Abstract
Objectives/Introduction
Demand for nursing home (NH) care by patients with endstage renal disease (ESRD) is likely to increase with growing numbers of older adults initiating chronic dialysis. We completed a systematic review to summarize the literature on NH residents with ESRD.
Methods
MEDLINE, CINAHL, EMBASE, and relevant conference proceedings were searched to identify articles using the following MESH terms or related key words in the title or abstract: “residential facilities”, “renal dialysis”, “renal replacement therapy”, and “chronic kidney failure”. We selected case control, cohort studies, and clinical trials that included older adults with ESRD (defined as those receiving chronic dialysis or those with Stage 5 chronic kidney disease (CKD)) living in residential care facilities. We abstracted information on study design, quality, and results.
Results
Of 198 unique citations identified by the search strategy, 14 articles met eligibility criteria. The majority of articles were multicenter studies that were conducted in the 1990s. One study focused on patients with Stage 5 CKD, and the remaining thirteen studies focused on chronic dialysis patients of which eight studies included only peritoneal dialysis (PD) patients, four studies included both PD and hemodialysis (HD) patients, and one study included only HD patients. All studies were observational, no clinical trials were identified, and study design limitations and heterogeneity within study populations were common. Summarizing results across these studies suggests that NH residents with ESRD have limited survival, particularly early after dialysis initiation. Functional impairment is highly prevalent in this population and independently associated with poor outcomes.
Conclusions
NH residents with ESRD appear to be a particularly vulnerable population, but current information on their prevalence, characteristics, and outcomes is limited. Further research is needed to provide a better understanding of modifiable predictors of survival and functional decline in this population.
Keywords: renal dialysis, skilled nursing facilities, aged
Objectives/Introduction
Adults aged 75 years and older comprise the fastest growing segment of the population initiating dialysis each year1. Many of these older adults with end-stage renal disease (ESRD) have a high burden of comorbidity and disability and are at high risk for nursing home (NH) placement. In addition, it is estimated that nearly half of all long-term care NH residents have chronic kidney disease (CKD)2. For both of these reasons, the projected need for short or long term NH care among older adults with ESRD is expected to increase. Patients with ESRD often have disease-specific management needs, and are at especially high risk for complications and functional decline after acute hospitalizations3. Thus, caring for frail patients with ESRD may be particularly challenging for NH staff4.
To better understand what information is currently available about NH residents with ESRD and their outcomes, we conducted a systematic review to answer the following questions: (1) “What is the prevalence of ESRD among residents of NHs?” (2) “What outcomes have been described in NH residents with ESRD?” and (3) “What are the predictors or interventions that influence these outcomes? “
Methods
Search Strategy
We searched MEDLINE, CINAHL, and EMBASE for articles about patients with ESRD who reside in NHs on March 27, 2012. The following medical subject headings (MESH) search terms were combined: “residential facilities” AND “renal dialysis” (or “dialysis”, “renal replacement therapy”) OR “chronic kidney failure”. Articles were included if the words “end stage renal disease”, “end-stage kidney disease”, “dialysis”, or “nursing home” were found in the title or abstract text. Articles were limited to the English language. These key words were also used to identify studies in conference proceedings from the American Medical Directors Association (2010–2012), the American Geriatrics Society (2010–2012), and the American Society of Nephrology (2009–2011). Finally, we hand searched reference lists of studies that met the inclusion criteria.
Selection of Studies
We sought to identify case-control studies, cohort studies, and clinical trials with study populations including patients with ESRD located in NHs worldwide. The ESRD population of interest included dialysis patients or National Kidney Foundation Stage 5 CKD patients who were not receiving dialysis because these groups have similar prognoses. In order to be inclusive, we chose not to specify intervention type, comparison group, and outcomes of interest. Two reviewers independently screened the titles and abstracts to identify studies that met our inclusion criteria. Studies with CKD patients were included if results were reported by CKD stage to allow for extraction of relevant data on patients with Stage 5 CKD. Studies without abstracts were excluded. We then reviewed the full text of the remaining studies. We excluded case reports, case-series, and literature reviews.
Data extraction and Synthesis
With a structured abstraction tool, two reviewers independently extracted information on study design, population, intervention (or exposure), comparison group, outcomes of interest, and significant predictors of those outcomes (if assessed). We assessed the validity of study findings using published criteria for observational studies5. We compared the independently abstracted information and all discrepancies were resolved by consensus.
Data tables were constructed with studies grouped by research question and design. We qualitatively evaluated individual studies for similarities and differences in study design, results, and methodological issues. Then, we synthesized data for specific outcomes commonly measured across the studies. A meta-analysis could not be performed due to heterogeneity in study design, participants, and outcome measurement.
Results
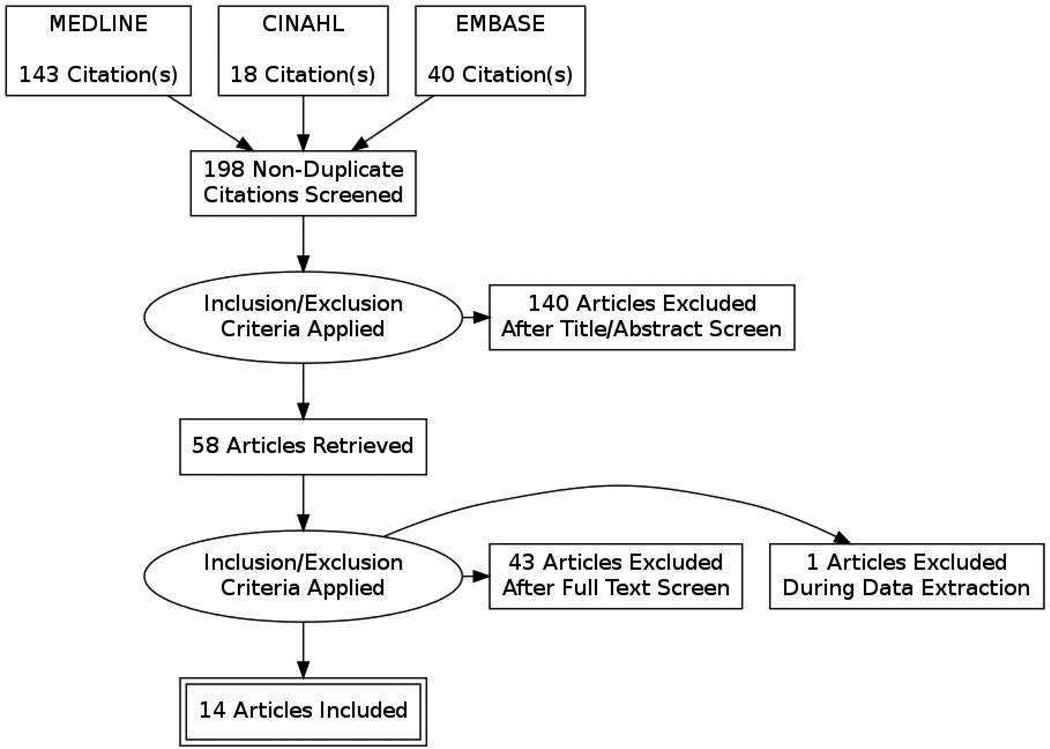
Our search identified 198 unique citations, of which 58 underwent full text review, and 14 met all inclusion criteria (Figure 1). We excluded one study during the data extraction phase, because all subjects were included in a subsequent, larger study captured in our review6,7.
