Introduction
Since most ovarian cancer (OvCa) patients present at a late stage, when metastasis has already occurred, the study of early events in peritoneal dissemination is difficult. One problem has been the lack of adequate model systems for the study of ovarian tumor transformation and metastasis1, 2. Current models in use include co-cultures, whole tissue cultures, immunocompromised and genetic mouse models. All of these have unique advantages; however none of them replicates the human in vivo situation. The development and use of a 3D organotypic model of OvCa has the potential to bridge the gap between the current models of OvCa and the human disease.
Ovarian Cancer Metastasis
Most patients with OvCa present with advanced disease metastatic to the peritoneum. Despite aggressive surgery and chemotherapy, patients with intra-abdominal widely disseminated OvCa rarely achieve long term cures3. The key to improved treatment of OvCa is a better understanding of the molecular mechanisms governing peritoneal dissemination.
OvCa metastasis is predominantly confined to the abdominal cavity and, unlike breast, colon or lung cancer, is rarely metastasizing hematogenously. Once an ovarian epithelial cell undergoes neoplastic transformation, it can freely disseminate throughout the peritoneal cavity carried by the flow of peritoneal fluid which is resorbed by the mesothelial cells of the omentum and peritoneum. Most published studies have focused on metastasis of OvCa cells to the abdominal peritoneum, under the assumption that similar mechanisms are involved in metastasis to the omentum. Given the differences in the structure of omentum and peritoneum, and that the omentum per se is the target of metastasis in 80% of advanced stage cases, a model system focusing on the omentum is a vital, but currently under studied area of focus4. Since most OvCa patients present at a late stage, when metastasis has already occurred, the study of early events in peritoneal dissemination is difficult. The potential of a tumor cell to metastasize depends on many factors, including interactions with the microenvironment at the metastatic site, which can promote cell adhesion, growth, survival, angiogenesis, and invasion5. Adequate model systems, therefore, are vital to address the study of early regulatory steps in metastasis1, 2. Traditional models that have been widely used include whole tissue cultures, co-culture and immunocompromised and genetic mouse models, all of which have their unique advantages and disadvantages. While no model has comprehensively been able to recapitulate the tumor microenvironment to study early steps in metastasis, our newly described 3D organotypic model of OvCa6 has the potential to bridge the gap between the existing models of OvCa and the nascent human environment.
Charactersitics of the Ovarian Cancer Microenvironment in the Omentum
The omentum is a visceral fold of the peritoneum which is divided into two regions, an adipose-rich and a membranous/translucent region. The adipose-rich region, also known as the greater omentum, makes up most of the human omentum and is a fatty apron-like structure that is positioned on top of the abdominal organs like a blanket7–9. Its clinical function is to provide protection from blunt trauma, to promote healing, to wall off intra-abdominal infection, store lipids, and act as a regulator for fluid exchange12. The normal human omentum and peritoneum are covered by a confluent layer of epithelial mesothelial cells (see Figure 1). This surface mesothelium, which is the first contact site for OvCa cells, forms a low-friction, nonadhesive surface and selective barrier, which is involved in fluid metabolism between the abdominal cavity, the interstitial space and the vasculature10. Tight junction proteins (zonula occludens protein 1, occludin and claudin-1) and the adherens junction protein β-catenin, hold the continuous single layer of mesothelial cells together11. A number of adhesion molecules play an integral role in normal mesothelial cell physiology. The integrins, for instance, play a important role in mesothelial cell-cell and cell-ECM adhesions12. Adhesion of OvCa cells to mesothelial cells is also mediated by different proteases, notably MMP-2, and CD446, 13–16. In addition, mesothelial cells secrete fibronectin, which plays a role in the adhesion and invasion of OvCa cells6, 16–18. Fibroblasts, which are embedded in the layer of extra-cellular matrix (ECM) underlying the mesothelial cells (see Figure 1), constitute another major cell type that interacts with OvCa during adhesion and invasion to the peritoneal cavity. Many reports confirmed the hypothesis that stromal fibroblasts play an important role in cancer cell progression and metastasis (reviewed in19 and20).
Figure 1.
Histology of human omentum and peritoneum. Hematoxylin and eosin staining of human omentum at 100x (A) and 200x (B). Trichrome staining for the detection of collagen in human omentum (C) and peritoneum (D). Collagen fibers are stained in blue and cells are stained in red. MC, mesothelial cells; F, fibroblasts.
The mesothelial cell layer is attached to a basement membrane (BM) predominantly composed of collagen I and IV, fibronectin, vitronectin, and laminin18, 21, 22. OvCa cells have a predisposition to adhere to collagen23–25, an interaction which can be blocked with an α2β1-integrin antibody25. The laboratory of Dr. Sharon Stack has, as early as 1994, shown that collagen I is important in OvCa progression and invasion23, 26–32. In the omentum and peritoneum, a thick network of collagen fibers are entwined between fibroblasts just below the mesothelium as shown in paraffin embedded human tissue stained with trichrome to detect collagen (see Figure 1C & D).
Models to Study Ovarian Cancer Biology
Most models used to study OvCa utilize human OvCa cell lines established from ascites or pleural effusions which grow on plastic as flat monolayer in two-dimensions (2D). The advantages of using cell lines are that they are readily renewable sources, amenable to genetic manipulation and relatively inexpensive. The small number of OvCa cell lines available have allowed for extensive characterization by many laboratories. Indeed, studies using OvCa cell lines have made invaluable contributions to our understanding of ovarian tumor biology, especially of the signaling pathways involved in this disease33, 34. However, 2D models have clear limitations lacking the micro environmental context to study the earliest stages of metastasis as they occur in vivo35, 36. Ovarian tumors are not composed exclusively of malignant cells, but of a mixture of fibroblasts, mesothelial cells and inflammatory cells. Therefore, 2D cultures on plastic do not mimic the complexities of the “tumor microenvironment” and the crosstalk that may take place between and amongst components of the microenvironment. Hence, the results obtained using them may not be as relevant to the clinical situation as they could be. Incremental improvements have been made to OvCa 2D cultures by co-culturing the cancer cells on one ECM protein or with one stromal cell type. However, these stromal cells are often from a site other than the peritoneum/omentum (e.g. foreskin fibroblasts) or from a different species altogether (e.g. rat) raising the question of tissue or host specificity37–39.
