Abstract
Objective
To determine the effects of maternal vitamin A supplementation from preconception through postpartum on cognitive and motor development of children at 10–13 years of age in rural Nepal.
Design
Follow-up assessment of children born to women randomly assigned by a village to receive either supplemental vitamin A (7000 µg retinol equivalents) or placebo weekly during a continuous 3.5-year period from 1994–1997. The participants came from 12 wards, a subset of 270 wards in the original trial. Trained staff tested children for cognition by the Universal Nonverbal Intelligence Test (UNIT) and motor ability using four subtests from the Movement Assessment Battery for Children (MABC). Data on schooling, home environment and nutritional and socioeconomic status were also collected.
Setting
Southern plains district of Sarlahi, Nepal.
Participants
390 Nepalese children 10–13 years of age.
Main outcome measures
Raw scores on UNIT and square-root transformed scores on an abridged version of the MABC tests, expressed as cluster-summarised (mean±SD) values to account for the design of the original trial.
Results
There were no differences in UNIT (79.61±5.99 vs 80.69±6.71) or MABC (2.64±0.07 vs 2.49±0.09) test scores in children whose mothers were exposed to vitamin A vs placebo (mean differences: −1.07, 95% CI −7.10 to 9.26, p=0.78; 0.15, 95% CI 0.43 to −0.08, p=0.15), respectively. More children in the placebo group had repeated a grade in school (28% of placebo vs 16.7% of vitamin A, p=0.01).
Conclusions
Preconceptional to postpartum maternal vitamin A supplementation, in an undernourished setting, does not improve cognition or motor development at ages 10–13 years.
Keywords: Public health
Article summary.
Article focus
This article examines the effects of early vitamin A supplementation on cognitive and motor skills in school-aged children. The participants were born to women who participated in a randomised, placebo-controlled vitamin A supplementation trial before, during and after pregnancy.
Animal studies have suggested that early vitamin A deficiency can permanently impair cognitive and motor function. We hypothesised that children born to women in the placebo group would have poorer scores on UNIT and MABC than their counterparts in the vitamin A group.
Our results provide no evidence that early vitamin A supplementation improves cognitive or motor skills in 10-year-old to 13-year-old-children in rural Nepal.
Key messages
The period between conception and age 2 is an important one for human central nervous system development. Deficiency of essential nutrients can harm the fetus; no research to date has examined the long-term effect of early vitamin A deficiency on children's cognitive or motor skills.
This study suggests no benefit of maternal vitamin A supplementation on cognitive or motor skills in 10-year-old to 13-year-old offspring.
This research suggests that central nervous system development may be protected in vitamin A deficiency, possibly because vitamin A is preferentially allocated to the developing fetus.
Strengths and limitations of this study
This study draws on the strength of the original randomised trial design. The participants were exposed to vitamin A deficiency or supplementation during a developmental critical window.
Psychometric tests were conducted by trained master’s level psychologists, whose works were continuously monitored by school psychologists at the Penn State University.
One of the study's main weaknesses is that the participants were tested relatively late in childhood; it is possible that early differences in ability might have grown less detectable over time.
Introduction
Risk for cognitive and motor impairment begins in the womb, where nutritional restrictions on embryofetal tissue growth, differentiation, development and maturation may disturb growth, maturation and function of the central nervous system.1 Neural myelination and synaptogenesis are underway before 24 weeks’ gestational age, neural tube formation and cell migration are complete before the third trimester of pregnancy, and basic structures of the brain formed before birth.2 As complex structures and functions extend as a cascade, disruptions to nascent systems by nutritional and other insults can be amplified with age.3
Embryofetal development is sensitive to gestational deficiency in vitamin A,4 most evident in animals by gross anomalies in organ and tissue morphogenesis, development, structure and function.5–7 Retinoic acid, the oxidised metabolite and transcriptional ligand of vitamin A, regulates the expression of Hox genes that are essential in neural tube formation,8 the development of neurons, and in synaptic signalling that underlies learning and memory.9–13 Developing neural tissue in the mouse is especially sensitive to variation in retinoic acid exposure near parturition, a time analogous to the second trimester of human gestation, when deficiency can induce behavioural problems and mild structural damage within the limbic cortex.14
Chronic dietary vitamin A deficiency is common among pregnant women in poor countries,15 16 leading to concern that it may have adverse consequences for cognitive and motor development of the fetus. There are suggestions from sparse human data that fetal vitamin A deficiency may adversely affect childhood development. In a study of healthy Chinese mothers and newborns, a positive relationship was observed between cord blood retinol level and motor skill achievements by age 2.17 In Indonesia, newborn vitamin A supplementation resulted in modest improvement in motor development scores after 3 years,18 while vitamin A supplementation to HIV-positive Tanzanian women exerted no detectable changes in infant psychomotor development.19 There remains, however, a need for additional causal data on early life vitamin A exposure and neurocognitive and motor function.
This study is the first to examine effects of routine, maternal vitamin A supplementation before, during and following pregnancy, provided via a randomised, placebo-controlled trial, on child cognitive and motor ability. The trial was conducted in a chronically undernourished population where supplementation with vitamin A or β-carotene reduced maternal mortality,20 biochemical vitamin A deficiency,21 night blindness22 and morbidity,23 but had no overall effect on infant mortality.24 In the long term, at 9–13 years of age, the intervention has been shown to have improved lung size25 and innate aspects of immunity,26 but has not affected biomarker risk factors for cardiovascular diseases.27
Methods
Selection and description of sample
This study followed a sample of children born to women who participated in a cluster-randomised, placebo-controlled trial of vitamin A or β-carotene supplementation before, during and after pregnancy. The trial, known as the Nepal Nutrition Intervention Project Sarlahi-2 (NNIPS-2), ran from March 1994 through September 1997; details of the trial have been described elsewhere.20 Briefly, married women of reproductive age were randomly assigned to the treatment group by the ward, an administrative subdivision of the village development committee (VDC). The women received weekly, oral doses of vitamin A (23 300 IU or 7000 μg retinol equivalents) as retinyl palmitate, 42 mg of all-trans β-carotene (∼7000 μg retinol equivalents at a 6 : 1 conversion ratio), arithmetically equivalent to a daily recommended dietary allowance,28 or a placebo. The supplements were identical, opaque, gelatin capsules, all of which also contained 5 mg dl-α-tocopherol as a preservative.20 Within the trial catchment area, a subset of three contiguous VDCs (Haripur, Netragunj and Kabalisi), comprising 27 wards (9 per supplement arm), were designated as a substudy area. The women in the substudy area gave blood and urine samples during pregnancy and at 3 months postpartum; they were also weighed and measured during the first trimester of pregnancy. Infants in the substudy area were weighed and measured soon after birth and at 3 and 6 months of age.
