Abstract
We compared the efficacy of the novel sodium-hydrogen exchanger (NHE-1) inhibitor AVE4454B with cariporide for resuscitation from ventricular fibrillation (VF) assessing effects on left ventricular myocardial distensibility during chest compression, myocardial function after return of spontaneous circulation, and survival. Three groups of ten rats each were subjected to 10 minutes of untreated VF and resuscitation attempted by providing chest compression for up to 8 minutes with the depth of compression adjusted to attain an aortic diastolic pressure between 26 and 28 mmHg (to secure a coronary perfusion pressure above 20 mmHg), followed by electrical shocks. Rats received AVE4454B (1 mg/kg), cariporide (1 mg/kg), or vehicle control immediately before chest compression. We observed that NHE-1 inhibition preserved left ventricular myocardial distensibility during chest compression evidenced by less depth of compression required to attain the target aortic diastolic pressure corresponding to (mean ± SD) 14.1 ± 1.1 mm in the AVE4454B group (p < 0.001 vs control), 15.0 ± 1.4 mm in the cariporide group (p < 0.01 vs control), and 17.0 ± 1.2 mm in controls. When the depth of compression was related to the coronary perfusion pressure generated (CPP/Depth ratio) – an index of left ventricular distensibility – only cariporide group attained statistical significance. Post-resuscitation, both compounds ameliorated myocardial dysfunction evidenced by lesser reductions in mean aortic pressure and +dP/dtmax and earlier normalization of left ventricular end-diastolic pressure increases. This effect was associated with improved survival corresponding to 55% in the AVE4454B group (NS) and 70% in the cariporide group (p < 0.01 vs control by Gehan-Breslow analysis). There was an inverse correlation between plasma cytochrome c and indices of left ventricular function at post-resuscitation 240 minutes suggesting that NHE-1 inhibition exerts beneficial effects in part by attenuating mitochondrial injury. We conclude that cariporide is more effective than AVE4454B for resuscitation from cardiac arrest given its more prominent effect on preserving left ventricular myocardial distensibility and promoting survival.
INTRODUCTION
Cessation of coronary blood flow after onset of cardiac arrest prompts rapid development of myocardial ischemia leading to intense intracellular acidosis (1-3). Intracellular acidosis activates the sarcolemmal sodium-hydrogen exchanger isoform-1 (NHE-1) initiating an electro-neutral Na+–H+ exchange that brings Na+ into the cell (4,5). During the ensuing resuscitation effort, the typical coronary blood flow produced by current cardiopulmonary resuscitation (CPR) techniques is not sufficient to reverse myocardial ischemia. However, such coronary blood flow perfuses the myocardium with blood that typically has normal pH, washing-out protons accumulated in the extracellular space during the preceding no-flow interval, thus intensifying the sarcolemmal Na+–H+ exchange rate and the resulting Na+ entry (4,6,7). Na+ accumulates inside the cell because the Na+-K+ ATPase activity is concomitantly reduced (8) resulting in prominent increases in intracellular Na+ (5). The increased intracellular Na+, in turn, drives sarcolemmal Ca2+ influx through reverse mode operation of the sarcolemmal Na+–Ca2+ exchanger leading to cytosolic and mitochondrial Ca2+ overload (5,9). Mitochondrial Ca2+ overload can worsen cell injury in part by compromising its capability to sustain oxidative phosphorylation (10) and by promoting the release of pro-apoptotic factors (11).
This mechanism of injury is highly relevant to the global myocardial ischemia of cardiac arrest and the subsequent reperfusion injury that occurs during the resuscitation effort (12). Extensive work in our laboratory, using various animal models of cardiac arrest and resuscitation (5,7,12-22), demonstrates multiple myocardial benefits associated with administration of NHE-1 inhibitors given at the beginning of the resuscitation effort and therefore given coincident with the onset of reperfusion injury but before reversal of myocardial ischemia which occurs only after return of spontaneous circulation. CPR generates coronary blood flows that typically fail to reverse myocardial ischemia. Functionally, these benefits manifest by preservation of left ventricular myocardial distensibility leading to hemodynamically more effective chest compression (15,17,18), attenuation of reperfusion arrhythmias preventing episodes of refibrillation (15,16,21), and amelioration of post-resuscitation left ventricular systolic and diastolic dysfunction enabling greater hemodynamic stability (15,20,21). Mechanistically, these benefits are linked to attenuation of cytosolic Na+ overload (5,7), attenuation of mitochondrial Ca2+ accumulation (5), and preservation of mitochondrial bioenergetic function (20) and are accompanied by lesser increases in plasma troponin I (22).
Most of the aforementioned studies were conducted using NHE-1 inhibitors being developed for eventual clinical use, with cariporide leading the group for myocardial protection during acute coronary events and during coronary artery bypass graft surgery. Unfortunately, development of cariporide was halted by unexpected decreases in survival after coronary artery bypass graft surgery associated with increased cerebrovascular occlusive events despite statistically significant reduction in the rate of post-operative myocardial infarction in the EXPEDITION trial (23).
With the intent of circumventing possible adverse effects of cariporide, Sanofi-Aventis initiated development of a novel NHE-1 inhibitor known as AVE4454B. In previous studies, we reported that AVE4454B elicited the expected myocardial benefits of NHE-1 inhibitors during resuscitation from ventricular fibrillation (VF) in a rat model (5). In the present study we compared the effects of AVE4454B with those of cariporide on left ventricular myocardial distensibility, recurrence of VF, post-resuscitation myocardial dysfunction, and survival at 240 minutes post-resuscitation. We included a control group and conducted two independent analyses; one comparing the three groups to identify possible differences between NHE-1 inhibitors and one comparing the two NHE-1 inhibitors combined versus control in order to assess the effects of NHE-1 inhibition (i.e., class effect) gaining additional statistical power. We also included measurements of plasma cytochrome c which we have recently proposed as a novel biomarker of mitochondrial injury after resuscitation from cardiac arrest (24).
METHODS
These studies were approved by our Institutional Animal Care and Utilization Committee and were conducted in accordance with institutional guidelines.
Animal Preparation
Adult male Sprague-Dawley rats (477 to 556 grams) were anesthetized by intraperitoneal injection of sodium pentobarbital (45 mg/kg) supplemented (10 mg/kg) at 30 minute intervals if required. Core temperature was maintained between 36.5°C and 37.5°C using an infrared heating lamp. A conventional lead II electrocardiogram was recorded through subcutaneous needles.
Through the right femoral artery a thermocouple microprobe (IT-23, Physitemp) was advanced into the thoracic aorta and used to measure core blood temperature and thermodilution cardiac output. Through the right femoral vein a PE50 tube was advanced into the right atrium and used to measure blood pressure, sample blood, and delivery of drugs. Through the left femoral artery, a PE50 catheter was advanced into the abdominal aorta and used to measure blood pressure and sample blood. Through the right carotid artery, a high-fidelity microtip catheter (Scisense Inc.) was advanced into the left ventricle and used to measure left ventricular pressure. Through the left external jugular vein, a PE50 catheter was advanced into the right atrium and used for injection of the thermal tracer required for thermodilution cardiac output. Through the right jugular vein, a 3F catheter (C-PUM-301J, Cook) was advanced into the right atrium and a pre-curved guidewire advanced through its lumen into the right ventricle. It was used for induction of VF. A 5F catheter was orally advanced into the trachea and used for positive pressure ventilation after placement was verified with an infrared CO2 analyzer.
