Abstract
Cardiac myocyte function is dependent on the synchronized movements of Ca2+ into and out of the cell, as well as between the cytosol and sarcoplasmic reticulum. These movements determine cardiac rhythm and regulate excitation–contraction coupling. Ca2+ cycling is mediated by a number of critical Ca2+-handling proteins and transporters, such as L-type Ca2+ channels (LTCCs) and sodium/calcium exchangers in the sarcolemma, and sarcoplasmic/endoplasmic reticulum calcium ATPase 2a (SERCA2a), ryanodine receptors, and cardiac phospholamban in the sarcoplasmic reticulum. The entry of Ca2+ into the cytosol through LTCCs activates the release of Ca2+ from the sarcoplasmic reticulum through ryanodine receptor channels and initiates myocyte contraction, whereas SERCA2a and cardiac phospholamban have a key role in sarcoplasmic reticulum Ca2+ sequesteration and myocyte relaxation. Excitation–contraction coupling is regulated by phosphorylation of Ca2+-handling proteins. Abnormalities in sarcoplasmic reticulum Ca2+ cycling are hallmarks of heart failure and contribute to the pathophysiology and progression of this disease. Correcting impaired intracellular Ca2+ cycling is a promising new approach for the treatment of heart failure. Novel therapeutic strategies that enhance myocyte Ca2+ homeostasis could prevent and reverse adverse cardiac remodeling and improve clinical outcomes in patients with heart failure.
Introduction
The incidence of congestive heart failure is increasing worldwide, despite important advances in pharmacological and device therapies.1–3 Therefore, novel treatment strategies are urgently needed. An improved understanding of the molecular mechanisms involved in the pathogenesis of heart failure has led to the identification of new therapeutic targets, such as the Ca2+-handling proteins in the sarcoplasmic reticulum.
Ca2+ has critical functions as a second messenger in a number of signaling pathways in all cell types.4,5 Unlike other second messengers, Ca2+ is stored intracellularly. The membranes of the endoplasmic reticulum (and the sarcoplasmic reticulum in muscle cells), act as a major reservoir for intracellular Ca2+. Cytosolic Ca2+ is maintained at basal levels by the actions of Ca2+ channels, ATPase pumps, exchangers, transporters, and Ca2+-binding proteins. In the heart, Ca2+ regulates electrical signals that determine cardiac rhythm and excitation–contraction coupling, which converts the electrical stimulus to muscle contraction. Ca2+ might also regulate cardiac remodeling and apoptotic and necrotic cell death by activating enzymes and transcriptional gene regulation (Box 1).
Box 1. The role of Ca2+ in cardiac myocytes.
Cytosolic levels of Ca2+ are regulated by various Ca2+ channels, ATPase pumps, Ca2+-binding proteins, and Ca2+ transporters, which determine when and to what extent Ca2+ influx and efflux occur.
Signal transduction
Ca2+ is a critical second messenger in G-protein-coupled pathways that induce gene transcription
These pathways affect cardiac cell function, by activating Ca2+-regulated enzymes, and might also regulate the cardiac cell population by inducing hypertrophy, necrosis, or apoptosis
Heart rhythm
Ca2+ regulates electrical signals that determine the cardiac rhythm via ion currents and exchangers
β-adrenergic stimulation results in increased Ca2+ entry into the cell, which ultimately leads to increased rates of myofilament contraction and relaxation
An increase in external Ca2+ concentration induces shorting of phase 2 of the cardiac action potential, and consequently reduces the action potential duration
After a delay, K+ channels reopen, allowing diffusion of K+ out of the cell, which causes repolarization to the resting state and reduces the duration of the action potential
Excitation–contraction coupling converts the electrical stimulus provided by the arrival of an action potential to a mechanical response, muscular contraction
Ca2+ release from the sarcoplasmic reticulum into the cytosol is required for muscle contraction: cytosolic Ca2+ levels activate the myofilaments and modulate their contractile properties
Mitochondrial function
Cytosolic Ca2+ levels influence energy production and respiration, as ATP produced by the mitochondria is required for both muscle contraction and relaxation
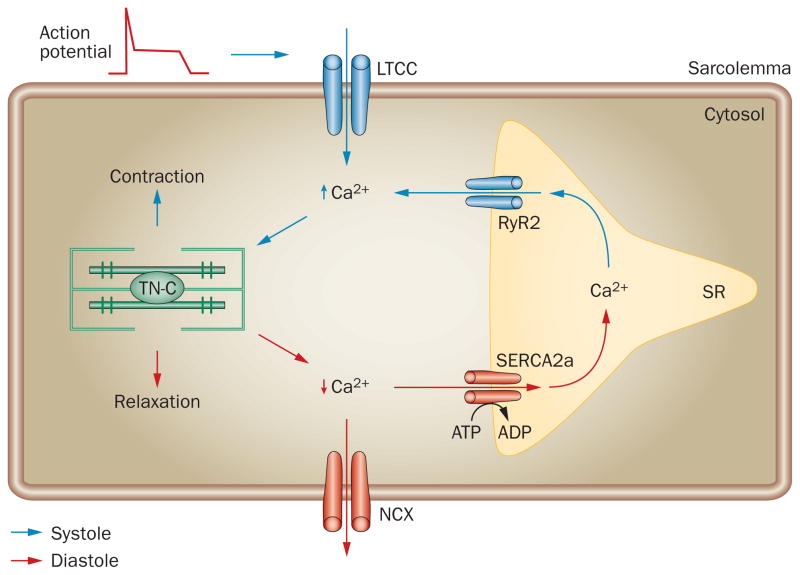
Rapid changes in levels of intracellular Ca2+ are crucial for heart muscle contraction (systole) and relaxation (diastole). The contractile function of individual cardiac myocytes is controlled by excitation–contraction coupling (Figure 1). The arrival of an action potential depolarizes the sarcolemma and enables a small amount of extracellular Ca2+ to diffuse into the cell through voltage-dependent L-type calcium channels (LTCC). This Ca2+ influx triggers Ca2+ release from the sarcoplasmic reticulum through ryanodine receptor (RyR) channels. Intracellular Ca2+ levels then rapidly increase and Ca2+ binds to troponin C, resulting in muscular contraction. During cardiac relaxation, Ca2+ is removed from the cytosol by two main mechanisms: extrusion through the sarcolemma and reuptake into the sarcoplasmic reticulum. Extrusion occurs through sodium/calcium exchangers (NCXs) and reuptake is mediated mainly via activation of the cardiac isoform of sarcoplasmic/endoplasmic reticulum calcium ATPase 2, SERCA2a.
Figure 1.
Excitation–contraction coupling. During systole, the action potential depolarizes the sarcolemma. This depolarization enables a small amount of extracellular Ca2+ to enter the cytosol through the LTCCs. Ca2+ entry triggers the release of Ca2+ from the SR through RyR2 channels. The intracellular Ca2+ concentration increases and binding of Ca2+ to TN-C activates myofilaments, resulting in muscle contraction. Removal of cytosolic Ca2+ during diastole is primarily facilitated by SERCA2a, which returns Ca2+ to the SR. Some Ca2+ also exits the cell through NCX. The decrease in intracellular Ca2+ leads to dissociation of Ca2+ from TN-C and muscle relaxation. Abbreviations: LTCC, voltage-dependent L-type Ca2+ channel; NCX, Na+/Ca2+ exchanger; RyR2, ryanodine receptor 2; SERCA2, sarcoplasmic/endoplasmic reticulum calcium ATPase 2; SR, sarcoplasmic reticulum; TN-C, troponin C.
Excitation–contraction coupling is modulated by other important second messengers, such as cyclic AMP (cAMP). This molecule is upregulated via activation of adenylate cyclase in response to β-adrenergic stimulation coupled with stimulatory G-protein activation. cAMP activates phosphatases and a series of protein kinases, including cAMP-dependent protein kinase (PKA),6,7 which in turn activate β-adrenergic receptor (β-AR) signaling pathways.8–10 PKA phosphorylates several targets that have important roles in excitation–contraction coupling. For example, phosphorylation of LTCC leads to increases in both Ca2+ current and force generation;11,12 phosphorylation of the SERCA2a inhibitor, cardiac phospholamban, reduces SERCA2a inhibition, which results in an increase in Ca2+ resequestration into the sarcoplasmic reticulum;13–15 and phosphorylation of troponin I reduces the sensitivity of myofilaments to Ca2+, leading to enhanced Ca2+ resequestration and diastolic relaxation.16–18 β-adrenergic stimulation, therefore, increases the rates of both contraction (positive inotropy) and relaxation (positive lusitropy) in cardiac myocytes.19 The PKA-induced increase in Ca2+ current and amelioration of SERCA2a inhibition are major factors that enhance cardiac contractility.20
Defects in every stage of excitation–contraction coupling lead to diastolic and systolic abnormalities, as well as an increased propensity for ventricular arrhythmias, all of which have all been reported in patients with heart failure. These defects are the result of altered expression or function of proteins that are required for the maintenance of Ca2+ homeostasis. In this Review, we explain the association between abnormalities in Ca2+ handling in the sarcoplasmic reticulum and heart failure, and discuss the therapeutic potential of targeting key proteins involved in this process. β-ARs and adenylate cyclase are possible targets for the treatment of heart failure that have been reviewed elsewhere;21,22 as such, they are mentioned only briefly in this Review.
Impaired Ca2+ cycling in heart failure
The primary cause of heart failure is diastolic dysfunction resulting from impairments in myocardial relaxation, ventricular filling, or both. Diastolic dysfunction is also associated with systolic dysfunction. At the cellular level, the causes of impaired cardiac myocyte relaxation include cytosolic Ca2+ overload, myofilament structural changes or dysfunction, and neurohormonal activation.
In many patients with heart failure, excitation– contraction coupling is defective. A number of abnormalities of receptors, pumps, and regulatory proteins that are involved in Ca2+ cycling have been shown in these patients. These abnormalities lead to a prolongation of the cytosolic Ca2+ transient time and, in patients with end-stage heart failure, an increase in end-diastolic Ca2+ concentration.23 Such changes, which are often a result of a decrease in the density or function of SERCA2a in the sarcoplasmic reticulum, impair systolic and diastolic function. An increased level of intracellular Na+ in heart failure myocytes can contribute to increased cytosolic Ca2+ loading via NCX. However, upregulation of NCX function, which might also occur in heart failure, could partially compensate for SERCA2a downregulation with respect to diastolic function, but would not affect systolic function.
In chronic heart failure, sustained activation of the β-AR and downstream signaling pathways have adverse effects on cardiac cells. At the molecular level, β-AR dysfunction is characterized by a reduction of β1-AR density, resulting in an increased β2-AR: β1-AR ratio and uncoupling of β-ARs from G-protein complexes.24,25 This desensitization is mediated by elevated G-protein receptor kinase activity.6,26 PKA and calcium/calmodulin-dependent protein kinase type II (CaMKII) are key effectors downstream of β-AR stimulation, and their activity might affect target proteins, resulting in inappropriate or maladaptive responses.27,28
Increases in diastolic Ca2+ level can activate Ca2+-sensitive signaling pathways in cardiac myocytes. Pathological cardiac hypertrophy is driven by a number of Ca2+-sensitive signaling pathways, including the calcineurin–NFAT29 and CaMKII–histone deacetylase30 pathways. The sarcoplasmic reticulum Ca2+ sensor, stromal interaction molecule 1 (STIM1), might also activate prohypertrophic, calcium-sensitive signaling pathways.31,32
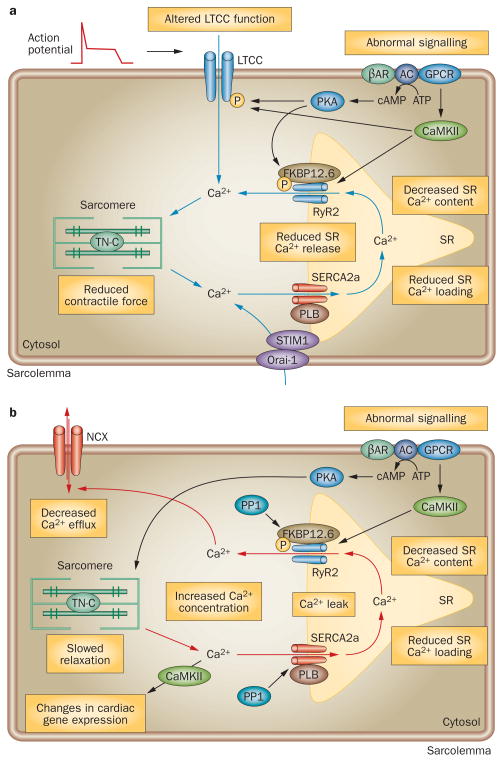
In heart failure, intracellular Ca2+ overload can result from excessive Ca2+ entry into the cytosol, either from the sarcoplasmic reticulum or from outside the cell. Alternatively, a decrease in Ca2+ efflux, or inadequate Ca2+ reuptake by the sarcoplasmic reticulum during diastole can result in intracellular Ca2+ overload in this condition (Figure 2).
Figure 2.
Abnormal intracellular Ca2+ handling in failing cardiomyocytes results in reduced contractile force and prolonged relaxation. a. Reduced SR Ca2+ storage and release cause abnormal systolic cardiomyocyte function. Abnormal β-AR and GPCR signaling increase the expression of PKA and CaMKII, which hyperphosphorylate and alter the function of RyR2 and LTCC. FKBP12.6 also contributes to stabilization of the RyR2 open probability. Impaired SERCA2a function decreases SR Ca2+ loading, resulting in reduced SR Ca2+ content and release and, therefore, reduced contractility. Alterations in LTCC function and STIM1–ORAI1-mediated store-operated Ca2+ influx can also result in abnormal Ca2+ handling during systole. b. Reduced SR Ca2+ resequestration is a key abnormality in diastole. Decreased SERCA2a activity, downregulation of SERCA2a, a decreased PLB:SERCA2a ratio, or PLB hypophosphorylation cause prolonged intracellular Ca2+ transients, reduced SR Ca2+ loading, and slowed cardiomyocyte relaxation. An increase in levels of NCX might be a compensatory response to prevent Ca2+ overloading. While increased NCX activity would be initially adaptive, excessive or sustained NCX activation could contribute to decreased sarcoplasmic reticulum Ca2+ content by removing cytosolic Ca2+, and reducing systolic Ca2+ transients and contractile function. SR Ca2+ leak resulting from impaired RyR2 function and increased expression of PP1 can reduce SR Ca2+ content and increase cytosolic Ca2+ concentration during diastole. On activation, the β-AR–AC–GPCR complex synthesizes cAMP, which activates PKA. Hyperactive PKA decreases the Ca2+ sensitivity of the myofilaments and prolongs relaxation. Intracellular Ca2+ overload stimulates CaMKII, which contributes to SR diastolic Ca2+ leak by hyperphosphorylating RyR2 and induces the transduction of pathological Ca2+ signaling. Abbreviations: AC, adenylate cyclase; β-AR, β-adrenergic receptor; CaMKII, calcium and calmodulin-dependent protein kinase type II; FKBP12.6, FK506 binding protein; GPCR, G-protein-coupled receptor; LTCC, voltage-dependent L-type Ca2+ channel; NCX, Na+/Ca2+ exchanger; Orai-1, calcium-release activated calcium channel protein 1; P, phosphorylation; PLB, cardiac phospholamban; PP1, protein phosphatase 1; RyR2, ryanodine receptor 2; SERCA2a, sarcoplasmic/endoplasmic reticulum calcium ATPase 2a; STIM1, stromal interaction molecule 1.
