Abstract
Carbon monoxide (CO), a low molecular weight gas, is a ubiquitous environmental product of organic combustion, which is also produced endogenously in the body, as the byproduct of heme metabolism. CO binds to hemoglobin, resulting in decreased oxygen delivery to bodily tissues at toxicological concentrations. At physiological concentrations, CO may have endogenous roles as a potential signaling mediator in vascular function and cellular homeostasis. Exhaled CO (eCO), similar to exhaled nitric oxide (eNO), has been evaluated as a candidate breath biomarker of pathophysiological states, including smoking status, and inflammatory diseases of the lung and other organs. eCO values have been evaluated as potential indicators of inflammation in asthma, stable COPD and exacerbations, cystic fibrosis, lung cancer, or during surgery or critical care. The utility of eCO as a marker of inflammation, and potential diagnostic value remains incompletely characterized. Among other candidate “medicinal gases” with therapeutic potential, (e.g., NO and H2S), CO has been shown to act as an effective anti-inflammatory agent in preclinical animal models of inflammatory disease, acute lung injury, sepsis, ischemia/reperfusion injury and organ graft rejection. Current and future clinical trials will evaluate the clinical applicability of this gas as a biomarker and/or therapeutic in human disease.
Keywords: Carbon Monoxide, Exhaled Breath, Heme Oxygenase-1
Introduction
Carbon monoxide (CO), and other similar low molecular weight gases including nitric oxide (NO), and hydrogen sulfide (H2S) have gained much recent attention as “medically-relevant gases” [1-8], with respect to three major areas of investigation: (I) as endogenously produced biological mediators that may have physiological significance in the regulation of cellular and tissue homeostasis; (II) as exhaled substances on the breath that can be detected and evaluated as potential biomarkers of inflammation or disease, and (III) as candidate inhalation gaseous therapeutics for lung diseases and other disorders.
(I) Gases as physiological mediators
The discovery of nitric oxide (NO) as an endogenously produced gas identical to the vascular mediator previously identified as “endothelial derived relaxing factor” heralded the beginning of the era of physiologically relevant gases [9-10]. NO arises endogenously as the product of constitutive and inducible nitric oxide synthase enzymes (NOS), and can exert physiological functions on vascular tone and homeostasis [11]. These effects were found to be mediated largely through activation of guanylate cyclase (sGC) and production of 3′,5′-cyclic guanosine monophosphate (cGMP) [12-13]. CO has recognized for a longer period as an endogenously produced “waste” gas that is produced primarily as the byproduct of hemoglobin turnover [14-15]. CO is a natural product of the heme catalytic activity of heme oxygenase (HO) enzymes [16]. Recent studies over the last decades have sought to determine a physiological significance of endogenous CO production, including putative roles of this gas as a mediator of vascular function [17], inflammation [18], and neuronal signaling [19-20]. A third endogenously produced gaseous molecule H2S has been recently been demonstrated in mammalian systems [7].
(II) Gases in Exhaled Breath Testing
Analysis of exhaled breath promises a non-invasive and practical approach for the evaluation of human health and disease. Exhaled breath testing relies on the principle that human breath contains a number of dissolved or gaseous substances, the concentrations of which may vary with the physical environment, diet, and wellness state of the individual. Sampling and analyses of these substances in breath may be used to construct an analytical profile that may be employed to diagnose diseases of the lung or other organs, or to monitor the effectiveness of pharmaceutical interventions [21-26].
Exhaled breath monitoring may include various analytical approaches including the analysis of exhaled breath condensates for pH or chemical composition [26-27], the analysis of volatile organic compounds on breath by gas chromatography/mass spectrometry [28-29], and the electrochemical detection of trace gases in exhaled air, including NO and CO [30-36].
To date, both NO and CO have been implicated as possible indicators of lung and/or systemic inflammation, or oxidative stress. In this regard, both exhaled NO (eNO) [30-33] or exhaled CO (eCO) [32-36] have been widely studied as putative inflammatory markers of disease, with applications in respiratory diseases including asthma [30-32, 35-37], chronic obstructive pulmonary disease (COPD) [33], cystic fibrosis [38], bronchiectasis [39] as well as in systemic conditions such as sepsis [40-41], and diabetes [42]. Recent studies have measured eCO as a general marker of inflammation in critically ill, surgical or organ transplant patients [43-47]. Finally, eCO has been proposed as a useful breath test for validating current smoking status of patients [33, 48]. This review will therefore discuss studies that either support or refute the validity of eCO as a predictor of disease.
(III) Gases in Molecular Medicine
The lung, which by nature acts as a portal of entry for air, primarily for the delivery of oxygen to the systemic circulation, naturally serves as the ideal target for inhalation therapeutics. The prospect of utilizing gases as potential molecular medicine represents a viable and practical experimental approach to therapy. In this regard, NO remains one of the most widely studied medical gases. Inhaled NO (iNO) acts as a selective pulmonary vasodilator. iNO has been investigated for its therapeutic potential in diseases such as acute respiratory distress syndrome (ARDS), and pulmonary hypertension [49-52], and has been used clinically for the treatment of persistent pulmonary hypertension of the newborn [53]. By comparison, CO has been indicated as an inhalation or ex vivo therapeutic on the basis of extensive preclinical animal testing in rodents and higher animals. The therapeutic effects of CO have been studied in models of acute lung injury (ALI) and inflammation, ischemia/reperfusion (I/R) injury, sepsis, organ transplantation, and others (Reviewed in Refs. [1-5, 54-55]). These effects of CO have been related to the modulation of inflammation, as discussed in the following sections, and may also include a number of other mechanisms, including pro-/anti-apoptotic effects, immunomodulatory effects, anti-/pro-oxidative effects, and effects on systemic circulation [1-5] .
With respect to these three focus areas, this review will focus primarily on CO, with a discussion of the biological significance of CO as an endogenously produced gas and as a possible physiological mediator of cellular homeostasis. Emphasis will be placed on medical applications of CO, with respect to its potential as a breath biomarker for applications in clinical diagnostics and therapeutic monitoring, and also as an experimental inhalation therapeutic for pulmonary and inflammatory diseases.
Biological Sources of Carbon Monoxide
Environmental CO
CO represents a toxic inhalation hazard, and a common contaminant of indoor and outdoor air. Environmental CO arises primarily as the product of organic combustion processes, including the burning of wood, coal, gas, and tobacco [56]. Indoor levels of CO range from 0.5-5 parts per million (ppm) but may reach higher values (up to 30 ppm). Ambient CO values may be higher near sources of combustion, alongside roadways, or in heavily populated urban areas. For example, CO levels near high traffic areas can reach 10-50 ppm [57]. Cigarette smoke (which contains ~4% CO by volume) represents a significant source of ambient CO, which may yield up to 20 mg of CO per cigarette, depending on the type of cigarette [58].
Systemic CO
Carbon monoxide (CO) arises in the human body as the natural product of hemoprotein turnover, most of which originates from circulating hemoglobin [14-15, 59-61]. Under physiological conditions the rate of endogenous CO production has been estimated at ~18 μmol CO per hour [15]. Turnover of other hemoprotein species may also contribute to endogenous systemic CO production as a function of relative abundance and turnover rate, and these include myoglobin, cytochrome p-450, catalase, and others [62].
CO produced systemically forms a complex with native hemoglobin (Hb) to form the cherry red pigment carboxyhemoglobin (COHb), with an affinity for Hb ~245 times that of oxygen. COHb acts as a sink for endogenously produced or inhaled CO [56]. The basal COHb level in man is approximately 0.1-1% in the absence of environmental contamination or smoking. Habitation of heavily populated urban areas with high levels of ambient CO, such as originating from automobile exhaust may increase this background [63]. Habitual smokers may have COHb values typically between 5-15% or higher [64]. COHb levels of greater than 20% are typically associated with symptoms of clinical toxicity, which may lead to neurotoxicity, cognitive impairment, unconsciousness, and death at chronic or elevated concentrations [65-66].
Heme, the iron containing, and oxygen-binding co-factor of cellular hemeoproteins (e.g., Hb) is degraded by the heme oxygenase (HO: E.C: 1:14:99:3) enzyme system [16]. Whereas heme-b is the major substrate of HO, other forms of heme (e.g., heme-a, heme-c) may also serve as substrates [62]. The HO enzymes bind heme and catalyze the cleavage of the α-methene bridge, resulting in tetrapyrrole ring opening and oxidation to generate the bile pigment biliverdin-IXα, with concomitant release of equimolar amounts of ferrous iron and CO. The reaction utilizes 3 moles of O2 per heme molecule oxidized and requires electrons from the NADPH cytochrome p450 reductase system [16]. Bilirubin-IXα is then reduced to biliverdin-IXα by NADPH: biliverdin reductase [67]. Although the HO reaction represents the major enzymatic source of CO, a minor component of endogenous CO may arise from non-heme sources, although this fraction is incompletely characterized. For example, CO may arise as a by-product of lipid oxidation or as the product of the metabolism of certain xenobiotics (e.g. methylene chloride) by the cytochrome p-450 system [68].
Heme oxygenases consist of two major isozymes (HO-1, and HO-2), each the product of distinct genes [62, 69]. HO-1, the inducible isozyme, represents a major cellular stress response [70]. The transcriptional induction of HO-1 responds to many extracellular stress signals, belonging to diverse chemical and physical categories. In particular, the response can be elicited by the natural enzymatic substrate heme, itself a pro-oxidant compound, as well as by oxidants, heavy metals and thiol-reactive substances, natural antioxidants, and fluctuations in ambient oxygen tension [1, 71]. HO-1 represents a general inducible response to oxidative or pro-inflammatory stress. HO-2, a constitutive isozyme, does not typically respond to transcriptional induction with some exceptions (e.g., glucocorticoids) [72]. HO-2 is constitutively expressed in most tissues, with high values reported for testis, liver, brain, and vascular tissue [62].
Carbon Monoxide in Exhaled Breath
Source of Exhaled CO
The origins of eCO likely reflect a systemic elimination process through diffusion of CO from the pulmonary circulation through the alveolae. In this regard eCO values may be related in part to HbCO values. Since HO-1 gene expression and subsequent activity, a source of CO, can be induced by inflammation or systemic stress, there may be a relationship between increased heme metabolic rate and increases in HbCO values, and/or eCO values. In addition to systemic production, however, a significant fraction of eCO may originate directly in the airways and nasal passages [39, 73]. eCO may arise as the product of inducible HO-1 activity in the airway and nasal epithelium, as well as in alveolar macrophages, endothelial cells and other lung cell types, as the consequence of local inflammation or oxidative stress [39, 73].
Detection of CO
The eCO levels in exhaled breath are most commonly measured with electrochemical (chemiluminescence) technology [24, 74-76]. The values thus obtained correlate with parallel gas chromatographic analysis and these sensors are sensitive in the 1-500 ppm range. Current analytical devices are portable which makes them ideal for clinical use [77]. More recent CO detection systems suitable for clinical measurements include a gas sensor adapted from a controlled potential electrolysis method, which is sensitive to 0.1 ppm [77]. At the experimental stage, several novel techniques based on infrared laser spectroscopic methods have been recently developed which report enhanced sensitivity for CO in the parts per billion (ppb) range. Variations on these techniques include cavity leak out spectroscopy (CALOS), integrated cavity output spectroscopy (ICOS), cavity ring-down spectroscopy (CRDS) and quantum cascade laser absorption spectroscopy (QCLAS) [78-84].
Increasing the sensitivity of gas detection apparatus would provide definite advantage with respect to analytical measurements. However this endeavor must be reconciled with other factors, including portability, practicality for clinical use, and cost effectiveness. Nevertheless, a recent study has demonstrated the application of laser spectrometric methods to clinical monitoring of respiratory disease patients, with the implementation of a portable compact apparatus [85].