Participants in the current follow-up study were drawn from 12 wards, 6 each from the vitamin A and placebo groups in two of the three substudy VDCs (Haripur and Netragunj). The participants from the β-carotene arms were excluded for cost and logistical reasons, and because there was no prior basis to posit an effect of β-carotene, beyond its potential as a provitamin A carotenoid.29 The participants in this study were chosen from among those alive and enumerated during a project census in 2006, and had been registered by the fall of 2007 into a follow-up study.25–27 Among women who had contributed >1 pregnancy and live born child to the original NNIPS-2 trial, only the eldest surviving study child was defined a priori as being eligible for the current investigation.
The children studied were exposed in utero and postnatally through breast milk intake to a weekly supplemental supply of vitamin A, calculated to meet maternal dietary recommendations during pregnancy and lactation,29 or to placebo (serving as controls). Beyond 6 months of age, the national, semiannual vitamin A supplementation programme, implemented from 1995 onwards, offered children in the Sarlahi district supplements containing 100 000 IU up to 11 months, and 200 000 IU from 12 to ∼60 months of age, with coverage routinely reaching ≥80%.30 The study children were therefore likely to have started receiving vitamin A periodically through the national programme from age 6 months onwards, but there are no individual records of their programme participation.
Objective and outcomes
This study hypothesised that, by school age, children born to mothers routinely supplemented with vitamin A would perform better on tests of cognitive and motor ability than children born to placebo-supplemented women. The cognitive ability was measured by the child's performance on the Universal Nonverbal Intelligence Test (UNIT) and motor ability by an abridged version (using four subtests) of the Movement Assessment Battery for Children (MABC).
UNIT is a non-verbal test designed to be accessible to children who may be at a disadvantage in traditional intelligence testing because they do not speak the same language as the tester.31 It is composed of six subtests: spatial memory, symbolic memory, cube design, object memory, analogical reasoning and mazes. The participants in this study did not take the analogical reasoning test because pretesting determined that the pictures used in this test were not familiar to Nepalese children. The testers summed the raw scores on each subtest to yield a total raw score. UNIT is a relatively new intelligence test, and this study was the first known use of UNIT in Nepal.
MABC assesses the spectrum of gross and fine motor skills. The participants in this study took four of the eight possible MABC subtests designed for children aged 11–12 years. The trained research assistants scored the 10-year-old participants (n=10) on the 11-year-old standards and the 13-year-olds (n=63) on the 12-year-old standards. The tests given in this study were the peg turing test, the flower trail test, the walking backwards test and the one-handed ball catching test. The MABC tests are scored on a scale of 0 to 5, where 0 is the best possible score and 5 is the worst. The testers summed the scores on each subtest to yield a total raw score.
All psychological testing was conducted by five research assistants, recruited from social science master's programmes at Tribhuvan University and trained and supervised by psychologists from Penn State University in the scoring and administration of UNIT and MABC. The testers were masked to the participant's treatment group. As the purpose of this study was to compare two groups of children, not to diagnose learning or motor problems or calculate intelligence quotients, a lack of test validation data from Nepal was not a serious limitation.
Sample size
Our sample size was determined by considerations of both a minimum detectable difference between groups and logistical constraints associated with enrolling and testing participants. We hypothesised that early life vitamin A supplementation could yield differences of 40% of an SD based on standardised distributions obtainable from UNIT and MABC, with 80% power, and arbitrarily assumed a design effect associated with ward randomisation of 2 and a 5% refusal rate. These assumptions led us to a required sample size of ∼210 children per group, for a total N=420. The sample included all resident children (n=47) in the 12-ward substudy area whose mothers were documented to have had night blindness, a clinical symptom of moderate vitamin A deficiency, during the original trial. The remaining 373 children were drawn from a unified random sample distribution generated using the RANUNI command in SAS V.9.0 (SAS Institute, Cary, North Carolina, USA).
Data collection and informed consent
Fieldwork was carried out from January to September 2008. The study staff visited the participants at their project-verified address, introduced themselves, explained the study and obtained consent from each child’s parent. The interviewer then conducted a series of structured interviews with the mother on the child's health and school history, the family's socioeconomic status, and conducted an early adolescent version of the Home Observation for Measurement of Environment (HOME inventory) and the Ten Questions Plus disability screening.32 33 After these interviews, each mother and child were invited to visit a local, central testing site in the next few days.
Testing rooms had natural and electric lights, fans in the warm weather and heaters in the cool winter months. The study staff brought the sampled child with a parent or guardian to the test site by car. At the test site, study staff read a consent form to the parent or guardian, and assent to the child, whose verbal responses were recorded. The children and their mothers were then fed a snack to ensure that no participant would be distracted by hunger during testing.
At the central site, the participants were weighed on a digital scale (Model 881, Seca, Hamburg, Germany, read to the nearest 100 g) and height was measured using a stationary board with a portable head block (Shorr Products, Olney, Maryland, USA), read to the nearest 0.1 cm. A staff member drew a capillary blood sample from the middle finger of each child's non-dominant hand using a BD Genie Lancet (BD Franklin Lakes, New Jersey, USA), and collected the blood in a microcuvette for haemoglobin testing in a B-Hemoglobin Analyzer (HemoCue, Lake Forest, California, USA).