Figure 1. Flow Diagram of study selection.
The characteristics and main findings of included studies are described in Table 1. The majority of studies (9 of 14) encompassed study periods between 1985 and 2000. Most studies had multicenter cohorts from various NHs located in the United States, while two studies examined outcomes in NHs located in Toronto, Canada8,9. Mean subject age ranged from 63 to 83 years. Eight of the studies focused only on residents receiving peritoneal dialysis (PD), one study included patients receiving hemodialysis (HD), and four studies included patients receiving either HD or PD. One study reported results specific to CKD Stage 5 patients who were not receiving dialysis, but this study only provided data regarding prevalence of Stage 5 CKD and no clinical outcomes10. No clinical trials were identified and all studies were observational. Nine studies used a prospective cohort design to follow patients for clinical outcomes over time. Of the remaining studies, there were four retrospective chart reviews and one cross-sectional study.
Table 1. Characteristics of Studies.
| Reference | Study Design |
Cohort and Facility Characteristics |
Outcome Measures | Main Findings |
|---|---|---|---|---|
| Anderson et al.11 | Prospective | 228 PD and HD | Prevalence; | Point – prevalence: 0.4–0.6% of ESRD network population lived in NH |
| (1993) | cohort | patients | Survival rate; | |
| Mean age= 65.5 | Discharge Disposition) | Survival rates from Date of NH admission: | ||
| 6 months- 56% | ||||
| Multiple NHs in 4 | 12 months- 42% | |||
| states: VA, WV, MD, | ||||
| DC | 12 month survival (Discharged vs. not discharged): 66% vs. 36%, p<0.05 | |||
| Predictors of poor survival: | ||||
| age>75 (reference (ref): age< 75): HR 1.7 (1.4–2.1) | ||||
| PD (ref: HD): HR 2.0 (1.6–2.6) | ||||
| ADL <8 (ref: ADL>8) HR 1.7 (1.5–2.5) | ||||
| Discharge home:17% | ||||
| Anderson et al.6 (1997) | Prospective | 109 PD patients | Survival rate; | Survival rates from Date of NH admission: |
| cohort | Mean age = 62.7 | Discharge Disposition; | 6 months - 52% | |
| Dialysis-Related Outcomes | 12 months -37% | |||
| A 233-bed academic | ||||
| nursing home in | Predictors of poor survival: | |||
| Baltimore, MD | Age >75 (ref: age<75): HR 4.75 ( 2.56–8.86); | |||
| ADL (ref: 1 ADL point lower): HR 0.91 (0.87–0.96) | ||||
| CAD: HR 1.65 (1.09–2.5) | ||||
| Decubitus Ulcer: HR 1.84 (1.15–2.92) | ||||
| Discharge home: 37%; | ||||
| Hospitalization rate: 22.4 days per patient-year | ||||
| Predictors of discharge home: | ||||
| Rehab admission: OR 19.14 (5.16, 71) | ||||
| ADL score >8 (ref: ADL score <8): OR 4.16 (1.37–12.66) | ||||
| Peritonitis rate:1 episodes per 10 patient-months | ||||
| Exit-site infection rate: 1 episode per 60 patient-months | ||||
| Anderson et al.14 | Prospective | 109 PD patients | Survival rate; | Survival rates from Date of NH admission: |
| (2006) | cohort | Mean age = 62.7 | Advance Care Planning | 6 months: 51% |
| 12 months: 37% | ||||
| A 233-bed academic | ||||
| nursing home in | 12 month survival (DNAR vs. no DNAR): 21% vs. 44%, p<0.02 | |||
| Baltimore, MD | ||||
| Proportion with advance directives: 99% | ||||
| DNAR: 36%; DNH: 13% | ||||
| Predictors of DNAR status: | ||||
| Older age: OR 1.04 (1.0007, 1.09) | ||||
| CAD: OR 4.24 (1.49, 12.02) | ||||
| Lower ADL score: OR 1.22 (1.08, 1.38) | ||||
| Carey et al.12 | Prospective | 84 PD patients | Survival rate; | Cumulative Survival Rates from Date of NH admission: |
| (2001) | cohort | Mean age = 65.3 | Discharge Disposition; | 6 months – 50% |
| Dialysis-Related Outcomes | 12 months – 40% | |||
| 10 community based | ||||
| facilities in | Discharge home:37%; | |||
| New Haven, CT | Hospitalization rate: 44.6 days per patient-year | |||
| Peritonitis Rate: 1 episode per 9.6 patient-months | ||||
| Dharmarajan et al.10 | Cross- | 323 NH residents | Prevalence | Prevalence of Stage 5 CKD: 0.9% |
| (2010) | sectional | Mean age = 83.4 | ||
| study | ||||
| A 350 bed urban | ||||
| long-term care facility | ||||
| in | ||||
| Bronx, NY | ||||
| Hariprasad et al.19 | Retrospective | 9 PD patients | Dialysis-Related Outcomes | Peritonitis Rate: 1 episode in 131 patient-months |
| (1995) | chart review | Mean age = NR | ||
| A community based | ||||
| facility in Hornell, NY | ||||
| Kurella Tamura et al.16 | Prospective | 3702 incident dialysis | Survival rate; | Survival Rates from Date of HD initiation: |
| (2009) | cohort | patients (PD and HD) | Functional Impairment | 6 months - 50% |
| Mean age = 73.4 | 12 months - 25% | |||