Assessment of Smoking Status
eCO has been proposed as a practical, non-invasive diagnostic tool for health professionals to assess and monitor smoking status, in particular, in patients who deny or underestimate smoking status in self-evaluation [32, 35, 48, 86-87]. Recent studies suggest that eCO measurements may represent a reliable and cost-effective alternative to testing of urinary cotinine, as a method for monitoring smoking cessation [88]. eCO values are typically increased in smokers with normal lung function relative to non-smokers [48]. The eCO appears to correlate with the frequency and number of cigarettes smoked and may vary with the type of tobacco, cigarette, or smoking method [89-90]. In a comprehensive survey of 322 subjects, a mean value of 17 ppm was reported for normal smokers relative to recorded values of ~3 ppm and ~5 ppm for non-smokers and passive smokers, respectively [48]. This study and two other reports of outpatient smokers have suggested optimal cutoff values in the range 6.0-6.5 ppm to distinguish smokers from nonsmokers; values in excess of which may indicate recurrent smoking with 90-96% sensitivity and 80-93% specificity [48, 86, 91]. It should be noted that despite these approximations, eCO values exceeding 7 ppm have been reported among non-smokers living in heavily urbanized areas [92]. Measured eCO values were found to decay exponentially with time elapsed since the last incidence of smoking with a half-life of approximately 4.3 hrs [93]. Recent studies suggest that eCO values may be useful to discriminate between recent smoking (<8 hrs since last incidence) and short term abstinence (>8 hrs) among current smokers, and have proposed an arbitrary cutoff value of 12 ppm for this purpose [93]. As the presence of underlying pulmonary diseases associated with inflammatory responses, such as asthma and COPD (discussed in the subsequent sections) may impact eCO values, some reports have also suggested that cutoff values for smoking cessation should be adjusted higher for patients with these diseases [94]. By comparison eNO values were reported to decline in smokers relative to non-smokers with or without COPD [33, 95].
COPD
COPD is a disease defined by progressive airflow limitation that is not fully reversible, associated with an abnormal inflammatory response of the lungs to noxious particles or gases [96-97]. Worldwide, cigarette smoking remains the principle cause of COPD, which develops in ~15-20% of smokers. However, environmental and occupational exposures to particulate air pollution, chronic infections, and asthma, may also contribute to COPD progression [98]. Cigarette smoke (CS) is a complex mixture, contains low molecular weight gases (e.g., nitrogen oxides NO/NO2, and CO), as well as ~4,500 chemicals which are present in or generated during combustion of tobacco, including free radicals, oxidants, aldehydes, heavy metals (e.g., arsenic, cadmium, lead) and aromatic hydrocarbons [99]. The underlying mechanisms of COPD pathogenesis remain incompletely understood, but may include protease/anti-protease imbalance, increased oxidative stress, and pulmonary cell apoptosis [98, 100-102].
With respect to exhaled breath analysis, COPD patients had higher measured eCO values than non-smokers without COPD. Among COPD patients, current smokers at the time of study had higher eCO values than former smokers [33]. However, eCO values did not correlate with lung function measurements assessed by forced expiratory volume in 1 second (FEV1), nor with eNO values. Measured eNO values were also higher in COPD patients than controls; however, eNO levels were reported higher among COPD non-smokers than COPD smokers. eNO values also negatively correlated with lung function as assessed by FEV1 [33]. In the Copenhagen Heart Study, eCO values among smokers had no correlation to lung function or lung function decline [90]. Recent studies conclude that eCO levels may also correlate with exacerbations in chronic lung disease in children [103]. eCO values were also measured in α-1 anti-trypsin (α1-AT) deficiency, a genetic cause of emphysema associated with increased neutrophil elastase activity and lung tissue destruction which arises independently of smoking status. In α1-AT deficiency patients, eCO levels were found to be decreased compared to those of healthy subjects or those with smoking-associated COPD, despite the presence of pulmonary inflammation in this disease [104].
Comparative HbCO measurements have also been made to evaluate endogenous CO production in COPD. Higher arterial HbCO were measured in ex-smoking stable COPD patients relative to healthy ex-smoking patients. The increases in HbCO correlated with COPD disease severity with greater HbCO levels recorded in severe COPD, compared to moderately severe disease. HbCO levels were further elevated in patients with COPD exacerbations relative to stable disease [105]. Increased HbCO correlated with increased eCO at moderate stages of COPD severity but did not correlate at advanced stage. This apparent conundrum was interpreted to represent declined lung/airways production of CO or decreased alveolar function and CO diffusion capacity in severe COPD, in combination with increased systemic CO production [105].
HO-1, the enzyme primarily responsible for endogenous CO production, may be implicated in the pathogenesis of COPD. HO-1 may confer protection against the pro-oxidant and pro-apoptotic effects of cigarette smoke in epithelial cells [106-107], and also protect in vivo against experimental emphysema [108]. Elevated levels of HO-1 were reported in the alveolar spaces of chronic smokers with and without COPD relative to non-smokers [109]. Increased HO-1 staining in inflammatory cells was reported during exacerbations of severe COPD relative to stable disease [110]. In contrast, decreased HO-1 expression was observed in alveolar macrophages of severe COPD patients relative to those of smokers without lung function impairment [111]. In a study which controlled for smoking status, ex-smoking COPD patients were reported to have reduced HO-1 expression in alveolar macrophages relative to healthy ex-smokers [112]. These results suggest that elevations of eCO observed in COPD are not necessarily correlated with HO-1 activation, especially in advanced disease, as reported for asthmatics [37].
Asthma and airways disease
Asthma is a chronic inflammatory disease of the airways that results in an airflow limitation that is partially reversible either spontaneously or by therapy. The persistent inflammation associated with this disease causes airway hypersensitivity to allergens and irritants [113]. With respect to eCO measurements in asthmatics, eCO values were significantly higher in non-steroid treated asthmatic patients compared with healthy subjects, whereas these values returned to baseline in patients receiving corticosteroids [37]. Acute asthma exacerbations caused increased eCO values associated with a reduction in peak expiratory flow rate. These changes were reversible by oral glucocorticoids [114]. Moreover, eCO levels in asthma patients on β-agonists were decreased in response to a four week regimen of inhaled corticosteroid therapy and correlated with decreases in sputum eosinophil counts [115]. In atopic asthma patients, eCO values did not correlate with lung functional measurements in a cross-sectional analysis. However a longitudinal trend towards correlation of eCO values with bronchial reactivity was described [116]. In allergic asthmatics, eCO increased in response to allergen challenge that preceded the peak decline in lung function as assessed by FEV1 [117]. The eCO values in these patients were not sensitive to stimuli that reduced FEV1, including histamine, or to subsequent recovery by β-agonists, suggesting that eCO values did correlate to airway function [117]. Differential effects of methacholine and allergen challenges on the levels of eCO and eNO were studied in a cohort of adult subjects with atopic asthma. Bronchospasm negatively modulated eCO and eNO values, whereas the inflammatory stimulus of allergen exposure increased eNO levels [118].
In bronchial asthma patients, eCO levels correlated with arterial HbCO levels [119-120]. The HbCO levels increased at the time of exacerbation, and declined to control levels in response to oral steroid treatment [119]. HbCO concentrations significantly correlated with FEV1. Furthermore, arterio-venous HbCO differences were higher in patients with bronchial asthma than in control subjects [120]. Increased HO-1 expression was detected in alveolar macrophages of patients with recent asthma exacerbations relative to those of healthy controls or patients undergoing steroid treatment [37]. This observation was associated with increased bilirubin levels in the induced sputum, and correlated with increases in eCO values in non-steroid treated asthmatics [37]. However, another study has also reported no change in HO-1 expression in airway macrophages and epithelium of mild asthmatics relative to healthy subjects, and no further change with extended inhaled steroid treatment [121].
Additional studies have examined exhaled breath gases in pediatric asthma patients. Increased eCO and eNO levels have been documented in children with persistent asthma, relative to healthy controls. Furthermore eNO, but not eCO, was significantly higher in patients with infrequent episodic asthma [122]. In childhood asthma patients, eCO values were found to be independent of expiratory flow rate [123]. Recent studies in preschool children report that eCO values were higher during asthma attacks relative to levels recorded in asymptomatic asthma subjects or healthy controls, and these values were normalized during combination inhalation therapy with salbutamol and sodium cromoglycate [124]. eCO levels were also found to correlate with allergic seasonal rhinitis, which returned to control values at the end of the allergen season [125-126].
Despite reports suggesting eCO measurement as a useful biomarker of asthma exacerbation and response to steroid therapy, several studies have reported negative findings with respect to eCO flux in asthma patients [116, 121, 127]. For example, no change in eCO was recorded in asthmatics following 30 days of inhaled corticosteroids, despite decreased airway eosinophil content and bronchial responsiveness to metacholine [121]. The explanation for variable results across studies is not entirely clear but may be related to differences in the sensitivity of the apparatus used, ambient background levels, and sampling size.
A recent literature analysis concluded that eCO levels generally increase in asthmatics relative to normal patients, and are responsive to treatment with corticosteroids, yet only partially reflect disease severity [128]. However, eCO levels do not distinguish between degrees of disease control or between steroid-treated and steroid-free patients in cross-sectional analysis [128]. In conclusion, eCO may still represent a potentially useful biomarker of airway inflammation and oxidative stress in non-smoking asthma patients [128].
eCO in Cystic Fibrosis
Cystic fibrosis is a genetic disorder characterized by aberrant mucous accumulation in the airways, which can cause pulmonary damage associated with secondary infections [129]. Measured eCO levels were higher in untreated vs. oral steroid-treated CF patients [38]. Furthermore, eCO increased in patients during exacerbations of CF, correlating to decline of FEV1, with normalization of the eCO levels after treatment [130]. Both eCO and exhaled ethane, a marker of oxidative stress, were elevated in non-steroid-treated CF patients, when compared to healthy controls, and these values were reduced by steroids [131]. Increased eCO was detected in children with CF, relative to healthy controls. Exercise testing further increased eCO in CF children and correlated with the degree of oxyhemoglobin desaturation [132]. In contrast, several studies have reported negative findings with respect to differences between eCO values of CF patients relative to healthy controls [123, 127].
eCO in Lung transplantation
Bronchiolitis obliterans syndrome (BOS) is the leading cause of death after lung transplantation. BOS affects 50-60% of all cases within the first five years following surgery with a ~30% mortality rate. The pathology of BOS involves mononuclear cell-predominant inflammation and scarring of the small airways resulting in altered lung function. Preventive therapeutic interventions are now available, but airflow limitation is not detectable early on during the course of the disease. The helium or nitrogen single breath washout technique is currently used to predict early BOS but the low sensitivity of the test limits is clinical value [133]. Increased eCO and eNO levels were detected in 65 patients with BOS [134]. When eCO and eNO was measured in combination with helium single breath washout it was highly discriminative of early (stage 0-1) BOS. The authors concluded that the combined testing has a high negative predictive value and it can be used as a non-invasive screening tool to exclude BOS following transplantation. Increased eCO levels also correlated with elevated neutrophil granulocyte count and pro-inflammatory cytokine levels in bronchoalveolar lavage fluid collected from lung transplant patients [46].
eCO in Respiratory Infections, Surgery, and Critical Care
The potential application of eCO for the non-invasive monitoring of patients with infectious disease or critical illness has also been explored. Elevated eCO levels were reported in lower respiratory tract infections, and these were decreased by antibiotic therapy [135]. Median eCO levels were higher among patients with bronchioectasis, a condition related to increased neutrophil inflammation, regardless of steroid treatment. However, eCO did not correlate with HbCO in this condition [39]. Furthermore, eCO levels in upper respiratory tract infections were higher when compared to healthy controls [123, 126, 136]. In patients with pneumonia, higher HbCO levels can be measured at the onset of illness, with decreasing values to control levels after antibiotic treatment [119].