VF and Resuscitation Protocol
VF was induced by a 60 Hz alternating current delivered to the right ventricular endocardium (0.10 to 0.70 mA). The current was discontinued after 3 minutes and VF allowed to persist untreated for an additional 7 minutes (total 10 minutes). Fifteen seconds before completion of the 10-minute interval of untreated VF, rats were randomized to receive a right atrial bolus of a vehicle or an NHE-1 inhibitor as described in the “Experimental Groups and Drugs” section.
Chest compression was then initiated using a pneumatically driven and electronically controlled piston device programmed to deliver 200 compressions per minute. The depth of compression was adjusted to attain an aortic diastolic pressure between 26 and 28 mmHg securing a coronary perfusion pressure greater than the 20 mmHg threshold required for successful resuscitation in rats (25). The depth of compression was recorded using a displacement transducer (DSPL, World Precision Instruments). Positive pressure ventilations were provided using 100% oxygen with a volume-controlled ventilator (683, Harvard Apparatus) set to deliver 25 unsynchronized breaths per minute at a tidal volume of 6 ml/kg. After 8 minutes of chest compression, electrical defibrillation was attempted by delivering a maximum of two 3-J biphasic transthoracic electrical shocks (Smart Biphasic Heartstream XL M4735A; Agilent Technologies). If VF persisted or an organized electrical rhythm with a mean aortic pressure of < 25 mmHg ensued, chest compression was resumed for 30 seconds. The defibrillation-compression sequence was repeated for a maximum of 3 additional cycles, increasing the energy of individual shocks to 5-J and then to 7-J for the last 2 cycles. Return of spontaneous circulation was defined as the return of an organized cardiac rhythm with a mean aortic pressure of ≥ 60 mmHg for ≥ 5 minutes. After successful return of spontaneous circulation, the ventilation rate was increased to 60 breaths per minute using 100% oxygen for the initial 15 minutes and 50% oxygen for the remaining post-resuscitation interval. Resuscitated rats were monitored for 240 minutes. Rats were euthanized after the monitoring period by intravenous administration of pentobarbital (150 mg/kg).
Experimental Groups and Drugs
Three groups of 10 rats each received either 1 mg/kg of AVE4454B, 1 mg/kg of cariporide, or a control solution corresponding to AVE4454B vehicle in 5 rats and cariporide vehicle in the other 5 rats, all in a volume of 1 ml/kg. The agent or control solution was given into the right atrium as a bolus immediately before starting chest compression. AVE4454B and cariporide were provided by Sanofi-Aventis. AVE4454B was dissolved in 1.8% glycine buffer (pH 4.0) to a final concentration of 1 mg/ml. For AVE4454B vehicle control, mannitol was also dissolved in 1.8% glycine buffer (pH 4.0) to a final concentration of 1.3 mg/ml to ensure equal osmolarity. Cariporide was dissolved in 0.9% NaCl to a final concentration of 1 mg/ml. For cariporide, the vehicle control was 0.9% NaCl. The dose of cariporide was chosen based on previous studies demonstrating beneficial cardiac effects in the same rat model (13). The dose of AVE4454B was chosen based on recommendations by the manufacturer Sanofi-Aventis and previous studies demonstrating functional myocardial benefits elicited with the chosen dose (5).
In previous studies we reported a rate of spontaneous defibrillation nearing 90 % with the administration of cariporide (18) but not with the administration of AVE4454B (5). Thus, to ensure comparable duration of VF among groups, the experiments were conducted in blocks of three with the first experiment assigned to cariporide and the remaining two experiments randomized to AVE4454B or control. Within each block, the initial electrical shock in the AVE4454B group and in the control group was timed to coincide with the time at which spontaneous defibrillation occurred in the preceding rat treated with cariporide or at 8 minutes of chest compression if spontaneous defibrillation did not occur. The AVE4454B or control assignment was randomized after having completed the cariporide experiment.
Other Chemicals
Acetonitrile, mannitol, rat heart cytochrome c, and trifluoro-acetic acid were purchased from Sigma.
Measurements
Hemodynamic variables
Signals were acquired using various transducer systems, processed using signal conditioners (BIOPAC Systems), sampled at 250 scans per second, digitized using a 16-bit data acquisition board (AT-MIO-16XE-50; National Instruments), and analyzed using custom developed programs in LabVIEW (National Instruments). The coronary perfusion pressure during resuscitation was calculated as the pressure difference between the aorta and right atrium at the end of chest relaxation. The depth of compression was recorded continuously during the interval of chest compression and used in relation to the coronary perfusion pressure to assess changes in left ventricular distensibility as previously reported (18). Left ventricular pressures were processed to obtain the maximal rate of pressure rise (+dP/dtmax) and the relaxation time constant (Tau). Cardiac output was measured after right atrial bolus injection of 200 μl of 0.9% NaCl at room temperature with the thermodilution curve recorded in the descending thoracic aorta and analyzed using a custom-developed system and software in LabVIEW (National Instruments). The cardiac output was normalized to body weight and reported as cardiac index in ml/min per kg body weight. The left ventricular stroke work index (LVSWI) was calculated by multiplying the stroke volume index by the difference between the systolic and diastolic left ventricular pressures and reported in the work units gram-force × meter normalized to body weight (gf·m/kg) multiplying mmHg·ml by 1.36×10-3. For variables related to left ventricular and hemodynamic function, only data from rats with a mean aortic pressure > 40 mmHg were included to avoid confounders stemming from low perfusion pressures, typically observed before demise.
Plasma cytochrome c
Arterial blood samples (200 μl) were collected into heparinized syringes and centrifuged at 5,000 rpm (2,320g) for 10 minutes at 4°C (Sorvall Biofuge Stratos, Heraeus). The supernatant (plasma) was frozen at -80°C for subsequent analysis using reverse-phase high performance liquid chromatography as described previously (24).
Statistical analysis
The data were subjected to two independent analyses; one to test for differences between the AVE4454B and cariporide groups combined (denoted as NHE-1 inhibition [NHEI]) and control and another to test for differences among AVE4454B, cariporide, and the control groups. For continuous variables, one-way ANOVA was used when testing differences among groups using the Holm-Sidak's method for multiple comparisons if overall differences were detected and Student's t-test when comparing differences between control and NHEI. Alternative nonparametric tests were used if the data failed tests for normality or equal variance. The strength of association between variables of interest was analyzed using Pearson's product moment correlation test. Differences in survival were analyzed using the Gehan-Breslow test. The data were presented as mean ± SD in the text and tables and mean ± SEM in figures. A two-tail value of p < 0.05 was considered significant.
RESULTS
There were no statistically significant differences among groups at baseline.