Xenograft mouse models are the second most widely used research models for the study of OvCa and were first described by Rygaard in 1969. They are powerful tools for investigating late stage OvCa metastasis and for testing new therapies40. However, they have several deficiencies that make extrapolation to human tumor biology problematic. Human cancer cells are injected into a young, immunocompromised mouse host which lacks factors, such as IL-8, that are present in humans. Moreover, the mouse omentum is anatomically adjacent to the pancreas and has a different histological appearance than human omentum7. These differences may be just one reason why only 27% of chemotherapeutic agents showing efficiency in a xenograft model have ultimately clinical activity in Phase II clinical trials41.
Given the deficiencies of 2D cell cultures and xenograft mouse models we and others hypothesized that establishment of a 3D organotypic model of OvCa metastasis might be a better method to study the interaction of cancer cells with their omental microenvironment. The advantages of this model are that it contains all the key regulatory components of a particular metastatic site, the omentum, is reconstituted from primary human cells and that it can be easily manipulated and evaluated.
Current In Vitro Models of Ovarian Cancer
A number of co-cultures have been established (see Figure 2) using OvCa cell lines and primary cells, which were added to a matrix as either single cells or as tissue-like aggregates (spheroids). 3D matrices have been generated from purified molecules such as collagen type I and from native extra-cellular matrices42. In 1987, Niedbala et al. were the first to utilize primary human OvCa cells to investigate degradation of ECM from bovine corneal endothelial cells (see Figure 2A)43. Kanemoto et al. investigated the adhesion, invasion, and colony formation of OVCAR-3 cells to a number of ECMs and reported that a RGD peptide inhibited adhesion to fibronectin and colony formation44. Rieppi et al. used conditioned media from mesothelial cells and found that it stimulated OvCa invasion through fibronectin17.
Figure 2.
Concepts of various 3D models to study OvCa cell adhesion/invasion. (A) OvCa cells cultured on a thin layer of ECM, (B) OvCa cells cultured on a thick layer of collagen , (C) OvCa cells cultured on a single layer of mesothelial cells or fibroblasts, (D) OvCa cells cultured on a single layer of mesothelial cells plated on ECM, (E) OvCa cells cultured on 3D organotypic model of human omentum. The 3D model is composed of fibroblasts embedded in a thick ECM layer and covered by primary human mesothelial cells.
The laboratories of Dr. Sharon Stack23, 26–32 and Dr. Amy Skubitz15, 45–52 have made very significant contributions to our understanding of integrin function in the interaction of OvCa cells with the ECM. Casey et al. blocked adhesion to fibronectin and spheroid formation in an OvCa cell line with a β1-integrin blocking antibody51. Burleson et al. quantified OvCa cell spheroid, ascites and cell line-derived, adhesion to and desegregation on a variety of ECMs45–47. They found that ascites-derived OvCa spheroids adhere to fibronectin and collagen type I, with reduced adhesion to collagen type IV and laminin, which can be partially inhibited by treatment with a β1-integrin antibody46. Dr. Barbolina from the Stack laboratory cultured OvCa cell lines in collagen (see Figure 2B)53 and found that OvCa cell lines grown in a 3D collagen microenvironment showed increased expression of the transcription factor early growth response protein and subsequently membrane type 1 matrix metalloproteinase expression when compared to cells grown on a thin layer of collagen (2D)27.
Several studies have explored OvCa cell adhesion and invasion through mesothelial cell monolayers (see Figure 2C)15, 54, 55. Lessan et al. discovered that CD44 and β1-integrin mediate OvCa cell adhesion to an immortalized peritoneal mesothelial cell line (LP9)15. Suzuki et al. found that treatment with an immunoglobin M that recognized a glycoprotein on the cell surface inhibited OvCa cell adhesion to mesothelial cells54. Kishikawa et al. investigated colony formation and invasion of six OvCa cell lines that interacted with primary omental mesothelial cells55. They classified the OvCa cells into two groups, adhesive-type and invasive-type, based on their interaction with mesothelial cells. Interestingly, blocking antibodies to α2- and β1-integrins only inhibited invasion of the invasive-type cancer cells through the mesothelial monolayer. Casey et al. employed an in vitro assay to measure OvCa cell line invasion through permeabilized mesothelial cell line monolayers50. Using a dye based assay they found an inhibitory effect of a β1-integrin antibody, hyaluronan, and GM6001, a MMP inhibitor, on OvCa cell invasion through mesothelial cells50.
In the latest studies, Burleson and colleagues investigated OvCa cell spheroids, ascites or cell line-derived, dissemination on and invasion into normal human mesothelial cell monolayers45–47. They found that ascites-derived spheroids adhere to live, but not fixed, human mesothelial cell monolayers, and this adhesion is partially inhibited by a β1-integrin antibody46.
These previously described studies investigated the interaction of OvCa cells either with an ECM or with mesothelial cells alone, with the exception of a very elegant study published in 1985. Niedbala et al. investigated the interactions of human OvCa tumor cells with human mesothelial cells grown on ECM (see Figure 2D)56. Primary human mesothelial cells were cultured on top of bovine corneal endothelial cell – derived ECM. Then, radiolabeled ascites-derived primary human OvCa cells were added and attachment measured56. Results of this study indicated that OvCa cells exhibit a more rapid and firmer attachment to ECM than to mesothelial cells or to plastic alone56.
3D Models of Carcinogenesis
The rationale for growing cells in 3D is that such a culture provides an environment that more closely mimics the human omentum, possibly making the results more relevant to understanding the biology of OvCa in humans. Epithelial cells grown in 3D exhibit a number of features comparable to their in vivo counterparts. When primary human cells at low passage number are used to assemble the 3D culture it is likely to be the closest approximation to the human in vivo situation currently known.
Cultures of cancer cells grown in 3D extra-cellular matrices began with the work of Bissell, Petersen and colleagues57–64. Culturing immortalized breast cells in 3D leads to lobule formation very similar to the normal breast lobules seen in a human breast 64. One of the most important early findings of these studies was that epithelial cells cultured within a thick ECM undergo glandular differentiation, whereas cells cultured on a thin ECM on tissue culture plastic do not65. The study of breast tumor cells in 3D also allowed investigators to unravel the importance of cell-adhesion proteins in modulating the tumor phenotype. For example α2β1-integrin is constitutively expressed in normal ductal mammary cells but lost once they transform into a malignant breast cancer66. Re-expression of α2β1-integrin restores the ability of cancer cells to differentiate into glands, reversing the malignant phenotype.
