Abstract
We investigated the diagnostic value of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) for restaging of treated uterine cervix squamous cell cancer with tumor maker elevation that was not explained by other conventional evaluation. We enrolled 32 cases who underwent PET/CT for the restaging of treated cervical cancer with tumor marker elevation that was not explained by recent conventional evaluation. All enrolled cases had squamous cell carcinoma. Increased tumor markers included squamous cell carcinoma antigen (SCC Ag) and carcinoembryonic antigen (CEA). PET/CT findings were determined by pathologic confirmation or clinical follow-up. We compared PET/CT accuracy and clinical parameters including normalization of tumor markers in both the SCC Ag elevation group and the CEA elevation group. The sensitivity, specificity, positive predictive value, and negative predictive value of PET/CT in detecting recurrence were 100%, 83.3%, 82.4%, and 100%, respectively. Accuracy was significantly different between the SCC Ag elevation group and the CEA elevation group (p=0.0169). PET/CT with SCC Ag elevation was more accurate (100%) than PET/CT with CEA elevation (66.7%). Normalization of tumor markers was observed more often in the SCC Ag elevation group than in the CEA elevation group (p=0.0429). PET/CT showed high negative predictive value and sensitivity in the restaging of cervical cancer with unexplained tumor marker elevation. PET/CT was more accurate in patients with SCC Ag elevation than in those with CEA elevation.
Keywords: Fluorodeoxyglucose F18, Positron-emission tomography and computed tomography, Recurrence
INTRODUCTION
Uterine cervical cancer is one of the most common malignancies in the world.1 During the past several years, the treatment of cervical cancer has progressed significantly. These advances were mostly in the early and accurate detection of noninvasive cancer, resulting in a decrease in total mortality. However, the mortality of invasive cancer has not changed for the past 30 years.2 Recurrence of cervix carcinoma usually occurs within 2 years after treatment.3-5 Reported 5-year recurrence and 5-year overall mortality rates are 28% and 27.8%, respectively.6
Early and accurate detection of recurrence is a significant factor in treatment strategy and overall survival.7,8 Also, early detection of recurrence is important in asymptomatic patients.9 Follow-up for treated cervical cancer includes conventional investigation such as physical examination, serum tumor markers, Papanicolaou smear (Pap smear), and radiological evaluation such as computed tomography (CT) or magnetic resonance imaging (MRI). However, the methodology for such follow-up has not been standardized.10 Moreover, no validated tumor markers are available for follow-up of cervical cancer.11
During follow-up, an elevated tumor marker can be the first sign of recurrence.10 Elevated squamous cell carcinoma antigen (SCC Ag) is detected in 71% to 85% of patients before or at the same time as detection of evidence of recurrence. The median lead time is 2 to 7.8 months from the time of SCC Ag elevation to the diagnosis of recurrence.12-14 Therefore, there might be a time gap from tumor marker elevation to detection of recurrence. More specifically, when a tumor marker is elevated without evidence of recurrence or metastasis in other evaluation, such as physical examination, Pap smear, or radiologic evaluation such as CT or MRI, it is referred to as "unexplained tumor marker elevation."
So far, there have been several reports regarding the role of 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) in this "unexplained tumor marker elevation" in cervical cancer.15,16 However, these reports used PET instead of PET/CT. We therefore performed the present study to assess the clinical impact of 18F-FDG PET/CT (PET/CT) for unexplained tumor marker elevation of treated cervical cancer patients.
MATERIALS AND METHODS
1. Patients
The study was carried out in accordance with the Declaration of Helsinki. This clinical study was a retrospective observation study. We enrolled patients with a history of cervical cancer who underwent PET/CT for unexplained tumor marker elevation between May 2004 and June 2009. Eligibility requirements for this study were as follows: 1) patients with a history of uterine cervix malignancy who went into complete remission after treatment, 2) pathology of squamous cell carcinoma, 3) elevation of tumor marker during follow-up after complete remission of cervical cancer, and 4) clinical and radiological evaluation that did not reveal the reason for tumor marker elevation.
Physical examination and Pap smear were done in all cases. Radiological evaluation, such as contrast-enhanced abdomen CT or pelvic MRI were done before PET/CT in nine cases. None of the enrolled cases showed evidence of recurrence or metastasis in those conventional evaluations. Further details are described in Table 1.
TABLE 1.
