Abstract
Rats selectively bred for high saccharin consumption (HiS) self-administer more oral ethanol and i.v. cocaine than those selectively bred for low saccharin consumption (LoS). Male and female drug-seeking-prone (HiS) and –resistant (LoS) rats were used in the present experiment to test the prediction that cocaine-induced locomotor activity and sensitization varied with sex and their selective breeding status (HiS and LoS). All rats were intermittently exposed over 2 weeks to pairs of sequential saline and cocaine injections, separated by 45 min. The first 5 pairs of injections, each separated by 2-3 days (10-12 days total), were given to examine the development of cocaine-induced locomotor activity and the development of locomotor sensitization, which was determined by comparing the effects of cocaine injection 1 with injection 6, which was given 2 weeks after the 5 pairs of intermittent injections. Results indicated that after the first injection pair (saline, cocaine) the HiS and LoS groups did not differ (saline vs. cocaine) in locomotor activity; however, after cocaine injections 1, 5 and 6, HiS females were more active than HiS males and LoS females. There were also significant phenotype differences (HiS > LoS) in locomotor activity after cocaine injections 5 and 6. There was only a weak sensitization effect in cocaine-induced locomotor activity in HiS females after cocaine injection 5 (compared to 1); however it was not present after injection 6 or in other groups. The lack of a strong sensitization effect under these temporal and dose conditions was inconsistent with previous reports. However, the results showing HiS > LoS and females > males on cocaine-induced activity measures are consistent with several measures of cocaine-seeking behavior (acquisition, maintenance, escalation, extinction, and reinstatement), and they suggest that cocaine-induced locomotor activity and sensitization are behavioral markers of drug-seeking phenotypes.
Keywords: Cocaine-induced, Drug-seeking, Female, Locomotor activity, Male, Saccharin intake, Selectively-bred, Sensitization, Sex differences
1. Introduction
Individual differences in locomotor activity induced by a novel environment and by psychomotor stimulants are predictive of subsequent drug self-administration (Piazza et al., 1989, 1990, 2000). However, the relationship between stimulant-induced locomotor sensitization and compulsive drug-seeking behavior is less clear. While recent studies have established a connection between sensitization and reinstatement of drug seeking, they have not found a clear relationship between another form of compulsive drug seeking, escalation (Ahmed and Cador, 2006; Ben-Shahar et al., 2004). However, others have found a relationship between escalation of cocaine self-administration and sensitization when additional cocaine doses and other activity measures (e.g., stereotypic head movements), thought to represent increased drug effects, were taken into account (Ferrario et al., 2005). In the present study, novelty reactivity, cocaine-induced locomotor activity, and sensitization were examined and compared in male and female rats that had been selectively bred to be drug-seeking-prone or -resistant based on their high (HiS) or low (LoS) intake of saccharin and previous demonstrations of drug-seeking behavior (Carroll et al., 2002; Dess et al., 1998; Perry et al. 2007b). The purpose of this work was to determine whether rats with well-established differences in reward-seeking behavior, reinforced by both drugs and preferred dietary substances, express similar differences in stimulant-induced locomotor activity and sensitization. HiS (vs. LoS) rats have exhibited elevated acquisition, escalation, dysregulation, extinction, and reinstatement of cocaine-seeking behavior (Carroll et al., 2002, 2007; Perry et al., 2007b), impulsivity for food (Perry et al., 2007b), intake of ethanol (Dess et al., 1998), and acquisition of heroin self-administration (Carroll et al., 2002). The HiS and LoS lines of rats offer an opportunity to examine the predictive relationship between drug-rewarded behavior and locomotor sensitization.
Several drug-seeking-prone and –resistant phenotypes have been identified by selecting the behavioral extremes from a randomly chosen group of outbred rats and studying models of compulsive of drug-seeking behavior. Examples of drug-seeking-prone behaviors are voluntary or forced reactivity to a novel environment (Piazza et al., 1989, 1990, 2000; Bardo et al., 1996; Klebaur and Bardo, 1999; Klebaur et al., 2001; Mantsch et al., 2001; Cain et al., 2004), novelty choice (Cain et al., 2005), wheel-running (Larson and Carroll 2005a), higher intake of saccharin- and sucrose-adulterated foods or liquids (Gahtan et al., 1996; Gosnell, 2000; Gosnell and Krahn, 1992; Gosnell et al., 1995) and dietary fat (Marks-Kaufman and Lipeles, 1982; Krahn and Gosnell, 1991), impulsiveness for food reward (Perry et al. 2005, 2007c), and stress reactivity (Piazza and LeMoal, 1996; Homberg et al., 2002). This approach to identifying drug abuse vulnerability factors can also be accomplished by selective breeding for the phenotype of interest, and similar results have emerged in the case of saccharin intake and drug self-administration (Dess et al., 1998; Carroll et al., 2002, 2006; Perry et al., 2007a,b).
