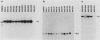Abstract
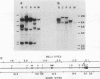
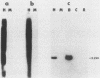
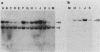
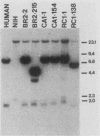
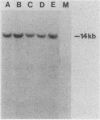
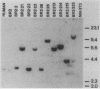
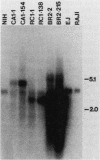
Three activated cellular raf genes have been detected by transfection of NIH 3T3 cells with human tumor DNAs. Blot hybridization analysis indicated that all three transforming raf genes had recombined with non-raf sequences in the vicinity of raf exon 7-intron 7, resulting in the deletion of about 40% of the normal coding sequence from the raf amino terminus. By cloning sequences upstream of the truncated raf loci we have shown that the rearrangements involve the fusion of three different 5' non-raf human sequences to the human raf gene. No rearrangements could be detected in the raf loci of the three original human tumor DNAs, suggesting that the raf genes were activated by DNA rearrangements occurring during transfection. Significant overexpression of raf mRNA was not evident in two of the three transformant lines, indicating that raf overexpression is not necessary and 5' truncation alone may be sufficient to activate the transforming potential of cellular raf genes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birchmeier C., Birnbaum D., Waitches G., Fasano O., Wigler M. Characterization of an activated human ros gene. Mol Cell Biol. 1986 Sep;6(9):3109–3116. doi: 10.1128/mcb.6.9.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner T. I., Kerby S. B., Sutrave P., Gunnell M. A., Mark G., Rapp U. R. Structure and biological activity of human homologs of the raf/mil oncogene. Mol Cell Biol. 1985 Jun;5(6):1400–1407. doi: 10.1128/mcb.5.6.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner T. I., Oppermann H., Seeburg P., Kerby S. B., Gunnell M. A., Young A. C., Rapp U. R. The complete coding sequence of the human raf oncogene and the corresponding structure of the c-raf-1 gene. Nucleic Acids Res. 1986 Jan 24;14(2):1009–1015. doi: 10.1093/nar/14.2.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. M., Okenquist S., Silverman L. Transforming activity of DNA of chemically transformed and normal cells. Nature. 1980 Apr 3;284(5755):418–421. doi: 10.1038/284418a0. [DOI] [PubMed] [Google Scholar]
- Copeland N. G., Cooper G. M. Transfection by exogenous and endogenous murine retrovirus DNAs. Cell. 1979 Feb;16(2):347–356. doi: 10.1016/0092-8674(79)90011-4. [DOI] [PubMed] [Google Scholar]
- Copeland N. G., Zelenetz A. D., Copper G. M. Transformation by subgenomic fragments of Rous sarcoma virus DNA. Cell. 1980 Apr;19(4):863–870. doi: 10.1016/0092-8674(80)90077-x. [DOI] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Fukui M., Yamamoto T., Kawai S., Maruo K., Toyoshima K. Detection of a raf-related and two other transforming DNA sequences in human tumors maintained in nude mice. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5954–5958. doi: 10.1073/pnas.82.17.5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Ishikawa F., Takaku F., Hayashi K., Nagao M., Sugimura T. Activation of rat c-raf during transfection of hepatocellular carcinoma DNA. Proc Natl Acad Sci U S A. 1986 May;83(10):3209–3212. doi: 10.1073/pnas.83.10.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa F., Takaku F., Ochiai M., Hayashi K., Hirohashi S., Terada M., Takayama S., Nagao M., Sugimura T. Activated c-raf gene in a rat hepatocellular carcinoma induced by 2-amino-3-methylimidazo[4,5-f]quinoline. Biochem Biophys Res Commun. 1985 Oct 15;132(1):186–192. doi: 10.1016/0006-291x(85)91005-8. [DOI] [PubMed] [Google Scholar]
- Jansen H. W., Lurz R., Bister K., Bonner T. I., Mark G. E., Rapp U. R. Homologous cell-derived oncogenes in avian carcinoma virus MH2 and murine sarcoma virus 3611. Nature. 1984 Jan 19;307(5948):281–284. doi: 10.1038/307281a0. [DOI] [PubMed] [Google Scholar]