Characteristics of patients with elevated tumor markers
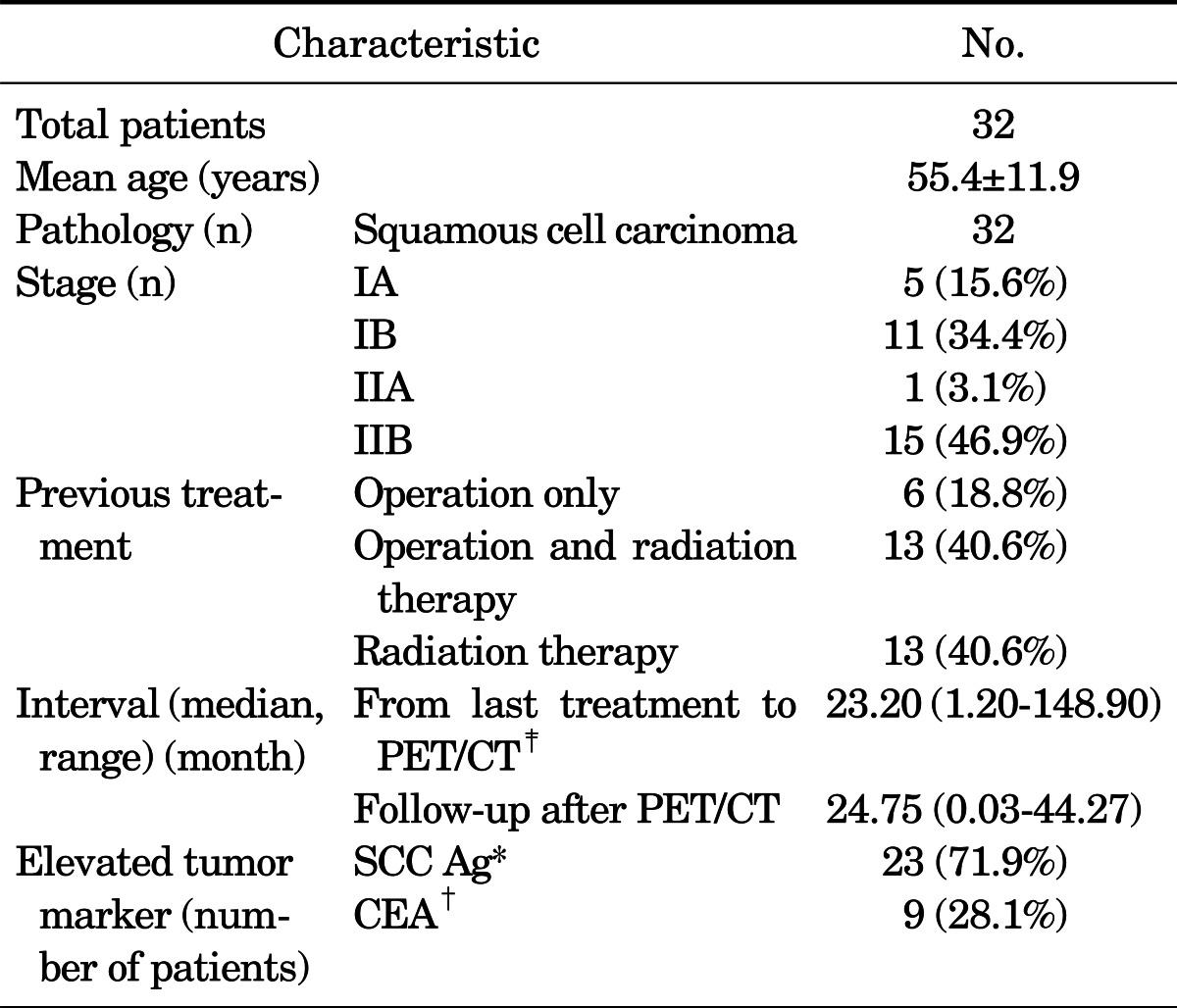
*Squamous cell carcinoma antigen, reference value: 0-1.5 ng/ml, †Carcinoembryonic antigen, reference value: 0-4.7 ng/ml, ‡Radiological evaluation was done before PET/CT and none of these cases showed metastatic lesions in these evaluations.
We excluded all cases in which the reasons for tumor marker evaluation were revealed in any previous evaluation, such as radiological or clinical evaluation before PET/CT.
2. Tumor markers
Choice of tumor markers was based on the decision of each clinician. Among the 32 enrolled cases, elevated tumor markers were SCC Ag or carcinoembryonic antigen (CEA). SCC Ag was measured by immunoradiometric assay (TFB. Inc., Tokyo, Japan) on a Gamma counter (Perkin Elmer, USA) in which the reference range was 1.5 ng/ml. CEA was measured by Elecsys CEA electrochemiluminescence assay on a Modular Analytics E170 system (Roche Diagnostics GmbH, Tokyo, Japan) in which the reference range was 4.7 ng/ml. Tumor marker measurement was done at each follow-up visit after treatment of the primary malignancy. All enrolled cases showed elevation of a tumor marker over the reference value on at least two serial measurements before PET/CT. Detailed information is described in Table 1.