Elevated eCO values have been measured in mechanically ventilated critical care subjects. In a clinical study of 29 patients on mechanical ventilation with 8 healthy non-smoking controls, average eCO values were higher in mechanically-ventilated patients relative to healthy subjects, and correlated with arterial HbCO levels, as well as with serum total and indirect bilirubin levels [77]. Another study of 30 ventilated patients also recorded an increase in eCO values when compared to a control group, whereas no correlations were found between eCO and arterial or venous HbCO [43]. Furthermore, eCO in mechanically-ventilated patients was shown to be responsive to increases in oxygen intake [44]. Increased eCO values were also recorded in mechanically-ventilated critically ill patients with sepsis, relative to ventilated but non-septic controls. Elevated eCO values on the first day of treatment correlated with the probability of patient survival [41]. In a study of non-smoking surgical patients, who had undergone general or spinal anesthesia, elevated mean eCO levels, as well as elevated arterial HbCO levels, were observed in post-surgical patients on the day after surgery relative to preoperative values, or later during recovery [45].
Additional Factors Influencing eCO measurements
Whereas eCO levels may be a reliable test of smoking status or pulmonary inflammation independently of each other, the predictive value of this test may be compromised in situations involving combinations of smoking, pulmonary disease, or high environmental background [35]. Recent studies indicate that eCO values may be slightly impacted by acute hyperventilation [137]. Interestingly, eCO may fluctuate with the ovarian cycle in women [138]. eCO levels increased after ovulation peaked in the premenstrual phase, whereas eNO values peaked at mid-cycle [138].
Carbon Monoxide in Therapeutics
The promise of therapies based on CO application originated with observations of protective effects in animal model studies and related in vitro experimentation which will be summarized in the following sections. In rodent models, inhalation of CO at low concentration (~250-500 ppm) has been shown to exert protective effects in acute lung injury (ALI) induced by high oxygen stress (hyperoxia) [139-140], mechanical ventilation [141-144], endotoxemia [18], ischemia/reperfusion injury [145-147], vascular injury [148], and graft rejection [148-150]. Additional applications of therapeutic CO have been discussed in other recent reviews [1, 3, 5].
General Mechanisms for Cytoprotective Effects of Carbon Monoxide
Many cellular studies modeling the therapeutic potential of CO have suggested roles of this gas as a signaling mediator of cellular homeostasis [151]. Similar to its cognate gas NO, CO has been proposed to act as a ligand and activator of soluble guanylate cyclase (sGC). CO forms a complex with the heme iron of sGC, inducing a conformational change leading to increased synthesis of guanosine 3′,-5′-monophosphate (cGMP). While both CO and NO bind to the heme iron of sGC, CO assumes a hexa-coordinate ligand structure, whereas the binding of NO is pentacoordinate, and involves the displacement of the axial histidine ligand of sGC [152]. Experimental evidence indicates that NO activates sGC in vitro and corresponding vasodilatory action in vivo with far greater potency [153]. Increased cGMP has been implicated in the vascular and neural effects of CO [17, 19, 151, 154]. CO may also regulate vascular function through the activation of calcium-dependent potassium channels Kca in vascular smooth muscle cells [155] These interactions may be relevant to airway epithelia during inhalation and exhalation of CO.
Cellular exposure to CO modulates several intrinsic signaling pathways. Of these, CO can stimulate activation of the mitogen activated protein kinases (MAPK), which are critical for the transduction or pro-inflammatory and/or stress signaling [18, 140, 145-146, 156]. CO was also found to modulate the STAT3 pathway and the cytoprotective phosphatydylinosital-3-kinase/Akt pathway in the context of protection against oxygen-dependent cell death [157].
Potent anti-inflammatory effects of CO were demonstrated in cultured macrophages [18]. Exogenous application of CO inhibited the secretion of pro-inflammatory cytokines in macrophages stimulated with bacterial lipopolysaccharide, related to activation of p38 MAPK [18], and also to inhibition of Toll-like receptor trafficking and activation [158], and enhanced PPAR–γ signaling [159]. CO when applied at low concentration, inhibited cell death caused by pro-apoptotic agents (e.g., TNFα) in endothelial cells, also through stimulation of the p38 MAPK pathway [156]. CO also been shown to exert anti-proliferative effects in vitro, with respect to the proliferation of vascular smooth muscle [160]. Collectively, these modulatory effects of CO on inflammatory, apoptosis and proliferative pathways all potentially contribute to the proposed therapeutic effects of this gas. Additional mechanisms underlying the cytoprotective effects of CO have been recently proposed. Among these include possible roles of this gas as stimulator of mitochondrial biogenesis [161-162], and as a regulator of extrinsic apoptotic pathways [163-164]. More recently, CO has been shown to positively regulate cellular macroautophagy, a cellular response to starvation that is involved in organelle turnover and nutrient recycling [165].
Acute Lung injury
Low doses of CO have been shown to provide protection against ALI in rodent models. The administration of CO (250 ppm) during hyperoxia exposure prolonged the survival of rats and mice subjected to a lethal dose of hyperoxia, and dramatically reduced histological indices of lung injury, including airway neutrophil infiltration, fibrin deposition, alveolar proteinosis, pulmonary edema, and apoptosis, relative to animals exposed to hyperoxia alone [139]. In mice, hyperoxia was shown to induce the expression of pro-inflammatory cytokines (i.e., TNFα, IL-1β, IL-6) and activate major MAPK pathways in lung tissue. The protection afforded by CO treatment against the lethal effects of hyperoxia correlated with the inhibited release of pro-inflammatory cytokines in BALF. The protective effects of CO in this model were found to depend on the MKK3/p38β MAPK pathway [140].
Ventilator-induced lung injury
The therapeutic potential of CO has been demonstrated in rodent models of ventilator-induced lung injury (VILI) [141-144], whereby inhaled CO reduced neutrophil granulocyte infiltration to the lung parenchyma and subsequent alveolar edema formation. Rats ventilated with an injurious (high tidal volume) ventilator setting in combination with an intraperitoneal LPS injection, exhibited lung injury. The inclusion of CO (250 ppm) during mechanical ventilation (MV) reduced the inflammatory cell count in bronchoalveolar lavage (BAL) fluid. In the absence of significant cardiovascular effects, CO dose-dependently decreased TNF-α and increased IL-10 in the BAL fluid [141]. CO application has also been reported to confer tissue protection in a mouse model of VILI, at moderate tidal volume ventilation [142-144]. In this model, MV caused significant lung injury reflected by increases in protein concentration, total cell and neutrophil counts in BAL fluid. CO reduced MV-induced cytokine and chemokine production and prevented lung injury during ventilation, involving the inhibition of MV-induced increases in BAL protein concentration and cell count, lung neutrophil recruitment, and pulmonary edema [142-144]. These effects were related in part to activation of caveolin-1 [143], activation of PPARγ, and inhibition of Egr-1 signaling [142]. These studies, taken together, suggest that MV in the presence of CO may provide protection in animal models of VILI. Further research is needed to elucidate the molecular mechanisms of VILI as well as the therapeutic potential of CO in these models.
Ischemia Reperfusion Injury
Tissue protective effects of CO have been shown experimentally in rodent models of organ I/R injury. Lung I/R caused by occlusion of the pulmonary artery causes lung apoptosis, involving caspase activation, expression changes in Bcl2 family proteins, cleavage of PARP, and mitochondrial cytochrome c release [145]. CO conferred tissue protection in rodents subjected to lung I/R injury, as evidenced by reduced markers of apoptosis, which depended on activation of the MKK3/p38 MAPK pathway [146]. In vivo studies using homozygous ho-1 knockout mice (hmox-1−/−) demonstrated that HO-1 deficiency conferred sensitivity to the lethal effects of lung I/R injury. Application of exogenous CO by inhalation compensated for the HO-1 deficiency in hmox-1−/− mice, and improved survival subsequent to pulmonary I/R [147]. The protection provided by CO involved the stimulation of fibrinolysis, by the cGMP-dependent inhibition of plasminogen activator inhibitor-1, a macrophage–derived activator of smooth muscle cell proliferation [147]. CO also inhibited fibrin deposition and improved circulation in ischemic lungs [166]. These protective effects were related to the inhibited expression of the pro-inflammatory transcription factor Egr-1, and the subsequent downregulation of Egr-1 target genes, which contribute to inflammatory or pro-thrombotic processes [166].
Organ Transplantation
CO has been widely studied as a potential therapeutic in organ transplant applications. CO has a demonstrated potential for reducing transplant associated I/R injury and reducing the probability of graft rejection when applied at low concentration, as an adjuvant to organ preservation fluid or when applied to donors or recipients in gaseous form at low concentration. The application of CO has now been shown to confer protection in several organ transplantation models, including vascular [148], cardiac [149], small intestine [167], kidney, [168], liver [169], and lung transplantation [150, 170-171]. In a rat model of orthotopic left lung transplantation, the transplanted lungs developed severe intra-alveolar hemorrhage and intra-vascular coagulation. Exogenous application of CO (500 ppm), markedly preserved the graft, and reduced hemorrhage, fibrosis, and thrombosis after transplantation. Furthermore, CO inhibited lung cell apoptosis and downregulated lung and pro-inflammatory cytokine and growth factor production which were induced during transplantation [150]. Furthermore, protection against I/R and inflammatory injury was reduced in syngeneic rat orthotopic lung transplantation by inhalation exposure to either the donor or the recipient [170]. Delivery of CO to lung grafts by saturation of the preservation media reduced I/R injury and inflammation in syngeneic rat orthotopic lung transplantation [171]. CO treatment conferred protection during cardiovascular transplantation. When transplant recipients of aortic grafts were maintained in a CO environment (250 ppm) with preconditioning, these animals displayed reduced intimal hyperplasia, and reduced accumulation of leukocytes, macrophages, and T cells in the graft [148]. Saturation of the organ buffer with CO gas prevented cold I/R injury during subsequent intestinal transplantation, implicating the ex vivo application of CO as an alternative therapeutic strategy [172]. In recent studies, inclusion of the water-soluble carbon monoxide releasing molecule (CORM)-3 in the preservation fluid improved cardiac function following cardiac transplantation [173]. The inhibition of apoptosis and inflammation may provide the major mechanisms by which CO protects transplanted organs from dysfunction and failure [174], though improvement of blood circulation by CO within the reperfused transplanted organ [148, 174-175], as well as anti-proliferative [148] effects have also been proposed.
Sepsis
Anti-inflammatory effects of CO have been demonstrated in a rodent model of endotoxemia [18], and recently recapitulated in a swine model of endotoxin challenge. In the later model, CO reduced the development of disseminated intravascular coagulation and diminished serum levels of the pro-inflammatory IL-1β in response to LPS, and induced IL-10 after LPS challenge [176]. Similar, though less significant effects were noted in a non-human primate model subjected to LPS [177]. Similar to inhalation CO, cytoprotective effects have been achieved with pharmacological application of CO releasing molecules (CORMs). CORMs can be used to deliver small amounts of CO to biological systems in a controlled manner and are emerging as an experimental therapy for sepsis [5]. CORMs reduce cytokine release in LPS-stimulated macrophages [178], and decrease inflammatory responses and oxidative stress in LPS-stimulated endothelial cells [179]. In vivo, CORMs attenuate systemic inflammation and pro-adhesive vascular cell properties in septic and thermally-injured mice by reducing nuclear factor-κB activation, protein expression of ICAM-1, and tissue granulocyte infiltration [180-181]. CORM-3 has been shown to prevent re-occurrence of sepsis. CORM-2 prolongs survival and reduces inflammation and CORM-3 reduces liver injury after CLP [181-182]. Recent studies on the protective effects of CORMs in murine sepsis were related to stimulation of mitochondrial biogenesis through the Nrf2/Akt axis [183]. These studies taken together have demonstrated that the CORM-dependent release of CO can reduce mortality in septic mice, suggesting that CORMs could be used therapeutically to prevent organ dysfunction and death in sepsis.
