Abstract
Purpose
The purpose of study was to compare glycemic control using glycated hemoglobin levels (HbA1c) in diabetic patients with chronic generalized periodontitis (CGP) undergoing scaling and root planing (SRP) with and without systemic doxycycline.
Methods
Fifty subjects with type 2 diabetes mellitus (T2DM) and CGP receiving antidiabetic therapy were selected for study. The selected subjects were randomly assigned to two groups (test group [TG] and control group [CG]) comprising 25 patients each. The TG received SRP followed by systemic doxycycline. The CG received treatment with SRP only. The periodontal parameters were recorded at baseline (day zero), and every 1 month for 4 months and included probing depth, clinical attachment level, plaque index, gingival index, and HbA1c level were recorded at baseline (day zero) and at the end of 4 months.
Results
A statistically significant effect was demonstrated for the periodontal parameters for both the TG and CG. HbA1c values did not show a statistically significant difference in the treatment group as compared to the CG.
Conclusions
The authors concluded that nonsurgical periodontal therapy improved glycemic control in patients with T2DM in both groups, but no statistical difference was observed with adjunctive systemic doxycycline therapy. A further study with a larger sample size is required.
Keywords: Chronic periodontitis, Diabetes mellitus, Glycosylated hemoglobin A, Periodontal debridement
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a complex metabolic disease that can result in various complications in human subjects [1].
The prevalence of periodontal diseases in individuals with T2DM is significantly higher than in non-T2DM counterparts. A large body of literature addresses the interrelationship between periodontal diseases and diabetes mellitus [2,3]. Periodontal disease has been noted to be the sixth complication of DM [4]. DM is also considered to be a risk factor for periodontitis, especially in subjects with poor glycemic control. Evidence-based literature lays emphasis on the effect of periodontal infections on diabetic complications and glycemic control, suggesting a two-way relationship between periodontitis and DM [5]. Studies have indicated a skewed glycemic control in DM patients with severe periodontitis [6].
It has been observed that scaling and root planing (SRP) has positive effects on the glycemic control of subjects with T2DM [7]. However, other clinical trials have shown the existence of infectious microbial agents, which are notorious for tissue invasion. These infectious microbial agents are persistent and are not eliminated from the tissue by SRP alone [8]. Thus, SRP in moderate to severe periodontal pockets and good oral hygiene in patients with T2DM may not be sufficient to promote optimal periodontal health or enhanced glycemic control [8,9]. The use of systemic antibiotics can be effective in decreasing the total count of bacteria in periodontal infection, with ensuing down-regulation of the inflammatory mediators leading to an improvement of glycemic control in T2DM subjects.
Administration of doxycycline and chemically modified tetracycline has salutary effects on the glycated hemoglobin level (HbA1c) and periodontal parameters. These positive effects may be attributed to their antibacterial and anticollagenase properties [10].
The present study was undertaken in order to determine the efficacy of nonsurgical periodontal therapy (NSPT) with or without systemic doxycycline on the periodontal health and HbA1c levels of patients with T2DM and chronic generalized periodontitis (CGP).
MATERIALS AND METHODS
Subjects
This study was conducted in the Department of Periodontics, Tatyasaheb Kore Dental College and Research Centre, New Pargaon. Fifty subjects fulfilling the inclusion criteria were selected from the outpatient department of the Department of Periodontics. The study was reviewed and approved by the Institutional Review Board.
Inclusion criteria
Male and female subjects, with age groups ranging from 30 to 70 years
Subjects with T2DM and CGP receiving antidiabetic therapy
Subjects presenting with no evident, major diabetic complications
Subjects with no history of any systemic antibiotic administration within the previous 6 months
Subjects with no history of any periodontal treatment 6 months prior to the study
Exclusion criteria
Subjects with any systemic disease other than T2DM
Tobacco use in any form
Pregnant female subjects
Alcohol consumers
The 50 subjects consisted of 34 males and 16 females, within the age range of 30 to 70 years. The test group (TG) consisted of 25 subjects, 16 males and 9 females, all of whom underwent full mouth SRP and antibiotic administration (doxycycline 100 mg once in day prescribed for 15 days). The control group (CG) consisted of 25 subjects, 18 males and 7 females, subjected only to full mouth SRP.
To assess the effect of the periodontal treatment on glycemic control, no change in medication or diet was made for either of the groups during the study period. None of the groups received any additional guidance in managing their diabetic status.
Periodontal treatment
The periodontal examination and SRP was performed by a single surgeon. Periodontal measurements were recorded by a single examiner with the help of the University of North Carolina 15 probe.
Data collection
The periodontal parameters were recorded at baseline (day 0) and at 1, 2, 3, and 4 months following the periodontal treatment in both of the groups. The parameters recorded were plaque index (PI) and gingival index (GI) around each tooth according to the criteria [11], probing depth (PD), gingival recession (GR), and clinical attachment level (CAL). Recordings were made from the buccal, lingual, and two interproximal surfaces of each tooth.
For assessment of HbA1c, a venous blood sample was taken from each patient and HbA1c levels were recorded at day 0 and 4 months following the periodontal treatment in both TG and CG. HbA1c level assessments were performed in a private laboratory.
