Abstract
Epithelial cells and most adherent normal cells rely on adhesion-dependent, integrin-mediated survival signals from the extracellular matrix (ECM) to survive. When these cells are deprived of adhesion to the ECM, they undergo a specific form of apoptosis termed “anoikis.” In contrast, malignant cells have attained mechanisms to enable them to survive in the absence of adhesion. This acquisition of anoikis resistance allows tumor cells to grow in an anchorage-independent manner and achieve metastatic disease. Recent studies have identified the mitochondrial Bcl2-inhibitor of transcription (Bit1) protein as part of a novel anoikis pathway. This review will focus on the biological function of Bit1 in the anoikis process, the underlying molecular mechanism of Bit1 apoptotic function, and its role in tumor metastasis.
Keywords: apoptosis, anoikis, integrins, metastasis
1. Introduction
A key hallmark of cancer cells is to evade anoikis or detachment-induced apoptosis [1]. By allowing cancer cells to survive in inappropriate places and to disseminate through the body, anoikis resistance is a critical determinant of tumor progression and metastasis and represents as a critical barrier to cancer treatment [2]. Hence, one important therapeutic strategy in curtailing tumor aggressiveness and metastasis is to restore or induce anoikis sensitivity in cancer cells. This experimental approach requires a detailed understanding of the molecular mechanisms of the anoikis-mediated cell death pathway in order to identify novel cell death effectors that can serve as therapeutic targets to overcome the anoikis resistance of tumor cells. In this review, we describe the molecular discovery and recent findings of the novel Bit1 anoikis pathway [3].
Bit1 is a 179-residue mitochondrial protein which appears to be part of a previously unknown apoptosis pathway that is regulated by integrin-mediated cell attachment [3]. As an anoikis effector, Bit1 is released from mitochondria in cells cultured in suspension and forms a complex with the transcriptional regulator protein Amino-terminal Enhancer of Split (AES). The formation of the Bit1-AES complex initiates a caspase-independent form of apoptosis. Integrin-mediated cell adhesion appears to be the only upstream anti-apoptotic treatment that can rescue cells from apoptosis induced by Bit1. Knowledge of the molecular workings and significance of the Bit1 apoptotic pathway may help design new ways of initiating anoikis for therapeutic purposes.
2. Anoikis: Apoptosis Following Loss of Anchorage-dependent Survival Signaling
Integrins are heterodimeric transmembrane proteins consisting of α and β subunits and function as receptors to many extracellular matrix (ECM) proteins, such as fibronectin and laminin. As mediators of cell-ECM interaction, integrins not only provide physical links with the cytoskeleton but also transduce signals from the ECM to the cell that are important for diverse cellular processes including migration, proliferation, and survival [1,2,4–6]. An enormous amount of literature exists documenting the central role of integrins in suppressing apoptosis in attached cells by eliciting anti-apoptotic and prosurvival signals from the ECM [2,6]. Indeed, loss of integrin-mediated attachment to the ECM in anchorage-dependent normal cells (such as epithelial and endothelial cells) leads to induction of apoptosis, which is termed as anoikis [1]. On the other hand, malignant or transformed cells are less dependent on integrin-mediated survival signals. Hence, such cells are anoikis resistant and are able to survive in an anchorage-independent manner.
Integrins are categorized depending on their α or β subunit composition. There at least four types of integrins (α5β1, αvβ3, α1β1 and α6β1) that have been shown to play a role in cell survival [7–10] in various cellular models. These specific integrins have differing abilities to protect cells from apoptosis and anoikis, suggesting that they utilize diverse signaling pathways. Their downstream pathways or molecules are diverse which include the focal adhesion kinase (FAK) [11], Src kinase [12], integrin-linked kinase (ILK) [13], the phosphatidylinositol 3-kinase (PI3K)/Akt [14], the extracellular signal regulated kinase (ERK) /mitogen activated protein kinase [15], and the antiapoptotic Bcl2 protein [7,16]. Activation or induction of expression of these signaling molecules has been shown to attenuate or block cellular anoikis, and it is therefore not surprising that some of these molecules have been found to upregulated or activated in malignant cells.