Human CO therapy
Among the first published attempts at applying CO to humans in a clinical setting, Mayr et al. studied the effects of CO inhalation on systemic inflammation during experimental human endotoxemia. In a randomized, double-blinded, placebo-controlled, two-way cross-over trial, experimental endotoxemia was induced in healthy volunteers by injection of 2 ng/kg LPS. The potential anti-inflammatory effects of CO inhalation were investigated by inhalation of 500 ppm CO (leading to an increase in CO-Hb from 1.2% to 7%) versus synthetic air as a placebo for 1 h. CO inhalation had no effect on the inflammatory response as measured by systemic cytokine production (TNF-α, IL-6, IL-8, IL-1α and IL-1β). In this study, no adverse side effects of CO inhalation were observed [184]. However, given the limited scope of this initial trial, and the protective characteristics of CO application in many animal models of sepsis, further more detailed clinical trials are required to reach a verdict on the efficacy of CO for reducing inflammation in septic patients. In contrast, a recent clinical study demonstrates the feasibility of administering inhaled CO to humans with COPD [185]. In this study, ex-smoking patients with stable COPD were subjected to CO inhalation (100-125 ppm for 2 hours/day for 4 days), which increased CO-Hb levels to 4.5%. Inhalation of CO by patients with stable COPD led to trends in reduction of sputum eosinophils and improvement of methacholine responsiveness [185].
Conclusions and Future Directions
The monitoring of CO, NO and other volatile components in the breath has the advantage over other biomarker-based clinical tests in that these methods are non-invasive and practical for bedside measurements. Measured eCO levels have been reported to change in several inflammatory lung diseases, and respond generally to anti-inflammatory therapies. CO is a common contaminant of outdoor and indoor air and thus measurements of CO must be carefully controlled and account for ambient levels.
Measurement of eCO shows demonstrated potential as a diagnostic test for tobacco consumption and for validation of smoking cessation. Furthermore, eCO may be useful as a readout system for monitoring the effectiveness of anti-inflammatory therapeutics. eCO testing may especially show continued potential for monitoring inflammation and/or oxidative stress in controlled environments were tobacco use and ambient contamination may be of limited concern, such as in intensive care or post-surgical recovery situations. On the other hand eCO may be of limited use as a diagnostic tool in complex diseases such as COPD and asthma, especially in the presence of confounding variables such as continued smoking, and ambient environmental pollutants.
Despite improvements in the standardization and sensitivity of methods to detect eCO, the predictive value of eCO measurements as a diagnostic tool in human diseases remains uncertain. Further clinical studies may refine clinical applications for eCO, and may further elucidate human respiratory physiology and metabolism.
With respect to therapeutic applications, CO remains a candidate for gaseous therapy for lung diseases and other inflammatory diseases. To date therapeutic effects of this gas have been demonstrated in animal model studies [1, 5]. Differences in lung physiological responses to CO between rodents, large animals (e.g., non-human primates) and humans require further investigation. Further experiments are required to confirm the safety and efficacy of CO inhalation as a treatment for inflammatory lung diseases. Pharmacological application of CO using CORM technology provides an attractive alternative to inhalation gas [5]. As with inhaled CO, consideration of the toxicological and physiological properties of CORMs, including possible effects on hemodynamics, must be understood before employing CORMs as clinical therapy. Currently, unlike inhaled NO, which has been approved for pediatric applications, the clinical applications for inhaled CO or CORMs remain prospective. The clinical applicability of inhaled CO as a therapeutic in diseases such as sepsis, renal transplantation, pulmonary fibrosis, and pulmonary hypertension, awaits the outcome of current unpublished preclinical testing and projected clinical trials.
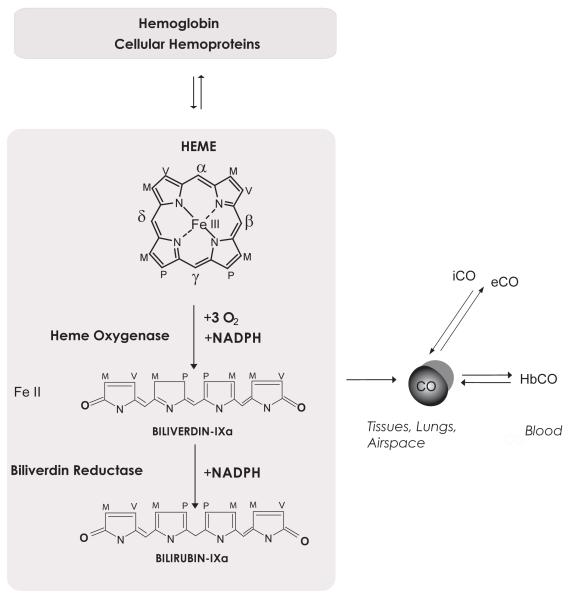
Figure 1.
CO is produced endogenously in the body, as the by-product of the turnover of hemoglobin and other hemoproteins. Heme, which is used as a prosthetic co-factor for hemoproteins, is degraded by the heme oxygenase (HO) enzyme system. HO catalyzes the rate-limiting step in heme degradation. The reaction produces biliverdin-IXα, carbon monoxide (CO), and ferrous iron (Fe II), and requires 3 mol molecular oxygen and NADPH. Biliverdin-IXα produced in the HO reaction is reduced to bilirubin-IXα by biliverdin reductase (Side chains are labeled as M=Methyl, V=Vinyl, P=Propionate). In the blood, CO binds hemoglobin to form carboxyhemoglobin (HbCO). CO eventually diffuses to the lung where it is eliminated as exhaled CO (eCO). CO may also be inhaled (iCO) with ambient air, as in the case of accidental or occupational exposure, or as a component of therapy, as discussed in this review.
Acknowledgements
This work was funded by NIH grants P01 HL108801 and R01 HL079904 to AMKC. Dr. Ryter received additional salary support from the Brigham and Women’s Hospital and Lovelace Respiratory Research Institute consortium for joint lung research.
References
- 1.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 2.Ryter SW, Morse D, Choi AM. Carbon monoxide: to boldly go where NO has gone before. Sci STKE. 2004;230:RE6. doi: 10.1126/stke.2302004re6. [DOI] [PubMed] [Google Scholar]
- 3.Wu L, Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005;57:585–630. doi: 10.1124/pr.57.4.3. [DOI] [PubMed] [Google Scholar]
- 4.Ryter SW, Otterbein LE. Carbon monoxide in biology and medicine. Bioessays. 2004;26:270–280. doi: 10.1002/bies.20005. [DOI] [PubMed] [Google Scholar]
- 5.Motterlini R, Otterbein LE. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov. 2010;9:728–743. doi: 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
- 6.Gentile MA. Inhaled medical gases: more to breathe than oxygen. Respir Care. 2011;56:1341–1357. doi: 10.4187/respcare.01442. [DOI] [PubMed] [Google Scholar]
- 7.Whiteman M, Le Trionnaire S, Chopra M, Fox B, Whatmore J. Emerging role of hydrogen sulfide in health and disease: critical appraisal of biomarkers and pharmacological tools. Clin Sci (Lond) 2011;121:459–488. doi: 10.1042/CS20110267. [DOI] [PubMed] [Google Scholar]
- 8.Siriussawakul A, Chen LI, Lang JD. Medical gases: a novel strategy for attenuating ischemia-reperfusion injury in organ transplantation? J Transplant. 2012;2012:819382. doi: 10.1155/2012/819382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ignarro LJ, Byrns RE, Buga GM, Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987;61:866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- 11.Moncada S, Palmer RMJ, Higgs E. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 12.Ignarro LJ. Nitric oxide: a unique endogenous signaling molecule in vascular biology. Biosci Rep. 1999;19:51–71. doi: 10.1023/a:1020150124721. [DOI] [PubMed] [Google Scholar]
- 13.Snyder SA, Bredt DS. Biological roles of nitric oxide. Sci Am. 1992 May;:68–77. doi: 10.1038/scientificamerican0592-68. [DOI] [PubMed] [Google Scholar]
- 14.Sjostrand T. Endogenous production of carbon monoxide in man under normal and pathophysiological conditions. Scand J Clin Lab Invest. 1949;1:201–214. [Google Scholar]
- 15.Coburn RF, Blakemore WS, Forster RE. Endogenous carbon monoxide production in man. J Clin Invest. 1963;42:1172–1178. doi: 10.1172/JCI104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tenhunen R, Marver HS, Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969;244:6388–6394. [PubMed] [Google Scholar]
- 17.Durante W, Johnson FK, Johnson RA. Role of carbon monoxide in cardiovascular function. J Cell Mol Med. 2006;10:672–686. doi: 10.1111/j.1582-4934.2006.tb00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 19.Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 20.Hanafy KA, Oh J, Otterbein LE. Carbon monoxide and the brain: Time to rethink the dogma. Curr Pharm Des. 2012 Oct 18; doi: 10.2174/1381612811319150013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dweik RA, Amann A. Exhaled breath analysis: the new frontier in medical testing. J Breath Res. 2008;2:030301. doi: 10.1088/1752-7163/2/3/030301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amann A, Spanĕl P, Smith D. Breath analysis: the approach towards clinical applications. Mini Rev Med Chem. 2007;7:115–129. doi: 10.2174/138955707779802606. [DOI] [PubMed] [Google Scholar]
- 23.Risby TH, Solga SF. Current status of clinical breath analysis. Applied Phys B-Lasers and Optics. 2006;85:421–426. [Google Scholar]
- 24.Kharitonov SA, Barnes PJ. Biomarkers of some pulmonary diseases in exhaled breath. Biomarkers. 2002;7:1–32. doi: 10.1080/13547500110104233. [DOI] [PubMed] [Google Scholar]
- 25.Kharitonov SA, Barnes PJ. Exhaled markers of inflammation. Curr Op Allergy Clin Immunol. 2001;1:217–224. doi: 10.1097/01.all.0000011017.58506.f1. [DOI] [PubMed] [Google Scholar]
- 26.Buszewski B, Kesy M, Ligor T, Amann A. Human exhaled air analytics: biomarkers of diseases. Biomed Chromatog BMC. 2007;21:553–566. doi: 10.1002/bmc.835. [DOI] [PubMed] [Google Scholar]
- 27.Vass G, Huszár E, Barát E, Horváth I. Exhaled breath condensate and its analysis--a new method in pulmonology. Orv Hetil. 2003;2003:144, 2517–2524. [PubMed] [Google Scholar]
- 28.Mazzone PJ. Analysis of volatile organic compounds in the exhaled breath for the diagnosis of lung cancer. J Thorac Oncol. 2008;3:774–780. doi: 10.1097/JTO.0b013e31817c7439. [DOI] [PubMed] [Google Scholar]
