Abstract
Rationale
There is growing evidence that the myocardium responds to injury by recruiting c-kit+ cardiac progenitor cells to the damage tissue. Even though the ability of exogenously introducing c-kit+ cells to injured myocardium has been established, the capability of recruiting these cells through modulation of local signaling pathways by gene transfer has not been tested.
Objective
To determine whether stem cell factor gene transfer mediates cardiac regeneration in a rat myocardial infarction model, through survival and recruitment of c-kit+ progenitors and cell-cycle activation in cardiomyocytes, and explore the mechanisms involved.
Methods and Results
Infarct size, cardiac function, cardiac progenitor cells recruitment, fibrosis, and cardiomyocyte cell-cycle activation were measured at different time points in controls (n=10) and upon stem cell factor gene transfer (n=13) after myocardial infarction. We found a regenerative response because of stem cell factor overexpression characterized by an enhancement in cardiac hemodynamic function: an improvement in survival; a reduction in fibrosis, infarct size and apoptosis; an increase in cardiac c-kit+ progenitor cells recruitment to the injured area; an increase in cardiomyocyte cell-cycle activation; and Wnt/β-catenin pathway induction.
Conclusions
Stem cell factor gene transfer induces c-kit+ stem/progenitor cell expansion in situ and cardiomyocyte proliferation, which may represent a new therapeutic strategy to reverse adverse remodeling after myocardial infarction.
Keywords: cardiac myocyte regeneration, gene transfer, myocardial infarction, stem cell factor
The recent demonstration of adult human cardiac renewal1 and identification and extensive characterization of c-kit+ cardiac stem and progenitor cells suggests that the heart is not terminally differentiated but an organ with regenerative potential. These results provide hope for development of therapeutic strategies to augment the limited regenerative process for the failing heart. We sought to enhance the limited endogenous repair process of the myocardium after injury using methods easily translated into clinical practice, by locally expanding c-kit+ cells2 using SCF (stem cell factor) gene transfer. Despite the fact that c-kit has been used extensively as a cell surface marker and much studied with respect to cells homing to infarcted myocardium, together with its ligand, SCF, the tyrosine kinase receptor c-kit is a key proliferation-controlling protein, driving not only the recruitment but the expansion of a number of stem cell types, including hematopoietic, neuronal, germ, and cardiac.4–6 SCF binding induces c-kit dimerization, activation of its intrinsic tyrosine kinase, and autophosphorylation leading to downstream signaling,7 including the Wnt-β-catenin pathway.8 Increased expression of SCF occurs naturally in response to myocardial infarction (MI), which has been shown to mediate migration of c-kit+ cardiac and bone marrow (BM) cells2 via activation of p38 mitogen-activated protein kinase,9 driven by infiltrating macrophages.10 Genetically mutant mice deficient in c-kit signaling (W/Wv) fare worse after MI, and transgenic mice overexpressing SCF in a cardiac-specific manner fare better after MI than their wild-type littermates.4,11,12 SCF has been implicated in promoting the reverse remodeling observed after left ventricular assist device implantation.13
Recently, dramatic improvements were reported in patients with ischemic cardiomyopathy, after intracoronary infusion of autologous c-kit+ cardiac stem cells (CSCs).14 This trial underscored the importance of c-kit+ cells in cardiac reparation. More recently cardiosphere (which contain a significant amount of c-kit+ cells) injection in patients with heart failure was also shown to improve clinical parameters in the injected patients.15
We therefore undertook an alternative strategy consisting of SCF adenoviral gene transfer into the infarcted myocardium in rats, to test the potential of SCF to recruit c-kit+ cells from cardiac and BM origin. In addition, we tested whether this strategy would lead to enhanced cardiac repair, function, and survival and finally to define whether SCF has an effect on cardiomyocyte proliferation and cell-cycle reentry.
Methods
SCF adenoviruses were injected in rats to enhance cardiac repair after MI. Molecular and functional approaches were performed to assess cardiac regeneration after SCF therapy. Detailed methods are provided in the Online Data Supplement.
Results
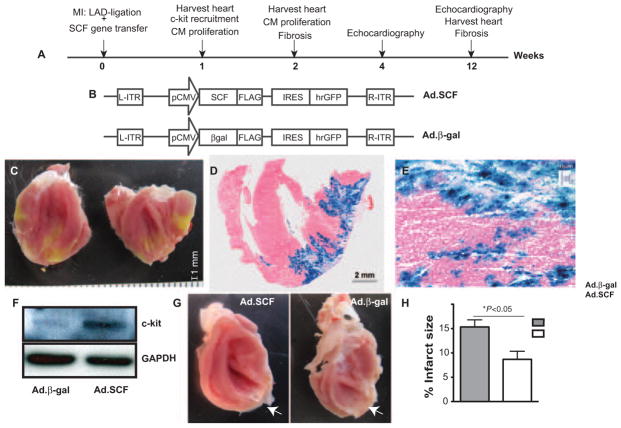
SCF Gene Transfer Decreases Infarct Size After LAD-Ligation
SCF alternative splicing leads to 2 isoforms, a soluble and a membrane-bound form. Stimulation with the soluble protein leads to rapid and transient activation, autophosphorylation, and fast degradation of SCF receptor, c-kit; whereas stimulation with the membrane-associated form leads to more sustained activation by preventing receptor–ligand complex internalization.7 Our strategy consisted of using adenoviral-mediated gene transfer of SCF as a therapy to promote cardiac regenerative mechanisms in rats undergoing MI. Myocardial regeneration and function was assessed 1, 2, 4, and 12 weeks post-MI (Figure 1A). Therefore, to maximize the proliferative signal for c-kit+ cells, we generated recombinant adenoviruses expressing SCF membrane-bound form and green fluorescent protein (Ad.SCF). Similarly, we generated adenoviruses containing β-gal and green fluorescent protein, used as controls (Ad.β-gal; Figure 1B). By permanent ligation of the left anterior descending coronary artery (LAD), we induced the MI and later, Ad.SCF and Ad.β-gal adenoviruses were delivered into the periinfarct left ventricle (LV). Gene transfer was confirmed 1 week post-MI by β-galactosidase, c-kit, and green fluorescent protein expression in controls and SCF-treated rats (Figure 1C–1F; Online Figure I). After SCF overexpression a reduction in the scar area was detected compared with controls (Figure 1G; Online Figure II). Cardiac magnetic resonance imaging was used to measure cardiac morphology and function at baseline (2 days) and 2 and 4 weeks post-MI. A significant reduction in infarct size, normalized to total LV, was detected after SCF therapy 2 weeks post-MI (15.3%±1.4% in Ad.β-gal versus 8.6%±1.6% in Ad.SCF; P<0.05) and 4 weeks post-MI (17.7%±0.3% in Ad.β-gal versus 7.6%±1.2% in Ad.SCF; P<0.01) (Figure 1H; Online Figure IIIA and IIIB). Thus, after SCF gene transfer, no significant differences in ejection fraction (EF) was found at baseline (2 days post-MI) between controls and SCF-treated rats (62.0%±0.5% in Ad.β-gal versus 63.5%±1.2% in Ad.SCF; P=0.31). Although a nonsignificant EF improvement was detected after SCF therapy 2 weeks post-MI (48.7%±0.8% Ad.β-gal versus 56.5%±2.9% Ad.SCF; P=0.06), we showed a significant improvement 4 weeks post-MI (48.3%±0.4% Ad.β-gal versus 58.4%±0.6% Ad.SCF; P<0.01; Online Figure IIIC). These preliminary observations indicate that SCF might be playing a role in enhancing cardiac protection after MI.