Effects on Left Ventricular Myocardial Distensibility during Chest Compression
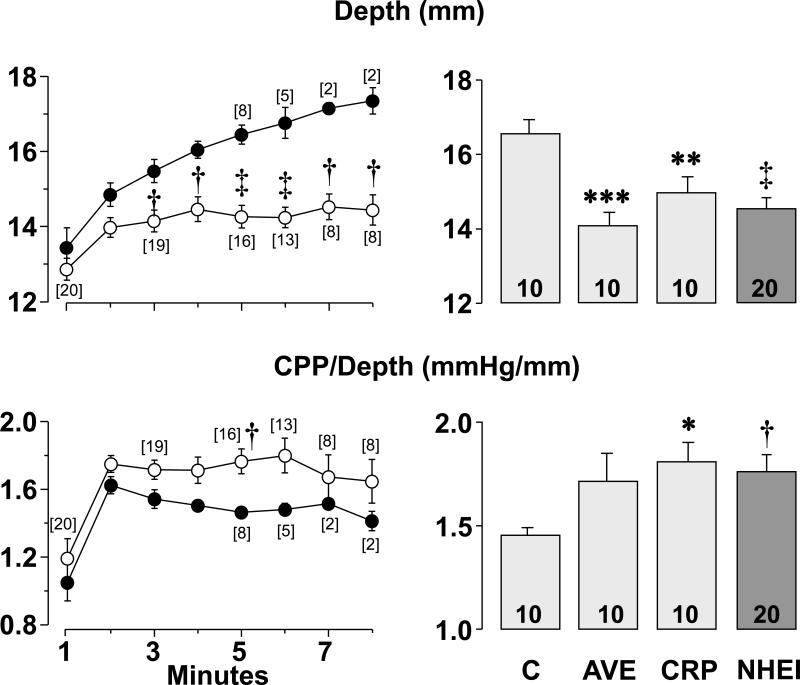
The depth of compression required to maintain the aortic diastolic pressure at the set target of 26 to 28 mmHg was significantly lower in the combined NHEI group starting from the 3rd minute of chest compression until the end of compression (Figure 1, top left graph) without significant differences between individual NHE-1 inhibitors. The depth of compression at the last minute of chest compression was significantly lower in rats treated with AVE4454B, cariporide, or their combination compared with control rats (Figure 1, top right graph). The ratio between the coronary perfusion pressure and the compression depth was higher in the combined NHEI group attaining statistical significance at the 5th minute of chest compression (Figure 1, bottom left graph), again without significant differences between individual NHE-1 inhibitors. The ratio between the coronary perfusion pressure and the compression depth at the last minute of compression was significantly higher in rats treated with cariporide and in the combined group compared with controls (Figure 1, bottom right graph).
Figure 1.
The depth of chest compression (Depth) and ratio between coronary perfusion pressure and depth of compression (CPP/Depth) are shown in rats that received vehicle control solution (C), the NHE-1 inhibitor AVE4454B (AVE), or cariporide (CRP) just before starting chest compression. NHEI = AVE and CRP groups combined. The line graphs on the left depict Depth and CPP/Depth throughout chest compression in the NHEI group (○) compared with the control group (●). The numbers in brackets denote rats remaining in VF during chest compression as explained in the “Experimental groups and drugs” section. One rat in the cariporide group spontaneously defibrillated coincident with the end of the 8th minute of chest compression and therefore did not require electrical shocks but was included for the aggregate measurement of coronary perfusion pressure and depth of compression. The bar graphs on the right depict the effects of the interventions at the last minute of chest compression before termination of VF spontaneously or by delivery of electrical shocks, which occurred between minute 3 and minute 8 of chest compression. Values are means ± SEM. †p < 0.01, ‡p < 0.001 vs control by Student's t-test; **p < 0.01, ***p < 0.001 vs control by one-way ANOVA using Holm-Sidak's test for multiple comparisons; *p < 0.05 vs control by one-way ANOVA using Dunn's test for multiple comparisons.
Effects on Defibrillation
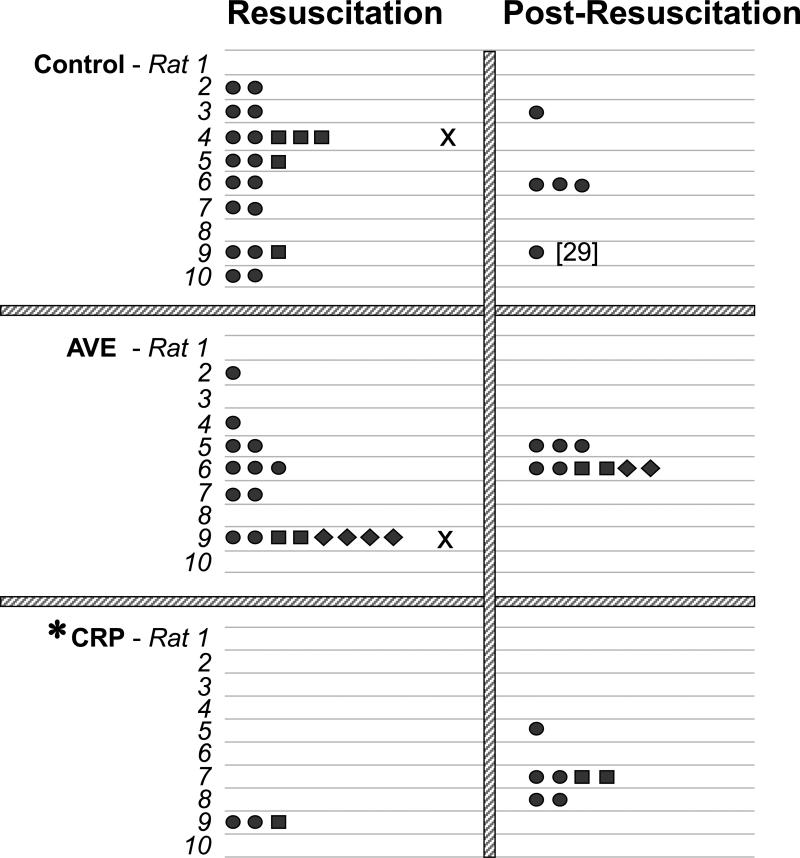
The number of electrical shocks and the energy of the shock required to terminate VF and reestablish spontaneous circulation are shown in Figure 2. All rats were successfully resuscitated in the cariporide group whereas one rat in the AVE4454B group and one in the control group were not resuscitated displaying pulseless electrical activity. Spontaneous defibrillation with return of spontaneous circulation occurred before completion of the 8-minute interval of chest compression in four AVE4454B and in nine cariporide treated rats. Given the effort to synchronize the time of initial shock delivery to the cariporide group, the duration of VF was comparable among groups corresponding to 1037 ± 37 seconds in the AVE4454B group, 963 ± 34 seconds in the cariporide group, and 1001 ± 41 seconds in the control group. The slightly longer duration in the AVE4454B and control groups was not statistically significant but attributed to failure of the initial electrical shock to terminate VF leading to additional time in VF. Given the higher rate of spontaneous defibrillation, fewer electrical shocks and less cumulative energy were required in the cariporide group to reestablish spontaneous circulation (Figure 2). Within the initial 15 minutes post-resuscitation, additional electrical shocks were required to reverse recurrent episodes of VF in two rats from the AVE4454B group, three rats from the cariporide group, and three rats from the control group (Figure 2).