In addition to drug-self-administration, behavior maintained by preferred dietary items (e.g., sweet, carbohydrate, fat) has many features in common with drug-seeking behavior, such as excessive and escalating behavior (Colantuoni et al., 2001, 2002; Lattanzio and Eikelboom, 2003; Corwin and Hajnal, 2005), withdrawal effects (Colantuoni et al., 2002; Stoffel and Kraft, 2004), sensitization (Avena and Hoebel, 2003a,b), and cross-sensitization (Gosnell, 2005; Vitale et al., 2003). For example, repeated exposure to sweetened liquids sensitizes rats to the locomotor effects of cocaine (Gosnell, 2005) or amphetamine (Avena and Hoebel, 2003a,b) as well as conditioned place preference induced by fentanyl or amphetamine (Vitale et al., 2003).
Behavior motivated by preferred foods also shares many of the same neurobiological mechanisms and neuroadaptations as drug-motivated behavior, such as common reward circuitry (Jonas, 1990; Hoebel et al., 1999; Colantuoni et al., 2001, 2002; Levine et al., 2003; Ahmed, 2005; Di Chiara, 2005; Ferrario et al., 2005; Kelley et al., 2005; Nestler, 2005; Volkow and Li, 2005) and early gene effects (Kelley et al. 2005; Nestler 2005) that may account for the relationship between intake of preferred foods and drug motivated behavior. A relationship between avidity for preferred dietary items, foods, drug-seeking behavior, cocaine-induced activity, and sensitization would provide an animal model supporting the notion of a more general “reward-addiction” in humans that encompasses and interchanges with behaviors motivated by both drug and nondrug events (e.g., Volkow and Wise, 2005).
Behavioral sensitization, which has been defined in rodents as an enhanced drug-induced locomotor response following repeated intermittent psychostimulant administration (see reviews by Robinson and Becker, 1986; Kalivas and Stewart, 1991; Robinson and Berridge, 1993, 2003; Spanagel, 1995; Wolf, 1998, 2002), can persist for long periods of time, even following the cessation of any drug withdrawal effects. Sensitization develops to the psychomotor stimulating (Robinson and Berridge, 1993), incentive motivational (Robinson and Berridge, 2003; Vezina, 2004), as well as drug-seeking and -reinforcing (Hooks et al. 1994; Phillips and DiCiano, 1996; de Vries et al., 1998, 2002; Paterson and Markou, 2003) effects of drugs. The neurobiological bases for these phenomena have been discussed and reviewed by several groups (Robinson and Becker, 1986; Kalivas and Stewart, 1991; Robinson and Berridge, 1993, 2003; Spanagel, 1995; Wolf, 1998, 2002). The midbrain dopaminergic pathways, particularly the projection from the ventral tegmental area to the nucleus accumbens, are thought to be critical for both the development and expression of drug-induced locomotor activity and drug self-administration (Vezina, 2004).
While much evidence links sensitization and drug-seeking behavior, a causal effect has not been established. For example, one of the hallmarks of cocaine addiction, escalation of cocaine self-administration with extended exposure, was related to stimulant-induced (Ferrario et al., 2005) sensitization when sensitization was measured with several cocaine doses and specific behavioral measures (e.g., stereotypic head movements), while others reported a dissociation between escalation of cocaine use and cocaine-induced locomotor sensitization using locomotor activity counts as a measure (Ben-Shahar et al., 2004; Ahmed and Cador, 2006). In contrast, sensitization has been consistently related to another form of compulsive drug-seeking, reinstatement (Ahmed and Cador, 2006; BenShahar et al. 2004). Thus, examining cocaine-induced and cocaine-sensitized locomotor activity in rats with different phenotypic profiles (drug-seeking-prone and -resistant) may be another way to test the hypothesis that drug-sensitized locomotor activity is related to compulsive drug-seeking behavior. The specific hypothesis to be tested in this study is that if cocaine-induced locomotor activity and subsequent development of sensitization are predictive compulsive drug-seeking behavior, then rats that are genetically predisposed toward excessive and compulsive cocaine- and other reward-seeking behaviors would show elevated cocaine-induced locomotor activity and sensitization.
Typically, sensitization and drug self-administration studies have been conducted in male rats, although several studies with both male and female rats indicate that females are more prone than males to cocaine-induced locomotor sensitization (Laviola et al., 1995; Quinones-Jenab et al., 1999; Gulley et al., 2003; Festa and Quinones-Jenab, 2004) and various forms of drug self-administration (e.g., Carroll et al., 2004, Festa and Quinones-Jenab, 2004; Lynch et al., 2002; Roth et al., 2004). Furthermore, the basis of these sex differences may be dependent on estrogen elevating and progesterone decreasing drug-seeking behavior across all phases of addiction that have been modeled in animals (Anker et al., 2006; Carroll et al., 2004, Festa and Quinones-Jenab, 2004; Larson et al. 2007; Roth et al., 2004). Thus, another question to be addressed in the present experiment was whether there are sex differences in HiS and LoS rats in locomotor responsivity to cocaine and sensitization after repeated exposure. It is also hypothesized that if females rank higher than males on measures of drug-seeking behavior, then cocaine-induced locomotor activity and sensitization should be elevated in females (vs. males). Additionally, this analysis allows us to determine whether rats with dual vulnerabililty (e.g., HiS, female) may be most likely to exhibit cocaine induced activity and sensitization compared with male HiS and male and female LoS rats that would be presumed to have one (HiS male, LoS female) or no (LoS male) vulnerability factors (underlined).