3. Molecular Pathways of Anoikis
The induction of the anoikis process can be classified by the interplay of two critical apoptotic pathways, the mitochondrial (intrinsic) and cell death receptor (extrinsic) pathways. In anoikis proficient cells, detachment from the ECM leads to disengagement of integrin-mediated attachment and subsequent loss of phosphorylation and inactivation of key downstream survival effectors (FAK, PI3K/Akt, ERK and MAP kinase). Inactivation of these integrin intermediates results in recruitment and activation of the pro-apoptotic members of the BCL family of proteins (Bax and Bak) which trigger mitochondrial dysfunction, cytochrome c release, downstream caspase activation, and eventual apoptosis [2,17]. Consistent with the role of the mitochondrial pathway in anoikis, cell detachment has also been shown to downregulate the expression of Bcl-xL, an anti-apoptotic Bcl family member that guards mitochondrial integrity [18], and such Bcl-xL suppression further compounds the mitochondrial-dependent caspase activation loop and apoptosis. The death receptor (intrinsic) pathway, which is activated by the binding of death ligands (Fas or TRAIL) to death receptor, hinges upon the formation of the death inducing signal complex (DISC) and upon FAS-associated death domain (FADD) dependent activation of caspase 8 [19,20]. The FADD-mediated caspase 8 activation results in the cleavage and activation of Bid, which destabilizes the mitochondrial membrane leading to apoptosis. Previous studies demonstrating the upregulation of Fas ligand [21] and specific caspase 8 activation [22] following loss of cell attachment in normal adherent cells indicate the important role of the intrinsic pathway to the anoikis process. The intrinsic pathway appears to be dysfunctional in malignant cells in part through failure to achieve death receptor-induced caspase 8 activation.
As described above, both extrinsic and intrinsic apoptotic pathways merge at and rely on the mitochondrial-dependent caspase activation loop to effect cell death, pointing to caspase signaling as a critical mechanism to trigger anoikis. Hence, tumors that possess defects in the regulation of caspase activation may conceivably be more resistant to undergo anoikis. Interestingly, certain metastatic tumors which exhibit inefficient caspase activation through overexpression of Bcl-2 or inactivation of caspase- 3 were found to be equally susceptible to undergo anoikis as their non-metastatic counterparts [23]. Such evidence indicates the existence of an alternative caspase-independent mode of anoikis. Indeed, Ruoslahti and colleagues have shown that integrins may regulate apoptosis through a caspase-independent mechanism [3]. In particular, loss of integrin-mediated attachment results in the release of mitochondrial Bit1 protein to the cytoplasm where it forms a complex with the transcriptional coregulator AES and subsequently induces a caspase-independent mode of apoptosis.
4. The Bit1 anoikis pathway
4.1. Screening and identification of the Bit1 protein
Jan and colleagues (2004) had previously established that the integrin-dependent FAK/PI3K/AKT survival pathway involved increased expression of Bcl2 [3]. In search of novel regulators of anoikis, they screened for cDNAs that regulate the Bcl2 anti-apoptotic pathway using the Bcl2 reporter system. One of the clones revealed by the screen encodes a novel 179-residue protein that is highly conserved from bacteria to human [24]. Based on its ability to reduce Bcl-2 promoter activity, this protein was named Bit1 (Bcl2, inhibitor of transcription). The archaeon and eukaryotic Bit1 contains an N-terminal mitochondrial localization sequence (MLS) which is absent in the bacterial homologue. Detailed crystallographic studies showed that human Bit1 contains a peptidyl-tRNA hydrolase domain [25], and hence it functions as a peptidyl-tRNA hydrolase 2 enzyme that may be important in protein translation. In adherent human cells, native mitochondrial Bit1 also serves as a mediator of integrin-dependent survival signals [26]. Interestingly, the endogenous Bit1 protein was also found to be localized in the endoplasmic reticulum membrane (ER) microdomains and Golgi complex, functioning as a regulator of the early secretory pathway [27].
4.2. Molecular characterization of Bit1 apoptotic function
Because of its hydrophobic N-terminal MLS sequence, Bit1 has been suggested to be a mitochondrial protein. Indeed, endogenous Bit1 of normal and transformed human adherent cells was found to be in the mitochondria, as evidenced by its colocalization with known mitochondrial markers and its presence in the heavy membrane (mitochondrial) fraction [3]. Like many other mitochondrial proteins, the Bit1 protein was found to be apoptotic when it is released to the cytoplasm. The Bit1 apoptotic activity was first observed in HEK-293T cells transfected with the Bit1 construct containing an N-terminal myc tag [3]. Interestingly, the N-terminally myc tagged Bit1 was localized in the cytoplasm and triggered significant apoptosis as evidenced by DNA fragmentation and annexin V (early marker of apoptosis) positivity. The unexpected cytosolic expression of Bit1 was due to disruption of the N-terminal MLS via the insertion of an N-terminal tag. In agreement with the notion that cytosol localization is required for its apoptotic activity, cells transfected with the C-terminally myc tagged Bit1 construct, which targets Bit1 to the mitochondria, did not exhibit any detectable apoptosis [3].
