Abstract
Intrapericardial drug delivery is a promising procedure, with the ability to localize therapeutics with the heart. Gelfoam particles are nontoxic, inexpensive, nonimmunogenic and biodegradable compounds that can be used to deliver therapeutic agents. We developed a new percutaneous approach method for intrapericardial injection, puncturing the pericardial sac safely under fluoroscopy and intravascular ultrasound (IVUS) guidance. In a porcine model of myocardial infarction (MI), we deployed gelfoam particles carrying either (a) autologous mesenchymal stem cells (MSCs) or (b) an adenovirus encoding enhanced green fluorescent protein (eGFP) 48 h post-MI. The presence of MSCs and viral infection at the infarct zone was confirmed by immunoflourescence and PCR. Puncture was performed successfully in 16 animals. Using IVUS, we successfully determined the size of the pericardial space before the puncture, and safely accessed that space in setting of pericardial effusion and also adhesions induced by the MI. Intrapericardial injection of gelfoam was safe and reliable. Presence of the MSCs and eGFP expression from adenovirus in the myocardium were confirmed after delivery. Our novel percutaneous approach to deliver (stem-) cells or adenovirus was safe and efficient in this pre-clinical model. IVUS-guided delivery is a minimally invasive procedure that seems to be a promising new strategy to deliver therapeutic agents locally to the heart.
Keywords: pericardium, adenovirus, mesenchymal stem cells, gelfoam
INTRODUCTION
One of the major challenges in the pharmacological treatment of heart diseases is to achieve delivery of suitable concentrations of therapeutic agents to the specific target site.1,2 Various approaches for local delivery to the heart include intramyocardial injections, epicardial deposition, and intracoronary or transvascular application.3,4 The efficacy of intramyocardial injection is limited by retention and survival rates of 2% or less.5 Epicardial deposition of cell-populated sheets is more effective, but is highly invasive.4 In the setting of vascular obstruction, such as myocardial infarction (MI), reduced local blood supply can significantly impair targeted agent delivery via the vasculature. Therefore, the development of a local, minimally invasive delivery system independent of the vascular status may offer an attractive alternative to existing delivery strategies.
The pericardium encloses the whole heart, creating a small and relatively isolated fluid-filled compartment; in sheep the pericardial fluid volume is about 8 ml, with an estimated turnover time ranging from 5.4 to 7.2 h.6 This enclosed space offers an ideal candidate for local delivery of a number of agents to the heart. For example, nitric oxide donors were successfully applied to this area and demonstrated a systemic effect.7 Furthermore, adenoviral-mediated gene transfer applied to the pericardium induced sustained VEGF165 expression in the myocardium.8 In a study by Lazarous et al.9 pericardial administration of bFGF resulted in substantial cardiac bFGF delivery, as 19% of the substance was present in the heart compared with 0.5% using traditional intravenous administration. In a rabbit model, the presence of bFGF in the intrapericarial sac enhanced new epicardial small-vessel growth.10 Even a single intrapericardial injection of bFGF in a porcine model of MI resulted in functionally significant myocardial angiogenesis.11 Given that these agents have been successfully applied to the pericardial sac with single injections, prolonged delivery of these agents might prove to be even more beneficial.
Gelfoam is an Food and Drug Administration approved absorbable sponge material that is clinically used in surgical and interventional procedures as a hemostatic substrate; it is inexpensive and nonallergenic.12 It is prepared from purified pork skin gelatin granules by a thermal treatment method. Gelatin sponge particles have been used for almost 40 years in interventional radiology as a temporary occlusive agent.13 When in contact with water the polymer swells but it does not dissolve,14 whereas when in contact with soft tissues in vivo it is absorbed in 4 to 6 weeks.12 When combined with PLGA microspheres, implanted gelfoam sponges carrying paclitaxel enabled slow and continuous release because of the biodegradable properties of the sponge, and released microspheres were successfully detected in the lymphatic system of the animal.15 In vivo gelatin sponges with beta-tri-calcium phosphate were even shown to retain bone morphogenetic protein-2 over a time period of 28 days.16 These release properties make gelfoam a suitable candidate for drug delivery in the pericardial space.
RESULTS
In preliminary experiments for this study, commercially available gelfoam patches were attached directly to the epicardial surface during a small, lateral thoracotomy. This approach led to severe adhesions to the lung and other structures of the chest cavity (Figure 1a). Therefore, we turned our attention to manufacturing gelfoam particles and establishing a safe route of administration.
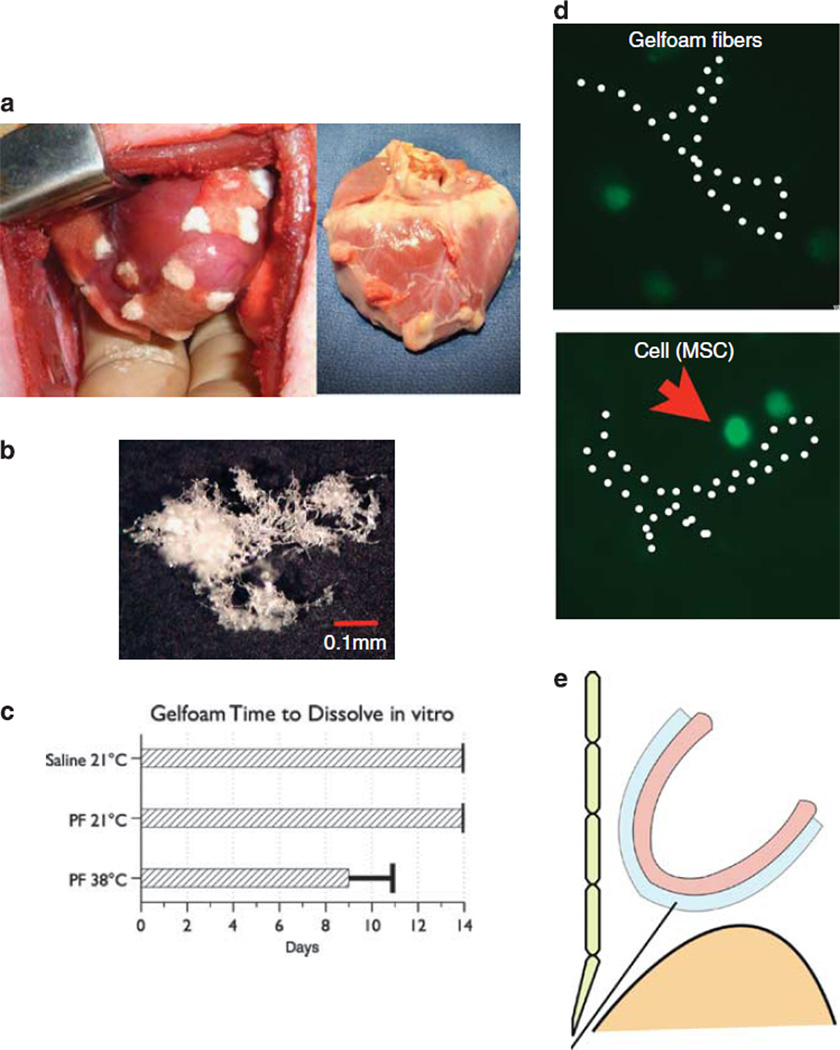
Figure 1.