Figure 1. Study design.
A, Experimental timeline. Our strategy consisted of using stem cell factor (SCF) as a therapy in cardiac regeneration in rats undergoing myocardial infarction (MI) after delivering adenoviruses expressing SCF membrane-bound form and green fluorescent protein (Ad.SCF) or adenoviruses containing β-gal and green fluorescent protein (Ad.β-gal) recombinant adenoviruses in the peri-infarct area. Myocardial regeneration and function was assessed at 1, 2, 4, and 12 weeks post-MI. B, Recombinant Ad.SCF and Ad.β-gal adenoviruses were generated after cloning SCF membrane isoform and β-gal gene sequences, respectively, into an adenoviral plasmid containing GFP as a reporter gene. C–E, Detection of gene transfer within the myocardium 1 week post-MI. Distribution of the viral infection was confirmed by GFP expression, imaged through digital photography (C) and by X-gal staining (blue) (D and E) in control rats (Ad.β-gal). F, Analysis of c-kit receptor expression by western blot (WB), in lysates from control and Ad.SCF-treated hearts. α-GAPDH was used as protein loading control. G and H, Infarct size (white arrows) (G) was measured by cardiac magnetic resonance imaging 2 weeks post-MI (15.3±1.4% Ad.β-gal vs 8.6±1.6% Ad.SCF; *P<0.05; H). LAD indicates left anterior descending coronary artery; IRES, internal ribosomal entry site.
SCF Improves Survival and Cardiac Function
Previous studies show that human SCF transgene expression in mice induces c-kit receptor activation, promoting an improvement in survival and cardiac function after MI.12 Survival was monitored at 3 months postoperation in sham, control, and Ad.SCF-treated groups (n=5, n=10, and n=13, respectively). Sham groups survival was 100%, whereas MI led to an increase in mortality. SCF gene transfer resulted in increased survival post-MI compared with controls (90% Ad.SCF versus 65% Ad.β-gal; P=0.07) (Figure 2A). We next evaluated the impact that SCF overexpression had on cardiac structure, analyzing cardiac function and LV dimension by echocardiography. SCF administration enhanced LV function at 1 and 3 months post-MI (Figure 2B and 2C). LAD ligation led to a time-dependent decrease in the fraction of shortening and EF, as well as an increase in LV chamber dimensions. However, the reduction in fraction of shortening attributable to MI was ameliorated after SCF overexpression (n=13) was compared with controls (n=10) at 1 month post-MI (46±8% Ad.SCF versus 30±7% Ad.β-gal; P<0.001). Similarly, EF decrease attributable to MI was improved after Ad.SCF administration at 1 month post-MI (67±14% Ad.SCF versus 51±11% Ad.β-gal; P<0.001) (Figure 2D). SCF overexpression also caused an improvement in fraction of shortening (43±6% Ad.SCF versus 29±8 Ad.β-gal; P<0.0001) and EF (64±7% Ad.SCF versus 46±16% Ad.β-gal; P<0.001) at 3 months post-MI (Figure 2E). Furthermore, LV chamber dimensions were also increased because of MI, although SCF overexpression attenuated the MI-induced increase in LV end-systolic and end-diastolic volume (Table). These results suggest that SCF administration continue to improve LV function 3 months after MI.
Figure 2. Cardiac function in the stem cell factor (SCF)-treated myocardium.
A, Kaplan–Meier survival analysis in sham (n=5), adenoviruses containing β-gal and green fluorescent protein (Ad.β-gal)–treated group (n=10), and adenoviruses expressing SCF membrane-bound form and green fluorescent protein (Ad.SCF)-treated groups (n=13). Although there was no statistical significance, a tendency toward an increase is detected in survival after SCF treatment compared with controls 3 months postmyocardial infarction (MI; 90% Ad.SCF vs 65% Ad.β-gal; P=0.07 vs Ad.β-gal). B–E, Echocardiographic measurements performed 1 and 3 months post-MI. Representative parameters short-axis M-mode view, in Ad.SCF-treated and control (Ad.β-gal) rats at 1 (B) and 3 months post-MI (C). Fractional shortening (FS) and ejection fraction (EF) 1 month (D) and 3 months (E) after Ad.SCF overexpression.
Table.
Effects of Myocardial Regeneration on Ad.SCF-Treated Rats
| Parameter, unit |
P<0.001
|
|||||
|---|---|---|---|---|---|---|
| Sham 1 mo | Ad.βgal 1 mo | Ad.SCF 1 mo | Sham 3 mo | Ad.βgal 3 mo | Ad.SCF 3 mo | |
| IVSd, mm | 1.8±0.03 | 1.8±0.4 | 2.1±0.5 | 2.0±0.04 | 2.0±0.5 | 2.2±0.5 |
| IVSs, mm | 4.1±0.3 | 2.6±0.9 | 3.5±0.9 | 4.5±0.04 | 2.9±0.8 | 3.5±0.6 |
| LVIDd, mm | 7.3±0.2 | 8.7±1.0 | 7.8±0.5 | 6.9±0.4 | 9.4±1.1 | 8.0±0.8 |
| LVIDs, mm | 2.8±0.2 | 6.1±0.9 | 4.2±0.8 | 2.6±0.4 | 6.5±1.4 | 4.6±0.9 |
| LVPWd, mm | 1.9±0 | 2.4±0.4 | 2.4±0.3 | 2.2±0.1 | 2.4±0.5 | 2.6±0.2 |
| LVPWs, mm | 3.6±0.03 | 3.4±0.6 | 3.8±0.3 | 3.8±0.02 | 3.9±0.5 | 4.0±0.3 |
| FS, % | 61±5 | 29±7 | 46±8 | 62±4 | 28±8 | 43±6 |
| EDV, mL | 0.44±0.05 | 0.88±0.27 | 0.66±0.16 | 0.52±0.14 | 1.15±0.39 | 0.71±0.17 |
| ESV, mL | 0.05±0.03 | 0.43±0.17 | 0.23±0.14 | 0.10±0.03 | 0.63±0.31 | 0.26±0.1 |
| EF, % | 88±5 | 51±11 | 67±14 | 79±2 | 46±16 | 64±7 |
Echocardiographic measurements. All values represent mean±SD.
