Abstract
Background
Survival of mice after K. pneumoniae infection and phagocytosis by alveolar macrophages (AMs), in the presence or absence of ozone (O3) exposure prior to infection is sex dependent.
Objectives
Study the role of gonadal hormones, 5α-dihydrotestosterone (DHT) and 17β-estradiol (E2) on mouse survival after filtered air (FA) or O3 exposure.
Methods
Gonadectomized female (GxF) and male (GxM) mice implanted with control or hormone pellets (DHT in GxF, or E2 in GxM), exposed to O3 (2 ppm, 3h) or FA and K. pneumoniae-infected were monitored for survival.
Results
Survival in GxF was identical after FA or O3 exposure; in GxM O3-exposure resulted in lower survival vs. FA. In O3-exposed females gonadectomy resulted in increased survival compared to intact females or to GxM+E2. A similar effect was observed in GxF+DHT. The combined negative effect of oxidative stress and hormone on survival was higher for E2.
Conclusions
Gonadectomy eliminated (females) or minimized (males) the previously observed sex-differences in survival in response to oxidative stress, and hormone treatment restored them. These findings indicate that gonadal hormones and/or oxidative stress have a significant effect on mouse survival.
Keywords: Surfactant protein-A, pneumonia, 17β-Estradiol, 5α-DHT, ozone, sex differences
INTRODUCTION
Respiratory diseases are a major cause of morbidity, mortality, and an economic burden worldwide (1, 2). Pneumonia is a major health problem, and a significant cause of infectious disease-related morbidity and mortality (3, 4). The lung interfaces with the environment and, hence, comes in contact with pollutants, such as oxidant gases and particulates, making it particularly prone to oxidant-mediated cellular damage.
Gaseous pollutants, including ozone (O3), as well as particulates and diesel exhaust particles are known to form reactive oxygen species, such as superoxide anion, hydrogen peroxide, and hydroxyl radicals. In the lung, pollutant effects include decreases in pulmonary function, airway inflammation and hyper-reactivity, compromised immune function and an increased risk of respiratory infection incidence and exacerbation of lung disease and mortality (5–9). The negative effects of O3 span a wide range of processes and functions. These include: impaired innate pulmonary host defense (5); decreases in survival of animals infected with Klebsiella pneumoniae and in phagocytic ability of alveolar macrophages (AMs) (10, 11); lung inflammation and severity of extrapulmonary lesions (12); changes in lung inflammatory mediators, tissue damage, and oxidative protein modification (13); cellular function impairment of a macrophage-like cell line (THP-1) (14); and changes of surfactant protein-A (SP-A) function, a host defense molecule (15).
Sex differences have been observed in lung function and disease susceptibility (16), and in risk, incidence, and pathogenesis of various lung diseases (17–22). Human and animal studies showed increased incidence of respiratory infections and severity of pneumonia (23–26), pulmonary fibrosis (27) and other interstitial lung diseases (28) in males. Male mice compared to females showed greater airway hyper-responsiveness and bronchoalveolar lavage fluid (BALF) total leukocytes, polymorphonuclear cells, and TNF-α levels (29) following LPS administration. Clinical studies show that women are less likely to develop most types of pneumonia, and generally experience a more favorable outcome after respiratory infection than men (23, 30).
Following K. pneumoniae infection, survival of male mice is lower than that of female mice, but after O3-induced oxidative stress, female survival becomes lower than that of males (10). More pronounced lung inflammation was also observed in O3-exposed infected females compared to male counterparts, and more extrapulmonary lesions in FA-exposed infected males compared to females (12). The phagocytic ability of AMs isolated from O3-exposed female mice showed a more pronounced reduction than those from males (10, 11). We hypothesized that gonadal hormones are responsible for sex differences in the susceptibility to respiratory infection by K. pneumoniae with prior exposure to FA or O3 and the resulting oxidative stress. We tested the effect of gonadal hormones, DHT and E2, in response to respiratory infection and in the presence or absence of O3-induced oxidative stress in mice. We studied whether removal of gonads alone or subsequent hormone treatment and O3 or filtered air (FA) exposure affects mouse survival after K. pneumoniae infection. We followed survival over 14 days to study whether sex differences in susceptibility to oxidative stress and infection were due to hormones. To distinguish between effects of circulating gonadal hormones vs. the potential effects of sexual differentiation on anatomic and physiologic airway differences between the sexes (31), gonadectomized females received DHT and males received E2.
METHODS
Animals
Male and female C57BL/6 mice (Jackson Laboratory; Bar Harbor, ME) were used at 10 weeks of age at the start of the experiment. The Pennsylvania State University Institutional Animal Care and Use Committee approved all procedures.