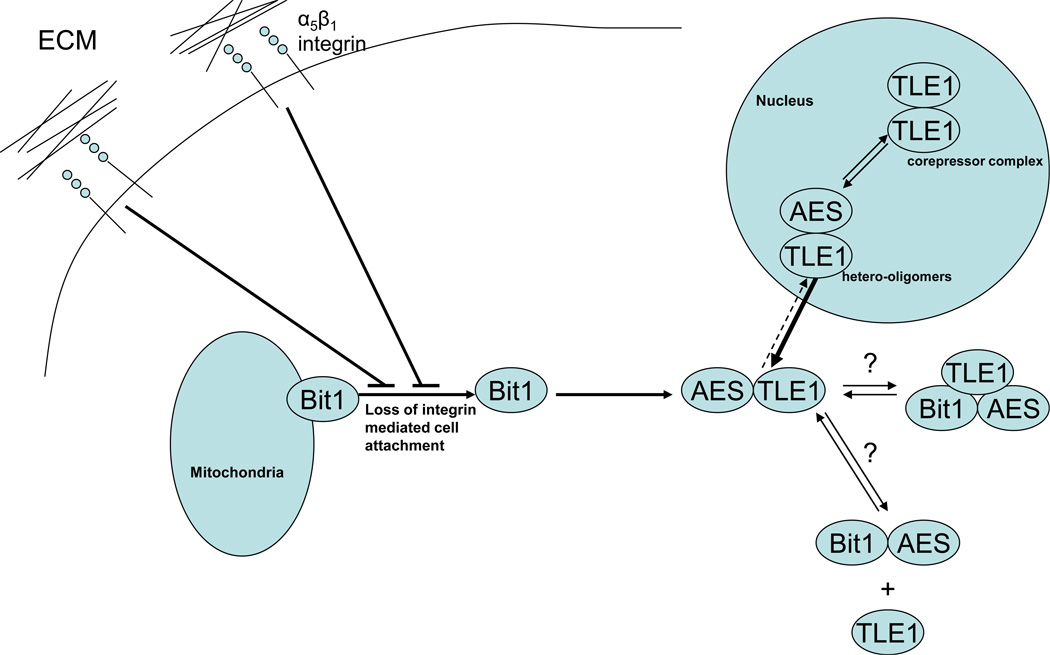
What is the molecular mechanism governing the Bit1 apoptotic function? Upon treatment with apoptosis-inducing agents such as staurosporin, or following culture in suspension, mitochondrial Bit1 is released to the cytoplasm where it forms a complex with the groucho transcriptional regulator AES protein (Fig. 1) and subsequently induces a caspase-independent form of apoptosis [3]. The biological interaction between Bit1 and AES and the formation of the Bit1-AES complex in the cytoplasm seem to be the trigger to induce cellular apoptosis. In agreement with this notion, HOP92 cells that lack AES expression are resistant to Bit1 apoptosis, and conversely, endogenous Bit1 expression-lacking HEK-293T cells do not exhibit apoptosis when transfected with AES. Two factors (α5β1-mediated attachment to fibronectin and the groucho related TLE1 corepressor protein) that are effective in inhibiting Bit1 apoptosis significantly reduce the levels of Bit1-AES complex, further indicating that this complex is a critical mediator of the Bit1 apoptotic pathway [3].
Figure 1.
Bit1 as an anoikis effector. In intact adherent cells, Bit1 is localized in the mitochondria. Following loss of integrin-mediated cell attachment, Bit1 is released from the mitochondria to the cytosol, forms a complex with the transcriptional regulator Amino-terminal Enhancer Split (AES) protein, and induces apoptosis [3]. Although the exact mechanism of Bit1-mediated apoptosis is yet to be characterized, the extramitochondrial Bit1 is likely to turn off the survival promoting function of the nuclear corepressor TLE1 protein, through competitive channeling of nuclear TLE1/AES hetero-oligomers to the cytoplasm. Cytoplasmic expression of Bit1 or its cell death domain (CDD) induces translocation of nuclear TLE1 to the cytoplasm in an AES-dependent manner [28], suggesting that the Bit1-AES complex may direct the sequestration of TLE1/AES hetero-oligomers from the nucleus to the cytoplasm, likely through Bit1/AES/TLE1 tricomplex formation. The existence of such a tri-complex, however, has not yet been demonstrated.