In preliminary exploratory studies, gelfoam patches applied directly to the epicardial surface of the swine heart lead to severe adhesions (a), left panel depicts gelfoam patches on the epicardial surface at the time of placement; right panel shows adhesions on the epicardial surface of the heart harvested 1 week after the procedure. Sponges of commercially available gelfoam can be rasped into small particles that appear cotton-like under the microscope (b). In vitro gelfoam particles can dissolve in pericardial fluid, but not in saline (c). MSCs within the three-dimensional (3D) scaffold of gelfoam fibers in the cell culture dish (d). The pericardial sac is approached by substernal puncture, safely bypassing the liver under flouroscopic guidance (e).
We created gelfoam particles by rasping a block of gelfoam with a commercially available bone rasp. Particles were collected and gas sterilized before injection. The particles measured between 1 and 4 mm in size. The cotton-like structure of the particles became visible under light microscopy (Figure 1b). When in contact with water the gelfoam transformed into a thick, slurry paste that was pumped rapidly several times between two connected 10 ml syringes.
To determine whether the gelfoam would dissolve in pericardial fluid in vitro, we harvested fresh samples of pericardial fluid and added gelfoam particles before placing the samples at 38 °C in a shaker in order to simulate the movement of the heart. Control samples were stored at room temperature and in saline solution (Figure 1c). The gelfoam dissolved completely after about 8.4 days in the samples that were kept in the incubator. After 14 days of observation, the control samples did not noticeably dissolve.
Before starting the in vivo experiments, we tested the survival of mesenchymal stem cells (MSCs) labeled with enhanced green fluorescent protein (eGFP) in the gelfoam matrix using the methods described above but maintaining the MSC/gelfoam mix in a cell culture dish in the incubator and changing the culture media biweekly. Under these conditions, cells were visible within the three-dimensional structure of the gelfoam for up to 14 days (Figure 1d).
For our large animal in vivo studies, we developed a fluoroscopic-guided approach to the pericardial sac (Figure 1e). The procedure allows us to precisely position the catheter over the anterior wall of the left ventricle before injection of the gelfoam (Figure 2a).
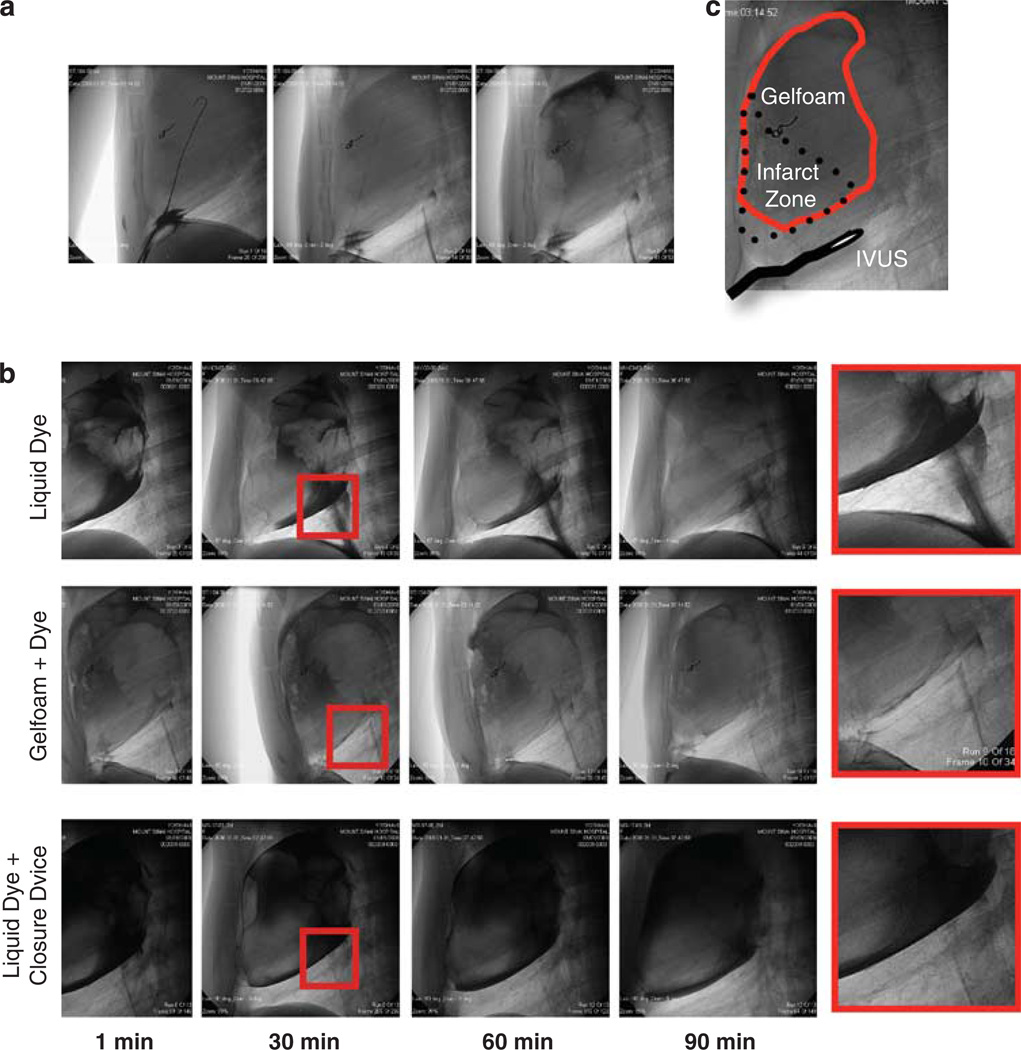
Figure 2.