While the research assistants conducted the psychological tests on children, the trained study staff conducted Raven's coloured progressive matrices, one of the oldest and most validated tests in cognitive testing, on the children's mothers.34
Quality control
The field supervisors reviewed all forms for completeness, and identified and corrected errors before sending the forms to project headquarters in Kathmandu for data entry. If the data entry operator identified an error, he returned the form to the field for correction.
Every UNIT and MABC test was video recorded using a Sanyo C40 video camera mounted on a tripod; videos were downloaded onto a Dell laptop computer after every test session. Once in every 2 weeks, videos from each of the testers were randomly selected, burnt to a DVD and sent to Penn State University where a psychologist reviewed the administration for scoring and technique.
Analysis
The child's height-for-age z-score (HAZ) and body mass index (BMI)-for-age z-score were calculated using the WHO National Center for Health Statistics (NCHS) 2006 growth reference.35
We compared characteristics between vitamin A and placebo groups using χ2 tests or one-way analysis of variance with two-sided p values. We compared the cluster-summarised test scores for vitamin A and placebo group children. An unpaired, two-sided test statistic that accounted for clustering was calculated using the formula described by Moulton and Hayes36:
 |
where cp is the number of clusters in the placebo group, cVA the number of clusters in the vitamin A group,36 and s can be calculated as the square-root of the variance, s2:
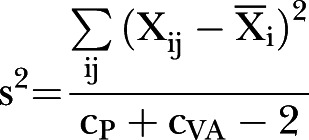 |
where Xij is the jth cluster mean of the i group, and Xi the mean of all six of the i group clusters.36 A CI for the difference in means was computed using the formula:
 |
where tv,0.025 is the 2.5% of a t distribution with v=cPl + cVA − 2 degrees of freedom.36
A two-step variation of this technique was used to estimate the treatment effect in an adjusted model that controlled for influences of potential confounders that were unevenly distributed between the two groups. First, a multivariate model was fitted without a term for the treatment group. The residuals of this model were saved and summarised by clusters. The cluster-averaged residual for both treatment groups were compared using the method described, where  is the cluster-averaged residual from a multivariate regression, used in place of the cluster-averaged mean in the method described above.
is the cluster-averaged residual from a multivariate regression, used in place of the cluster-averaged mean in the method described above.
Stata V.9.0 (Stata Corp, College Station, Texas, USA) was used for all analysis.
Results
Comparisons related to follow-up
During the NNIPS-2 trial, 751 children were born to vitamin A and placebo-recipient mothers in the 12 substudy wards in Haripur and Netragunj. The children who had died prior to the end of the trial in September 1997 (n=64) and children who exited the original trial alive but whose study area residence could not be verified during the project census in 2006 (n=154) were removed from the sampling frame. Further, children whose mother's histories of gestational night blindness during the trial were missing (n=31) were excluded from the sampling frame because night blindness is an indicator of vitamin A deficiency and was considered an essential exposure of interest for other research questions in this study. Finally, children who were born during the trial but were not their mother's first enrolled birth in the study (n=27) were also excluded from the sampling frame to avoid the possibility of within-family clustering. Figure 1 shows the flow of participants starting from those children alive, accounted for and eligible for the study in 2007.
Figure 1.
The study sample showing children from the vitamin A and placebo groups in Haripur and Netragunj village development committees, starting with children alive and accounted for in 2007.
Of the 420 children chosen for the study sample, 24 (5.7%) were not met after repeated visits, 5 (1.2%) refused to participate and 1 (0.23%) had died, leaving 390 (92.3%) who had enrolled. Of the 390 enrolled children, 382 came to the testing site for UNIT and MABC. One of these children was not testable because of Down's syndrome; three of them could not complete the abridged MABC because of injuries. In total, 381 (98% of those enrolled) children completed UNIT and 378 (97%) completed MABC.
Participant's description
The children who participated in the study were more likely to have come from land-owning families than children who were not studied because of having not been met, having refused or having died (83.7% vs 59.3%, p<0.05; data not shown). This trend, however, was only evident in the vitamin A group (among whom the percentages were 84.6 vs 52.9, respectively, p<0.01; data not shown).
Comparisons between participants of the randomised supplement group
The vitamin A and placebo groups of tested children were similar on measures of socioeconomic and nutritional status assessed during the original NNIPS-2 trial, the one important and expected difference being that a higher proportion of children whose mothers had been night blind during pregnancy were in the placebo vs vitamin A group (15.5% vs 8.2%, p=0.02). The children in the placebo group also had more missing maternal (50.6% vs 40%, p=0.04) and neonatal anthropometry (31.6% vs 18.8%, p<0.01) data than in the vitamin A group (table 1). However, as neither maternal nor neonatal anthropometry was a covariate of interest, these missing data did not bias the analyses.
Table 1.