The mechanism of how the Bit1-AES complex induces apoptosis remains to be determined. Based on our previous and recent data, we proposed a model whereby Bit1 may switch off the survival promoting gene-transcription program mediated by the groucho related TLE1 corepressor protein. Through its association with AES in the cytosol, the mitochondrial released Bit1 may channel any pre-existing nuclear AES-TLE1 hetero-oligomers to the cytoplasm and subsequently lower nuclear TLE1 levels (Fig. 1). Consistent with this model, cytoplasmic localized Bit1 or its cell death domain (CDD) triggers significant re-localization of nuclear TLE1 to the cytoplasm in an AES dependent manner [28]. The sequestration of nuclear TLE1 to the cytoplasm and/or loss of nuclear TLE1 hetero-oligomers may disallow the transcriptional regulatory activity of TLE1. In further agreement with the proposed model, counteracting the TLE1 nuclear to cytoplasmic shuttling via exogenous nuclear TLE1 expression counteracts Bit1 apoptosis [28]. Although the TLE1 transcriptional complex that confers cell survival that remains to be characterized, the TLE1 corepressor has been shown to be antiapoptotic and oncogenic in several cellular model systems [29–31]. Characterization of the anti-apoptotic TLE1 transcriptional program and its regulation by the Bit1/AES complex will yield important clues that will aid in our understanding of the Bit1 apoptotic pathway. In particular, identification of the TLE1 downstream target genes may provide the basis or mechanism on how the Bit1/AES complex triggers apoptosis. It remains a possibility that loss of the TLE1 corepressor function as induced by the Bit1/AES axis may lead to upregulation of expression of nuclear enzymes such as endonucleases which can facilitate DNA fragmentation and nuclear apoptosis.
Consistent with our model that Bit1 functions as an antagonist to the survival promoting TLE1 transcriptional program is the inhibitory effect of Bit1 on TLE1-induced Bcl2 expression. Promoter analysis studies showed that TLE1 enhances BcL-2 activity while Bit1/AES has suppressive effect on the BcL-2 promoter activity [3]. Our unpublished microarray data also indicate that the mRNAs of two additional anti-apoptotic proteins, HSP-70 [32] and thymosin β [33,34] are down-regulated by Bit1/AES and up-regulated by TLE1. These findings indicate potential gene regulatory function of the Bit1/AES axis likely through its regulation of the TLE1 transcriptional activity.
Another area that remains to be investigated is the regulatory mechanism underlying mitochondrial Bit1 release following an apoptotic insult. The release of mitochondrial Bit1 to the cytoplasm (like other mitochondrial resident proteins) may be a consequence of mitochondrial membrane permeabilization and dysfunction. Cell death stimuli such as loss of integrin-mediated cell attachment can recruit and activate pro-apoptotic Bcl family members to destabilize mitochondrial membrane, leading to cytoplasmic Bit1 release and subsequent Bit1 apoptotic activity. Hence, survival pathways such as PI3/AKT and ERK2 signaling and overexpression of antiapoptotic Bcl proteins (Bcl-2 and Bcl-xL) that guard mitochondrial integrity can conceivably protect cells from Bit1 apoptosis through prevention of Bit1 release. Further mechanistic studies addressing how these cellular factors (caspases and Bcl protein family members) regulate Bit1 translocation to cytoplasm will undoubtedly yield important insights on Bit1 apoptotic function.
The Bit1 cell death function may represent a unique apoptotic mechanism based on three features. First, once Bit1 is released to the cytoplasm, Bit1 induces apoptosis that appears to be independent from caspase activation based on the following findings: 1) absence of PARP cleavage in the lysates of Bit1 transfected cells, 2) caspase inhibitor proteins crmA and XIA had no effect on apoptosis induced by Bit1, and 3) the chemical pan-caspase inhibitor, z-VAD-fmk, was ineffective against Bit1 apoptosis [3,28]. Second, the apoptotic function of cytoplasmic Bit1 is unresponsive to several anti-apoptotic factors including treatment with PI-3K, Akt, Bcl-2, and Bcl-xl [3]. These antiapoptotic molecules, while capable of maintaining mitochondrial integrity and preventing the release of mitochondrial Bit1, are ineffective in blocking the apoptosis function of Bit1 once it is released in the cytoplasm (post-mitochondrial stage). Third, the only upstream factor that can effectively block Bit1 apoptotic activity upon its release from the mitochondria is integrin-mediated cell attachment. In particular, attachment to fibronectin via the α5β1 integrin provides the most protection against Bit1 apoptosis [3] by reducing the formation of the pro-apoptotic Bit1-AES complex. This finding raises the possibility that suppression of the Bit1 apoptotic pathway may underlie the ability of integrin signaling to prevent cell death caused by loss of cell attachment.