In the present experiment, baseline and cocaine-induced (acute and chronic) locomotor activity were measured in male and female LoS and HiS rats after repeated, intermittent saline and cocaine injections using a circular locomotor track (Piazza et al., 1989) and a sensitization procedure described by Kim and Vezina (2002). It was predicted that HiS rats would show greater cocaine-induced locomotor activity and sensitization than LoS rats, and females would exceed males within the HiS and LoS inbred groups.
2.Methods
2.1. Animals
Twenty-four experimentally naïve male and female rats were distributed into 4 groups of 6 rats each consisting of selectively-bred male, female, HiS and LoS rats from generations 20 to 23 of selective breeding. The HiS and LoS lines had been developed through pairings of rats with extreme HiS and LoS phenotype scores with no sibling, half-sibling, or first-cousin matings according to methods of Dess and Minor (1996). The breeding process, which was initiated at Occidental College (Los Angeles, CA, USA), has been continued in our laboratory at the University of Minnesota since 2002. Every 4-6 generations there is outbreeding with rats from the background strain (Sprague Dawley) to maintain the vigor of the HiS and LoS lines. Rats were housed in a dedicated breeding or holding room with food and water available ad libitum. During the experiment, the body weights of female rats averaged 292 g, and male rats averaged 428 g. HiS rats were slightly (4-6%) but not significantly, heavier than their LoS counterparts.
Before the experiment, rats were pair-housed in plastic cages with free access to food and water, and after the experiment began, they were singlely housed in plastic cages with ad lib food and water when not in the testing apparatus. The breeding and experimental rooms were temperature- (24° C) and humidity-controlled with a 12-hr light/dark cycle with lights on at 6:00 a.m. Experimental use of these animals was approved by the University of Minnesota Institutional Animal Care and Use Committee (IACUC, Protocol No. 0410A64760), laboratory facilities were accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC), and accepted principles of animal care (National Research Council, 2003) were followed.
2.2. Apparatus
A circular (inner diameter 46 cm; outer diameter 71 cm) locomotor device similar to that described by Piazza et al. (1989, 1990) was used to measure locomotor activity. The walls of the track were 25 cm high and 5 cm above the floor of the track, 4 infrared (IR) sensors (SE612CV, Banner Engineering Corp., Minneapolis, MN) were mounted on the outer wall at 0°, 90°, 180°, and 270°. An activity response, or beam crossing, was counted as a nonrepetitive IR beam break. Two or more successive beam breaks at the same beam were counted only as one. Sensors were connected to a VersaMax programmable logic controller (IC200UDR001, GE Fanuc Automation, Charlottesville, VA), and data were recorded using PCs and VersaPro software (GE Fanuc Automation, Charlottesville, VA).
2.3. Drugs
Cocaine HCl was obtained from the National Institute on Drug Abuse, Research Triangle Institute, Research Triangle Park, NC). It was dissolved in sterile saline and refrigerated until it was used for injection. Filled syringes were allowed to reach room temperature before each injection.
2.4. Procedure
Rats received 6 pairs of i.p. saline and cocaine injections, the first 5 were administered every 2 or 3 days, until 5 pairs were administered, and the 6th injection pair took place 2 weeks after the fifth. Behavior resulting from the rat’s first i.p. injection (saline) and 45 min exposure to the locomotor track was considered novelty reactivity, while the subsequent exposures constituted baseline locomotor activity. After each saline injection and its 45-min locomotor activity period, rats were administered cocaine (10 mg/kg, i.p.) and monitored in the locomotor track for another 45-min period. While locomotor activity was monitored after injection pairs 1, 5 and 6, the animals were not monitored in the locomotor track during these injection pairs (2, 3, and 4) due to the availability of only 2 tracks and the time-consuming process. The purpose of injection series 2-4 was to induce the development of locomotor sensitization by repeated, intermittent cocaine administration. The development of locomotor sensitization was assessed by comparing the locomotor responses to saline and cocaine during injection series 1 vs. 5. Finally, the expression of locomotor sensitization was examined by comparing locomotor responses after saline and cocaine injection pairs 5 and 6 (which occurred 2 weeks after 5).
Ten to 14 days after all experimental procedures were completed, a saccharin intake phenotype score was obtained from each rat. This test was conducted after the locomotor response experiment was completed to allow for verification of phenotypes without influencing drug-related behaviors by introducing saccharin. The saccharin score was obtained by giving each rat a 24-h 2-bottle choice test with 0.1% (wt/vol) saccharin and water to measure saccharin intake (Dess et al. 1998). Water consumption used in the calculation was from a previous 24-h 2-bottle test when only water was present. The equation used to obtain the saccharin phenotype score was:
A positive score indicated a preference for saccharin (vs. water), a negative score – an aversion, and zero – indifference. HiS rats typically have saccharin phenotype scores in the 30-50 range, and LoS rats’ scores usually range from 0-20 (Carroll et al., 2006; Perry et al., 2006a,b). Basic information on the group sizes, body weights, and saccharin phenotype scores is shown in Table 1. Females’ saccharin scores were more variable than males’ and they extended across a greater range; however, there were no sex differences. HiS females and HiS males’ scores were significantly higher than their LoS counterparts (p<0.05, females; p<0.01, males).