SCF indicates stem cell factor; Ad.SCF, adenoviruses expressing SCF membrane-bound form and green fluorescent protein; Ad.βgal, adenoviruses containing β-gal and green fluorescent protein; IVSd, interventricular septal thickness at diastole; IVSs, interventricular septal thickness at systole; LVIDd, left ventricular internal dimension at diastole; LVIDd, left ventricular internal dimension at systole; LVPWd, left ventricular posterior wall at diastole; LVPWs, left ventricular posterior wall at systole; FS, fraction of shortening; EDV, end-diastolic volume; ESV, end-systolic volume; EF, ejection fraction.
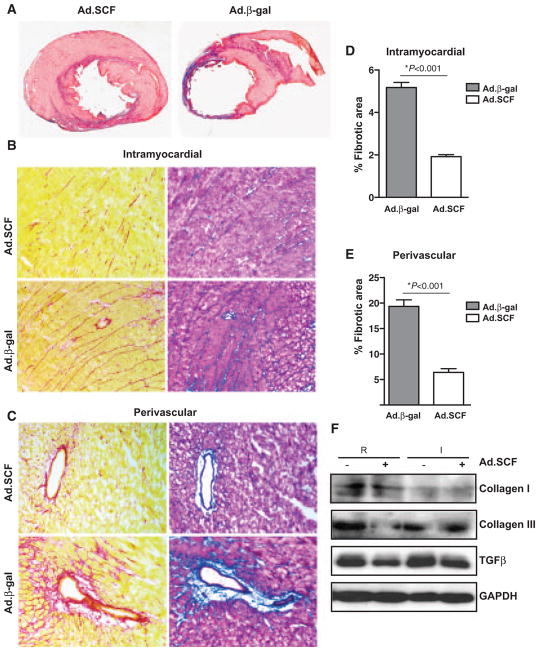
SCF Reduces Fibrosis in the Infarct Heart
The beneficial effects of SCF in improving cardiac function and survival after MI result from recruiting endothelial progenitor cells and decreasing cardiac remodeling.16 An important hallmark in fibrosis is the increase in collagen and other extracellular matrix components in the myocardium17 and the release of transforming growth factor-β (TGF-β) by active myofibroblasts.18,19 Consistent with the cardiac magnetic resonance imaging and echocardiography data, SCF therapy decreased LV wall thinning, increasing viable tissue within the risk region 1 month post-MI (Figure 3A). Thus, a reduction in fibrosis was detected after SCF overexpression. The collagenous area in the pericardial region was significantly decreased in SCF-treated rats (5.1%±0.2% Ad.β-gal versus 2.0%±0.1% Ad.SCF; P<0.0001). Similarly, the fibrotic area around the perivascular region was markedly reduced after SCF therapy (19.3%±1.2% Ad.β-gal versus 6.4%±0.7% Ad.SCF; P<0.001; Figure 3B–3E). Moreover, collagen content measured by Sircol Collagen Assay 3 months post-MI showed a significant decrease in the infarcted (44.8%±4.1% Ad.β-gal versus 27.8%±4.1% Ad.SCF; P<0.05) and remote regions (26.2%±1.8% Ad.β-gal versus 18.7%±2.0% Ad.SCF; P<0.05) after SCF overexpression (Online Figure IVA and IVB). Along with the progression of fibrosis, the expression of TGF-β was decreased in both remote and infarcted area after SCF therapy. In contrast, collagen III expression was only decreased in the remote area, and no significant differences were found in collagen I expression levels after SCF therapy (Figure 3F; Online Figure IVC and IVD). Together, these data suggest that SCF therapy might play a protective role against fibrosis replacement by reducing scar formation, restoring partially the structural integrity of the infarcted heart.
Figure 3. Cardiac fibrosis is decreased after stem cell factor (SCF) overexpression.
A, Representative Masson trichrome–stained myocardial sections from control and SCF-treated rats 1 month post-myocardial infarction (MI). Blue, scar tissue; red, viable myocardium. B–E, Intramyocardial (B) and periovascular fibrosis (C) analyzed by Picrosirius Red and Masson Trichrome staining 1 month post-MI. D and E, Effect of SCF overexpression in intramyocardial (D) (5.1%±0.2% in adenoviruses containing β-gal and green fluorescent protein [Ad.β-gal] vs 2.0%±0.1% in adenoviruses expressing SCF membrane-bound form and green fluorescent protein [Ad.SCF]; P<0.0001) and perivascular fibrosis (E) (19.3%±1.2% in Ad.β-gal vs 6.4%±0.7% in Ad.SCF; P<0.001). F, Analysis of collagen I, III, and transforming growth factor-β (TGF-β) expression by WB in cardiac lysates from remote (R) and infarcted (I) regions 3 months post-MI.
SCF Gene Transfer Augments Cardiac Progenitor Cell Recruitment to the Ischemic Myocardium
The SCF receptor, c-kit, is used as a marker to identify several types of adult stem cells, including those in cardiac, hematopoietic, brain, liver pancreatic, and lung tissue, and its expression is the primary characteristic of cardiac lineage commitment.20–22 The membrane-assotiated SCF isoform induces more persistent c-kit receptor activation, promoting cell survival by recruiting c-kit+ stem cells.23 We then asked whether Ad.SCF therapy could enhance the exogenous and endogenous recruitment of cardiac progenitors cells (CPC) and CSCs to the infarcted myocardium promoting the recovery of the injured heart. Our results confirmed our hypothesis, showing that Ad.SCF overexpression resulted in a 4-fold increase of c-kit+ cells compared with sham-operated (69±39 cells/mm3 in sham versus 307±11 cells/mm3 in Ad.SCF; P<0.05) or control (64±17 cells/mm3 in Ad.β-gal versus 307±11 cells/mm3 in Ad.SCF; P<0.05) groups 1 week post-MI (Figure 4A and 4B). To further confirm the faithful increase of CPCs, cardiac c-kit+ population was purified by fluorescence-activated cell sorting from the whole heart. SCF overexpression resulted in higher absolute numbers of c-kit+ cells within the heart (1:10000) compared with control (1:37000) or sham (1:55000) groups (Figure 4C). We next determined whether SCF expression was altering the hematopoietic c-kit+ population. The lack of lineage markers (Lin−) and c-kit+ expression was analyzed by fluorescence-activated cell sorting in hematopoietic stem cells (Lin− c-kit+) from blood and BM cells 1 week post-MI. No differences were found in circulating or BM-derived cells after SCF overexpression (Figure 4D).
Figure 4. Cardiac c-kit+ population is increased at 1 week post-myocardial infarction (MI).
A, Confocal images represent c-kit membrane staining in section hearts. B, A 4-fold increase in c-kit+ cells is detected after stem cell factor (SCF) therapy. C, Cardiac c-kit+ cells detection by fluorescence-activated cell (FACS) sorting. D, Analysis of hematopoietic stem cell population (Lin− c-kit+) by FACS in bone marrow and circulating blood cells shows no changes because of SCF overexpression. E and F, Cardiac c-kit+ population is gated for c-kit marker and early cardiac markers (GATA4, MEF2, MEF2C, and Nkx2.5) (E) or differentiated expression markers (eosinophils and high affinity IgE receptor [FcεRIα], multidrug resistant protein (MDR)-1, CD45, and α-sarcomeric actin [α-SA]) (F) are analyzed by FACS. G, Confocal images represent c-kit and CD45 membrane costaining in SCF-treated hearts. Ad.SCF indicates adenoviruses expressing SCF membrane-bound form and green fluorescent protein; Ad.β-gal, adenoviruses containing β-gal and green fluorescent protein.
