27,224 (20.6) |
| 2008 | 38,765 (29.3) |
| 2009 | 46,154 (34.5) |
| Pathologic findings* | |
| Normal/unremarkable | 104,682 (79.1) |
| Duodenitis | 11,732 (8.9) |
| Mild intraepithelial lymphocytosis | 657 (0.5) |
| Intraepithelial lymphocytosis | 5944 (4.5) |
| Blunted villi | 819 (0.6) |
| Flat villi | 628 (0.5) |
| Indication* | |
| Anemia | 25,628 (19.4) |
| Diarrhea | 22,689 (17) |
| Dyspepsia | 17,854 (13.5) |
| Heartburn | 24,714 (18.7) |
| Weight loss | 10,464 (7.9) |
| Suspected celiac disease/malabsorption | 10,808 (8.2) |
| Gross findings* | |
| Normal | 21,804 (16.5) |
| Abnormal: | 8,411 (6.4) |
| Duodenitis | 5,936 (4.5) |
| Scalloping | 527 (0.4) |
| Decreased folds | 16 (0.01) |
| Erythematous | 2229 (1.7) |
Sum does not equal total sample size due to missing data points.
Marsh I or II lesions were noted in 5,944 individuals (4.5%), whereas Marsh IIIA was found in 819 (0.6%) and Marsh IIIB/C was found in 628 (0.5%). When defining a pathological diagnosis of CD as blunted or flat villi (Marsh IIIA/B/C), a total of 1,447 individuals (1.1%) were categorized as having CD.
The most common number of small bowel specimens submitted during upper endoscopy was 2 (histogram, Figure 1). The mean (±SD) number of specimens submitted was 3.1 (±1.6) and the median number submitted was 3. Of the 132,352 patients undergoing upper endoscopy with small bowel biopsy, ≥4 small bowel specimens were submitted in 45,995 (35%). The proportion of patients with ≥4 specimens submitted during endoscopy increased from 33.8% in 2006 to 37.2% in 2009 (p for trend <0.0001).
Figure 1.
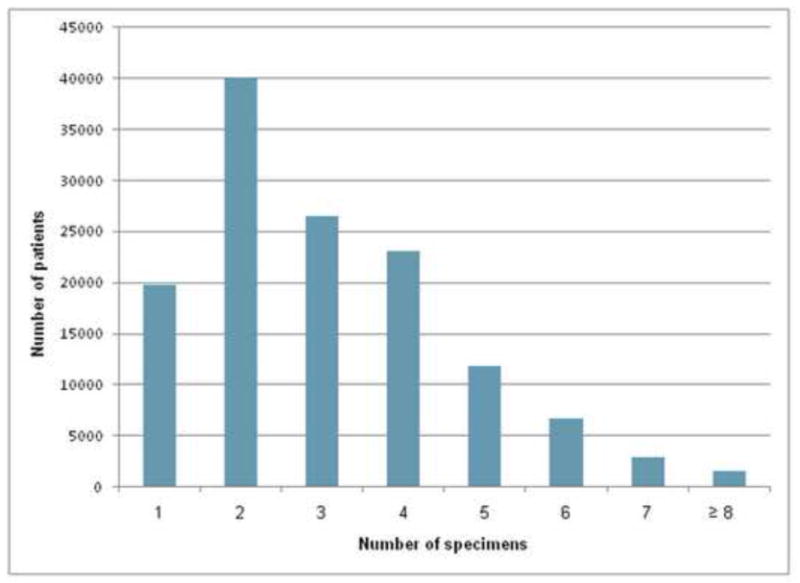
Histogram of number of specimens of small bowel biopsies among individuals not known to have celiac disease undergoing upper gastrointestinal endoscopy with duodenal biopsy (n=132,352)
Of the 45,995 individuals with ≥4 specimens submitted, a pathologic diagnosis of CD was present in 824 (1.8%), whereas among the 86,357 patients in whom <4 specimens were submitted, CD was present in 623 (0.7%, p<0.0001). When treated as a continuous variable, the number of specimens submitted was directly correlated with the probability of a pathologic diagnosis of CD (Figure 2). Biopsy of the duodenal bulb was performed in 10% of all patients; inclusion of a bulbar biopsy was not associated with an increased proportion of adherence to ≥4 small bowel specimens (p=0.4309), nor was it associated with an increased probability of a pathological diagnosis of CD (OR 0.93 95% CI 0.78–1.11, p=0.4373).