Early characteristics of the tested participants* of the treatment group, data collected during NNIPS-2 trial, 1994–1997
| Characteristics | Vitamin A |
Placebo |
||
|---|---|---|---|---|
| N=207 |
N=174 |
|||
| N | Per cent | n | Per cent | |
| Participant child | ||||
| Male | 99 | (47.8) | 81 | (46.6) |
| Born 1994 or 1995 | 85 | (41.1) | 70 | (40.2) |
| Born 1996 or 1997 | 122 | (58.9) | 104 | (59.8) |
| Missing neonatal anthropometry2 | 39 | (18.8) | 55 | (31.6) |
| Participant mother | ||||
| 66 | (31.9) | 59 | (33.9) | |
| Mother ever XN† | 17 | (8.2) | 27 | (15.5) |
| Missing maternal anthropometry2 | 83 | (40.1) | 88 | (50.6) |
| Household SES | ||||
| Missing SES | 10 | (4.8) | 8 | (4.6) |
| High caste Hindu | 20 | (10.2) | 22 | (13.3) |
| Mother can read | 23 | (11.7) | 18 | (10.8) |
| Father can read | 110 | (55.8) | 93 | (56.0) |
| Family has a radio | 64 | (32.5) | 55 | (33.1) |
| Family owns any land | 170 | (86.3) | 137 | (82.5) |
| Thatch roof house | 62 | (31.5) | 40 | (24.1) |
| Tile roof house | 133 | (67.5) | 126 | (75.9) |
| Salt ID≥30 ppm‡ | 121 | (67.2) | 99 | (66.9) |
| N |
 (SD) (SD) |
n |
 (SD) (SD) |
|
| Neonatal anthropometry§ | ||||
| Weight-for-age z-score | 167 | −1.66 (1.12) | 118 | −1.66 (1.00) |
| Length-for-age z-score | 167 | −1.46 (1.23) | 119 | −1.37 (1.10) |
| Head circumference-for-age z-score | 164 | −1.61 (1.10) | 117 | −1.61 (1.21) |
| Maternal anthropometry¶ | ||||
| Height (m) | 124 | 151.72 (5.18) | 86 | 151.40 (5.56) |
| BMI (kg/m2) | 124 | 19.31 (2.12) | 86 | 19.62 (2.11) |
*N=381 children who participated in UNIT, including 3 injured children who could not complete MABC.
†p<0.05 χ2 test with 1 degree of freedom.
‡n=53 missing, 27 vitamin A, 26 placebo.
§Children were weighed and measured in their homes between 3 and 71 days after birth, mean age 12.2 days, median age 10 days.
¶Women were weighed and measured during the first trimester of pregnancy, 1994–1997.
BMI, body mass index; MABC, Movement Assessment Battery for Children; NNIPS-2, the Nepal Nutrition Intervention Project Sarlahi-2; salt ID, salt iodine; SES, socioeconomic status; UNIT, Universal Nonverbal Intelligence Test.
The comparable proportions of children in the vitamin A and placebo groups came from two-parent families (94.2% vs 90.8%, respectively), and started school on time (59.4% vs 60.3%, respectively). A higher proportion of children in the placebo group had ever repeated a grade (28%) than children born to vitamin A-recipient mothers (16.7%, p=0.01). Fifty-five per cent of children in both groups were stunted, suggesting comparable levels of chronic undernutrition, although children born to mothers in the placebo group had a lower prevalence of anaemia (26% vs 36.6%, p=0.03) and lower exposure to adequately iodised salt in their home, based on tested concentrations of ≥30 ppm (78% vs 85.7%, p=0.05), than in the vitamin A group. The measures of household sanitation and socioeconomic status did not differ between groups, except that controls were slightly less likely to live in households with an improved water source (tap or tube well; 87.4% vs 99%, p<0.01). At the time of testing, control children were also younger than those in the vitamin A group (52.3% vs 62.3% were >12 years, p<0.01). The proportion of children tested by season also varied by supplement group, because children living in the same wards were tested at the same time (table 2).
Table 2.
Contemporary characteristics of the tested participants* of the treatment group, data collected during cognitive and motor development study, 2008
| Characteristic | Vitamin A N=207 |
Placebo N=174 |
||
|---|---|---|---|---|
| N | Per cent | n | % | |
| Sex | ||||
| Male | 99 | 47.8 | 81 | 46.6 |
| Age at testing1 | ||||
| 10 and 11 | 78 | 37.7 | 83 | 47.7 |
| 12 and 132 | 129 | 62.3 | 91 | 52.3 |
| Schooling | ||||
| No school | 16 | 7.7 | 17 | 9.8 |
| Started school ≤6 years | 123 | 59.4 | 105 | 60.3 |
| Started school >6 years | 68 | 32.9 | 52 | 29.9 |
| Ever repeated a grade* | 32 | 16.7 | 44 | 28.0 |
| Testing season† | ||||
| UNIT tested January–March | 17 | 8.2 | 79 | 45.4 |
| UNIT tested April–June | 116 | 56.0 | 19 | 10.9 |
| UNIT tested July–September | 74 | 35.7 | 76 | 43.7 |
| Nutritional status | ||||
| Stunted | 114 | 55.1 | 96 | 55.2 |
| Anaemicठ| 75 | 36.6 | 45 | 26.0 |
| Salt iodine ≥30 ppm3¶ | 174 | 85.7 | 135 | 78.0 |
| Contemporary SES** | ||||
| Has a latrine | 47 | 22.7 | 49 | 28.2 |
| Tube well or water tap2 | 205 | 99.0 | 152 | 87.4 |
| House ≥2 rooms | 161 | 77.8 | 141 | 81.0 |
| Tile roof | 182 | 87.9 | 153 | 87.4 |
| Thatch roof | 21 | 10.1 | 15 | 8.6 |
| Radio | 80 | 38.6 | 78 | 44.8 |
| Wristwatch | 126 | 60.9 | 110 | 63.2 |
| Family | ||||
| Lives with both biological parents | 169 | 43.2 | 222 | 56.8 |
 |
(SD) |  |
(SD) | |
| Height-for-age z-score†† | −2.05 | (1.01) | −2.08 | (1.00) |
| BMI-for-age z-score1 | −1.80 | (0.99) | −1.66 | (0.93) |
| HOME inventory score | 18.46 | (5.89) | 18.64 | (6.21) |
| Household size | 6.30 | (2.43) | 6.41 | (2.45) |
| Number of siblings at home‡‡ | 2.84 | (1.47) | 2.76 | (1.28) |
| Mother's Raven's coloured matrices score§§ | 17.78 | (5.15) | 16.94 | (4.89) |
*N=381 children who participated in UNIT, including 3 injured children who could not complete MABC.
†p≤0.01 χ2 test with 1 degree of freedom comparing vitamin A with placebo.