3. 18F-FDG PET/CT
Whole-body imaging was performed by using a combined PET/CT scanner (Discovery ST PET/CT system, General Electric Medical Systems, Milwaukee, WI). After fasting for 6 hours, all patients received 20 mg of intravenous furosemide, continuous intravenous hydration, and placement of a Foley catheter. 18F-FDG (375 MBq) was injected intravenously in a quiet environment. Sixty minutes after FDG administration, imaging was done. Initially, a low-dose CT scan (Helical, 8-slice, 120 Kvp, 10-130 mAs, and 3.79 slice thickness) was performed for attenuation correction. A whole-body emission PET scan was performed immediately after the low-dose CT scan, with a 3 minutes acquisition per bed position with the use of a three-dimensional acquisition mode. Attenuation-corrected PET images were reconstructed.
4. Image interpretation
All PET/CT images were analyzed in consensus by two experienced nuclear medicine physicians. Clinical history and results of previous radiological studies were available. The PET/CT images were interpreted as no metabolic evidence of recurrence when there was no abnormal 18F-FDG uptake in any region. Hypermetabolic lesions were classified on the basis of shape (focal, linear, and diffuse), size, location, and the SUV value (over max SUV 2.5). Recurred lesions were suggested when focal uptake was higher than that of the surrounding tissue and when it was not related to physiologic uptake.
5. Histologic/cytologic confirmation and follow-up after PET/CT
To confirm the lesions, either clinical evaluation and tissue biopsy or radiological or metabolic evaluation was done. For PET-negative cases, short-term follow-up with remeasurement of tumor marker, routine Pap smear, imaging studies, and clinical evaluation were done for all cases. The imaging studies included were abdomen CT or pelvic MRI or 18F-FDG PET/CT. In addition, follow-up of serum tumor markers was done.
6. Data analysis and statistics
On the basis of the follow-up of each PET/CT case, the cases were divided into metastasis and no metastasis groups. Also, the cases were classified as true-positive, true-negative, false-positive, and false-negative. Analysis of the sensitivity, specificity, accuracy, positive predictive value, negative predictive value, positive likelihood ratio, and negative likelihood ratio of PET/CT for detecting recurrence was done.
We compared PET/CT accuracy and clinical parameters including normalization of tumor markers in both the SCC Ag elevation group and the CEA elevation group. Also, we compared clinical parameters in the recurrence and no recurrence groups. For continuous data, we used the Mann-Whitney test. For categorical data, we used Fisher's exact test. p values<0.05 were considered statistically significant. We used MedCalc statistical software (MedCalc Software, Mariakerke, Belgium; version 11.3.1.0).
RESULTS
A total of 32 patients with a history of cervical cancer underwent PET/CT for unexplained tumor marker elevation (Table 1). In these patients, overall sensitivity, specificity, positive predictive value, and negative predictive value of PET/CT for detecting recurrence were 100%, 83.3%, 82.4%, and 100%, respectively (Table 2).
TABLE 2.