Figure 2.
Shown are the number and energy level of electrical shocks required to terminate ventricular fibrillation (Resuscitation) and the number and energy level of electrical shocks required in the event that VF recurred after successful defibrillation with return of spontaneous circulation (Post-Resuscitation), in rats that received control solution, AVE4454B (AVE), or cariporide (CRP). The number in brackets denotes number of shocks. Rats marked with “x” failed to regain spontaneous circulation. Symbols ●, ■, and ◆ denote 3, 5, and 7 joules respectively. *p < 0.05 vs control by one-way ANOVA using Dunn's test for multiple comparisons for resuscitation shocks.
Effects on Left Ventricular Function and Survival
Left ventricular systolic and diastolic dysfunction was evident after return of spontaneous circulation in each group as previously reported (13) and manifested by reductions in mean aortic pressure, LVSWI, and +dP/dtmax along with increases in Tau and left ventricular end diastolic pressure (Table 1). However, the magnitude of such dysfunction was less in rats that received an NHE-1 inhibitor, evidenced by lesser reductions in mean aortic pressure, +dP/dtmax, and lesser increases in left ventricular diastolic pressure without significant differences between individual NHE-1 inhibitors (Table 1).
Table 1.
Left Ventricular Function
| Baseline | Post-Resuscitation | |||||
|---|---|---|---|---|---|---|
| -10 min | 30 min | 60 min | 120 min | 240 min | ||
| Control | 132±10 | 77±15[7] | 91±17[5] | 80±27 | 116[1] | |
| AVE | 130±9 | 99±11[8]a1 | 94±23 | 106±15[6] | 101±24[4] | |
| MAP, mmHg | CRP | 123±5 | 86±14 | 78±24 | 87±16[8] | 86±20[6] |
| NHEI | 127±8 | 92±14[18]b | 85±24 | 95±18[14] | 92±22[10] | |
| Control | 10±1 | 13±4[7] | 11±1[5] | 11±2 | 12[1] | |
| Tau, msec | AVE | 10±1 | 13±3[8] | 11±3 | 11±2[6] | 11±2[4] |
| CRP | 9±2 | 11±3 | 11±3 | 13±3[8] | 11±4[6] | |
| NHEI | 9±2 | 12±3[18] | 11±3 | 12±3[14] | 11±3[10] | |
| Control | 0.78±0.14 | 0.27±0.08[6] | 0.23±0.09[5] | 0.18±0.15 | 0.54[1] | |
| LVSWI, gf·m/kg | AVE | 0.85±0.13 | 0.43±0.12[8]a2 | 0.32±0.14 | 0.27±0.07[6] | 0.22±0.08[4] |
| CRP | 0.67±0.21 | 0.31±0.10 | 0.29±0.13 | 0.26±0.11[8] | 0.24±0.08[6] | |
| NHEI | 0.76±0.19 | 0.36±0.12[18] | 0.30±0.13 | 0.27±0.09[14] | 0.23±0.08[10] | |
| Control | 8.4±0.9 | 3.6±0.9[7] | 4.8±1.3[5] | 4.4±1.1 | 6.7[1] | |
| +dP/dtmax, 103 mmHg/s | AVE | 9.3±1.6 | 4.7±0.7[8] | 5.2±0.9 | 5.7±0.7[6] | 5.8±1.1[4] |
| CRP | 8.2±1.1 | 4.2±0.8 | 4.4±1.4 | 4.6±0.9[8] | 4.9±1.0[6] | |
| NHEI | 8.7±1.4 | 4.4±0.8[18]b | 4.7±1.3 | 5.1±0.9[14] | 5.3±1.1[10] | |
| Control | 2±2 | 4±1[7] | 4±1[5] | 3±2 | 6[1] | |
| LVEDP, mmHg | AVE | 4±4 | 4±4[8] | 4±4 | 3±3[6] | 3±3[4] |
| CRP | 2±1 | 2±3 | 1±2a3 | 2±1[8] | 2±1[6] | |
| NHEI | 3±3 | 3±3[18] | 2±3b | 3±2[14] | 3±2[10] | |
Left ventricular function in rats randomized to receive control solution, AVE4454B (AVE), or cariporide (CRP) before chest compression. MAP = Mean aortic pressure; LVSWI = Left ventricular stroke work index; +dP/dtmax = Maximal rate of left ventricular pressure rise; LVDP = Left ventricular end diastolic pressure; NHEI = AVE and CRP groups combined. Numbers in brackets indicate when the sample size decreased from the initial number or from the preceding sample size. Technical problems precluded measuring cardiac output and thus calculating LVSWI in one control rat at post-resuscitation 30 minutes.
p <0.01,
p <0.05 vs control by one-way ANOVA and Holm-Sidak's test for multiple comparisons
p<0.05 vs control by one-way ANOVA and Dunn's test for multiple comparisons.
p<0.05 vs control by Student's t-test.
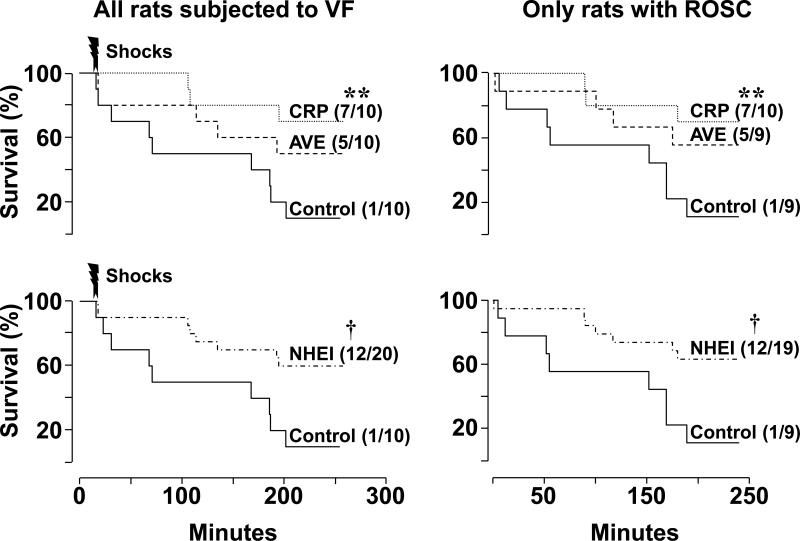
A survival benefit was evident for the combined NHEI groups compared with control. However, when individually NHE-1 inhibitors and control were compared, only the cariporide group showed statistically significant improvement in survival compared with the control group (Figure 3).
Figure 3.
Survival curves in rats that received vehicle control solution, AVE4454B (AVE), or cariporide (CRP). The graphs on the left depict survival curves for all rats subjected to ventricular fibrillation and graphs on the right only for rats that had return of spontaneous circulation (ROSC) after defibrillation. The graphs on the top depict survival for the individual interventions and on the bottom survival for the AVE and CRP groups combined (NHEI). The number of rats that survived for post-resuscitation 240-minute interval relative to the corresponding cohort is shown for each curve in parentheses. **p < 0.01 vs control by Gehan-Breslow analysis using Holm-Sidak's test for multiple comparisons; †p = 0.01 vs control by Gehan-Breslow analysis.