| National sample of | Maintenance of baseline functional status: | |||
| NHs identified by | 6 months - 27% | |||
| linkage of MDS and | 12 months -13% | |||
| USRDS datasets | ||||
| Predictors of maintenance of functional status: | ||||
| Older age (per 10yr): OR 0.6 (0.5–0.7) | ||||
| White race: OR 0.7 (0.5–0.9) | ||||
| Cerebrovascular disease: OR 0.7 (0.5–0.9) | ||||
| Dementia: OR 0.6 (0.4–0.9) | ||||
| Hospitalization at start: OR 0.6(0.5–0.8) | ||||
| Albumin <3.5g/dL: OR 0.6(0.4–0.8) | ||||
| Kurella Tamura et al. 18 | Prospective | 2402 incident dialysis | Cognitive Impairment | Proportion of patients with early dialysis initiation = 18% |
| (2010) | cohort | patients (PD and HD) | Dialysis-Related Outcomes | |
| Mean age = 73 | Predictors of early dialysis initiation: | |||
| Edema: OR 1.54 (1.24–1.92) | ||||
| National sample of | Dyspnea: OR 1.95 (1.40–2.73) | |||
| NHs identified by | Moderate to severe cognitive impairment: OR 0.84 (0.67, 1.04) | |||
| linkage of MDS and | Decline in cognitive function: OR 1.48 ( 1.17–1.88) | |||
| USRDS datasets | ||||
| Meddy 17 | Prospective | 32 PD patients | Discharge Disposition; | Discharge from NH: 6% |
| (1995) | cohort | Median age = 71 | Dialysis-Related Outcomes | Peritonitis rate: 1 episode per 15 patient-months |
| A 220 bed for-profit | ||||
| nursing home in | ||||
| Brooklyn NY | ||||
| Reddy et al.13 | Prospective | 271 HD patients | Survival rate; | Survival rates from Date of NH admission: |
| (2007) | cohort | Mean age = 70.5 | Discharge Dispositoin | 6 months - 38% |
| 12 months -26% | ||||
| 5 NHs in Chicago, IL | Median = 4.1 mo | |||
| Survival rates from Date of HD initiation: | ||||
| 6 months - 75% | ||||
| 12 months - 66% | ||||
| Median = 3.4 mo | ||||
| Median Survival by dialysis vintage: | ||||
| New (< 3 months of dialysis): 3.4 months | ||||
| Intermediate (3–12 months of dialysis): 3.5months | ||||
| Established (> 12 months of dialysis): 5.1 months (vs. New, p<0.01; vs. | ||||
| Intermediate, p=0.01) | ||||
| Discharge from NH: 37% | ||||
| New vs. Established: 28% vs. 48%, p=0.01 | ||||
| Taskapan et al. 9 | Retrospective | 38 PD patients | Survival rate; | Survival rates from Date of NH admission: |
| (2010) | chart review | Mean age = 77.3 | Dialysis-Related Outcomes | 6 months – 89.5% |
| 12 months – 60.5% | ||||
| 3 NHs in | ||||
| Scarborough, Toronto | Survival rates from Date of PD initiation: | |||
| 6 months –89.5 % | ||||
| 12 months – 76.3% | ||||
| Peritonitis Rate: 1 episode in 40.3 patient-months | ||||
| Exit-site infection rate: 1 episode per 42.4 patient-months | ||||
| Troidle et al.20 | Retrospective | 77 PD patients | Dialysis-Related Outcomes | Peritonitis rate: 1 episode per 19.8 patient-months |
| (2008) | chart review | Mean age = 66 | ||
| Characteristics of peritonitis population (vs. no peritonitis) : | ||||
| Unknown number of | Proportion of African-American race: 41% vs. 23%, p < 0.05 | |||
| Nursing homes close | Average length of NH stay: 106 vs. 77 days, p < 0.05 | |||
| to a dialysis unit | ||||
| located in New | ||||
| Haven, CT | ||||
| USRDS15 | Prospective | 3,748 incident dialysis | Survival rate; | Survival rates from Date of HD initiation: |
| (2011) | cohort | patients (PD and HD) | Functional Impairment | 6 months - 26%; |
| Mean age = 84.2 | 12 months – 14% | |||
| National sample of | Predictors of Death at 6 months(among all NH residents): | |||
| NHs identified by | Recent Dialysis Initiation (ref: non-CKD NH residents): HR 2.54 (2.36- | |||
| linkage of MDS and | 2.73) | |||
| USRDS datasets | ||||
| Maintenance of baseline functional status: | ||||
| 6 months - 10% | ||||
| 12 months - 6% | ||||
| Maintenance of baseline cognitive scores: | ||||
| 6 months – 17% | ||||
| 12 months – 10% | ||||
| Predictor of maintenance of functional status at 6 months: | ||||
| Recent Dialysis Initiation (ref: non-CKD NH residents): OR 0.69 (0.69 | ||||
| 0.58–0.82) | ||||
| Wang et al. 8 | Retrospective | 8 PD patients | Dialysis-Related Outcomes | Peritonitis rate: |
| (2002) | chart review | Mean age = 77.3 | 1 episodes per 7.5 patient-months | |
| A community based | ||||
| facility in Toronto | ||||
Abbreviations: PD, peritoneal dialysis; HD, hemodialysis; NH, nursing home; ESRD, end-stage renal disease; ADL, activities of daily living; HR, hazard ratio; CAD, coronary artery disease; SD, standard deviation; DNAR, do not attempt resuscitation; DNH, do not hospitalize; OR, odds ratio; CKD, chronic kidney disease; NR, not reported; MDS, Minimum Data Set; USRDS, United States Renal Data System.