Table 1.
Group Information
| Group | n | Weight (g) (±SEM) |
Saccharin Phenotype Score (± SEM) |
|---|---|---|---|
| LoS F | 6 | 287 (± 15) | 1.8 (± 4.5) |
| HiS F | 6 | 304 (± 12) | 43.5 (± 12.0)* |
| LoS M | 6 | 418 (± 32) | 11.2 (± 6.2) |
| HiS M | 6 | 437 (± 24) | 29.7 (± 4.1)** |
HiS > LoS
Female p < 0.05
Male p < 0.01
2.6 Data analyses
Dependent measures were the number of consecutive, nonrepetitive IR beam breaks during each 45-min period following the first, fifth, and sixth (2 week delay) injection pairs (saline, cocaine). The time course of locomotor activity was also examined in HiS vs. LoS and female vs. male rats by comparing them over 5-min intervals of the 45-min sessions following cocaine injections 1, 5, and 6. The mean total beam break data were analyzed with separate 3-way mixed factors repeated measure (sex × phenotype × injection number) ANOVAs for saline and cocaine injections. Additional 3-way mixed-factor, repeated measure ANOVAs compared treatment (saline vs. cocaine), phenotype (HiS vs. LoS) and sex after each injection series (1, 5, and 6). The time-course data were analyzed with 2-way, mixed-factors repeated measures ANOVAs comparing phenotype or sex and 5-min intervals as the repeated measure. Fisher’s LSD Protected t-tests were used for post hoc comparisons. Results were considered statistically significant if p<0.05.
3. Results
3.1. Novelty reactivity and baseline cocaine-induced locomotor activity
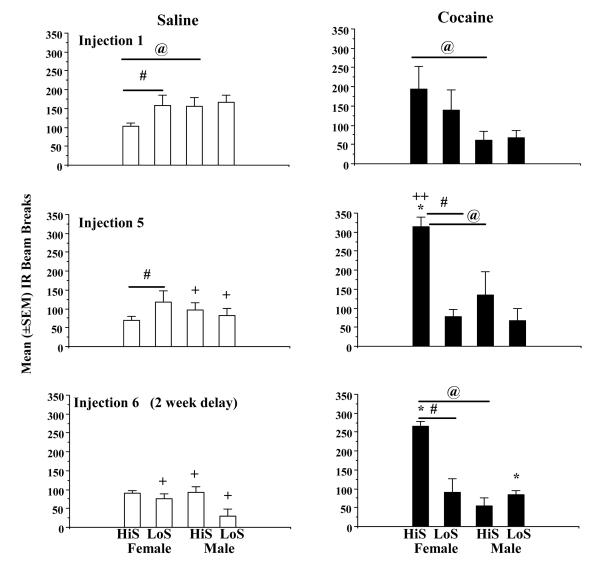
Figure 1 shows the mean total number of IR beam breaks per 45-min session as a function of saline or cocaine pretreatment in the 4 groups. The first injection (saline top left frame) was an indicator of the rats’ reactivity to the novel locomotor activity apparatus, while the second injection that occurred 45 min later was representative of the baseline reactivity to cocaine. A 3-way ANOVA comparing treatment (saline vs. cocaine), phenotype (HiS vs. LoS), and sex revealed no significant treatment (saline vs. cocaine), phenotype, or sex differences between saline and cocaine injection 1. The saline-cocaine injection 5 series, which is portrayed in the center frames showed a significantly higher overall rate of locomotor activity after cocaine (center, right) than saline (center, left) (F1,47 = 9.3045, p<0.01) (indicated by *), and the HiS female group was the main contributor to this effect. There was a significant phenotype (F1,47 = 8.6327, p<0.01) effect (LoS > HiS) (indicated by #), and a phenotype × treatment interaction (F1,47 = 21.523, p<0.0005), and HiS females > HiS males in locomotor responses after cocaine injection 5 (indicated by @). Injection series 6, which occurred 2 weeks later yielded a similar treatment effect (cocaine vs. saline) (F1,47 = 23.6132, p<0.0001), with a significant effect of phenotype (HiS > LoS) (F1,47 = 12.6684, p<0.005) (as shown by #), and sex (F>M) (F1,47 = 17.865, p<.0005) (indicated by @). Again, the post-hoc comparison indicated that the HiS female group’s locomotor responding was greater after cocaine than saline (indicated by *).
Fig. 1.