Figure 2.
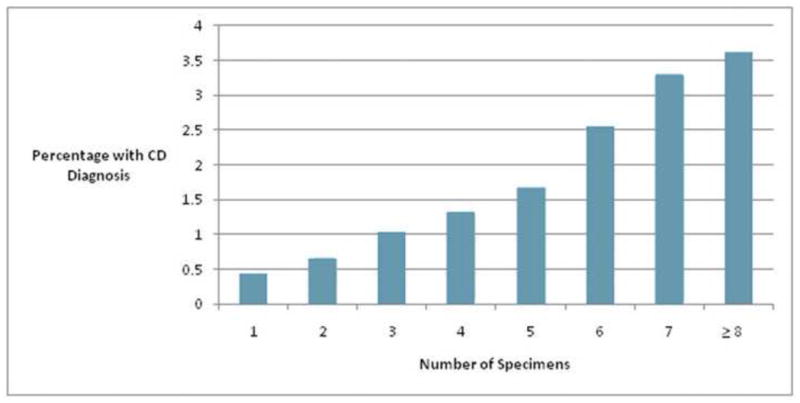
Number of specimens submitted and the probability of the diagnosis of celiac disease (Marsh IIIA/B/C, p for trend <0.0001).
Those with abnormal gross duodenal findings on endoscopy had an increased prevalence of CD (3.2% vs. 0.7%, OR 4.64, 95% CI 3.80–5.67). The relationship between adherence to the standard of ≥4 specimens submitted and a pathologic diagnosis of CD stratified by gross endoscopic findings is presented in Table 2. Gross endoscopic findings modified the association between number of specimens submitted and the prevalence of CD (Breslow-Day test for homogeneity of odds ratios: p=0.0015). This relationship was greater for those with abnormal gross findings (OR 3.67 95% CI 2.86–4.72) than for those with normal gross findings (OR 1.91 95% CI 1.38–2.63).
Table 2.
Association of number of specimens submitted with the diagnosis of celiac disease, stratified by gross appearance.
| Outcome | <4 specimens (%) | ≥4 specimens (%) | OR (95% CI) | p value |
|---|---|---|---|---|
|
| ||||
| All patients (n=132,352) | 86,357 (62.3) | 45,995 (34.8) | -- | -- |
|
| ||||
| Normal gross appearance (n=21,804) | 12,866 (59) | 8,938 (41) | ||
| Intraepithelial lymphocytosis | 579 (4.43) | 484 (5.42) | 1.24 (1.09–1.40) | 0.0009 |
| Blunted villi | 45 (0.35) | 57 (0.64) | 1.83 (1.24–2.71) | 0.0022 |
| Flat villi | 21 (0.16) | 30 (0.34) | 2.06 (1.18–3.60) | 0.0095 |
| Marsh IIIA/B/C | 66 (0.51) | 87 (0.97) | 1.91 (1.38–2.63)* | <0.0001 |
|
| ||||
| Abnormal gross appearance (n=8,411) | 5,755 (68.4) | 2,656 (31.6) | -- | |
| Intraepithelial lymphocytosis | 181 (3.15) | 128 (4.82) | 1.56 (1.24–1.96) | 0.0001 |
| Blunted villi | 61 (1.06) | 82 (3.09) | 2.97 (2.13–4.16) | <0.0001 |
| Flat villi | 41 (0.71) | 83 (3.13) | 4.50 (3.08–6.55) | <0.0001 |
| Marsh IIIA/B/C | 102 (1.77) | 165 (6.21) | 3.67 (2.86–4.72)* | <0.0001 |
Breslow-Day test for homogeneity of odds ratios: chi-square 10.0245, df=1; p=0.0015
Among physicians who submitted duodenal biopsies to this laboratory in at least 10 upper endoscopies during the study period (n=1,243), the adherence rate to the proposed standard varied widely (mean adherence rate 38%, standard deviation 27.8%).19% of providers had an adherence rate between 0 and 10%.