- 29.Ligor M, Ligor T, Bajtarevic A, Ager C, Pienz M, Klieber M, Denz H, Fiegl M, Hilbe W, Weiss W, Lukas P, Jamnig H, Hackl M, Buszewski B, Miekisch W, Schubert J, Amann A. Determination of volatile organic compounds in exhaled breath of patients with lung cancer using solid phase microextraction and gas chromatography mass spectrometry. Clin Chem Lab Med. 2009;47:550–560. doi: 10.1515/CCLM.2009.133. [DOI] [PubMed] [Google Scholar]
- 30.Barnes PJ, Dweik RA, Gelb AF, Gibson PG, George SC, Grasemann H, Pavord ID, Ratjen F, Silkoff PE, Taylor DR, Zamel N. Exhaled nitric oxide in pulmonary diseases: a comprehensive review. Chest. 2010;138:682–692. doi: 10.1378/chest.09-2090. [DOI] [PubMed] [Google Scholar]
- 31.Taylor DR, Pijnenburg MW, Smith AD, De Jongste JC. Exhaled nitric oxide measurements: clinical application and interpretation. Thorax. 2006;61:817–827. doi: 10.1136/thx.2005.056093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antus B, Horvath I. Exhaled nitric oxide and carbon monoxide in respiratory diseases. J Breath Res. 2007;1:024002. doi: 10.1088/1752-7155/1/2/024002. [DOI] [PubMed] [Google Scholar]
- 33.Montuschi P, Kharitonov SA, Barnes PJ. Exhaled carbon monoxide and nitric oxide in COPD. Chest. 2001;120:496–501. doi: 10.1378/chest.120.2.496. [DOI] [PubMed] [Google Scholar]
- 34.Ryter SW, Sethi JM. Exhaled carbon monoxide as a biomarker of inflammatory lung disease. J Breath Res. 2007;1:026004. doi: 10.1088/1752-7155/1/2/026004. [DOI] [PubMed] [Google Scholar]
- 35.Gajdocsy R, Horvath I. Exhaled carbon monoxide in airway diseases: from research findings to clinical relevance. J Breath Res. 2010;4:047102. doi: 10.1088/1752-7155/4/4/047102. [DOI] [PubMed] [Google Scholar]
- 36.Horvath I, MacNee W, Kelly FJ, Dekhuijzen PN, Phillips M, Doring G, Choi AM, Yamaya M, Bach FH, Willis D, Donnelly LE, Chung KF, Barnes PJ. Haemoxygenase-1 induction and exhaled markers of oxidative stress in lung diseases, summary of the ERS Research Seminar in Budapest, Hungary, September, 1999. Eur Resp J. 2001;18:420–430. doi: 10.1183/09031936.01.00231201. [DOI] [PubMed] [Google Scholar]
- 37.Horvath I, Donnelly LE, Kiss A, Paredi P, Kharitonov SA, Barnes PJ. Raised levels of exhaled carbon monoxide are associated with an increased expression of heme oxygenase-1 in airway macrophages in asthma: a new marker of oxidative stress. Thorax. 1998;53:668–672. doi: 10.1136/thx.53.8.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paredi P, Shah PL, Montuschi P, Sullivan P, Hodson ME, Kharitonov SA, Barnes PJ. Increased carbon monoxide in exhaled air of patients with cystic fibrosis. Thorax. 1999;54:917–920. doi: 10.1136/thx.54.10.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horvath I, Loukides S, Wodehouse T, Kharitonov SA, Cole PJ, Barnes PJ. Increased levels of exhaled carbon monoxide in bronchiectasis: a new marker of oxidative stress. Thorax. 1998;53:867–870. doi: 10.1136/thx.53.10.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morimatsu H, Takahashi T, Matsusaki T, Hayashi M, Matsumi J, Shimizu H, Matsumi M, Morita K. An increase in exhaled CO concentration in systemic inflammation/sepsis. J Breath Res. 2010;4:047103. doi: 10.1088/1752-7155/4/4/047103. [DOI] [PubMed] [Google Scholar]
- 41.Zegdi R, Perrin D, Burdin M, Boiteau R, Tenaillon A. Increased endogenous carbon monoxide production in severe sepsis. Intensive Care Med. 2002;28:793–796. doi: 10.1007/s00134-002-1269-7. [DOI] [PubMed] [Google Scholar]
- 42.Paredi P, Biernacki W, Invernizzi G, Kharitonov SA, Barnes PJ. Exhaled carbon monoxide levels elevated in diabetes and correlated with glucose concentration in blood: a new test for monitoring the disease? Chest. 1999;116:1007–1011. doi: 10.1378/chest.116.4.1007. [DOI] [PubMed] [Google Scholar]
- 43.Scharte M, Bone HG, Van Aken H, Meyer J. Increased carbon monoxide in exhaled air of critically ill patients. Biochem Biophys Res Commun. 2000;267:423–426. doi: 10.1006/bbrc.1999.1936. [DOI] [PubMed] [Google Scholar]
- 44.Zegdi R, Caïd R, Van De Louw A, Perrin D, Burdin M, Boiteau R, Tenaillon A. Exhaled carbon monoxide in mechanically ventilated critically ill patients: influence of inspired oxygen fraction. Intensive Care Med. 2000;26:1228–1231. doi: 10.1007/s001340000590. [DOI] [PubMed] [Google Scholar]
- 45.Hayashi M, Takahashi T, Morimatsu H, Fujii H, Taga N, Mizobuchi S, Matsumi M, Katayama H, Yokoyama M, Taniguchi M, Morita K. Increased carbon monoxide concentration in exhaled air after surgery and anesthesia. Anesth Analg. 2004;99:444–448. doi: 10.1213/01.ANE.0000123821.51802.F3. [DOI] [PubMed] [Google Scholar]
- 46.Vos R, Cordemans C, Vanaudenaerde BM, De Vleeschauwer SI, Schoonis A, Van Raemdonck DE, Dupont LJ, Verleden GM. Exhaled carbon monoxide as a noninvasive marker of airway neutrophilia after lung transplantation. Transplantation. 2009;87:1579–1583. doi: 10.1097/TP.0b013e3181a4e69c. [DOI] [PubMed] [Google Scholar]
- 47.Matsusaki T, Morimatsu H, Takahashi T, Matsumi M, Sato K, Kaku R, Sato T, Yagi T, Tanaka N, Morita K. Increased exhaled carbon monoxide concentration during living donor liver transplantation. Int J Mol Med. 2008;21:75–81. [PubMed] [Google Scholar]
- 48.Deveci SE, Deveci F, Acik Y, Ozan AT. The measurement of exhaled carbon monoxide in healthy smokers and non-smokers. Respir Med. 2004;98:551–556. doi: 10.1016/j.rmed.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 49.Ichinose F, Roberts JD, Jr, Zapol WM. Inhaled nitric oxide: a selective pulmonary vasodilator: current uses and therapeutic potential. Circulation. 2004;109:3106–3111. doi: 10.1161/01.CIR.0000134595.80170.62. [DOI] [PubMed] [Google Scholar]
- 50.Frostell C, Fratacci MD, Wain JC, Jones R, Zapol WM. Inhaled nitric oxide. A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation. 1991;83:2038–2047. doi: 10.1161/01.cir.83.6.2038. [DOI] [PubMed] [Google Scholar]
- 51.Rossaint R, Falke KJ, López F, Slama K, Pison U, Zapol WM. Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med. 1993;328:399–405. doi: 10.1056/NEJM199302113280605. [DOI] [PubMed] [Google Scholar]
- 52.Bloch KD, Ichinose F, Roberts JD, Jr, Zapol WM. Inhaled NO as a therapeutic agent. Cardiovasc Res. 2007;75:339–348. doi: 10.1016/j.cardiores.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Porta NF, Steinhorn RH. Pulmonary vasodilator therapy in the NICU: inhaled nitric oxide, sildenafil, and other pulmonary vasodilating agents. Clin Perinatol. 2012;39:149–164. doi: 10.1016/j.clp.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ryter SW, Choi AM. Heme oxygenase-1/carbon monoxide: novel therapeutic strategies in critical care medicine. Curr Drug Targets. 2010;11:1485–1494. doi: 10.2174/1389450111009011485. [DOI] [PubMed] [Google Scholar]
- 55.Ryter SW, Choi AM. Heme oxygenase-1/carbon monoxide: from metabolism to molecular therapy. Am J Respir Cell Mol Biol. 2009;41:251–60. doi: 10.1165/rcmb.2009-0170TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Von Burg R. Carbon monoxide. J Appl Toxicol. 1999;19:379–386. doi: 10.1002/(sici)1099-1263(199909/10)19:5<379::aid-jat563>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 57.World Health Organization . Carbon monoxide. In: WHO, editor. Air quality guidelines global update 2005 Copenhagen. World Health Organization; Denmark: 2005. p. 13. [Google Scholar]
- 58.Calafat AM, Polzin GM, Saylor J, Richter P, Ashley DL, Watson CH. Determination of tar, nicotine, and carbon monoxide yields in the mainstream smoke of selected international cigarettes. Tob Control. 2004;13:45–51. doi: 10.1136/tc.2003.003673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sjostrand T. The formation of carbon monoxide by in vitro decomposition of haemoglobin in bile pigments. Acta Physiol Scand. 1952;26:328–333. doi: 10.1111/j.1748-1716.1952.tb00913.x. [DOI] [PubMed] [Google Scholar]
- 60.Sjostrand T. Formation of carbon monoxide in connexion with haemoglobin catabolism. Nature. 1951;168:1118–1119. doi: 10.1038/1681118a0. [DOI] [PubMed] [Google Scholar]
- 61.Coburn RF, Williams WJ, Kahn SB. Endogenous carbon monoxide production in patients with hemolytic anemia. J Clin Invest. 1966;45:460–468. doi: 10.1172/JCI105360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 63.Rudra CB, Williams MA, Sheppard L, Koenig JQ, Schiff MA, Frederick IO, Dills R. Relation of whole blood carboxyhemoglobin concentration to ambient carbon monoxide exposure estimated using regression. Am J Epidemiol. 2010;171:942–951. doi: 10.1093/aje/kwq022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Russell MA. Blood carboxyhaemoglobin changes during tobacco smoking. Postgrad Med J. 1973;49:684–687. doi: 10.1136/pgmj.49.576.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gorman D, Drewry A, Huang YL, Sames C. The clinical toxicology of carbon monoxide. Toxicology. 2003;187:25–38. doi: 10.1016/s0300-483x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 66.Piantadosi CA. Diagnosis and treatment of carbon monoxide poisoning. Respir Care Clin N Am. 1999;5:183–202. [PubMed] [Google Scholar]
- 67.Tenhunen R, Ross ME, Marver HS, Schmid R. Reduced nicotinamide-adenine dinucleotide phosphate dependent biliverdin reductase: partial purification and characterization. Biochemistry. 1970;9:298–303. doi: 10.1021/bi00804a016. [DOI] [PubMed] [Google Scholar]
- 68.Vreman HJ, Wong RJ, Stevenson DK. In: Carbon monoxide in breath, blood, and other tissues, in Carbon monoxide toxicity. Penney DG, editor. CRC Press; Boca Raton: 2000. pp. 19–60. [Google Scholar]
- 69.Maines MD, Trakshel GM, Kutty RK. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J Biol Chem. 1986;261:411–419. [PubMed] [Google Scholar]
- 70.Keyse SM, Tyrrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci U S A. 1989;86:99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ryter SW, Kim HP, Nakahira K, Zuckerbraun BS, Morse D, Choi AM. Protective functions of heme oxygenase-1 and carbon monoxide in the respiratory system. Antioxid Redox Signal. 2007;9:2157–2173. doi: 10.1089/ars.2007.1811. [DOI] [PubMed] [Google Scholar]
- 72.McCoubrey WK, Jr, Maines MD. The structure, organization and differential expression of the gene encoding rat heme oxygenase-2. Gene. 1994;139:155–161. doi: 10.1016/0378-1119(94)90749-8. [DOI] [PubMed] [Google Scholar]