PET/CT performance in treated cervix malignancy with unexplained tumor marker elevation (n=32)*
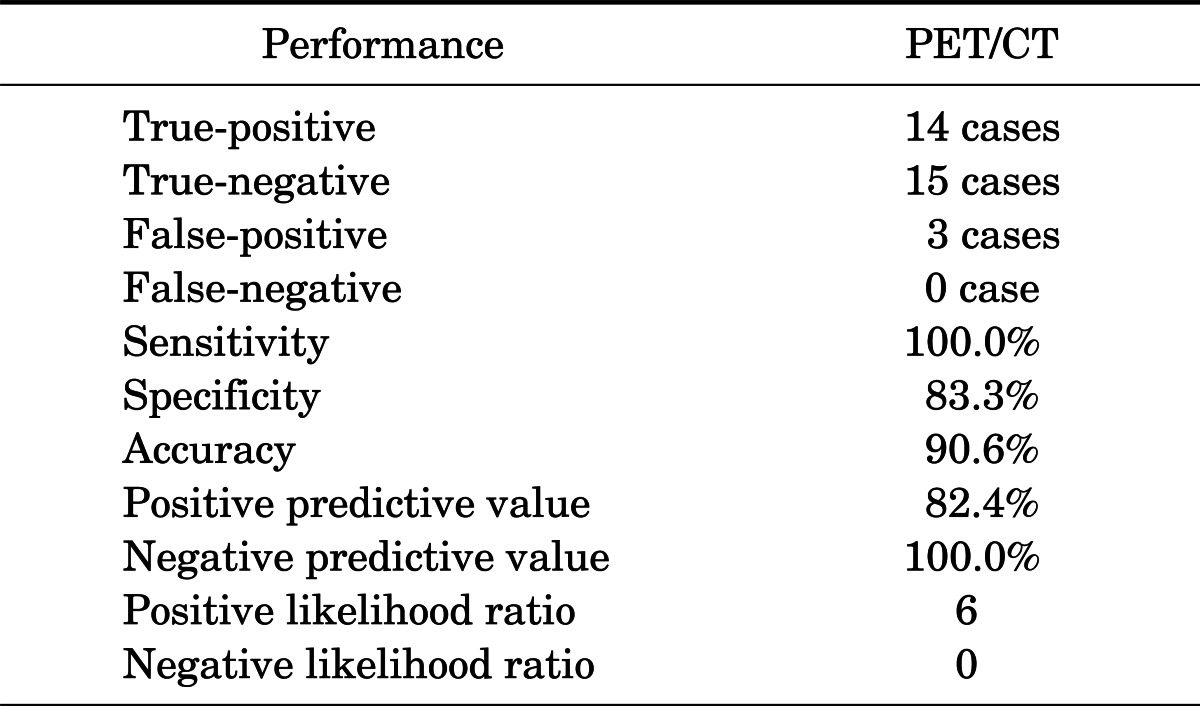
*Disease prevalence, 73.8% (23.4-62.3%). PET/CT: 18F-FDG PET/CT.
1. PET true-positive group
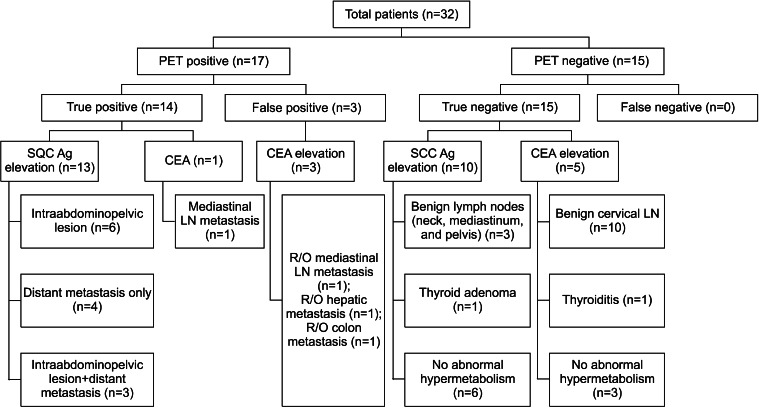
Among the 32 total cases of unexplained tumor marker elevation of treated cervical cancer, 17 cases (53.1%) showed suspicious lesions suggesting recurrence or metastasis (defined as PET-positive cases). The number of cases are described in Fig. 1. Fourteen of the cases were proven to be true positives (Figs. 2, 3). These lesions were abdominopelvic lesions (6 cases), distant metastases (4 cases: spleen, mediastinal lymph node, supraclavicular lymph node, and lung), and both abdominopelvic and distant lesions (3 cases). Six of them were confirmed by tissue biopsy. The other 11 cases showed disease progression on serial radiological evaluation (CT, MRI, or PET/CT) with persistent or elevated tumor markers.
FIG. 1.
Summary of 18F-FDG PET/CT findings and follow-up results in the 32 cases of tumor marker elevation of treated cervix malignancy. LN: lymph node.
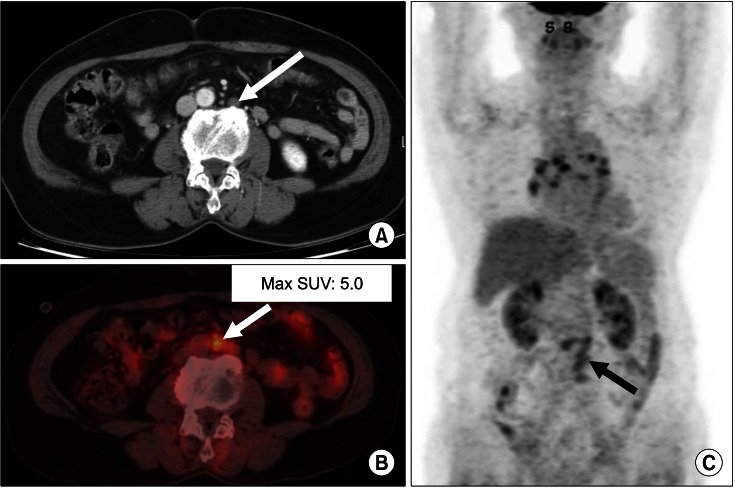
FIG. 2.