Adherence varied by indication (Table 3), with highest rates among examinations performed for evaluation of diarrhea (43.9%) and lowest levels of adherence among procedures in which the indication was heartburn/GERD (30.0%). Among the different indications, the diagnostic yield of submitting ≥4 specimens was variable (Table 3) but remained significantly associated with increased odds of diagnosing CD for every indication. Of note, among individuals whose only indication was malabsorption or suspected CD (n=3,261), adherence to this quality standard occurred in 38.5% of examinations.
Table 3.
Submission of ≥4 versus <4 specimens according to indication, and odds ratio of a diagnosis of CD when ≥4 specimens are submitted.
| Indication | Number (%) with ≥4 specimens submitted | OR for diagnosis of CD when ≥4 specimens submitted |
|---|---|---|
| Anemia | 9,695 (37.8) | 2.65 (2.13–3.30) |
| Diarrhea | 9,489 (43.9) | 1.86 (1.46–2.37) |
| Dyspepsia | 4,627 (33.0) | 2.94 (1.94–4.43) |
| Heartburn | 4,923 (30.0) | 1.84 (1.33–2.55) |
| Weight loss | 2,091 (38.8) | 1.83 (1.08–3.11) |
| Suspected celiac disease/malabsorption | 1,256 (38.5) | 7.37 (4.70–11.57) |
The results of GEE multivariate analysis of factors associated with the submission of ≥4 specimens during upper endoscopy while adjusting for clustering by individual provider are shown in Table 4. Patient age was associated with decreased odds of adherence, with individuals over 80 having the lowest odds of adherence compared to those younger than 30 (OR 0.67 95% CI 0.57–0.78). Clinical indication for endoscopy was significantly associated with the number of specimens submitted, with increased adherence to submitting ≥4 specimens among individuals with diarrhea (OR 1.20 95% CI 1.10–1.30) and malabsorption (OR 1.42 95% CI 1.10–1.85) and decreased adherence among patients undergoing endoscopy for dyspepsia (OR 0.78 95% CI 0.72–0.86) and heartburn (OR 0.78 95% CI 0.70–0.87). Abnormal gross findings were associated with decreased odds of submitting ≥4 specimens (OR 0.75 95% CI 0.69–0.81). The modest temporal trend of increased adherence to submitting ≥4 specimens remained significant in this multivariate analysis (OR for 2009 compared to 2006: 1.51, 95% CI 1.22–1.88).
Table 4.
GEE multivariate analysis of factors associated with the submission of ≥4 specimens, adjusting for clustering by individual provider.
| Characteristic | Odds Ratio | 95% CI | p value |
|---|---|---|---|
| Age (years) | <0.0001 | ||
| <30 | 1.0 | -- | -- |
| 30–39 | 1.08 | 0.79–1.48 | 0.6208 |
| 40–49 | 0.96 | 0.85–1.09 | 0.5572 |
| 50–59 | 0.94 | 0.84–1.05 | 0.2881 |
| 60–69 | 0.85 | 0.76–0.96 | 0.0062 |
| 70–79 | 0.82 | 0.73–0.92 | 0.0011 |
| ≥80 | 0.67 | 0.57–0.78 | <0.0001 |
| Gender | |||
| Male | 1.0 | -- | -- |
| Female | 1.05 | 0.99–1.12 | 0.1207 |
| Indication | <0.0001 | ||
| Anemia | 1.0 | -- | -- |
| Diarrhea | 1.20 | 1.10–1.30 | <0.0001 |
| Dyspepsia | 0.78 | 0.72–0.86 | <0.0001 |
| Heartburn | 0.78 | 0.70–0.87 | <0.0001 |
| Weight loss | 1.04 | 0.91–1.19 | 0.5674 |
| Suspected celiac disease/malabsorption | 1.42 | 1.10–1.85 | 0.008 |
| Gross findings | |||
| Normal | 1.0 | -- | -- |
| Abnormal | 0.75 | 0.69–0.81 | <0.0001 |
| Year | 0.0095 | ||
| 2006 | 1.0 | -- | -- |
| 2007 | 1.28 | 1.03–1.60 | 0.0288 |
| 2008 | 1.38 | 1.11–1.72 | 0.0035 |
| 2009 | 1.51 | 1.22–1.88 | 0.0002 |
Acronyms
CD: Celiac Disease
DISCUSSION
In this analysis of a national pathology database of duodenal biopsies, 35% of patients had ≥4 specimens submitted during upper endoscopy., Adherence to this proposed standard 1, 13 remained low even among those patients with malabsorption/suspected CD, with fewer than 40% of such patients having ≥4 specimens submitted. Regardless of indication, adherence to this proposed quality standard was associated with an increased rate of CD diagnosis.