Statistical analysis
Descriptive and inferential statistical analysis was carried out in the present study. Results of continuous measurements were presented as mean±standard deviation (min-max) and results of categorical measurements were presented in as number (%). Significance was assessed at the 5% level of significance. The following assumptions on data were made:
1) Dependent variables should be normally distributed.
2) Samples drawn from the population should be random.
3) Cases of the samples should be independent.
The Student's t-test (two tailed, independent) was used to find the significance of study parameters on a continuous scale between the two groups and intergroup analysis on metric parameters. Levene's test for homogeneity of variance was performed to assess the homogeneity of variance and a Student's t-test (two tailed, dependent) was used to find the significance of study parameters on a continuous scale within each group. A chi-squared test was used to find the significance of study parameters on a categorical scale between two or more 'groups' Pearson correlation was found between HbA1c and clinical variables.
All of the calculations were performed using statistical software, namely SAS ver. 9.2 (SAS Institute Inc., Cary, NC, USA) and SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
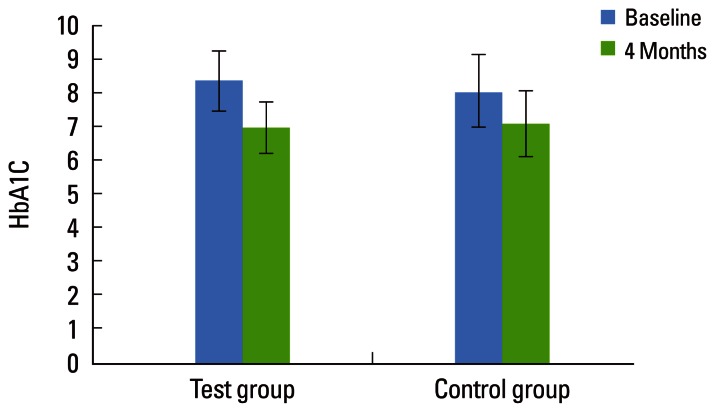
A total of 50 subjects (34 males and 16 females) were included in the study. Only 42 subjects (31 males and 11 females) completed the study in adherence to the prescribed protocol. At baseline, the TG and CG had similar mean values for age and gender distribution. There was no statistically significant difference in HbA1c levels between the two groups at baseline (P=0.312). The TG showed a significant reduction in the HbA1c (%) level from 8.38%±0.89% to 7.00%±0.76% after 4 months and the CG showed a significant reduction from 8.06%±1.10% to 7.11%±0.99% after 4 months. Both the groups showed a statistically significant difference in HbA1c (%) at 4 months (P<0.001). However, there were no significant differences between the two groups (P=0.710) after 4 months, as shown in Table 1 and Fig. 1.
Table 1.
Comparative evaluation of glycated hemoglobin levels (HbA1c) in the test and control groups.
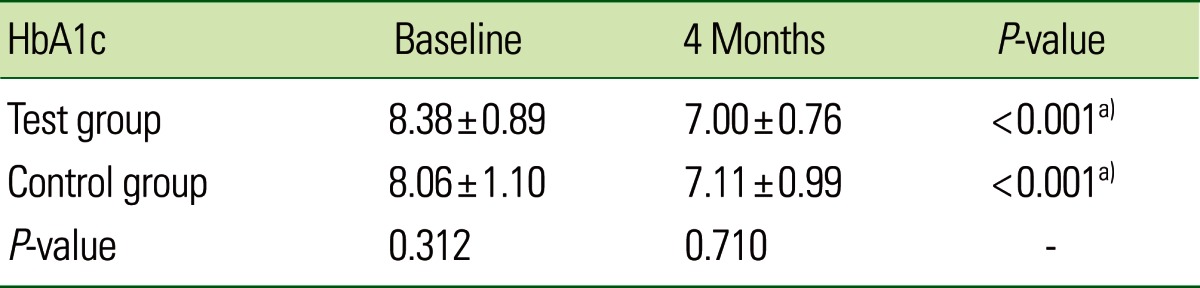
Values are presented as mean±standard deviation.
a)Strongly significant (P≤0.01).
Figure 1.
Comparative evaluation of glycated hemoglobin (HbA1c) levels in the test and control groups.