Excessive Ca2+ entry into the cytosol
In cardiac myocytes, Ca2+ influx occurs almost entirely through voltage-activated LTCCs. These multisubunit complexes consist of a pore-forming α1C subunit, and the auxiliary β, α2δ, and γ subunits,33,34 which traffic the α1C subunit to the sarcolemma and modulate the voltage dependence of channel gating.35 Ca2+ influx through LTCCs triggers sarcoplasmic reticulum Ca2+ release, which determines the rate and magnitude of myocyte contraction via a process called Ca2+-induced Ca2+ release. LTCC-mediated Ca2+ influx is also associated with cardiac hypertrophic signaling pathways; therefore, abnormalities in LTCC activity and expression can cause cardiac dysfunction. In mice, disruption of cardiac LTCC subunit genes caused defects in cardiac function and vascular remodeling that resulted in embryonic death.36,37 Consistent with a dominant role of LTCC in Ca2+ transport, pharmacological blockade of LTCC effectively inhibited cardiac remodeling after pressure overload38 and prevented cardiomyopathy in murine models.39 Most animal models of heart failure have shown either no change or a slight decrease in the whole-cell density of LTCC current in the myocardium.40 A murine study showed that a decrease in LTCC current caused a compensatory increase in Ca2+ leakage from the sarcoplasmic reticulum via ryanodine receptor 2 (RyR2), resulting in pathological cardiac hypertrophy and heart failure through calcineurin–NFAT signaling.41 A reduction in expression of LTCC and Ca2+ current has not been definitively established in human heart failure. However, the single-channel activity of LTCC was significantly increased in human failing ventricular myocytes compared with nonfailing control myo-cytes.42 The basal level of LTCC phosphorylation was also increased in failing myocardium, suggesting that LTCC phosphorylation might contribute to the blunted inotropic effects of adrenergic stimulation in the failing human heart.42,43 Clinical trials have shown either no effect, or a detrimental effect, of LTCC-blocking agents on the survival of patients with heart failure.44 Whether LTCC-inhibitors could be used to treat heart failure is, therefore, unclear.
Several groups of researchers have proposed that store-operated Ca2+ entry (SOCE, also known as capacitative Ca2+ entry)—a mechanism through which calcium-release-activated calcium (CRAC) channels in the plasma membrane sense depletion of intracellular Ca2+ and open to allow influx of Ca2+ from the extracellular reservoir—may be enhanced in pathological remodeling.45 SOCE is the major Ca2+ entry mechanism in nonexcitable cells that lack voltage-gated Ca2+ channels, such as mast cells and T cells.46 The process has an important role in signal transduction that controls Ca2+ homeostasis, cell differentiation, and secretion.46 SOCE compensates for the loss of Ca2+ that is transported out of the cell by sarcolemmal Ca2+ ATPase and the NCX, and ensures proper Ca2+ refilling of the endoplasmic reticulum.47 The pore-forming subunit, CRAC channel protein 1 (also known as protein orai-1), is a well-characterized component of CRAC channels in plasma membranes.48
The sarcoplasmic reticulum Ca2+ sensor STIM1 has a role in the detection of Ca2+ depletion and the activation of CRAC channels.49–51 STIM1 is predominantly located in the endoplasmic or sarcoplasmic reticulum membrane. Functional EF-hand domains in the endoplasmic or sarcoplasmic reticulum luminal region of the molecule bind Ca2+ and detect Ca2+ depletion.52,53 Dissociation of Ca2+ from the EF-hand domains leads to rapid oligomerization of STIM1,52,54 relocalization of the molecule to the junction between the plasma membrane and the endoplasmic or sarcoplasmic reticulum, and activation of CRAC channels in the plasma membrane.53,55 STIM1 directly interacts with CRAC channel protein 1, the pore-forming subunit of the CRAC channels, results in the assembly of an active tetrameric complex that opens the Ca2+ channel and allows Ca2+ influx into the cytosol.56 Ca2+ influx activates calcineurin–NFAT signaling and induces transcriptional activation of immune response and prohypertrophic genes.57,58
SOCE has been identified in excitable cells, including skeletal muscle fibers59–61 and smooth muscle cells.62 In smooth and skeletal muscle cells, SOCE might be mediated by the comparatively nonselective, canonical transient receptor potential (TRPC) channels.63,64 STIM1 directly interacts with TRPC channels, but whether STIM1-mediated gating of these channels is direct or indirect is unclear. The role of CRAC channel protein 1 in SOCE in excitable cells is also not fully understood. However, there is evidence for a role of STIM1 in SOCE-mediated cardiac pathology. In neonatal cardiac myocytes, STIM1 was required for in vitro hypertrophic responses induced by autocrine/paracrine growth factors and neurotransmitters (such as endothelin-1, phenyl-ephrine, and angiotensin II).31,65 In adult rat cardiac myocytes, STIM1 overexpression increased SOCE current and activated pathological hypertrophic responses.32 By contrast, depletion of cardiac STIM1 prevented cardio-myocyte hypertrophy and decreased NFAT activation in rats.32 However, whether STIM1 modulation has a role in the development of heart failure remains unclear.
Reduced Ca2+ efflux from the cytosol
As well as acting as a Ca2+ reservoir, the sarcoplasmic reticulum actively maintains cytosolic Ca2+ concentrations during contraction–relaxation of cardiac myocytes. In humans and higher mammals, sarcoplasmic reticulum Ca2+ uptake is mainly regulated by SERCA2a. This Ca2+ pump has an important role in restoring sarcoplasmic reticulum Ca2+ levels in diastole and terminating Ca2+- dependent force activation via Ca2+ release into the cytosol through RyR2 in systole. In failing human myocardium, the force-frequency response is blunted, possibly as a result of defective activity or expression of SERCA2a.66–68 An increase in end-diastolic cytosolic Ca2+ levels and prolongation of Ca2+ transient during diastole were shown in cardiac myocytes isolated from patients with end-stage heart failure.69,70 Such abnormalities are associated with the development of enhanced diastolic tension.
In mammals, three different genes (ATP2A1, ATP2A2, and ATP2A3) encode SERCA protein. The phylo-genetically oldest isoform, SERCA2, is the predominant variant and three different splice transcripts have been reported: SERCA2a, SERCA2b, and SERCA2c. These transcripts differ at their C-terminus and have distinct tissue distributions. SERCA2a, which has a four-aminoacid tail and 10 transmembrane domains, is selectively expressed in the heart and in slow-twitch muscle. By contrast, SERCA2b is ubiquitously expressed and has a 49-amino-acid tail. This C-terminal extension, which has highly hydrophobic properties, might form an additional endoplasmic or sarcoplasmic reticulum transmembrane domain,71 which could explain why SERCA2b has a higher affinity for Ca2+ than SERCA2a.72 Both SERCA2a and SERCA2b bind cardiac phospholamban and they do not differ in their sensitivity to the SERCA inhibitor thapsigargin.73 The third variant, SERCA2c, has a 61-base-pair intronic sequence between exons 20 and 21 of SERCA2a, and a short tail of six amino acids. SERCA2c has been detected in human monocytes and fetal hearts74 and is also expressed in skeletal muscle and cardiomyocytes.75
No shift in the relative amounts of the SERCA2 iso-forms has been detected in failing human hearts.76 However, in animal models of hypertrophic77–79 and ischemic80,81 heart failure, a decrease in the rate of sarcoplasmic reticulum Ca2+ uptake as well as low levels of SERCA2a have been shown. Furthermore, a reduction in SERCA2a activity with a resulting decrease in sarcoplasmic reticulum Ca2+ uptake is a consistent feature of failing cardiac myocytes in humans. Data from several studies on explanted failing human hearts (obtained during transplantation procedures) suggest that sarcoplasmic reticulum Ca2+ transport function is impaired in end-stage human heart failure. Decreases in the level of SERCA2a mRNA, protein, or activity correlate strongly with decreased myocardial function and impaired contractile force–frequency relationships.68,82,83
SERCA2a activity is tightly regulated by the small inhibitory peptide cardiac phospholamban. This protein binds to SERCA2a and induces a conformational change that reduces the Ca2+-binding affinity of SERCA2a and the rate of Ca2+ uptake by the sarcoplasmic reticulum.84,85 In most failing human hearts, cardiac phospholamban protein expression is unaltered,86,87 but a decrease in the level of cardiac phospholamban mRNA has been reported in patients with dilated or ischemic cardio-myopathies.88,89 Interestingly, the decrease in levels of SERCA2a in failing myocardium is greater than the decrease in levels of cardiac phospholamban;90 therefore, the ratio of cardiac phospholamban to SERCA2a increases, resulting in increased inhibition of SERCA2a. A reduction in phosphorylation of cardiac phospholamban can also increase SERCA2a inhibition in failing myocardium.91 In animal models of heart failure, phosphorylation of cardiac phospholamban was reduced at either Ser16,92 Thr17,93,94 or both.95 Similarly, reduced phosphorylation of cardiac phospholamban at Ser16, but no change in phosphorylation at Thr17, was shown in samples from failing human hearts.92,96 The reduction in phosphorylation at Ser16 correlated with a decrease in the sensitivity of SERCA2a to high Ca2+ levels in human failing myocardium.92,96 Cardiac phospholamban is dephosphorylated by protein phosphatase 1 (PP1)97 and, as we will discuss in more detail later in this Review, increased PP1 activity has been shown in animal models of heart failure.98 These data suggest that the level of phospholamban phosphorylation might depend on the severity of heart failure, and could be affected by β-AR signaling pathways through alterations in the activation of protein kinase A (PKA).
Mutations in the PLN gene, which encodes cardiac phospholamban, are associated with human heart failure. In patients with familial dilated cardiomyopathy, mutations in the coding region of the PLN gene have been identified.97 PLN mutations, such as an arginine to cysteine substitution in codon 9 (Arg9Cys) and an arginine deletion in codon 14 (Arg14del), might enhance cardiac phospholamban-mediated inhibition of SERCA2a activity.99–101 Transgenic mice that expressed the Arg9Cys mutant developed terminal heart failure that resulted in premature death.100 In addition, two more mutations at Arg9 (Arg–Leu and Arg–His substitutions) were identified in a cohort of patients with heart failure.99 A mutation in the coding region of the PLN gene that resulted in a termination codon (Leu39stop) was identified in two families with hereditary heart failure.102 Patients who were homozygous for this mutation, which prevented phospholamban expression, developed dilated cardiomyopathy and heart failure.102
NCX has a major role in the extrusion of Ca2+ through the sarcolemma into the extracellular space and contributes to Ca2+ removal during mammalian cardiac myocyte relaxation.103 Some studies have shown increased NCX expression in failing human hearts.104,105 In human heart failure, NCX upregulation might compensate, at least in part, for any decline in SERCA2a activity because an increase in NCX activity might accelerate the removal of Ca2+ from failing cardiac myocytes during relaxation. However, another study showed no change in NCX expression in myocardium from terminally failing human hearts.106 A study in which the level of NCX mRNA in myocardium from failing human hearts was analyzed showed no correlation between NCX mRNA expression and the severity of disease.107 NCX mRNA was upregulated only in end-stage heart failure.107 However, NCX mRNA levels do not always indicate the level of NCX protein expression or activity.108,109 Although increased NCX activity in heart failure might initially be adaptive, excessive or sustained NCX activation could contribute to a further decrease in the Ca2+ content of the sarcoplasmic reticulum as a result of increased cytosolic Ca2+ removal. This decrease could lead to diminished systolic Ca2+ transients, and reduced contractile function. Some studies have suggested that NCX is involved in Ca2+ influx, which might occur via a reverse mode in the presence of high levels of intra-cellular Na+ and positive membrane potentials.110,111 In the forward mode, NCX transports Na+ into the cell and reduces diastolic Ca2+ levels by extruding Ca2+. However, during the action potential and/or when intracellular Na+ concentration is increased, NCX can function in a reverse mode and transport Ca2+ into the cell. This Ca2+ influx might promote sarcoplasmic Ca2+ loading and, together with the LTCC, modulate the release of Ca2+ from the sarcoplasmic reticulum, resulting in an increase in intracellular Ca2+ concentration.
Reduced sarcoplasmic reticulum Ca2+ content
A reduction in Ca2+ uptake by the sarcoplasmic reticulum reduces the amount of stored Ca2+ available for release into the cytosol. In addition, decreased Ca2+ storage reduces excitation–contraction coupling and contributes to dysfunction of systolic contractility.112
The most abundant acidic sarcoplasmic reticulum protein is calsequestrin (CASQ). In humans, two isoforms of CASQ have been identified; CASQ-1 is found in skeletal muscle, and CASQ-2 in cardiac muscle.113 CASQ-2 is primarily responsible for the Ca2+ storage capacity of the myocardial sarcoplasmic reticulum. The protein has a high capacity and a moderate affinity for Ca2+ and acts as a Ca2+ buffer that decreases the concentration of free luminal Ca2+ to facilitate further SERCA2a-mediated Ca2+ uptake into the sarcoplasmic reticulum.113 The large Ca2+ buffering range of CASQ-2 facilitates the large storage capacity of the sarcoplasmic reticulum as well as the rapid release of Ca2+ into the cytosol.113 In addition, CASQ-2 has a role in regulating the open probability of RyR2 channels and, therefore, the amount of Ca2+ release from the sarcoplasmic reticulum.114,115 The levels of CASQ-2 mRNA and protein do not change substantially in failing human hearts. However, CASQ-2 is associated with certain types of arrhythmia, such as catecholaminergic polymorphic ventricular tachycardia (CPVT), which is characterized by stress-induced or exercise-induced ventricular tachycardias that lead to sudden cardiac death at young ages (9 ± 4 years).116 In mice, CASQ-2 knockout caused an autosomal-recessive form of CPVT, although Ca2+ transients in cardiac myocytes were unaltered.117 Mutations in CASQ2, the gene that encodes CASQ-2, also cause CPVT.118 In humans, a missense mutation in a highly conserved region of CASQ2 that resulted in an amino acid change from aspartic acid to histidine at codon 307 (Asp307His) caused autosomal-recessive CPVT.118 The Asp307His mutation also caused RyR2 dysfunction and a CVPT phenotype in a mouse model.119
Cardiac CASQ-2 is glycosylated and then trimmed by a series of mannosidases located within distinct intra-cellular compartments.120 This glycosylation prevents premature polymerization of CASQ-2, and ensures transport of the protein to the sarcoplasmic reticulum. Interestingly, defective glycosylation of CASQ-2 was identified in canine ventricles following rapid-pacing-induced heart failure.120 The levels of glycan forms of CASQ-2 that were characteristic of a junctional sarcoplasmic reticulum location were reduced by 80%, and the level of the mannose 8,9 CASQ-2 glycoform, characteristic of a rough endoplasmic reticulum location, was increased twofold in failing canine hearts compared with healthy controls.120 Data from CASQ-2 trafficking studies in adult rats also suggested that abnormal glycosylation might result in retention of CASQ-2 in the rough endoplasmic reticulum of cardiac myocytes.121,122 These findings suggest that defective post-translational processing of CASQ-2 might lead to impaired CASQ-2 cellular compartmentalization during heart failure.
Triadin and junctin are transmembrane sarcoplasmic reticulum proteins that form a complex with CASQ-2 and RyR2. This complex, which anchors CASQ-2 to the luminal membrane in close proximity to the Ca2+-release channel, might have an important role in the regulation of Ca2+ release from the sarcoplasmic reticulum. Triadin and junctin might be involved in luminal Ca2+ sensing by mediating the interaction between CASQ-2 and RyR2,123 and might be required for the normal operation of Ca2+ release. The levels of triadin and junctin are reduced in failing human myocardium.124 However, whether this reduction causes heart failure, or if the downregulation is an adaptive response that improves Ca2+ handling in failing cardiac myocytes, is not clear. Overexpression of triadin in adult rat ventricular myocytes increased RyR2 channel activity and arrhythmia.125 Downregulation of junctin in failing human hearts might reduce sarcoplasmic Ca2+ leak in response to chronic β-adrenergic stimulation and improve cardiac function.126 Cardiac-specific overexpression of β1-AR in transgenic mice resulted in a decrease in junctin protein levels and progression of hypertrophy.126,127 Abnormalities in sarcoplasmic reticulum protein trafficking and assembly of the Ca2+-release complex, which result in impaired Ca2+ release from the sarcoplasmic reticulum, might contribute to heart failure.