‡p≤0.01 χ2 test with 2 degrees of freedom comparing vitamin A with placebo.
§n=3 missing.
¶n=5 missing.
**Data shown are percentage of respondents answering yes to the question, “Does your household or anyone in your household have _____.”
††Calculated using the WHO 2006 Growth Reference.35 WMGRS. WHO Child Growth Standards: head circumference-for-age, arm circumference-for-age, triceps skinfold-for-age and subscapular skinfold-for-age: methods and development. Geneva: WHO, 2007.
‡‡Includes siblings, step and half siblings.
§§n=23 missing, 14 vitamin A and 9 placebo.
BMI, body mass index; HOME, Home Observation for Measurement of Environment; MABC, Movement Assessment Battery for Children; SES, socioeconomic status; UNIT, Universal Nonverbal Intelligence Test.
The children were generally stunted (mean HAZ=−2.06, SD=1.00) and thin (mean BMI-for-age z-score=−1.74, SD=0.96). They came from large families (mean household members=6.35, SD=2.43) with several siblings at home (mean number of siblings at home=2.81, SD=1.38). These estimates did not vary between groups, the estimated maternal cognitive ability (mean Raven's coloured matrices score=17.39, SD=5.04) or the quality of the participants’ home environment (mean HOME inventory score=18.54, SD=6.03); see table 2.
Outcome measures
The UNIT scores were normally distributed in both groups (mean=79.81, SD=26.47), with an intracluster correlation of 0.02. The MABC score (mean 6.38, range 0–20 and IQR 3.00, 9.50) was right skewed; the square-root transformation (√(1+raw MABC score)) was used in analysis ( =2.59, SD=0.81). The intracluster correlation for the transformed MABC score was 0.03.
=2.59, SD=0.81). The intracluster correlation for the transformed MABC score was 0.03.
The unadjusted, cluster-level average UNIT scores were comparable in both groups (difference=−1.07 points, 95%CI −7.10 to 9.26, p=0.78; figure 2 and table 3). There was also no difference in the MABC performance (difference=0.18 square-root transformed units, 95% CI −0.08 to 0.43, p=0.15; figure 3 and table 3). Similarly, in regression analysis, which controlled for imbalances between treatment groups (age, anaemia, water source, household salt iodine and test season), there was no difference in the UNIT adjusted mean residual (difference −1.59 points, 95%CI −10.96 to 7.79, p=0.72) or the residual transformed MABC score (difference 0.06 transformed units, 95%CI −0.29 to 0.17, p=0.59) in children born to vitamin A versus placebo supplemented mothers (table 3).
Figure 2.
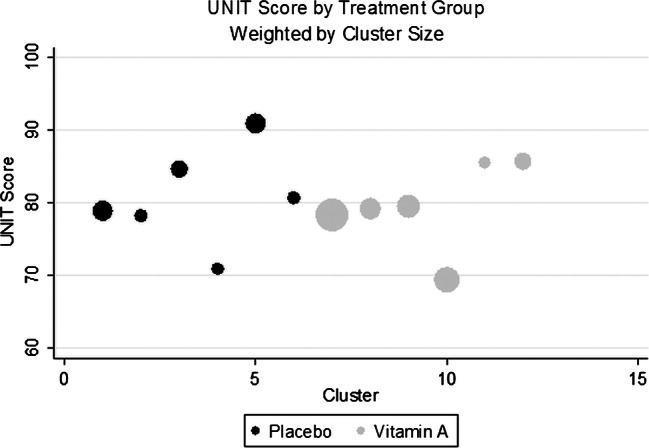
The cluster-summarised Universal Nonverbal Intelligence Test (UNIT) scores by the cluster and treatment group, size of the bubble corresponds to the number of children in each cluster.
Table 3.
The average cluster-summarised UNIT and MABC scores and cluster-summarised residuals from a regression controlling for possible confounders
| Vitamin A | Placebo | Effect estimate* | 95% CI | p Value | |
|---|---|---|---|---|---|
| Number of clusters | 6 | 6 | |||
| UNIT test | |||||
| Number of participants | 207 | 174 | |||
| Mean of cluster mean UNIT scores | 79.61 | 80.69 | −1.07 | (−7.10 to 9.26) | 0.78 |
| Adjusted mean residual1 | 1.21 | −0.37 | −1.59 | (−10.96 to 7.79) | 0.72 |
| MABC test | |||||
| Number of participants | 205 | 173 | |||
| Mean of cluster mean √(MABC score+1) | 2.64 | 2.46 | 0.18 | (−0.08 to 0.43) | 0.15 |
| Adjusted mean residuals† | 0.005 | −0.05 | 0.06 | (−0.29 to 0.17) | 0.59 |
*The effect estimate is the difference in scores or adjusted residuals comparing vitamin A with placebo.
†Based on residuals from a linear regression predicting the UNIT scores and transformed MABC scores and controlling for imbalances between groups in age, anaemia, water source, household salt iodine and test season.
N=3 missing because of refusing the capillary blood sample.
MABC, Movement Assessment Battery for Children; UNIT, Universal Nonverbal Intelligence Test.
Figure 3.
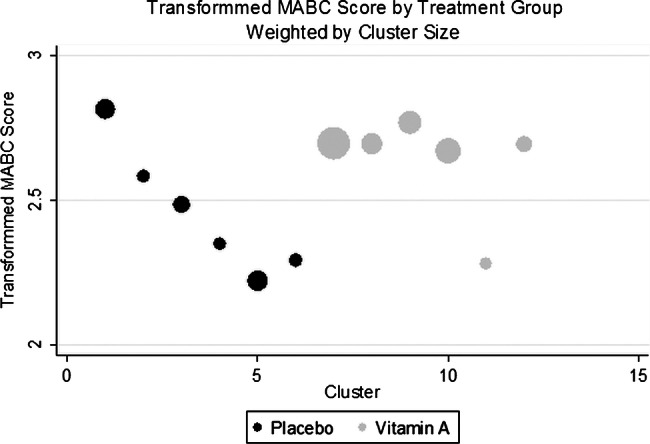
The cluster-summarised transformed Movement Assessment Battery for Children (MABC) score by the cluster and treatment group, size of the bubble corresponds to the number of children in each cluster.