Periodontal parameters
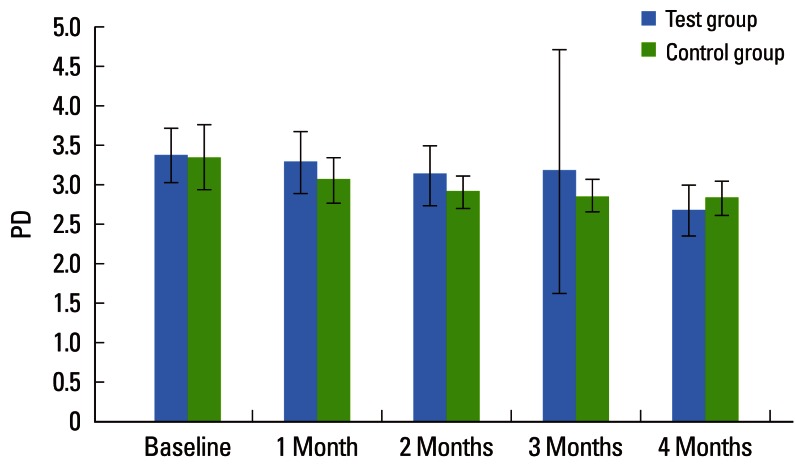
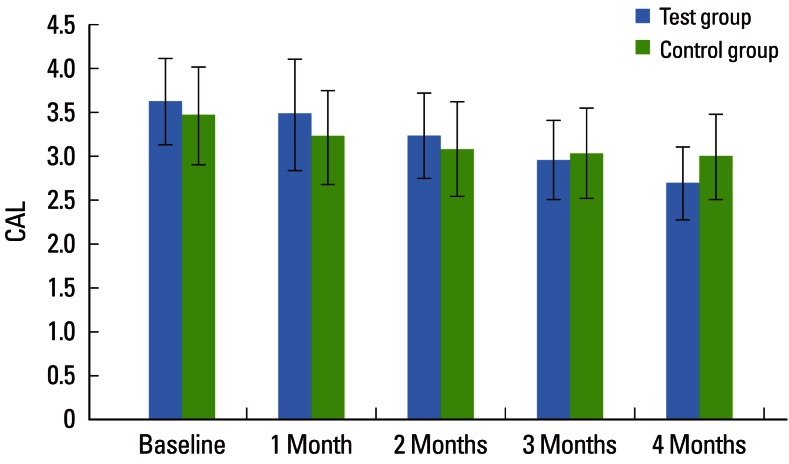
Both the TG and CG showed significant improvement in periodontal parameters over the experimental period. The mean PD for the TG at baseline was 3.36±0.35, which had fallen significantly by 1 and 2 months to 3.28±0.39 and 3.12±0.38, respectively, and after 3 months it was 3.16±1.54, which was not statistically significant (P=0.113). A further significant reduction in PD was seen after 4 months to 2.67±0.32, which was statistically significant. The mean PD for the CG at baseline, 1, 2, 3, and 4 months was 3.34±0.40, 3.05±0.30, 2.90±0.21, 2.85±0.20, and 2.82±0.21, respectively, which was statistically significant (P<0.001). The mean PD between the two groups showed a statistically significant difference at 4 months (P=0.076). These findings indicate that the TG (2.67±0.32) showed a greater reduction in PD than did the CG (2.82±0.21) after 4 months (Table 2, Fig. 2). The mean CAL for the TG at baseline 1, 2, 3, and 4 months was 3.62±0.50, 3.47±0.63, 3.23±0.48, 2.96±0.45, and 2.69±0.42, respectively, which was statistically significant (P<0.001).
Table 2.
Comparative evaluation of probing depth (PD) in two groups of patients studied.
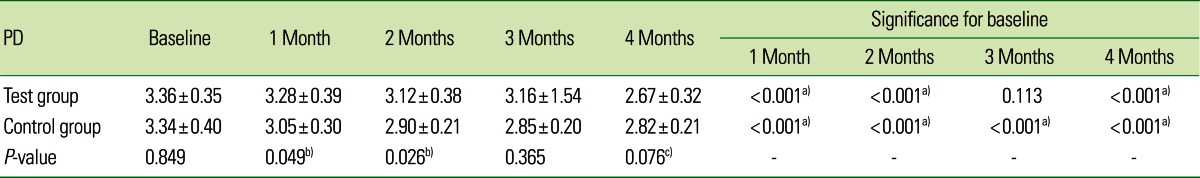
Values are presented as mean±standard deviation.
a)Strongly significant (P≤0.01). b)Moderately significant (0.01<P≤0.05). c)Suggestive significance (0.05<P<0.10).
Figure 2.
Comparative evaluation of probing depth (PD) in the test and control groups.