Under fluoroscopic guidance, a wire, followed by a catheter is inserted into the pericardium and positioning is confirmed by contrast dye bolus injection (a). Fluoroscopic images of liquid dye, gelfoam mixed with dye and liquid dye after closure of the puncture site to assess possible leakage (b). Position of the injected gelfoam as well as the IVUS probe in relation to the infarct zone (c).
We confirmed the presence of the gelfoam in the pericardium by mixing the gelfoam with 50% saline and 50% contrast dye before injection. Fluoroscopic pictures were acquired every 10min for up to 90 min to assess the amount of leakage after removal of the catheter (Figure 2b). Leakage occurred to a large extent when only liquid contrast dye was injected. This effect is presumably enhanced by gravity in combination with the higher density of the liquid dye. In contrast, almost no gelfoam was visible in the chest cavity, even when the puncture site was not closed. In order to further improve our approach, we performed these procedures using a Starclose SE vascular closure device (Abbott, Abbott Park, IL, USA) to seal the pericardium. This strategy resulted in elimination of any visible leakage, even after injection of pure liquid contrast dye.
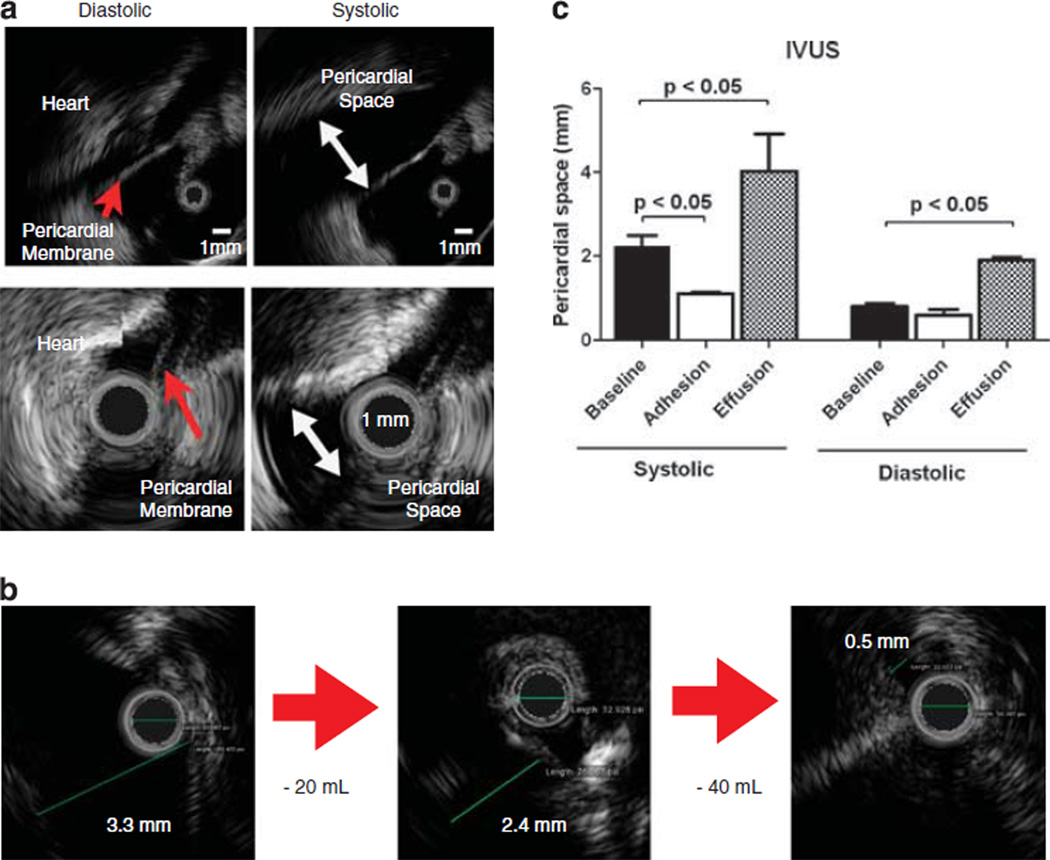
The distribution of the gelfoam in relation to the infarct zone is depicted in Figure 2c. To increase safety of the percutaneous puncture, we further established an intravascular ultrasound (IVUS)-guided approach. The IVUS probe is advanced after the subxiphoid access and positioned next to the pericardial sac at the proposed puncture site (Figure 2c). The ultrasound probe at the tip of the catheter produces a real-time picture to inspect the size of the space between the heart and the pericardial membrane in diastole and systole (Figure 3a). The distance between the heart and the pericardial membrane can become altered from post-infarction effusion (upper panel) or adhesion (lower panel). With this instrument, we were able to visualize a decrease in pericardial space because of severe pericardial effusion (Figure 3b) with removal of two times 20 ml of fluid. Statistical analysis showed significant differences in the setting of severe adhesion and pericardial effusion vs the pericardial space of a naive animal in systole (baseline: 2.1 ± 0.2 mm vs adhesion: 0.9 ± 0.08 mm vs effusion: 3.98 ± 1.1 mm; P<0.05) (Figure 3c).