Sarcoplasmic reticulum histidine-rich calcium-binding protein (HRC) interacts with triadin and SERCA2a, and might have a role in both Ca2+ release and uptake.127 In adult rat cardiac myocytes, overexpression of HRC increased the Ca2+ storage capacity of the sarcoplasmic reticulum but decreased sarcoplasmic reticulum Ca2+ release and the Ca2+ transient duration, resulting in a reduction in contractility.128 HRC expression is significantly reduced in failing human and animal hearts.128 This reduction might be a compensatory mechanism that increases Ca2+ release from the sarcoplasmic reticulum in an attempt to improve cardiac function during heart failure.128 A HRC gene polymorphism, which results in the amino acid change Ser96Ala, was associated with ventricular arrhythmias in patients with idiopathic dilated cardiomyopathy.129
S100 calcium binding protein A1 (S100A1), a member of the S100 family of proteins that contain EF-hand Ca2+-binding motifs, is located in the sarcoplasmic reticulum, sarcomere, and mitochondria, and is highly expressed in cardiac myocytes.130 S100A1 stabilizes RyR2 in diastole and augments Ca2+ release during systole. S100A1 also increases SERCA2a activity during the relaxation phase.131 Myocardial levels of S100A1 are decreased in patients with heart failure as a result of cardio-myopathy.132 Gain-of-function and loss-of-function experiments have been used to characterize the in vivo and ex vivo positive inotropic effects of S100A1.133–135 Cardiac-restricted overexpression of S100A1 in transgenic mice had chronic cardiac inotropic effects that were independent of β-adrenergic signaling,133 and an increase in S100A1 protein levels in isolated adult and neonatal murine cardiac myocytes enhanced their systolic and diastolic performance through modulation of cellular Ca2+ handling.133,134 By contrast, S100A1-deficient mice showed severely impaired inotropic and lusitropic responses to β-adrenergic stimulation and acute or chronic hemodynamic stress.135 These impairments were at least partly caused by a reduction in cardiac Ca2+ sensitivity.135 A decrease in S100A1 protein levels might, therefore, contribute to Ca2+ dysregulation and impaired contractile function in human heart failure. Interestingly, myocardial contractility is also regulated by energy metabolism and S100A1 interacts with mitochondrial ATP synthase, resulting in an increase in the activity of the enzyme and increased production of ATP.136 These data suggest that S100A1 might also have a role in cardiac metabolism. However, further investigation of the potential mechanism of S100A1-mediated metabolism in cardiac myocytes is required.
Sarcoplasmic reticulum Ca2+ leakage
Ca2+ channels within the sarcoplasmic reticulum are nonselective and have high-conductance cation transport properties, which facilitate rapid, localized Ca2+ release. Abnormalities in the structure and function of the sarcoplasmic reticulum Ca2+ channels have been associated with cardiac arrhythmia and human heart failure.137,138
Release of Ca2+ from the sarcoplasmic reticulum into the cytosol, which is required for contractile activation of the heart, is mediated by RyR2.139 The RyR2 tetramer is a massive macromolecular signaling complex that binds kinases and phosphatases as well as adaptor and targeting proteins.140 Most of these regulatory proteins, including calmodulin, CaMKII, FKBP 12.6, PKA, PP1, and calcineurin, bind to the cytosolic region of RyR2, whereas triadin and junctin bind to the luminal sarcoplasmic region.123,141–147 Several studies have shown increased sarcoplasmic reticulum Ca2+ leak during diastole as a result of RyR2 dysfunction in heart failure.148,149 This diastolic Ca2+ leak depletes Ca2+ stores and presumably impairs the contractility of cardiac myocytes.
Expression of RyR2 is unchanged in failing human hearts.150,151 However, several studies showed an increased level of PKA-mediated phosphorylation of RyR2 in human heart failure.152,153 Chronic hyperactivity of β-AR signaling might lead to hyperactivation of PKA and subsequent hyperphosphorylation of RyR2 in failing hearts. However, the effect of RyR2 hyperphosphorylation on channel activation is not clear. Some studies showed that PKA-mediated phosphorylation of RyR2 activated the channel,152,153 whereas other studies showed that the open channel probability of RyR2 was slightly decreased in response to phosphorylation.154,155 Alternatively, PKA-mediated phosphorylation might destabilize RyR2 channels by inducing dissociation of the accessory protein FKBP12.6.153 PKA levels are unchanged in patients with heart failure, but levels of phosphatases in the RyR2 protein complex, such as PP1 and protein phosphatase 2A (PP2A), are reduced. These changes resulted in a reduced rate of dephosphorylation of RyR2 (at Ser2,809 in rabbit and Ser2,808 in humans and mice) and dissociation of FKBP12.6 from the RyR2 complex.153,156 FKBP12.6 depletion increases the Ca2+ sensitivity of RyR2 and results in leakage of Ca2+ from the sarcoplasmic reticulum during diastole. Abnormal interactions between FKBP12.6 and RyR2 have been shown in left-sided heart failure.157,158 Impaired binding of FKBP12.6 to RyR2 might also result in diastolic Ca2+ leakage from the sarcoplasmic reticulum, which could potentially initiate ventricular arrhythmias. However, further work is required to elucidate the mechanisms by which PKA modulates RyR2 channel activation in intact cells and to clarify the effect of FKBP12.6 binding on single-channel RyR2 function.
Several missense mutations in cardiac RyR2 have been identified in patients with CPVT.159 A pathogenic role of the RyR2 mutation Arg4497Cys was shown in a conditional knock-in mouse model.160 The arrhythmia morphology, arrhythmia severity, and incomplete responses to β-blockers, of Arg4497Cys mutant mice were similar to that of patients with CPVT.
Altered Ca2+-activated signaling
Abnormal Ca2+ cycling can affect multiple signaling pathways in the heart and result in hypertrophic growth and cardiac remodeling, which frequently progress to heart failure. Changes in the morphological and functional properties of the myocardium ultimately require reprogramming of cardiac myocyte gene expression.161
Calcineurin (also known as Ca2+/calmodulin dependent protein phosphatase 2B) is a serine/threonine phosphatase that has a key role in coupling Ca2+ signaling to cellular responses, through interaction with NFAT.162 Increases in intracellular Ca2+ levels activate calcineurin phosphatase activity. The activated protein binds and dephosphorylates NFAT, which translocates from the cytosol to the nucleus and induces gene expression.163,164 In the heart, NFAT interacts directly with the cardiac-restricted zinc finger transcription factor GATA4, which regulates expression of hypertrophic genes, including β-myosin heavy chain, atrial natriuretic factor, and B-type natriuretic peptide.165,166 In mice, constitutive activation of calcineurin resulted in progressive decompensation, dilatation, and the development of heart failure.167,168 This progression recapitulated the development of hypertrophic cardiomyopathy in human heart failure.167,168 However, the effect of pharmacological inhibition of calcineurin (using cyclosporine A) on hypertrophy in rat and mouse models is unclear. Two studies showed that treatment with cyclosporine A prevented or attenuated cardiac hypertrophy in aortic-banded mice,169,170 but there was no evidence that the drug inhibited cardiac hypertrophy in other studies that used the same experimental model.171,172 High-dose cyclosporine A treatment might result in severe adverse effects that could mask the therapeutic effects of the drug.168 A complete understanding of the role of the calcineurin–NFAT pathway in the progression of hypertrophy is required to facilitate the development of antihypertrophic drugs.
CaMKII can also be activated in response to increased levels of intracellular Ca2+.173 The protein has a role in multiple cellular processes that are important in the transition from early structural heart disease to heart failure and sudden death,173 including regulation of hypertrophic growth (mediated by class II histone deacetylase), MEF2 transcriptional activity, and inflammatory responses via activation of NFκB.173 Consistent with the role of CaMKII in the regulation of hypertrophic gene expression, CaMKII expression and activity correlated with the progression of heart failure in patients with structural heart disease post-myocardial infarction.174,175 Overexpression of CaMKIIδ in the hearts of transgenic mice induced a hypertrophic phenotype that rapidly transitioned to dilated cardiomyopathy and abnormal intracellular Ca2+ homeostasis,176 resembling the remodeled myocardium in human heart failure. By contrast, CaMKII inhibition in animal models, by either genetic or pharmacological approaches, prevented phenotypic changes that are associated with heart failure, such as arrhythmias, hypertrophy, and myocardial dysfunction.177–179
Importantly, CaMKII might have a role in the regulation of myocyte Ca2+ homeostasis.180 The protein can phosphorylate and, therefore, modulate the function of key Ca2+-handling proteins, such as cardiac phospholamban, RyR2, and LTCC.173 CaMKII-dependent phosphorylation of cardiac phospholamban at Thr17 can lead to an increase SERCA2a activity and thereby accelerate sarcoplasmic reticulum Ca2+ transport.181 Cardiac phospholamban is phosphorylated at Ser16 by PKA and at Thr17 by CaMKII, but the relative importance of these dual phosphorylation sites in the regulation of cardiac contractility is difficult to assess. A number of studies showed that phosphorylation at Ser16 was required to allow phosphorylation at Thr17 and so maximize the contractile effects of β-adrenergic stimulation.182–184 However, Thr17 phosphorylation that was independent of Ser16 phosphorylation has also been reported.185,186 In mice, targeted inhibition of CaMKII activity using an inhibitory peptide resulted in a substantial decrease in phosphorylation of phospholamban at Thr17, but had no effect on phosphorylation at Ser16.186 The mice developed dilated heart failure.186
CaMKII can phosphorylate RyR2 at Ser2,808 (or Ser2,809, depending on species)—which is also a PKA phosphorylation site—and at other sites, including Ser2,815. This phosphorylation increases the activity of the RyR2 channels.187 Endogenouse CaMKII increased the amount of Ca2+ release from the sarcoplasmic reticulum for a given sarcoplasmic reticulum Ca2+ content in intact cardiomyocytes.188 Consistent with this finding, transgenic mice overexpressing CaMKIIδc (the major cardiac isoform) had an increased Ca2+ spark frequency (indicative of diastolic spontaneous SR Ca2+ release) and low diastolic Ca2+ content compared with wild-type mice.189 Overexpression of CaMKIIδc causes heart failure.189
CaMKII also associates with LTCC complexes and increases the open probability of the channels, resulting in an increase in Ca2+ current and augmentation of cellular Ca2+ signaling via a process called facilitation.190 CaMKII-dependent facilitation of LTCCs requires Thr498 phosphorylation in the β2a subunit to enhance regulatory phosphorylation.191 A role of CaMKII in the development of early after-depolarization and arrhythmias through LTCC activation has been shown in mouse models.190,192 Increases in the speed of sarcoplasmic reticulum Ca2+ uptake and release as a result of CaMKII-mediated phosphorylation of Ca2+-handling proteins might provide more time for diastolic filling of the ventricles at higher heart rates.
Potential therapeutic strategies
Improvements in knowledge of Ca2+ dynamics in health and disease have led to increased understanding of the therapeutic potential of targeting Ca2+-handling proteins. A number of pharmacological and gene therapy strategies aimed at restoring the function of the sarcoplasmic reticulum—either by increasing Ca2+ uptake or preventing Ca2+ leakage—have been investigated (Table 1).
Table 1.
Therapies for heart failure that target Ca2+-handling proteins
| Target | Strategy | Effects in heart failure | Clinical stage of development |
|---|---|---|---|
| Restoration of Ca2+ uptake into the sarcoplasmic reticulum | |||
| SERCA2a | Increasing expression by AAV1-mediated gene delivery | Rescues depressed myocardial contractility and increases survival | Clinical trials: Phase I/II CUPID209 demonstrated safety and efficacy in patients with advanced heart failure, Phase II NCT00454818210 is ongoing |
| SUMO1 | Increasing expression by manipulation of post-translationally modified protein | Improves SERCA2a activity and stability | Beneficial effects of AAV9-mediated SUMO1 gene delivery shown in mouse models233 |
| Phospholamban | Decreasing expression by ablation or depletion using dominant-negative molecules | Increases SERCA2a activity Reduces phospholamban-mediated SERCA2a inhibition |
Beneficial in experimental animal models211–213,215 Outcome in human heart failure is unclear |
| IPP-1 | Overexpression of constitutively active form (IPP-1c) | Conditional amplification of β-adrenergic signaling Enhanced phospholamban phosphorylation |
Beneficial effects of IPP-1 gene transfer shown in murine models of heart failure225 |
| Prevention of Ca2+ leakage from the sarcoplasmic reticulum | |||
| RyR2 | Channel stabilization Decreased probability of open channels |
Prevention of sarcoplasmic reticulum dysfunction and possible antiarrhythmic effects | Agents that stabilize RyR2, such as JTV-519 and S44121, are being tested in animal models262,263 and in muscle preparations from patients with heart failure and arrhythmias247 |
| CaMKII | Inhibition of CaMKII hyperactivity | Prevention of pathological β-AR mediated signaling Prevention of arrhythmia |
Inhibition of CaMKII using inhibitory peptides or small molecules beneficial in animal models of structural heart disease177–179 |
| S100A1 | Overexpression | Stabilization of RyR2 in diastole and reduction in arrhythmic risk Increased SERCA2a activity |
Safety and efficacy shown in preclinical large animal model261 as well as in human cardiomyocytes230 Additional beneficial effects shown in a rat model of myocardial infarction when S100A1 gene therapy was combined with β-AR blockers259 |
Abbreviations: AAV, adeno-associated virus; β-AR, β-adrenergic receptor; CaMKII, calcium/calmodulin-dependent protein kinase type II; IPP-1, endogenous protein phosphatase 1 regulatory subunit 1A; RyR2, ryanodine receptor 2; S100A1, S100 calcium binding protein A1; SERCA2a, sarcoplasmic and endoplasmic reticulum calcium ATPase 2a; SUMO-1, small ubiquitin-related modifier 1.
Restoring Ca2+ uptake
Modulation of SERCA2a activity is critical for improving Ca2+ uptake by the sarcoplasmic reticulum. SERCA2a overexpression leads to an improvement in cardiac function in many animal models of heart failure. Transgenic rats with increased SERCA2a expression showed enhanced contractility and better survival after pressure overload than wild-type rats.193 Similarly, initial data indicated that SERCA2a restoration by gene transfer increased contractility in isolated failing human cardiac myocytes.194 These data, which have been validated in independent animal studies,195,196 support the hypothesis that in vivo gene delivery of SERCA2a could rescue the heart failure phenotype. Furthermore, SERCA2a overexpression in failing rat hearts increased the phosphocreatine:ATP ratio197 and decreased the oxygen cost of left ventricular contraction.198 These findings suggest that restoration of sarcoplasmic reticulum Ca2+ transport could normalize energy metabolism and utilization in failing hearts.