This study evaluated the recommended practice of submitting ≥4 specimens when a diagnosis of CD is under consideration. 1, 13 This proposed guideline is new and subject to debate. As one recent review stated, “the optimal method of obtaining biopsies in patients with celiac disease is controversial.”20 This proposed guideline has not been established prospectively, and this recommendation stemmed instead from the observation that the histopathologic abnormalities of CD are patchy and can be missed entirely if an insufficient quantity of specimens is submitted. The recommendation was subsequently supported by a single-center retrospective study of 93 patients with CD, which found that four specimens led to a positive diagnosis in 100% of patients, whereas two specimens were diagnostic in only 90% of patients. 14 Those authors concluded that at least 4 duodenal biopsy specimens should be taken to rule out CD. A second study, investigating 56 patients with known CD, 15 found that three biopsy specimens were sufficient as long as one specimen was obtained from the duodenal bulb; however, five biopsy specimens were necessary to recognize the most severe extent of villous atrophy. These studies are limited by their small sample size and single-center settings.
To our knowledge, no previous study has evaluated the diagnostic yield of submitting ≥4 specimens for patients without known CD in accordance with these proposed guidelines. The incremental yield of submitting ≥4 specimens has not been evaluated in a population undergoing endoscopy for a variety of indications, in which only a small proportion of patients will have celiac disease, and in which such patients may have a more patchy distribution of pathologic abnormalities. Moreover, adherence was low even to those who consider ≥3 specimens to be satisfactory,20 as the most common submitted number of specimens was 2 (Figure 1).
These results indicate that this proposed standard appears to be slowly diffusing into clinical practice, as the proportion of individuals undergoing duodenal biopsy who have ≥4 specimens submitted has increased between the years 2006 and 2009. Nevertheless, this practice was performed in the minority of patients even in 2009, when only 37% of patients had ≥4 specimens submitted. Guidelines are adopted by physicians at variable rates, and at times this variability creates new racial or socioeconomic disparities.21 In our study we did not have access to socioeconomic or racial data to determine whether these individual patient characteristics were associated with the submission of the recommended number of specimens.
In this study, the incremental diagnostic yield of submitting ≥4 specimens was large, as the proportion of patients diagnosed with CD was doubled when ≥4 specimens were submitted. This incremental yield varied by indication, and was greatest when the indication was malabsorption/suspected CD (OR 7.37 95% CI 4.70–11.57) or anemia (OR 2.65 95% CI 2.13–3.30). However, submitting ≥4 specimens also increased the diagnostic yield of CD even when the indication was GERD (OR 1.84 95% CI 1.33–2.55). We therefore conclude that although the increased diagnostic yield of adherence varies in magnitude, it is present and should be adhered to regardless of indication.
Why were ≥4 specimens submitted only 35% of the time? One possibility is that this proposed guidline is new and not fully accepted.,1, 13,20Another possibility is that knowledge of the appropriate amount of specimens to submit is not yet widespread. This explanation is supported by the finding that the submission of ≥4 specimens has modestly increased over time (OR for 2009 vs. 2006 1.58 95% CI 1.27–1.97). A third contributing factor is the extra time involved in submitting additional specimens. The most common number of specimens submitted in this dataset was 2 (Figure 1). Two specimens can usually be collected using one pass of the biopsy forceps. A second pass of the forceps, done for the purpose of collecting additional specimens, increases the length of the procedure. Although the amount of time for an additional pass of the biopsy forceps for additional biopsies is low (approximately 1 minute), the incremental yield of this additional time taken was heretofore unknown. Given the high incremental yield in the present analysis (resulting in a doubling of the proportion of patients with a pathological diagnosis of CD), the proposed standard of submitting ≥4 specimens appears to be justified.