Locomotor activity during 45-min sessions for LoS F (n=6), HiS F (n=6), LoS M (n=6), and HiS M (n=6) rats following saline (left frames, open bars), then cocaine (10 mg/kg, i.p.) (right frames, filled bars) injections 1, 5, and 6 (2 week delay). Data represent the mean (±SEM) number of infrared (IR) beam breaks (positioned every 90° in a circular locomotor track). Asterisks represent significant within-group (locomotor stimulant effects saline vs. cocaine differences) at p<0.05. Horizontal bars with a pound sign represent a significant phenotype (HiS vs. LoS) differences at p<0.05. @ signs represent significant sex differences (F>M or M>F at p<0.05). A + sign represents a significant (p<0.05) decrease in locomotor activity relative to saline injection 1, evidence of a novelty effect after the first saline injection and exposure to the locomotor track. A double plus sign (++) indicates a significant (p<0.05) increase in cocaine-induced locomotor activity, evidence of a sensitization effect.
The left side of Figure 1 reveals changes in locomotor activity across the 4 groups as a result of repeated saline injections. A 3-way repeated measures ANOVA comparing phenotype, sex, and injection number (1, 5, 6) for saline or cocaine infusions revealed no significant main effect due to sex or phenotype; however, there was a significant effect of injection number on saline-induced locomotor activity (F(2,71) = 29.137, p<0.0001) and significant 2-way interactions with phenotype (F(2,71) = 3.7248, p<0.05), indicated by #, and sex (F(2,71) = 7.2308, p<0.05) shown by @. Post-hoc 2-group comparisons indicated that these differences were due to HiS males showing more activity than HiS females after saline injection 1 and LoS females > HiS females after saline injection 5 (ps<0.05). The main effect due to saline injections was due to elevated novelty reactivity shown as elevated locomotor behavior after injection 1 compared with 5 (LoS and HiS males) or after 1 vs. 6 (LoS females, LoS males and HiS males) (ps<0.05). This novelty effect is shown by (+) signs.
3.2 Development and expression of cocaine-induced locomotor sensitization
The right side of Figure 1 shows the mean number of total IR beam breaks per 45-min session as a function of cocaine injection number in the 4 groups. There was a significant main effect due to phenotype (F1,71 = 11.5627, p<0.005) and sex (F1,71 = 17.8284, p<0.001), as well as a significant 2-way interaction between phenotype and injection number (F2,71 = 4.2038, p<0.05). Post hoc 2-group contrasts revealed significant phenotype differences (HiS>LoS) in females (shown by #) after cocaine injections 5 and 6 (p<0.05) and sex differences (females > males) in HiS rats after cocaine injections 1, 5, and 6. Evidence for the development of cocaine-induced locomotor sensitization was found in female HiS rats that showed greater locomotor activity after injection 5 vs. injection 1 (p<0.05) as indicated by (++). Although activity was nearly as high after injection 6, as it was after 5, in this group, a comparison of cocaine injection 1 vs. 6 failed to reach statistical significance, indicating that sensitization had not persisted for another 2 weeks, and there were no other significant differences in cocaine-induced locomotor responding across cocaine injections 1, 5, and 6 in the other 3 groups.
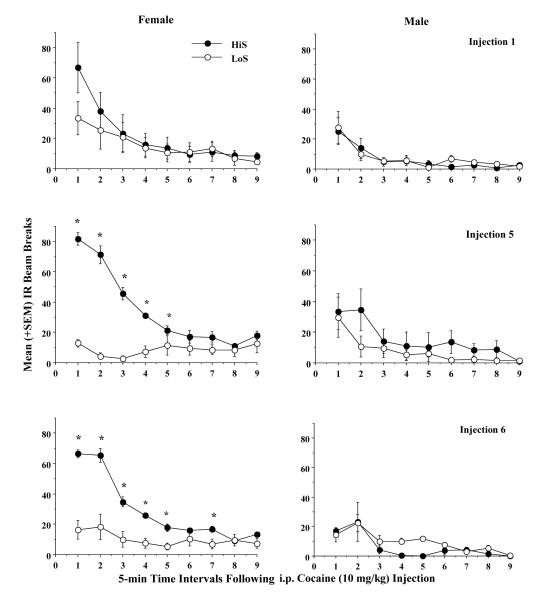
When the 45- min locomotor activity sessions were analyzed for differences in locomotor responding over their 9, 5-min bins, there was a significant effect of time interval for all 3 saline and all 3 cocaine injections with the number of IR beam breaks decreasing over the 45 min period (p<0.005, Fs not shown). Figure 2 illustrates the time course data after cocaine injections 1, 5, and 6 to compare HiS and LoS rats over the 9, 5-minute intervals that constitute the 45-min session. In females, after cocaine injection 1 (top left), there was no phenotype effect or phenotype × time interaction in the baseline response to cocaine. However, after cocaine injection 5 (center left) there was a significant main effect of phenotype (F1,107 = 58.8793, p<0.0001) and a phenotype × time interaction (F8,107 = 32.6431, p<0.0001). Cocaine-induced locomotor responding was greater in HiS (vs. LoS) rats for the first 25 min of the 45-min session (*). Similarly, after cocaine injection 6 (lower left), there was a significant main effect of phenotype (F1,107 = 20.366, p<0.001) and a phenotype × time interaction (F8,107 = 47.418, p<0.0001), with greater cocaine-induced locomotor activity in HiS (vs. LoS) females during 6 of the 9 intervals (5, 10, 15, 20, 25, and 35 min). In the right frames, HiS and LoS male rats’ locomotor activity was compared during the same intervals, and there was no significant main effect of phenotype or phenotype × time interactions after cocaine injections 1, 5, or 6. Thus, phenotype (HiS>LoS) contributed to the development and expression of cocaine-induced locomotor sensitization in females but not in males. After the saline injections, there were no significant sex or phenotype time course differences in locomotor activity across the 9, 5-min intervals for injections 1, 5, or 6 (data not shown). In fact, the time course patterns for saline for all groups were very similar to those shown in Figure 2 for LoS females, and HiS and LoS males with most of the locomotor responding occurring during the first 5-10 minutes of the 45-min session.