A comparable anatomic situation to that of OvCa is skin cancer. Melanomas grow and invade on a surface, the skin, just as OvCa grows and invades the surfaces lining the peritoneal cavity. A human skin reconstruct model was pioneered by the laboratory of Dr. Meenhard Herlyn combining collagen type I, fibroblasts, and keratinocytes with cancer cells to simulate human melanoma67–69. Haass et al. manipulated the multiple layers of the 3D skin model to identify key cell-cell adhesion and cell communication molecules involved in the development of melanoma, and found that E-cadherin, N-cadherin and integrins are involved in melanoma growth and invasion69.
Designing the 3D Organotypic Model of Ovarian Cancer
Since the peritoneum and omentum are the major sites of OvCa metastasis, we sought to develop a 3D organotypic model to mimic the key components of the peritoneal/omental surface microenvironment (see Figure 2E)6. Our first step was to review and characterize the normal histology of the superficial layer of normal human peritoneum/omentum. The surface of the omentum is covered by a layer of mesothelial cells on top of a matrix containing collagen fibers and primary fibroblasts (see Figure 1C). To mimic the human omentum as closely a possible in 3D we extracted primary human fibroblasts and mesothelial cells from nondiseased human omentum removed at surgery from the same patient. The 3D model was assembled by embedding early passage primary human fibroblasts in ECM (collagen type I) (see Figure 3A), which was covered by a layer of early passage primary human mesothelial cells (see Figure 3B). The 1:8 ratio of fibroblasts to mesothelial cells was extrapolated studying normal human omentum. Collagen I was used as the matrix since it is the major structural ECM protein present in the omentum (see Figure 1C ) 23, 26–31.
Figure 3.
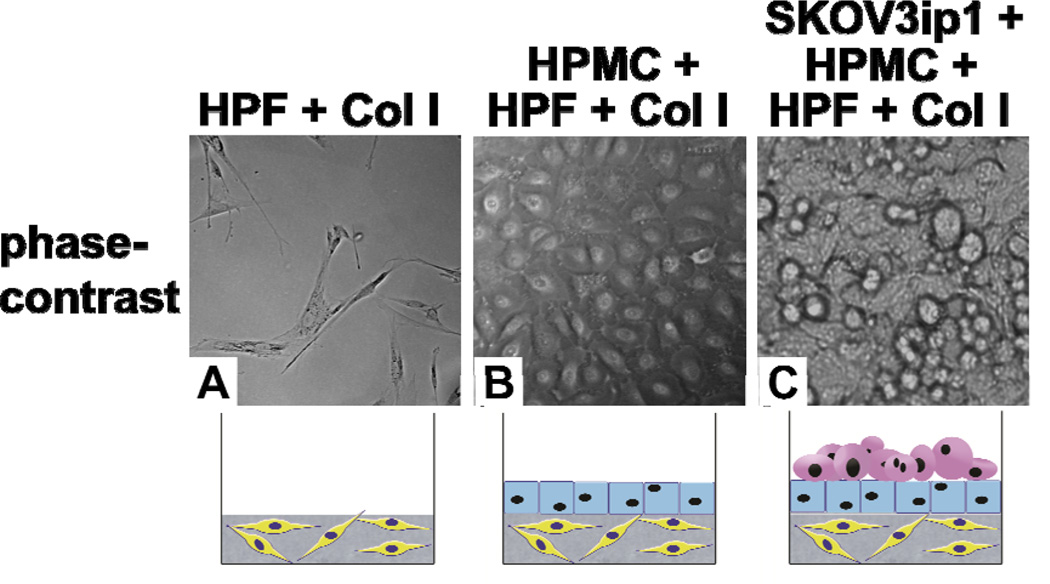
Construction of a 3D organotypic model of OvCa. Phase-contrast pictures of (A) fibroblasts in a collagen gel, (B) a confluent layer of mesothelial cells overlaying fibroblasts in a collagen gel, and (C) SKOV3ip1 OvCa cells adhering to the 3D model. Col I, collagen I; HPMC, human primary mesothelial cells; HPF, human primary fibroblasts.
In order to understand early OvCa metastasis, we cultivated OvCa cells with the 3D organotypic model of the peritoneal microenvironment (see Figure 2E and 3C) and optimized the model to investigate OvCa cell adhesion and invasion. To investigate adhesion, a thin layer of collagen I (0.5mg/0.3cm2) was used, while a thicker layer of collagen (15mg/0.3cm2) was applied to the 3D organotypic model to investigate invasion. After addition of OvCa cells, the histologic appearance of the 3D organotypic model mimicked microscopic metastases to the omentum from patients with OvCa (see Figure 4)6. Two different OvCa cell lines (HeyA8, SKOV3.ip1) and primary human OvCa cells were added to the 3D organotypic model to evaluate the contribution of various cell types and ECMs to OvCa cell adhesion and invasion. Direct contact of OvCa cells with mesothelial cells inhibited adhesion and invasion, when compared to OvCa cell interactions with fibroblasts and ECMs (see Figure 5)6. The principal inhibitory effect of mesothelial cells on OvCa cell adhesion and invasion is mediated by direct cancer cell – mesothelial cell contact because pre-treatment of cancer cells with mesothelial cell conditioned media only minimally inhibited adhesion and invasion6. This finding suggests that the mesothelial cell layer in the peritoneum and omentum serves as a protective layer blocking initial OvCa metastasis.
Figure 4.
A 3D organotypic model of OvCa metastasis. Hematoxylin and eosin staining of normal human omentum surface (A) and early OvCa cell metastasis to the omentum (B & C). First, OvCa cells attach and proliferate on the surface of the omentum (closed arrows B & C). Second, OvCa cells invade the basal membrane and invade the omental tissue (dashed arrow C). Hematoxylin and eosin staining of the 3D organotypic model without cancer cells (D) and with SKOV3ip1 OvCa cells for 24 (E) and 120 hours (F). First, OvCa cells attach and proliferate on the surface of the 3D organotypic model (closed arrow E). Second, OvCa cells invade the basal membrane of the 3D organotypic model (dashed arrow F). MC, mesothelial cells; F, fibroblasts; Col I, collagen I; HPMC, human primary mesothelial cells; HPF, human primary fibroblasts.
Figure 5.