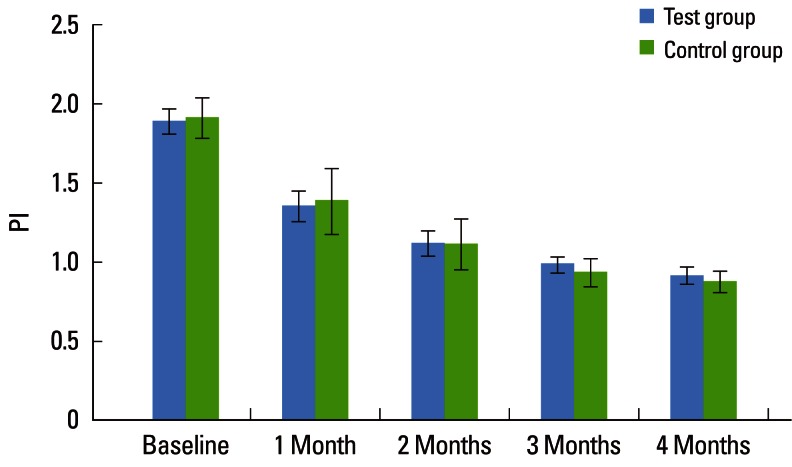
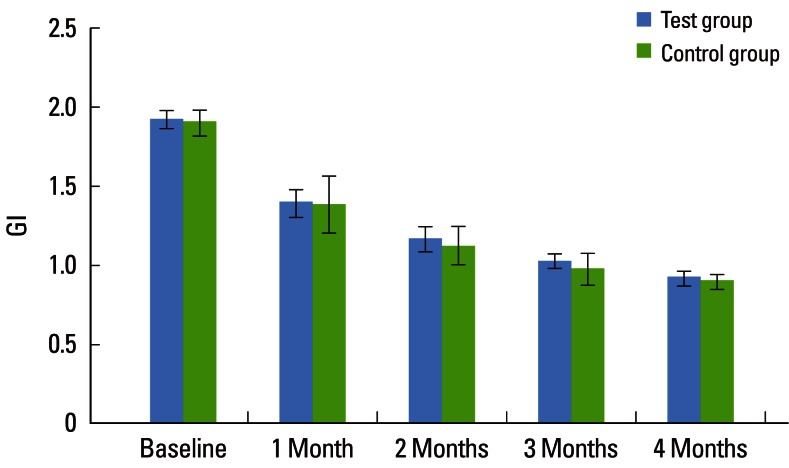
The mean CAL for the CG at baseline, 1, 2, 3, and 4 months was 3.46±0.56, 3.22±0.54, 3.08±0.54, 3.03±0.51, and 2.99±0.49, respectively, which was statistically significant (P<0.001). On intergroup comparison between the TG and CG, there was a greater reduction in CAL in the TG (2.69±0.42) than in the CG (2.99±0.49), suggestive of a statistically significant difference (P=0.042) as shown in Table 3 and Fig. 3. The mean PI for the TG at baseline, 1, 2, 3, and 4 months was 1.88±0.08, 1.35±0.10, 1.12±0.08, 0.98±0.05, and 0.91±0.05, respectively, which was statistically significant (P<0.001). For the CG, the mean PI at baseline, 1, 2, 3, and 4 months was 1.91±0.13, 1.38±0.21, 1.11±0.16, 0.93±0.09, and 0.87±0.07, respectively, which was statistically significant (P<0.001). The PI showed a statistically significant difference between the two groups (P<0.001) as shown in Table 4 and Fig. 4. The mean GI at baseline, 1, 2, 3, and 4 months for both of the groups is given in Table 5 and Fig. 5. The mean GI for the TG at baseline, 1, 2, 3, and 4 months was 1.92±0.06, 1.39±0.09, 1.16±0.08, 1.02±0.04, and 0.91±0.05, respectively, which was statistically significant (P<0.001). The mean GI for the CG at baseline, 1, 2, 3, and 4 months was 1.90±0.08, 1.38±0.18, 1.12±0.12, 0.97±0.10, and 0.89±0.05, respectively, which was statistically significant (P<0.001). However, the differences between the TG and CG were not statistically significant at any interval (P=0.219) as shown in Table 5 and Fig. 5.
Table 3.
Comparative evaluation of clinical attachment level (CAL) in the test and control groups.
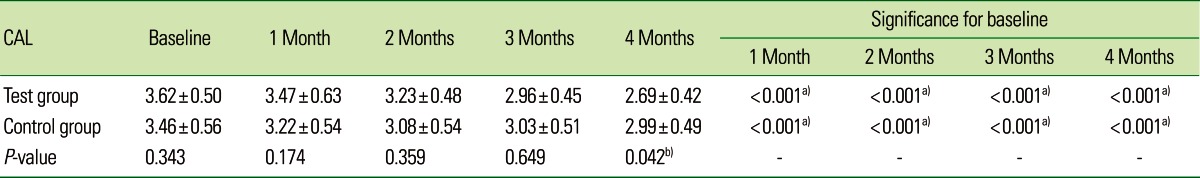
Values are presented as mean±standard deviation.
a)Strongly significant (P≤0.01). b)Moderately significant (0.01<P≤0.05).
Figure 3.
Comparative evaluation of clinical attachment level (CAL) in the test and control groups.
Table 4.
Comparative evaluation of the plaque index (PI) in the test and control groups.
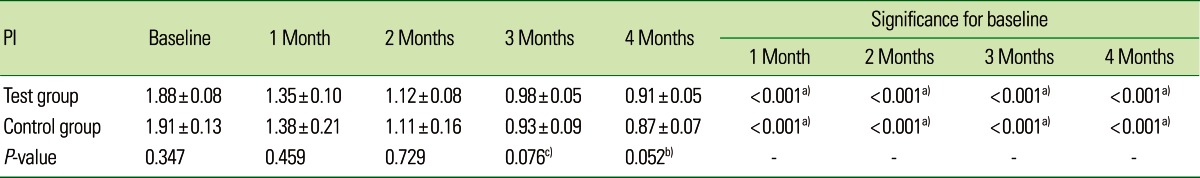
Values are presented as mean±standard deviation.
a)Strongly significant (P≤0.01). b)Moderately significant (0.01<P≤0.05). c)Suggestive significance (0.05<P<0.10).
Figure 4.
Comparative evaluation of the plaque index (PI) in the test and control groups.
Table 5.
Comparative evaluation of gingival index (GI) in the test and control groups.