Figure 3.
With the ultrasound probe at the tip of the catheter, the size of the space between the heart and the pericardial membrane can be assessed precisely in diastole and systole. Lower panels show a smaller range of variation in the size of the pericardial space in a pig with severe pericardial adhesions compared with a control pig in the upper panels (a). Note different scales for upper and lower panels. The real-time pictures from the IVUS system show an immediate decrease in the pericardial space by removal of pericardial fluid in the presence of effusion (b). Pericardial space was measured in naive pigs (baseline) and in animals after MI showing either pericardial effusion or adhesions (c); the latter were confirmed during thoracotomy (n=4).
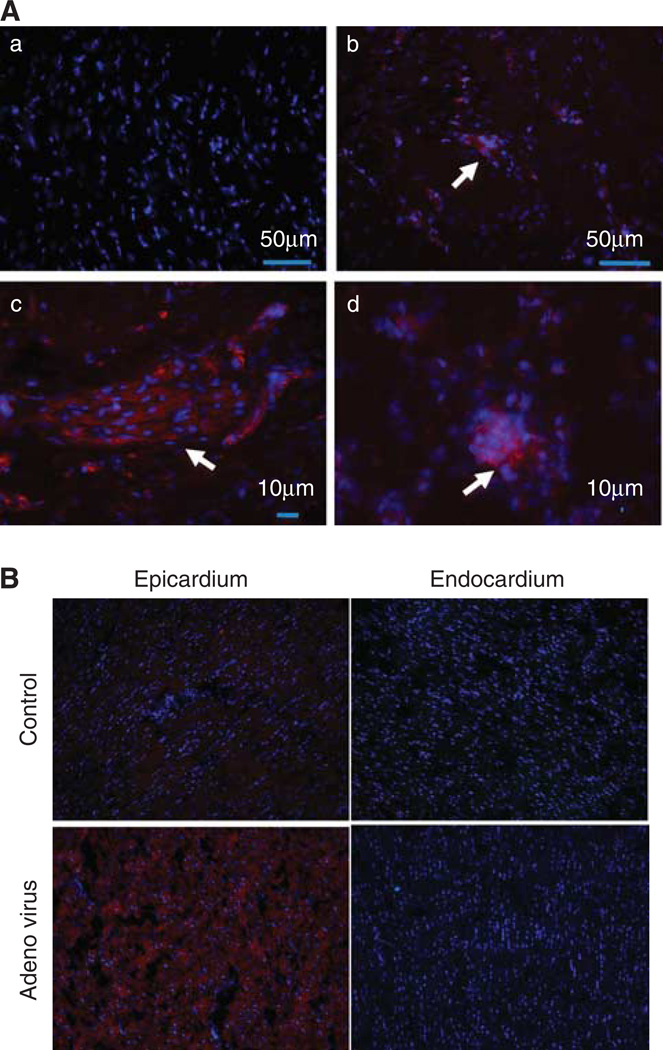
We performed PCR and immunocytochemistry on the myocardial tissue samples in order to assess the efficacy of our delivery method. Fluorescent microscopy images obtained from tissue harvested one week after MSC delivery (n=2) demonstrated the successful engraftment of eGFP-labeled MSCs in the peri-infarct area and scar (Figure 4A). The presence of MSCs in the peri-infarct area was demonstrated by the presence of clusters of cells stained positive for eGFP.
Figure 4.
Fluorescent microscopy images demonstrate the engraftment of eGFP-labeled MSCs in the peri-infarct area and scar. (A) Immunostaining performed with anti-GFP rabbit immunoglobulin G (IgG), and secondary antibody goat anti rabbit IgG (Texas Red) and nuclei counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). (a) Representative image of control sample, negative for eGFP, demonstrate only counterstained blue nuclei. (b, c) The presence of MSCs in the peri-infarct area is demonstrated by the presence of clusters of cells stained positive for eGFP using a secondary antibody conjugated to a red fluoropore (arrow). (d) MSCs (GFP+) identified in scar tissue (arrow). (B) Fluorescent microscopy of eGFP transfection.
In the animals that received adenoviral injection, fluorescent microscopy demonstrated the presence of eGFP-positive cells in the epicardial layer of the anterior, peri-infarct area, while no expression was found in other layers of the myocardium (Figure 4B).
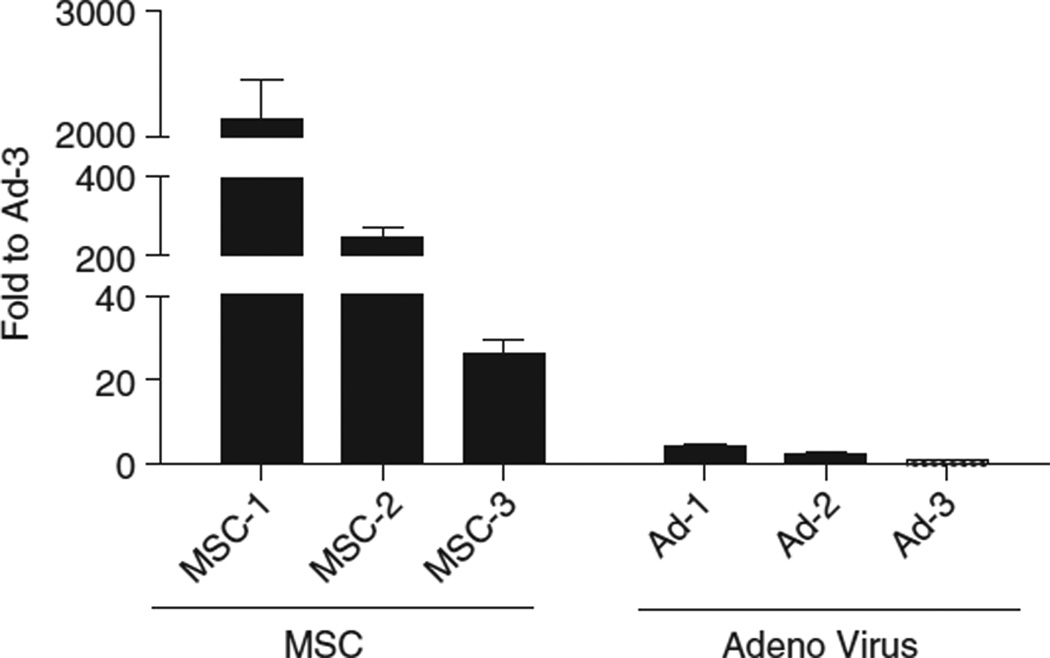
To assess the gene transfer efficiency of a new percutaneous approach method for intrapericardial injection, epicardial layer tissue samples were used to determine eGFP expression by quantitative realtime PCR. As shown in Figure 5, both groups, the MSC and the adenovirus-injected, express eGFP. The MSC group expresses high levels of eGFP compared with the adenoviral group. Overall, the new percutaneous approach for myocardial delivery is proficient in achieving gene expression both in the mRNA and the protein level in the MSC group and in the mRNA level in the adenoviral group.