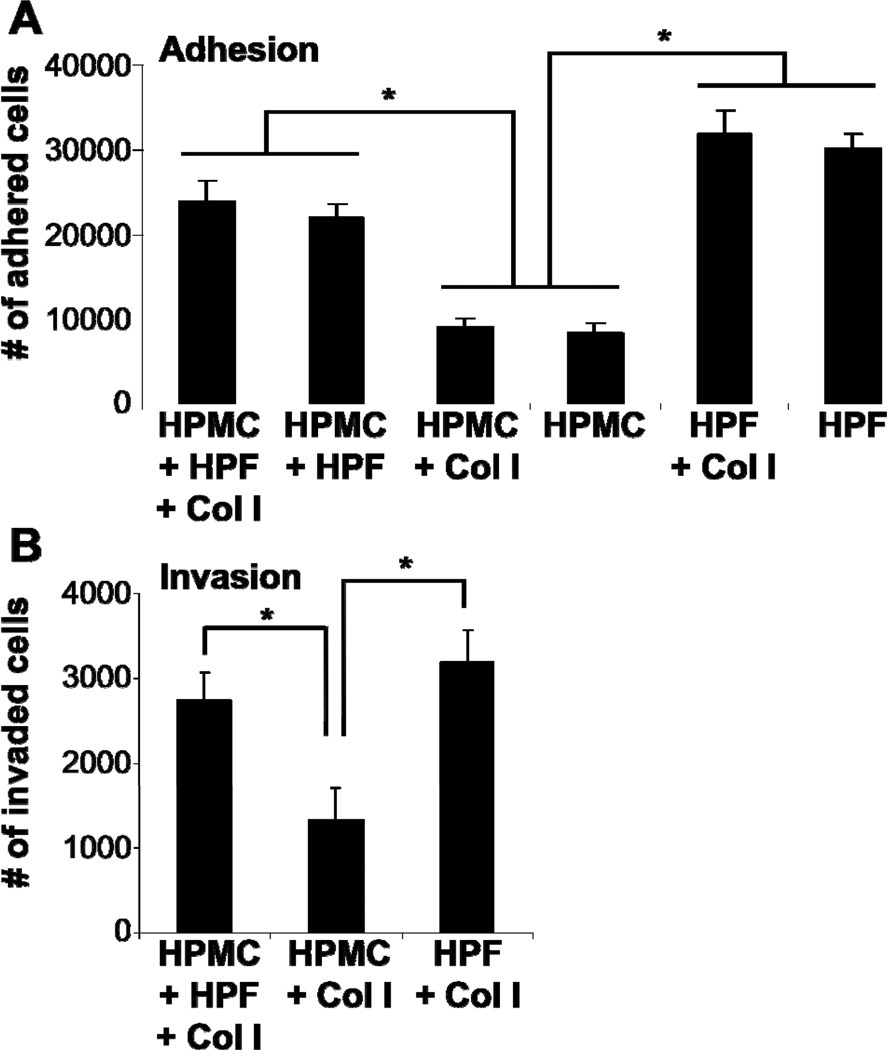
Mesothelial cells inhibit, while fibroblasts induce OvCa cell adhesion and invasion. Adhesion assays were performed with fluorescently-labeled HeyA8 cells for 4 hours to primary human fibroblasts, mesothelial cells or a combination of both with or without collagen I (A). Invasion assays were performed with fluorescently-labeled HeyA8 cells for 24 hours to primary human fibroblasts on collagen I, mesothelial cells on collagen I, or the 3D organotypic model (B). * (p<0.01) denotes a significant change in number of bound or invaded cells between treatments. Each bar represents the average of 3 wells and standard deviation. Col I, collagen I; HPMC, human primary mesothelial cells; HPF, human primary fibroblasts.
Stromal fibroblasts play an important role in cancer cell progression19, 20. They undergo a transformation from quiescent cells to proliferating and excessively matrix-producing cells, a process known as ‘fibroblast activation’, which results in cancer-associated fibroblasts. To determine the role of omental fibroblasts in omental metastasis we studied the adhesion and invasion of cancer cells in both the presence and absence of primary human omental fibroblasts. In contrast to our findings with mesothelial cells, OvCa cells are significantly more adhesive and invasive when cultured on fibroblasts (see Figure 5). Moreover, fibroblast-conditioned media increased OvCa cell adhesion and invasion6. Therefore, we saw an increase in OvCa cell adhesive and invasive activity which involved both soluble mediators and direct interaction(s) between tumor cells and fibroblasts.
Our findings from testing the 3D organotypic model of OvCa metastasis imply that the stromal cells are more important than the ECM in determining OvCa cell attachment and invasion. However, we can not exclude the possibility that the effect of stromal cells is, at least in part, mediated by other ECM proteins that are laid down by the mesothelial cells or the fibroblasts during the time the 3D organotypic model is assembled. Mesothelial cells secrete fibronectin and collagen, which are also part of the omental basement membrane underlying the mesothelial cells25. In the 3D organotypic model, stimulation of invasion by collagen I can be abrogated by mesothelial cells or further induced by fibroblasts, suggesting that, at least in the early phases of omental metastases, stromal cells modulate the pro-invasive signals of the ECM.
A third of OvCa patients present with ascites70 containing OvCa cells as single cells, aggregates or as spheroids71. Ascites fluid can either stimulate or inhibit OvCa cell invasion, proliferation, spheroid formation and gene expression71. Similarly, we confirmed that ascites fluid either increased or decreased OvCa cell adhesion to the 3D organotypic model depending on individual cases (see Figure 6A). However, OvCa cells were significantly more invasive through the 3D organotypic model when stimulated with ascitic fluid (see Figure 6).
Figure 6.
The effect of ascites on OvCa cell adhesion and invasion. Treatment of SKOV3ip1 OvCa cells with ascites during adhesion to (A) and invasion through (B) the 3D organotypic model. Three independent patient ascites samples were tested. * (p<0.01) denotes a significant change in number of bound or invaded cells between treatments. Each bar represents the average of 3 wells and standard deviation. Hematoxylin and eosin staining of the 3D organotypic model with SKOV3ip1 OvCa cells for 48 hours in absence (C) or presence (D) of ascites. Col I, collagen I; HPMC, human primary mesothelial cells; HPF, human primary fibroblasts.
In our next study, we sought to determine if the 3D organotypic model could be used to unravel novel molecular mechanisms involved in metastasis of ovarian cancer to the omentum and peritoneum. The 3D culture was assembled with fibroblasts, mesothelial cells, and ECM (see Figure 4) and fluorescently labeled OvCa cells added and allowed to interact. The co-culture was harvested and the labeled OvCa cells sorted by FACS, allowing us to study the molecular changes in OvCa cells occurring after adhesion to the 3D culture. We found that after adhesion MMP-2 is transcriptionally upregulated in OvCa. Cancer cell derived MMP-2 then cleaved the ECM proteins, fibronectin and vitronectin allowing for even stronger adhesion of OvCa cells to the ECM fragments through αβ1 and αvβ3-integrins22.