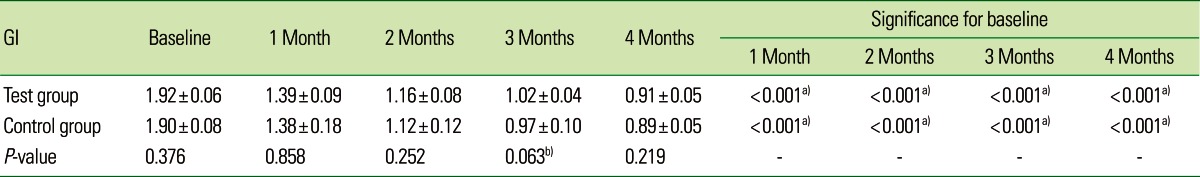
Values are presented as mean±standard deviation.
a)Strongly significant (P≤0.01). b)Suggestive significance (0.05<P<0.10).
Figure 5.
Comparative evaluation of gingival index (GI) in the test and control groups.
DISCUSSION
The inter-relationship between periodontitis and DM is strongly supported in the literature [12]. There is evidence that periodontal infection adversely affects glycemic control in DM [13,14]. The literature also describes a two-way relationship between periodontal disease and DM [5]. Both DM and periodontitis are connected through a common mechanism involving an escalation in proinflammatory factors, such as cytokines and adipokines, and the existence of a compromised neutrophil function. Periodontitis and DM are reckoned to elicit a state of systemic inflammation, with both of the conditions coupled by a perturbed inflammatory and immune status [15,16]. Various studies have corroborated that intensive periodontal therapy may ameliorate periodontal parameters and improve glycemic control [1,17,18]. There is evidence to support the role of NSPT in the improvement of glycemic control [1,17,19]. However, contradictory opinions exist in the literature concerning the appropriate time for assessing the healing response to NSPT. Morrison et al. [20] and Lowenguth and Greenstein [21] proposed an evaluation period of 1 month. Badersten et al. [22] observed that significant changes in periodontal pocket depths of 4-7 mm are revealed in the first 4-5 months, whereas in deep periodontal pockets, gauging 12 mm, a gradual resolution takes place, spanning over a period of 12 months. In the present study, the majority of patients had a mean PD of around 3-5 mm, and the response was evaluated after 4 months. The assessment was performed every 1 month to evaluate the response to NSPT and oral hygiene maintenance. The monitored periodontal clinical parameters in the present study showed improvements after NSPT and are described below.
On intragroup comparison of the PI and GI for the TG and CG, the results of this study showed a statistically significant difference from baseline to 4 months. On an intergroup comparison of TG and CG, the PI showed a statistically significant difference, whereas the GI did not show any statistically significant difference. These results are in agreement with Promsudthi et al. [23], Kiran et al. [17], and O'Connell et al. [24].
On intragroup comparison, the mean PD in the TG and CG fell significantly from baseline to 4 months. On intergroup comparison between the TG and CG, the TG showed a greater reduction in PD compared to the CG. Grossi et al. [25] and Promsudthi et al. [23] also observed similar results. In contradiction to the present study, Llambes et al. [26] observed no significant changes in the PD after 3 months. On intragroup comparison, within the TG and CG, the mean CAL showed a significant gain from baseline to 4 months. On inter-group comparison, there was a significantly greater gain in CAL in the TG as compared to CG. The present study is in agreement with Grossi et al. [25] and Promsudthi et al. [23], who reported a significant gain in CAL after 3 months. These results are suggestive of the optimal resolution of periodontal lesions with healing in the TG receiving antimicrobials, when compared to the CG, treated with SRP alone [25]. However, some studies have reported conflicting results and failed to observe any significant gain in the CAL in the group receiving SRP plus doxycycline and the group treated with SRP after 3 months [24,26]. This significant reduction in PD and gain in CAL can be explained by the antimicrobial and additional anticollagenase effects of doxycycline [25,27]. The rationale for the selection of doxycycline as an adjunct to SRP is supported by the spectrum of the drug, its ability to achieve higher concentrations in the gingival crevicular fluid, and its host modulating effect. Doxycycline inhibits matrix metalloproteinases, thus downregulating the host-mediated periodontal destruction [27,28].
Some studies have failed to detect an enhanced effect of doxycycline on the mean PD and CAL values [24,26,29]. The mean values of PD and CAL are refuted as ideal parameters, to assess the positive effects of doxycycline for the following reasons:
1) Shallow pockets are less likely to respond to NSPT, and the mean values will also be included in the recall visits.
2) The inability of the antibiotics to be effective in deep pockets renders it necessary to check the effect of the antibiotic in deeper pockets separately. Deep pockets exhibit the most favorable change after beginning NSPT [30,31]. PD changes after therapy are more common in the sites with deep pockets.
The hypothesis that glycemic control (HbA1c) can be improved by the successful treatment of periodontitis associated with systemic antibiotics as adjunctive therapy [19,25,29] was substantiated by the present study. In addition to improvement in the periodontal parameters, there was a significant reduction in HbA1c levels.