We further examined outcomes stratifying children by whether they had attended school and never repeated a grade (n=269) or had repeated at least one grade (n=76), for which differences across groups existed, reflecting either an imbalance or a previously unspecified supplement effect, but for which inclusion as a covariate could lead to overadjustment in cognitive outcome measures. The children who never attended school (n=33) were excluded from this analysis. There was no difference in the UNIT scores between vitamin A and placebo group children regardless of whether or not they repeated a grade (table 4). However, among the children who never repeated a grade, children in the placebo group scored better than those in the vitamin A group on MABC by 0.36 transformed points, equivalent to 44% of SD (95%CI 0.05 to 0.67, p=0.03).
Table 4.
The average cluster-summarised UNIT and MABC scores among children who repeated a grade (n=76), and those who never repeated a grade (n=272)
| Vitamin A | Placebo | Effect estimate* | 95% CI | p Value | |
|---|---|---|---|---|---|
| Repeaters | |||||
| Number of clusters | 5 | 6 | |||
| Number of participants | 32 | 44 | |||
| √(MABC+1) mean of cluster means | 2.58 | 2.73 | −0.15 | (−0.60 to 0.31) | 0.49 |
| UNIT mean of cluster means | 77.43 | 81.09 | −3.65 | (−14.07 to 6.77) | 0.45 |
| Non-repeaters | |||||
| Number of clusters | 6 | 6 | |||
| Number of participants | 159 | 113 | |||
| √(MABC+1) mean of cluster means† | 2.59 | 2.23 | 0.36 | (0.05 to 0.67) | 0.03 |
| UNIT mean of cluster means | 85.33 | 82.85 | −2.48 | (−14.19, 9.23) | 0.64 |
| Never attended school | |||||
| Number of clusters | 6 | 4 | |||
| Number of participants | 18 | 17 | |||
| √(MABC+1) mean of cluster means‡ | 3.27 | 3.52 | −0.25 | (−0.99 to 0.50) | 0.47 |
| UNIT mean of cluster means | 41.72 | 40.72 | 1.00 | (−24.25 to 26.25) | 0.93 |
*The effect estimate is the difference in scores or adjusted residuals comparing vitamin A with placebo.
†N=1 MABC score missing due to injury from the placebo group.
‡N=1 MABC score missing due to injury from the placebo group.
MABC, Movement Assessment Battery for Children; UNIT, Universal Nonverbal Intelligence Test.
Discussion
In this rural, undernourished setting of southern Nepal, we observed no effect of weekly maternal vitamin A receipt before, during and after pregnancy on cognitive or motor skill development among 10-year-old to 13-year-old offspring. The lack of effect is unlikely to be due to a lack of vitamin A deficiency among pregnant women; ∼20% of mothers were noted to have a serum retinol concentration below 0.70 µmol/l, a prevalence that fell to ∼3% with supplementation.20 Gestational night blindness of ∼10% also responded to vitamin A supplementation,37 and the risk of maternal death was reduced by ∼40% through supplementation.20 There was no lack of cognitive or motor effect, which is likely to be owing to the poor response to supplementation among the offspring; infants born to supplemented women had a higher serum retinol than infants born to placebo mothers by 3 months of age.24 The children born to supplemented women also had better lung function (as measured by the forced expiratory volume at 1 s and forced vital capacity)25 and raised natural antibody levels26 in childhood, further evidence of a physiological effect.
The hypothesised effect of gestational vitamin A supplementation on cognition and motor development is based on sound theory. Animal models have hinted at a special sensitivity of the emerging motor system to retinoic acid.38 39 The developing cerebellum and motor neurons appear to be especially vulnerable to fluctuations in retinoic acid concentration.40 One explanation for the lack of effect is that the participants in this study have passed the age at which differences in their early development are measurable. The human studies that have suggested a role for vitamin A in cognitive and motor development are limited to much younger children.17 18 Another possible explanation for our results is that differences between groups were attenuated by routine supplementation from the national vitamin A programme started in 1995.30 Given the wide CIs for experimental measures of cognitive and motor development, and the potential for brain plasticity, the supplementation programme might have attenuated a treatment effect, allowing placebo group children to regain the nutritional losses of their early life. There are also a host of other nutritional influences on cognitive development, some of which might obscure the relationship between early vitamin A deficiency and developmental outcomes. Iron and zinc deficiencies, both common in Nepal, can also influence child development, as can deficiencies of essential fatty acids.1 The high prevalence of stunting in this sample suggests that the participants’ diets are severely lacking in multiple micronutrients and macronutrients. These deficiencies, working individually or in combination, might also have attenuated between-group differences.
There is also the possibility that the study lacked statistical power to detect a difference between groups, given the modest number of clusters represented in the sample. Though the study had the power to detect a difference of about 40% of SD in the test score, a smaller difference in mean scores might have been undetected. Similarly, this study recruited children from among those alive and accounted for in the study area in 2006. We found that vitamin A group children in the sampling frame were from more affluent families than their counterparts in the placebo group, as indicated by measures of wealth collected during the NNIPS-2 trial. However, the adjusted analysis that controlled for socioeconomic status also found no difference between groups.
Another possible explanation for the lack of difference between groups is that UNIT and MABC are insufficiently precise to pick up on subtle differences in vitamin A nutriture. This is possible, despite the fact that both instruments estimate ability using a diverse cross-section of tests that draw on widely different cognitive and motor functions. Both tests were adapted for use in rural Nepal, but these adaptations were unlikely to adversely affect test validity. Furthermore, research assistants with master’s degrees in social sciences conducted the tests; they were trained, standardised and regularly monitored by professional psychologists. All testing was performed in central sites selected for a calm setting that would enable the children's concentration and adequate attention to testing. We consider it unlikely that procedural flaws could explain the lack of effect.