We observed a marked variability between individual endoscopists with regard to the proportion of examinations in which the recommended number of specimens was submitted. Although the mean adherence rate among providers was 38.3%, the most common percentage adherence per individual was between 0 and 10%. The wide variability in adherence to this recommendation is reminiscent of the variability of a familiar quality indicator in gastroenterology, the adenoma detection rate in screening colonoscopy. 22 The discovery of that variability and associated predictive factors such as colonoscope withdrawal time,23 has led to a focus on high quality colonoscopy as a priority for the profession of gastroenterology. 24 The findings in the present study, of low adherence to a recommendation in the face of a high diagnostic yield of submitting ≥4 specimens, should spur efforts to increase adherence to this standard.
This study has several limitations. This was a retrospective analysis of a pathology tissue database, which has nevertheless yielded high-quality analyses of gastrointestinal epidemiology and quality measures.25–26 In this database we did not have access to key variables that influence the likelihood of CD, such as data regarding family history of CD or serology results in all the patients. Those with positive CD serologies, (i.e., noted in the clinical indication field), were classified in the “suspected CD” indication category; this variable was included in the multivariate analysis. Information regarding the type of sedation used during the procedure and degree of sedation, which may have impacted the ability to obtain ≥4 specimens, was not available. The diagnosis of CD in this study was based strictly on histopathologic findings, and reliance on histology alone has been criticized for its lack of specificity.27 For this reason, we considered only the most severe histopathologic changes (Marsh III lesions) as CD, excluding the increasingly common report of increased intraepithelial lymphocytosis, so as to maximize the specificity of the outcome in this analysis. Certain providers may have a particular interest or expertise in CD, and thus are more likely to submit ≥4 specimens. We therefore performed GEE multivariate analysis, adjusting for clustering by individual provider. The fact that the association between the number of specimens submitted and the diagnosis of CD is magnified when examining those high pre-test probability strata (such as gross abnormal appearance or indication of suspected CD/malabsorption) supports the argument that the relationship between submitting ≥4 specimens and an increased probability of CD is causal and robust.
We conclude that the minority of individuals undergoing upper gastrointestinal endoscopy with duodenal biopsy in the United States has ≥4 specimens submitted during the procedure. Even among those patients with an indication for endoscopy of malabsorption or suspected CD (including positive serologies), adherence to this proposed standard occurred in only 38.5% of examinations. The additional diagnostic yield of submitting ≥4 specimens varies by indication and gross appearance, but is in all cases associated with an increased probability of a diagnosis of CD. Given the high incremental yield of submitting 4 or more specimens, efforts to increase adherence to this standard are warranted.
Acknowledgments
Financial Support
Robert M. Genta is employed by Caris Life Sciences, Irving, TX.
Funding sources: None
Footnotes
No other potential conflicts of interest exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–43. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 2.Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286–92. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 3.Murray JA, Van Dyke C, Plevak MF, Dierkhising RA, Zinsmeister AR, Melton LJ., 3rd Trends in the identification and clinical features of celiac disease in a North American community, 1950–2001. Clin Gastroenterol Hepatol. 2003;1:19–27. doi: 10.1053/jcgh.2003.50004. [DOI] [PubMed] [Google Scholar]
- 4.Ludvigsson JF, Montgomery SM, Ekbom A, Brandt L, Granath F. Small-intestinal histopathology and mortality risk in celiac disease. JAMA. 2009;302:1171–8. doi: 10.1001/jama.2009.1320. [DOI] [PubMed] [Google Scholar]
- 5.Green PH, Fleischauer AT, Bhagat G, Goyal R, Jabri B, Neugut AI. Risk of malignancy in patients with celiac disease. Am J Med. 2003;115:191–5. doi: 10.1016/s0002-9343(03)00302-4. [DOI] [PubMed] [Google Scholar]