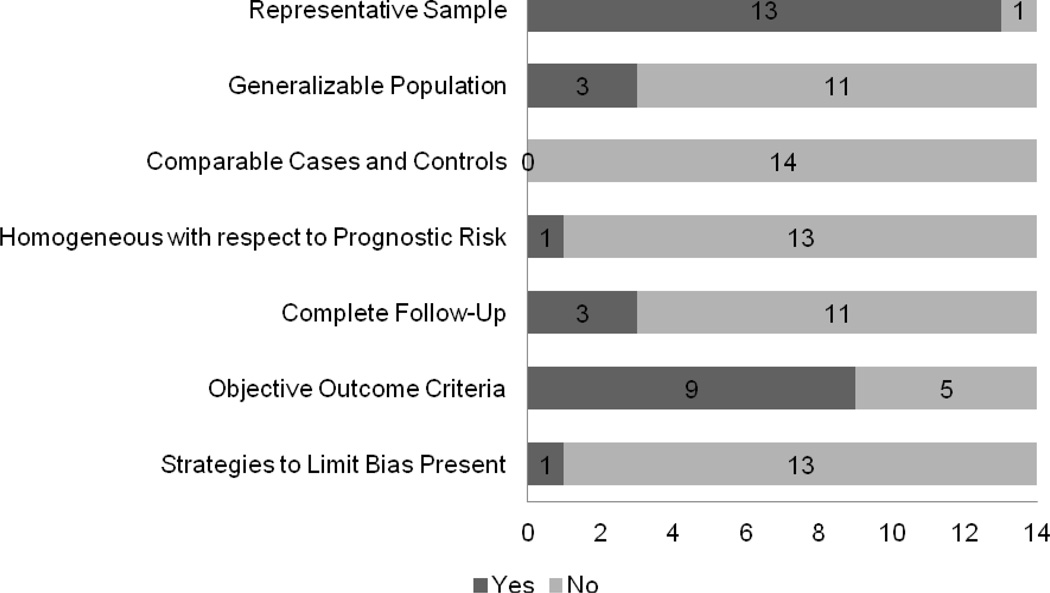
In Figure 2, we summarize the risk of bias in these studies. A majority of studies did not meet all criteria for validity for observational studies. Specifically, none of the studies included a control group, study populations’ baseline characteristics were often heterogeneous at the beginning of follow-up, and there was potential for bias in the assessment of outcome. Specifically, criteria for measuring peritonitis rates were subject to measurement bias, and missing data strategies were not reported for studies using administrative data.
Figure 2. Frequency of Observational Study Validity Criteria across Included Studies.
This is a bar graph of the frequency of validity criteria met by studies included in this review. Each study’s validity was evaluated by criteria for prospective studies6. The number of studies that met each criterion is delineated by the dark grey bar.
Outcome measures for included studies were grouped into the following categories: 1) prevalence of ESRD; 2) survival; 3) NH discharge disposition; 4) functional impairment; 5) cognitive impairment; 6) dialysis-related outcomes and 7) advance care planning. A summary of the outcome categories’ key findings and remaining gaps in evidence is shown in Table 2. We describe the data from the studies and relevant critical appraisal by outcome category below.
Table 2. Key Findings and Remaining Gaps in Literature by Outcome Category.
| Outcome | Key Findings | What Remains Unknown |
|---|---|---|
| Prevalence | ~ 0.4–0.6% of the ESRD population lives in NHs (data derived from 4 states, from 1990) |
Current prevalence of ESRD in NHs and related facility characteristics |
| Survival Rates | 1 year survival rate is 26–42% from NH admission New dialysis patients have lower survival rates Key predictors of survival: age and functional status |
How survival compares to non-ESRD residents Modifiable risk factors of mortality |
| Nursing Home Discharge Disposition |
% Discharged Home: 6–37% Hospitalization Rates for Resident on PD: 22–44 days per patient-year Key predictor of NH discharge: functional status |
How disposition differs from non- ESRD residents and by dialysis modality |
| Functional Impairment | 80% of ESRD residents have decline in ADLs and cognition 12 months after dialysis initiation |
Modifiable risk factors of functional impairment in ESRD residents |
| Cognitive Impairment | 90% of ESRD residents have decline in cognition 12 months after dialysis initiation |
Modifiable risk factors of cognitive impairment in ESRD residents |
| Dialysis-Related Outcomes |
Average Peritonitis Rate: 1 episode per 17 patient-months | Risk factors for peritonitis that are unique to NH residents |
| Advance Care Planning | 36% have Do Not Attempt Resuscitation Orders 2–17% Withdraw From Dialysis |
Extent of coordination of Advance Directives with Dialysis Units |
Abbreviations: ESRD, end-stage renal disease; NH, nursing home; PD, peritoneal dialysis.
Prevalence of Nursing Home Residence among ESRD patients, and of ESRD in Nursing Homes
The studies included in this review provide insufficient data to estimate the size of the population of NH residents with ESRD. Using survey results obtained from dialysis unit social workers, one 1993 study found that 0.4–0.6% of all ESRD patients across four states (VA, WV, MD, DC) lived in NHs with an admission incidence rate of 152 patients per year11. Another study reported the proportion of NH residents with ESRD and found nearly 1% of residents in a single 350-bed urban facility had Stage 5 CKD10. These two studies suggest that a small proportion of residents within each NH have ESRD; however, these results are more than five years old and represent a relatively small number of NHs, and thus may not reflect the current prevalence of NH residents with ESRD.