In many small-animal studies, adenoviral delivery of SERCA2a has been used to restore SERCA2a expression in failing hearts.199,200 However, expression of adenovirally delivered transgenes is short-lived and the adenoviral vector induces an inflammatory response.201 To avoid these problems, new vectors, such as various recombinant adeno-associated virus (AAV) serotypes, are now being used. These vectors are nonpathogenic, nonreplicative, and nonintegrating.202
In pigs with volume-overload heart failure, an increase in sarcoplasmic reticulum Ca2+ uptake via AAV-mediated SERCA2a gene transfer was beneficial for maintenance of cardiac function.196 In this study, the viral vector containing SERCA2a was administered by intracoronary artery infusion, and ventricular remodeling and improvements in cardiac parameters (including ejection fraction and peak left ventricular pressure rate) were observed 2 months after SERCA2a gene transfer. Improvements in left ventricular function have also been reported in sheep with rapid-pacing-induced heart failure.203
Other animal studies have shown an association between SERCA2a overexpression and reduced frequency of arrhythmias.195,204–206 However, in one study involving a rat model in which SERCA2a was constitutively overexpressed, investigators found a delay in myocardial failure after myocardial infarction in rats at a cost of increased acute arrhythmias.207 Notably, though, this latter study did not involve gene transfer of SERCA2a and the overexpression of SERCA2a was not evident at the protein level. One of the early concerns with SERCA2a overexpression was that, with additional SERCA2a pumps, the SR would trigger calcium release and cause arrhythmias, especially if the RyR2 are leaky.208 However, experimental models have shown that SERCA2a overexpression decreased ventricular arrhythmias in both small204 and large195 animal models of ischemia reperfusion. More-recent studies have shown that overexpression of SERCA2a suppresses electrical alter-nans,205 interrupting an important pathway leading to cardiac fibrillation, and decrease SR Ca2+ leak in a model of advanced heart failure.206 In a rat model of heart failure induced by myocardial infarction, SERCA2a gene therapy stabilized sarcoplasmic reticulum Ca2+ load, reduced RyR2 phosphorylation, and decreased Ca2+ leakage from the sarcoplasmic reticulum.206 These data suggest that SERCA2a has positive inotropic and lusitropic effects, and SERCA2a gene therapy could have antiarrhythmic effects in patients with heart failure.
CUPID (Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease), the first clinical trial of AAV1 delivery of SERCA2a in patients with advanced heart failure, began in 2007. The main objective was to investigate the safety and biological effects of restoring SERCA2a activity in patients with advanced heart failure. Phase I was an open-label, sequential-dose-escalation study in nine patients with advanced heart failure (NYHA class III or IV).209 AAV1–SERCA2a was administered by single coronary artery infusion at doses ranging from 1.4 × 1011–3.0 × 1012 DNAse-resistant particles (DRP). There were no unexpected safety concerns and several patients showed improvements in a number of relevant parameters, including heart failure symptoms and cardiac function, at 6-month follow-up. The phase I trial, therefore, demonstrated safety and efficacy of the therapy despite the small number of enrolled patients.
Phase II of the CUPID trial was a double-blind, placebo-controlled, randomized study of 39 patients with advanced heart failure.210 In this study, the participants received either placebo or one of three doses of AAV1–SERCA2: 6 × 1011 DRP (low-dose), 3 × 1012 DRP (mid-dose) or 1 × 1013 DRP (high-dose). Each dose group contained eight patients. At 6 months of follow-up, the patients treated with high-dose AAV1–SERCA2a showed improvement or stabilization of heart failure symptoms (assessed using the NYHA classification and Minnesota Living With Heart Failure Questionnaire), functional status (assessed using a 6 min walk test and measurement of maximal oxygen consumption), and hemodynamic performance (left ventricular ejection fraction and left ventricular end-systolic volume), as well as a decrease in levels of the heart failure biomarker N-terminal B-type natriuretic propeptide. At 12 months follow-up, a significant decrease in cardiovascular events (hospitalizations related to heart failure, episodes of worsening of heart failure, death, or the requirement for left ventricular assist devices or cardiac transplant) was evident in the patients who received high-dose AAV1–SERCA2a compared with those who received placebo (HR = 0.12, P = 0.003). Moreover, during the 12-month study, the estimated average duration of cardiovascular hospitalizations was considerably shorter for patients in the high-dose AAV1–SERCA2a group than in the placebo group (0.4 days versus 4.5 days). The primary end point success criteria were defined as achieving efficacy in either the group-level analysis, with improvement in at least two of the four efficacy domains (symptomatic, functional, biomarker, and left ventricular function or remodeling), the individual-level analysis, or Kaplan–Meier analysis of the outcome end point (time to death, or requirement for ventricular assist device or transplantation). These analyses showed that treatment with high-dose AAV1–SERCA2a was better than placebo. Statistical significance for all three criteria was defined as P <0.2, rather than P <0.05, because additional requirements were imposed on the study outcomes to control for the probability of obtaining positive study results by chance alone.
Two clinical trials of AAV1–SERCA2a therapy in patients with heart failure are ongoing. A randomized, double-blind study of 16 patients who have received a left ventricular assist device for an accepted clinical indication is being carried out in the UK to evaluate the safety and feasibility of AAV1–SERCA2a therapy, and a Phase 2 trial in patients with severe heart failure is being conducted in France to evaluate structural changes in response to the therapy.
Reducing SERCA2a inhibition by targeting cardiac phospholamban is an alternative strategy for increasing SERCA2a activity. Cardiac phospholamban modulation using antisense RNA,211 a dominant-negative phospholamban mutant,212 or cardiac-phospholamban-targeted antibodies can increase SERCA2a activity.213 However, pharmacological approaches to cardiac phospholamban modulation have not been successful because of non-specificity of the pharmacological agents.91 An in vitro study showed that siRNA-mediated inhibition was not sufficient for modulation of cardiac phospholamban in cardiac myocytes, perhaps owing to poor stability of the RNA transcript.214 However, myocardial phospholamban expression silencing, using an AAV9 vector encoding a short hairpin RNA, resulted in heart failure rescue, increased SERCA2a activity, and improved left ventricular function in aortic-banded rats.215 This result suggests that AAV-mediated RNA-interference therapies might be beneficial for patients with cardiac hypertrophy and heart failure.
The serine/threonine phosphatases PP1 and PP2 have a role in regulation of cardiac contractility.216 In particular, the negative regulator of Ca2+ cycling PP1, which localizes to the sarcoplasmic reticulum, has been implicated in the regulation of the β-AR response.217 Dephosphorylation of phospholamban restores inhibition of SERCA, and inhibition of Ca2+ uptake into the sarcoplasmic reticulum218 and an increase in PP1 activity219,220 was shown in failing human hearts. PP1 is regulated by endogenous protein phosphatase 1 regulatory subunit 1A (IPP-1; also known as protein phosphatase inhibitor 1).221 IPP-1 is activated by PKA-mediated phosphorylation at Thr35 and specifically inhibits PP1 activity. The protein could, therefore, act an amplifier of β-adrenergic responses in the heart.222,223 In heart failure, IPP-1 expression is decreased and the protein is dephosphorylated and inactive.224 The decrease in the level of active IPP-1 results in a reduction in PP1 inhibition, activation of cardiac phospholamban, and inhibition of SERCA2a activity.221 PP1 inhibitors could, therefore, potentially be used to increase SERCA2a activity in patients with heart failure. In rats with heart failure, this approach enhanced contractility under basal conditions and after β-adrenergic stimulation.225 In another study, inducible overexpression of IPP-1c in mouse hearts increased cardiac phospholamban phosphorylation at Ser16, but had no effect on phosphorylation of RyR2 at Ser2,808, troponin I at Ser23 or Ser24, or myosin binding protein C at Ser282.226 These data confirm that IPP-1c specifically inhibits cardiac phospholamban. In addition, IPP-1c expression improved postischemic cardiac performance226 and had cardioprotective effects (namely, attenuation of the endoplasmic reticulum stress response) in adult transgenic mice.226 IPP-1c overexpression enhanced cardiac contractility in young mice, but resulted in contractile dysfunction and ventricular dilatation in response to β-adrenergic stimulation in aged mice.227 By contrast, IPP-1c-mediated, PP1-specific inhibition increased Ca2+ uptake into the sarcoplasmic reticulum without direct modification of sarcoplasmic reticulum Ca2+ release in rat cardiac myocytes.228 These data suggest that IPP-1c could be an interesting new target for gene therapy in heart failure.
The post-translational modification of small ubiquitin- related modifier 1 (SUMO-1) could be targeted to increase SERCA2a activity. SUMO-1 is a unique polypeptide that covalently conjugates to target proteins. This binding, termed SUMOylation, regulates many aspects of protein function, such as subcellular localization, protein–protein interactions, and transcriptional activity.229 A number of target proteins that are regulated by SUMOylation have been implicated in human disease.230 For example, a decrease in lamin A SUMOylation could be a causative factor in familial dilated cardiomyopathy; two different mutations in lamin A have been identified in patients with this disease.231,232 These mutations, which cause the amino-acid substitutions Glu203Gly and Glu203Lys, are likely to lead to defective lamin A SUMOylation as well as alterations in nuclear morphology and the subcellular localization of lamin A.232 We have shown that the activity and stability of SERCA2a in cardiac myocytes is modulated by SUMO-1, and that cardiac expression of SUMO-1 is significantly reduced in humans and animals with heart failure.233 Cardiac-specific overexpression of SUMO-1 in transgenic mice restored SERCA2a function, improved hemodynamic performance, and significantly increased survival following thoracic aortic constriction.233 Consistent with these findings, a long-term cardiac benefit of SUMO-1 over-expression (using AAV9-mediated SUMO1 gene delivery) has been shown in mouse models of heart failure. Transgene-mediated SUMO-1 overexpression rescued pressure-overload-induced cardiac dysfunction concomitantly with increased SERCA2a function and improved left ventricular dilatation, functional deterioration, and hemodynamic performance 2 months after gene transfer (4 months after thoracic aortic constriction).233 SUMO-1 overexpression also significantly reduced mortality among the mice with heart failure (P <0.05).233 Depletion of cardiac SUMO-1 using AAV9-mediated delivery of a small hairpin RNA in normal mice resulted in a decline in SERCA2a function and expression, and a dramatic increase in mortality.233 Importantly, these beneficial effects of SUMO-1 overexpression did not occur when SERCA2a was downregulated,233 suggesting a SERCA2a-dependent role of SUMO-1 in cardiac function. Further studies are required to define the molecular mechanism of SERCA2a regulation by SUMOylation and to investigate the association between low SUMO-1 expression and heart failure.
Preventing Ca2+ leak
Hyperphosphorylation of RyR2 by PKA or CaMKII destabilizes the channel and might cause channel dysfunction.234,235 Two strategies for enhancing RyR2 stabilization—increasing FKBP12.6 binding and reducing hyperphosphorylation—have been investigated. Several animal studies have been carried out to investigate the effects of FKBP12.6 overexpression in failing hearts. In one study, adenovirus-mediated FKBP12.6 over-expression reduced Ca2+ leakage from the sarcoplasmic reticulum as well as Ca2+ spark frequency and amplitude in isolated rabbit cardiac myocytes.236 In another study, cardiac-specific overexpression of FKBP12.6 in mice led to significantly improved cardiac function after myocardial infarction.237 Improved cardiac function was also shown in transgenic mice that overexpressed a mutant form of FKBP12.6 with a high binding affinity for hyper-phosphorylated RyR2 in vitro.237 These results support the hypothesis that increased binding of FKBP12.6 to RyR2 might improve cardiac function in heart failure.237
Pharmacological strategies aimed at reducing Ca2+ leakage through RyR2 have also been investigated. These strategies involve the use of agents that interact with RyR2 and inhibit Ca2+ release from the sarcoplasmic reticulum, either by altering the gating of the RyR2 channel or controlling ion translocation. Unfortunately, most of the classic RyR2-targeting drugs, such as dantrolene and flecainide, have unacceptable adverse effects or lack long-term efficacy.238,239 JTV-519 (also known as K201) is a promising new drug with cardioprotective effects.240 The drug is a 1,4-benzothiazepine derivative that has structural similarities to diltiazem, a voltage-dependent LTCC blocker used in the treatment of hypertension and some types of arrhythmias.241 JTV-519 has cardioprotective and antiarrhythmic properties.242,243 The agent improved the contractility of paced hearts and prevented development of heart failure in dogs, probably by preventing dissociation of FKBP12.6 from RyR2 and, therefore, improving defective channel gating.244 JTV-519 treatment also increased FKBP12.6 binding to hyperphosphorylated RyR2 and improved cardiac function in dogs with pacing-induced heart failure.244 In guinea pigs245 and rats,246 JTV-519 prevented myocardial injury caused by ischemia, and provided more-effective myocardial protection than Ca2+-channel blockers and 247 In one study, the presence of FKBP12.6 β1-AR blockers. was not required for JTV-519 function and the drug did not prevent delayed after-depolarizations and ventricular arrhythmias in a mouse model of CPVT.248 The suggestion that FKBP12.6 might be necessary for RyR2 conformational stabilization during the closed state is still controversial. However, JTV-519 has clear beneficial effects in heart failure and most studies have shown that the drug improves arrhythmia induced by defective RyR2 protein.
Rycals (CPU0213, S107, and S44121) are drugs that stabilize RyR2–FKBP complexes and inhibit Ca2+ leakage from the sarcoplasmic reticulum; they could, therefore, also have beneficial effects in patients with heart failure.249 In one study, the rycal S107 reduced biochemical and histological evidence of skeletal muscle damage, improved skeletal muscle function, and increased exercise performance in wild-type mice subjected to intensive exercise and in a mouse model of Duchenne muscular dystrophy.250 To our knowledge, cardioprotective effects of rycal drugs have not yet been shown. However, the efficacy of S44121 is currently being evaluated in patients with chronic heart failure who are at risk of ventricular arrhythmias.251 Suppression of CaMKII hyperactivity after chronic β-AR stimulation has been suggested as a therapy for heart failure. However, whether elevated CaMKII activity is a causative factor in the development of heart failure or a consequence of the disease is not clear.252 Pharmacological inhibition of CaMKII using KN-92 and KN-93 reduced RyR2 phosphorylation and Ca2+ leak from the sarcoplasmic reticulum in human myocardium.253 However, these drugs are not specific inhibitors of CaMKII, but rather inhibit all CaMK iso-forms, as well as LTCC.254 Peptide inhibitors of CaMKII have low potency as well as poor specificity (they also inhibit other CaMKs and PKA).255 Cardiac-specific CaMKII inhibitors that could be used to treat patients with steady-state heart failure are not currently available.
Another potential target for heart failure therapy is the RyR2-stabilizing protein, S100A1. Reduced S100A1 expression has been implicated in cardiomyopathies,256 and animal models have shown the potential of S100A1 gene therapy for treatment of heart failure.257–259 For example, in rat models of heart failure, AAV6-mediated overexpression of S100A1 improved cardiac function parameters, including ejection fraction, and reversed left ventricular remodeling.259 Adenovirus-mediated S100A1 overexpression also improved the function of failing cardiac myocytes isolated from patients with ischemic heart failure.260 This result suggests that S100A1 could be a therapeutic target in human heart failure. The safety and efficacy of coronary venous AAV9–S100A1 delivery has been shown in a pig model of postischemic heart failure; contractile function and cardiac remodeling were significantly improved.261 However, this system has not yet been evaluated in human heart failure.
Conclusions
Improved understanding of the mechanisms of Ca2+ cycling in cardiac cells has led to the development of novel therapeutic strategies for heart failure. These strategies include the targeting of proteins implicated in Ca2+ cycling abnormalities, such as Ca2+-handling proteins in the sarcoplasmic reticulum. Targeting these proteins to enhance Ca2+ uptake into the sarcoplasmic reticulum or block sarcoplasmic reticulum Ca2+ leakage could have substantial therapeutic benefits in patients with heart failure. Several novel therapies are currently being evaluated in animal models, and the beneficial effects of SERCA2a-targeted therapies in patients with heart failure have been shown in clinical trials. A focus on Ca2+-handling proteins will ultimately guide the future development of novel treatment modalities for patients with heart failure.
Key points.