Gonadectomy and hormone treatment
Animals were anesthetized with an intramuscular injection of Ketamine-HCl (Ketazet 90mg/kg) and Xylazine (Xila-Ject 10mg/kg), and gonads were surgically removed. In females, the effect of gonadectomy was confirmed by vaginal smear and comparison of serum E2 levels, before and two weeks after ovariectomy. In males, effectiveness of gonadectomy was assessed after two weeks, by comparing the size and weight of seminal vesicles, and by comparing serum DHT levels with those of intact mice of the same age. Animals were monitored twice daily, and experiments were done two weeks after surgery. Hormone determinations were performed at Penn State core endocrine laboratory (E2: solid-phase radioimmunoassay kit, Siemens; DHT: ELISA, ALPCO Diagnostics).
To distinguish between effects of circulating gonadal hormones vs. the potential effects of anatomic and physiologic airway differences due to sexual differentiation (31) on survival, females received DHT and males received E2. We used DHT (as opposed to Testosterone) because it is the active androgen that does not get converted to E2.
Pellets were from Innovative Research of America (Rockville, MD). Control (vehicle) pellets contained the matrix alone, and hormone pellets contained matrix plus active product (E2: 0.006 mg/pellet, 60 day release; DHT: 5 mg/pellet, 60 day release). One week after gonadectomy, pellets were implanted subcutaneously on the lateral neck side between the ear and shoulder via a small incision (approximately 3 mm). GxM were implanted with E2 pellets (GxM+E2, n=50) or control pellets (GxM+CoP, n=50), and GxF received DHT pellets (GxF+DHT, n=50) or control pellets (GxF+CoP, n=50).
Exposure to O3 or FA
One week after pellet implantation each group (n=5 mice/experiment; 5 experiments/group) was exposed for 3h to either FA or O3 (2 ppm), in different chambers as described (10, 11, 13), and intratracheally infected immediately after exposure to FA or O3 (Figure 1).
Figure 1. Experimental model.
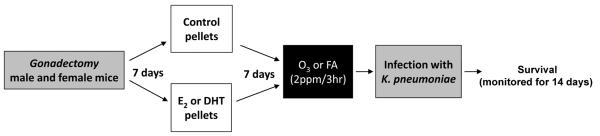
Male and female mice (C57BL/6; 10 weeks old) were gonadectomized. One week later, control or hormone pellets were implanted (E2: 0.006 mg/pellet, DHT: 5 mg/pellet). One week after pellet implantation, mice were exposed to either FA or O3 (2 ppm, 3h), and intratracheally infected with K. pneumoniae (450 CFU/mouse). Survival was monitored for 14 days.
Preparation of bacteria and infection of mice
K. pneumoniae (ATCC 43816) was obtained from the American Tissue Culture Collection (Rockville, MD). Bacteria were prepared as described (10). Infection was performed with ~450 CFU/mouse. Mice were monitored twice a day and the numbers of surviving and deceased mice were recorded. Deaths during the first 12h post-infection were considered to be due to the surgical procedure rather than to infection and these mice were excluded from the study (six animals).
Statistics
Sigmastat® software was used for all analyses. Kaplan-Meier survival curves were analyzed by log-rank test for cumulative survival (14-day period), and with Fisher's Exact test (daily survival). Mean survival, standard deviation, and standard error were calculated for five independent experiments for each group (n=5 mice/group). One Way ANOVA and Tukey's test for multiple comparisons were used to compare mean survival between multiple groups. Z-test was used to compare ratios of animals surviving after different conditions. Results were considered significant when p<0.05.
RESULTS
To study the effects of gonadal hormones on acute lung injury after oxidative stress and experimental pneumonia, we compared the survival rate of gonadectomized male and female mice with and without hormone treatment after O3 or FA (control) exposure and K. pneumoniae infection. An outline of the experimental design is shown in Figure 1. Because the main focus of this study is on circulating gonadal hormones and their effect on survival after infection, we used E2 implants in males and DHT implants in females in order to eliminate potential differences in survival resulting from anatomic and/or physiologic differences in male and female airways.
Confirmation of completeness of gonadectomy
In intact females, serum E2 levels ranged between 21–249 pg/ml, depending on the stage of the estrous cycle (confirmed by vaginal smear cytology). Two weeks after gonadectomy, a decrease in E2 levels to less than 10 pg/ml, and vaginal cytology showing absence of cornified epithelium, confirmed successful elimination of ovarian hormones.
In males, the weight of seminal vesicles ranged from 0.177–0.215 g in intact males and decreased to 0.06–0.08 g two weeks after gonadectomy (Table 1A). Serum DHT levels in intact males ranged between 113–164 pg/ml and decreased to 23–41 pg/ml two weeks after gonadectomy (Table 1A). Together, the reduction in seminal vesicle weight and serum DHT levels confirmed successful elimination of testicular hormones.
TABLE 1A.