- Jelinek W. R., Toomey T. P., Leinwand L., Duncan C. H., Biro P. A., Choudary P. V., Weissman S. M., Rubin C. M., Houck C. M., Deininger P. L. Ubiquitous, interspersed repeated sequences in mammalian genomes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1398–1402. doi: 10.1073/pnas.77.3.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan N. C., Flordellis C. S., Mark G. E., Duesberg P. H., Papas T. S. Nucleotide sequence of avian carcinoma virus MH2: two potential onc genes, one related to avian virus MC29 and the other related to murine sarcoma virus 3611. Proc Natl Acad Sci U S A. 1984 May;81(10):3000–3004. doi: 10.1073/pnas.81.10.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempnauer K. H., Ramsay G., Bishop J. M., Moscovici M. G., Moscovici C., McGrath J. P., Levinson A. D. The product of the retroviral transforming gene v-myb is a truncated version of the protein encoded by the cellular oncogene c-myb. Cell. 1983 Jun;33(2):345–355. doi: 10.1016/0092-8674(83)90416-6. [DOI] [PubMed] [Google Scholar]
- Knopf J. L., Lee M. H., Sultzman L. A., Kriz R. W., Loomis C. R., Hewick R. M., Bell R. M. Cloning and expression of multiple protein kinase C cDNAs. Cell. 1986 Aug 15;46(4):491–502. doi: 10.1016/0092-8674(86)90874-3. [DOI] [PubMed] [Google Scholar]
- Martin-Zanca D., Hughes S. H., Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. 1986 Feb 27-Mar 5Nature. 319(6056):743–748. doi: 10.1038/319743a0. [DOI] [PubMed] [Google Scholar]
- Moelling K., Heimann B., Beimling P., Rapp U. R., Sander T. Serine- and threonine-specific protein kinase activities of purified gag-mil and gag-raf proteins. Nature. 1984 Dec 6;312(5994):558–561. doi: 10.1038/312558a0. [DOI] [PubMed] [Google Scholar]
- Mölders H., Defesche J., Müller D., Bonner T. I., Rapp U. R., Müller R. Integration of transfected LTR sequences into the c-raf proto-oncogene: activation by promoter insertion. EMBO J. 1985 Mar;4(3):693–698. doi: 10.1002/j.1460-2075.1985.tb03685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Müller D. Co-transfection of normal NIH/3T3 DNA and retroval LTR sequences: a novel strategy for the detection of potential c-onc genes. EMBO J. 1984 May;3(5):1121–1127. doi: 10.1002/j.1460-2075.1984.tb01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naharro G., Robbins K. C., Reddy E. P. Gene product of v-fgr onc: hybrid protein containing a portion of actin and a tyrosine-specific protein kinase. Science. 1984 Jan 6;223(4631):63–66. doi: 10.1126/science.6318314. [DOI] [PubMed] [Google Scholar]
- Park M., Dean M., Cooper C. S., Schmidt M., O'Brien S. J., Blair D. G., Vande Woude G. F. Mechanism of met oncogene activation. Cell. 1986 Jun 20;45(6):895–904. doi: 10.1016/0092-8674(86)90564-7. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Coussens L., Totty N., Rhee L., Young S., Chen E., Stabel S., Waterfield M. D., Ullrich A. The complete primary structure of protein kinase C--the major phorbol ester receptor. Science. 1986 Aug 22;233(4766):853–859. doi: 10.1126/science.3755547. [DOI] [PubMed] [Google Scholar]
- Schäfer R., Griegel S., Dubbert M. A., Willecke K. Unstable transformation of mouse 3T3 cells by transfection with DNA from normal human lymphocytes. EMBO J. 1984 Mar;3(3):659–663. doi: 10.1002/j.1460-2075.1984.tb01863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K., Nakatsu Y., Sekiguchi M., Hokamura K., Tanaka K., Terada M., Sugimura T. Molecular cloning of an activated human oncogene, homologous to v-raf, from primary stomach cancer. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5641–5645. doi: 10.1073/pnas.82.17.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutrave P., Bonner T. I., Rapp U. R., Jansen H. W., Patschinsky T., Bister K. Nucleotide sequence of avian retroviral oncogene v-mil: homologue of murine retroviral oncogene v-raf. Nature. 1984 May 3;309(5963):85–88. doi: 10.1038/309085a0. [DOI] [PubMed] [Google Scholar]
- Tahara S. M., Traugh J. A. Differential activation of two protease-activated protein kinases from reticulocytes by a Ca2+-stimulated protease and identification of phosphorylated translational components. Eur J Biochem. 1982 Aug;126(2):395–399. doi: 10.1111/j.1432-1033.1982.tb06793.x. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Ritz J., Cooper G. M. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell. 1985 Sep;42(2):581–588. doi: 10.1016/0092-8674(85)90115-1. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Ledley F., Goff S., Lee R., Groner Y., Baltimore D. The mouse c-abl locus: molecular cloning and characterization. Cell. 1984 Feb;36(2):349–356. doi: 10.1016/0092-8674(84)90228-9. [DOI] [PubMed] [Google Scholar]
- Young D., Waitches G., Birchmeier C., Fasano O., Wigler M. Isolation and characterization of a new cellular oncogene encoding a protein with multiple potential transmembrane domains. Cell. 1986 Jun 6;45(5):711–719. doi: 10.1016/0092-8674(86)90785-3. [DOI] [PubMed] [Google Scholar]