Figure 5.
Expression levels of eGFP in tissues derived from myocardium injected with gelfoam particles carrying either autologous MSCs or and adenovirus carrying the eGFP transgene. Expression levels by quantitative reverse transcriptase (qRT)-PCR show that the MSC group expresses high levels of eGFP, whereas the adenovirus group shows significant but lower expression. Fold changes were calculated using the lowest expressing sample (Ad3-RBX26) as a reference.
DISCUSSION
We sought to develop a minimally invasive strategy to deliver gelfoam as a releasing platform for local biological agents to the heart. As a carrier for therapeutic agents, gelfoam has demonstrated suitable release kinetics in vivo. Therapeutic agents targeting the heart may have unwanted systemic side effects, which could limit their effective-ness. For this reason, developing a method that can localize such biological agents and confine them in an enclosed area is highly desirable. The concept of gelfoam sponges as a carrier for a therapeutic agent targeting an infarcted area of the myocardium was shown previously in a small animal model.17 We initially adapted this concept from the small animal model to the swine large animal model by attaching gelfoam sponges to the peri-infarcted area with fibrin glue. Performing a large lateral thoracotomy, the heart was stripped of from the pericardium and the extent of the infracted area visualized as it was demarcated 48 h after the infarct. The obvious advantage of this method was the exact placement of the patches on the target area. However, accessing the scar and/or infarct area required that we manipulate and lift the heart, which resulted in severe arrhythmia and hypotension. Three out of five pigs died during the procedure from adverse events.
To minimize invasiveness of the procedure and avoid damaging the pericardial sac, we developed a gelfoam particle approach. The cotton-like appearance of the particles explains why they cling together easily when dry, thereby forming larger aggregates. These findings are consistent with the literature12 that gelfoam swells but does not dissolve in fluids. The observed degradation of these samples in pericardial fluid at body temperature is presumably caused by enzymatic reactions.
Post-injection and 1-month follow-up cardiac echocardiography did not reveal any signs of pericardial effusion. Owing to the minimally invasive character of the procedure, adhesions to other tissues such as the lungs were minimal. The pigs were killed 1 month after injection and the pericardium could be easily dissected from the heart, with minor adhesions present at the apex area of the infarcted left ventricle. Overall, the mortality of the injection in the high-risk period immediately following MI was remarkably low; none of the 16 animals that underwent the puncture died during the procedure.
Using the IVUS system, we were able to quantify the size of the pericardial space. Guidance by IVUS helps ensure the safety of the pericardial puncture. Using this real-time imaging technology, placement of the puncture can be chosen to avoid rare but possible complications in the case of pre-existing adhesions or other reasons that may prevent a safe and effective puncture.
Application of MSCs has been shown in multiple clinical studies to improve cardiac function after MI,18,19 although the mechanism are still not fully understood.20,21 Transplantation of autologous MSCs, as used in our study, has the advantage of avoiding the immune response or even rejection from the host.22 After injection of MSCs mixed with gelfoam into the pericardial space, we were able to show evidence of engraftment of these cells in the peri-infarcted area. To our knowledge, this is the first demonstration of pericardial cell transfer using a gelfoam scaffold for stem cells. Recently, Kondoh et al. demonstrated the successful transfer of myoblasts in the setting of dilated cardiomyopathy using a cell sheet applied to the surface of the heart.4,23–25 These approaches underscore the fact that cardiac function can be preserved or improved utilizing cell transfer techniques from the cardiac surface or pericardial sac.
Our adenoviral infection led to eGFP expression in the epicardial layer of the anterior and inferior segments of the left ventricle. The adenovirus-injected group shows relatively low expression levels, which nonetheless is in agreement with the literature that adenoviral vectors can only sustain transient expression. The MSC group shows high levels of expression maintained even 1 month after the delivery of the gelfoam particles, which makes the proposed method favorable for cell therapy applications. Effective, but superficial infection of cardiomyocytes with a pericardial approach has been shown before.26 Nevertheless, we are reporting here the first percutaneous pericardial access in the large animal in combination with viral infection.
A limitation of this study, and the pericardial approach in general, is the fact that a systemic exposure cannot be completely prevented because of lymphatic drainage of the pericardial fluid.6 Therefore, re-circulation of the labeled MSCs through the vascular system and consecutive migration into the peri-infarcted area might be possible. However, the fact that we used a total occlusion model, with a coil blocking the coronary vessel, makes this rather unlikely compared with the direct migration of cells from gelfoam in contact with the epicardial surface.
CONCLUSION
In this study, we demonstrate for the first time the feasibility of injecting liquified gelfoam particles associated with biological agents into the pericardial space as a potential therapeutic strategy for targeted delivery to the myocardium. Using IVUS guidance for additional safety, this method proved to be successful in delivering MSCs and adenovirus to the infarcted heart. We believe the described percutaneous approach holds great potential for preclinical research where various agents can be delivered to the heart, especially in the field of cardiac cellular therapy.