Confirmation of gonadectomy and hormone treatment.
| Seminal vesicle weight (g) | Serum DHT (pg/ml) | |
|---|---|---|
| Intact male | 0.18–0.22 | 113–164 |
| GxM | 0.06–0.08 | 23–41 |
| GxF+DHT (5mg/pellet) | NA | 117–228 |
Comparison of seminal vesicles weight (g) in intact and gonadectomized male (GxM) mice with control pellets. Serum DHT levels (pg/ml) in intact males, GxM or gonadectomized females (GxF) implanted with DHT pellets (n=5/group) were determined by ELISA.
Hormone treatment
In gonadectomized females implanted with DHT pellets (5 mg/pellet), serum DHT levels were comparable to those of intact males (117–228 pg/ml) two weeks after implantation (Table 1A). Circulating levels of E2 in GxM implanted with pellets containing different doses of E2 were measured (Table 1B). Two weeks after implantation with 0.006 mg pellets, circulating E2 levels were 112–243 pg/ml, values comparable to female mice in estrus (32). However, higher doses of E2 (>0.012mg/pellet) resulted in higher E2 serum levels and caused urinary retention and bladder enlargement in GxM. Therefore, pellets of 0.006 mg of E2 were used for further experiments.
TABLE 1B.
Serum E2 levels (pg/ml) in gonadectomized males implanted with E2 pellets
| E2 pellet dose (mg/pellet) | Serum E2 levels (pg/ml) |
|---|---|
| 0.25 | 1580–2808 |
| 0.1 | 628–730 |
| 0.05 | 451–539 |
| 0.0125 | 319–451 |
| 0.006 | 112–243 |
Serum E2 levels (pg/ml), measured by RIA, in gonadectomized males two weeks after implantation with different E2 pellets doses (mg/pellet) (n=3/dose).
Survival after gonadectomy and O3 or FA exposure
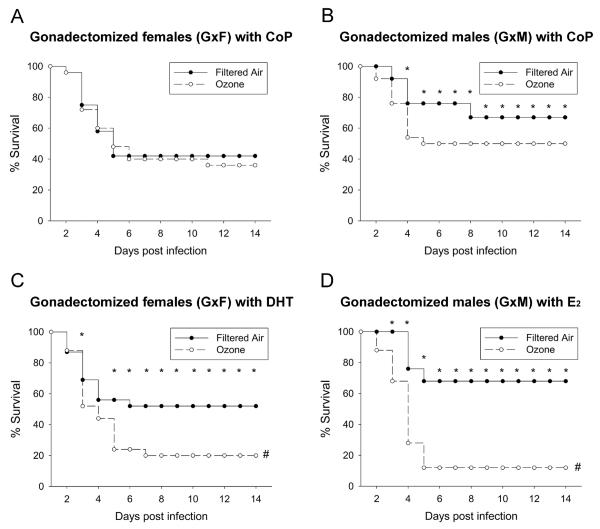
On day 14, the mean survival of GxF mice implanted with control pellets following infection with K. pneumoniae was similar whether the mice were pre-exposed to FA (control) or O3 (42% (10/24) vs. 36% (9/25), Figure 2A). However, mean survival evaluated 14 days after gonadectomy and infection was reduced in GxM with control pellets, by pre-exposure to O3 as compared to FA (50% (12/24) vs. 67% (16/24), p<0.05) (Figure 2B). Thus, gonadectomy eliminated entirely the differences in survival attributable to O3-exposure demonstrated previously (10) in females, but not in males.
Figure 2. Gonadectomy and hormone treatment affect survival after O3 or FA exposure and K. pneumoniae infection.
Survival of GxF (panel A) or GxM (panel B) mice implanted with control pellets (CoP) or hormone pellets (DHT in GxF, panel C; E2 in GxM, panel D) was monitored for 14 days after exposure to O3 or FA and infection. Significant differences are indicated for daily survival (*: p<0.05) or survival after 14 days (#: p<0.05) (n=25/group).
Survival after hormone treatment and FA or O3 exposure
Treatment of gonadectomized mice with steroid hormones (vs. control pellets) significantly decreased survival of O3-exposed and infected females and males (Figure 2, panels C and D). The 14-day survival of infected FA-exposed GxF implanted with DHT was 52% (12/23) vs. 20% (5/25) of O3-exposed (p<0.05), and GxM+E2 survival was 68% (17/25) in FA vs. 12% (3/25) for O3 exposed animals (Figure 2, panels C and D).