CPCs have been characterized as a heterogeneous cell population in a state of differentiation and commitment to cardiac lineage, scoring negative for α-sarcomeric actin but positive for GATA4, Nkx2.5, or MEF2 expression markers.24 Cardiac c-kit+ population was gated and examined for coexpression of transcription factors expressed at early and late stages of cardiomyocyte development. Despite this heterogeneity, an increase in MEF2, MEF2C, Nkx2.5, and GATA4 early cardiac expression markers was detected after Ad.SCF therapy in gated c-kit+ cells (Figure 4E). Furthermore, a decrease in α-sarcomeric actin expression, a later cardiac-specific marker, was also observed after SCF overexpression (Figure 4F). By contrast, the cardiac c-kit+ subset was mainly negative for mast cells (eosinophils and high affinity IgE receptor [FcεRIα])25–27 and hematopoietic stem cell (multidrug resistant protein-1) markers.28 In resting normal hearts, c-kit+ cardiac stem/progenitors are CD45−24; however, in failing hearts, c-kit+CD45+ cells predominate.29,30 Our data showed a predominant c-kit+ population coexpressing the CD45+ hematopoietic lineage marker, suggesting an exogenous BM origin post-MI.31,32 Although our results do not exclude the possibility of BM-derived c-kit+ cell recruitment in the infarcted myocardium, we have also shown the existence of a small subpopulation of cardiac c-kit+ cells that might have cardiomyogenic and cardioprotective effects (Figure 4F and 4G). The increase of c-kit+ subset coexpressing Nkx2.5, MEF2, or MEF2C together with the decrease in α-sarcomeric actin expression, suggests an accumulation of immature c-kit+ population on SCF administration. These results confirm the heterogeneity of c-kit population, as well as its different stage of commitment to cardiac lineage.
SCF Induces Proliferation of Cardiomyocytes in the Ischemic Heart
SCF is a growth factor that promotes the proliferation of different stem cells populations, including cardiac, hematopoietic, neuronal, and germ,4–6 together with c-kit+ cells’ recruitment to injured myocardium.12 The accumulation of c-kit+CPCs led us to investigate the role that SCF was playing in cardiomyocyte cell-cycle activation by evaluating their potential to divide and undergo DNA synthesis at 1 week post-MI. Cardiomyocyte potential to divide was assessed by colocalization of phosphohistone H3 (P-H3) and Ki67 together with α-sarcomeric actin marker. As expected, a global increase of P-H3 and Ki67-positive cardiomyocytes was detected in the periinfarct zone of the Ad.SCF-treated hearts at 1 week (Figure 5A; Online Figure V). Similarly, cardiomyocyte cytokinesis was assessed by colocalization of Aurora B kinase together with α-sarcomeric actin marker. Upon SCF therapy, Aurora B kinase–positive cardiomyocytes were detected in the periinfarct zone 1 week post-MI (Online Figure VI). As expected from these data, an increase in cardiomyocytes undergoing DNA synthesis at the time of bromodeoxyuridine labeling was detected in SCF-treated hearts compared with controls. The number of total bromodeoxyuridine-labeled nuclei (9.5±1.3% Ad.SCF versus 3.8±0.3% Ad.β-gal; P=0.001) and bromodeoxyuridine-labeled cardiomyocytes (3.3±0.4% Ad.SCF versus 1.3±0.3% Ad.β-gal; P=0.002) were significantly greater after SCF overexpression (Figure 5A and 5B; Online Figure V). Thus, an enhancement in the expression levels of proliferative markers was detected after SCF treatment by Western blot analysis. CyclinD1, proliferating cell nuclear Ag, and P-H3 expression was upregulated in the infarcted region 1 week post-Ad.SCF therapy. However, no demonstrable changes in cyclin D1, proliferating cell nuclear Ag, or P-H3 expression were found in the remote region (Figure 5C). The enhancement in ventricular function suggests that SCF could be inducing a protective response, enhancing cell survival. Using terminal deoxynucleotidyl transferase dUTP nick end labeling staining apoptotic cells were 1 week post-MI. The density of apoptotic cells per area in the border zone was significantly lower after SCF overexpression compared with controls (7.0%±0.5% in Ad.β-gal versus 3.5%±2.1% in Ad.SCF; P<0.0001; Figure 5D and 5E). Thus, caspase 3 activity was decreased upon SCF therapy in the infarcted area (Figure 5F). Together, these data suggest a protective role of SCF in preventing apoptosis.
Figure 5. Stem cell factor (SCF) therapy induces cardiomyocyte cell-cycle activation 1 week post-MI.
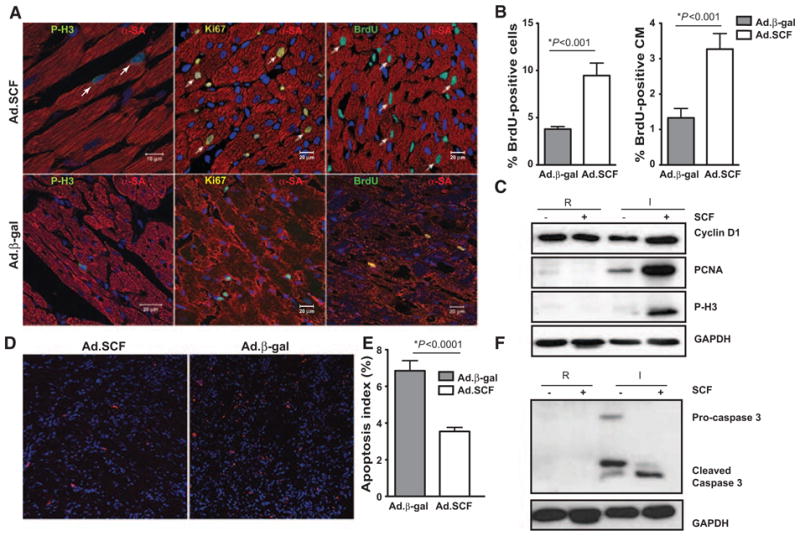
A, Cardiomyocytes cell-cycle reentry (arrows) analyzed by costaining with α-sarcomeric actin (α-SA) and phosphohistone 3 (P-H3) or Ki67 proliferative markers. DNA synthesis is detected by bromodeoxyuridine (BrdU) incorporation. B, An increase of BrdU+ cells (10.1±1.4% adenoviruses expressing SCF membrane-bound form and green fluorescent protein [Ad.SCF] vs 3.7±0.3% adenoviruses containing β-gal and green fluorescent protein [Ad.β-gal]; *P<0.001; left) and BrdU+α-SA+ cells (CM) (3.8±0.3% Ad.SCF vs 1.8±0.2% Ad.β-gal, *P<0.001; right) is shown in the border area after SCF therapy. C, Cyclin D1, proliferating cell nuclear Ag (PCNA), and P-H3 proliferation expression markers in lysates from remote (R) and infarcted (I) regions. D–F, SCF prevent apoptosis in the infarcted myocardium. Apoptotic cells detection by terminal deoxynucleotidyl transferase dUTP nick end labeling staining (red) in the border area (D). Apoptotic index (%) analysis by TUNEL (E) (7.0%±0.5% Ad.β-gal vs 3.5%±2.1% Ad.SCF; *P<0.0001). Caspase 3 activity analysis by western blot (WB) (F).