Effects on Plasma Lactate and Cytochrome c Levels
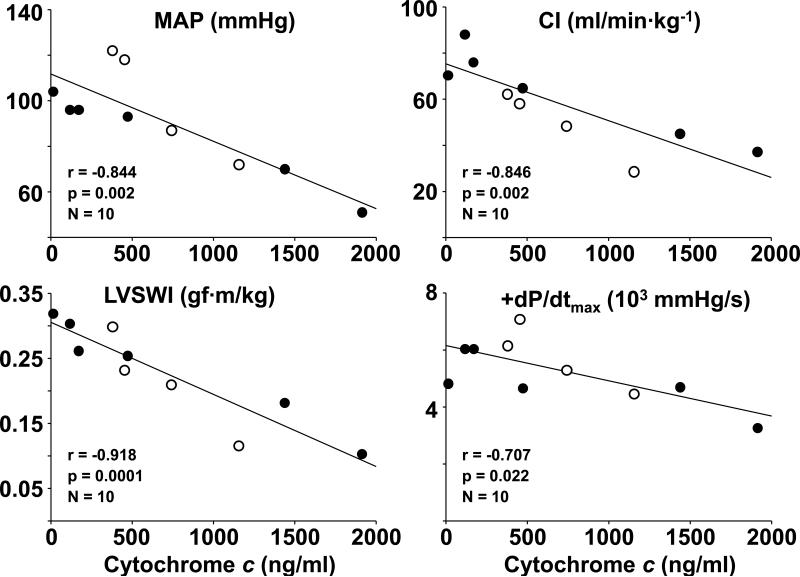
AVE4454B and cariporide attenuated increases in plasma lactate levels post-resuscitation demonstrating statistically significant differences at 120 minutes (Table 2). The levels of cytochrome c were lower in AVE4454B and cariporide but the differences were not statistically significant (Table 2). The levels of plasma cytochrome c measured at 240 minutes post-resuscitation in ten rats that were hemodynamically stable with a mean aortic pressure > 40 mmHg (four in the AVE4454B group and six in the cariporide group) demonstrated to be inversely correlated with the mean aortic pressure, cardiac index, LVSWI, and +dP/dtmax (Figure 4).
Table 2.
Plasma lactate and cytochrome c level
| Baseline | Post-Resuscitation | |||||
|---|---|---|---|---|---|---|
| -10 min | 30 min | 60 min | 120 min | 240 min | ||
| Control | 1.1±0.2 | 9.9±6.1[7] | 4.9±2.3[5] | 5.6±2.9 | 1.5[1] | |
| Lactate (Ao), mmol/L | AVE | 1.3±0.3 | 7.4±3.5[7] | 5.0±3.3 | 2.7±0.7[5]a2 | 3.6±0.1[4] |
| CRP | 1.3±0.3 | 6.7±2.6 | 4.5±2.3 | 3.5±1.0[8]a2 | 3.7±1.9[6] | |
| NHEI | 1.3±0.3 | 6.9±2.9[17] | 4.7±2.7 | 3.2±0.9[13] | 3.6±1.4[10] | |
| Control | 276±493 | 642±712[7] | 787±378[5] | 880±567 | 884[1] | |
| Plasma cytochrome c, ng/ml | AVE | 203±499 | 415±321[8] | 445±319 | 859±668[6] | 668±352[4] |
| CRP | 210±246 | 331±443 | 504±541 | 502±454[8] | 674±795[6] | |
| NHEI | 207±383 | 368±385[18] | 478±445 | 655±563[14] | 671±627[10] | |
Plasma lactate and cytochrome c levels in rats randomized to receive control solution, AVE4454B (AVE), or cariporide (CRP) just before chest compression. Ao = Aorta; NHEI = AVE and CRP groups combined. Numbers in brackets indicate when the sample size decreased from the initial number or from the preceding sample size. Technical problems precluded measuring lactate in one AVE4454B rat at post-resuscitation 30, 60, and 120 minutes.
p<0.05 vs control by one-way ANOVA and Holm-Sidak's test for multiple comparisons.
Figure 4.
Scatterplots depicting the relationship between plasma cytochrome c and mean aortic pressure (MAP), cardiac index (CI), left ventricular stroke work index (LVSWI), and the maximal rate of left ventricular pressure rise (+dP/dtmax) at 240 minutes post-resuscitation in rats treated with AVE4454B (○) or cariporide (●). There was only one survival in the control group at 240 minutes and was removed from the analysis.
DISCUSSION
The present study conducted in a rat model of VF and closed-chest resuscitation demonstrated that the novel NHE-1 inhibitor AVE4454B and the established NHE-1 inhibitor cariporide given at the start of chest compression elicited myocardial effects beneficial for successful resuscitation from cardiac arrest with cariporide demonstrating greater efficacy than AVE4454B.
Effects on Left Ventricular Myocardial Distensibility
Studies in various animal models of cardiac arrest have shown progressive left ventricular wall thickening with progressive left ventricular cavity reduction during resuscitation without changes in the left ventricular pressure measured between compressions, consistent with reductions in left ventricular myocardial distensibility (15,20,26). Reduction in left ventricular myocardial distensibility negatively impacts the hemodynamic efficacy of chest compression. As blood returns to the heart during the relaxation phase of chest compression, distensible ventricles are critically important to secure adequate preload for subsequent compressions. This phenomenon has also been reported in humans suffering out-of-hospital cardiac arrest and who received open-chest direct manual cardiac compression after failure of closed-chest resuscitation (27). In 36 patients, a “firm” myocardium was noted affecting predominantly the left ventricle. In other 23 patients, the myocardium was “soft.” The presence of a “firm” myocardium limited the generation of forward blood flow evidenced by a lower end-tidal CO2 tension (PETCO2) – a surrogate measurement of blood flow during cardiac resuscitation (28,29). Hearts with “very firm” myocardium never regained spontaneous contractions. Hearts with “less firm” myocardium showed some – albeit insufficient – spontaneous contractions. Hearts with “soft” myocardium regained contractions and were able to generate a peripheral pulse in most instances.
Reduction in left ventricular myocardial distensibility occurs during the interval in which myocardial blood flow is being generated by artificial means (e.g., chest compression) – not during the preceding interval of no-flow – and it is a manifestation of reperfusion injury. We have previously linked decreases in left ventricular myocardial distensibility to Na+ induced mitochondrial Ca2+ overload leading to reductions in mitochondrial bioenergetic function evidenced by sharp decreases in the myocardial capability for rephosphorylation of creatine accompanied by intense generation of lactic acid (20). We have also shown that administration of NHE-1 inhibitors can preserve left ventricular myocardial distensibility through effects associated with attenuation of mitochondrial Ca2+ overload and preservation of bioenergetic function (5,20).