Survival Rates for Nursing Home Residents with ESRD
Twelve month survival rates ranged from 14% to 61% and appeared to be better in patients who had been on dialysis for a longer period of time. In five prospective U.S. studies, survival rates were established from the date of NH admission6,11–14. Across these studies, 6 month survival ranged from 38% to 56%, while 12 month survival ranged from 26 to 42%. The lowest survival rates (i.e., 38%) were reported by Reddy et al. in a study of HD patients in which nearly half of the cohort (47%) had initiated HD within 3 months of NH admission13. A higher survival rate (i.e., 56%) was reported in a mixed cohort of HD and PD patients in whom only 25% had initiated dialysis within 3 months of admission11, while the most favorable survival rate (i.e., 61%) was found in a Canadian population with a median dialysis duration of 31 months9. This association was further characterized by Reddy et al. in an analysis of survival time according to length of time on HD13. In this study, NH residents who had initiated HD within 3 months of NH admission had a lower 12 month survival (20%) than NH residents who had initiated HD at least 12 months before NH admission (36%)(3.4 months vs. 5.1 months, p <0.01). Survival among long-term residents who initiated dialysis while residing in a NH was assessed in two studies15,16. From the date of dialysis initiation, survival rates were 26–59% at 6 months and 14- 42% at 12 months, with lower survival rates in the study with an older cohort (mean age 84 vs. 73). The wide range in survival rates reflects the heterogeneity in study design and baseline characteristics across cohorts; however the findings suggest an association between the duration of dialysis and survival, perhaps reflecting a healthy survivor effect.
Survival rates in NH residents may also be associated with dialysis modality. In two cohorts of PD patients, 6 month and 12 month survival rate ranges were 50–52% and 37–40%, respectively6,12. In contrast, survival rates of HD patients reported by Reddy et al. at 6 month and 12 month were lower, 38% and 26%, respectively13. Direct comparisons of survival rates across these studies are limited because the patients differed by comorbidities and location, and the study periods varied from 1980s to 2000s6,12,13. In a cohort of HD and PD patients, PD patients had a greater risk of death than HD patients (HR 2.0 (1.6–2.6)) 11. Also, PD patients who switched to HD had longer survival than other PD patients (32 vs. 11 months) with similar baseline clinical characteristics6, but confounding by indication may limit the validity of this finding. Overall, data are conflicting on whether dialysis modality confers a survival benefit in this population, and randomized allocation or high quality comparative effectiveness studies are needed.
Predictors of poor survival among NH residents with ESRD have not been extensively studied but, include age, functional impairment, and comorbidities such as coronary artery disease (CAD) and decubitus ulcers. The 2010 Annual Data Report from the United States Renal Data System (USRDS) reports that residents with recent dialysis initiation had at least 2 times greater risk of death compared to residents without CKD15. Among these NH residents with ESRD, survival depended heavily on age. Specifically, the one year survival rate ranged from 19% in the 65–74 year age group to 10% in the 85+ year age group. In studies reporting the results of multivariable analyses, older age and lower ADL scores were consistently found to be independent predictors of lower survival6,11. Other reported predictors of lower survival include CAD and decubitus ulcers6. Given the heterogeneity of subjects within and across these studies, these predictors of poor survival likely represent only a portion of all possible explanatory variables.
Lastly, two cohort studies of PD patients provided information on cause of death. In both studies, cardiovascular disease was the most common cause of death (40%), but Anderson et al. found 23% died from infection, while Carey et al. only found 12% with infection-related deaths6,12. These data are subject to observer bias and needs to be validated through additional studies.
Nursing Home Discharge Disposition
Little is known about discharge disposition after NH care among residents with ESRD. Five studies considered the outcome of discharge to home17 and the proportion of ESRD short-term residents discharged to home ranged from 6% to 37%6,11–13,17. Baseline clinical characteristics were not reported in all studies, so it is unclear why there was such a large variation in rates of discharge to home. Two PD cohort studies reported rates of hospitalization which ranged from 22–44 days per patient-year6,12, and no studies examined rates of rehospitalization. Peritonitis and other infections were the primary reasons for hospitalization. The significance of these findings is limited because these were small, heterogeneous cohorts from selected NHs.
Some of the predictors of survival (i.e., functional status and dialysis duration) may also predict discharge to home. A cohort study of PD patients found that functional independence was a predictor of discharge to home, in addition the presence of diabetes, CAD, or age over 75 years reduced the likelihood of discharge home6. Also, Reddy et al. found that a larger proportion of established HD patients were discharged home compared to residents with recent HD initiation (48% vs. 28%, p =0.01)13. Neither study included both PD and HD patients.
Functional Impairment
The prevalence of functional impairment in NH residents increases after dialysis initiation. Two studies assessed change in functional status after dialysis initiation in a national sample of long-term residents15,16. Kurella Tamura et al. found that the median Minimum Data Set- Activities of Daily Living (MDS-ADL) score increased from 12 to 16 (indicating greater dependence) after 3 months of dialysis16. In the first month after dialysis initiation, average MDS-ADL scores increased by nearly 2 points, and subsequently, the rate of increase in MDSADL score was slower. By 6 and 12 months, only 30% and 13% of residents maintained their baseline MDS-ADL score, respectively. In a more recent study in an older cohort, the proportion of residents that maintained functional status was 10% at 6 months and 6% at 12 months after dialysis initiation15. Kurella Tamura et al. examined predictors of loss of functional status, and found the following characteristics to be independently associated with this outcome: older age, white race, cerebrovascular disease, dementia, hospitalization at time of dialysis initiation, or low serum albumin levels16. Because these studies are national cohorts, these findings likely apply to a majority of residents who undergo dialysis initiation.
Cognitive Impairment
Cognitive impairment is a predictor of timing of dialysis initiation, but it also worsens after dialysis initiation. Two studies examined the relationship between cognition and dialysis initiation15,18. Using the same national cohort of NH residents starting dialysis, Kurella Tamura et al. found that long-term care residents with moderate to severe cognitive impairment were less likely to start dialysis early; however, a recent decline in cognitive function was associated with increased odds of early dialysis initiation18. The USRDS study found that residents starting dialysis had higher cognitive scores compared to residents with a diagnosis of CKD diagnosis identified in Medicare claims data at baseline15. However, after 2 months, cognitive scores declined more in residents receiving chronic dialysis than in residents with a diagnosis of CKD (percentage with worse memory score: 10% vs. 18%, percentage with worse executive function score: 9% vs. 14%, percentage with worse verbal skills score: 5% vs. 7%). The average proportion of NH residents with recent dialysis initiation that maintained their baseline cognitive scores by 6 and 12 months was 17% and 10%, respectively. Given these studies are national cohorts; the associations between cognition and dialysis initiation described in these studies are likely pertinent to additional NH residents who develop renal failure.