Fig. 2.
Comparisons of phenotype differences (HiS, filled circles vs. LoS, open circles) in cocaine-induced locomotor activity in female (left frames) and male (right frames) rats. Data represent the mean (±SEM) number of IR beam breaks distributed over 5-min intervals over the 45-min session following cocaine injections 1, 5, and 6 (saline injections are not included). Asterisks indicate significant (p<0.05) differences between HiS and LoS rats.
4. Discussion
The HiS female rats showed a greater locomotor response after cocaine administration (injection 1) than LoS females and HiS males. Repeated intermittent exposure to cocaine injections also produced a weak cocaine-induced locomotor sensitization. A locomotor sensitization effect was found only in HiS females, and only after cocaine injection 5, which took place 10-12 days after injection 1. A significant sensitization effect was not found after injection 6 in any of the groups, which was 2 weeks after injection 5. The lack of a significant sensitization effect in HiS females after injection 6 (2 weeks after injection 1) could have been due to the small number of rats used, and the high variability of the baseline measure (injection 1). The mean IR beam breaks increased from 190 to 270 (compared to 315 after injection 5); however, that increase that occurred 24–26 days after injection 1 failed to reach significance, and this was inconsistent with previous reports in which sensitization effects to 10 mg/kg cocaine lasted several weeks. It is also possible that compulsive drug-seeking that is seen with HiS rats (vs. LoS), and females (vs. males) is not related to locomotor sensitization in these selectively-bred rats. Furthermore the selective breeding process may have resulted in weaker sensitization overall, than found in the outbred rats from earlier studies. Others have shown mixed results in attempts to compare compulsive drug-seeking and sensitization. There is a connection between sensitization and cocaine-primed reinstatement (Ahmed and Cador 2006; Ben-Shahar et al., 2004), but with escalation of cocaine intake as sensitization effect was reported only under limited conditions of counting head movements rather than locomotor activity (Ferrario et al. 2005). Furthermore, it was reported in a recent study that heroin sensitization was also related to reinstatement but not escalation using a range of conditions (Lenoir and Ahmed, 2007).
The modest sensitization effect found in the present study after injection 5 (10-12 days after injection 1) in HiS females concurs with other reports that sensitization is more robust in female vs. male rodents (Laviola et al., 1995; Quinones-Jenab et al., 1999; Gulley et al., 2003; Festa and Quinones-Jenab., 2004), and this may be related to differential neuroendocrine, physiological, and pharmacodynamic processes between males and females (Cirulli and Laviola, 2000). The sex differences in the present study also agree with findings from previous studies regarding other aspects of reward-seeking behavior in which female rats displayed greater acquisition (Lynch and Carroll, 1999b), escalation (Roth et al., 2004), impulsiveness (Perry et al., 2007b,c), and reinstatement (Lynch and Carroll, 1999b) in regard to cocaine-seeking behavior than males. Paradoxically, the HiS female group showed less novelty reactivity after saline injection 1 and less locomotor activity after saline injection 5 than the other 3 groups, suggesting that avidity for saccharin and locomotor activity and reactivity are not related or are negatively related (VanderWeele et al., 2002).
The present results showing that HiS female rats had a greater locomotor response to cocaine than LoS female and both HiS and LoS male rats after all 3 cocaine injections are consistent with phenotype differences found in previous cocaine self-administration studies (Carroll et al., 2002, 2006; Perry et al., 2006, 2007a,b). These studies showed that HiS rats exceeded LoS rats in cocaine self-administration, and females self-administered more than males within those phenotypes. Previous work with the HiS and LoS strains has indicated that HiS rats self-administered more ethanol (Dess et al. 1998), and cocaine (Carroll et al., 2002, Perry et al., 2007a,b), and heroin during acquisition (Carroll et al., 2002) than LoS rats. HiS rats also showed greater escalation of i.v. cocaine self-administration during extended sessions (Perry et al. 2007b), greater dysregulation in cocaine dose self-selection (Carroll et al., 2006), elevated extinction when cocaine access was discontinued, and more cocaine-primed reinstatement of behavior that was previously reinforced by cocaine (Perry et al., 2006) compared to their LoS counterparts. Under maintenance conditions, HiS rats were also more impulsive for food reward (but not cocaine) when given a choice between a small-immediate vs. a large-delayed amount (Perry et al., 2007b). However, it is important to note that the dampened reactivity to cocaine in LoS and male rats compared to HiS females was not reflected in a generally lower reactivity to novelty suggesting that the sex and phenotype differences were specific to the effects of cocaine. The other 3 groups were more reactive than HiS females to novelty of the locomotor apparatus after the first injection (saline).