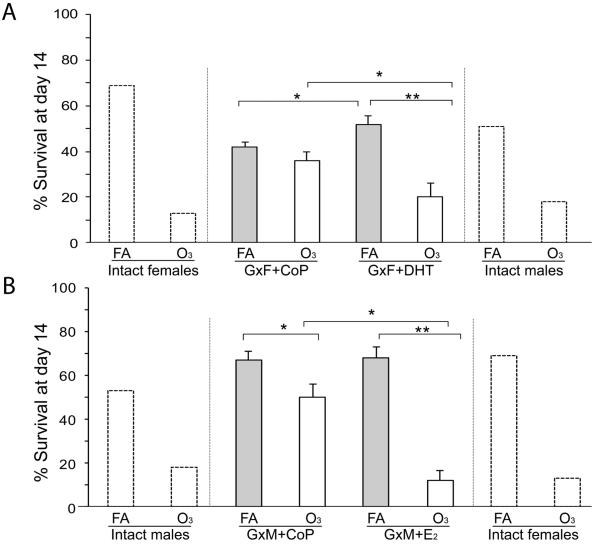
In FA-exposed mice, survival of GxM implanted with E2 pellets did not differ significantly from GxM with control pellets (68% GxM+E2 vs. 67% GxM+CoP). However, implanting GxM with E2 pellets drastically reduced survival when mice were pre-exposed to O3 (12% GxM+E2 vs. 50% GxM+CoP) (Figure 2, panels B and D). Moreover, while in FA-exposed mice survival at day 14 for GxF+DHT was increased compared to those with control pellets (52% GxF+DHT vs. 42% GxF+CoP, p<0.05), it was markedly decreased after O3 exposure (20% GxF+DHT vs. 38% GxF+CoP, p<0.05) (Figure 2, panels A and C). Survival of GxF implanted with DHT was also similar to that of intact males and GxM implanted with E2 was similar to that of intact females, after O3 or FA exposure and infection. Survival at day 14 among all groups in male and female mice, and (for comparison purposes) data of intact mice from our previous study (10) are shown in Figure 3. Moreover, differences in daily survival, by analyzing the proportions of O3-exposed GxM+E2 or GxF+DHT surviving vs. FA-exposed GxM+E2 or GxF+DHT surviving, respectively, for each day are shown in Figure 4. After O3 exposure, the negative effect of E2 on survival was significantly higher than that of DHT from days 4–14, and mimicked that of intact males and females (10).
Figure 3. Survival after 14 days of male and female mice pre-exposed to O3 or FA infected with K. pneumoniae.
Comparison of survival at day 14 among (A) gonadectomized females (GxF) with control (CoP) or DHT pellets, or (B) gonadectomized males (GxM) with control or E2 pellets. Significant differences are shown (*: p<0.05, **: p<0.001). Bar shows mean + SEM (n=25/group). Survival of intact mice data on extreme right and left (dotted lines) from our published work (10), presented here for illustrative purposes.
Figure 4. Daily survival rates of GxF+DHT and GxM+E2 exposed to ozone after infection.
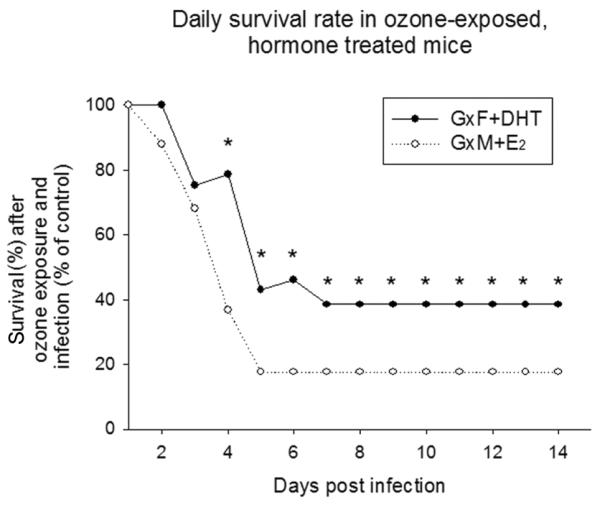
Survival of gonadectomized mice treated with sex hormones was monitored after K. pneumoniae infection, as described earlier. Survival of mice exposed to FA was set as 100%, and the proportional survival of the corresponding O3-exposed group was calculated as a percent of the control group (O3/FA × 100%). Proportions (i.e. O3/FA absolute survival rates) were compared with z-test, as described in Methods. Significant differences are indicated (*: p<0.05, n=25).
DISCUSSION
This study provides evidence that gonadal hormones affect survival of mice infected with K. pneumoniae, and demonstrates an interaction between lung infection and O3-induced oxidative stress. Previous data generated in our laboratory, with intact animals from the same strain, bred in the same conditions as the ones used in the present study, and exposed to identical FA/O3 conditions have shown sex differences in survival after K. pneumoniae infection (10). Here, we studied the hypothesis that gonadal hormones play a role in the sex differences observed in survival. Comparison of the present mouse survival data with our published data (10) indicated that gonadectomy can eliminate the previously observed differences in female mice infected with K. pneumoniae, and reduce the differences observed in males pre-exposed to FA or O3. Treatment of GxF and GxM mice with gonadal hormones of the opposite sex significantly reduced survival of infected mice pre-exposed to O3, but not of mice pre-exposed to FA. Moreover, the survival of GxM treated with E2 was similar to that of intact females, and survival of GxF treated with DHT was similar to that of intact males (10). However, in the presence of oxidative stress, E2 had a significantly higher negative effect on survival than DHT. Together, these findings lead us to propose a role for gonadal steroid hormones, DHT and E2, in the altered survival following lung infection in the presence or absence of O3-induced oxidative stress.