In another project, our laboratory used the 3D culture to determine which integrin is important for the initial step of OvCa metastasis. OvCa cells were treated with antibodies against the most common integrins and adhesion was measured. We reported that α5-integrin, but not αvβ3-integrin, plays an important role in the initial adhesion of OvCa cells to the 3D culture18. These findings were confirmed in vivo when inhibition with the α5-integrin blocked adhesion to mouse peritoneum and omentum and decreased tumor growth and metastasis in a xenograft model of OvCa.
We believe that the organotypic omental or peritoneal 3D culture presents a number of advantages. The 3D culture can be assembled to be histologically very similar to the superficial layers of human omentum (see Figure 4) or peritoneum. It can be assembled with primary human omental mesothelial cells and primary human omental fibroblasts, approximating the human tissue as close as possible. While we have not yet done so, it is certainly feasible to extract mesothelial cells, fibroblasts and OvCa cells from the same patient and study patient specific metastatic biology. After extracting primary cells from many patients we found the results from various preparations of primary cells to be very reproducible (e.g. inhibition of OvCa cell adhesion and invasion by mesothelial cells). This model can also be used as a predictive preclinical model that allows for drug testing and might allow to predict if an ovarian cancer cell will respond to therapy. The fact that inhibition of adhesion with an MMP inhibitor22 or an α5-integrin antibody18 could be confirmed in other models show that drug sensitivity patterns can be recapitulated in the 3D organotypic model. From our perspective the chief strength of the 3D culture may be that labeled OvCa cells can be added to the culture and that after interaction, cancer cells can be isolated and used to study signaling, cell cycle progression, protease expression or other early regulatory pathways6.
Clearly, there are limitations to the herein described omental/peritoneal 3D culture. While it attempts to mimic the omental surface, it is only an approximation of the in vivo omentum. The 3D model lacks the host immune cells, vasculature, and other ECM proteins present in vivo. The culture is viable only for one week and thus, provides reproducible conditions only for a short period of time (see Figure 4). Given the intrinsic complexity of the invasion process this limits study of invasion to its earliest steps, such as the breakdown of the basement membrane or the early interaction with stromal cells. Also, only a limited number of investigators have access and approval to use human peritoneum or omental tissue for the isolation of primary cells, which from our perspective is necessary for a close approximation to the in vivo situation. Still, the model can be assembled by isolating mesothelial cells and fibroblasts from mouse or rat omentum/peritoneum, by using immortalized mesothelial (e.g. LP-9)15 or fibroblast39 cell lines. Alternatively, a full organ culture could be used to study ovarian cancer metastasis, as is described by Hotary et al.72. For this set-up, full rat peritoneum is stripped of overlying mesothelial cells and the basement membrane is cultured in a transwell dish.
Future Perspectives
Because of its modular concept, the 3D organotypic model of OvCa metastasis allows for the manipulation of individual culture conditions. The contribution of endothelial cells, inflammatory cells, and ECMs present in human peritoneum and/or omentum could be analyzed by including them into the culture. These cells have been shown in other cancers to contribute to the metastatic process 73. Another feature of the 3D model is that it could allow investigators to study OvCa spheroid adhesion and invasion, which appears to differ from the adhesion and invasion of single cells. Lu M. et al. found that A2780 cells grown in spheroids were more resistant to an anticancer drug associated with the overexpression of anti-apoptosis protein bcl-2 and the down-regulation of caspase-3 activity74. Gene expression analysis of 3 OvCa models from OvCa tumors and ascites showed diverse expression profiles between cells grown as spheroids, xenografts or monolayer cultures75. A third feature of the 3D organotypic model is that is could allow investigators to study Type I and Type II OvCa adhesion, invasion and proliferation and the molecular mechanisms involved in these pathways. Most recently, Kurman et al. have proposed to sub classify OvCa into two major sub-types after correlation of molecular genetic studies with clinical and histopathologic findings76. Type I cancers are slow growing tumors which are often confined to the ovary and harbor mutations in KRAS, BRAF, PTEN, and beta-catenin. Type II cancers are highly aggressive tumors that are highly metastatic and often have p53 mutations and a high level of genetic instability. The 3D model could be utilized to compare adhesion, proliferation and invasion in Type I and Type OvCa cells. Notably, the 3D organotypic model may enable us to discover unknown mechanisms of ovarian tumor metastasis to mesothelial cell covered surfaces. It is hoped that by identifying key molecules involved in the attachment, growth, or invasion of OvCa cells, researchers will discover new OvCa specific targets and, ultimately, develop new therapies, even site specific therapies.
Conclusions
The spread of cancer cells within the abdominal cavity leads to innumerable tumor nodules which can cause a significant tumor burden, bowel obstruction, and will ultimately lead to death. The omentum and peritoneum, which covers the entire abdominal cavity, provides a rich medium for the attachment, spread, growth, and invasion of OvCa cells. To make further progress in the treatment of OvCa, a better understanding of OvCa metastasis is absolutely essential. The 3D organotypic model of OvCa metastasis provides a physiologically relevant approximation to the human omentum and peritoneum allowing researchers to investigate various aspects of tumor biology regulated by the peritoneal microenvironment. We are hopeful that with all the models currently available that are best used together; we will advance our understanding of OvCa dissemination and find new therapies that regulate metastasis, thereby prolonging the survival of OvCa patients.
Acknowledgements
The development of the 3D ovarian cancer culture was supported over the years through grants to Ernst Lengyel from the Gynecologic Cancer Foundation (2005/2006 GCF/Molly Cade Ovarian Cancer Research Grant), the Ovarian Cancer Research Fund (OCRF, Liz Tilberis Scholars Program), and the NCI (R01 CA111882). Ernst Lengyel holds a Clinical Scientist Award in Translational Research from the Burroughs Wellcome Fund.
Hillary A. Kenny was supported by a Penny Severns Breast, Cervical, and Ovarian Cancer Research postdoctoral fellowship from the Illinois Department of Public Health and a Graduate Training Program in Cancer Biology postdoctoral fellowship through the University of Chicago (NIH/NCI 5T32 CA09594). Marion Zillhardt was supported by the Graduate Training Program in Cancer Biology through the University of Chicago (NIH/NCI T32 CA09594).
The authors would like to thank Gail Isenberg (University of Chicago) for her graphic designs and editorial expertise.