In the presented study we demonstrated capability of both AVE4454B and cariporide to preserve left ventricular myocardial distensibility. We investigated this phenomenon by measuring the depth of compression required to generate a predetermined coronary perfusion pressure; an approach that we had developed earlier in the same rat model using fluorescent microspheres to measure total and regional blood flow (18). In these earlier studies, administration of cariporide allowed the generation of equal or higher total and regional blood flow by chest compression with less depth of compression; an effect that was not mediated by effects on peripheral vascular resistance which remains unchanged after administration of cariporide (17). Accordingly, maintaining left ventricular myocardial distensibility during chest compression enabled a given depth of compression to generate a coronary perfusion pressure higher than if the left ventricle had become progressively less distensible or to generate a predetermined coronary perfusion pressure with less depth of compression as shown for AVE4454B and cariporide in Figure 1. In the present study, however, when the depth of compression was normalized to coronary perfusion pressure – i.e., CPP/Depth ratio – only the cariporide group attained statistical difference suggesting that cariporide was more effective than AVE4454B.
Thus, independent of potential differences in magnitude between AVE4454B and cariporide as that of other NHE-1 inhibitors (20), it can be concluded that NHE-1 inhibitors can maintain left ventricular myocardial distensibility and thereby prevent a rightward shift of the flow-depth relationship during chest compression, representing a novel mechanism of action that can be used to improve the hemodynamic efficacy of chest compression. This effect, however, is not limited to NHE-1 inhibitors and recent studies by our group indicate that similar functional myocardial effects can be elicited by administration of erythropoietin (30,31).
Effects on Post-Resuscitation Myocardial Function and Survival
Variable degrees of systolic (32-35) and diastolic (15,36) left ventricular dysfunction develop after resuscitation from cardiac arrest. If severe and persistent, myocardial dysfunction may preclude reestablishment of stable circulation and contribute to the approximately 40% fatality rate reported in victims of out-hospital cardiac arrest before admission to a hospital (34). In the present study, rats that received AVE4454B or cariporide had less myocardial dysfunction during the early post-resuscitation interval. This beneficial effect was also evidenced by faster normalization of increases in arterial lactate produced by the whole body ischemia precipitated by cardiac arrest.
Less post-resuscitation myocardial dysfunction likely contributed to improved survival during the 240-minute post-resuscitation observation interval. However, cariporide was more effective than AVE4454B in securing survival as shown in Figure 3. Additional benefits, elsewhere, accounting for the differences in survival between compounds cannot be excluded given the systemic nature of the injury precipitated by cardiac arrest and resuscitation and the ubiquitness of NHE-1 activation as a mechanism of injury, known to affect the brain (37), liver (38), kidney (39), and leukocyte function (40) during episodes of ischemia and reperfusion. The compounds differ in their potency and selectivity with the possibility that cariporide at the dose given could recruit additional non-specific effects that are beneficial to resuscitation as discussed below.
Evidence Supporting that Mitochondria are Involved
We have previously reported that AVE4454B given to rats during chest compression attenuates increases in left ventricular mitochondrial Ca2+ (5). Zoniporide – another NHE-1 inhibitor – given to swine during simulated resuscitation attenuates decreases in myocardial creatine-phosphate/creatine ratio and myocardial increases in lactate consistent with preservation of mitochondrial bioenergetic function (20). In a similar rat model of VF and closed-chest resuscitation we reported that cytochrome c translocates to the cytosol accompanied by activation of the mitochondrial apoptotic pathway in myocardial tissue at 240 minutes post-resuscitation (24) and that cytochrome c is also released to the bloodstream attaining levels that are inversely related to survival (24). Cytochrome c is a mitochondrial protein located in the intermembrane space that plays a key role in energy metabolism by enabling electron transport from respiratory complex III to respiratory complex IV. Release of cytochrome c to the cytosol – which typically occurs when mitochondria are injured – activates the intrinsic apoptotic pathway as we reported in our rat model (22).
Accordingly, the preceding work supports the idea that mitochondria are injured during resuscitation from cardiac arrest and that such injury is characterized – among other effects – by release of cytochrome c to the cytosol and bloodstream. The work also supports the idea that mitochondrial injury can be attenuated by NHE-1 inhibitors. In the present study, we found that plasma cytochrome c was inversely correlated with left ventricular function at 240 minutes post-resuscitation (time at which the average left ventricular stroke work index was only 30% of baseline) suggesting a possible relationship between the severity of left ventricular dysfunction and the severity of mitochondrial injury.
However, further work is required to determine the nature of this relationship. For example, it is possible that plasma cytochrome c is contributed by various organs given the global nature of cardiac arrest and resuscitation with the levels reflecting the severity of such global injury with the heart being one of various organs proportionally affected.
Differences between AVE4454B and Cariporide
AVE4454B has been shown to have high potency and high selectivity for NHE-1 (IC50, 0.051 μM) compared with NHE-2 (IC50, 7.6 μM) and NHE-3 and -5 (no inhibition at 10 μM) using human NHE subtypes. The IC50 of cariporide for NHE-1 is similar at 0.074 μM but the selectivity is less (41). Cariporide promoted a higher rate of spontaneous defibrillation than AVE4454B and had a better effect on survival. We have previously proposed that potential additional beneficial effects of cariporide may stem from non-selective effects of cariporide over other ion transport mechanisms, especially considering the pharmacokinetics of bolus dosing during resuscitation. In a previous study in pigs, a bolus dose of cariporide (3 mg/kg) given at the start of chest compression yielded plasma levels more than three orders of magnitude the concentration needed to completely inhibit NHE-1 activity in isolated cardiomyocytes (10 μM) and to inhibit slowly inactivating Na+ currents (42), which can also confer protection during ischemia and reperfusion.
We tested AVE4454B to determine whether its efficacy for resuscitation is comparable to that of cariporide, assuming that cariporide would be less likely to be developed clinically because of the results of the EXPEDITION trial (23). However, our level of concern for the potential side effects of cariporide applied to resuscitation is low for a number of reasons. First, the mode of administration for resuscitation would differ substantially (single-bolus vs infusion for 48 hours). Second, the EXPEDITION trial indeed confirmed a cardiac benefit that was significant at day 5 and persisted at 6 months; whereas, the effect on mortality was significant only at day 5 but not at 6 months. Third, the effect on mortality at day 5 (increased from 1.5 % to 2.2%) – if confirmed and applicable to resuscitation – would be of debatable clinical relevance when applied to resuscitation from cardiac arrest given the currently poor survival rates of 5% and a resuscitation benefit expected to substantially offset such potential side effect.
Conclusions
Both compounds – AVE4454B and cariporide – elicited beneficial myocardial effects for resuscitation from cardiac arrest presumably by protecting mitochondrial integrity and function. Both compounds were able to preserve left ventricular myocardial distensibility during closed-chest resuscitation – which enabled hemodynamically more effective closed-chest resuscitation – and attenuated post-resuscitation myocardial dysfunction. However, cariporide was more effective than AVE4454B based on more favorable effects on left ventricular myocardial distensibility, spontaneous defibrillation, and survival rates which could stem from its non-specific effects on other ion transport mechanisms playing a role in sarcolemmal Na+ entry.
Acknowledgments
Financial support:
National Institutes of Health R01 HL71728 (to RJG); VA Merit Review Grant (to RJG).