Oxidative stress caused by exposure to environmental agents (i.e. O3) has been shown to affect pulmonary function in humans (33), and the negative consequences of ozone on health have been shown to generate a major economic burden (34). Although the animal model used in this study does not allow for extrapolation of effects of chronic O3 exposure, it is rather remarkable that a single (acute) exposure to O3 has long lasting effects, as assessed here by the effects on survival rate. To better extrapolate findings from animal models to humans it is necessary that animals are exposed to higher O3 concentrations, because higher O3 doses are required for rodents versus humans to reach comparable amounts of O3 concentration in the distal lung (35). Moreover, resting rodents exposed to 2 ppm of O3 display comparable or lower levels of various BAL parameters than exercising humans with considerable lower O3 exposures (0.4 ppm, the equivalent of an extremely high atmospheric concentration) (33, 36).
Alveolar macrophages constitute an important first line of host defense against lung infection. Oxidative stress severely compromises the phagocytic ability of AMs (10, 11), and lung inflammation (12). The interaction between gonadal hormones and oxidative stress appears to be complex, as illustrated by the effects of gonadectomy, hormone treatment, and O3 exposure. Whether AM-mediated mechanisms, e.g. impaired phagocytosis, inflammation, other, are contributing factors for the observed sex differences in response to K. pneumoniae infection after O3 or FA exposure remain to be determined.
In several animal models, male and female gonadal hormones have been previously implicated in sex differences in respiratory anatomy (31, 37), physiology (29, 38), in responses to diverse pathogens (29), and in lung inflammatory response (16, 39–41). In animal models, gonadal hormones exhibit the following effects: 1) alter markers of oxidative stress (42), 2) modulate oxidative stress response (43), 3) regulate synthesis of antioxidants (44), 4) modulate cytokine production, 5) regulate expression of macrophage surface receptors, such as CD14 and TLR4, and 6) contribute to sex differences in immune response under normal and pathologic conditions (45–47). In the present study, removal of gonadal hormones improved survival after O3 exposure of both male and female mice and hormone treatment of gonadectomized mice decreased survival after O3 exposure. The fact that in the clinical course of our infection model, sex-specific patterns could be reversed by giving E2 to GxM and DHT to GxF mice indicates that the observed sex differences may not be due to structural sexual dimorphism, but rather a result of the influence of these hormones.
Female sex has been generally associated with protection against infection (25, 26, 39). In the present study, gonadectomy in FA-exposed females decreased survival, indicating that female gonadal hormones (E2 and progesterone) are protective, consisting with previous findings and clinical data (30). Furthermore, E2 treatment in gonadectomized males increased survival to levels similar to intact females (10), indicating that E2 alone is protective against infection under these experimental conditions. In contrast, gonadectomy in ozone exposed females resulted in increased survival compared to intact females (10) or to E2 treatment in GxM, indicating that in the presence of ozone-induced oxidative stress, the protective effect of E2 is lost or minimized. A similar effect was observed in GxF treated with DHT pellets, indicating that a combination of gonadal hormones and oxidative stress increases susceptibility to infection. Together, these findings point to a role of gonadal hormones in response to pathogens after lung oxidative stress.
Previous studies of acute lung injury are consistent with the above interpretation. Androgens appeared to be detrimental in the pathogenesis of lipopolysaccharide (LPS)-induced airway inflammation in a mouse model (25, 29). While gonadectomy caused a reduction in LPS-induced airway hyper-responsiveness and airway inflammation, exogenous testosterone administered to intact female mice increased their inflammatory responses to levels similar to those of intact males (29), and increased their susceptibility to Mycobacterium infection (25). In murine respiratory mycoplasmosis (26), surgical removal of reproductive organs reduced the severity of the disease as well as the CFU recovered from lungs of male mice. In cystic fibrosis patients, exposure to high levels of O3 and other air pollutants increased the risk of pulmonary exacerbations (48), and females had worse pulmonary function and survival than males (49). In addition, in a cystic fibrosis mouse model, exogenous E2 increased the inflammatory response and the severity of pneumonia caused by Pseudomonas aeruginosa (50).