Reference List
- 1.Tan D, Agarwal R, Kaye SB. Mechanisms of transcoelomic metastasis in ovarian cancer. Lancet. 2006;7:925–934. doi: 10.1016/S1470-2045(06)70939-1. [DOI] [PubMed] [Google Scholar]
- 2.Landen C, Birrer MJ, Sood AK. Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol. 2008;26(6):995–1005. doi: 10.1200/JCO.2006.07.9970. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal R, Kaye S. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3(7):502–516. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 4.Doig T, Monaghan H. Sampling the omentum in ovarian neoplasia: When one block is enough. Int J Gynecol Cancer. 2006;16:36–40. doi: 10.1111/j.1525-1438.2006.00273.x. [DOI] [PubMed] [Google Scholar]
- 5.Fidler IJ. The pathogenesis of cancer metastasis: the "seed and soil" hypothesis revisited. Nat Rev Cancer. 2003;5:355–366. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 6.Kenny HA, Krausz T, Yamada SD, Lengyel E. Development of an organotypic peritoneal three-dimensional culture to study peritoneal attachment of ovarian cancer cells. Int J Cancer. 2007;121(7):1463–1472. doi: 10.1002/ijc.22874. [DOI] [PubMed] [Google Scholar]
- 7.Wilkoxz S, Ireland G, Khwaja N, et al. A comparative study of the structure of human and murine greater omentum. Anat Embryo. 2005;209:251–261. doi: 10.1007/s00429-004-0446-6. [DOI] [PubMed] [Google Scholar]
- 8.Liebermann-Meffert D. The greater omentum, anatomy, embryology, and surgical applications. Surg Clin North Am. 2000;80(1):275–293. doi: 10.1016/s0039-6109(05)70406-0. [DOI] [PubMed] [Google Scholar]
- 9.Lieberman-Meffert D. The greater omentum, anatomy, physiology, pathology and surgery with a historical survey. Berlin Heidelberg New York: Springer; 1985. pp. 3–30. [Google Scholar]
- 10.Daya D, McCaughy WT. Pathology of the peritoneum: A review of selected topics. Semin Diagn Pathology. 1991;8(4):277–289. [PubMed] [Google Scholar]
- 11.Leung JC, Chan LY, Li FF, et al. Glucose degradation products downregulate ZO-1 expression in human peritoneal mesothelial cells: the role of VEGF. Nephrol Dial Transplant. 2005;20(7):1336–1349. doi: 10.1093/ndt/gfh814. [DOI] [PubMed] [Google Scholar]
- 12.Liaw YS, Yu CJ, Shun CT, et al. Expression of integrins in human cultured mesothelial cells: the roles in cell-to-extracellular matrix adhesion and inhibition by RGD-containing peptide. Respir Med. 2001;95(3):221–226. doi: 10.1053/rmed.2000.1026. [DOI] [PubMed] [Google Scholar]
- 13.Strobel T, Swanson L, Cannistra SA. In vivo inhibition of CD44 limits intraabdominal spread of a human ovarian cancer xenograft in nude mice: A novel role for CD 44 in the process of peritoneal implantation. Cancer Res. 1997 Apr 1;57:1228–1232. [PubMed] [Google Scholar]
- 14.Strobel T, Cannistra SA. β1-integrins partly mediate binding of ovarian cancer cells to peritoneal mesothelium in vitro. Gynecol Oncol. 1999;73:362–367. doi: 10.1006/gyno.1999.5388. [DOI] [PubMed] [Google Scholar]
- 15.Lessan K, Aguiar D, Oegema TR, Siebenson L, Skubitz AP. CD44 and β1 integrin mediate ovarian carcinoma cell adhesion to peritoneal mesothelial cells. Am J Pathol. 1999;154(5):1525–1537. doi: 10.1016/s0002-9440(10)65406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed N, Riley C, Rice G, Quinn M. Role of integrin receptors for fibronectin, collagen and laminin in the regulation of ovarian carcinoma functions in response to a matrix microenvironment. Clin Exp Metastasis. 2005;22:391–402. doi: 10.1007/s10585-005-1262-y. [DOI] [PubMed] [Google Scholar]
- 17.Rieppi M, Vergani V, Gatto C, et al. Mesothelial cells induce the motility of human ovarian carcinoma cells. Int J Cancer. 1999;80:303–307. doi: 10.1002/(sici)1097-0215(19990118)80:2<303::aid-ijc21>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Sawada K, Radjabi AR, Bhaskar V, et al. Loss of E-cadherin promotes ovarian cancer metastasis via alpha 5-integrin, which is a therapeutic target. Cancer Res. 2008;68(7):2329–2339. doi: 10.1158/0008-5472.CAN-07-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nature. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 20.Mueller M, Fusenig N. Friends or foes - Bipolar effects of the tumour stroma in cancer. Nature Reviews. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 21.Witz C, Montoya-Rodriguez I, Cho S, Centonze V, Bonewald L, Schenken R. Composition of the extracellular matrix of the peritoneum. J Soc Gynecol Investig. 2001;8(5):299–304. doi: 10.1016/s1071-5576(01)00122-8. [DOI] [PubMed] [Google Scholar]
- 22.Kenny HA, Kaur S, Coussens L, Lengyel E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J Clin Invest. 2008;118(4):1367–1379. doi: 10.1172/JCI33775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moser TL, Pizzo SV, Bafetti L, Fishman DA, Stack MS. Evidence for preferential adhesion of ovarian epithelial carcinoma cells to type I collagen mediated by the α2β1 integrin. Int J Cancer. 1996;67:695–701. doi: 10.1002/(SICI)1097-0215(19960904)67:5<695::AID-IJC18>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Zhu G, Risteli J, Puistola U, Kauppila A, Risteli L. Progressive ovarian carcinoma induces synthesis of type 1 and type III procollagens in the tumor tissue and peritoneal cavity. Cancer Res. 1993;53:5028–5032. [PubMed] [Google Scholar]
- 25.Cannistra SA, Ottensmeier C, Niloff J, Orta B, DiCarlo J. Expression and function of β1 and αvβ3 integrins in ovarian cancer. Gynecol Oncol. 1995;58:216–225. doi: 10.1006/gyno.1995.1214. [DOI] [PubMed] [Google Scholar]
- 26.Symowicz J, Adley BP, Gleason KJ, et al. Engagement of collagen-binding integrins promotes matrix metalloproteinase-9-dependent E-cadherin ectodomain shedding in ovarian carcinoma cells. Cancer Res. 2007;67(5):2030–2039. doi: 10.1158/0008-5472.CAN-06-2808. [DOI] [PubMed] [Google Scholar]