To assess the impact of cardiomyocyte proliferation on LV hypertrophy because of SCF therapy, we measured LV weight (LVW) and heart weight (HW) to body weight (BW) ratio at 2 weeks post-MI. After measuring the ratio of LVW/BW and HW/BW after MI and SCF administration, we found a trend toward a decrease in LVW/BW after MI (0.20%±0.04% Ad.β-gal versus 0.23%±0.01% sham, P=NS) and HW/BW (0.25%±0.05% Ad.β-gal versus 0.30%±0.02% sham), which was reversed after SCF administration in LVW/BW (0.23%±0.04% Ad.SCF versus 0.23%±0.01% sham; P=NS) and in HW/BW (0.28%±0.05% Ad.SCF versus 0.30%±0.02% sham). We next assessed the tranverse diameter of cardiomyocytes, by analyzing the minimum cross-sectional diameter at the nuclear level, to minimize the effects of orientation on cardiomyocyte size measurements.33 Significant smaller myocyte cross-sectional diameters detected by vinculine staining were found after SCF treatment (34.05±0.27 μm Ad.SCF versus 41.65±0.40 μm Ad.β-gal; P<0.0001). Echo analysis of cardiac wall thickness also documented reduction in cardiac hypertrophy in SCF-treated rats (Table; Online Figure VII).
These results showing decreased LV mass decrease along with reduced cardiomyocyte size are all concordant with decreased LV hypertrophy detected after SCF administration. Together, these data suggest that SCF over expression promotes cardiac regeneration by inducing newly dividing cardiomyocytes and preventing hypertrophy and apoptosis of existing cardiac cells in the ischemic heart.
Wnt/β-Catenin Pathway Is Activated Upon SCF Overexpression
We next examined the molecular and cellular mechanisms responsible for cardiac progenitor and stem cell expansion in the ischemic myocardium after Ad.SCF overexpression. Wnt signaling has been shown to promote the self-renewal and differentiation of undifferentiated cells including c-kit+ hematopoietic stem cell34 and Isl1+ cardiovascular progenitor cells,35 enhancing also the differentiation of c-kit+ adipose-derived murine stromal vascular cells into cardiac myocytes.8 The accumulation of c-kit+ cells, together with the proliferation of cardiomyocytes after SCF treatment and MI, led us to study Wnt/β-catenin signaling in the context of cardiac regeneration. The hallmark of canonical Wnt pathway activation is β-catenin accumulation in the cytoplasm and its translocation to the nucleus.36 An increase in β-catenin stabilization was observed after SCF overexpression in remote and infarcted regions (Figure 6A). We next determined whether Wnt signaling might be regulating genes involved in c-kit+ stem cell self-renewal and proliferation, such as Notch1, HoxB4, and cyclin D1.34,37,38 By using real-time polymerase chain reaction analysis on cardiac extracts on either SCF-treated or control hearts, we found that Notch1, HoxB4, and cyclin D1 were upregulated an average of 2, 6, and 7-fold, respectively, in the remote area and 1.2-, 2.2-, and 2-fold, respectively, in the infarcted area upon SCF administration (Figure 6B; Online Table I).
Figure 6. Proposed mechanism: Wnt/β-catenin pathway is activated in adenoviruses expressing stem cell factor (SCF) membrane-bound form and green fluorescent protein (Ad.SCF)-treated rats.

A, β-catenin accumulation is observed in the remote (R) and infarcted (I) area 2 weeks after SCF overexpression. B, RNA isolated from remote (R) and infarcted (I) regions from controls and Ad.SCF-treated rats was reverse transcribed and Notch1, HoxB4, and cyclin D1 expression analyzed by quantitative real-time polymerase chain reaction. An upregulation of Notch1, HoxB4, and cyclin D1 is detected 2 weeks after SCF therapy. C, β-catenin and phospho-GSK3β increased expression is detected in isolated cardiomyocytes after SCF treatment. D, After myocardial infarction (MI), SCF therapy promotes cardiac repair by enhancing survival and left ventricle (LV) function and by preventing remodeling and apoptosis of existing cardiac cells. We hypothesize that SCF overexpression through c-kit receptor activation induces Wnt signaling activation in a paracrine way, promoting cardiac progenitors cells (CPCs) recruitment and cardiomyocytes (CM) cell-cycle activation and survival in the ischemic myocardium. RFU indicates relative fluorescence units.
Furthermore, Wnt pathway activation was also detected because of SCF overexpression in isolated cardiomyocytes (Online Figure VIII) through glycogen synthase kinase 3β phosphorylation and β-catenin accumulation in the cytoplasm (Figure 6C). We hypothesize that SCF administration may be inducing Wnt signaling activation in either CPCs by promoting their recruitment and self-renewal and also in cardiomyocytes by inducing their reentry into cell-cycle after MI (Figure 6D).
Discussion
Recent studies have shown that stem cells may be able to replace damaged tissue and potentially treat cancer, Type 1 diabetes mellitus, Parkinson disease, Huntington disease, and cardiac failure along with many other diseases.39 Clinical experience from the first ≈1000 patients who have received stem cell therapy for acute MI or chronic heart failure indicates a favorable safety profile with modest improvement in cardiac function and structural remodeling. However, cell therapy challenges remain formidable40 and include isolation of the appropriate cell, low efficiency of engraftment, expansion of the cells ex vivo, and ethical and regulatory hurdles. In the last decade, gene therapy has reemerged with novel vectors that are safer and more targeted to various diseased states. In fact clinical gene therapy for cardiovascular diseases may be able to fulfill an unmet need for effective therapies in heart failure.41
The identification of the resident c-kit+ CSC population in the adult heart has demonstrated the regenerative potential of the adult heart.42–44 The need to activate in situ the intrinsic cardiac regenerative capacity suggests the use of growth factors, cytokines, and drugs to promote cardiac repair. Alternatively, BM progenitor cells may migrate to the myocardium leading to the formation of new myocyte progeny.45 Several studies have investigated the increased cardiac progenitor recruitment after SCF soluble protein administration,41 overexpression in transplanted cells11 and in transgenic mice.12
These experimental results were recently tested in a clinical trial, whereby intracoronary infusion of autologous c-kit+ cells in patients with ischemic cardiomyopathy resulted in significant improvement in systolic function and a reduction in infarct size.14 These encouraging clinical results emphasize the importance of recruiting c-kit+ cells in the setting of ischemic cardiomyopathy.