True-positive case (lymph node metastasis). A 72-year-old female had a cervical malignancy (squamous cell carcinoma) treated with chemoradiation therapy. Four months after the last treatment, serum SCC Ag was elevated (8.8 ng/ml). Pelvic CT (A) was done, but no metastatic lesions were detected. After 1 month, 18F-FDG PET/CT was done (B, C). Hypermetabolic lymph nodes with max SUV 5.0 were detected in the paraaortic area (B, C arrows). These lymph nodes were supposed to be benign lesions in the previous CT (A, arrow). Recurrence was clinically confirmed by follow-up CT studies and chemotherapy was done for this patient.
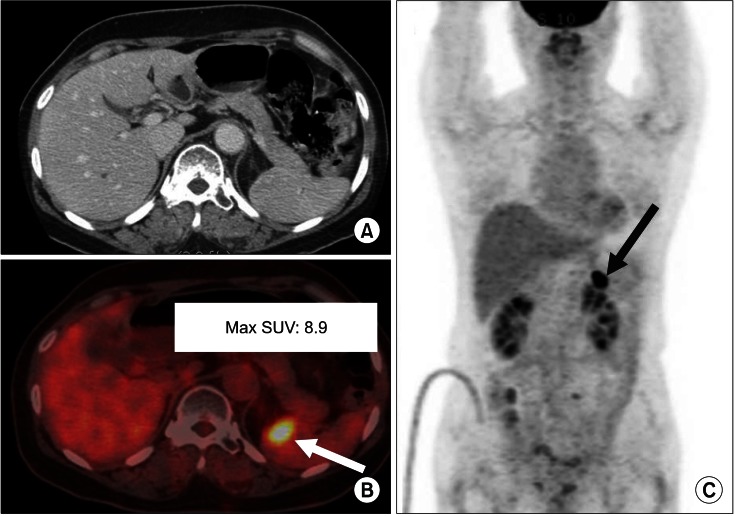
FIG. 3.
True-positive case (splenic metastasis). A 54-year-old female had treated cervical cancer (squamous cell carcinoma). One year later, serum SCC Ag was elevated (from 1.4 to 3.2 ng/ml in 1 month). Contrast-enhanced abdomen CT (A) was done but recurrence of metastasis was not revealed. After 1 week, 18F-FDG PET/CT (B, C) detected a hypermetabolic lesion of max SUV 8.9 in the spleen (B, C arrows). Metastasis was confirmed by biopsy.
2. PET false-positive group
There were three false-positive cases. PET/CT indicated metastatic lesions in the mediastinal lymph nodes and malignant lesions in the liver and colon. The elevated tumor marker was CEA in these three cases. In a case of a colonic lesion, colonoscopy and biopsy were done, which revealed radiation-induced inflammation. In a case of mediastinal lymph node lesions, the patient had undergone cholecystectomy a week before PET/CT. There were several hypermetabolic, high-attenuated lymph nodes in the mediastinum and hilum. The left paratracheal lymph nodes were considered as recurrence owing to lower CT attenuation. However, follow-up with chest CT revealed that this was a false-positive. In a case of hepatic hypermetabolism, pathologic evaluation was not done. However, radiological evaluation such as chest CT and hepatic MRI revealed no definite pathologic disease in those regions. In all of these false-positive cases, routine evaluation including physical examination and Pap smear also showed no evidence of recurrence.
3. PET true-negative group
The other 15 cases of PET/CT (46.9%) did not show any metabolic evidence of recurrence or metastasis (Fig. 1). After more than 2 years (in this subgroup, 20.89 months on average) of follow-up, all of these cases were confirmed as true negatives. PET/CT did not show any abnormal FDG uptake in nine cases. The other six cases showed benign lesions on PET/CT. Further details are described in Tables 2 and 3.
TABLE 3.