Other behavioral phenotypes differing on the drug-seeking-prone and –-resistant continuum have been studied by others, and similar results have been presented. For example, Ranaldi et al., (2001) showed that inbred Nijemegen rats that are high (HR) and low (LR) responders for ethanol, like the inbred Lewis (LEW) and Fischer 344 (F344), respectively, had different rates of acquisition of cocaine self-administration (HR > LR; LEW > F344); however, when these rats were pretreated with cocaine before lateral hypothalamic brain self-stimulation sessions, the elevating effects of cocaine on stimulation thresholds (reward) were similar across groups, suggesting that the HR - LR differences were due to more to the testing situations than the reinforcing effects of cocaine. In other studies, LEW rats exceeded F344 rats in locomotor activity after repeated cocaine administration, in cocaine-induced conditioned place preference (Kosten et al., 1994) and in their oral intake of cocaine, opioids, and sedatives (George and Goldberg, 1988; Suzuki et al., 1987; 1988a,b, 1992). Alcohol-naïve offspring of rats selectively bred to prefer (P) alcohol self-administered more alcohol (Li et al., 1993), and also nicotine (Le et al., 2006) and sucrose (Stewart et al., 1994; Eiler et al., 1995; Sutton et al., 2006), than those bred not to prefer alcohol (NP) rats. Others have shown that behavioral sensitization to sweetened food cross-sensitizes to cocaine and morphine intake (Le Merrer and Stephens, 2006), and wheel-running (Werme et al., 2002). Furthermore, LEW, but not F344 rats, developed compulsive, running (Werme et al., 1999). Thus, shared genetic vulnerability may underlie several forms of reward-seeking behavior, and it is likely that there is a polygenetic basis (George and Goldberg, 1989; Kosten et al., 1994).
The genetic makeup of the HiS LoS and other behaviorally-selected rat lines that are predisposed to be drug-seeking-prone or -resistant, respectively, may rely on a number of different underlying mechanisms, such as different endogenous or environmentally-induced neuroadaptations, differential sensitivity to rewarding stimuli, sensitivity to environmental stimuli in general, responsivity to novelty, stress, or other factors and their combinations. Recent evidence links behavioral sensitization with structural changes in neuroplasticity (Ferrario et al., 2005; Li et al., 2003, 2004), and these changes may be different in males, females, HiS vs. LoS rats and other reward-seeking-prone and – resistant groups. If the neuroadaptations that underlie the long-lasting effects of cocaine-induced locomotor sensitization are important for the enduring (relapse) and irreversible (escalation) processes that occur during drug addiction, that may explain the present results that groups that are known to differ on drug-motivated behavior (e.g., HiS > LoS, females > males) show corresponding differences in cocaine-induced locomotor activity and under limited conditions, sensitization.
Response to novelty or behavioral reactivity has been shown to be related to subsequent drug self-administration behaviors, particularly psychostimulant self-administration (Cools and Gingras, 1998; Piazza et al., 1989, 1998). For example, rats with high novelty-induced locomotor activity (high responders, HR) acquired i.v. amphetamine (Hooks et al., 1991a; Piazza et al., 1989, 1990) and cocaine (Hooks et al. 1991b; Mantsch et al., 2001; Piazza et al., 1998) self-administration more readily than low responders (LR), showing a potential connection between intake of sweetened substances and drugs, the HR rats also consumed greater amounts of sucrose than LR rats (Cools and Gingras, 1998). The elevated response to novelty and corresponding vulnerability to drug self-administration in rats has been suggested to be analogous to high sensation-seeking behaviors in humans (Dellu et al., 1996). In the present study, reactivity to the novel environment was not related to the HiS, female characteristic of greater cocaine-reactivity, as would be predicted. However, reactivity to the novel effects of cocaine (cocaine injection 1) was related to the drug-seeking phenotype (HiS, female) than responsivity to the novel environment (saline injection 1). While previous research indicates that locomotor activity in a novel environment is predictive of drug self-administration (e.g., Mantsch et al., 2001; Piazza et al., 1989, 1990), rats in the present study were randomly selected, and subsequent behaviors (locomotor, drug effects) were correlated. The difference in the present study is that the rats were already selected (selectively bred) for a different behavior (saccharin intake), and that difference may be independent from or overshadowed by the locomotor differences in predicting the effects of cocaine. If more rats had been tested, so they could have been stratified into HiS (HR, LR) and LoS (HR, LR), on novelty reactivity (R), differences may have emerged. Similar negative findings on the predictability of locomotor activity and behavioral phenotype were reported for rats selected for high (HiI) and low (LoI) impulsivity when a delay-discounting task for food (Perry et al. 2005, 2007c) was used or a 5-choice serial reaction time task of sustained visual attention (Dalley et al. 2007). While impulsivity (like saccharin intake in HiS rats) predicted cocaine-seeking behavior, locomotor reactivity was not related to impulsivity for food, saccharin intake, or cocaine-maintained responding in these rats.