A decrease in the levels of HbA1c and collagen degradation, following doxycycline or chemically modified tetracycline treatment, was illustrated in a diabetic rat model [10]. It has been proposed that tetracycline and its congeners inhibit the glycation of proteins in DM, acting by the anticollagenase property. Tetracycline and its congeners prevent the activation of latent pro-MMP, de-escalate MMP expression, and counteract oxidative mechanisms responsible for periodontal tissue destruction [32,33]. This evidence has provided the basis for a therapeutic approach to controlling periodontal disease in individuals with DM using tetracycline and their derivatives [34]. In the present study, the authors did not observe any adverse effects in the TG, and systemic doxycycline (100 mg/day for 15 days) was well tolerated by the subjects in the TG. These findings were similar to those of Llambes et al. [26] who used the same drug regimen. In the present study, we selected HbA1c as a parameter for metabolic control instead of urine or plasma glucose because these parameters can show the level at a specific time of sampling but this value can alter within a few minutes due to various factors including diet, physical activity, and medication. Unlike HbA1c measurements, these parameters are not appropriate indicators for long term glycemic control. HbA1c provides an estimate of the average glucose level over the 30 to 90 days preceding the test and it does not account for short term fluctuations in plasma glucose levels [35]. Reduction in levels of HbA1c in the TG and CG showed significant improvement from baseline to 4 months. However, on an intergroup comparison between the TG and CG, there was no significant difference. Grossi et al. [25] noted a significant reduction in the HbA1c level in the study group treated with doxycycline although this reduction was not significant after 3 months of evaluation. Similar results were found in the present study, as the reduction in the HbA1c level in the TG and CG did not fall significantly on evaluation after 4 months. Doxycycline may act against periodontal bacteria, resulting in a favorable effect. However, these changes may be ineffectual after a 3 months period, due to the diminution of the antimicrobial effect of the agent. The results of the present study are in accordance to that reported by Promsudthi et al. [23], Jones et al. [36], and O'Connell et al. [24]. These authors failed to establish a significant reduction in HbA1c levels, when the TG (SRP+doxycycline) and CG (SRP only) were compared, after 3 months. Contrary to the above mentioned studies, Llambes et al. [26] and Singh et al. [37] demonstrated a significant reduction in HbA1c levels in the TG (SRP+doxycycline) compared the CG (SRP only) at 3 months. The possible causes for the lack of significant differences in HbA1c levels between the TG and CG have been discussed in light of scientific evidence such as the higher reduction in HbA1c levels seen in patients with a higher initial level of HbA1c [38], but in the present study, we did not use HbA1c levels as part of the inclusion criteria of this study. Treatment with systemic antibiotics can yield notable results with regard to HbA1c, since antibiotics may counteract systemic sources of infection or inflammation in addition to their local positive effects, ensuing in the decrease in HbA1c. It is acknowledged that the HbA1c level is affected by systemic inflammation, because there is an increase in the levels of inflammatory markers and mediators such as C-reactive protein, interleukin-6, plasminogen activator inhibitor-1, and tumor necrosis factor-α. These factors may fortify insulin resistance and further increase HbA1c levels [39,40]. In sum, given these possible causes, changes seen in HbA1c may be less conspicuous after the 3 months evaluation period, owing to the reduction of the antimicrobial drug effect [25].
This study found a statistically significant association between clinical improvement in the periodontal condition and improved metabolic control of diabetes in both the TG and CG. Moreover, on intergroup comparison between the TG and CG, adjunctive use of systemic doxycycline showed improvement in the CAL and PD, which was statistically significant, but the effect on the HbA1c level was not statistically significant. The authors reiterate on the importance of the coherent efforts between oral health care professionals and general physicians/diabetologists for the systematic management of subjects with DM and periodontitis.
ACKNOWLEDGEMENTS
The authors wish to thank Dr. K. P. Suresh, Scientist (Biostatistics), Project Directorate on Animal Disease Monitoring and Surveillance, Bangalore.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Stewart JE, Wager KA, Friedlander AH, Zadeh HH. The effect of periodontal treatment on glycemic control in patients with type 2 diabetes mellitus. J Clin Periodontol. 2001;28:306–310. doi: 10.1034/j.1600-051x.2001.028004306.x. [DOI] [PubMed] [Google Scholar]