In summary, the study of the impact of gonadal hormones, DHT and E2, on survival of mice revealed that: 1) removal of gonadal hormones eliminated (females) or minimized (males) differences in survival of animals exposed to O3 compared to those exposed to FA, 2) treatment with DHT or E2 negatively affected survival in response to oxidative stress and infection, with the E2 effect being stronger than the DHT, 3) survival of GxF treated with DHT and of GxM treated with E2 was similar to that observed previously with intact males and females, respectively (10), indicating a role of DHT and E2 in mechanisms associated with survival in this model.
CONCLUSION
Gonadal hormones and oxidative stress caused by O3 influence mouse susceptibility to K. pneumoniae infection.
ACKNOWLEDGEMENTS
This work was supported by the National Institute of Environmental Health Sciences ES09882 (JF) and by a grant from the Children's Miracle Network at Penn State College of Medicine (DSP).
Footnotes
DECLARATION OF INTERESTS The authors declare no competing financial interests.
REFERENCES
- 1.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370(9589):765–73. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 2.Braman SS. The global burden of asthma. Chest. 2006;130(1 Suppl):4S–12S. doi: 10.1378/chest.130.1_suppl.4S. [DOI] [PubMed] [Google Scholar]
- 3.Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year? Lancet. 2003;361(9376):2226–34. doi: 10.1016/S0140-6736(03)13779-8. [DOI] [PubMed] [Google Scholar]
- 4.Waterer G, Wunderink R. Respiratory infections: a current and future threat. Respirology. 2009;14(5):651–5. doi: 10.1111/j.1440-1843.2009.01554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hollingsworth JW, Kleeberger SR, Foster WM. Ozone and pulmonary innate immunity. Proc Am Thorac Soc. 2007;4(3):240–6. doi: 10.1513/pats.200701-023AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jerrett M, Burnett RT, Pope CA, 3rd, Ito K, Thurston G, Krewski D, et al. Long-term ozone exposure and mortality. N Engl J Med. 2009;360(11):1085–95. doi: 10.1056/NEJMoa0803894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen B, Kan H. Air pollution and population health: a global challenge. Environ Health Prev Med. 2008;13(2):94–101. doi: 10.1007/s12199-007-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kampa M, Castanas E. Human health effects of air pollution. Environ Pollut. 2008;151(2):362–7. doi: 10.1016/j.envpol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Kim CS, Alexis NE, Rappold AG, Kehrl H, Hazucha MJ, Lay JC, et al. Lung Function and Inflammatory Responses in Healthy Young Adults Exposed to 0.06 ppm Ozone for 6.6 Hours. Am J Respir Crit Care Med. 2011;183(9):1215–21. doi: 10.1164/rccm.201011-1813OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mikerov AN, Gan X, Umstead TM, Miller L, Chinchilli VM, Phelps DS, et al. Sex differences in the impact of ozone on survival and alveolar macrophage function of mice after Klebsiella pneumoniae infection. Respir Res. 2008;9:24. doi: 10.1186/1465-9921-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikerov AN, Haque R, Gan X, Guo X, Phelps DS, Floros J. Ablation of SP-A has a negative impact on the susceptibility of mice to Klebsiella pneumoniae infection after ozone exposure: sex differences. Respir Res. 2008;9:77. doi: 10.1186/1465-9921-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikerov AN, Cooper TK, Wang G, Hu S, Umstead TM, Phelps DS, et al. Histopathologic evaluation of lung and extrapulmonary tissues show sex differences in Klebsiella pneumoniae - infected mice under different exposure conditions. Int J Physiol Pathophysiol Pharmacol. 2011;3(3):176–90. [PMC free article] [PubMed] [Google Scholar]
- 13.Haque R, Umstead TM, Ponnuru P, Guo X, Hawgood S, Phelps DS, et al. Role of surfactant protein-A (SP-A) in lung injury in response to acute ozone exposure of SP-A deficient mice. Toxicol Appl Pharmacol. 2007;220(1):72–82. doi: 10.1016/j.taap.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janic B, Umstead TM, Phelps DS, Floros J. Modulatory effects of ozone on THP-1 cells in response to SP-A stimulation. Am J Physiol Lung Cell Mol Physiol. 2005;288(2):L317–25. doi: 10.1152/ajplung.00125.2004. [DOI] [PubMed] [Google Scholar]