4.3. Bit1 as an anoikis effector
The ability of integrin-mediated attachment to effectively block Bit1 apoptosis indicates that Bit1 may play a critical role in anoikis as a “guardian of anchorage dependence” [3]. Indeed, since the discovery of the Bit1 protein, numerous data have emerged demonstrating Bit1 as an effector of anoikis in both normal (human mammary epithelial cell line MCF10A [3], human umbilical vein endothelial cells HUVEC[36] and transformed cell lines (HEK-293T [3], HeLa [3,36], MCF-7 [37], MDA-MB-231[28]). The role of Bit1 in the anoikis process was originally examined by increasing the expression of mitochondrial Bit1 expression in HEK-293T cells via transfection with a mitochondrial localized Bit1 or vector construct, and the cells susceptibility to undergo apoptosis in attached and detached conditions was examined [3]. While the Bit1 and vector transfected cells exhibited similar levels of spontaneous apoptosis when grown attached to a culture dish, detachment induced a significantly higher level of apoptosis in Bit1 transfected cells than in control cells. In agreement with a requirement of cytosolic Bit1 to induce apoptosis, Bit1 was released from mitochondria and formed a complex with AES only in cells cultured in suspension, not in attached conditions [3]. Similar anoikis induction was also observed upon exogenous expression of mitochondrial Bit1 in the highly aggressive MDA-MB-231 breast cancer cell line [28,37]. To further investigate the role of Bit1 in the anoikis process, the effect of disabling the Bit1 apoptotic pathway in suspension-induced apoptosis was examined. Downregulation of endogenous Bit1 expression via siRNA and shRNA technology decreased the sensitivity of normal (MCF10A [3], HUVEC [35] and cancer (HeLa [36],MCF-7 [37]) cells to anoikis. Taken together, these findings indicate Bit1 is a critical effector of the anoikis cell death pathway.
Considering that anoikis insensitivity is a primary determinant of transformation [2], malignant cells are likely to circumvent the Bit1 pathway to become anoikis resistant and anchorage-independent. Hence, it will be interesting to see if Bit1 expression is downregulated or if its inhibitor TLE1 protein is overexpressed in advanced stages of cancer. Recently, it has been shown that Bit1 expression is significantly downregulated in a fraction of invasive breast carcinomas as compared to normal breast tissue and noninvasive ductal carcinoma in situ (DCIS) lesions [28,37]. In addition, TLE1 has been shown to be elevated and is considered as a putative oncogene in human lung carcinoma [31]. Elevated TLE1 expression is an unfavorable prognostic marker in lymphoma [38] and sarcoma [39]. Although the exact role of TLE1 in cancer progression remains to be determined, TLE1 may function to protect cancer cells against anoikis. Indeed, TLE1 has recently been shown to suppress anoikis in breast cancer cells, and that its inhibitory effect on anoikis is in part through circumventing the Bit1-mediated anoikis pathway [28].
Experimental data on the signaling mechanisms underlying the Bit1 anoikis pathway have begun to emerge. Recently, the Erk MAP kinase pathway was found to be one of the downstream effectors of Bit1. Cultured HeLa [36] and MCF7 [37] cells in which Bit1 had been downregulated and mouse embryo fibroblasts from Bit1 knockout mice [36] exhibited enhanced Erk activation. Interestingly, the Bit1 knockdown cells did not exhibit increased upstream MEK activation, but instead showed decreased Erk phosphatase activity. The increased Erk activation contributed in part to the enhanced anoikis resistance of the Bit1 knockdown cells, since forced inhibition of the Erk pathway partially restored their anoikis sensitivity. Another important mechanism that can regulate Bit1 anoikis function is the translocation efficiency of Bit1 from the mitochondria to the cytosol in response to the loss of integrin-mediated adhesion. PKD can phosphorylate the Bit1 S5 residue which is located within the N-terminal MLS [35]. Phosphorylation of the Bit1 S5 residue resulted in mitochondrial import inefficiency and subsequent increase in Bit1 cytosolic localization. Indeed, activation of PKD enhanced the mitochondrial release of Bit1 with concomitant induction of the Bit1 anoikis function.