- 73.Andersson JA, Uddman R, Cardell LO. Carbon monoxide is endogenously produced in the human nose and paranasal sinuses. J Allergy Clin Immunol. 2000;105:269–273. doi: 10.1016/s0091-6749(00)90075-7. [DOI] [PubMed] [Google Scholar]
- 74.Vreman HJ, Stevenson DK, Oh W, Fanaroff AA, Wright LL, Lemons JA, Wright E, Shankaran S, Tyson JE, Korones SB, Bauer CR, Stoll BJ, Papile L-A, Donovan EF, Ehrenkranz RA. Semiportable electrochemical instrument for determining carbon monoxide in breath. Clin Chem. 1994;40:1927–1933. [PubMed] [Google Scholar]
- 75.Vreman HJ, Baxter LM, Stone RT, Stevenson DK. Evaluation of a fully automated end-tidal carbon monoxide instrument for breath analysis. Clin Chem. 1996;42:50–56. [PubMed] [Google Scholar]
- 76.Kharitonov SA, Barnes PJ. Exhaled markers of pulmonary disease. Am J Respir Crit Care Med. 2001;163:1693–722. doi: 10.1164/ajrccm.163.7.2009041. [DOI] [PubMed] [Google Scholar]
- 77.Morimatsu H, Takahashi T, Maeshima K, Inoue K, Kawakami T, Shimizu H, Takeuchi M, Yokoyama M, Katayama H, Morita K. Increased heme catabolism in critically ill patients: correlation among exhaled carbon monoxide, arterial carboxyhemoglobin, and serum bilirubin IXalpha concentrations. Am J Physiol Lung Cell Mol Physiol. 2006;290:L114–L119. doi: 10.1152/ajplung.00031.2005. [DOI] [PubMed] [Google Scholar]
- 78.Engel GS, Drisdell WS, Keutsch FN, Moyer EJ, Anderson JG. Ultrasensitive near-infrared integrated cavity output spectroscopy technique for detection of CO at 1.57 microm: new sensitivity limits for absorption measurements in passive optical cavities. Appl Opt. 2006;45:9221–9229. doi: 10.1364/ao.45.009221. [DOI] [PubMed] [Google Scholar]
- 79.Popp A, Muller F, Kuhnemann F, Schiller S, von Basum G, Dahnke H, Hering P, Murtz M. Ultra-sensitive mid-infrared cavity leak-out spectroscopy using a cw optical parametric oscillator. Applied Physics B-Lasers and Optics. 2002;75:751–754. [Google Scholar]
- 80.Murtz M, Halmer D, Horstjann M, Thelen S, Hering P. Ultrasensitive trace gas detection for biomedical applications. Spectrochim Acta A Mol Biomol Spectrosc. 2006;63:963–969. doi: 10.1016/j.saa.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 81.Fritsch T, Hering P, Murtz M. Infrared laser spectroscopy for online recording of exhaled carbon monoxide-a progress report. J Breath Res. 2007;1:014002. doi: 10.1088/1752-7155/1/1/014002. [DOI] [PubMed] [Google Scholar]
- 82.Sowa M, Murtz M, Hering P. Mid-infrared laser spectroscopy for online analysis of exhaled CO. J Breath Res. 2010;4:047101. doi: 10.1088/1752-7155/4/4/047101. [DOI] [PubMed] [Google Scholar]
- 83.Shorter JH, Nelson DD, Barry McManus J, Zahniser MS, Milton DK. Multicomponent breath analysis with infrared absorption using room-temperature quantum cascade lasers. IEEE Sens J. 2009;10:76–84. doi: 10.1109/JSEN.2009.2035764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nwaboh JA, Persijn S, Heinrich K, Sowa M, Hering P, Werhahn O. QCLAS and CRDS-Based CO Quantification as aimed at in breath measurements. Int J Spectroscopy. 2012;2012:894841. [Google Scholar]
- 85.Shorter JH, Nelson DD, McManus JB, Zahniser MS, Sama SR, Milton DK. Clinical study of multiple breath biomarkers of asthma and COPD (NO, CO(2), CO and N(2)O) by infrared laser spectroscopy. J Breath Res. 2011;5:037108. doi: 10.1088/1752-7155/5/3/037108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Middleton ET, Morice AH. Breath carbon monoxide as an indication of smoking habit. Chest. 2000;117:758–763. doi: 10.1378/chest.117.3.758. [DOI] [PubMed] [Google Scholar]
- 87.Chatkin J, Fritscher L, de Abreu C, Cavalet-Blanco D, Chatkin G, Wagner M, Fritscher C. Exhaled carbon monoxide as a marker for evaluating smoking abstinence in a Brazilian population sample. Prim Care Respir J. 2007;16:36–40. doi: 10.3132/pcrj.2007.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fritz M, Wallner R, Grohs U, Kemmler G, Saria A, Zernig G. Comparable sensitivities of urine cotinine and breath carbon monoxide at follow-up time points of three months or more in a smoking cessation trial. Pharmacology. 2010;85:234–240. doi: 10.1159/000280435. [DOI] [PubMed] [Google Scholar]
- 89.Hung J, Lin CH, Wang JD, Chan CC. Exhaled carbon monoxide level as an indicator of cigarette consumption in a workplace cessation program in Taiwan. J Formos Med Assoc. 2006;105:210–213. doi: 10.1016/S0929-6646(09)60307-7. [DOI] [PubMed] [Google Scholar]
- 90.Fabricius P, Scharling H, Lokke A, Vestbo J, Lange P. Exhaled CO, a predictor of lung function? Respir Med. 2007;101:581–6. doi: 10.1016/j.rmed.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 91.Deveci SE, Deveci F, Acik Y, Ozan AT. The measurement of exhaled carbon monoxide in healthy smokers and non-smokers. Respir Med. 2004;98:551–556. doi: 10.1016/j.rmed.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 92.Jones AY, Lam PK. End-expiratory carbon monoxide levels in healthy subjects living in a densely populated urban environment. Sci Total Environ. 2006;354:150–156. doi: 10.1016/j.scitotenv.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 93.Sandberg A, Sköld CM, Grunewald J, Eklund A, Wheelock ÅM. Assessing recent smoking status by measuring exhaled carbon monoxide levels. PLoS One. 2011;6:e28864. doi: 10.1371/journal.pone.0028864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sato S, Nishimura K, Koyama H, Tsukino M, Oga T, Hajiro T, Mishima M. Optimal cutoff level of breath carbon monoxide for assessing smoking status in patients with asthma and COPD. Chest. 2003;124:1749–1754. doi: 10.1378/chest.124.5.1749. [DOI] [PubMed] [Google Scholar]
- 95.Kharitonov SA, Robbins RA, Yates D, Keatings V, Barnes PJ. Acute and chronic effects of cigarette smoking on exhaled nitric oxide. Am J Respir Crit Care Med. 1995;152:609–612. doi: 10.1164/ajrccm.152.2.7543345. [DOI] [PubMed] [Google Scholar]
- 96.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 97.Barnes PJ. Chronic obstructive pulmonary disease. NEJM. 2000;343:269–280. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- 98.Bowler RP, Barnes PJ, Crapo JD. The role of oxidative stress in chronic obstructive pulmonary disease. COPD. 2004;1:255–277. doi: 10.1081/copd-200027031. [DOI] [PubMed] [Google Scholar]
- 99.Church DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect. 1985;64:111–126. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tuder RM, Yoshida T, Arap W, Pasqualini R, Petrache I. State of the art. Cellular and molecular mechanisms of alveolar destruction in emphysema: an evolutionary perspective. Proc Am Thorac Soc. 2006;3:503–510. doi: 10.1513/pats.200603-054MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Park JW, Ryter SW, Choi AM. Functional significance of apoptosis in chronic obstructive pulmonary disease. COPD. 2007;4:347–353. doi: 10.1080/15412550701603775. [DOI] [PubMed] [Google Scholar]
- 102.Henson PM, Vandivier RW, Douglas IS. Cell death, remodeling, and repair in chronic obstructive pulmonary disease? Proc Am Thorac Soc. 2006;3:713–717. doi: 10.1513/pats.200605-104SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abd El, Khalek KA, El Seify MY, Youssef OI, Badr MM. Diagnostic value of exhaled carbon monoxide as an early marker of exacerbation in children with chronic lung diseases. ISRN Pediatr. 2012;2012:859873. doi: 10.5402/2012/859873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Machado RF, Stoller JK, Laskowski D, Zheng S, Lupica JA, Dweik RA, Erzurum SC. Low levels of nitric oxide and carbon monoxide in alpha 1-antitrypsin deficiency. J Appl Physiol. 2002;93:2038–2043. doi: 10.1152/japplphysiol.00659.2002. [DOI] [PubMed] [Google Scholar]
- 105.Yasuda H, Yamaya M, Nakayama K, Ebihara S, Sasaki T, Okinaga S, Inoue D, Asada M, Nemoto M, Sasaki H. Increased arterial carboxyhemoglobin concentrations in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:1246–1251. doi: 10.1164/rccm.200407-914OC. [DOI] [PubMed] [Google Scholar]
- 106.Slebos DJ, Ryter SW, van der Toorn M, Liu F, Guo F, Baty CJ, Karlsson JM, Watkins SC, Kim HP, Wang X, Lee JS, Postma DS, Kauffman HF, Choi AM. Mitochondrial localization and function of heme oxygenase-1 in cigarette smoke-induced cell death. Am J Respir Cell Mol Biol. 2007;36:409–417. doi: 10.1165/rcmb.2006-0214OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 107.Kim HP, Wang X, Chen ZH, Lee SJ, Huang MH, Wang Y, Ryter SW, Choi AM. Autophagic proteins regulate cigarette smoke-induced apoptosis: protective role of heme oxygenase-1. Autophagy. 2008;4:887–895. doi: 10.4161/auto.6767. [DOI] [PubMed] [Google Scholar]
- 108.Shinohara T, Kaneko T, Nagashima Y, Ueda A, Tagawa A, Ishigatsubo Y. Adenovirus-mediated transfer and overexpression of heme oxygenase 1 cDNA in lungs attenuates elastase-induced pulmonary emphysema in mice. Hum Gene Ther. 2005;16:318–327. doi: 10.1089/hum.2005.16.318. [DOI] [PubMed] [Google Scholar]
- 109.Maestrelli P, El Messlemani AH, De Fina O, Nowicki Y, Saetta M, Mapp C, Fabbri LM. Increased expression of heme oxygenase (HO)-1 in alveolar spaces and HO-2 in alveolar walls of smokers. Am J Respir Crit Care Med. 2001;164:1508–1513. doi: 10.1164/ajrccm.164.8.2011083. [DOI] [PubMed] [Google Scholar]
- 110.Tsoumakidou M, Tzanakis N, Chrysofakis G, Siafakas NM. Nitrosative stress, heme oxygenase-1 expression and airway inflammation during severe exacerbations of COPD. Chest. 2005;127:1911–2928. doi: 10.1378/chest.127.6.1911. [DOI] [PubMed] [Google Scholar]
- 111.Maestrelli P, Paska C, Saetta M, Turato G, Nowicki Y, Monti S, Formichi B, Miniati M, Fabbri LM. Decreased haem oxygenase-1 and increased inducible nitric oxide synthase in the lung of severe COPD patients. Eur Respir J. 2003;21:971–976. doi: 10.1183/09031936.03.00098203. [DOI] [PubMed] [Google Scholar]
- 112.Slebos DJ, Kerstjens HA, Rutgers SR, Kauffman HF, Choi AM, Postma DS. Haem oxygenase-1 expression is diminished in alveolar macrophages of patients with COPD. Eur Respir J. 2004;23:652–653. doi: 10.1183/09031936.04.00127904. [DOI] [PubMed] [Google Scholar]