- 6.West J, Logan RF, Smith CJ, Hubbard RB, Card TR. Malignancy and mortality in people with coeliac disease: population based cohort study. BMJ. 2004;329:716–9. doi: 10.1136/bmj.38169.486701.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Logan RF, Rifkind EA, Turner ID, Ferguson A. Mortality in celiac disease. Gastroenterology. 1989;97:265–71. doi: 10.1016/0016-5085(89)90060-7. [DOI] [PubMed] [Google Scholar]
- 8.Anderson LA, McMillan SA, Watson RG, et al. Malignancy and mortality in a population-based cohort of patients with coeliac disease or “gluten sensitivity”. World J Gastroenterol. 2007;13:146–51. doi: 10.3748/wjg.v13.i1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Askling J, Linet M, Gridley G, Halstensen TS, Ekstrom K, Ekbom A. Cancer incidence in a population-based cohort of individuals hospitalized with celiac disease or dermatitis herpetiformis. Gastroenterology. 2002;123:1428–35. doi: 10.1053/gast.2002.36585. [DOI] [PubMed] [Google Scholar]
- 10.Viljamaa M, Kaukinen K, Pukkala E, Hervonen K, Reunala T, Collin P. Malignancies and mortality in patients with coeliac disease and dermatitis herpetiformis: 30-year population-based study. Dig Liver Dis. 2006;38:374–80. doi: 10.1016/j.dld.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Card TR, West J, Holmes GK. Risk of malignancy in diagnosed coeliac disease: a 24-year prospective, population-based, cohort study. Aliment Pharmacol Ther. 2004;20:769–75. doi: 10.1111/j.1365-2036.2004.02177.x. [DOI] [PubMed] [Google Scholar]
- 12.Harewood GC, Holub JL, Lieberman DA. Variation in small bowel biopsy performance among diverse endoscopy settings: results from a national endoscopic database. Am J Gastroenterol. 2004;99:1790–4. doi: 10.1111/j.1572-0241.2004.40176.x. [DOI] [PubMed] [Google Scholar]
- 13.Rostom A, Murray JA, Kagnoff MF. American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease. Gastroenterology. 2006;131:1981–2002. doi: 10.1053/j.gastro.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Pais WP, Duerksen DR, Pettigrew NM, Bernstein CN. How many duodenal biopsy specimens are required to make a diagnosis of celiac disease? Gastrointest Endosc. 2008;67:1082–7. doi: 10.1016/j.gie.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Hopper AD, Cross SS, Sanders DS. Patchy villous atrophy in adult patients with suspected gluten-sensitive enteropathy: is a multiple duodenal biopsy strategy appropriate? Endoscopy. 2008;40:219–24. doi: 10.1055/s-2007-995361. [DOI] [PubMed] [Google Scholar]
- 16.Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’) Gastroenterology. 1992;102:330–54. [PubMed] [Google Scholar]
- 17.Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185–94. doi: 10.1097/00042737-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Carmack SW, Genta RM. The diagnostic value of the duodenal biopsy: a clinico-pathologic analysis of 28,000 patients. Dig Liver Dis. 2010;42:485–9. doi: 10.1016/j.dld.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Genta RM, Kinsey RS, Singhal A, Suterwala S. Gastric foveolar metaplasia and gastric heterotopia in the duodenum: no evidence of an etiologic role for Helicobacter pylori. Hum Pathol. 2010;41:1593–600. doi: 10.1016/j.humpath.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Yantiss RK, Odze RD. Optimal approach to obtaining mucosal biopsies for assessment of inflammatory disorders of the gastrointestinal tract. Am J Gastroenterol. 2009;104:774–83. doi: 10.1038/ajg.2008.108. [DOI] [PubMed] [Google Scholar]
- 21.McBride RB, Lebwohl B, Hershman DL, Neugut AI. Impact of socioeconomic status on extent of lymph node dissection for colon cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:738–45. doi: 10.1158/1055-9965.EPI-09-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen SC, Rex DK. Endoscopist can be more powerful than age and male gender in predicting adenoma detection at colonoscopy. Am J Gastroenterol. 2007;102:856–61. doi: 10.1111/j.1572-0241.2006.01054.x. [DOI] [PubMed] [Google Scholar]
- 23.Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533–41. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 24.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104:739–50. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 25.Sonnenberg A, Lash RH, Genta RM. A National Study of H. pylori Infection in Gastric Biopsy Specimens. Gastroenterology. 2010 doi: 10.1053/j.gastro.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 26.Abrams JA, Kapel RC, Lindberg GM, et al. Adherence to biopsy guidelines for Barrett’s esophagus surveillance in the community setting in the United States. Clin Gastroenterol Hepatol. 2009;7:736–42. doi: 10.1016/j.cgh.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hassall E. Not everything is celiac disease. Gastrointest Endosc. 2010;72:569–71. doi: 10.1016/j.gie.2010.02.030. [DOI] [PubMed] [Google Scholar]