However, we sought to develop a clinically relevant local expression system for the membrane-bound SCF isoform to optimally stimulate cardiac repair after experimental MI. Furthermore, the administration of SCF membrane-bound form, as opposed to the soluble form, limited the expansion of mast cells in BM and blood, an attribute which has severely limited the clinical use of the soluble form of the molecule (Stemgen; ancestim). Our results show that SCF gene transfer stimulates a regenerative response after MI that appears to restore the injured heart and function.
In the present study, we used intramyocardial SCF adenoviral gene transfer to enhance cardiac repair after LAD ligation by recruiting and expanding resident c-kit+ cardiac progenitor/stem cells. Our studies have shown several significant findings. Using transient SCF expression, we observed an increase in cardiac c-kit+ cell recruitment after MI. Increased c-kit+ CPC population in SCF hearts is demonstrated by 2 independent techniques: cell counting by histology and sorting flow cytometry. These CPCs are a mixed population containing not only CSCs and precursors but also cells committed to other lineages. Although the majority population of c-kit+ cells are presumably endogenous,24 we do observe a high enrichment of c-kit+CD45+ fraction post-MI,29,30 suggesting a BM origin for many of the CPCs isolated. This conclusion is also consistent with the studies reporting that BM progenitor cells are mobilized after MI, homing to injured areas promoting cardiac repair.31,46 Regardless of cell origin, the increase in c-kit+ cells in SCF-treated hearts, whether CSC, BM cells, or a combination, does mediate a beneficial effect on survival, cardiac structure, and ventricular function after MI.
Previous studies show that cardiomyocytes self-renew though proliferation of endogenous c-kit+ CPCs,24 maintaining the cardiomyocyte turnover in the adult heart after cell loss.47 However, BM progenitor cells can also populate the heart, adopting a cardiomyogenic phenotype as a source of cardiomyocyte self-renewal.48 Our results show that SCF stimulates the regenerative potential of the heart tissue by inducing cardiomyocyte cell-cycle activation, undergoing DNA synthesis 1 week post-MI. It is possible that the significant increase of P-H3-, Ki67-, and Aurora B kinase–labeled cardiomyocytes that occurs after SCF administration may be the result of resident c-kit+CPCs49 or c-kit+ BM progenitor cells differentiating into cardiomyocytes31 or stimulation of adult cardiomyocytes to reenter the cell-cycle and divide.50 Our data support some or all of these mechanisms. For instance, 1 week post-MI we found c-kit+ cells coexpressing GATA4, Nkx2.5, MEF2, or MEF2C, which support their potential to differentiate into cardiomyocytes. Thus, we also found by immunohistochemistry a pool of cardiac c-kit+ CD45− cells, suggesting that the myocardium harbors a pool of resident CPCs after SCF treatment. However, we also show c-kit+ cells coexpressing CD45 suggesting that this pool of cells may be derived from the BM via the blood stream, reflecting their exogenous origin. Furthermore, we demonstrated endogenous regeneration by the increase in bromodeoxyuridine incorporation in cardiomyocytes, after SCF treatment. Nevertheless, to quantify the contribution of each potential source to newly regenerated cardiomyocytes is beyond this study. An additional variable that may have influenced cardiac regeneration could be the salutary effect that SCF has on enhancing survival by preventing apoptosis in the viable myocardium. Terminal deoxynucleotidyl transferase dUTP nick end labeling staining demonstrated that the density of apoptotic cells was reduced in the border area after SCF therapy, reflecting the cardioprotection capacity of SCF. The decrease in apoptosis in SCF-treated rats probably played a role in reducing infarct size and improving cardiac function. SCF overexpression was found to lead to a greater proportion of viable tissue in the surviving myocardium and a 2-fold decrease in the infarcted area. This decrease was mainly related to a reduction in infarct wall thinning and, in parallel, to a decrease in collagen content and fibrotic area after SCF overexpression, promoting an enhancement in cardiac performance.
In this study we provide evidence that involvement of Wnt/β-catenin signaling may occur in cardiomyocyte and CPC pool after MI because of SCF overexpression. Wnt signaling is an important pathway in stem cell behavior,51,52 cardiac development,53 and myocardial repair.54 Additionally, Wnt signaling has been shown to promote the self-renewal and differentiation of c-kit+ hematopoietic stem cell34 and Isl1+ cardiovascular progenitor cells.35 The stimulation of c-kit+ cell recruitment and cardiomyocyte proliferation after SCF overexpression led us to study Wnt signaling in the context of cardiac regeneration after MI. The fact that isolated cardiomyocytes in the presence of SCF increase β-catenin stabilization and GSK-3β phosphorylation indicates that Wnt signaling may be activated in cardiomyocyte population, which may also include undifferentiated CPCs. Aditionally, Notch1, HoxB4, and cyclin D1, Wnt target genes,34,38 are upregulated in the myocardium after SCF therapy. The function of cyclin D1 in facilitating the initiation of DNA synthesis and reentry into cell-cycle of quiescent or senescent cell, suggests that Wnt pathway activation resulting from SCF therapy might be inducing S-phase reentry of cardiomyocytes. Collectively, these observations suggest that SCF therapy may have a role in inducing Wnt signaling activation after MI in CSCs, CPCs, and cardiomyocytes, and therefore, it should be studied for a role in CSC therapies. It will be of particular interest to identify specific Wnt signals that might drive the activation of the pathway.
In the current study, we have used a permanent LAD ligation inducing a dead area around the MI. Four major conclusions emanate from this study: (1) SCF therapy improves survival, cardiac function, and LV wall thickness in the border area after MI by decreasing infarct size and remodeling and preventing apoptosis; (2) SCF administration activates the resident and exogenous CPCs in adult rat myocardium fostering the regeneration of cardiomyocytes; (3) SCF overexpression improves cardiomyocyte survival, inducing their potential to cell-cycle reentry after MI; and (4) the effects of SCF overexpression is still measurable 2 months after its administration, suggesting the existence of a feedback loop triggered by external stimuli, such as Wnt signaling, which could explain the long duration of the regenerative cardiac response after MI.
In summary, these results provide the proof that SCF gene therapy promotes cardiac regeneration in the injured myocardium and may offer a novel strategy for patients with ischemic cardiomyopathy, as well as other diseases in which c-kit+ stem cells have demonstrated use. The ability to induce cardiac repair with essential signaling molecules allows for a strategy to both dissect CPC niches effects and selectively induce cardiomyocyte cell-cycle reentry useful in myocardial regeneration treatments.
Supplementary Material
Novelty and Significance.
What Is Known?
Stem cell factor (SCF), a c-kit receptor ligand, is involved in the proliferation and recruitment of several stem cells populations.
Cardiac progenitor cells traffic to the injured myocardium after myocardial infarction (MI).