Ca2+ cycling defects in cardiac myocytes are a hallmark of heart failure
Ca2+-handling abnormalities in failing cardiac myocytes include reduced Ca2+ uptake, decreased sarcoplasmic reticulum Ca2+ sequestration, and defective Ca2+ release from the sarcoplasmic reticulum, resulting in cytosolic Ca2+ overload
Changes in the expression and activity of Ca2+-handling proteins have been described in patients with chronic heart failure
Restoration of sarcoplasmic reticulum Ca2+ uptake through activation of sarcoplasmic/endoplasmic reticulum calcium ATPase 2a is a promising strategy for the treatment of patients with heart failure
Reducing Ca2+ leakage from the sarcoplasmic reticulum by targeting calcium/calmodulin-dependent protein kinase type II or stabilizing ryanodine receptors is also a promising strategy for treatment of patients with heart failure
Review criteria.
A search for original articles published between 1970 and 2012 and focusing on the sarcoplasmic reticulum was performed in MEDLINE and PubMed. The search terms used were “sarcoplasmic reticulum”, “calcium”, “SERCA2a”, and “ryanodine receptor”, alone and in combination. All articles identified were English-language, full-text papers. We also searched the reference lists of identified articles for further relevant papers.
Acknowledgments
This study was supported by National Institutes of Health Grants HL100396 and NIH/NHLBI Contract HHSN268201000045C.
Footnotes
Competing interests
R. J. Hajjar declares an association with the following company: Celladon Corporation. See the article online for full details of the relationship. The other authors declare no competing interests.
Author contributions
All authors researched the data and wrote the article. R. J. Hajjar reviewed and edited the manuscript before submission.
References
- 1.de Giuli F, et al. Incidence and outcome of persons with a clinical diagnosis of heart failure in a general practice population of 696,884 in the United Kingdom. Eur J Heart Fail. 2005;7:295–302. doi: 10.1016/j.ejheart.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 2.Sliwa K, Damasceno A, Mayosi BM. Epidemiology and etiology of cardiomyopathy in Africa. Circulation. 2005;112:3577–3583. doi: 10.1161/CIRCULATIONAHA.105.542894. [DOI] [PubMed] [Google Scholar]
- 3.Jiang H, Ge J. Epidemiology and clinical management of cardiomyopathies and heart failure in China. Heart. 2009;95:1727–1731. doi: 10.1136/hrt.2008.150177. [DOI] [PubMed] [Google Scholar]
- 4.Clapham DE. Calcium signaling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 5.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 6.Rockman HA, Koch WJ, Lefkowitz RJ. Seven transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 7.Antos CL, et al. Dilated cardiomyopathy and sudden death resulting from constitutive activation of protein kinase A. Circ Res. 2001;89:997–1004. doi: 10.1161/hh2301.100003. [DOI] [PubMed] [Google Scholar]
- 8.Feldman RD, Gros R. New insights into the regulation of cAMP synthesis beyond GPCR/G protein activation: implications in cardiovascular regulation. Life Sci. 2007;81:267–271. doi: 10.1016/j.lfs.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Wittkopper K, Dobrev D, Eschenhagen T, El-Armouche A. Phosphatase-1 inhibitor-1 in physiological and pathological β-adrenoceptor signalling. Cardiovasc Res. 2011;91:392–401. doi: 10.1093/cvr/cvr058. [DOI] [PubMed] [Google Scholar]
- 10.Reinkober J, et al. Targeting GRK2 by gene therapy for heart failure: benefits above β-blockade. Gene Ther. 2012;19:686–693. doi: 10.1038/gt.2012.9. [DOI] [PubMed] [Google Scholar]
- 11.Sculptoreanu A, Rotman E, Takahashi M, Scheuer T, Catterall WA. Voltage-dependent potentiation of the activity of cardiac L-type calcium channel α1 subunits due to phosphorylation by cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1993;90:10135–10139. doi: 10.1073/pnas.90.21.10135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamp TJ, Hell JW. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res. 2000;87:1095–1102. doi: 10.1161/01.res.87.12.1095. [DOI] [PubMed] [Google Scholar]
- 13.Colyer J, Wang JH. Dependence of cardiac sarcoplasmic reticulum calcium pump activity on the phosphorylation status of phospholamban. J Biol Chem. 1991;266:17486–17493. [PubMed] [Google Scholar]
- 14.Haghighi K, Gregory KN, Kranias EG. Sarcoplasmic reticulum Ca-ATPase-phospholamban interactions and dilated cardiomyopathy. Biochem Biophys Res Commun. 2004;322:1214–1222. doi: 10.1016/j.bbrc.2004.07.164. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z, Akin BL, Jones LR. Mechanism of reversal of phospholamban inhibition of the cardiac Ca2+-ATPase by protein kinase A and by anti-phospholamban monoclonal antibody 2D12. J Biol Chem. 2007;282:20968–20976. doi: 10.1074/jbc.M703516200. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Desantiago J, Chu G, Kranias EG, Bers DM. Phosphorylation of phospholamban and troponin I in β-adrenergic-induced acceleration of cardiac relaxation. Am J Physiol Heart Circ Physiol. 2000;278:H769–H779. doi: 10.1152/ajpheart.2000.278.3.H769. [DOI] [PubMed] [Google Scholar]
- 17.Kentish JC, et al. Phosphorylation of troponin I by protein kinase A accelerates relaxation and crossbridge cycle kinetics in mouse ventricular muscle. Circ Res. 2001;88:1059–1065. doi: 10.1161/hh1001.091640. [DOI] [PubMed] [Google Scholar]
- 18.Pena JR, Wolska BM. Troponin I phosphorylation plays an important role in the relaxant effect of β-adrenergic stimulation in mouse hearts. Cardiovasc Res. 2004;61:756–763. doi: 10.1016/j.cardiores.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of β-adrenergic signaling in heart failure? Circ Res. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 20.Lyon AR, et al. Plasticity of surface structures and β2-adrenergic receptor localization in failing ventricular cardiomyocytes during recovery from heart failure. Circ Heart Fail. 2012;5:357–365. doi: 10.1161/CIRCHEARTFAILURE.111.964692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tilley DG, Rockman HA. Role of β-adrenergic receptor signaling and desensitization in heart failure: new concepts and prospects for treatment. Expert Rev Cardiovasc Ther. 2006;4:417–432. doi: 10.1586/14779072.4.3.417. [DOI] [PubMed] [Google Scholar]
- 22.Ho D, Yan L, Iwatsubo K, Vatner DE, Vatner SF. Modulation of β-adrenergic receptor signaling in heart failure and longevity: targeting adenylyl cyclase type 5. Heart Fail Rev. 2010;15:495–512. doi: 10.1007/s10741-010-9183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perreault CL, Bing OH, Brooks WW, Ransil BJ, Morgan JP. Differential effects of cardiac hypertrophy and failure on right versus left ventricular calcium activation. Circ Res. 1990;67:707–712. doi: 10.1161/01.res.67.3.707. [DOI] [PubMed] [Google Scholar]
- 24.Ungerer M, Bohm M, Elce JS, Erdmann E, Lohse MJ. Altered expression of β-adrenergic receptor kinase and β1-adrenergic receptors in the failing human heart. Circulation. 1993;87:454–463. doi: 10.1161/01.cir.87.2.454. [DOI] [PubMed] [Google Scholar]
- 25.Kiuchi K, et al. Myocardial β-adrenergic receptor function during the development of pacing-induced heart failure. J Clin Invest. 1993;91:907–914. doi: 10.1172/JCI116312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi DJ, Koch WJ, Hunter JJ, Rockman HA. Mechanism of β-adrenergic receptor desensitization in cardiac hypertrophy is increased β-adrenergic receptor kinase. J Biol Chem. 1997;272:17223–17229. doi: 10.1074/jbc.272.27.17223. [DOI] [PubMed] [Google Scholar]
- 27.Woo AY, Xiao RP. β-adrenergic receptor subtype signaling in heart: from bench to bedside. Acta Pharmacol Sin. 2012;33:335–341. doi: 10.1038/aps.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grimm M, Brown JH. β-adrenergic receptor signaling in the heart: role of CaMKII. J Mol Cell Cardiol. 2009;48:322–330. doi: 10.1016/j.yjmcc.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilkins BJ, et al. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 30.Zhang CL, et al. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell. 2002;110:479–488. doi: 10.1016/s0092-8674(02)00861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voelkers M, et al. Orai1 and Stim1 regulate normal and hypertrophic growth in cardiomyocytes. J Mol Cell Cardiol. 2010;48:1329–1334. doi: 10.1016/j.yjmcc.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hulot JS, et al. Critical role for stromal interaction molecule 1 in cardiac hypertrophy. Circulation. 2011;124:796–805. doi: 10.1161/CIRCULATIONAHA.111.031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buraei Z, Yang J. The β subunit of voltage-gated Ca2+ channels. Physiol Rev. 2010;90:1461–1506. doi: 10.1152/physrev.00057.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andrade A, et al. The subunit augments α2δ functional expression and modifies the pharmacology of CaV1.3 L-type channels. Cell Calcium. 2009;46:282–292. doi: 10.1016/j.ceca.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benitah JP, Alvarez JL, Gomez AM. L-type Ca2+ current in ventricular cardiomyocytes. J Mol Cell Cardiol. 2010;48:26–36. doi: 10.1016/j.yjmcc.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 36.Seisenberger C, et al. Functional embryonic cardiomyocytes after disruption of the L-type α1C (Cav1.2) calcium channel gene in the mouse. J Biol Chem. 2000;275:39193–39199. doi: 10.1074/jbc.M006467200. [DOI] [PubMed] [Google Scholar]
- 37.Weissgerber P, et al. Reduced cardiac L-type Ca2+ current in CaVβ2−/− embryos impairs cardiac development and contraction with secondary defects in vascular maturation. Circ Res. 2006;99:749–757. doi: 10.1161/01.RES.0000243978.15182.c1. [DOI] [PubMed] [Google Scholar]
- 38.Semsarian C, et al. The L-type calcium channel inhibitor diltiazem prevents cardiomyopathy in a mouse model. J Clin Invest. 2002;109:1013–1020. doi: 10.1172/JCI14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liao Y, et al. Benidipine, a long-acting calcium channel blocker, inhibits cardiac remodeling in pressure-overloaded mice. Cardiovasc Res. 2005;65:879–888. doi: 10.1016/j.cardiores.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 40.Mukherjee R, Spinale FG. L-type calcium channel abundance and function with cardiac hypertrophy and failure: a review. J Mol Cell Cardiol. 1998;30:1899–1916. doi: 10.1006/jmcc.1998.0755. [DOI] [PubMed] [Google Scholar]
- 41.Goonasekera SA, et al. Decreased cardiac L-type Ca2+ channel activity induces hypertrophy and heart failure in mice. J Clin Invest. 2012;122:280–290. doi: 10.1172/JCI58227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schroder F, et al. Increased availability and open probability of single L-type calcium channels from failing compared with nonfailing human ventricle. Circulation. 1998;98:969–976. doi: 10.1161/01.cir.98.10.969. [DOI] [PubMed] [Google Scholar]
- 43.Chen X, et al. L-type Ca2+ channel density and regulation are altered in failing human ventricular myocytes and recover after support with mechanical assist devices. Circ Res. 2002;91:517–524. doi: 10.1161/01.res.0000033988.13062.7c. [DOI] [PubMed] [Google Scholar]
- 44.Mahe I, Chassany O, Grenard AS, Caulin C, Bergmann JF. Defining the role of calcium channel antagonists in heart failure due to systolic dysfunction. Am J Cardiovasc Drugs. 2003;3:33–41. doi: 10.2165/00129784-200303010-00004. [DOI] [PubMed] [Google Scholar]
- 45.Luo X, et al. STIM1-dependent store-operated Ca2+ entry is required for pathological cardiac hypertrophy. J Mol Cell Cardiol. 2012;52:136–147. doi: 10.1016/j.yjmcc.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 47.Vig M, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prakriya M, et al. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 49.Liou J, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roos J, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang SL, et al. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci USA. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell. 2008;135:110–122. doi: 10.1016/j.cell.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 53.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: an initiation mechanism for capacitive Ca2+ entry. J Biol Chem. 2006;281:35855–35862. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- 55.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park CY, et al. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gwack Y, et al. A genome-wide drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT. Nature. 2006;441:646–650. doi: 10.1038/nature04631. [DOI] [PubMed] [Google Scholar]
- 58.Aubart FC, et al. RNA interference targeting STIM1 suppresses vascular smooth muscle cell proliferation and neointima formation in the rat. Mol Ther. 2009;17:455–462. doi: 10.1038/mt.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kurebayashi N, Ogawa Y. Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J Physiol. 2001;533:185–199. doi: 10.1111/j.1469-7793.2001.0185b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pan Z, et al. Dysfunction of store-operated calcium channel in muscle cells lacking mg29. Nat Cell Biol. 2002;4:379–383. doi: 10.1038/ncb788. [DOI] [PubMed] [Google Scholar]
- 61.Launikonis BS, Barnes M, Stephenson DG. Identification of the coupling between skeletal muscle store-operated Ca2+ entry and the inositol trisphosphate receptor. Proc Natl Acad Sci USA. 2003;100:2941–2944. doi: 10.1073/pnas.0536227100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gibson A, McFadzean I, Wallace P, Wayman CP. Capacitative Ca2+ entry and the regulation of smooth muscle tone. Trends Pharmacol Sci. 1998;19:266–269. doi: 10.1016/s0165-6147(98)01222-x. [DOI] [PubMed] [Google Scholar]
- 63.Leung FP, Yung LM, Yao X, Laher I, Huang Y. Store-operated calcium entry in vascular smooth muscle. Br J Pharmacol. 2008;153:846–857. doi: 10.1038/sj.bjp.0707455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zanou N, et al. Role of TRPC1 channel in skeletal muscle function. Am J Physiol Cell Physiol. 2010;298:C149–C162. doi: 10.1152/ajpcell.00241.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ohba T, et al. Essential role of STIM1 in the development of cardiomyocyte hypertrophy. Biochem Biophys Res Commun. 2009;389:172–176. doi: 10.1016/j.bbrc.2009.08.117. [DOI] [PubMed] [Google Scholar]
- 66.Mulieri LA, Hasenfuss G, Leavitt B, Allen PD, Alpert NR. Altered myocardial force-frequency relation in human heart failure. Circulation. 1992;85:1743–1750. doi: 10.1161/01.cir.85.5.1743. [DOI] [PubMed] [Google Scholar]
- 67.Pieske B, et al. Alterations in intracellular calcium handling associated with the inverse force-frequency relation in human dilated cardiomyopathy. Circulation. 1995;92:1169–1178. doi: 10.1161/01.cir.92.5.1169. [DOI] [PubMed] [Google Scholar]
- 68.Mercadier JJ, et al. Altered sarcoplasmic reticulum Ca2+-ATPase gene expression in the human ventricle during end-stage heart failure. J Clin Invest. 1990;85:305–309. doi: 10.1172/JCI114429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morgan JP. Abnormal intracellular modulation of calcium as a major cause of cardiac contractile dysfunction. N Engl J Med. 1991;325:625–632. doi: 10.1056/NEJM199108293250906. [DOI] [PubMed] [Google Scholar]