5. Bit1 as a suppressor of metastasis
Anoikis resistance is a determinant of transformation and tumor metastatic potential [2]. Hence, disruption of the Bit1 pathway may increase the tumorigenic and/or metastatic proficiency of malignant cells, at least in part by providing cancer cells with an alternative way of resisting anoikis. To address the potential role of Bit1 in tumorigenicity, stable control and Bit1 knockdown pools of cells derived from the mouse melanoma B16F1 cell line were injected subcutaneously into nude mice [37]. The control and Bit1 knockdown cells exhibited similar tumor growth kinetics. Interestingly, the lungs of the mice with tumors from the Bit1 knockdown pool showed increased incidence of metastatic colonies as compared to the lungs of control tumor bearing mice. To validate these findings, an experimental metastasis assay was performed wherein Bit1 knockdown and control B16F1 cells were injected intravenously into nude mice. The lungs of mice that received injections of Bit1 knockdown cells showed increased numbers and larger sizes of metastatic foci. Downregulation of endogenous Bit1 expression in the MCF7 cell line also resulted in enhanced metastasis using the experimental metastasis assay. In agreement with these results, exogenous expression of mitochondrial Bit1 in the highly aggressive and metastatic melanoma B16F10 cell line significantly attenuated the formation of metastatic colonies in the lungs using an experimental metastasis assay [37]. Taken together, these findings indicate that Bit1 is a suppressor of metastasis.
The signaling pathways downstream of Bit1 in inhibiting tumor metastasis remain to be explored. Given the inhibitory effect of Bit1 on the Erk pathway whose sustained activation has been implicated in several steps of the metastatic process [40], the role of Erk activation in the enhanced metastasis of Bit1 knockdown cells was examined by immunohistochemistry staining of the metastatic tumor foci for active Erk [37]. The metastatic foci from Bit1 knockdown B16F1 cells showed increased phosphor-Erk staining as compared to that of control cells, suggesting that activation of Erk may be an important molecular event contributing to the enhanced metastatic potential of Bit1 knockdown cells. However, additional studies are needed to determine whether the observed elevated Erk activity in Bit1 knockdown cells directly contributes to enhanced metastasis. For example, does inhibiting Erk activity via siRNA-mediated Erk downregulation or treatment with Erk inhibitors reverse the increased metastatic potential of Bit1 knockdown cells? Knowledge of the mechanism underlying Erk regulation by Bit1 may yield clues as to the importance of the Erk pathway in the Bit1 inhibitory effect on metastasis.
6. Conclusions and future directions
The molecular circuitry and key signaling effectors of the anoikis mode of cell death have started to be unraveled. It has been established that following loss of cell attachment both the extrinsic death receptor and intrinsic mitochondrial pathways are activated through a caspase-dependent mechanism. The identification and characterization of the mitochondrial Bit1 protein indicates the existence of an alternative caspase-independent cell death machinery that is activated following loss of cell attachment. In light of increasing data documenting that tumor cells exhibit disabled or inefficient caspase activity, triggering anoikis sensitivity in tumor cells via the caspase-independent mechanism may represent a viable and effective strategy. Based on its pronounced inhibitory effect on anoikis resistance, which is a primary determinant of the metastatic process, Bit1 can be used as an adjunct therapeutic agent to specifically block metastasis. The suitability of Bit1 as a therapeutic target, however, hinges on several important considerations including knowledge of the specific requirements of Bit1 in normal cell physiology, the expression pattern of Bit1 in normal tissues, and the regulation of Bit1 expression among the different cell types within the tumor microenvironment.
Acknowledgments
This work was supported by Louisiana Cancer Research Consortium (LCRC) Start up Grant (HB), NIH RCMI G12RR026250-03 Grant (to Xavier University of Losuiana), NIH SC2CA153382 grant (HB), NIH 1R15CA158677-01A1 (HB), Deparment of Defense Breast Cancer Program Grant W81XWH-08-10727 (ER), and Cancer Center Support Grant CA30199 (to the Sanford-Burnham Medical Research Institute).