- 113.Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368:804–813. doi: 10.1016/S0140-6736(06)69290-8. [DOI] [PubMed] [Google Scholar]
- 114.Yamaya M, Sekizawa K, Ishizuka S, Monma M, Sasaki H. Exhaled carbon monoxide levels during treatment of acute asthma. Eur Respir J. 1999;13:757–760. doi: 10.1034/j.1399-3003.1999.13d10.x. [DOI] [PubMed] [Google Scholar]
- 115.Zayasu K, Sekizawa K, Okinaga S, Yamaya M, Ohrui T, Sasaki H. Increased carbon monoxide in exhaled air of asthmatic patients. Am J Respir Crit Care Med. 1997;156:1140–1143. doi: 10.1164/ajrccm.156.4.96-08056. [DOI] [PubMed] [Google Scholar]
- 116.Pearson P, Lewis S, Britton J, Fogarty A. Exhaled carbon monoxide levels in atopic asthma: a longitudinal study. Respir Med. 2005;99:1292–1296. doi: 10.1016/j.rmed.2005.02.042. [DOI] [PubMed] [Google Scholar]
- 117.Paredi P, Leckie MJ, Horvath I, Allegra L, Kharitonov SA, Barnes PJ. Changes in exhaled carbon monoxide and nitric oxide levels following allergen challenge in patients with asthma. Eur Respir J. 1999;13:48–52. doi: 10.1183/09031936.99.13104899. [DOI] [PubMed] [Google Scholar]
- 118.Sethi JM, White AM, Patel SA, Dinella JV, Calhoun WJ, Choi AM. Bronchoprovocation testing in asthma: effect on exhaled monoxides. J Breath Res. 2010;4:047104. doi: 10.1088/1752-7155/4/4/047104. [DOI] [PubMed] [Google Scholar]
- 119.Yasuda H, Yamaya M, Yanai M, Ohrui T, Sasaki H. Increased blood carboxyhaemoglobin concentrations in inflammatory pulmonary diseases. Thorax. 2002;57:779–783. doi: 10.1136/thorax.57.9.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yasuda H, Sasaki T, Yamaya M, Ebihara S, Maruyama M, Kanda A, Sasaki H. Increased arteriovenous carboxyhemoglobin differences in patients with inflammatory pulmonary diseases. Chest. 2004;125:2160–2168. doi: 10.1378/chest.125.6.2160. [DOI] [PubMed] [Google Scholar]
- 121.Lim S, Groneberg D, Fischer A, Oates T, Caramori G, Mattos W, Adcock I, Barnes PJ, Chung KF. Expression of heme oxygenase isoenzymes 1 and 2 in normal and asthmatic airways: effect of inhaled corticosteroids. Am J Respir Crit Care Med. 2000;162:1912–1918. doi: 10.1164/ajrccm.162.5.9909081. [DOI] [PubMed] [Google Scholar]
- 122.Uasuf CG, Jatakanon A, James A, Kharitonov SA, Wilson NM, Barnes PJ. Exhaled carbon monoxide in childhood asthma. J Pediatr. 1999;135:569–574. doi: 10.1016/s0022-3476(99)70054-5. [DOI] [PubMed] [Google Scholar]
- 123.Beck-Ripp J, Latzin P, Griese M. Exhaled carbon monoxide is not flow dependent in children with cystic fibrosis and asthma. Eur J Med Res. 2004;9:518–522. [PubMed] [Google Scholar]
- 124.Ohara Y, Ohara T, Ohrui T, Morikawa T, Asamura T, Sasaki H, Arai H. Exhaled carbon monoxide levels in preschool-age children with episodic asthma. Pediatr Int. 2012;54:227–232. doi: 10.1111/j.1442-200X.2011.03515.x. [DOI] [PubMed] [Google Scholar]
- 125.Monma M, Yamaya M, Sekizawa K, Ikeda K, Suzuki N, Kikuchi T, Takasaka T, Sasaki H. Increased carbon monoxide in exhaled air of patients with seasonal allergic rhinitis. Clin Exp Allergy. 1999;29:1537–1541. doi: 10.1046/j.1365-2222.1999.00684.x. [DOI] [PubMed] [Google Scholar]
- 126.Andersson JA, Uddman R, Cardell LO. Increased carbon monoxide levels in the nasal airways of subjects with a history of seasonal allergic rhinitis and in patients with upper respiratory tract infection. Clin Exp Allergy. 2002;32:224–227. doi: 10.1046/j.1365-2222.2002.00532.x. [DOI] [PubMed] [Google Scholar]
- 127.Zetterquist W, Marteus H, Johannesson M, Nordval SL, Ihre E, Lundberg JO, Alving K. Exhaled carbon monoxide is not elevated in patients with asthma or cystic fibrosis. Eur Respir J. 2002;20:92–99. doi: 10.1183/09031936.02.00245302. [DOI] [PubMed] [Google Scholar]
- 128.Zhang J, Yao X, Yu R, Bai J, Sun Y, Huang M, Adcock IM, Barnes PJ. Exhaled carbon monoxide in asthmatics: a meta-analysis. Respir Res. 2010;11:50. doi: 10.1186/1465-9921-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ratjen F, Doring G. Cystic fibrosis. Lancet. 2003;361:681–689. doi: 10.1016/S0140-6736(03)12567-6. [DOI] [PubMed] [Google Scholar]
- 130.Antuni JD, Kharitonov SA, Hughes D, Hodson ME, Barnes PJ. Increase in exhaled carbon monoxide during exacerbations of cystic fibrosis. Thorax. 2000;55:138–142. doi: 10.1136/thorax.55.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Paredi P, Kharitonov SA, Leak D, Shah PL, Cramer D, Hodson ME, Barnes PJ. Exhaled ethane is elevated in cystic fibrosis and correlates with carbon monoxide levels and airway obstruction. Am J Respir Crit Care Med. 2000;161:1247–1251. doi: 10.1164/ajrccm.161.4.9906122. [DOI] [PubMed] [Google Scholar]
- 132.Horvath I, Borka P, Apor P, Kollai M. Exhaled carbon monoxide concentration increases after exercise in children with cystic fibrosis. Acta Physiol Hung. 1999;86:237–244. [PubMed] [Google Scholar]
- 133.Van Muylem A, Verbanck S, Estenne M. Monitoring the lung periphery of transplanted lungs. Respir Physiol Neurobiol. 2005;148:141–151. doi: 10.1016/j.resp.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 134.Van Muylem A, Knoop C, Estenne M. Early detection of chronic pulmonary allograft dysfunction by exhaled biomarkers. Am J Respir Crit Care Med. 2007;175:731–736. doi: 10.1164/rccm.200609-1301OC. [DOI] [PubMed] [Google Scholar]
- 135.Biernacki WA, Kharitonov SA, Barnes PJ. Exhaled carbon monoxide in patients with lower respiratory tract infection. Respir Med. 2001;95:1003–1005. doi: 10.1053/rmed.2001.1196. [DOI] [PubMed] [Google Scholar]
- 136.Yamaya M, Sekizawa K, Ishizuka S, Monma M, Mizuta K, Sasaki H. Increased carbon monoxide in exhaled air of subjects with upper respiratory tract infections. Am J Respir Crit Care Med. 1998;158:311–314. doi: 10.1164/ajrccm.158.1.9711066. [DOI] [PubMed] [Google Scholar]
- 137.Cavaliere F, Volpe C, Gargaruti R, Poscia A, Di Donato M, Grieco G, Moscato U. Effects of acute hypoventilation and hyperventilation on exhaled carbon monoxide measurement in healthy volunteers. BMC Pulm Med. 2009;9:51. doi: 10.1186/1471-2466-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Antczak A, Ciebiada M, Kharitonov SA, Gorski P, Barnes PJ. Inflammatory markers: exhaled nitric oxide and carbon monoxide during the ovarian cycle. Inflammation. 2012;35:554–559. doi: 10.1007/s10753-011-9345-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Otterbein LE, Mantell LL, Choi AM. Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol. 1999;276:L688–L694. doi: 10.1152/ajplung.1999.276.4.L688. [DOI] [PubMed] [Google Scholar]
- 140.Otterbein LE, Otterbein SL, Ifedigbo E, Liu F, Morse DE, Fearns C, Ulevitch RJ, Knickelbein R, Flavell RA, Choi AM. MKK3 mitogen-activated protein kinase pathway mediates carbon monoxide-induced protection against oxidant-induced lung injury. Am J Pathol. 2003;163:2555–2563. doi: 10.1016/S0002-9440(10)63610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dolinay T, Szilasi M, Liu M, Choi AM. Inhaled carbon monoxide confers antiinflammatory effects against ventilator-induced lung injury. Am J Respir Crit Care Med. 2004;170:613–620. doi: 10.1164/rccm.200401-023OC. [DOI] [PubMed] [Google Scholar]
- 142.Hoetzel A, Dolinay T, Vallbracht S, Zhang Y, Kim HP, Ifedigbo E, Alber S, Kaynar AM, Schmidt R, Ryter SW, Choi AM. Carbon monoxide protects against ventilator-induced lung injury via PPAR-gamma and inhibition of Egr-1. Am J Respir Crit Care Med. 2008:1771223–1232. doi: 10.1164/rccm.200708-1265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hoetzel A, Schmidt R, Vallbracht S, Goebel U, Dolinay T, Kim HP, Ifedigbo E, Ryter SW, Choi AM. Carbon monoxide prevents ventilator-induced lung injury via caveolin-1. Crit Care Med. 2009;37:1708–1715. doi: 10.1097/CCM.0b013e31819efa31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Faller S, Foeckler M, Strosing KM, Spassov S, Ryter SW, Buerkle H, Loop T, Schmidt R, Hoetzel A. Kinetic effects of carbon monoxide inhalation on tissue protection in ventilator-induced lung injury. Lab Invest. 2012;92:999–1012. doi: 10.1038/labinvest.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang X, Shan P, Alam J, Davis RJ, Flavell RA, Lee PJ. Carbon monoxide modulates Fas/Fas ligand, caspases, and Bcl-2 family proteins via the p38alpha mitogen-activated protein kinase pathway during ischemia-reperfusion lung injury. J Biol Chem. 2003;278:22061–22070. doi: 10.1074/jbc.M301858200. [DOI] [PubMed] [Google Scholar]
- 146.Zhang X, Shan P, Otterbein LE, Alam J, Flavell RA, Davis RJ, Choi AM, Lee PJ. Carbon monoxide inhibition of apoptosis during ischemia-reperfusion lung injury is dependent on the p38 mitogen-activated protein kinase pathway and involves caspase 3. J Biol Chem. 2003;278:1248–1258. doi: 10.1074/jbc.M208419200. [DOI] [PubMed] [Google Scholar]
- 147.Fujita T, Toda K, Karimova A, Yan SF, Naka Y, Yet SF, Pinsky DJ. Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by derepression of fibrinolysis. Nat Med. 2001;7:598–604. doi: 10.1038/87929. [DOI] [PubMed] [Google Scholar]
- 148.Otterbein LE, Zuckerbraun BS, Haga M, Liu F, Song R, Usheva A, Stachulak C, Bodyak N, Smith RN, Csizmadia E, Tyagi S, Akamatsu Y, Flavell RJ, Billiar TR, Tzeng E, Bach FH, Choi AM, Soares MP. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat Med. 2003;9:183–190. doi: 10.1038/nm817. [DOI] [PubMed] [Google Scholar]
- 149.Sato K, Balla J, Otterbein L, Smith RN, Brouard S, Lin Y, Csizmadia E, Sevigny J, Robson SC, Vercellotti G, Choi AM, Bach FH, Soares MP. Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J Immunol. 2001;166:4185–4194. doi: 10.4049/jimmunol.166.6.4185. [DOI] [PubMed] [Google Scholar]
- 150.Song R, Kubo M, Morse D, Zhou Z, Zhang X, Dauber JH, Fabisiak J, Alber SM, Watkins SC, Zuckerbraun BS, Otterbein LE, Ning W, Oury TD, Lee PJ, McCurry KR, Choi AM. Carbon monoxide induces cytoprotection in rat orthotopic lung transplantation via anti-inflammatory and anti-apoptotic effects. Am J Pathol. 2003;163:231–242. doi: 10.1016/S0002-9440(10)63646-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kim HP, Ryter SW, Choi AM. CO as a cellular signaling molecule. Annu Rev Pharmacol Toxicol. 2006;46:411–449. doi: 10.1146/annurev.pharmtox.46.120604.141053. [DOI] [PubMed] [Google Scholar]