MATERIALS AND METHODS
Experimental study
The study was performed in accordance with the Guidelines for the Care and Use of Laboratory Animals and was approved by the Subcommittee on Research Animal Care at Mount Sinai School of Medicine. Yorkshire swine were anesthetized using Atropine 0.04mg kg−1 and Telazol (tiletamine/zolazepam) 6.0 mg kg−1. Animals were intubated and ventilated with 100% oxygen. General anesthesia was maintained with Propofol 5 mg kg−1 h−1 throughout the interventional procedures. Surgical procedures such as the bone marrow puncture and the thoracotomy were performed under anesthesia with isoflurane (1–2%) mixed with 98% oxygen. For MI generation, we introduced an 8F sheath into the femoral artery and cannulated the left anterior descending artery with an 8F hockey stick guiding catheter (Cordis Infiniti, Johnson and Johnson, New Brunswick, NJ, USA). After injecting 100 µg nitroglycerin and obtaining a baseline coronary angiogram, we placed a platinum embolic coil (0.035 in, 40 mm length, 5×3-mm diameter, Cook Medical Inc., Bloomington, IN, USA) using a 4F amplatz right catheter (Cordis Infiniti, Johnson and Johnson) into the left anterior descending after the takeoff of the first diagonal branch, thus occluding 2/3 of the left anterior descending tributary, determined by coronary angiography.
Gelfoam particles
We adapted a previously reported controlled release system,17 consisting of solid gelfoam (Pfizer, New York, NY, USA), for minimally invasive delivery by preparing a gelfoam slurry. As a first step, we tested the ability of gelfoam to dissolve in saline and in pericardial fluid, obtained immediately before incubating it with the gelfoam. The 1 ml plastic tubes (BD, Franklin Lakes, NJ, USA) were stored either at room temperature or in an incubator (37°C and 5% CO2).
Pericardial injection of gelfoam particles
In order to make this approach suitable for preclinical testing, we developed a percutaneous method of accessing the pericardial space and injecting the liquified gelfoam. Injections were performed 48 h after MI. Under fluoroscopic guidance, we advanced a 18G puncture needle (Cook Medical) under the sternum toward the pericardium. After confirming successful puncture with a small bolus injection of contrast dye, we placed a wire in the pericardial space. After puncture, clear pericardial fluid could be aspirated. Only in a few cases the fluid contained small amounts of blood, which had no implications for the safety of the procedure. We then placed a 8F vascular sheath over the wire and thereafter inserted a 5 French amplatz right catheter and positioned it over the anterior wall of the left ventricle.
Intravascular ultrasound
Measurement of the distance between the pericardial sac and the heart was performed with a Galaxy IVUS catheter (Boston Scientific, Ample Groove, MN, USA). After subxyphoid puncture, the ultrasound probe was advanced over the 8F sheath toward the pericardial sac. The procedure was performed under fluoroscopic guidance and during breath-hold.
Porcine bone marrow-derived MSC isolation and labeling
Four weeks before the scheduled gelfoam/stem cell pericardial injection procedure, bone marrow was harvested from the posterior iliac crest of each pig. All surgical procedures were performed with the animals under general anesthesia and maintained on a ventilator. Autologous bone marrow-derived MSCs were isolated and expanded in culture following established protocols.27–29 The bone marrow aspirate (12 ml) was processed by density gradient centrifugation with Ficoll Paque-PLUS (GE Healthcare Biosciences, Piscataway, NJ, USA); the buffy coat containing the mononuclear cells was collected, and after wash with Hanks balanced salt solution, and centrifugation at 500 g for 10min, the cell pellet was resuspended in Dulbecco’s modified Eagle’s medium low glucose (1 gl−1) (Sigma, St Louis, MO, USA) supplemented with 10% fetal bovine serum. Cells were plated on tissue culture treated dishes and maintained at 37°C and 5% carbon dioxide. The media was changed every 72 h, cells were trypsinized when they reached 80–90% confluency and re-seeded in T-175 flasks with vented cap. When cells reached passage 3, they were labeled to express GFP via infection with adenovirus carrying the reporter gene eGFP; 72 h after infection, eGFP expression was confirmed under fluorescent microscopy.
Gelfoam/MSC constructs
Gelfoam sponge was used as a scaffold for seeding MSC by an adaptation of previously described protocols.27,30,31 The gelfoam particles (1–4 mm) were placed in a 50 ml polystyrene conical tube with Dulbecco’s modified Eagle’s medium and hydrated for 2 h on a VariMix Shaker (Thermo Fisher Scientific, Waltham, MA, USA), at room temperature. The culture media was decanted and the gelfoam was dabbed dry on a gauze, and transferred to a 50 ml tube containing a suspension of 6×107 MSC–eGFP-labeled cells in 3 ml of Dulbecco’s modified Eagle’s medium, and incubated for 2h at 37°C and 5% CO2. The gelfoam/MSC construct was transferred to a 10ml syringe by removing and replacing the plunger, and was immediately used for in vivo implantation. For the preliminary in vitro testing the construct was transferred onto a cell culture dish and incubated at 37°C.
Viral vector
Recombinant adenoviral vectors were produced using standard techniques.32 Briefly, the eGFP gene was subcloned from the pEGFP-C1 vector (Clontech, Mountain View, CA, USA) to the pShuttle-cytomegalovirus vector of the AdEasy system.33 The resulting construct, which carries the eGFP gene under the control of the cytomegalovirus promoter, was transformed to the BJ5182-AD-1 (Stratagene, Santa Clara, CA, USA), which are stably transfected with the pAdEasy-1 plasmid, which carries the adenoviral genes necessary for amplification in the HEK293 genes. These pShuttle-cytomegalovirus-eGFPC1 and pAdEasy-1 undergo homologous recombination to produce a plasmid construct containing the cytomegalovirus-eGFP-C1 cassette and the adenoviral genes. The resulting construct was transformed to HEK293 cells and the resulting adenoviral virus was used for four rounds of amplification. The large-scale adenoviral product was purified using CsCl gradient ultracentrifugation and was quantified by plaque assay.32 The concentration was determined as 1013 particle-forming units ml−1.