- 70.Lindner M, Erdmann E, Beuckelmann DJ. Calcium content of the sarcoplasmic reticulum in isolated ventricular myocytes from patients with terminal heart failure. J Mol Cell Cardiol. 1998;30:743–749. doi: 10.1006/jmcc.1997.0626. [DOI] [PubMed] [Google Scholar]
- 71.Campbell AM, Kessler PD, Fambrough DM. The alternative carboxyl termini of avian cardiac and brain sarcoplasmic reticulum/endoplasmic reticulum Ca2+-ATPases are on opposite sides of the membrane. J Biol Chem. 1992;267:9321–9325. [PubMed] [Google Scholar]
- 72.Verboomen H, Wuytack F, Van den Bosch L, Mertens L, Casteels R. The functional importance of the extreme C-terminal tail in the gene 2 organellar Ca2+-transport ATPase (SERCA2a/b) Biochem J. 1994;303:979–984. doi: 10.1042/bj3030979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Verboomen H, Wuytack F, De Smedt H, Himpens B, Casteels R. Functional difference between SERCA2a and SERCA2b Ca2+ pumps and their modulation by phospholamban. Biochem J. 1992;286:591–595. doi: 10.1042/bj2860591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gélébart P, Martin V, Enouf J, Papp B. Identification of a new SERCA2 splice variant regulated during monocytic differentiation. Biochem Biophys Res Commun. 2003;303:676–684. doi: 10.1016/s0006-291x(03)00405-4. [DOI] [PubMed] [Google Scholar]
- 75.Dally S, et al. Ca2+-ATPases in non-failing and failing heart: evidence for a novel cardiac sarco/endoplasmic reticulum Ca2+-ATPase 2 isoform (SERCA2c) Biochem J. 2006;395:249–258. doi: 10.1042/BJ20051427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arai M, Matsui H, Periasamy M. Sarcoplasmic reticulum gene expression in cardiac hypertrophy and heart failure. Circ Res. 1994;74:555–564. doi: 10.1161/01.res.74.4.555. [DOI] [PubMed] [Google Scholar]
- 77.Feldman AM, Weinberg EO, Ray PE, Lorell BH. Selective changes in cardiac gene expression during compensated hypertrophy and the transition to cardiac decompensation in rats with chronic aortic banding. Circ Res. 1993;73:184–192. doi: 10.1161/01.res.73.1.184. [DOI] [PubMed] [Google Scholar]
- 78.Kiss E, Ball NA, Kranias EG, Walsh RA. Differential changes in cardiac phospholamban and sarcoplasmic reticular Ca2+-ATPase protein levels. Effects on Ca2+ transport and mechanics in compensated pressure-overload hypertrophy and congestive heart failure. Circ Res. 1995;77:759–764. doi: 10.1161/01.res.77.4.759. [DOI] [PubMed] [Google Scholar]
- 79.Zarain-Herzberg A, Rupp H, Elimban V, Dhalla NS. Modification of sarcoplasmic reticulum gene expression in pressure overload cardiac hypertrophy by etomoxir. FASEB J. 1996;10:1303–1309. doi: 10.1096/fasebj.10.11.8836044. [DOI] [PubMed] [Google Scholar]
- 80.Zarain-Herzberg A, Afzal N, Elimban V, Dhalla NS. Decreased expression of cardiac sarcoplasmic reticulum Ca2+-pump ATPase in congestive heart failure due to myocardial infarction. Mol Cell Biochem. 1996;163–164:285–290. doi: 10.1007/BF00408669. [DOI] [PubMed] [Google Scholar]
- 81.Guo X, Chapman D, Dhalla NS. Partial prevention of changes in SR gene expression in congestive heart failure due to myocardial infarction by enalapril or losartan. Mol Cell Biochem. 2003;254:163–172. doi: 10.1023/a:1027321130997. [DOI] [PubMed] [Google Scholar]
- 82.Arai M, Alpert NR, MacLennan DH, Barton P, Periasamy M. Alterations in sarcoplasmic reticulum gene expression in human heart failure. A possible mechanism for alterations in systolic and diastolic properties of the failing myocardium. Circ Res. 1993;72:463–469. doi: 10.1161/01.res.72.2.463. [DOI] [PubMed] [Google Scholar]
- 83.Hasenfuss G, et al. Relation between myocardial function and expression of sarcoplasmic reticulum Ca2+-ATPase in failing and nonfailing human myocardium. Circ Res. 1994;75:434–442. doi: 10.1161/01.res.75.3.434. [DOI] [PubMed] [Google Scholar]
- 84.Koss KL, Kranias EG. Phospholamban: a prominent regulator of myocardial contractility. Circ Res. 1996;79:1059–1063. doi: 10.1161/01.res.79.6.1059. [DOI] [PubMed] [Google Scholar]
- 85.Brittsan AG, Kranias EG. Phospholamban and cardiac contractile function. J Mol Cell Cardiol. 2000;32:2131–2139. doi: 10.1006/jmcc.2000.1270. [DOI] [PubMed] [Google Scholar]
- 86.Movsesian MA, Karimi M, Green K, Jones LR. Ca2+-transporting ATPase, phospholamban, and calsequestrin levels in nonfailing and failing human myocardium. Circulation. 1994;90:653–657. doi: 10.1161/01.cir.90.2.653. [DOI] [PubMed] [Google Scholar]
- 87.Schwinger RH, et al. Unchanged protein levels of SERCA II and phospholamban but reduced Ca2+ uptake and Ca2+-ATPase activity of cardiac sarcoplasmic reticulum from dilated cardiomyopathy patients compared with patients with nonfailing hearts. Circulation. 1995;92:3220–3228. doi: 10.1161/01.cir.92.11.3220. [DOI] [PubMed] [Google Scholar]
- 88.Linck B, et al. Messenger RNA expression and immunological quantification of phospholamban and SR-Ca2+-ATPase in failing and nonfailing human hearts. Cardiovasc Res. 1996;31:625–632. [PubMed] [Google Scholar]
- 89.Flesch M, et al. Sarcoplasmic reticulum Ca2+ ATPase and phospholamban mRNA and protein levels in end-stage heart failure due to ischemic or dilated cardiomyopathy. J Mol Med (Berl) 1996;74:321–332. doi: 10.1007/BF00207509. [DOI] [PubMed] [Google Scholar]
- 90.Meyer M, et al. Alterations of sarcoplasmic reticulum proteins in failing human dilated cardiomyopathy. Circulation. 1995;92:778–784. doi: 10.1161/01.cir.92.4.778. [DOI] [PubMed] [Google Scholar]
- 91.Kranias EG, Hajjar RJ. Modulation of cardiac contractility by the phopholamban/SERCA2a regulatome. Circ Res. 2012;110:1646–1660. doi: 10.1161/CIRCRESAHA.111.259754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sande JB, et al. Reduced level of serine 16 phosphorylated phospholamban in the failing rat myocardium: a major contributor to reduced SERCA2 activity. Cardiovasc Res. 2002;53:382–391. doi: 10.1016/s0008-6363(01)00489-8. [DOI] [PubMed] [Google Scholar]
- 93.Netticadan T, Temsah RM, Kawabata K, Dhalla NS. Sarcoplasmic reticulum Ca2+/Calmodulin-dependent protein kinase is altered in heart failure. Circ Res. 2000;86:596–605. doi: 10.1161/01.res.86.5.596. [DOI] [PubMed] [Google Scholar]
- 94.Mishra S, Sabbah HN, Jain JC, Gupta RC. Reduced Ca2+-calmodulin-dependent protein kinase activity and expression in LV myocardium of dogs with heart failure. Am J Physiol Heart Circ Physiol. 2003;284:H876–H883. doi: 10.1152/ajpheart.00266.2002. [DOI] [PubMed] [Google Scholar]
- 95.Huang B, Wang S, Qin D, Boutjdir M, El-Sherif N. Diminished basal phosphorylation level of phospholamban in the postinfarction remodeled rat ventricle: role of β-adrenergic pathway, Gi protein, phosphodiesterase, and phosphatases. Circ Res. 1999;85:848–855. doi: 10.1161/01.res.85.9.848. [DOI] [PubMed] [Google Scholar]
- 96.Schwinger RH, et al. Reduced Ca2+-sensitivity of SERCA2a in failing human myocardium due to reduced serin-16 phospholamban phosphorylation. J Mol Cell Cardiol. 1999;31:479–491. doi: 10.1006/jmcc.1998.0897. [DOI] [PubMed] [Google Scholar]
- 97.Nicolaou P, Hajjar RJ, Kranias EG. Role of protein phosphatase-1 inhibitor-1 in cardiac physiology and pathophysiology. J Mol Cell Cardiol. 2009;47:365–371. doi: 10.1016/j.yjmcc.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yamada M, et al. Inhibition of protein phosphatase 1 by inhibitor-2 gene delivery ameliorates heart failure progression in genetic cardiomyopathy. FASEB J. 2006;20:1197–1199. doi: 10.1096/fj.05-5299fje. [DOI] [PubMed] [Google Scholar]
- 99.Medeiros A, et al. Mutations in the human phospholamban gene in patients with heart failure. Am Heart J. 2011;162:1088.e1–1095.e1. doi: 10.1016/j.ahj.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 100.Schmitt JP, et al. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science. 2003;299:1410–1413. doi: 10.1126/science.1081578. [DOI] [PubMed] [Google Scholar]
- 101.Haghighi K, et al. A mutation in the human phospholamban gene, deleting arginine 14, results in lethal, hereditary cardiomyopathy. Proc Natl Acad Sci USA. 2006;103:1388–1393. doi: 10.1073/pnas.0510519103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Haghighi K, et al. Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J Clin Invest. 2003;111:869–876. doi: 10.1172/JCI17892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bers DM, Weber CR. Na/Ca exchange function in intact ventricular myocytes. Ann N Y Acad Sci. 2002;976:500–512. doi: 10.1111/j.1749-6632.2002.tb04784.x. [DOI] [PubMed] [Google Scholar]
- 104.Studer R, et al. Gene expression of the cardiac Na+-Ca2+ exchanger in end-stage human heart failure. Circ Res. 1994;75:443–453. doi: 10.1161/01.res.75.3.443. [DOI] [PubMed] [Google Scholar]
- 105.Hasenfuss G, et al. Relationship between Na+-Ca2+-exchanger protein levels and diastolic function of failing human myocardium. Circulation. 1999;99:641–648. doi: 10.1161/01.cir.99.5.641. [DOI] [PubMed] [Google Scholar]
- 106.Schwinger RH, et al. Reduced sodium pump α1, α3, and β1-isoform protein levels and Na+, K+-ATPase activity but unchanged Na+-Ca2+ exchanger protein levels in human heart failure. Circulation. 1999;99:2105–2112. doi: 10.1161/01.cir.99.16.2105. [DOI] [PubMed] [Google Scholar]
- 107.Piper C, et al. Is myocardial Na+/Ca2+ exchanger transcription a marker for different stages of myocardial dysfunction? Quantitative polymerase chain reaction of the messenger RNA in endomyocardial biopsies of patients with heart failure. J Am Coll Cardiol. 2000;36:233–241. doi: 10.1016/s0735-1097(00)00703-8. [DOI] [PubMed] [Google Scholar]
- 108.Flesch M, et al. Evidence for functional relevance of an enhanced expression of the Na+–Ca2+ exchanger in failing human myocardium. Circulation. 1996;94:992–1002. doi: 10.1161/01.cir.94.5.992. [DOI] [PubMed] [Google Scholar]
- 109.Reinecke H, Studer R, Vetter R, Holtz I, Drexler H. Cardiac Na+/Ca2+ exchange activity in patients with end-stage heart failure. Cardiovasc Res. 1996;31:48–54. [PubMed] [Google Scholar]
- 110.Piacentino V, 3rd, et al. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res. 2003;92:651–658. doi: 10.1161/01.RES.0000062469.83985.9B. [DOI] [PubMed] [Google Scholar]
- 111.Weber CR, Piacentino V, 3rd, Houser SR, Bers DM. Dynamic regulation of sodium/calcium exchange function in human heart failure. Circulation. 2003;108:2224–2229. doi: 10.1161/01.CIR.0000095274.72486.94. [DOI] [PubMed] [Google Scholar]
- 112.Bers DM, Eisner DA, Valdivia HH. Sarcoplasmic reticulum Ca2+ and heart failure: roles of diastolic leak and Ca2+ transport. Circ Res. 2003;93:487–490. doi: 10.1161/01.RES.0000091871.54907.6B. [DOI] [PubMed] [Google Scholar]
- 113.Györke S, Stevens SC, Terentyev D. Cardiac calsequestrin: quest inside the SR. J Physiol. 2009;587 (Pt 13):3091–3094. doi: 10.1113/jphysiol.2009.172049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Györke S, Terentyev D. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc Res. 2008;77:245–255. doi: 10.1093/cvr/cvm038. [DOI] [PubMed] [Google Scholar]
- 115.Knollmann BC. New roles of calsequestrin and triadin in cardiac muscle. J Physiol. 2009;587:3081–3087. doi: 10.1113/jphysiol.2009.172098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kontula K, et al. Catecholaminergic polymorphic ventricular tachycardia: recent mechanistic insights. Cardiovasc Res. 2005;67:379–387. doi: 10.1016/j.cardiores.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 117.Postma AV, et al. Absence of calsequestrin 2 causes severe forms of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2002;91:e21–e26. doi: 10.1161/01.res.0000038886.18992.6b. [DOI] [PubMed] [Google Scholar]
- 118.Lahat H, et al. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am J Hum Genet. 2001;69:1378–1384. doi: 10.1086/324565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Song L, et al. Calsequestrin 2 (CASQ2) mutations increase expression of calreticulin and ryanodine receptors, causing catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2007;117:1814–1823. doi: 10.1172/JCI31080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kiarash A, et al. Defective glycosylation of calsequestrin in heart failure. Cardiovasc Res. 2004;63:264–272. doi: 10.1016/j.cardiores.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 121.Houle TD, Ram ML, McMurray WJ, Cala SE. Different endoplasmic reticulum trafficking and processing pathways for calsequestrin (CSQ) and epitope-tagged CSQ. Exp Cell Res. 2006;312:4150–4161. doi: 10.1016/j.yexcr.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 122.McFarland TP, Milstein ML, Cala SE. Rough endoplasmic reticulum to junctional sarcoplasmic reticulum trafficking of calsequestrin in adult cardiomyocytes. J Mol Cell Cardiol. 2010;49:556–564. doi: 10.1016/j.yjmcc.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang L, Kelley J, Schmeisser G, Kobayashi YM, Jones LR. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J Biol Chem. 1997;272:23389–23397. doi: 10.1074/jbc.272.37.23389. [DOI] [PubMed] [Google Scholar]
- 124.Gergs U, et al. On the role of junctin in cardiac Ca2+ handling, contractility, and heart failure. Am J Physiol Heart Circ Physiol. 2007;293:H728–H734. doi: 10.1152/ajpheart.01187.2006. [DOI] [PubMed] [Google Scholar]
- 125.Terentyev D, et al. Triadin overexpression stimulates excitation–contraction coupling and increases predisposition to cellular arrhythmia in cardiac myocytes. Circ Res. 2005;96:651–658. doi: 10.1161/01.RES.0000160609.98948.25. [DOI] [PubMed] [Google Scholar]
- 126.Engelhardt S, et al. Early impairment of calcium handling and altered expression of junctin in hearts of mice overexpressing the β1-adrenergic receptor. FASEB J. 2001;15:2718–2720. doi: 10.1096/fj.01-0107fje. [DOI] [PubMed] [Google Scholar]
- 127.Pritchard TJ, Kranias EG. Junctin and the histidine-rich Ca2+ binding protein: potential roles in heart failure and arrhythmogenesis. J Physiol. 2009;587:3125–3133. doi: 10.1113/jphysiol.2009.172171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fan GC, Gregory KN, Zhao W, Park WJ, Kranias EG. Regulation of myocardial function by histidine-rich, calcium-binding protein. Am J Physiol Heart Circ Physiol. 2004;287:H1705–H1711. doi: 10.1152/ajpheart.01211.2003. [DOI] [PubMed] [Google Scholar]
- 129.Arvanitis DA, et al. The Ser96Ala variant in histidine-rich calcium-binding protein is associated with life-threatening ventricular arrhythmias in idiopathic dilated cardiomyopathy. Eur Heart J. 2008;29:2514–2525. doi: 10.1093/eurheartj/ehn328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kiewitz R, Lyons GE, Schafer BW, Heizmann CW. Transcriptional regulation of S100A1 and expression during mouse heart development. Biochim Biophys Acta. 2000;1498:207–219. doi: 10.1016/s0167-4889(00)00097-5. [DOI] [PubMed] [Google Scholar]