Abbreviations
- Bit1
Bcl-2 inhibitor of transcription 1
- TLE1
Transducing-like Enhancer of split 1
- AES
Amino-terminal Enhancer of Split
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
None
References
- 1.Frisch SM, Frances H. Disruption of epithelial cell-matrix interactions induce apoptosis. J. Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frisch SM, Ruoslahti E. Integrins and anoikis. Curr. Opin. Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- 3.Jan Y, Matter M, Pai JT, Chen YL, Pilch J, Komatsu M, Ong E, Fukuda M, Ruoslahti E. A mitochondrial protein, Bit1, mediates apoptosis regulated by integrins and Groucho/TLE corepressors. Cell. 2004;116:751–762. doi: 10.1016/s0092-8674(04)00204-1. [DOI] [PubMed] [Google Scholar]
- 4.Boudreau NH, Jones PL, et al. Extracellular matrix and integrin signaling: the shape of things to come. Biochemical Journal. 1999;339:481–488. [PMC free article] [PubMed] [Google Scholar]
- 5.Reddig RJ, Juliano RL. Clinging to life: cell to matrix adhesion and cell survival. Cancer and Metastasis Reviews. 2005;24:425–439. doi: 10.1007/s10555-005-5134-3. [DOI] [PubMed] [Google Scholar]
- 6.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z, Vuori K, Reed JC, Ruoslahti E. The alpha 5 beta 1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc. Natl. Acad. Sci. USA. 1995;92:6161–6165. doi: 10.1073/pnas.92.13.6161. 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Brien V, Frisch SM, Juliano RL. Expression of the integrin alpha 5 subunit in HT29 colon carcinoma cells suppresses apoptosis triggered by serum deprivation. Exp. Cell Res. 1996;224:208–213. doi: 10.1006/excr.1996.0130. [DOI] [PubMed] [Google Scholar]
- 9.Matter ML, Zhang Z, Nordstedt C, Ruoslahti E. The alpha 5 beta 1 integrin mediates elimination of amyloid-beta peptide and protects against apoptosis. J. Cell. Biol. 1998;141:1019–1030. doi: 10.1083/jcb.141.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brassard DL, Maxwell E, Malkowski M, Nagabhushan TL, Kumar CC, Armstrong L. Integrin alpha(v)beta(3)-mediated activation of apoptosis. Exp. Cell Res. 1999;251:33–45. doi: 10.1006/excr.1999.4559. [DOI] [PubMed] [Google Scholar]
- 11.Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PV. Control of adhesion-dependent cell survival by focal adhesion kinase. J. Cell Biol. 1996;134:793–399. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23:7906–7909. doi: 10.1038/sj.onc.1208160. [DOI] [PubMed] [Google Scholar]
- 13.Bottcher BT, Lange A, Fassler R. How ILK and kindlins cooperate to orchestrate integrin signaling. Curr. Opin. Cell Biol. 2009;21:670–675. doi: 10.1016/j.ceb.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne PH, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins NL, Reginato MJ, Paulus JK, Sgroi DC, Labaer J, Brugge JS. G1/S cell cycle arrest provides anoikis resistance through Erk-mediated Bim suppression. Mol Cell Biol. 2005;25:5281–5291. doi: 10.1128/MCB.25.12.5282-5291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matter MA, Ruoslahti E. A signaling pathway from the α5β1 and αvβ3 integrins that elevates Bcl-2 transcription. J. Biol. Chem. 2001;276:27757–27763. doi: 10.1074/jbc.M102014200. [DOI] [PubMed] [Google Scholar]
- 17.Frisch SM, Screaton RA. Anoikis Mechanisms. Curr. Opin. Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 18.Rosen K, Rak J, Leung T, Dean NM, Kerbel RS, Filmus J. Activated Ras prevents downregulation of Bcl-X(L) triggered by detachment from the extracellular matrix, A mechanism of Ras-induced resistance to anoikis in intestinal epithelial cells. J. Cell Biol. 2000;149:447–456. doi: 10.1083/jcb.149.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frisch SM. Evidence for a function of death-receptor-related, death-domain-containing proteins in anoikis. Curr. Biol. 1999;9:1047–1049. doi: 10.1016/s0960-9822(99)80455-2. [DOI] [PubMed] [Google Scholar]
- 20.Rytomaa M, Martins LM, Downward J. Involvement of FADD and caspase-8 signalling in detachment-induced apoptosis. Curr. Biol. 1999;9:1043–1046. doi: 10.1016/s0960-9822(99)80454-0. [DOI] [PubMed] [Google Scholar]
- 21.Rosen K, Shi W, Calabretta B, Filmus J. Cell detachment triggers p38 mitogen-activated protein kinase-dependent overexpression of Fas ligand, A novel mechanism of Anoikis of intestinal epithelial cells. J. Biol. Chem. 2002;277:46123–46130. doi: 10.1074/jbc.M207883200. [DOI] [PubMed] [Google Scholar]