Gene transfer and stem cell–based therapies have been successfully used in clinical trials, enhancing cardiac function after heart failure.
What New Information Does This Article Contribute?
SCF therapy enhanced survival, left ventricle function, and cardiac regeneration after MI and was associated with a smaller infarct size, remodeling, and apoptosis.
SCF administration promotes cardiac progenitor cell recruitment and cardiomyocyte cell-cycle reentry after MI through Wnt signaling activation.
Gene transfer and stem cell–based combination therapy have successfully induced cardiac regeneration in rats and may have the potential to promote tissue restoration in patients with heart failure.
Gene transfer and stem cell–based therapies have been developed to replace the loss of cardiac mass after heart failure. The current study demonstrates that SCF gene transfer has the potential to promote cardiac repair after MI through cardiac progenitor cells recruitment and cardiomyocyte cell-cycle reentry. Our results also provide evidence that local SCF gene transfer induces cardiac regeneration by enhancing survival and left ventricle function after MI, and by decreasing infarct size, hypertrophy, fibrosis, and apoptosis of existing cardiac cells, in the ischemic heart. These findings introduce a novel therapeutic approach for heart failure, through a combination of gene transfer and stem cell–based therapy.
Acknowledgments
This paper is dedicated to Mikel Yaniz-Galende who taught us to be persistent and patient in pursuing our goals and who also inspired this study.
Sources of Funding
This work was supported by the National Heart, Lung, and Blood Institute as a Program of Excellence in Nanotechnology (PEN) Award, Contract #HHSN268201000045C; by Leducq Foundation through the network (Dr Hajjar) and by grants from the US National Institutes of Health: r01 HL093183, HL088434, HL071753, HL080498, HL083156, and P20HL100396 (Dr Hajjar).
Non-standard Abbreviations and Acronyms
- BM
bone marrow
- CSCs
cardiac stem cells
- EF
ejection fraction
- LAD
left anterior descending coronary artery
- MI
myocardial infarction
- P-H3
phosphohistone H3
- SCF
stem cell factor
Footnotes
Disclosures
None.
References
- 1.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisén J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lutz M, Rosenberg M, Kiessling F, Eckstein V, Heger T, Krebs J, Ho AD, Katus HA, Frey N. Local injection of stem cell factor (SCF) improves myocardial homing of systemically delivered c-kit + bone marrow-derived stem cells. Cardiovasc Res. 2008;77:143–150. doi: 10.1093/cvr/cvm027. [DOI] [PubMed] [Google Scholar]
- 3.Zsebo KM, Williams DA, Geissler EN, Broudy VC, Martin FH, Atkins HL, Hsu RY, Birkett NC, Okino KH, Murdock DC. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990;63:213–224. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]
- 4.Ayach BB, Yoshimitsu M, Dawood F, Sun M, Arab S, Chen M, Higuchi K, Siatskas C, Lee P, Lim H, Zhang J, Cukerman E, Stanford WL, Medin JA, Liu PP. Stem cell factor receptor induces progenitor and natural killer cell-mediated cardiac survival and repair after myocardial infarction. Proc Natl Acad Sci USA. 2006;103:2304–2309. doi: 10.1073/pnas.0510997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith MA, Pallister CJ, Smith JG. Stem cell factor: biology and relevance to clinical practice. Acta Haematol. 2001;105:143–150. doi: 10.1159/000046556. [DOI] [PubMed] [Google Scholar]
- 6.Keshet E, Itin A, Fischman K, Nir U. The testis-specific transcript (ferT) of the tyrosine kinase FER is expressed during spermatogenesis in a stage-specific manner. Mol Cell Biol. 1990;10:5021–5025. doi: 10.1128/mcb.10.9.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rönnstrand L. Signal transduction via the stem cell factor receptor/c-Kit. Cell Mol Life Sci. 2004;61:2535–2548. doi: 10.1007/s00018-004-4189-6. [DOI] [PubMed] [Google Scholar]
- 8.Palpant NJ, Yasuda S, MacDougald O, Metzger JM. Non-canonical Wnt signaling enhances differentiation of Sca1+/c-kit+ adipose-derived murine stromal vascular cells into spontaneously beating cardiac myocytes. J Mol Cell Cardiol. 2007;43:362–370. doi: 10.1016/j.yjmcc.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuang D, Zhao X, Xiao G, Ni J, Feng Y, Wu R, Wang G. Stem cell factor/c-kit signaling mediated cardiac stem cell migration via activation of p38 MAPK. Basic Res Cardiol. 2008;103:265–273. doi: 10.1007/s00395-007-0690-z. [DOI] [PubMed] [Google Scholar]
- 10.Frangogiannis NG, Perrard JL, Mendoza LH, Burns AR, Lindsey ML, Ballantyne CM, Michael LH, Smith CW, Entman ML. Stem cell factor induction is associated with mast cell accumulation after canine myocardial ischemia and reperfusion. Circulation. 1998;98:687–698. doi: 10.1161/01.cir.98.7.687. [DOI] [PubMed] [Google Scholar]
- 11.Fazel S, Chen L, Weisel RD, Angoulvant D, Seneviratne C, Fazel A, Cheung P, Lam J, Fedak PW, Yau TM, Li RK. Cell transplantation preserves cardiac function after infarction by infarct stabilization: augmentation by stem cell factor. J Thorac Cardiovasc Surg. 2005;130:1310. doi: 10.1016/j.jtcvs.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Xiang FL, Lu X, Hammoud L, Zhu P, Chidiac P, Robbins J, Feng Q. Cardiomyocyte-specific overexpression of human stem cell factor improves cardiac function and survival after myocardial infarction in mice. Circulation. 2009;120:1065–74. doi: 10.1161/CIRCULATIONAHA.108.839068. 9 p following 1074. [DOI] [PubMed] [Google Scholar]
- 13.Jahanyar J, Youker KA, Torre-Amione G, Koerner MM, Bruckner B, Noon GP, Loebe M. Increased expression of stem cell factor and its receptor after left ventricular assist device support: a potential novel target for therapeutic interventions in heart failure. J Heart Lung Transplant. 2008;27:701–709. doi: 10.1016/j.healun.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Bolli R, Chugh AR, D’Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marbán L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marbán E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohtsuka M, Takano H, Zou Y, Toko H, Akazawa H, Qin Y, Suzuki M, Hasegawa H, Nakaya H, Komuro I. Cytokine therapy prevents left ventricular remodeling and dysfunction after myocardial infarction through neovascularization. FASEB J. 2004;18:851–853. doi: 10.1096/fj.03-0637fje. [DOI] [PubMed] [Google Scholar]
- 17.Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117:568–575. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Creemers EE, Pinto YM. Molecular mechanisms that control interstitial fibrosis in the pressure-overloaded heart. Cardiovasc Res. 2011;89:265–272. doi: 10.1093/cvr/cvq308. [DOI] [PubMed] [Google Scholar]