Dialysis-Related Outcomes
Early dialysis initiation in NH residents is not common, but it is more likely to occur when residents exhibit symptoms associated with renal failure. In her national study of NH residents on dialysis, Kurella Tamura et al. found that 18% of residents initiated dialysis early (eGFR was greater than or equal to 15ml/min/1.73m2)18. Signs and symptoms independently associated with early dialysis initiation include edema, dyspnea, and moderate to severe cognitive impairment. Although this national cohort study well characterized the prevalence of early dialysis initiation and related factors, they did not assess the impact of all probable related factors, such as metabolic abnormalities or pruritus.
No studies examined clinical outcome measures relevant to ESRD and hemodialysis (i.e., anemia, nutrition, bone disease, dialysis adequacy, or hemodialysis access type), although seven studies reported outcomes related to PD, with some consistent and some conflicting findings6,8,9,12,17,19,20. In these studies, NH staff were specifically trained to do PD exchanges, and in several studies, nurses from the dialysis unit were available for regular patient assessments and troubleshooting. Peritonitis rates (defined as effluent with greater than 100 white blood cells per milliliter in 4 of 7 studies6,8,12,20) varied markedly across studies, ranging from 1 episode per 7 patient-months to 1 episode per 131 patient-months. The lowest peritonitis rate was reported in a prospective study of nine PD patients whose length of stay was not reported19. Among the three other prospective studies, peritonitis rates were 1 episode per 10–15 patient-months, and these rates were reported to be higher than in the community6,12. In contrast, peritonitis rates from retrospective chart reviews ranged from 1 episode per 7 patient-months to 1 episode per 40 patient-months8,9,20. Direct comparisons of these rates are limited by variation in assessment of peritonitis and study design (prospective vs. retrospective), as well as differences in patient populations (Canadian vs. United States; NH characteristics; average length of stay). Additional PD outcomes were assessed in three studies6,9,12. Anderson et al. reported an exit-site infection rate of 1 episode per 60 patient-months, while a smaller, more recent cohort based in Canada had an exit-site infection rate of 1 episode per 42 patient-months6,9. Carey et al. found 10 exit-site infections but the rate was not reported, and the diagnostic criteria were not defined12. From these studies, the proportion of patients that transitioned to HD ranged from 5% to 14%; with half of transitions due to ultrafiltration failure, and a smaller proportion as a result of complicated peritonitis (20–25%)6,9,12. Troidle et al. found that African-American residents and those with longer NH stays were more likely to develop peritonitis20, but no additional analyses were performed to identify clinical characteristics associated with other outcomes related to PD. Overall, these reports of PD-related outcomes are informative, but the heterogeneity of study populations and measurement bias associated with peritonitis and exit-site infections limit the strength of this evidence.
Advance Care Planning
Residents with ESRD appear to have similar treatment preferences in the setting of lifelimiting illness as other patients residing in a NH. Advance care planning in NH residents with ESRD has been examined in one study that characterized patterns of advance care planning in a cohort of ESRD residents on PD14. Upon NH admission, 36% had “do not attempt resuscitation” (DNAR) orders. Residents with DNAR orders had lower 1-year survival compared to residents without DNAR orders, and these residents were more likely to be older, to have functional impairment, and to have CAD. Four studies reported rates of withdrawal from dialysis that ranged from 2–17%9,12–14, with most (86%) having multi-organ failure at the time of dialysis discontinuation12. This evidence seems consistent with preferences of other patients with considerable illness burdens, but the narrow scope of these individual studies limits the strength of these findings.
Discussion
In this systematic review, we summarize available evidence on the prevalence of ESRD and a range of clinical outcomes among NH residents with ESRD, including survival rates, discharge disposition, functional impairment, cognitive impairment, dialysis-related outcomes, and advance care planning. Despite using broad selection criteria, we found only fourteen studies of which none were clinical trials, a majority were conducted more than a decade ago, and each individual study possessed design limitations (i.e., absence of control groups, study population heterogeneity) that affect the validity of the evidence. The evidence from this review does reveal that NH residents with ESRD have limited survival and significant functional impairment, but it does not reveal how outcomes differ between residents with ESRD who do not undergo dialysis and those who do or how outcomes in residents with ESRD differ from other residents without CKD or less advanced stages of CKD. Although important questions about patients with ESRD and their outcomes in the NH setting remain unclear, this review does highlight key findings and identifies important targets for quality improvement and future research.
In our literature search, we found no current estimates of the prevalence of ESRD in NHs. One study reported that 0.4–0.6% of all dialysis patients at a given time were NH residents, but this study was conducted over 20 years ago and was limited to four states11. Additional work by Kurella Tamura et al. suggests that in a 30-month period (from June 1998 to October 2000), there were approximately 3700 incident dialysis patients in NHs, but does not provide data on the denominator population of prevalent NH residents at this time16. These findings likely underestimate the contemporary prevalence of ESRD patients residing in NHs due to growth of dialysis among older adults and shifting referral patterns for dialysis and long-term care. Accurate prevalence information is needed to evaluate trends in NH placement among ESRD patients and allow NH staff to plan for the distinct care needs of this potentially growing population. Future research should also describe the types of facilities that have a higher volume of ESRD patients.
Available studies suggest that residents receiving chronic dialysis have poor survival rates, with 1-year survival from the date of NH admission ranging from 26 to 42%. These survival rates are approximately 10–30% lower than those reported for more broadly defined populations of octogenarians and nonagenarians initiating dialysis21, and lower than overall rates reported for general NH populations22,23, although the lack of a relevant comparison group in most studies of ESRD residents makes it impossible to quantify the impact of ESRD on survival in a NH population. Survival does appear to be lower in patients newly started on dialysis, who may represent an important subgroup for future study or intervention. Given limited data on residents with Stage 5 CKD who do not initiate dialysis, this review does not elucidate whether dialysis improves survival in NH residents. For NH residents receiving dialysis, survival rates may differ by dialysis modality, although additional studies comparing dialysis modality while adjusting for baseline mortality risk are warranted. The generalizability of the survival rate estimates is also limited by heterogeneity in patient characteristics across studies. Still, this evidence suggests that medical directors should recognize that residents with ESRD will in general have very limited life expectancy, but this might vary depending on their functional status. Additional research is needed to identify predictors of survival that are modifiable, especially in residents who experience dialysis initiation. Matched sampling or propensity scoring could further clarify how survival rates differ from non-ESRD residents.