- 27.Barbolina MV, Adley BP, Ariztia EV, Liu Y, Stack MS. Microenvironmental regulation of membrane type 1 matrix metalloproteinase activity in ovarian carcinoma cells via collagen-induced EGR1 expression. J Biol Chem. 2007;282(7):4924–4931. doi: 10.1074/jbc.M608428200. [DOI] [PubMed] [Google Scholar]
- 28.Ellerbroek SM, Wu YI, Overall CM, Stack MS. Functional interplay between type I collagen and cell surface matrix metalloproteinase activity. J Biol Chem. 2001 Jul 6;276:24833–24842. doi: 10.1074/jbc.M005631200. [DOI] [PubMed] [Google Scholar]
- 29.Fishman DA, Liu Y, Ellerbroek SM, Stack MS. Lysophosphatidic acid promotes matrix metalloproteinase (MMP) activation and MMP-dependent invasion in ovarian cancer cells. Cancer Res. 2001;61(7):3194–3199. [PubMed] [Google Scholar]
- 30.Fishman DA, Kearns AS, Chilukuri K, et al. Metastatic dissemination of human ovarian epithelial carcinoma is promoted by a α2β1-integrin-mediated interaction with type I collagen. Invasion & Metastasis. 1998;18:15–26. doi: 10.1159/000024495. [DOI] [PubMed] [Google Scholar]
- 31.Ellerbroek SM, Fishman DA, Kearns AS, Bafetti L, Stack MS. Ovarian carcinoma regulation of matrix metalloproteinase-2 and membrane type 1 matrix metalloproteinase through β1 integrin. Cancer Res. 1999;59:1635–1641. [PubMed] [Google Scholar]
- 32.Moser TL, Young TN, Rodriguez GC, Pizzo SV, Bast RC, Stack MS. Secretion of extracellular matrix-degrading proteinases is increased in epithelial ovarian carcinoma. Int J Cancer. 1994;56:552–559. doi: 10.1002/ijc.2910560415. [DOI] [PubMed] [Google Scholar]
- 33.Cheng K, Lahad J, Kuo W, et al. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nature Med. 2004;10(11):1251–1256. doi: 10.1038/nm1125. [DOI] [PubMed] [Google Scholar]
- 34.Shayesteh L, Lu Y, Kuo W, et al. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999 Jan;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- 35.Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 2002 Oct 4;111:29–40. doi: 10.1016/s0092-8674(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 36.Beningo K, Dembo M, Wanf Yu. Responses of fibroblasts to anchorage of dorsal extracellular matrix receptors. Proc Natl Acad Sci USA. 2004;101(52):18024–18029. doi: 10.1073/pnas.0405747102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sawada M, Shii J, Akedo H, Tanizawa O. An experimental model for ovarian tumor invasion of cultured mesothelial cell monolayer. Lab Invest. 1994;70(3):333–338. [PubMed] [Google Scholar]
- 38.Westerlund A, Hujanen E, Puistola U, Turpeenniemi-Hujanen T. Fibroblasts stimulate human ovarian cancer cell invasion and expression of 72-kDa gelatinase A (MMP-2) Gynecol Oncol. 1997;67:76–82. doi: 10.1006/gyno.1997.4808. [DOI] [PubMed] [Google Scholar]
- 39.Boyd R, Balkwill F. MMP-2 release and activation in ovarian carcinoma: The role of fibroblasts. Br J Cancer. 1999;80:315–321. doi: 10.1038/sj.bjc.6690357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rygaard J, Povlsen CO. Heterotransplantation of a human malignant tumor to "Nude" mice. Acta Pathol Microbiol Scand. 1969;77(4):758–760. doi: 10.1111/j.1699-0463.1969.tb04520.x. [DOI] [PubMed] [Google Scholar]
- 41.Voskoglou-Nomikos T, Pater JL, Seymour L. Clinical predicitive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin Cancer Res. 2003;9:4227–4239. [PubMed] [Google Scholar]
- 42.Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression. Journal of Theoretical Biology. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 43.Niedbala MJ, Crickard K, Bernacki R. In vitro degradation of extracellular matrix by human ovarian carcinoma cells. Clin Exp Metastasis. 1987;5(2):181–197. doi: 10.1007/BF00058063. [DOI] [PubMed] [Google Scholar]
- 44.Kanemoto T, Martin GR, Hamilton TC, Fridman R. Effects of synthetic peptides and protease inhibitors on the interaction of a human ovarian cancer cell line (NIH:OVCAR-3) with a reconstituted basement membrane (matrigel) Invasion & Metastasis. 1991;11:84–92. [PubMed] [Google Scholar]
- 45.Burleson KM, Boente MP, Pambuccian SE, Skubitz AP. Dissaggregation and invasion of ovarian carcinoma ascites spheroids. J Transl Med. 2006;24:4–6. doi: 10.1186/1479-5876-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burleson KM, Casey RC, Skubitz KM, Pambuccian SE, Oegema TR, Skubitz AP. Ovarian carcinoma ascites spheroids adhere to extracellular matrix components and mesothelial cell monolayers. Gynecol Oncol. 2004;93:170–181. doi: 10.1016/j.ygyno.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 47.Burleson KM, Hansen LK, Skubitz AP. Ovarian carcinoma spheroids disaggregate on type I collagen and invade live human mesothelial cell monolayers. Clin Exp Metastasis. 2004;21(8):685–697. doi: 10.1007/s10585-004-5768-5. [DOI] [PubMed] [Google Scholar]
- 48.Casey RC, Skubitz AP. CD44 and β1 integrins mediate ovarian carcinoma cell migration toward extracellular matrix proteins. Clin Exp Metastasis. 2000;18:67–75. doi: 10.1023/a:1026519016213. [DOI] [PubMed] [Google Scholar]
- 49.Casey RC, Oegema TR, Skubitz KM, Pambuccian SE, Grindle SM, Skubitz AP. Cell membrane glycosylation mediates the adhesion, migration, and invasion of ovarian carcinoma cells. Clin Exp Metastasis. 2003;20(2):143–152. doi: 10.1023/a:1022670501667. [DOI] [PubMed] [Google Scholar]
- 50.Casey RC, Koch KA, Oegema TR, et al. Establishment of an in vitro assay to measure the invasion of ovarian carcinoma cells through mesothelial cell monolayers. Clin Exp Metastasis. 2003;20:343–356. doi: 10.1023/a:1024009131191. [DOI] [PubMed] [Google Scholar]
- 51.Casey RC, Burleson KM, Skubitz KM, et al. β1-integrins regulate the formation and adhesion of ovarian carcinoma multicellular spheroids. Am J Pathol. 2001 Dec;159:2071–2080. doi: 10.1016/s0002-9440(10)63058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skubitz AP, Bast RC, Wayner EA, Letourneau PC, Wilke MS. Expression of α6 and β4 integrins in serous ovarian carcinoma correlates with expression of the basement membrane protein laminin. Am J Pathol. 1996;148(5):1445–1461. [PMC free article] [PubMed] [Google Scholar]