Tumor marker normalization and benign disease in true-negative and false-positive cases (n=number of cases)
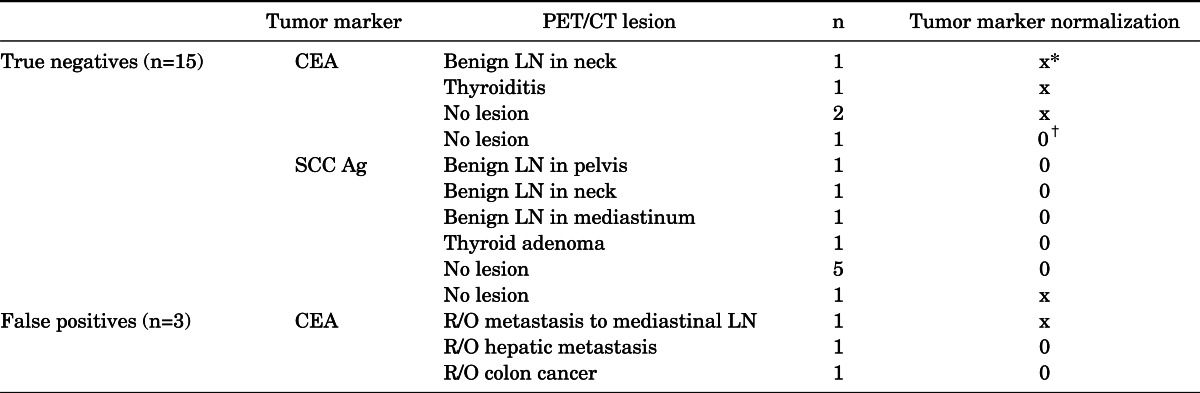
N: lymph node. *Did not normalized, †Normalized.
4. Comparison between groups: SCC Ag elevation group vs. CEA elevation group
The accuracy of PET/CT in detecting recurrence was significantly different between the SCC Ag elevation group and the CEA elevation group (p=0.017). PET/CT with SCC Ag elevation was more accurate (100%; 23/23) than PET/CT with CEA elevation (66.7%; 6/9).
5. Comparison between groups: PET true-positive group vs. false-positive group
The PET true-positive group (n=14) and PET false-positive group (n=3) had significantly different tumor marker elevation (p=0.006). The PET true-positive group had more SCC Ag elevation than CEA elevation. All three false-positive cases had CEA elevation.
The stage, age, and interval from last treatment and PET/CT were not significantly different between the two groups.
6. Normalization of tumor markers
Tumor markers in patients who were without recurrence showed variable changes (Table 3). In 10 cases of true negatives, tumor markers were normalized during follow-up (SCC Ag: 9 cases, CEA: 1 case). In the other five cases, persistent high levels of CEA (4 cases) or SCC Ag (1 case) were shown. In two false-positive cases, the tumor markers were normalized. However, the other one showed persistent levels of CEA. The normalization of tumor markers showed a statistically significant difference between the SCC Ag group and the CEA group. CEA more frequently remained at a high level compared with SCC Ag (p=0.043). The interval from last treatment and PET/CT and age were not significantly different between the two groups.
7. Comparison between groups: recurrence vs. no recurrence
Detection of recurrence was significantly different between the SCC Ag elevation group and the CEA elevation group (p=0.044). Also, stage at first diagnosis was significantly different between cases with recurrence and cases without recurrence (p=0.005).
In addition, stages of primary cancer were higher in the recurrence group than in the no recurrence group (p=0.005). This also differed between the PET true-positive and true-negative groups (p=0.002). However, tumor marker and stage were not significantly different between PET false-positive cases and PET true-positive cases.
The interval from last treatment and PET/CT and age were not significantly different between the two groups.
DISCUSSION
In our study, we revealed the good sensitivity (100%) and high negative predictive value (100%) of PET/CT in treated cervical cancer patients with unexplained tumor marker elevation. We found that the accuracy of PET/CT was significantly higher in the SCC Ag elevation group than in the CEA elevation group (100% vs. 66.7%, p=0.017). All of the false-positive cases were patients with CEA elevation.
This PET/CT study dealt with only unexplained tumor marker elevation in cervical cancer with more than 1 year of follow-up after PET/CT. There have been several reports regarding the impact of 18F-FDG in patients with serum tumor marker elevation for detecting recurrence of cervical cancer.10,15-18 However, those studies were done by PET (not PET/CT) or did not clearly exclude cases that were already proven to have recurrence or symptoms before PET/CT.10,15-18
The sensitivity and specificity of our study with PET/CT were higher than those of the study of Chang et al.15 with PET-only data (100% and 83.3% vs. 94% and 78%, respectively). These differences are probably due to the additional information acquired with PET/CT. However, PET/CT showed lower specificity (83.3%) compared with another study with PET (94.0%).16 The reason for this difference might be the differences in the tumor markers included. Both previous studies investigated only SCC Ag elevation. All three false-positive cases in our study were cases of CEA elevation. Thus, inclusion of CEA might have caused the decrease in specificity in our study. Also, the follow-up after the PET studies was not enough to detect false-negative cases, in contrast with the 1 year and 6 months of follow-up in our study.