- 19.van den Borne SW, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol. 2010;7:30–37. doi: 10.1038/nrcardio.2009.199. [DOI] [PubMed] [Google Scholar]
- 20.Ikuta K, Weissman IL. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc Natl Acad Sci USA. 1992;89:1502–1506. doi: 10.1073/pnas.89.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Rev. 2005;85:1373–1416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 22.Kajstura J, Rota M, Hall SR, et al. Evidence for human lung stem cells. N Engl J Med. 2011;364:1795–1806. doi: 10.1056/NEJMoa1101324. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Miyazawa K, Williams DA, Gotoh A, Nishimaki J, Broxmeyer HE, Toyama K. Membrane-bound Steel factor induces more persistent tyrosine kinase activation and longer life span of c-kit gene-encoded protein than its soluble form. Blood. 1995;85:641–649. [PubMed] [Google Scholar]
- 24.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 25.Tsai M, Shih LS, Newlands GF, Takeishi T, Langley KE, Zsebo KM, Miller HR, Geissler EN, Galli SJ. The rat c-kit ligand, stem cell factor, induces the development of connective tissue-type and mucosal mast cells in vivo. Analysis by anatomical distribution, histochemistry, and protease phenotype. J Exp Med. 1991;174:125–131. doi: 10.1084/jem.174.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wershil BK, Tsai M, Geissler EN, Zsebo KM, Galli SJ. The rat c-kit ligand, stem cell factor, induces c-kit receptor-dependent mouse mast cell activation in vivo. Evidence that signaling through the c-kit receptor can induce expression of cellular function. J Exp Med. 1992;175:245–255. doi: 10.1084/jem.175.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coleman JW, Holliday MR, Kimber I, Zsebo KM, Galli SJ. Regulation of mouse peritoneal mast cell secretory function by stem cell factor, IL-3 or IL-4. J Immunol. 1993;150:556–562. [PubMed] [Google Scholar]
- 28.Chaudhary PM, Roninson IB. Expression and activity of P-glycoprotein, a multidrug efflux pump, in human hematopoietic stem cells. Cell. 1991;66:85–94. doi: 10.1016/0092-8674(91)90141-k. [DOI] [PubMed] [Google Scholar]
- 29.Kubo H, Jaleel N, Kumarapeli A, Berretta RM, Bratinov G, Shan X, Wang H, Houser SR, Margulies KB. Increased cardiac myocyte progenitors in failing human hearts. Circulation. 2008;118:649–657. doi: 10.1161/CIRCULATIONAHA.107.761031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angert D, Berretta RM, Kubo H, Zhang H, Chen X, Wang W, Ogorek B, Barbe M, Houser SR. Repair of the injured adult heart involves new myocytes potentially derived from resident cardiac stem cells. Circ Res. 2011;108:1226–1237. doi: 10.1161/CIRCRESAHA.110.239046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 32.Deb A, Wang S, Skelding KA, Miller D, Simper D, Caplice NM. Bone marrow-derived cardiomyocytes are present in adult human heart: A study of gender-mismatched bone marrow transplantation patients. Circulation. 2003;107:1247–1249. doi: 10.1161/01.cir.0000061910.39145.f0. [DOI] [PubMed] [Google Scholar]
- 33.Scherrer-Crosbie M, Ullrich R, Bloch KD, Nakajima H, Nasseri B, Aretz HT, Lindsey ML, Vançon AC, Huang PL, Lee RT, Zapol WM, Picard MH. Endothelial nitric oxide synthase limits left ventricular remodeling after myocardial infarction in mice. Circulation. 2001;104:1286–1291. doi: 10.1161/hc3601.094298. [DOI] [PubMed] [Google Scholar]
- 34.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 35.Qyang Y, Martin-Puig S, Chiravuri M, et al. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway. Cell Stem Cell. 2007;1:165–179. doi: 10.1016/j.stem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 36.Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 37.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 38.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 39.Singec I, Jandial R, Crain A, Nikkhah G, Snyder EY. The leading edge of stem cell therapeutics. Annu Rev Med. 2007;58:313–328. doi: 10.1146/annurev.med.58.070605.115252. [DOI] [PubMed] [Google Scholar]
- 40.Bu L, Jiang X, Martin-Puig S, Caron L, Zhu S, Shao Y, Roberts DJ, Huang PL, Domian IJ, Chien KR. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature. 2009;460:113–117. doi: 10.1038/nature08191. [DOI] [PubMed] [Google Scholar]
- 41.Kuhlmann MT, Kirchhof P, Klocke R, Hasib L, Stypmann J, Fabritz L, Stelljes M, Tian W, Zwiener M, Mueller M, Kienast J, Breithardt G, Nikol S. G-CSF/SCF reduces inducible arrhythmias in the infarcted heart potentially via increased connexin43 expression and arteriogenesis. J Exp Med. 2006;203:87–97. doi: 10.1084/jem.20051151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin-Puig S, Wang Z, Chien KR. Lives of a heart cell: tracing the origins of cardiac progenitors. Cell Stem Cell. 2008;2:320–331. doi: 10.1016/j.stem.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453:322–329. doi: 10.1038/nature07040. [DOI] [PubMed] [Google Scholar]
- 44.Pouly J, Bruneval P, Mandet C, Proksch S, Peyrard S, Amrein C, Bousseaux V, Guillemain R, Deloche A, Fabiani JN, Menasché P. Cardiac stem cells in the real world. J Thorac Cardiovasc Surg. 2008;135:673–678. doi: 10.1016/j.jtcvs.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 45.Anversa P, Kajstura J, Leri A, Bolli R. Life and death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation. 2006;113:1451–1463. doi: 10.1161/CIRCULATIONAHA.105.595181. [DOI] [PubMed] [Google Scholar]
- 46.Mouquet F, Pfister O, Jain M, Oikonomopoulos A, Ngoy S, Summer R, Fine A, Liao R. Restoration of cardiac progenitor cells after myocardial infarction by self-proliferation and selective homing of bone marrow-derived stem cells. Circ Res. 2005;97:1090–1092. doi: 10.1161/01.RES.0000194330.66545.f5. [DOI] [PubMed] [Google Scholar]
- 47.Kajstura J, Gurusamy N, Ogórek B, et al. Myocyte turnover in the aging human heart. Circ Res. 2010;107:1374–1386. doi: 10.1161/CIRCRESAHA.110.231498. [DOI] [PubMed] [Google Scholar]
- 48.Rota M, Kajstura J, Hosoda T, et al. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci USA. 2007;104:17783–17788. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci USA. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kühn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13:962–969. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- 51.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 52.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 53.Cohen ED, Tian Y, Morrisey EE. Wnt signaling: an essential regulator of cardiovascular differentiation, morphogenesis and progenitor self-renewal. Development. 2008;135:789–798. doi: 10.1242/dev.016865. [DOI] [PubMed] [Google Scholar]
- 54.Oka T, Xu J, Molkentin JD. Re-employment of developmental transcription factors in adult heart disease. Semin Cell Dev Biol. 2007;18:117–131. doi: 10.1016/j.semcdb.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.