- 152.Stone JR, Marletta MA. Soluble guanylate cyclase from bovine lung: activation with nitric oxide and carbon monoxide and spectral characterization of the ferrous and ferric states. Biochemistry. 1994;33:5636–5640. doi: 10.1021/bi00184a036. [DOI] [PubMed] [Google Scholar]
- 153.Furchgott RF, Jothianandan D. Endothelium-dependent and -independent vasodilation involving cyclic GMP: relaxation induced by nitric oxide, carbon monoxide and light. Blood Vessels. 1991;28:52–61. doi: 10.1159/000158843. [DOI] [PubMed] [Google Scholar]
- 154.Zakhary R, Poss KD, Jaffrey SR, Ferris CD, Tonegawa S, Snyder SH. Targeted gene deletion of heme oxygenase 2 reveals neural role for carbon monoxide. Proc Natl Acad Sci U S A. 1997;94:14848–14853. doi: 10.1073/pnas.94.26.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang R. Resurgence of carbon monoxide: an endogenous gaseous vasorelaxing factor. Can J Physiol Pharmacol. 1998;76:1–15. doi: 10.1139/cjpp-76-1-1. [DOI] [PubMed] [Google Scholar]
- 156.Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, Soares MP. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med. 2000;192:1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang X, Shan P, Alam J, Fu XY, Lee PJ. Carbon monoxide differentially modulates STAT1 and STAT3 and inhibits apoptosis via a phosphatidylinositol 3-kinase/Akt and p38 kinase-dependent STAT3 pathway during anoxia-reoxygenation injury. J Biol Chem. 2005;280:8714–8721. doi: 10.1074/jbc.M408092200. [DOI] [PubMed] [Google Scholar]
- 158.Nakahira K, Kim HP, Geng XH, Nakao A, Wang X, Murase N, Drain PF, Wang X, Sasidhar M, Nabel EG, Takahashi T, Lukacs NW, Ryter SW, Morita K, Choi AM. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J Exp Med. 2006;203:2377–2389. doi: 10.1084/jem.20060845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bilban M, Bach FH, Otterbein SL, Ifedigbo E, d’Avila JC, Esterbauer H, Chin BY, Usheva A, Robson SC, Wagner O, Otterbein LE. Carbon monoxide orchestrates a protective response through PPARgamma. Immunity. 2006;24:601–610. doi: 10.1016/j.immuni.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 160.Morita T, Mitsialis SA, Koike H, Liu Y, Kourembanas S. Carbon monoxide controls the proliferation of hypoxic vascular smooth muscle cells. J Biol Chem. 1997;272:32804–32809. doi: 10.1074/jbc.272.52.32804. [DOI] [PubMed] [Google Scholar]
- 161.Piantadosi CA, Suliman HB. Redox regulation of mitochondrial biogenesis. Free Radic Biol Med. 2012;53:2043–2053. doi: 10.1016/j.freeradbiomed.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Suliman HB, Carraway MS, Tatro LG, Piantadosi CA. A new activating role for CO in cardiac mitochondrial biogenesis. J Cell Sci. 2007;120:299–308. doi: 10.1242/jcs.03318. [DOI] [PubMed] [Google Scholar]
- 163.Wang X, Wang Y, Kim HP, Nakahira K, Ryter SW, Choi AM. Carbon monoxide protects against hyperoxia-induced endothelial cell apoptosis by inhibiting reactive oxygen species formation. J Biol Chem. 2007;282:1718–1726. doi: 10.1074/jbc.M607610200. [DOI] [PubMed] [Google Scholar]
- 164.Wang X, Wang Y, Lee SJ, Kim HP, Choi AM, Ryter SW. Carbon monoxide inhibits Fas activating antibody-induced apoptosis in endothelial cells. Med Gas Res. 2011;1:8. doi: 10.1186/2045-9912-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 165.Lee SJ, Ryter SW, Xu JF, Nakahira K, Kim HP, Choi AM, Kim YS. Carbon monoxide activates autophagy via mitochondrial reactive oxygen species formation. Am J Respir Cell Mol Biol. 2011;45:867–873. doi: 10.1165/rcmb.2010-0352OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mishra S, Fujita T, Lama VN, Nam D, Liao H, Okada M, Minamoto K, Yoshikawa Y, Harada H, Pinsky DJ. Carbon monoxide rescues ischemic lungs by interrupting MAPK-driven expression of early growth response 1 gene and its downstream target genes. Proc Natl Acad Sci USA. 2006;13:5191–5196. doi: 10.1073/pnas.0600241103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nakao A, Kimizuka K, Stolz DB, Seda Neto J, Kaizu T, Choi AM, Uchiyama T, Zuckerbraun BS, Bauer AJ, Nalesnik MA, Otterbein LE, Geller DA, Murase N. Protective effect of carbon monoxide inhalation for cold-preserved small intestinal grafts. Surgery. 2003;134:285–292. doi: 10.1067/msy.2003.238. [DOI] [PubMed] [Google Scholar]
- 168.Faleo G, Neto JS, Kohmoto J, Tomiyama K, Shimizu H, Takahashi T, Wang Y, Sugimoto R, Choi AM, Stolz DB, Carrieri G, McCurry KR, Murase N, Nakao A. Carbon monoxide ameliorates renal cold ischemia-reperfusion injury with an upregulation of vascular endothelial growth factor by activation of hypoxia-inducible factor. Transplantation. 2008;85:1833–1840. doi: 10.1097/TP.0b013e31817c6f63. [DOI] [PubMed] [Google Scholar]
- 169.Kaizu T, Nakao A, Tsung A, Toyokawa H, Sahai R, Geller DA, Murase N. Carbon monoxide inhalation ameliorates cold ischemia/reperfusion injury after rat liver transplantation. Surgery. 2005;138:229–235. doi: 10.1016/j.surg.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 170.Kohmoto J, Nakao A, Kaizu T, Tsung A, Ikeda A, Tomiyama K, Billiar TR, Choi AM, Murase N, McCurry KR. Low-dose carbon monoxide inhalation prevents ischemia/reperfusion injury of transplanted rat lung grafts. Surgery. 2006;140:179–185. doi: 10.1016/j.surg.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 171.Kohmoto J, Nakao A, Sugimoto R, Wang Y, Zhan J, Ueda H, McCurry KR. Carbon monoxide-saturated preservation solution protects lung grafts from ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2008;136:1067–1075. doi: 10.1016/j.jtcvs.2008.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nakao A, Toyokawa H, Tsung A, Nalesnik MA, Stolz DB, Kohmoto J, Ikeda A, Tomiyama K, Harada T, Takahashi T, Yang R, Fink MP, Morita K, Choi AM, Murase N. Ex vivo application of carbon monoxide in University of Wisconsin solution to prevent intestinal cold ischemia/reperfusion injury. Am J Transplant. 2006;6:2243–2255. doi: 10.1111/j.1600-6143.2006.01465.x. [DOI] [PubMed] [Google Scholar]
- 173.Musameh MD, Green CJ, Mann BE, Fuller BJ, Motterlini R. Improved myocardial function after cold storage with preservation solution supplemented with a carbon monoxide-releasing molecule (CORM-3) J Heart Lung Transplant. 2007;26:1192–1198. doi: 10.1016/j.healun.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 174.Pannen BH, Köhler N, Hole B, Bauer M, Clemens MG, Geiger KK. J Clin Invest. 1998;102:1220–1228. doi: 10.1172/JCI3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Amersi F, Shen XD, Anselmo D, Melinek J, Iyer S, Southard DJ, Katori M, Volk HD, Busuttil RW, Buelow R, Kupiec-Weglinski JW. Ex vivo exposure to carbon monoxide prevents hepatic ischemia/reperfusion injury through p38 MAP kinase pathway. Hepatology. 2002;35:815–823. doi: 10.1053/jhep.2002.32467. [DOI] [PubMed] [Google Scholar]
- 176.Mazzola S, Forni M, Albertini M, Bacci ML, Zannoni A, Gentilini F, Lavitrano M, Bach FH, Otterbein LE, Clement MG. Carbon monoxide pretreatment prevents respiratory derangement and ameliorates hyperacute endotoxic shock in pigs. FASEB J. 2005;19:2045–2047. doi: 10.1096/fj.05-3782fje. [DOI] [PubMed] [Google Scholar]
- 177.Mitchell LA, Channell MM, Royer CM, Ryter SW, Choi AM, McDonald JD. Evaluation of inhaled carbon monoxide as an anti-inflammatory therapy in a nonhuman primate model of lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2010;299:L891–L897. doi: 10.1152/ajplung.00366.2009. [DOI] [PubMed] [Google Scholar]
- 178.Sawle P, Foresti R, Mann BE, Johnson JR, Green CJ, Motterlini R. Carbon monoxide-releasing molecules (CO-RMs) attenuate the inflammatory response elicited by lipopolysaccharide in RAW264.7 murine macrophages. Br J Pharmacol. 2005;145:800–810. doi: 10.1038/sj.bjp.0706241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sun B, Zou X, Chen Y, Zhang P, Shi G. Preconditioning of carbon monoxide releasing molecule-derived CO attenuates LPS-induced activation of HUVEC. Int J Biol Sci. 2008;4:270–278. doi: 10.7150/ijbs.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sun B, Sun H, Liu C, Shen J, Chen Z, Chen X. Role of CO-releasing molecules liberated CO in attenuating leukocytes sequestration and inflammatory responses in the lung of thermally injured mice. J Surg Res. 2007;139:128–135. doi: 10.1016/j.jss.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 181.Cepinskas G, Katada K, Bihari A, Potter RF. Carbon monoxide liberated from carbon monoxide-releasing molecule CORM-2 attenuates inflammation in the liver of septic mice. Am J Physiol Gastrointest Liver Physiol. 2008;294:G184–G191. doi: 10.1152/ajpgi.00348.2007. [DOI] [PubMed] [Google Scholar]
- 182.Mizuguchi S, Stephen J, Bihari R, Markovic N, Suehiro S, Capretta A, Potter RF, Cepinskas G. CORM-3-derived CO modulates polymorphonuclear leukocyte migration across the vascular endothelium by reducing levels of cell surface-bound elastase. Am J Physiol Heart Circ Physiol. 2009;297:H920–H929. doi: 10.1152/ajpheart.00305.2009. [DOI] [PubMed] [Google Scholar]
- 183.Lancel S, Hassoun SM, Favory R, Decoster B, Motterlini R, Neviere R. Carbon monoxide rescues mice from lethal sepsis by supporting mitochondrial energetic metabolism and activating mitochondrial biogenesis. J Pharmacol Exp Ther. 2009;329:641–648. doi: 10.1124/jpet.108.148049. [DOI] [PubMed] [Google Scholar]
- 184.Mayr FB, Spiel A, Leitner J, Marsik C, Germann P, Ullrich R, Wagner O, Jilma B. Effects of carbon monoxide inhalation during experimental endotoxemia in humans. Am J Respir Crit Care Med. 2006;171:354–360. doi: 10.1164/rccm.200404-446OC. [DOI] [PubMed] [Google Scholar]
- 185.Bathoorn E, Slebos DJ, Postma DS, Koeter GH, van Oosterhout AJ, van der Toorn M, Boezen HM, Kerstjens HA. Anti-inflammatory effects of inhaled carbon monoxide in patients with COPD: a pilot study. Eur Resp J. 2007;30:1131–1137. doi: 10.1183/09031936.00163206. [DOI] [PubMed] [Google Scholar]