ABBREVIATIONS
- +dP/dtmax
Maximal rate of left ventricular pressure rise
- AVE
AVE4454B
- CRP
Cariporide
- LVEDP
Left ventricular end diastolic pressure
- LVSWI
Left ventricular stroke work index
- MAP
Mean aortic pressure
- NHE-1
Sodium-hydrogen exchanger isoform 1
- NHEI
NHE-1 inhibition
- ROSC
Return of spontaneous circulation
- Tau
Relaxation time constant
- VF
Ventricular fibrillation
REFERENCES
- 1.von Planta M, Weil MH, Gazmuri RJ, Bisera J, Rackow EC. Myocardial acidosis associated with CO2 production during cardiac arrest and resuscitation. Circulation. 1989;80:684–692. doi: 10.1161/01.cir.80.3.684. [DOI] [PubMed] [Google Scholar]
- 2.Kette F, Weil MH, Gazmuri RJ, Bisera J, Rackow EC. Intramyocardial hypercarbic acidosis during cardiac arrest and resuscitation. Crit Care Med. 1993;21:901–906. doi: 10.1097/00003246-199306000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Noc M, Weil MH, Gazmuri RJ, Sun S, Bisera J, Tang W. Ventricular fibrillation voltage as a monitor of the effectiveness of cardiopulmonary resuscitation. J Lab Clin Med. 1994;124:421–426. [PubMed] [Google Scholar]
- 4.Karmazyn M, Sawyer M, Fliegel L. The na(+)/h(+) exchanger: a target for cardiac therapeutic intervention. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:323–335. doi: 10.2174/1568006054553417. [DOI] [PubMed] [Google Scholar]
- 5.Wang S, Radhakrishnan J, Ayoub IM, Kolarova JD, Taglieri DM, Gazmuri RJ. Limiting sarcolemmal Na+ entry during resuscitation from VF prevents excess mitochondrial Ca2+ accumulation and attenuates myocardial injury. J Appl Physiol. 2007;103:55–65. doi: 10.1152/japplphysiol.01167.2006. [DOI] [PubMed] [Google Scholar]
- 6.Imahashi K, Kusuoka H, Hashimoto K, Yoshioka J, Yamaguchi H, Nishimura T. Intracellular sodium accumulation during ischemia as the substrate for reperfusion injury. Circ Res. 1999;84:1401–1406. doi: 10.1161/01.res.84.12.1401. [DOI] [PubMed] [Google Scholar]
- 7.Gazmuri RJ, Hoffner E, Kalcheim J, Ho H, Patel M, Ayoub IM, Epstein M, Kingston S, Han Y. Myocardial protection during ventricular fibrillation by reduction of proton-driven sarcolemmal sodium influx. J Lab Clin Med. 2001;137:43–55. doi: 10.1067/mlc.2001.111693. [DOI] [PubMed] [Google Scholar]
- 8.Avkiran M, Ibuki C, Shimada Y, Haddock PS. Effects of acidic reperfusion on arrhythmias and Na(+)-K(+)-ATPase activity in regionally ischemic rat hearts. Am J Physiol. 1996;270:H957–H964. doi: 10.1152/ajpheart.1996.270.3.H957. [DOI] [PubMed] [Google Scholar]
- 9.An J, Varadarajan SG, Camara A, Chen Q, Novalija E, Gross GJ, Stowe DF. Blocking Na(+)/H(+) exchange reduces [Na(+)](i) and [Ca(2+)](i) load after ischemia and improves function in intact hearts. Am J Physiol. 2001;281:H2398–H2409. doi: 10.1152/ajpheart.2001.281.6.H2398. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto S, Matsui K, Ohashi N. Protective effect of Na+ /H+ exchange inhibitor, SM-20550, on impaired mitochondrial respiratory function and mitochondrial Ca2+ overload in ischemic/reperfused rat hearts. J Cardiovasc Pharmacol. 2002;39:569–575. doi: 10.1097/00005344-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Borutaite V, Brown GC. Mitochondria in apoptosis of ischemic heart. FEBS Lett. 2003;541:1–5. doi: 10.1016/s0014-5793(03)00278-3. [DOI] [PubMed] [Google Scholar]
- 12.Ayoub IM, Radhakrishnan J, Gazmuri RJ. Targeting mitochondria for resuscitation from cardiac arrest. Crit Care Med. 2008;36:S440–S446. doi: 10.1097/ccm.0b013e31818a89f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gazmuri RJ, Ayoub IM, Hoffner E, Kolarova JD. Successful ventricular defibrillation by the selective sodium-hydrogen exchanger isoform-1 inhibitor cariporide. Circulation. 2001;104:234–239. doi: 10.1161/01.cir.104.2.234. [DOI] [PubMed] [Google Scholar]
- 14.Gazmuri RJ, Ayoub IM, Kolarova JD, Karmazyn M. Myocardial protection during ventricular fibrillation by inhibition of the sodium-hydrogen exchanger isoform-1. Crit Care Med. 2002;30:S166–S171. doi: 10.1097/00003246-200204001-00010. [DOI] [PubMed] [Google Scholar]
- 15.Ayoub IM, Kolarova JD, Yi Z, Trevedi A, Deshmukh H, Lubell DL, Franz MR, Maldonado FA, Gazmuri RJ. Sodium-hydrogen exchange inhibition during ventricular fibrillation: Beneficial effects on ischemic contracture, action potential duration, reperfusion arrhythmias, myocardial function, and resuscitability. Circulation. 2003;107:1804–1809. doi: 10.1161/01.CIR.0000058704.45646.0D. [DOI] [PubMed] [Google Scholar]
- 16.Ayoub IM, Kolarova J, Kantola RL, Sanders R, Gazmuri RJ. Cariporide minimizes adverse myocardial effects of epinephrine during resuscitation from ventricular fibrillation. Crit Care Med. 2005;33:2599–2605. doi: 10.1097/01.ccm.0000186773.88576.83. [DOI] [PubMed] [Google Scholar]
- 17.Kolarova J, Yi Z, Ayoub IM, Gazmuri RJ. Cariporide potentiates the effects of epinephrine and vasopressin by nonvascular mechanisms during closed-chest resuscitation. Chest. 2005;127:1327–1334. doi: 10.1378/chest.127.4.1327. [DOI] [PubMed] [Google Scholar]
- 18.Kolarova JD, Ayoub IM, Gazmuri RJ. Cariporide enables hemodynamically more effective chest compression by leftward shift of its flow-depth relationship. Am J Physiol Heart Circ Physiol. 2005;288:H2904–H2911. doi: 10.1152/ajpheart.01181.2004. [DOI] [PubMed] [Google Scholar]
- 19.Gazmuri RJ, Ayoub IM. The case for sodium-hydrogen exchanger isoform-1 inhibition during cardiac resuscitation remains strong. Crit Care Med. 2006;34:1580–1582. doi: 10.1097/01.CCM.0000216687.86553.EC. [DOI] [PubMed] [Google Scholar]
- 20.Ayoub IM, Kolarova J, Kantola R, Radhakrishnan J, Gazmuri RJ. Zoniporide preserves left ventricular compliance during ventricular fibrillation and minimizes post-resuscitation myocardial dysfunction through benefits on energy metabolism. Crit Care Med. 2007;35:2329–2336. doi: 10.1097/01.ccm.0000280569.87413.74. [DOI] [PubMed] [Google Scholar]