- 22.Aoudjit F, Vuori K. Matrix attachment regulates Fas-induced apoptosis in endothelial cells: a role for c-flip and implications for anoikis. J. Cell Biol. 2001;152:633–643. doi: 10.1083/jcb.152.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bondar VM, McConkey DJ. Anoikis is regulated by BCL-2-independent pathways in human prostate carcinoma cells. Prostate. 2002;51:42–49. doi: 10.1002/pros.10070. [DOI] [PubMed] [Google Scholar]
- 24.Lai CH, Chou CY, Chang LY, Liu CS, Lin W. Identification of novel human genes evolutionarily conserved in Caenorhaditis elegans by comparative proteomics. Genome Research. 2001:703–713. doi: 10.1101/gr.10.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Pereda JM, et al. Crystal structure of a human peptidyl-tRNA hydrolase reveals a new fold and suggests basis for a bifunctional activity. J Biol Chem. 2004;279:8111–8115. doi: 10.1074/jbc.M311449200. [DOI] [PubMed] [Google Scholar]
- 26.Griffiths RS, et al. Bit-1 Mediates Integrin-dependent Cell Survival through Activation of the NFκB Pathway. J Biol Chem. 2011;286:14713–14723. doi: 10.1074/jbc.M111.228387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yi P, Nguyên DT, Higa-Nishiyama A, et al. MAPK scaffolding by BIT1 in the Golgi complex modulates stress resistance. J. Cell Sci. 2010;123:1060–1072. doi: 10.1242/jcs.059717. [DOI] [PubMed] [Google Scholar]
- 28.Brunquell C, Biliran H, Jennings S, Ireland SK, Chen R, Ruoslahti E. TLE1 is an anoikis regulator and is downregulated by Bit1 in breast cancer cells. Mol. Cancer Res. 2012;4 doi: 10.1158/1541-7786.MCR-12-0144. Published Online First September. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonderegger CK, Vogt PK. Binding of the corepressor TLE1 to Qin enhances Qin-mediated transformation of chicken embryo fibroblasts. Oncogene. 2003;22:1749–1757. doi: 10.1038/sj.onc.1206308. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Chen HM, Jaramillo E, Wang L, D’Mello SR, et al. Histone deacetylase-related protein inhibits AES-mediated neuronal cell death by direct interaction. J. Neuroscience Res. 2008;86:2423–2431. doi: 10.1002/jnr.21680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen T, van Tuyl M, Iyengar P, Jothy S, Post M, et al. Grg1 Acts as a Lung-Specific Oncogene in a Transgenic Mouse Model. Cancer Res. 2006;66:1294–1301. doi: 10.1158/0008-5472.CAN-05-1634. [DOI] [PubMed] [Google Scholar]
- 32.Nylandsted J, Rohde M, Brand K, Bastholm L, Elling F, Jäättelä M. Selective depletion of heat shock protein 70 (Hsp70) activates a tumor-specific death program that is independent of caspases and bypasses Bcl-2. Proc. Natl. Acad. Sci. USA. 2000;97:7871–7876. doi: 10.1073/pnas.97.14.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iguchi K, Usami Y, Hirano K, Hamatake M, Shibata M, Ishida R. Decreased thymosin beta4 in apoptosis induced by a variety of antitumor drugs. Biochem. Pharm. 1999;57:1105–1111. doi: 10.1016/s0006-2952(99)00030-1. [DOI] [PubMed] [Google Scholar]
- 34.Niu M, Nachmias VT. Increased resistance to apoptosis in cells overexpressing thymosin beta four: A role for focal adhesion kinase pp125FAK. Cell Adhesion Comm. 2000;7:311–320. doi: 10.3109/15419060009015002. [DOI] [PubMed] [Google Scholar]
- 35.Biliran H, Jan Y, Chen R, Pasquale EB, Ruoslahti E. Protein kinase D is a positive regulator of Bit1 apoptotic function. J. Biol. Chem. 2008;283:28029–28037. doi: 10.1074/jbc.M803139200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kairouz-Wahbe R, Biliran H, Luo X, Khor I, Wankell M, et al. Anoikis effector Bit1 negatively regulates Erk activity. Proc. Natl. Acad. Sci. USA. 2008;105:1528–1532. doi: 10.1073/pnas.0711357105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karmali PP, Brunquell C, Tram H, Ireland SK, Ruoslahti E, Biliran H. Metastasis of tumor cells is enhanced by downregulation of Bit1. PLoS ONE. 2011;6–8:e23840. doi: 10.1371/journal.pone.0023840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shipp MA, Ross KN, Tamayo P, Weng AP, Kutok JL, Aguiar RC, Gaasenbeek M, Angelo M, Reich M, Pinkus GS, Ray TS, Koval MA, Last KW, Norton A, Lister TA, Mesirov J, Neuberg DS, Lander ES, Aster JC, Golub TR. Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat. Med. 2002;8:68–74. doi: 10.1038/nm0102-68. [DOI] [PubMed] [Google Scholar]
- 39.Seo SW, Lee H, Lee HI, Kim HS. The role of TLE1 in synovial sarcoma. J. Orthop. Res. 2011;29:1131–1136. doi: 10.1002/jor.21318. [DOI] [PubMed] [Google Scholar]
- 40.Ward Y, Wang W, Woodhouse E, Linnoila I, Liotta L, Kelly K, et al. Signal pathways which promote invasion and metastasis: critical and distinct contributions of extracellular signal-regulated kinase and Ral-specific guanine exchange factor pathways. Mol Cell Biol. 2001;21:5958–5969. doi: 10.1128/MCB.21.17.5958-5969.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]