- 2.Oliver RC, Tervonen T. Diabetes: a risk factor for periodontitis in adults? J Periodontol. 1994;65(5 Suppl):530–538. doi: 10.1902/jop.1994.65.5s.530. [DOI] [PubMed] [Google Scholar]
- 3.Emrich LJ, Shlossman M, Genco RJ. Periodontal disease in non-insulin-dependent diabetes mellitus. J Periodontol. 1991;62:123–131. doi: 10.1902/jop.1991.62.2.123. [DOI] [PubMed] [Google Scholar]
- 4.Loe H. Periodontal disease: the sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329–334. [PubMed] [Google Scholar]
- 5.Grossi SG, Genco RJ. Periodontal disease and diabetes mellitus: a two-way relationship. Ann Periodontol. 1998;3:51–61. doi: 10.1902/annals.1998.3.1.51. [DOI] [PubMed] [Google Scholar]
- 6.Nelson RG, Shlossman M, Budding LM, Pettitt DJ, Saad MF, Genco RJ, et al. Periodontal disease and NIDDM in Pima Indians. Diabetes Care. 1990;13:836–840. doi: 10.2337/diacare.13.8.836. [DOI] [PubMed] [Google Scholar]
- 7.Gurav AN. Periodontal therapy: an adjuvant for glycemic control. Diabetes Metab Syndr. 2012;6:218–223. doi: 10.1016/j.dsx.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Smith GT, Greenbaum CJ, Johnson BD, Persson GR. Short-term responses to periodontal therapy in insulin-dependent diabetic patients. J Periodontol. 1996;67:794–802. doi: 10.1902/jop.1996.67.8.794. [DOI] [PubMed] [Google Scholar]
- 9.Christgau M, Palitzsch KD, Schmalz G, Kreiner U, Frenzel S. Healing response to non-surgical periodontal therapy in patients with diabetes mellitus: clinical, microbiological, and immunologic results. J Clin Periodontol. 1998;25:112–124. doi: 10.1111/j.1600-051x.1998.tb02417.x. [DOI] [PubMed] [Google Scholar]
- 10.Ryan M, Ramamurthy N, Golub LM. Six CMTs, modulate MMPs, and non-enzymatic glycosylation in diabetic rats [abstract] J Dent Res. 1995;74:138. [Google Scholar]
- 11.Loe H. The Gingival Index, the Plaque Index and the Retention Index Systems. J Periodontol. 1967;38(Suppl):610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 12.Mealey BL, Oates TW American Academy of Periodontology. Diabetes mellitus and periodontal diseases. J Periodontol. 2006;77:1289–1303. doi: 10.1902/jop.2006.050459. [DOI] [PubMed] [Google Scholar]
- 13.Taylor GW, Borgnakke WS. Periodontal disease: associations with diabetes, glycemic control and complications. Oral Dis. 2008;14:191–203. doi: 10.1111/j.1601-0825.2008.01442.x. [DOI] [PubMed] [Google Scholar]
- 14.Iacopino AM. Periodontitis and diabetes interrelationships: role of inflammation. Ann Periodontol. 2001;6:125–137. doi: 10.1902/annals.2001.6.1.125. [DOI] [PubMed] [Google Scholar]
- 15.Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E. Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol. 2001;72:1221–1227. doi: 10.1902/jop.2000.72.9.1221. [DOI] [PubMed] [Google Scholar]
- 16.Loos BG, Craandijk J, Hoek FJ, Wertheim-van Dillen PM, van der Velden U. Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J Periodontol. 2000;71:1528–1534. doi: 10.1902/jop.2000.71.10.1528. [DOI] [PubMed] [Google Scholar]
- 17.Kiran M, Arpak N, Unsal E, Erdogan MF. The effect of improved periodontal health on metabolic control in type 2 diabetes mellitus. J Clin Periodontol. 2005;32:266–272. doi: 10.1111/j.1600-051X.2005.00658.x. [DOI] [PubMed] [Google Scholar]
- 18.Janket SJ, Wightman A, Baird AE, Van Dyke TE, Jones JA. Does periodontal treatment improve glycemic control in diabetic patients? A meta-analysis of intervention studies. J Dent Res. 2005;84:1154–1159. doi: 10.1177/154405910508401212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwamoto Y, Nishimura F, Nakagawa M, Sugimoto H, Shikata K, Makino H, et al. The effect of antimicrobial periodontal treatment on circulating tumor necrosis factor-alpha and glycated hemoglobin level in patients with type 2 diabetes. J Periodontol. 2001;72:774–778. doi: 10.1902/jop.2001.72.6.774. [DOI] [PubMed] [Google Scholar]
- 20.Morrison EC, Ramfjord SP, Hill RW. Short-term effects of initial, nonsurgical periodontal treatment (hygienic phase) J Clin Periodontol. 1980;7:199–211. doi: 10.1111/j.1600-051x.1980.tb01963.x. [DOI] [PubMed] [Google Scholar]
- 21.Lowenguth RA, Greenstein G. Clinical and microbiological response to nonsurgical mechanical periodontal therapy. Periodontol 2000. 1995;9:14–22. doi: 10.1111/j.1600-0757.1995.tb00052.x. [DOI] [PubMed] [Google Scholar]
- 22.Badersten A, Nilveus R, Egelberg J. Effect of nonsurgical periodontal therapy. I. Moderately advanced periodontitis. J Clin Periodontol. 1981;8:57–72. doi: 10.1111/j.1600-051x.1981.tb02024.x. [DOI] [PubMed] [Google Scholar]
- 23.Promsudthi A, Pimapansri S, Deerochanawong C, Kanchanavasita W. The effect of periodontal therapy on uncontrolled type 2 diabetes mellitus in older subjects. Oral Dis. 2005;11:293–298. doi: 10.1111/j.1601-0825.2005.01119.x. [DOI] [PubMed] [Google Scholar]