It is known that community-dwelling older adults may experience loss of functional status after dialysis initiation24. Functional decline can lead to adverse events, such as falls. Among those who experience falls, mortality risk nearly doubles25. Studies in this review of NH residents also show strong associations between level of functional impairment and mortality 6,11. We did not find any information on whether residents undergoing dialysis who experience decline in functional status are less likely to be discharged than other residents, or whether their rehabilitation outcomes differ from other residents. A single study suggests that inpatient geriatric rehabilitation for dialysis patients may yield comparable outcomes to non-dialysis patients26, but that rehabilitation in residents receiving dialysis may be uniquely challenging because of the functional decline associated with dialysis initiation and the timing of dialysis treatments26,27. Given the findings of this review, NHs should monitor for functional decline in residents with ESRD and consider more frequent assessments of functional status and restorative activities to help residents adapt to new disabilities28. Several authors have suggested novel approaches towards rehabilitation in dialysis patients, such as inpatient rehabilitation with on-site dialysis29. Further research is needed to explore modifiable risk factors of functional and cognitive decline and strategies for rehabilitation in long-term residents undergoing dialysis initiation.
This systematic review also describes the available literature of ESRD residents undergoing PD. The management of PD patients in NHs has historically been difficult due to concerns including NH staff training, adequate staffing levels, medical liability, costs, and storage of dialysis supplies30. At the same time, travel requirements are generally much less onerous for patients receiving PD than for those receiving hemodialysis. The prospective studies included in this review suggest that that it is feasible to conduct PD in NHs with appropriate staff training and support6,12,17,19. While three studies found higher peritonitis rates in NH residents compared to in the community6,8,12, there are likely underlying differences in the health and comorbidities of these patients which explain at least part of the higher infection rate. These limitations also affect generalizability of data on hospitalization rates, exit site infections, and transitions to HD. These cohort studies were also conducted in 1990s and early 2000s, so they may not reflect the current prevalence and practices of PD in NHs. Given the potential for increased demand for PD in NHs, additional research should address the remaining challenges to providing PD services in NHs and whether there are risk factors for peritonitis that are unique to the NH population.
Despite the variety of outcomes described in this systematic review, there are significant gaps in the literature relevant to the management of residents with ESRD. None of the studies addressed dietary considerations for patients with ESRD, management of dialysis access, anemia, or issues related to transportation to dialysis units, and these care management issues may be barriers to NH admission30. Quality of life and personal experiences of residents with ESRD also were not addressed in the studies of this review. Predictors of adverse outcomes were examined in this review, but we did not identify significant modifiable risk factors as potential targets for improving these outcomes. While adverse outcomes in this frail population are common, advance care planning preferences were examined in only one study14. Trends in advance care planning need to be determined, along with the prevalence, management, and predictors of dialysis withdrawal and conservative management of ESRD across NHs. Addressing these questions and reporting of quality improvement projects on management of patients with ESRD could lead to development of best practices that limit avoidable hospitalizations and other adverse outcomes and reduce the perceived challenges of caring for residents with ESRD.
This systematic review has design considerations and limitations. First, we focused on NH residents with ESRD which included chronic dialysis patients or patients with Stage 5 CKD, and we excluded NH residents with earlier stages of CKD. Given the heterogeneity of CKD and related outcomes among older adults, the narrower eligibility criteria allowed for identification of findings that are specific to residents with ESRD. Finally, the risk for publication bias is an issue in any systematic review; we attempted to minimize publication bias by including conference proceedings in our search strategy.
Conclusion
Although the strength of evidence is limited, this review reveals that NH residents with ESRD have very high mortality rates and very high rates of functional decline, especially for those recently initiating dialysis. This review also reveals that there major knowledge gaps remain in the literature about this vulnerable population. These findings highlight a need for NHs to increase attention to the management of residents with ESRD, although studies developing and validating such approaches are needed. Further research will help inform the development of NH protocols and quality measures unique to this growing segment of the NH population.
Supplementary Material
Acknowledgements
This material is the result of work supported with resources and the use of facilities at the Durham VA Medical Center. This study is supported by the Claude D. Pepper Older Americans Independence Center (NIH P30 AG028716) and the Agency for Healthcare Research and Quality (T32HS019490). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs or the United States government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors report any potential conflicts of interest.