Unexplained tumor marker elevation might be a sign of recurrence even though conventional imaging modalities could not find lesions. In a previous report, an increasing SCC Ag level could precede by 8 months the detection of metastatic lesions in 46% to 92% of the cases.11 Detecting metabolic change by PET/CT before morphologic change might be a way to detect metastasis during this time gap. In our study, PET/CT found 9 cases with metastatic lesions in the abdominopelvic cavity. In these cases, CT or MRI, which were done before PET/CT, could not detect the metastasis (Fig. 2).
In addition to the detection of the metabolic changes, whole-body coverage in the follow-up of cervical cancer is another benefit of PET/CT. Fagundes et al.4 reported that the 10-year actuarial incidence of distant metastasis was 3% of stage IA, 16% of stage IB, 31% of stage IIA, 26% of stage IIB, 39% of stage III, and 75% of stage IVA. Frequent metastatic sites of cervical cancer are reported to be the lung, abdominal cavity, gastrointestinal tract, and liver.4 In our study, PET/CT revealed distant metastases to the spleen, mediastinal lymph node, supraclavicular lymph node, and lung, which were not covered by the patients' previous imaging studies.
Tumor markers can also be increased in serum in patients with nonmalignant disease. SCC Ag may be elevated in serum in pemphigus and renal failure.19-21 Also, the serum CEA level is affected by several factors including hepatic function and smoking. In our study, several benign diseases were revealed, such as thyroid adenoma, thyroiditis, and reactive lymph nodes. In some of these cases, the tumor marker elevation normalized during follow-up. However, some cases showed persistent tumor marker elevation (Fig. 1 and Table 3).
False-positives can occur on PET/CT. The gastrointestinal tract and liver can show physiologic hypermetabolism on PET/CT. These are known to be frequent metastatic lesions of cervical cancer.4 Also, reactive lymph nodes can show hypermetabolic activity on PET/CT. Lymph node involvement of cervix cancer was also reported in 22% of cases, predominantly to paraaortic, supraclavicular, and inguinal nodes.4 In our study, three cases of false-positive lesions involved the mediastinal lymph node, liver, and colon (Fig. 1).
The present study had limitations. The number of cases was small, resulting in difficulty in comparison between groups. Despite these limitations, however, the results of this study, which showed a high negative predictive value of PET/CT, may be good evidence for the application of PET/CT in routine clinical evaluation, especially in patients with a higher stage of cancer or unexplained SCC Ag elevation.
In conclusion, 18F-FDG PET/CT showed high negative predictive value and sensitivity in restaging of cervical cancer with unexplained tumor marker elevation. In addition, PET/CT was more accurate in patients with SCC Ag elevation than in those with CEA elevation.
References
- 1.Ellenson LH, Wu TC. Focus on endometrial and cervical cancer. Cancer Cell. 2004;5:533–538. doi: 10.1016/j.ccr.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 2.Oh SW, Kim SK. Clinical application of 18F-FDG PET in cervix cancer. Nucl Med Mol Imaging. 2008;42(suppl 1):101–109. [Google Scholar]
- 3.Brady LW, Perez CA, Bedwinek JM. Failure patterns in gynecologic cancer. Int J Radiat Oncol Biol Phys. 1986;12:549–557. doi: 10.1016/0360-3016(86)90062-3. [DOI] [PubMed] [Google Scholar]
- 4.Fagundes H, Perez CA, Grigsby PW, Lockett MA. Distant metastases after irradiation alone in carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1992;24:197–204. doi: 10.1016/0360-3016(92)90671-4. [DOI] [PubMed] [Google Scholar]
- 5.Perez CA, Breaux S, Madoc-Jones H, Bedwinek JM, Camel HM, Purdy JA, et al. Radiation therapy alone in the treatment of carcinoma of uterine cervix I Analysis of tumor recurrence. Cancer. 1983;51:1393–1402. doi: 10.1002/1097-0142(19830415)51:8<1393::aid-cncr2820510812>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 6.Benedet JL, Odicino F, Maisonneuve P, Beller U, Creasman WT, Heintz AP, et al. Carcinoma of the cervix uteri. J Epidemiol Biostat. 2001;6:7–43. [PubMed] [Google Scholar]