The post hoc analyses reported in this paper hinted at differences in motor skills after in utero vitamin A supplementation, but did so only after excluding children who never attended school and those who repeated a grade. This analysis does not include the entire spectrum of ability and social background in the population, and therefore should not be overinterpreted. Similarly, measures of cognitive and motor development are influenced by other factors that are difficult to measure, such as school and teacher quality. The children in this sample attended different schools; the differences in education and enrichment of their school environments may have introduced differences into the data that were not accounted for in the analysis.
In conclusion, in this vitamin A deficient setting, where maternal vitamin A supplementation reduced maternal night blindness and mortality, no long-term effects have been observed on the cognitive and motor skill development of prepubescent offspring. Given that some positive effects have been observed in other organ systems, specifically the lung25 and innate immune26 function, it may be that the central nervous system is relatively more protected during gestation under such marginally nourished conditions.
Supplementary Material
Acknowledgments
Apart from the authors, the following members of the Nepal study team helped in the successful implementation of the study: Sharda Ram Shrestha, Tirtha Raj Shakya, Gokarna Subedi, Uma Shankar Shah, Keshab Dhakal, Shishir Shrestha, Dhruba Khadka, Dhan Raj Lama. The authors would like to thank Darrell Mast and Andre Hackman for computer programming, and Monalisha Pradhan, Keshav Raj Mishra, Nar Bahadur Thapa, Sumeetra Dahal and Bikram Sherchan for conducting the Universal Nonverbal Intelligence Test (UNIT) and the Movement Assessment Battery for Children (MABC), and Elizabeth Haytmenek and Zachary Pusch for quality control.
Contributors: GJB was responsible for the analysis and drafting of the manuscript, and worked on field implementation. PC was the principal investigator of the neurocognitive follow-up study, procured funding for the study and participated in the development of the protocol and implementation, data interpretation, and provided comments on the manuscript. LEMK participated in the development of the protocol and implementation of the study, training, and helped with data interpretation and reviewed the manuscript. SCLC and SKK participated in field implementation, supervision and quality control of data collection and data entry, as well as a review of the manuscript. LW drew the participant sample. KPW was the principal investigator of the Nepal Nutrition Intervention Project Sarlahi-2 (NNIPS-2) trial, and he also provided comments on the manuscript.
Funding: The Bill and Melinda Gates Foundation, Seattle, Washington (Grant No. GH614), the National Institutes for Health (1 R01 HD050254–01A) the US Agency for International Development (Cooperative Agreement No, HRN-A-00–97–00015–00), and the Sight and Life Research Institute, Basel, Switzerland.
Competing interests: None.
Ethics approval: The Institutional Review Boards at the Institute of Medicine, Tribhuvan University, Kathmandu, Nepal and the Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA approved the research plan and consent forms for this study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
Abbreviation for Nepal Nutrition Intervention Project, Sarlahi, the second trial.
References
- 1.Walker SP, Wachs TD, Gardner JM, et al. Child development: risk factors for adverse outcomes in developing countries. Lancet 2007;369:145–57 [DOI] [PubMed] [Google Scholar]
- 2.Thompson RA, Nelson CA. Developmental science and the media. Early brain development. Am Psychol 2001;56:5–15 [DOI] [PubMed] [Google Scholar]
- 3.Wainwright PE, Colombo J. Nutrition and the development of cognitive functions: interpretation of behavioral studies in animals and human infants. Am J Clin Nutr 2006;84:961–70 [DOI] [PubMed] [Google Scholar]
- 4.McCaffery PJ, Adams J, Maden M, et al. Too much of a good thing: retinoic acid as an endogenous regulator of neural differentiation and exogenous teratogen. Eur J Neurosci 2003;18:457–72 [DOI] [PubMed] [Google Scholar]
- 5.Hale F. Pigs born without eye balls. In: Persaud TVN, ed. Problems of birth defects. The Netherlands: Springer, 1977:166–7 [Google Scholar]
- 6.Wilson JG, Barch S. Fetal death and maldevelopment resulting from maternal vitamin A deficiency in the rat. Proceedings of the Society for Experimental Biology and Medicine. Soc Exp Biol Med 1949;72:687–93 [DOI] [PubMed] [Google Scholar]
- 7.Wolbach SB, Howe PR. Tissue changes following deprivation of fat-soluble A vitamin. J Exp Med 1925;42:753–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenquist TH, Van Waes JG, Shaw GM, et al. Nutrient effects upon embryogenesis: folate, vitamin A and iodine. Nestle Nutrition workshop series. Paediatr Programme 2005;55:29–40 [DOI] [PubMed] [Google Scholar]
- 9.Chiang MY, Misner D, Kempermann G, et al. An essential role for retinoid receptors RARbeta and RXRgamma in long-term potentiation and depression. Neuron 1998;21:1353–61 [DOI] [PubMed] [Google Scholar]
- 10.Cocco S, Diaz G, Stancampiano R, et al. Vitamin A deficiency produces spatial learning and memory impairment in rats. Neuroscience 2002;115:475–82 [DOI] [PubMed] [Google Scholar]
- 11.Misner DL, Jacobs S, Shimizu Y, et al. Vitamin A deprivation results in reversible loss of hippocampal long-term synaptic plasticity. Proc Natl Acad Sci U S A 2001;98:11714–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Etchamendy N, Enderlin V, Marighetto A, et al. Vitamin A deficiency and relational memory deficit in adult mice: relationships with changes in brain retinoid signalling. Behav Brain Res 2003;145:37–49 [DOI] [PubMed] [Google Scholar]