Intrapericardial delivery and cell tracking
To test the feasibility of using the gelfoam intrapericardial injection as a method of delivery of stem cells, gelfoam/MSC constructs were created using MSC−GFP-labeled cells seeded on gelfoam particles and delivered through a percutaneous approach into the pericardial sac, using a 6Fr catheter. This procedure was performed 48 h after MI creation in two pigs that underwent bone marrow harvest 4 weeks before the planned MSC implantation procedure. One week after gelfoam/MSC implantation the animals were killed. To track the presence of MSC in the myocardium, the heart tissue was harvested and processed for immunohistochemistry and molecular analysis for identification of eGFP-positive cells.
Real-time quantitative reverse transcriptase-PCR
Relative gene expression was determined using two-step quantitative real-time PCR. Total RNA was isolated with TRIzol reagent (Invitrogen) followed by a clean-up step as described in the RNeasy Isolation kit (Qiagen) with on-column DNase I treatment to eliminate contaminating genomic DNA with RNase-free DNase Set (Qiagen). About 1 µg total RNA from each sample was reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) according to the manufacturer’s protocol. Primers for Serca2a were designed with the Primer Express software (forward: 5′-CTGTCCATGTCACTCCACTTCC-3′ reverse: 5′-AGCGGTTACTCCAGTATTGCAG-3′). Quantitative PCR reactions were performed with Power SYBR Green Master Mix (Applied Biosystems) on an ABI Prism 7500 Real-Time PCR System. The PCR protocol consisted of one cycle at 95 °C (10 min) followed by 40 cycles of 95 °C (15 s) and 60°C (1 min). Fold changes in gene expression were determined using the ΔΔCt method with normalization to glyceraldehyde 3-phosphate dehydrogenase endogenous control.
Immunohistochemistry was performed on frozen tissue sections (10µm) using anti-GFP rabbit immunoglobulin G as primary antibody (A11122, Invitrogen) and Texas Red goat anti-rabbit immunoglobulin G (T2767 Invitrogen) as secondary antibody followed by mounting media containing 4′,6-diamidino-2-phenylindole. PCR was performed using the following primers: forward 5′-TGACCCTGAAGTTCATCTGCACCA-3′, reverse 5′-TCTTGTAGTTGCCGTCGTCCTTGA-3′.
Statistical analyses
Numeric data are presented as mean ± s.e.m. We tested statistical significance with Student’s t-test and analysis of variance using Prism (Graph Pad, La Jolla, CA, USA) software. The α-value was set at 0.05.
ACKNOWLEDGEMENTS
We thank James Lough and Catherine McMahon for their excellent technical assistance. This work was supported by grants from the National Institutes of Health: R01HL078731, R01HL080498, R01HL083156, R01HL093183, R01HL088434 and P20HL100396 (RJH) and R21HL095980 (KDC), from the Transatlantic Leducq Foundation (RJH) and the National Heart, Lung, and Blood Institute, National Institutes of Health, as a Program of Excellence in Nanotechnology (PEN) Award, Contract #HHSN268201000045C. DL was supported by the German Research Foundation (DFG).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Hwang CW, Wu D, Edelman ER. Physiological transport forces govern drug distribution for stent-based delivery. Circulation. 2001;104:600–605. doi: 10.1161/hc3101.092214. [DOI] [PubMed] [Google Scholar]
- 2.Spadaccio C, Chello M, Trombetta M, Rainer A, Toyoda Y, Genovese JA. Drug releasing systems in cardiovascular tissue engineering. J Cell Mol Med. 2009;13:422–439. doi: 10.1111/j.1582-4934.2008.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kornowski R, Fuchs S, Leon MB, Epstein SE. Delivery strategies to achieve therapeutic myocardial angiogenesis. Circulation. 2000;101:454–458. doi: 10.1161/01.cir.101.4.454. [DOI] [PubMed] [Google Scholar]
- 4.Hamdi H, Furuta A, Bellamy V, Bel A, Puymirat E, Peyrard S, et al. Cell delivery: intramyocardial injections or epicardial deposition? A head-to-head comparison. Ann Thorac Surg. 2009;87:1196–1203. doi: 10.1016/j.athoracsur.2008.12.074. [DOI] [PubMed] [Google Scholar]
- 5.Fukushima S, Varela-Carver A, Coppen SR, Yamahara K, Felkin LE, Lee J, et al. Direct intramyocardial but not intracoronary injection of bone marrow cells induces ventricular arrhythmias in a rat chronic ischemic heart failure model. Circulation. 2007;115:2254–2261. doi: 10.1161/CIRCULATIONAHA.106.662577. [DOI] [PubMed] [Google Scholar]
- 6.Boulanger B, Yuan Z, Flessner M, Hay J, Johnston M. Pericardial fluid absorption into lymphatic vessels in sheep. Microvasc Res. 1999;57:174–186. doi: 10.1006/mvre.1998.2127. [DOI] [PubMed] [Google Scholar]
- 7.Waxman S, Moreno R, Rowe KA, Verrier RL. Persistent primary coronary dilation induced by transatrial delivery of nitroglycerin into the pericardial space: a novel approach for local cardiac drug delivery. J Am Coll Cardiol. 1999;33:2073–2077. doi: 10.1016/s0735-1097(99)00131-x. [DOI] [PubMed] [Google Scholar]
- 8.Lazarous DF, Shou M, Stiber JA, Hodge E, Thirumurti V, Goncalves L, et al. Adenoviral-mediated gene transfer induces sustained pericardial VEGF expression in dogs: effect on myocardial angiogenesis. Cardiovasc Res. 1999;44:294–302. doi: 10.1016/s0008-6363(99)00203-5. [DOI] [PubMed] [Google Scholar]
- 9.Lazarous DF, Shou M, Stiber JA, Dadhania DM, Thirumurti V, Hodge E, et al. Pharmacodynamics of basic fibroblast growth factor: route of administration determines myocardial and systemic distribution. Cardiovasc Res. 1997;36:78–85. doi: 10.1016/s0008-6363(97)00142-9. [DOI] [PubMed] [Google Scholar]