- 24.O'Connell PA, Taba M, Nomizo A, Foss Freitas MC, Suaid FA, Uyemura SA, et al. Effects of periodontal therapy on glycemic control and inflammatory markers. J Periodontol. 2008;79:774–783. doi: 10.1902/jop.2008.070250. [DOI] [PubMed] [Google Scholar]
- 25.Grossi SG, Skrepcinski FB, DeCaro T, Robertson DC, Ho AW, Dunford RG, et al. Treatment of periodontal disease in diabetics reduces glycated hemoglobin. J Periodontol. 1997;68:713–719. doi: 10.1902/jop.1997.68.8.713. [DOI] [PubMed] [Google Scholar]
- 26.Llambes F, Silvestre FJ, Hernandez-Mijares A, Guiha R, Caffesse R. Effect of non-surgical periodontal treatment with or without doxycycline on the periodontium of type 1 diabetic patients. J Clin Periodontol. 2005;32:915–920. doi: 10.1111/j.1600-051X.2005.00736.x. [DOI] [PubMed] [Google Scholar]
- 27.Sorsa T, Ingman T, Suomalainen K, Halinen S, Saari H, Konttinen YT, et al. Cellular source and tetracycline-inhibition of gingival crevicular fluid collagenase of patients with labile diabetes mellitus. J Clin Periodontol. 1992;19:146–149. doi: 10.1111/j.1600-051x.1992.tb00454.x. [DOI] [PubMed] [Google Scholar]
- 28.Koromantzos PA, Makrilakis K, Dereka X, Offenbacher S, Katsilambros N, Vrotsos IA, et al. Effect of non-surgical periodontal therapy on C-reactive protein, oxidative stress, and matrix metalloproteinase (MMP)-9 and MMP-2 levels in patients with type 2 diabetes: a randomized controlled study. J Periodontol. 2012;83:3–10. doi: 10.1902/jop.2011.110148. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigues DC, Taba MJ, Novaes AB, Souza SL, Grisi MF. Effect of non-surgical periodontal therapy on glycemic control in patients with type 2 diabetes mellitus. J Periodontol. 2003;74:1361–1367. doi: 10.1902/jop.2003.74.9.1361. [DOI] [PubMed] [Google Scholar]
- 30.Kaldahl WB, Kalkwarf KL, Patil KD, Molvar MP, Dyer JK. Long-term evaluation of periodontal therapy: I. Response to 4 therapeutic modalities. J Periodontol. 1996;67:93–102. doi: 10.1902/jop.1996.67.2.93. [DOI] [PubMed] [Google Scholar]
- 31.Ramfjord SP, Caffesse RG, Morrison EC, Hill RW, Kerry GJ, Appleberry EA, et al. 4 modalities of periodontal treatment compared over 5 years. J Clin Periodontol. 1987;14:445–452. doi: 10.1111/j.1600-051x.1987.tb02249.x. [DOI] [PubMed] [Google Scholar]
- 32.Sorsa T, Lindy O, Konttinen YT, Suomalainen K, Ingman T, Saari H, et al. Doxycycline in the protection of serum alpha-1-antitrypsin from human neutrophil collagenase and gelatinase. Antimicrob Agents Chemother. 1993;37:592–594. doi: 10.1128/aac.37.3.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanemaaijer R, Visser H, Koolwijk P, Sorsa T, Salo T, Golub LM, et al. Inhibition of MMP synthesis by doxycycline and chemically modified tetracyclines (CMTs) in human endothelial cells. Adv Dent Res. 1998;12:114–118. doi: 10.1177/08959374980120010301. [DOI] [PubMed] [Google Scholar]
- 34.Rifkin BR, Vernillo AT, Golub LM. Blocking periodontal disease progression by inhibiting tissue-destructive enzymes: a potential therapeutic role for tetracyclines and their chemically-modified analogs. J Periodontol. 1993;64(8 Suppl):819–827. doi: 10.1902/jop.1993.64.8s.819. [DOI] [PubMed] [Google Scholar]
- 35.Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care. 2002;25:275–278. doi: 10.2337/diacare.25.2.275. [DOI] [PubMed] [Google Scholar]
- 36.Jones JA, Miller DR, Wehler CJ, Rich S, Krall E, Christiansen CL, et al. Study design, recruitment, and baseline characteristics: the Department of Veterans Affairs Dental Diabetes Study. J Clin Periodontol. 2007;34:40–45. doi: 10.1111/j.1600-051X.2006.00998.x. [DOI] [PubMed] [Google Scholar]
- 37.Singh S, Kumar V, Kumar S, Subbappa A. The effect of periodontal therapy on the improvement of glycemic control in patients with type 2 diabetes mellitus: A randomized controlled clinical trial. Int J Diabetes Dev Ctries. 2008;28:38–44. doi: 10.4103/0973-3930.43097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koromantzos PA, Makrilakis K, Dereka X, Katsilambros N, Vrotsos IA, Madianos PN. A randomized, controlled trial on the effect of non-surgical periodontal therapy in patients with type 2 diabetes. Part I: effect on periodontal status and glycaemic control. J Clin Periodontol. 2011;38:142–147. doi: 10.1111/j.1600-051X.2010.01652.x. [DOI] [PubMed] [Google Scholar]
- 39.Moutsopoulos NM, Madianos PN. Low-grade inflammation in chronic infectious diseases: paradigm of periodontal infections. Ann N Y Acad Sci. 2006;1088:251–264. doi: 10.1196/annals.1366.032. [DOI] [PubMed] [Google Scholar]
- 40.Gurav AN. Periodontitis and insulin resistance: casual or causal relationship? Diabetes Metab J. 2012;36:404–411. doi: 10.4093/dmj.2012.36.6.404. [DOI] [PMC free article] [PubMed] [Google Scholar]