- 13.Jacobs S, Lie DC, DeCicco KL, et al. Retinoic acid is required early during adult neurogenesis in the dentate gyrus. Proc Natl Acad Sci U S A 2006;103:3902–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo T, Wagner E, Crandall JE, et al. A retinoic-acid critical period in the early postnatal mouse brain. Biol Psychiatry 2004;56:971–80 [DOI] [PubMed] [Google Scholar]
- 15.Underwood BA. Maternal vitamin A status and its importance in infancy and early childhood. Am J Clin Nutr 1994;59(2 Suppl):517S–22S [DOI] [PubMed] [Google Scholar]
- 16.Singh V, West KP., Jr Vitamin A deficiency and xerophthalmia among school-aged children in Southeastern Asia. Eur J Clin Nutr 2004;58:1342–9 [DOI] [PubMed] [Google Scholar]
- 17.Chen K, Zhang X, Wei XP, et al. Antioxidant vitamin status during pregnancy in relation to cognitive development in the first two years of life. Early Hum Dev 2009;85:421–7 [DOI] [PubMed] [Google Scholar]
- 18.Humphrey JH, Agoestina T, Juliana A, et al. Neonatal vitamin A supplementation: effect on development and growth at 3 y of age. Am J Clin Nutr 1998;68:109–17 [DOI] [PubMed] [Google Scholar]
- 19.McGrath N, Bellinger D, Robins J, et al. Effect of maternal multivitamin supplementation on the mental and psychomotor development of children who are born to HIV-1-infected mothers in Tanzania. Pediatrics 2006;117:e216–25 [DOI] [PubMed] [Google Scholar]
- 20.West KP, Jr, Katz J, Khatry SK, et al. Double blind, cluster randomised trial of low dose supplementation with vitamin A or beta carotene on mortality related to pregnancy in Nepal. The NNIPS-2 Study Group. BMJ 1999;318:570–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamini S, West KP, Jr, Wu L, et al. Circulating levels of retinol, tocopherol and carotenoid in Nepali pregnant and postpartum women following long-term beta-carotene and vitamin A supplementation. Eur J Clin Nutr 2001;55:252–9 [DOI] [PubMed] [Google Scholar]
- 22.Christian P, West KP, Jr, Khatry SK, et al. Night blindness of pregnancy in rural Nepal—nutritional and health risks. Int J Epidemiol 1998;27:231–7 [DOI] [PubMed] [Google Scholar]
- 23.Christian P, West KP, Jr, Khatry SK, et al. Vitamin A or beta-carotene supplementation reduces symptoms of illness in pregnant and lactating Nepali women. J Nutr 2000;130:2675–82 [DOI] [PubMed] [Google Scholar]
- 24.Katz J, West KP, Jr, Khatry SK, et al. Maternal low-dose vitamin A or beta-carotene supplementation has no effect on fetal loss and early infant mortality: a randomized cluster trial in Nepal. Am J Clin Nutr 2000;71:1570–6 [DOI] [PubMed] [Google Scholar]
- 25.Checkley W, West KP, Jr, Wise RA, et al. Maternal vitamin A supplementation and lung function in offspring. N Engl J Med 2010;362:1784–94 [DOI] [PubMed] [Google Scholar]
- 26.Palmer AC, Schulze KJ, West KP. Preconceptional through post-partum vitamin A supplementation increases natural antibody concentrations of offspring aged 9-13 years in rural Nepal. FASEB J 2011;25:333–7 [Google Scholar]
- 27.Christian P, Stewart CP. Maternal micronutrient deficiency, fetal development, and the risk of chronic disease. J Nutr 2010;140:437–45 [DOI] [PubMed] [Google Scholar]
- 28.Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: The National Academies Press, 2001 [PubMed] [Google Scholar]
- 29.Tang G. Bioconversion of dietary provitamin A carotenoids to vitamin A in humans. Am J Clin Nutr 2010;91:1468S–73S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nepal Family Health Program II. Technical Brief # 2: Vitamin A Supplements for Children.
- 31.Bracken BA MR. Universal Nonverbal Intelligence Test—Examiner's manual. Itasca, IL: Riverside, 1998 [Google Scholar]
- 32.Caldwell BM, Bradley RH. Home inventory administration manual. University of Arkansas for Medical Sciences, 2001 [Google Scholar]
- 33.Durkin MS, Davidson LL, Desai P, et al. Validity of the ten questions screened for childhood disability: results from population-based studies in Bangladesh, Jamaica, and Pakistan. Epidemiology 1994;5:283–9 [PubMed] [Google Scholar]
- 34.Raven J. The Raven progressive matrices: a review of national norming studies and ethnic and socioeconomic variation within the United States. J Educ Meas 1989;26:1–16 [Google Scholar]
- 35.WHO Multicentre Growth Reference Study WHO Child Growth Standards: head circumference-for-age, arm circumference-for-age, triceps skinfold-for-age and subscapular skinfold-for-age: methods and development. Geneva: World Health Organization, 2007 [Google Scholar]
- 36.Hayes RJ, Moulton LH. Cluster randomised trials. CHAPMAN & HALL, 2009 [Google Scholar]
- 37.Christian P, West KP, Jr, Khatry SK, et al. Vitamin A or beta-carotene supplementation reduces but does not eliminate maternal night blindness in Nepal. J Nutr 1998;128:1458–63 [DOI] [PubMed] [Google Scholar]
- 38.Coluccia A, Belfiore D, Bizzoca A, et al. Gestational all-trans retinoic acid treatment in the rat: neurofunctional changes and cerebellar phenotype. Neurotoxicol Teratol 2008;30:395–403 [DOI] [PubMed] [Google Scholar]
- 39.Coluccia A, Borracci P, Belfiore D, et al. Effects of early gestational all-trans retinoic acid treatment on motor skills: a longitudinal study in the offspring of Sprague-Dawley rats. Neurotoxicology 2008;29:1107–13 [DOI] [PubMed] [Google Scholar]
- 40.Maden M. Vitamin A and the developing embryo. Postgrad Med J 2001;77:489–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