- 21.Ayoub IM, Kolarova J, Gazmuri RJ. Cariporide given during resuscitation promotes return of electrically stable and mechanically competent cardiac activity. Resuscitation. 2009;81:106–110. doi: 10.1016/j.resuscitation.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radhakrishnan J, Ayoub IM, Gazmuri RJ. Activation of caspase-3 may not contribute to postresuscitation myocardial dysfunction. Am J Physiol Heart Circ Physiol. 2009;296:H1164–H1174. doi: 10.1152/ajpheart.00338.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mentzer RM, Jr., Bartels C, Bolli R, Boyce S, Buckberg GD, Chaitman B, Haverich A, Knight J, Menasche P, Myers ML, Nicolau J, Simoons M, Thulin L, Weisel RD. Sodium-hydrogen exchange inhibition by cariporide to reduce the risk of ischemic cardiac events in patients undergoing coronary artery bypass grafting: results of the EXPEDITION study. Ann Thorac Surg. 2008;85:1261–1270. doi: 10.1016/j.athoracsur.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 24.Radhakrishnan J, Wang S, Ayoub IM, Kolarova JD, Levine RF, Gazmuri RJ. Circulating levels of cytochrome c after resuscitation from cardiac arrest: a marker of mitochondrial injury and predictor of survival. Am J Physiol Heart Circ Physiol. 2007;292:H767–H775. doi: 10.1152/ajpheart.00468.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Planta I, Weil MH, von Planta M, Bisera J, Bruno S, Gazmuri RJ, Rackow EC. Cardiopulmonary resuscitation in the rat. J Appl Physiol. 1988;65:2641–2647. doi: 10.1152/jappl.1988.65.6.2641. [DOI] [PubMed] [Google Scholar]
- 26.Klouche K, Weil MH, Sun S, Tang W, Povoas HP, Kamohara T, Bisera J. Evolution of the stone heart after prolonged cardiac arrest. Chest. 2002;122:1006–1011. doi: 10.1378/chest.122.3.1006. [DOI] [PubMed] [Google Scholar]
- 27.Takino M, Okada Y. Firm myocardium in cardiopulmonary resuscitation. Resuscitation. 1996;33:101–106. doi: 10.1016/s0300-9572(96)00995-1. [DOI] [PubMed] [Google Scholar]
- 28.Sanders AB, Atlas M, Ewy GA, Kern KB, Bragg S. Expired PCO2 as an index of coronary perfusion pressure. Am J Emerg Med. 1985;3:147–149. doi: 10.1016/0735-6757(85)90039-7. [DOI] [PubMed] [Google Scholar]
- 29.Gudipati CV, Weil MH, Bisera J, Deshmukh HG, Rackow EC. Expired carbon dioxide: A noninvasive monitor of cardiopulmonary resuscitation. Circulation. 1988;77:234–239. doi: 10.1161/01.cir.77.1.234. [DOI] [PubMed] [Google Scholar]
- 30.Singh D, Kolarova JD, Wang S, Ayoub IM, Gazmuri RJ. Myocardial protection by erythropoietin during resuscitation from ventricular fibrillation. Am J Ther. 2007;14:361–368. doi: 10.1097/01.pap.0000249957.35673.f0. [DOI] [PubMed] [Google Scholar]
- 31.Grmec S, Strnad M, Kupnik D, Sinkovic A, Gazmuri RJ. Erythropoietin facilitates the return of spontaneous circulation and survival in victims of out-of-hospital cardiac arrest. Resuscitation. 2009;80:631–637. doi: 10.1016/j.resuscitation.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 32.Gazmuri RJ, Weil MH, Bisera J, Tang W, Fukui M, McKee D. Myocardial dysfunction after successful resuscitation from cardiac arrest. Crit Care Med. 1996;24:992–1000. doi: 10.1097/00003246-199606000-00020. [DOI] [PubMed] [Google Scholar]
- 33.Kern KB, Hilwig RW, Rhee KH, Berg RA. Myocardial dysfunction after resuscitation from cardiac arrest: An example of global myocardial stunning. J Am Coll Cardiol. 1996;28:232–240. doi: 10.1016/0735-1097(96)00130-1. [DOI] [PubMed] [Google Scholar]
- 34.Laurent I, Monchi M, Chiche JD, Joly LM, Spaulding C, Bourgeois B, Cariou A, Rozenberg A, Carli P, Weber S, Dhainaut JF. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol. 2002;40:2110–2116. doi: 10.1016/s0735-1097(02)02594-9. [DOI] [PubMed] [Google Scholar]
- 35.Ruiz-Bailen M, Aguayo dH, Ruiz-Navarro S, Diaz-Castellanos MA, Rucabado-Aguilar L, Gomez-Jimenez FJ, Martinez-Escobar S, Moreno RM, Fierro-Roson J. Reversible myocardial dysfunction after cardiopulmonary resuscitation. Resuscitation. 2005;66:175–181. doi: 10.1016/j.resuscitation.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 36.Xu T, Tang W, Ristagno G, Wang H, Sun S, Weil MH. Postresuscitation myocardial diastolic dysfunction following prolonged ventricular fibrillation and cardiopulmonary resuscitation. Crit Care Med. 2008;36:188–192. doi: 10.1097/01.CCM.0000295595.72955.7C. [DOI] [PubMed] [Google Scholar]
- 37.Phillis JW, Estevez AY, Guyot LL, O'Regan MH. 5-(N-Ethyl-N-isopropyl)-amiloride, an Na(+)-H(+) exchange inhibitor, protects gerbil hippocampal neurons from ischemic injury. Brain Res. 1999;839:199–202. doi: 10.1016/s0006-8993(99)01705-9. [DOI] [PubMed] [Google Scholar]
- 38.Pedersen SF. The Na+/H+ exchanger NHE1 in stress-induced signal transduction: implications for cell proliferation and cell death. Pflugers Arch. 2006;452:249–259. doi: 10.1007/s00424-006-0044-y. [DOI] [PubMed] [Google Scholar]
- 39.Schelling JR, bu Jawdeh BG. Regulation of cell survival by Na+/H+ exchanger-1. Am J Physiol Renal Physiol. 2008;295:F625–F632. doi: 10.1152/ajprenal.90212.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Redlin M, Werner J, Habazettl H, Griethe W, Kuppe H, Pries AR. Cariporide (HOE 642) attenuates leukocyte activation in ischemia and reperfusion. Anesth Analg. 2001;93:1472–1479. doi: 10.1097/00000539-200112000-00024. [DOI] [PubMed] [Google Scholar]
- 41.Palandoken H, By K, Hegde M, Harley WR, Gorin FA, Nantz MH. Amiloride peptide conjugates: prodrugs for sodium-proton exchange inhibition. J Pharmacol Exp Ther. 2005;312:961–967. doi: 10.1124/jpet.104.076984. [DOI] [PubMed] [Google Scholar]
- 42.Scholz W, Albus U, Counillon L, Gogelein H, Lang HJ, Linz W, Weichert A, Scholkens BA. Protective effects of HOE642, a selective sodium-hydrogen exchange subtype 1 inhibitor, on cardiac ischaemia and reperfusion. Cardiovasc Res. 1995;29:260–268. [PubMed] [Google Scholar]