- 15.Mikerov A, Umstead T, Gan X, Huang W, Guo X, Wang G, et al. Impact of ozone exposure on the phagocytic activity of human surfactant protein A (SP-A) and SP-A variants. Am J Physiol Lung Cell Mol Physiol. 2008;294(1):L121–30. doi: 10.1152/ajplung.00288.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Card JW, Zeldin DC. Hormonal influences on lung function and response to environmental agents: lessons from animal models of respiratory disease. Proc Am Thorac Soc. 2009;6(7):588–95. doi: 10.1513/pats.200904-020RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan V, Angus DC, Griffin MF, Clermont G, Scott Watson R, Linde-Zwirble WT. Hospitalized community-acquired pneumonia in the elderly: age- and sex-related patterns of care and outcome in the United States. Am J Respir Crit Care Med. 2002;165(6):766–72. doi: 10.1164/ajrccm.165.6.2103038. [DOI] [PubMed] [Google Scholar]
- 18.Falagas ME, Mourtzoukou EG, Vardakas KZ. Sex differences in the incidence and severity of respiratory tract infections. Respir Med. 2007;101(9):1845–63. doi: 10.1016/j.rmed.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Almqvist C, Worm M, Leynaert B. Impact of gender on asthma in childhood and adolescence: a GA2LEN review. Allergy. 2008;63(1):47–57. doi: 10.1111/j.1398-9995.2007.01524.x. PubMed PMID: 17822448. [DOI] [PubMed] [Google Scholar]
- 20.de Torres JP, Cote CG, Lopez MV, Casanova C, Diaz O, Marin JM, et al. Sex differences in mortality in patients with COPD. Eur Respir J. 2009;33(3):528–35. doi: 10.1183/09031936.00096108. [DOI] [PubMed] [Google Scholar]
- 21.Varkey AB. Chronic obstructive pulmonary disease in women: exploring gender differences. Curr Opin Pulm Med. 2004;10(2):98–103. doi: 10.1097/00063198-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Carey MA, Card JW, Voltz JW, Arbes SJ, Germolec DR, Korach KS, et al. It's all about sex: gender, lung development and lung disease. Trends Endocrinol Metab. 2007;18(8):308–13. doi: 10.1016/j.tem.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gannon CJ, Pasquale M, Tracy JK, McCarter RJ, Napolitano LM. Male gender is associated with increased risk for postinjury pneumonia. Shock. 2004;21(5):410–4. doi: 10.1097/00024382-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Loeb M, McGeer A, McArthur M, Walter S, Simor AE. Risk factors for pneumonia and other lower respiratory tract infections in elderly residents of long-term care facilities. Arch Intern Med. 1999;159(17):2058–64. doi: 10.1001/archinte.159.17.2058. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto Y, Saito H, Setogawa T, Tomioka H. Sex differences in host resistance to Mycobacterium marinum infection in mice. Infect Immun. 1991;59(11):4089–96. doi: 10.1128/iai.59.11.4089-4096.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yancey AL, Watson HL, Cartner SC, Simecka JW. Gender is a major factor in determining the severity of mycoplasma respiratory disease in mice. Infect Immun. 2001;69(5):2865–71. doi: 10.1128/IAI.69.5.2865-2871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voltz JW, Card JW, Carey MA, Degraff LM, Ferguson CD, Flake GP, et al. Male sex hormones exacerbate lung function impairment after bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2008;39(1):45–52. doi: 10.1165/rcmb.2007-0340OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carey MA, Card JW, Voltz JW, Germolec DR, Korach KS, Zeldin DC. The impact of sex and sex hormones on lung physiology and disease: lessons from animal studies. Am J Physiol Lung Cell Mol Physiol. 2007;293(2):L272–8. doi: 10.1152/ajplung.00174.2007. [DOI] [PubMed] [Google Scholar]
- 29.Card JW, Carey MA, Bradbury JA, DeGraff LM, Morgan DL, Moorman MP, et al. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J Immunol. 2006;177(1):621–30. doi: 10.4049/jimmunol.177.1.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gutierrez F, Masia M, Mirete C, Soldan B, Rodriguez JC, Padilla S, et al. The influence of age and gender on the population-based incidence of community-acquired pneumonia caused by different microbial pathogens. J Infect. 2006;53(3):166–74. doi: 10.1016/j.jinf.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Massaro GD, Mortola JP, Massaro D. Sexual dimorphism in the architecture of the lung's gas-exchange region. Proc Natl Acad Sci U S A. 1995;92(4):1105–7. doi: 10.1073/pnas.92.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Offner H, Adlard K, Zamora A, Vandenbark AA. Estrogen potentiates treatment with T-cell receptor protein of female mice with experimental encephalomyelitis. J Clin Invest. 2000;105(10):1465–72. doi: 10.1172/JCI9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKittrick T, Adams WC. Pulmonary function response to equivalent doses of ozone consequent to intermittent and continuous exercise. Arch Environ Health. 1995;50(2):153–8. doi: 10.1080/00039896.1995.9940892. [DOI] [PubMed] [Google Scholar]