- 131.Volkers M, et al. S100A1 decreases calcium spark frequency and alters their spatial characteristics in permeabilized adult ventricular cardiomyocytes. Cell Calcium. 2007;41:135–143. doi: 10.1016/j.ceca.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 132.Remppis A, et al. Altered expression of the Ca2+-binding protein S100A1 in human cardiomyopathy. Biochim Biophys Acta. 1996;1313:253–257. doi: 10.1016/0167-4889(96)00097-3. [DOI] [PubMed] [Google Scholar]
- 133.Most P, et al. Transgenic overexpression of the Ca2+-binding protein S100A1 in the heart leads to increased in vivo myocardial contractile performance. J Biol Chem. 2003;278:33809–33817. doi: 10.1074/jbc.M301788200. [DOI] [PubMed] [Google Scholar]
- 134.Kettlewell S, Most P, Currie S, Koch WJ, Smith GL. S100A1 increases the gain of excitation–contraction coupling in isolated rabbit ventricular cardiomyocytes. J Mol Cell Cardiol. 2005;39:900–910. doi: 10.1016/j.yjmcc.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 135.Du XJ, et al. Impaired cardiac contractility response to hemodynamic stress in S100A1-deficient mice. Mol Cell Biol. 2002;22:2821–2829. doi: 10.1128/MCB.22.8.2821-2829.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Boerries M, et al. Ca2+-dependent interaction of S100A1 with F1-ATPase leads to an increased ATP content in cardiomyocytes. Mol Cell Biol. 2007;27:4365–4373. doi: 10.1128/MCB.02045-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wehrens XH, Marks AR. Altered function and regulation of cardiac ryanodine receptors in cardiac disease. Trends Biochem Sci. 2003;28:671–678. doi: 10.1016/j.tibs.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 138.Kushnir A, Marks AR. The ryanodine receptor in cardiac physiology and disease. Adv Pharmacol. 2010;59:1–30. doi: 10.1016/S1054-3589(10)59001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Avila G, O’Connell KM, Groom LA, Dirksen RT. Ca2+ release through ryanodine receptors regulates skeletal muscle L-type Ca2+ channel expression. J Biol Chem. 2001;276:17732–17738. doi: 10.1074/jbc.M009685200. [DOI] [PubMed] [Google Scholar]
- 140.Lanner JT, Georgiou DK, Joshi AD, Hamilton SL. Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol. 2010;2:a003996. doi: 10.1101/cshperspect.a003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Song DW, Lee JG, Youn HS, Eom SH, Kim do H. Ryanodine receptor assembly: a novel systems biology approach to 3D mapping. Prog Biophys Mol Biol. 2011;105:145–161. doi: 10.1016/j.pbiomolbio.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 142.Balshaw DM, Xu L, Yamaguchi N, Pasek DA, Meissner G. Calmodulin binding and inhibition of cardiac muscle calcium release channel (ryanodine receptor) J Biol Chem. 2001;276:20144–20153. doi: 10.1074/jbc.M010771200. [DOI] [PubMed] [Google Scholar]
- 143.Zhang T, et al. The δC isoform of CAMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res. 2003;92:912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 144.Sharma MR, Jeyakumar LH, Fleischer S, Wagenknecht T. Three-dimensional visualization of FKBP12.6 binding to an open conformation of cardiac ryanodine receptor. Biophys J. 2006;90:164–172. doi: 10.1529/biophysj.105.063503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xiao B, et al. Ser-2030, but not Ser-2808, is the major phosphorylation site in cardiac ryanodine receptors responding to protein kinase A activation upon β-adrenergic stimulation in normal and failing hearts. Biochem J. 2006;396:7–16. doi: 10.1042/BJ20060116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Marx SO, et al. Phosphorylation-dependent regulation of ryanodine receptors: a novel role for leucine/isoleucine zippers. J Cell Biol. 2001;153:699–708. doi: 10.1083/jcb.153.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lee EH, et al. N-terminal region of FKBP12 is essential for binding to the skeletal ryanodine receptor. J Biol Chem. 2004;279:26481–26488. doi: 10.1074/jbc.M309574200. [DOI] [PubMed] [Google Scholar]
- 148.Shan J, et al. Role of chronic ryanodine receptor phosphorylation in heart failure and β-adrenergic receptor blockade in mice. J Clin Invest. 2010;120:4375–4387. doi: 10.1172/JCI37649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shannon TR, Lew WY. Diastolic release of calcium from the sarcoplasmic reticulum: a potential target for treating triggered arrhythmias and heart failure. J Am Coll Cardiol. 2009;53:2006–2008. doi: 10.1016/j.jacc.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 150.Go LO, et al. Differential regulation of two types of intracellular calcium release channels during end-stage heart failure. J Clin Invest. 1995;95:888–894. doi: 10.1172/JCI117739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sainte Beuve C, et al. Cardiac calcium release channel (ryanodine receptor) in control and cardiomyopathic human hearts: mRNA and protein contents are differentially regulated. J Mol Cell Cardiol. 1997;29:1237–1246. doi: 10.1006/jmcc.1996.0360. [DOI] [PubMed] [Google Scholar]
- 152.Hain J, Onoue H, Mayrleitner M, Fleischer S, Schindler H. Phosphorylation modulates the function of the calcium release channel of sarcoplasmic reticulum from cardiac muscle. J Biol Chem. 1995;270:2074–2081. doi: 10.1074/jbc.270.5.2074. [DOI] [PubMed] [Google Scholar]
- 153.Marx SO, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 154.Valdivia HH, Kaplan JH, Ellis-Davies GC, Lederer WJ. Rapid adaptation of cardiac ryanodine receptors: modulation by Mg2+ and phosphorylation. Science. 1995;267:1997–2000. doi: 10.1126/science.7701323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xiao B, et al. Characterization of a novel PKA phosphorylation site, serine-2030, reveals no PKA hyperphosphorylation of the cardiac ryanodine receptor in canine heart failure. Circ Res. 2005;96:847–855. doi: 10.1161/01.RES.0000163276.26083.e8. [DOI] [PubMed] [Google Scholar]
- 156.Reiken S, et al. PKA phosphorylation activates the calcium release channel (ryanodine receptor) in skeletal muscle: defective regulation in heart failure. J Cell Biol. 2003;160:919–928. doi: 10.1083/jcb.200211012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yano M, et al. Altered stoichiometry of FKBP12.6 versus ryanodine receptor as a cause of abnormal Ca2+ leak through ryanodine receptor in heart failure. Circulation. 2000;102:2131–2136. doi: 10.1161/01.cir.102.17.2131. [DOI] [PubMed] [Google Scholar]
- 158.Ono K, et al. Altered interaction of FKBP12.6 with ryanodine receptor as a cause of abnormal Ca2+ release in heart failure. Cardiovasc Res. 2000;48:323–331. doi: 10.1016/s0008-6363(00)00191-7. [DOI] [PubMed] [Google Scholar]
- 159.Priori SG, et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 160.Cerrone M, et al. Bidirectional ventricular tachycardia and fibrillation elicited in a knock-in mouse model carrier of a mutation in the cardiac ryanodine receptor. Circ Res. 2005;96:e77–e82. doi: 10.1161/01.RES.0000169067.51055.72. [DOI] [PubMed] [Google Scholar]
- 161.Nicol RL, Frey N, Olson EN. From the sarcomere to the nucleus: role of genetics and signaling in structural heart disease. Annu Rev Genomics Hum Genet. 2000;1:179–223. doi: 10.1146/annurev.genom.1.1.179. [DOI] [PubMed] [Google Scholar]
- 162.Li H, Rao A, Hogan PG. Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol. 2010;21:91–103. doi: 10.1016/j.tcb.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Molkentin JD. Calcineurin and beyond: cardiac hypertrophic signaling. Circ Res. 2000;87:731–738. doi: 10.1161/01.res.87.9.731. [DOI] [PubMed] [Google Scholar]
- 164.Molkentin JD. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res. 2004;63:467–475. doi: 10.1016/j.cardiores.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 165.Molkentin JD, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Molkentin JD, Olson EN. GATA4: a novel transcriptional regulator of cardiac hypertrophy? Circulation. 1997;96:3833–3835. [PubMed] [Google Scholar]
- 167.Lim HW, et al. Reversal of cardiac hypertrophy in transgenic disease models by calcineurin inhibition. J Mol Cell Cardiol. 2000;32:697–709. doi: 10.1006/jmcc.2000.1113. [DOI] [PubMed] [Google Scholar]
- 168.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 169.Meguro T, et al. Cyclosporine attenuates pressure-overload hypertrophy in mice while enhancing susceptibility to decompensation and heart failure. Circ Res. 1999;84:735–740. doi: 10.1161/01.res.84.6.735. [DOI] [PubMed] [Google Scholar]
- 170.Sussman MA, et al. Prevention of cardiac hypertrophy in mice by calcineurin inhibition. Science. 1998;281:1690–1693. doi: 10.1126/science.281.5383.1690. [DOI] [PubMed] [Google Scholar]
- 171.Ding B, et al. Pressure overload induces severe hypertrophy in mice treated with cyclosporine, an inhibitor of calcineurin. Circ Res. 1999;84:729–734. doi: 10.1161/01.res.84.6.729. [DOI] [PubMed] [Google Scholar]
- 172.Luo Z, Shyu KG, Gualberto A, Walsh K. Calcineurin inhibitors and cardiac hypertrophy. Nat Med. 1998;4:1092–1093. doi: 10.1038/2578. [DOI] [PubMed] [Google Scholar]
- 173.Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:468–473. doi: 10.1016/j.yjmcc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hoch B, Meyer R, Hetzer R, Krause EG, Karczewski P. Identification and expression of δ-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ Res. 1999;84:713–721. doi: 10.1161/01.res.84.6.713. [DOI] [PubMed] [Google Scholar]
- 175.Kirchhefer U, Schmitz W, Scholz H, Neumann J. Activity of cAMP-dependent protein kinase and Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human hearts. Cardiovasc Res. 1999;42:254–261. doi: 10.1016/s0008-6363(98)00296-x. [DOI] [PubMed] [Google Scholar]
- 176.Maier LS, et al. Transgenic CaMKIIδC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003;92:904–911. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 177.Zhang R, et al. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 178.Wu Y, et al. Suppression of dynamic Ca2+ transient responses to pacing in ventricular myocytes from mice with genetic calmodulin kinase II inhibition. J Mol Cell Cardiol. 2006;40:213–223. doi: 10.1016/j.yjmcc.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 179.Li J, et al. Calmodulin kinase II inhibition shortens action potential duration by upregulation of K+ currents. Circ Res. 2006;99:1092–1099. doi: 10.1161/01.RES.0000249369.71709.5c. [DOI] [PubMed] [Google Scholar]
- 180.Grueter CE, Colbran RJ, Anderson ME. CaMKII, an emerging molecular driver for calcium homeostasis, arrhythmias, and cardiac dysfunction. J Mol Med (Berl) 2007;85:5–14. doi: 10.1007/s00109-006-0125-6. [DOI] [PubMed] [Google Scholar]
- 181.Bartel S, et al. Phosphorylation of phospholamban at threonine-17 in the absence and presence of β-adrenergic stimulation in neonatal rat cardiomyocytes. J Mol Cell Cardiol. 2000;32:2173–2185. doi: 10.1006/jmcc.2000.1243. [DOI] [PubMed] [Google Scholar]
- 182.Hagemann D, Xiao RP. Dual site phospholamban phosphorylation and its physiological relevance in the heart. Trends Cardiovasc Med. 2002;12:51–56. doi: 10.1016/s1050-1738(01)00145-1. [DOI] [PubMed] [Google Scholar]
- 183.Mattiazzi A, Mundiña-Weilenmann C, Guoxiang C, Vittone L, Kranias E. Role of phospholamban phosphorylation on Thr17 in cardiac physiological and pathological conditions. Cardiovasc Res. 2005;68:366–375. doi: 10.1016/j.cardiores.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 184.Brixius K, Wollmer A, Bölck B, Mehlhorn U, Schwinger RH. Ser16- but not Thr17-phosphorylation of phospholamban influences frequency-dependent force generation in human myocardium. Pflugers Arch. 2003;447:150–157. doi: 10.1007/s00424-003-1163-3. [DOI] [PubMed] [Google Scholar]
- 185.Hagemann D, et al. Frequency-encoding Thr17 phospholamban phosphorylation is independent of Ser16 phosphorylation in cardiac myocytes. J Biol Chem. 2000;275:22532–22536. doi: 10.1074/jbc.C000253200. [DOI] [PubMed] [Google Scholar]
- 186.Ji Y, et al. Targeted inhibition of Ca2+/calmodulin-dependent protein kinase II in cardiac longitudinal sarcoplasmic reticulum results in decreased phospholamban phosphorylation at threonine 17. J Biol Chem. 2003;278:25063–25071. doi: 10.1074/jbc.M302193200. [DOI] [PubMed] [Google Scholar]
- 187.Wehrens XH, et al. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004;304:292–296. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- 188.Li L, Satoh H, Ginsburg KS, Bers DM. The effect of Ca2+-calmodulin-dependent protein kinase II on cardiac excitation-contraction coupling in ferret ventricular myocytes. J Physiol. 1997;501:17–31. doi: 10.1111/j.1469-7793.1997.017bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Maier LS, et al. Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003;92:904–911. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 190.Dzhura I, Wu Y, Colbran RJ, Balser JR, Anderson ME. Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nat Cell Biol. 2000;2:173–177. doi: 10.1038/35004052. [DOI] [PubMed] [Google Scholar]
- 191.Grueter CE, et al. L-type Ca2+ channel facilitation mediated by phosphorylation of the β subunit by CaMKII. Mol Cell. 2006;23:641–650. doi: 10.1016/j.molcel.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 192.Wu Y, et al. Calmodulin kinase II and arrhythmias in a mouse model of cardiac hypertrophy. Circulation. 2002;106:1288–1293. doi: 10.1161/01.cir.0000027583.73268.e7. [DOI] [PubMed] [Google Scholar]
- 193.Muller OJ, et al. Transgenic rat hearts overexpressing SERCA2a show improved contractility under baseline conditions and pressure overload. Cardiovasc Res. 2003;59:380–389. doi: 10.1016/s0008-6363(03)00429-2. [DOI] [PubMed] [Google Scholar]
- 194.del Monte F, et al. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation. 1999;100:2308–2311. doi: 10.1161/01.cir.100.23.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Prunier F, et al. Prevention of ventricular arrhythmias with sarcoplasmic reticulum Ca2+ ATPase pump overexpression in a porcine model of ischemia reperfusion. Circulation. 2008;118:614–624. doi: 10.1161/CIRCULATIONAHA.108.770883. [DOI] [PubMed] [Google Scholar]
- 196.Kawase Y, et al. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol. 2008;51:1112–1119. doi: 10.1016/j.jacc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 197.del Monte F, et al. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca2+-ATPase in a rat model of heart failure. Circulation. 2001;104:1424–1429. doi: 10.1161/hc3601.095574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sakata S, et al. Restoration of mechanical and energetic function in failing aortic-banded rat hearts by gene transfer of calcium cycling proteins. J Mol Cell Cardiol. 2007;42:852–861. doi: 10.1016/j.yjmcc.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Miyamoto MI, et al. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc Natl Acad Sci USA. 2000;97:793–798. doi: 10.1073/pnas.97.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.del Monte F, et al. Transcriptional changes following restoration of SERCA2a levels in failing rat hearts. FASEB J. 2004;18:1474–1476. doi: 10.1096/fj.04-1714fje. [DOI] [PubMed] [Google Scholar]