References
- 1.Collins AJ, Foley R, Herzog C, et al. Excerpts from the United States Renal Data System 2007 annual data report. Am J Kidney Dis. 2008 Jan;51(1 Suppl 1):S1–S320. doi: 10.1053/j.ajkd.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Schnelle J, Osterweil D, Globe D, et al. Chronic kidney disease, anemia, and the association between chronic kidney disease-related anemia and activities of daily living in older nursing home residents. J Am Med Dir Assoc. 2009 Feb;10(2):120–126. doi: 10.1016/j.jamda.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Holley JL. Palliative care in end-stage renal disease: illness trajectories, communication, and hospice use. Adv Chronic Kidney Dis. 2007 Oct;14(4):402–408. doi: 10.1053/j.ackd.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Sankarasubbaiyan S, Holley JL. An analysis of the increased demands placed on dialysis health care team members by functionally dependent hemodialysis patients. Am J Kidney Dis. 2000 Jun;35(6):1061–1067. doi: 10.1016/s0272-6386(00)70040-0. [DOI] [PubMed] [Google Scholar]
- 5.Guyatt G. Users' guides to the medical literature : essentials of evidence-based clinical practice. 2nd ed. New York: McGraw-Hill Medical; 2008. [Google Scholar]
- 6.Anderson JE. Ten years' experience with CAPD in a nursing home setting. Perit Dial Int. 1997 May-Jun;17(3):255–261. [PubMed] [Google Scholar]
- 7.Anderson JE, Sturgeon D, Lindsay J, et al. Use of continuous ambulatory peritoneal dialysis in a nursing home: patient characteristics, technique success, and survival predictors. Am J Kidney Dis. 1990 Aug;16(2):137–141. doi: 10.1016/s0272-6386(12)80568-3. [DOI] [PubMed] [Google Scholar]
- 8.Wang T, Izatt S, Dalglish C, et al. Peritoneal dialysis in the nursing home. Int Urol Nephrol. 2002;34(3):405–408. doi: 10.1023/a:1024478523252. [DOI] [PubMed] [Google Scholar]
- 9.Taskapan H, Tam P, Leblanc D, et al. Peritoneal dialysis in the nursing home. Int Urol Nephrol. 2010 Jun;42(2):545–551. doi: 10.1007/s11255-010-9714-y. [DOI] [PubMed] [Google Scholar]
- 10.Dharmarajan TS, Banik P, Kanagala M, et al. High prevalence of chronic kidney disease, anemia, and falls in an urban long-term care facility: relationship or coincidence? J Am Med Dir Assoc. 2010 May;11(4):297–299. doi: 10.1016/j.jamda.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Anderson JE, Kraus J, Sturgeon D. Incidence, prevalence, and outcomes of end-stage renal disease patients placed in nursing homes. Am J Kidney Dis. 1993 Jun;21(6):619–627. doi: 10.1016/s0272-6386(12)80034-5. [DOI] [PubMed] [Google Scholar]
- 12.Carey HB, Chorney W, Pherson K, et al. Continuous peritoneal dialysis and the extended care facility. Am J Kidney Dis. 2001 Mar;37(3):580–587. [PubMed] [Google Scholar]
- 13.Reddy NC, Korbet SM, Wozniak JA, et al. Staff-assisted nursing home haemodialysis: patient characteristics and outcomes. Nephrol Dial Transplant. 2007 May;22(5):1399–1406. doi: 10.1093/ndt/gfl809. [DOI] [PubMed] [Google Scholar]
- 14.Anderson JE, Sikorski I, Finucane TE. Advance care planning by or on behalf of peritoneal dialysis patients in long-term care. Am J Kidney Dis. 2006 Jul;48(1):122–127. doi: 10.1053/j.ajkd.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 15.Outcomes in the transition zone in nursing home patients with chronic kidney disease. American Journal of Kidney Diseases. 2011;57(1 SUPPL. 1):e99–e108. [Google Scholar]
- 16.Kurella Tamura M, Covinsky KE, Chertow GM, et al. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009 Oct 15;361(16):1539–1547. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meddy J. Developing a peritoneal dialysis unit in a long-term care setting. Dialysis and Transplantation. 1995;24(2) 62+64+67-68+90. [Google Scholar]
- 18.Kurella Tamura M, O'Hare AM, McCulloch CE, et al. Signs and symptoms associated with earlier dialysis initiation in nursing home residents. Am J Kidney Dis. 2010 Dec;56(6):1117–1126. doi: 10.1053/j.ajkd.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hariprasad MK, Teribury M, Dagon P, et al. Peritoneal dialysis nursing home experience. Dialysis and Transplantation. 1995;24(4):192. [Google Scholar]
- 20.Troidle LK, Gorban-Brennan N, Kliger AS, et al. Peritonitis in the extended-care facility. Adv Perit Dial. 1998;14:127–130. [PubMed] [Google Scholar]
- 21.Kurella M, Covinsky KE, Collins AJ, et al. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med. 2007 Feb 6;146(3):177–183. doi: 10.7326/0003-4819-146-3-200702060-00006. [DOI] [PubMed] [Google Scholar]
- 22.Flacker JM, Kiely DK. Mortality-related factors and 1-year survival in nursing home residents. J Am Geriatr Soc. 2003 Feb;51(2):213–221. doi: 10.1046/j.1532-5415.2003.51060.x. [DOI] [PubMed] [Google Scholar]
- 23.Hjaltadottir I, Hallberg IR, Ekwall AK, et al. Predicting mortality of residents at admission to nursing home: a longitudinal cohort study. BMC Health Serv Res. 2011;11:86. doi: 10.1186/1472-6963-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jassal SV, Chiu E, Hladunewich M. Loss of independence in patients starting dialysis at 80 years of age or older. N Engl J Med. 2009 Oct 15;361(16):1612–1613. doi: 10.1056/NEJMc0905289. [DOI] [PubMed] [Google Scholar]
- 25.Li M, Tomlinson G, Naglie G, et al. Geriatric comorbidities, such as falls, confer an independent mortality risk to elderly dialysis patients. Nephrol Dial Transplant. 2008 Apr;23(4):1396–1400. doi: 10.1093/ndt/gfm778. [DOI] [PubMed] [Google Scholar]
- 26.Farragher J, Jassal SV. Rehabilitation of the geriatric dialysis patient. Semin Dial. 2012 Nov;25(6):649–656. doi: 10.1111/sdi.12014. [DOI] [PubMed] [Google Scholar]
- 27.Forrest GP. Inpatient rehabilitation of patients requiring hemodialysis. Arch Phys Med Rehabil. 2004 Jan;85(1):51–53. doi: 10.1016/s0003-9993(03)00366-6. [DOI] [PubMed] [Google Scholar]
- 28.Cook WL, Jassal SV. Functional dependencies among the elderly on hemodialysis. Kidney Int. 2008 Jun;73(11):1289–1295. doi: 10.1038/ki.2008.62. [DOI] [PubMed] [Google Scholar]
- 29.Jassal SV, Chiu E, Li M. Geriatric hemodialysis rehabilitation care. Adv Chronic Kidney Dis. 2008 Apr;15(2):115–122. doi: 10.1053/j.ackd.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Smith-Wheelock L, Sink V. Caring for the nursing home resident on dialysis: a search for solutions. Adv Ren Replace Ther. 2000 Jan;7(1):78–84. doi: 10.1016/s1073-4449(00)70009-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.