- 10.Landau C, Jacobs AK, Haudenschild CC. Intrapericardial basic fibroblast growth factor induces myocardial angiogenesis in a rabbit model of chronic ischemia. Am Heart J. 1995;129:924–931. doi: 10.1016/0002-8703(95)90113-2. [DOI] [PubMed] [Google Scholar]
- 11.Laham RJ, Rezaee M, Post M, Novicki D, Sellke FW, Pearlman JD, et al. Intrapericardial delivery of fibroblast growth factor-2 induces neovascularization in a porcine model of chronic myocardial ischemia. J Pharmacol Exp Ther. 2000;292:795–802. [PubMed] [Google Scholar]
- 12.Hamalainen KM, Maatta E, Piirainen H, Marianne S, Vaisanen A, Ranta VP, et al. Roles of acid/base nature and molecular weight in drug release from matrices of gelfoam and monoisopropyl ester of poly(vinyl methyl ether-maleic anhydride) J Control Release. 1998;56:273–283. doi: 10.1016/s0168-3659(98)00094-7. [DOI] [PubMed] [Google Scholar]
- 13.Abada HT, Golzarian J. Gelatine sponge particles: handling characteristics for endovascular use. Tech Vasc Interv Radiol. 2007;10:257–260. doi: 10.1053/j.tvir.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Ofner CM, III, Schott H. Swelling studies of gelatin. I: gelatin without additives. J Pharm Sci. 1986;75:790–796. doi: 10.1002/jps.2600750814. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Meisner D, Kwong E, Wu XY, Johnston MR. A novel trans-lymphatic drug delivery system: implantable gelatin sponge impregnated with PLGA-paclitaxel microspheres. Biomaterials. 2007;28:3236–3244. doi: 10.1016/j.biomaterials.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi Y, Yamamoto M, Tabata Y. Enhanced osteoinduction by controlled release of bone morphogenetic protein-2 from biodegradable sponge composed of gelatin and beta-tricalcium phosphate. Biomaterials. 2005;26:4856–4865. doi: 10.1016/j.biomaterials.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Kühn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, et al. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13:962–969. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- 18.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 19.Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 20.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 21.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 22.Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond) 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kondoh H, Sawa Y, Miyagawa S, Sakakida-Kitagawa S, Memon IA, Kawaguchi N, et al. Longer preservation of cardiac performance by sheet-shaped myoblast implantation in dilated cardiomyopathic hamsters. Cardiovasc Res. 2006;69:466–475. doi: 10.1016/j.cardiores.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Derval N, Barandon L, Dufourcq P, Leroux L, Lamaziere JM, Daret D, et al. Epicardial deposition of endothelial progenitor and mesenchymal stem cells in a coated muscle patch after myocardial infarction in a murine model. Eur J Cardiothorac Surg. 2008;34:248–254. doi: 10.1016/j.ejcts.2008.03.058. [DOI] [PubMed] [Google Scholar]
- 25.Kutschka I, Chen IY, Kofidis T, Arai T, von Degenfeld G, Sheikh AY, et al. Collagen matrices enhance survival of transplanted cardiomyoblasts and contribute to functional improvement of ischemic rat hearts. Circulation. 2006;114(1 Suppl):I167–I173. doi: 10.1161/CIRCULATIONAHA.105.001297. [DOI] [PubMed] [Google Scholar]
- 26.Lamping KG, Rios CD, Chun JA, Ooboshi H, Davidson BL, Heistad DD. Intrapericardial administration of adenovirus for gene transfer. Am J Physiol. 1997;272(1 Part 2):H310–H317. doi: 10.1152/ajpheart.1997.272.1.H310. [DOI] [PubMed] [Google Scholar]
- 27.Quintavalla J, Uziel-Fusi S, Yin J, Boehnlein E, Pastor G, Blancuzzi V, et al. Fluorescently labeled mesenchymal stem cells (MSCs) maintain multilineage potential and can be detected following implantation into articular cartilage defects. Biomaterials. 2002;23:109–119. doi: 10.1016/s0142-9612(01)00086-2. [DOI] [PubMed] [Google Scholar]
- 28.Alhadlaq A, Mao JJ. Mesenchymal stem cells: isolation and therapeutics. Stem Cells Dev. 2004;13:436–448. doi: 10.1089/scd.2004.13.436. [DOI] [PubMed] [Google Scholar]
- 29.Makkar RR, Price MJ, Lill M, Frantzen M, Takizawa K, Kleisli T, et al. Intramyocardial injection of allogenic bone marrow-derived mesenchymal stem cells without immunosuppression preserves cardiac function in a porcine model of myocardial infarction. J Cardiovasc Pharmacol Ther. 2005;10:225–233. doi: 10.1177/107424840501000403. [DOI] [PubMed] [Google Scholar]
- 30.Babb JR, Ahn JI, Azar FM, Canale ST, Beaty JH. Transphyseal anterior cruciate ligament reconstruction using mesenchymal stem cells. Am J Sports Med. 2008;36:1164–1170. doi: 10.1177/0363546508314719. [DOI] [PubMed] [Google Scholar]
- 31.Rohanizadeh R, Swain MV, Mason RS. Gelatin sponges (gelfoam) as a scaffold for osteoblasts. J Mater Sci Mater Med. 2008;19:1173–1182. doi: 10.1007/s10856-007-3154-y. [DOI] [PubMed] [Google Scholar]
- 32.Luo J, Deng ZL, Luo X, Tang N, Song WX, Chen J, et al. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc. 2007;2:1236–1247. doi: 10.1038/nprot.2007.135. [DOI] [PubMed] [Google Scholar]
- 33.He TC, Chan TA, Vogelstein B, Kinzler KW. PPARdelta is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99:335–345. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]