- 53.Barbolina MV, Adley BP, Shea LD, Stack MS. Wilms tumor gene protein 1 is associated with ovarian cancer metastasis and modulates cell invasion. Cancer. 2007;112(7):1632–1641. doi: 10.1002/cncr.23341. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki N, Aoki D, Tamada Y, et al. HMOCC-1, a human monoclonal antibody that inhibits adhesion of ovarian cancer cells to human mesothelial cells. Gynecol Oncol. 2004;95:290–298. doi: 10.1016/j.ygyno.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 55.Kishikawa T, Sakamoto M, Ino Y, Kubushiro K, Nozawa S, Hirohashi S. Two distinct pattern of peritoneal involvement shown by in vitro and in vivo ovarian cancer dissemination models. Invasion & Metastasis. 1995;15:11–21. [PubMed] [Google Scholar]
- 56.Niedbala MJ, Crickard K, Bernacki R. Interactions of human ovarian tumor cells with human mesothelial cells grown on extracellular matrix. An in vitro model system for studying tumor cell adhesion and invasion. Exp Cell Res. 1985;160(2):499–513. doi: 10.1016/0014-4827(85)90197-1. [DOI] [PubMed] [Google Scholar]
- 57.Weaver VM, Fischer A, Peterson O, Bissell MJ. The importance of the microenvironment in breast cancer progression: recapitulation of mammary tumorigenesis using a unique human mammary epithelial cell model and a three-dimensional culture assay. Biochem Cell Biology. 1996;74(6):833–851. doi: 10.1139/o96-089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weaver VM, Petersen OW, Wang F, et al. Revision of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. The Journal of Cell Biology. 1997;137(1):231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park CC, Zhang H, Pallavicini M, et al. β1 Integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growths, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006;66(3):1526–1535. doi: 10.1158/0008-5472.CAN-05-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weaver VM, Howlett AR, Langton-Webster B, Petersen O, Bissell M. The development of a functionally relevant cell culture model of progressive human breast cancer. Sem Cancer Biol. 1995;6(3):175–184. doi: 10.1006/scbi.1995.0021. [DOI] [PubMed] [Google Scholar]
- 61.Wang F, Weaver VM, Petersen OW, et al. Reciprocal interactions between β1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: A different perspective in epithelial biology. Proc Natl Acad Sci USA. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rizki A, Weaver VM, Lee SY, et al. A human breast cell model of preinvasive to invasive transition. Cancer Res. 2008;68(5):1378–1387. doi: 10.1158/0008-5472.CAN-07-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee GY, Kenny PA, Lee EH, Bissel MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods. 2007;4(4):359–365. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peterson OW, Ronnov-Jessen L, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant breast epithelial cells. Proc Natl Acad Sci USA. 1992;89(19):9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roskelley CD, Desprez PY, Bissell MJ. Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemiacal signal transduction. Proc Natl Acad Sci USA. 1994;91:12378–12382. doi: 10.1073/pnas.91.26.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zutter MM, Santoro SA, Staatz WD, Tsung YL. Re-expression of the α2β1-integrin abrogates the malignant phenotype of breast carcinoma cells. Proc Natl Acad Sci USA. 1995;92:7411–7415. doi: 10.1073/pnas.92.16.7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berking C, Herlyn M. Human skin reconstruct models: A new application for studies of melanocyte and melanoma biology. Histo Histopath. 2001;16:669–674. doi: 10.14670/HH-16.669. [DOI] [PubMed] [Google Scholar]
- 68.Meier FE, Nesland M, Hsu M, et al. Human melanoma progression in skin reconstructs. Am J Pathol. 2000;156(1):193–200. doi: 10.1016/S0002-9440(10)64719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haass NK, Smalley KS, Li L, Hermes M. Adhesion, migration and communication in melanocytes and melanoma. Pigment Cell Res. 2005;18(3):150–159. doi: 10.1111/j.1600-0749.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- 70.Auersperg N, Ota T, Mitchell GW. Early events in ovarian epithelial carcinogenesis progress and problems in experimental approaches. Int J Gynecol Cancer. 2002;12:691–703. doi: 10.1046/j.1525-1438.2002.01152.x. [DOI] [PubMed] [Google Scholar]
- 71.Puiffe ML, Le Page C, Filali-Mouhim A, et al. Characterization of ovarian cancer ascites on cell invasion, proliferation, spheroid formation, and gene expression in an in vitro model of epithelial ovarian cancer. Neoplasia. 2007;9(10):820–829. doi: 10.1593/neo.07472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hotary KB, Li X, Allen E, Stevens SL, Weiss SJ. A cancer cell metalloprotease triad regulates the basement membrane transmigration program. Genes Dev. 2006;20:2673–2686. doi: 10.1101/gad.1451806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66(2):605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 74.Lu M, Gao R, Xiao L, Wang Z. Construction of three-dimensional in vitro culture model of ovarian carcinoma and the study of its multicellular drug resistance. J Huazhong Univ Sci Technolog Med Sci. 2006;26(6):741–743. doi: 10.1007/s11596-006-0632-2. [DOI] [PubMed] [Google Scholar]
- 75.Zietarska M, Maugard C, Filali-Mouhim A, et al. Molecular description of a 3D in vitro model for the study of epithelial ovarian cancer (EOC) Molecular Carcinogenesis. 2007;46:872–885. doi: 10.1002/mc.20315. [DOI] [PubMed] [Google Scholar]
- 76.Kurman R, Visvanathan K, Roden R, Wu TC, Shih I-M. Early detection and treatment of ovarian cancer: shifting from early stage to minimal volume of disease based on a new model of carcinogenesis. Journal of obstetrics and gynecology. 2008:351–356. doi: 10.1016/j.ajog.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]