- 201.Nayak S, Herzog RW. Progress and prospects: immune responses to viral vectors. Gene Ther. 2010;17:295–304. doi: 10.1038/gt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Asokan A, Schaffer DV, Samulski RJ. The AAV vector toolkit: poised at the clinical crossroads. Mol Ther. 2012;20:699–708. doi: 10.1038/mt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mariani JA, et al. Augmentation of left ventricular mechanics by recirculation-mediated AAV2/1-SERCA2a gene delivery in experimental heart failure. Eur J Heart Fail. 2011;13:247–253. doi: 10.1093/eurjhf/hfq234. [DOI] [PubMed] [Google Scholar]
- 204.del Monte F, et al. Abrogation of ventricular arrhythmias in a model of ischemia and reperfusion by targeting myocardial calcium cycling. Proc Natl Acad Sci USA. 2004;101:5622–5627. doi: 10.1073/pnas.0305778101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cutler MJ, Wan X, Laurita KR, Hajjar RJ, Rosenbaum DS. Targeted SERCA2a gene expression identifies molecular mechanism and therapeutic target for arrhythmogenic cardiac alternans. Circ Arrhythm Electrophysiol. 2009;2:686–694. doi: 10.1161/CIRCEP.109.863118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lyon AR, et al. SERCA2a gene transfer decreases sarcoplasmic reticulum calcium leak and reduces ventricular arrhythmias in a model of chronic heart failure. Circ Arrhythm Electrophysiol. 2011;4:362–372. doi: 10.1161/CIRCEP.110.961615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chen Y, et al. Constitutive cardiac overexpression of sarcoplasmic/endoplasmic reticulum Ca2+-ATPase delays myocardial failure after myocardial infarction in rats at a cost of increased acute arrhythmias. Circulation. 2004;109:1898–1903. doi: 10.1161/01.CIR.0000124230.60028.42. [DOI] [PubMed] [Google Scholar]
- 208.Davia K, et al. SERCA2a overexpression decreases the incidence of aftercontractions in adult rabbit ventricular myocytes. J Mol Cell Cardiol. 2001;33:1005–1015. doi: 10.1006/jmcc.2001.1368. [DOI] [PubMed] [Google Scholar]
- 209.Jaski BE, et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID trial), a first-in-human phase 1/2 clinical trial. J Card Fail. 2009;15:171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Jessup M, et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 2011;124:304–313. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Eizema K, et al. Adenovirus-based phospholamban antisense expression as a novel approach to improve cardiac contractile dysfunction: comparison of a constitutive viral versus an endothelin-1-responsive cardiac promoter. Circulation. 2000;101:2193–2199. doi: 10.1161/01.cir.101.18.2193. [DOI] [PubMed] [Google Scholar]
- 212.Hoshijima M, et al. Chronic suppression of heart-failure progression by a pseudophosphorylated mutant of phospholamban via in vivo cardiac rAAV gene delivery. Nat Med. 2002;8:864–871. doi: 10.1038/nm739. [DOI] [PubMed] [Google Scholar]
- 213.Dieterle T, et al. Gene transfer of a phospholamban-targeted antibody improves calcium handling and cardiac function in heart failure. Cardiovasc Res. 2005;67:678–688. doi: 10.1016/j.cardiores.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 214.Watanabe A, et al. Phospholamban ablation by RNA interference increases Ca2+ uptake into rat cardiac myocyte sarcoplasmic reticulum. J Mol Cell Cardiol. 2004;37:691–698. doi: 10.1016/j.yjmcc.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 215.Suckau L, et al. Long-term cardiac-targeted RNA interference for the treatment of heart failure restores cardiac function and reduces pathological hypertrophy. Circulation. 2009;119:1241–1252. doi: 10.1161/CIRCULATIONAHA.108.783852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Neumann J, et al. Evidence for physiological functions of protein phosphatases in the heart: evaluation with okadaic acid. Am J Physiol. 1993;265:H257–H266. doi: 10.1152/ajpheart.1993.265.1.H257. [DOI] [PubMed] [Google Scholar]
- 217.Hubbard MJ, Cohen P. On target with a new mechanism for the regulation of protein phosphorylation. Trends Biochem Sci. 1993;18:172–177. doi: 10.1016/0968-0004(93)90109-z. [DOI] [PubMed] [Google Scholar]
- 218.Steenaart NA, Ganim JR, Di Salvo J, Kranias EG. The phospholamban phosphatase associated with cardiac sarcoplasmic reticulum is a type 1 enzyme. Arch Biochem Biophys. 1992;293:17–24. doi: 10.1016/0003-9861(92)90359-5. [DOI] [PubMed] [Google Scholar]
- 219.Neumann J, et al. Increased expression of cardiac phosphatases in patients with end-stage heart failure. J Mol Cell Cardiol. 1997;29:265–272. doi: 10.1006/jmcc.1996.0271. [DOI] [PubMed] [Google Scholar]
- 220.Gupta RC, et al. Cardiac SR-coupled PP1 activity and expression are increased and inhibitor 1 protein expression is decreased in failing hearts. Am J Physiol Heart Circ Physiol. 2003;285:H2373–H2381. doi: 10.1152/ajpheart.00442.2003. [DOI] [PubMed] [Google Scholar]
- 221.Nicolaou P, Hajjar RJ, Kranias EG. Role of protein phosphatase-1 inhibitor-1 in cardiac physiology and pathophysiology. J Mol Cell Cardiol. 2009;47:365–371. doi: 10.1016/j.yjmcc.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Carr AN, et al. Type 1 phosphatase, a negative regulator of cardiac function. Mol Cell Biol. 2002;22:4124–4135. doi: 10.1128/MCB.22.12.4124-4135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.El-Armouche A, et al. Evidence for protein phosphatase inhibitor-1 playing an amplifier role in β-adrenergic signaling. FASEB J. 2003;17:437–439. doi: 10.1096/fj.02-0057fje. [DOI] [PubMed] [Google Scholar]
- 224.El-Armouche A, Pamminger T, Ditz D, Zolk O, Eschenhagen T. Decreased protein and phosphorylation level of the protein phosphatase inhibitor-1 in failing human hearts. Cardiovasc Res. 2004;61:87–93. doi: 10.1016/j.cardiores.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 225.Pathak A, et al. Enhancement of cardiac function and suppression of heart failure progression by inhibition of protein phosphatase 1. Circ Res. 2005;96:756–766. doi: 10.1161/01.RES.0000161256.85833.fa. [DOI] [PubMed] [Google Scholar]
- 226.Nicolaou P, et al. Inducible expression of active protein phosphatase-1 inhibitor-1 enhances basal cardiac function and protects against ischemia/reperfusion injury. Circ Res. 2009;104:1012–1020. doi: 10.1161/CIRCRESAHA.108.189811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wittköpper K, et al. Constitutively active phosphatase inhibitor-1 improves cardiac contractility in young mice but is deleterious after catecholaminergic stress and with aging. J Clin Invest. 2010;120:617–626. doi: 10.1172/JCI40545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kawashima H, et al. Protein phosphatase inhibitor-1 augments a protein kinase A- dependent increase in the Ca2+ loading of the sarcoplasmic reticulum without changing its Ca2+ release. Circ J. 2009;73:1133–1140. doi: 10.1253/circj.cj-08-0871. [DOI] [PubMed] [Google Scholar]
- 229.Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol. 2007;8:947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- 230.Woo CH, Abe J. SUMO—a post-translational modification with therapeutic potential? Curr Opin Pharmacol. 2010;10:146–155. doi: 10.1016/j.coph.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Jakobs PM, et al. Novel lamin A/C mutations in two families with dilated cardiomyopathy and conduction system disease. J Card Fail. 2001;7:249–256. doi: 10.1054/jcaf.2001.26339. [DOI] [PubMed] [Google Scholar]
- 232.Zhang YQ, Sarge KD. Sumoylation regulates lamin A function and is lost in lamin A mutants associated with familial cardiomyopathies. J Cell Biol. 2008;182:35–39. doi: 10.1083/jcb.200712124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kho C, et al. SUMO1-dependent modulation of SERCA2a in heart failure. Nature. 2011;477:601–605. doi: 10.1038/nature10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wehrens XH, et al. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci USA. 2006;103:511–518. doi: 10.1073/pnas.0510113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Belevych AE, et al. The relationship between arrhythmogenesis and impaired contractility in heart failure: role of altered ryanodine receptor function. Cardiovasc Res. 2011;90:493–502. doi: 10.1093/cvr/cvr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Loughrey CM, et al. Over-expression of FK506-binding protein FKBP12.6 alters excitation–contraction coupling in adult rabbit cardiomyocytes. J Physiol. 2004;556:919–934. doi: 10.1113/jphysiol.2003.057166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Huang F, Shan J, Reiken S, Wehrens XH, Marks AR. Analysis of calstabin2 (FKBP12.6)-ryanodine receptor interactions: rescue of heart failure by calstabin2 in mice. Proc Natl Acad Sci USA. 2006;103:3456–3461. doi: 10.1073/pnas.0511282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Xu L, Tripathy A, Pasek DA, Meissner G. Potential for pharmacology of ryanodine receptor/calcium release channels. Ann N Y Acad Sci. 1998;853:130–148. doi: 10.1111/j.1749-6632.1998.tb08262.x. [DOI] [PubMed] [Google Scholar]
- 239.McCauley MD, Wehrens XH. Targeting ryanodine receptors for anti-arrhythmic therapy. Acta Pharmacol Sin. 2011;32:749–757. doi: 10.1038/aps.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kaneko N. New 1,4-benzothiazepine derivative, K201, demonstrates cardioprotective effects against sudden cardiac cell death and intracellular calcium blocking action. Drug Dev Res. 1994;33:429–438. [Google Scholar]
- 241.Hunt DJ, et al. K201 (JTV519) suppresses spontaneous Ca2+ release and 3H ryanodine binding to RyR2 irrespective of FKBP12.6 association. Biochem J. 2007;404:431–438. doi: 10.1042/BJ20070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Inagaki K, Kihara Y, Izumi T, Sasayama S. The cardioprotective effects of a new 1,4-benzothiazepine derivative, JTV519, on ischemia/reperfusion-induced Ca2+ overload in isolated rat hearts. Cardiovasc Drugs Ther. 2000;14:489–495. doi: 10.1023/a:1007884905461. [DOI] [PubMed] [Google Scholar]
- 243.Kumagai K, Nakashima H, Gondo N, Saku K. Antiarrhythmic effects of JTV-519, a novel cardioprotective drug, on atrial fibrillation/flutter in a canine sterile pericarditis model. J Cardiovasc Electrophysiol. 2003;14:880–884. doi: 10.1046/j.1540-8167.2003.03050.x. [DOI] [PubMed] [Google Scholar]
- 244.Kohno M, et al. A new cardioprotective agent, JTV519, improves defective channel gating of ryanodine receptor in heart failure. Am J Physiol Heart Circ Physiol. 2003;284:H1035–H1042. doi: 10.1152/ajpheart.00722.2002. [DOI] [PubMed] [Google Scholar]
- 245.Ito K, et al. JTV-519, a novel cardioprotective agent, improves the contractile recovery after ischaemia-reperfusion in coronary perfused guinea-pig ventricular muscles. Br J Pharmacol. 2000;130:767–776. doi: 10.1038/sj.bjp.0703373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Inagaki K, et al. Anti-ischemic effect of a novel cardioprotective agent, JTV519, is mediated through specific activation of δ-isoform of protein kinase C in rat ventricular myocardium. Circulation. 2000;101:797–804. doi: 10.1161/01.cir.101.7.797. [DOI] [PubMed] [Google Scholar]
- 247.Toischer K, et al. K201 improves aspects of the contractile performance of human failing myocardium via reduction in Ca2+ leak from the sarcoplasmic reticulum. Basic Res Cardiol. 2010;105:279–287. doi: 10.1007/s00395-009-0057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Liu N, et al. Arrhythmogenesis in catecholaminergic polymorphic ventricular tachycardia: insights from a RyR2 R4496C knock-in mouse model. Circ Res. 2006;99:292–298. doi: 10.1161/01.RES.0000235869.50747.e1. [DOI] [PubMed] [Google Scholar]
- 249.Bellinger AM, et al. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med. 2009;15:325–330. doi: 10.1038/nm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Fauconnier J, et al. Leaky RyR2 trigger ventricular arrhythmias in Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 2010;107:1559–1564. doi: 10.1073/pnas.0908540107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.International Standard Randomised Controlled Trial Number Register. Controlled-trials.com; 2012. [online] http://www.controlled-trials.com/ISRCTN14227980. [Google Scholar]
- 252.Currie S, Elliott EB, Smith GL, Loughrey CM. Two candidates at the heart of dysfunction: the ryanodine receptor and calcium/calmodulin protein kinase II as potential targets for therapeutic intervention—an in vivo perspective. Pharmacol Ther. 2011;131:204–220. doi: 10.1016/j.pharmthera.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 253.Sossalla S, et al. Inhibition of elevated Ca2+/calmodulin-dependent protein kinase II improves contractility in human failing myocardium. Circ Res. 2010;107:1150–1161. doi: 10.1161/CIRCRESAHA.110.220418. [DOI] [PubMed] [Google Scholar]
- 254.Li G, Hidaka H, Wollheim CB. Inhibition of voltage-gated Ca2+ channels and insulin secretion in HIT cells by the Ca2+/calmodulin-dependent protein kinase II inhibitor KN-62: comparison with antagonists of calmodulin and L-type Ca2+ channels. Mol Pharmacol. 1992;42:489–488. [PubMed] [Google Scholar]
- 255.Hvalby O, et al. Specificity of protein kinase inhibitor peptides and induction of long-term potentiation. Proc Natl Acad Sci USA. 1994;91:4761–4765. doi: 10.1073/pnas.91.11.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Remppis A, et al. Altered expression of the Ca2+-binding protein S100A1 in human cardiomyopathy. Biochim Biophys Acta. 1996;1313:253–257. doi: 10.1016/0167-4889(96)00097-3. [DOI] [PubMed] [Google Scholar]
- 257.Most P, et al. Cardiac adenoviral S100A1 gene delivery rescues failing myocardium. J Clin Invest. 2004;114:1550–1563. doi: 10.1172/JCI21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Most P, et al. Cardiac S100A1 protein levels determine contractile performance and propensity toward heart failure after myocardial infarction. Circulation. 2006;114:1258–1268. doi: 10.1161/CIRCULATIONAHA.106.622415. [DOI] [PubMed] [Google Scholar]
- 259.Pleger ST, et al. Stable myocardial-specific AAV6–S100A1 gene therapy results in chronic functional heart failure rescue. Circulation. 2007;115:2506–2515. doi: 10.1161/CIRCULATIONAHA.106.671701. [DOI] [PubMed] [Google Scholar]
- 260.Brinks H, et al. S100A1 genetically targeted therapy reverses dysfunction of human failing cardiomyocytes. J Am Coll Cardiol. 2011;58:966–973. doi: 10.1016/j.jacc.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Pleger ST, et al. Cardiac AAV9-S100A1 gene therapy rescues post-ischemic heart failure in a preclinical large animal model. Sci Transl Med. 2011;3:92ra64. doi: 10.1126/scitranslmed.3002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Yano M, et al. FKBP12.6-mediated stabilization of calcium-release channel (ryanodine receptor) as a novel therapeutic strategy against heart failure. Circulation. 2003;107:477–484. doi: 10.1161/01.cir.0000044917.74408.be. [DOI] [PubMed] [Google Scholar]
- 263.Wehrens XH, et al. Enhancing calstabin binding to ryanodine receptors improves cardiac and skeletal muscle function. Proc Natl Acad Sci USA. 2005;102:9607–9612. doi: 10.1073/pnas.0500353102. [DOI] [PMC free article] [PubMed] [Google Scholar]