- 34.Umstead T, Phelps D. Floros J. The toll of ozone. Pneumon. 2011;24(1):20–3. [Google Scholar]
- 35.Hatch GE, Koren H, Aissa M. A method for comparison of animal and human alveolar dose and toxic effect of inhaled ozone. Health Phys. 1989;57(Suppl 1):37–40. doi: 10.1097/00004032-198907001-00004. [DOI] [PubMed] [Google Scholar]
- 36.Hatch GE, Slade R, Harris LP, McDonnell WF, Devlin RB, Koren HS, et al. Ozone dose and effect in humans and rats. A comparison using oxygen-18 labeling and bronchoalveolar lavage. Am J Respir Crit Care Med. 1994;150(3):676–83. doi: 10.1164/ajrccm.150.3.8087337. [DOI] [PubMed] [Google Scholar]
- 37.Massaro D, Massaro GD. Estrogen regulates pulmonary alveolar formation, loss, and regeneration in mice. Am J Physiol Lung Cell Mol Physiol. 2004;287(6):L1154–9. doi: 10.1152/ajplung.00228.2004. [DOI] [PubMed] [Google Scholar]
- 38.Reinhard C, Eder G, Fuchs H, Ziesenis A, Heyder J, Schulz H. Inbred strain variation in lung function. Mamm Genome. 2002;13(8):429–37. doi: 10.1007/s00335-002-3005-6. [DOI] [PubMed] [Google Scholar]
- 39.Speyer CL, Rancilio NJ, McClintock SD, Crawford JD, Gao H, Sarma JV, et al. Regulatory effects of estrogen on acute lung inflammation in mice. Am J Physiol Cell Physiol. 2005;288(4):C881–90. doi: 10.1152/ajpcell.00467.2004. [DOI] [PubMed] [Google Scholar]
- 40.Ligeiro de Oliveira AP, Oliveira-Filho RM, da Silva ZL, Borelli P, Tavares de Lima W. Regulation of allergic lung inflammation in rats: interaction between estradiol and corticosterone. Neuroimmunomodulation. 2004;11(1):20–7. doi: 10.1159/000072965. [DOI] [PubMed] [Google Scholar]
- 41.Shand FH, Langenbach SY, Keenan CR, Ma SP, Wheaton BJ, Schuliga MJ, et al. In vitro and in vivo evidence for anti-inflammatory properties of 2-methoxyestradiol. J Pharmacol Exp Ther. 2011;336(3):962–72. doi: 10.1124/jpet.110.174854. [DOI] [PubMed] [Google Scholar]
- 42.Crema LM, Diehl LA, Aguiar AP, Almeida L, Fontella FU, Pettenuzzo L, et al. Effects of chronic restraint stress and 17-β-estradiol replacement on oxidative stress in the spinal cord of ovariectomized female rats. Neurochem Res. 2010;35(11):1700–7. doi: 10.1007/s11064-010-0232-1. [DOI] [PubMed] [Google Scholar]
- 43.Prediger ME, Gamaro GD, Crema LM, Fontella FU, Dalmaz C. Estradiol protects against oxidative stress induced by chronic variate stress. Neurochem Res. 2004;29(10):1923–30. doi: 10.1023/b:nere.0000042219.98446.e7. [DOI] [PubMed] [Google Scholar]
- 44.Mancini A, Festa R, Di Donna V, Leone E, Littarru GP, Silvestrini A, et al. Hormones and antioxidant systems: role of pituitary and pituitary-dependent axes. J Endocrinol Invest. 2010;33(6):422–33. doi: 10.1007/BF03346615. [DOI] [PubMed] [Google Scholar]
- 45.Verthelyi D, Klinman DM. Sex hormone levels correlate with the activity of cytokine-secreting cells in vivo. Immunology. 2000;100(3):384–90. doi: 10.1046/j.1365-2567.2000.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kramer PR, Winger V, Kramer SF. 17beta-Estradiol utilizes the estrogen receptor to regulate CD16 expression in monocytes. Mol Cell Endocrinol. 2007;279(1–2):16–25. doi: 10.1016/j.mce.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rettew JA, Huet-Hudson YM, Marriott I. Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol Reprod. 2008;78(3):432–7. doi: 10.1095/biolreprod.107.063545. [DOI] [PubMed] [Google Scholar]
- 48.Goss CH, Newsom SA, Schildcrout JS, Sheppard L, Kaufman JD. Effect of ambient air pollution on pulmonary exacerbations and lung function in cystic fibrosis. Am J Respir Crit Care Med. 2004;169(7):816–21. doi: 10.1164/rccm.200306-779OC. [DOI] [PubMed] [Google Scholar]
- 49.Sims EJ, Green MW, Mehta A. Decreased lung function in female but not male subjects with established cystic fibrosis-related diabetes. Diabetes Care. 2005;28(7):1581–7. doi: 10.2337/diacare.28.7.1581. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Cela E, Gagnon S, Sweezey NB. Estrogen aggravates inflammation in Pseudomonas aeruginosa pneumonia in cystic fibrosis mice. Respir Res. 2010;11:166. doi: 10.1186/1465-9921-11-166. [DOI] [PMC free article] [PubMed] [Google Scholar]