- 7.Larson DM, Copeland LJ, Stringer CA, Gershenson DM, Malone JM, Jr, Edwards CL. Recurrent cervical carcinoma after radical hysterectomy. Gynecol Oncol. 1988;30:381–387. doi: 10.1016/0090-8258(88)90252-1. [DOI] [PubMed] [Google Scholar]
- 8.Irvin WP, Rice LW, Berkowitz RS. Advances in the management of endometrial adenocarcinoma. A review. J Reprod Med. 2002;47:173–189. [PubMed] [Google Scholar]
- 9.Bodurka-Bevers D, Morris M, Eifel PJ, Levenback C, Bevers MW, Lucas KR, et al. Posttherapy surveillance of women with cervical cancer: an outcomes analysis. Gynecol Oncol. 2000;78:187–193. doi: 10.1006/gyno.2000.5860. [DOI] [PubMed] [Google Scholar]
- 10.Sakurai H, Suzuki Y, Nonaka T, Ishikawa H, Shioya M, Kiyohara H, et al. FDG-PET in the detection of recurrence of uterine cervical carcinoma following radiation therapy--tumor volume and FDG uptake value. Gynecol Oncol. 2006;100:601–607. doi: 10.1016/j.ygyno.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 11.Gadducci A, Tana R, Cosio S, Genazzani AR. The serum assay of tumour markers in the prognostic evaluation, treatment monitoring and follow-up of patients with cervical cancer: a review of the literature. Crit Rev Oncol Hematol. 2008;66:10–20. doi: 10.1016/j.critrevonc.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Chan YM, Ng TY, Ngan HY, Wong LC. Monitoring of serum squamous cell carcinoma antigen levels in invasive cervical cancer: is it cost-effective? Gynecol Oncol. 2002;84:7–11. doi: 10.1006/gyno.2001.6497. [DOI] [PubMed] [Google Scholar]
- 13.Lozza L, Merola M, Fontanelli R, Stefanon B, Seregni E, Bombardieri E, et al. Cancer of the uterine cervix: clinical value of squamous cell carcinoma antigen (SCC) measurements. Anticancer Res. 1997;17:525–529. [PubMed] [Google Scholar]
- 14.Micke O, Prott FJ, Schäfer U, Tangerding S, Pötter R, Willich N. The impact of squamous cell carcinoma (SCC) antigen in the follow-up after radiotherapy in patients with cervical cancer. Anticancer Res. 2000;20:5113–5115. [PubMed] [Google Scholar]
- 15.Chang TC, Law KS, Hong JH, Lai CH, Ng KK, Hsueh S, et al. Positron emission tomography for unexplained elevation of serum squamous cell carcinoma antigen levels during follow-up for patients with cervical malignancies: a phase II study. Cancer. 2004;101:164–171. doi: 10.1002/cncr.20349. [DOI] [PubMed] [Google Scholar]
- 16.Chang WC, Hung YC, Lin CC, Shen YY, Kao CH. Usefulness of FDG-PET to detect recurrent cervical cancer based on asymptomatically elevated tumor marker serum levels--a preliminary report. Cancer Invest. 2004;22:180–184. doi: 10.1081/cnv-120030205. [DOI] [PubMed] [Google Scholar]
- 17.Chung HH, Jo H, Kang WJ, Kim JW, Park NH, Song YS, et al. Clinical impact of integrated PET/CT on the management of suspected cervical cancer recurrence. Gynecol Oncol. 2007;104:529–534. doi: 10.1016/j.ygyno.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Hu YY, Sun XR, Lin XP, Liang PY, Zhang X, Fan W. Application of 18F-FDG PET/CT in cervical cancer with elevated levels of serum squamous cell carcinoma antigen during the follow-up. Ai Zheng. 2009;28:994–999. doi: 10.5732/cjc.009.10097. [DOI] [PubMed] [Google Scholar]
- 19.Numa F, Takeda O, Nakata M, Nawata S, Tsunaga N, Hirabayashi K, et al. Tumor necrosis factor-alpha stimulates the production of squamous cell carcinoma antigen in normal squamous cells. Tumour Biol. 1996;17:97–101. doi: 10.1159/000217972. [DOI] [PubMed] [Google Scholar]
- 20.Cases A, Filella X, Molina R, Ballesta AM, Lopez-Pedret J, Revert L. Tumor markers in chronic renal failure and hemodialysis patients. Nephron. 1991;57:183–186. doi: 10.1159/000186247. [DOI] [PubMed] [Google Scholar]
- 21.Tramonti G, Ferdeghini M, Donadio C, Norpoth M, Annichiarico C, Bianchi R, et al. Renal function and serum concentration of five tumor markers (TATI, SCC, CYFRA 21-1, TPA, and TPS) in patients without evidence of neoplasia. Cancer Detect Prev. 2000;24:86–90. [PubMed] [Google Scholar]