Differences in emotionality between HiS and LoS rats may have also contributed to the differences shown in the present study as well as to other forms of cocaine-maintained behavior (acquisition, regulation, escalation, progressive-ratio schedule performance, and reinstatement). Emotionality is operationally defined in rats by placing them into an open field and evaluating their latency to emerge to the center, by quantifying their defacation, and by measuring their circulating corticosterone (CORT) levels (Ramos and Mormede, 1998). According to these procedures, HiS rats showed little or no emotionality; whereas, LoS rats showed high emotionality (Dess and Minor, 1996, Dess et al., 2000). Differences in emotionality could explain previous reports of HiS/LoS line differences, including ethanol intake (HiS > LoS) (Dess et al., 1998), acquisition of cocaine self-administration (HiS > LoS) (Carroll et al., 2002), elevated basal (LoS > HiS) and stress-induced (HiS = LoS) CORT levels (VanderWeele et al., 2002), acoustic startle amplitude (LoS > HiS) (VanderWeele et al., 2002), and taste reactivity (HiS > LoS) (Dess and Minor, 1996). Other groups with differing genetic propensities for drug-seeking behavior, such as those bred for high (HR) and low (LR) reactivity to a novel environment show differences in emotionality that are in the same direction (LR > HR) (Stead et al., 2006), highlighting the complex interactions among genetic background, environmental stimuli, and drug effects.
Other mechanisms that could explain the phenotype and sex differences described here, and in related studies, are sensitivity to stress (Goeders 2002), the rate of learning and ability to perform the operant task (Mitchell et al., 2005), the ability to associate stimuli with the reward, and other traits such as impulsivity for the reward (Dalley et al. 2007; Perry et al., 2005, 2007b,c). Future investigations of the unique behavioral and neurobiological characteristics of the HiS and LoS and other reward-seeking-prone- and resistant animals could provide information on the mechanisms underlying the interrelated findings and complex interactions that have emerged. While the present study was limited by small groups and a within-subject comparison of saline and cocaine effects on locomotor activity, the current findings concur with many other results regarding sex and the HiS, LoS phenotypes. This emphasizes the value of studying selectively bred reward-seeking-prone and –resistant animals (e.g., HiS, LoS), as it provides additional information regarding the heritability of the behaviors, how they differ from other traits, and offers applicability to humans.
In summary, cocaine-induced locomotor activity of HiS and LoS, female and male rats was examined, and the locomotor-stimulatory effects of cocaine were more pronounced in female HiS vs. LoS and male HiS and LoS rats. Repeated intermittent cocaine administration engendered locomotor sensitization in HiS female rats by the fifth injection (which occurred 10-12 days after the first), highlighting a potential phenotype × sex interaction in drug responsiveness; however, this effect was not significant after the 6th injection 2 weeks later. The findings indicated that the phenotype and sex differences between LoS and HiS rats in cocaine-induced locomotor sensitization were not due to baseline activity differences. Results also indicated that phenotype and sex differences (HiS > LoS and females > males) in cocaine-induced locomotor activity are consistent with similar differences in drug-seeking behavior in these selectively-bred lines during several phases of drug abuse. Thus, the present findings highlight multiple vulnerability factors, including sex, sweet intake, and cocaine-induced locomotor activity that may be useful for predicting drug abuse vulnerability.
Table 2.
Experimental procedure
| Injection | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Administered | S, C* | S, C | S, C | S, C | S, C | S, C |
| Timeline | Day 1 | 2-3 days later |
2-3 days later |
2-3 days later |
2-3 days later |
2-weeks after Injection 5 |
| Locomotor Track | Yes | No | No | No | Yes | Yes |
| Measure | Novelty (S) and baseline cocaine - induced locomotor activity (C) |
Development of cocaine-induced locomotor sensitization |
Expression of cocaine-induced locomotor sensitization |
|||
S, C saline then cocaine (10 mg/kg i.p.)
Acknowledgements
The authors wish to thank Drs. Erin Larson, Jennifer Newman, and Jennifer Perry for their editorial assistance and review of the manuscript and Justin Anker for his technical assistance and editorial comments. This work was supported by NIH/NIDA grants T32 DA007097-25 (ADM), R01 DA003240-23 (MEC) and K05 DA015267-06 (MEC)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Cador M. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharm. 2006;31:563–571. doi: 10.1038/sj.npp.1300834. [DOI] [PubMed] [Google Scholar]
- Ahmed SH. Imbalance between drug and non-drug reward availability: a major risk factor for addiction. Eur J Pharmacol. 2005;526:9–20. doi: 10.1016/j.ejphar.2005.09.036. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Larson EB, Carroll ME. Effects of progesterone and estrogen on reinstatement of cocaine seeking behavior. College on Problems of Drug Dependence; Scottsdale, AZ: Jun, 2006. [Google Scholar]
- Avena NM, Hoebel BG. A diet promoting sugar dependency causes behavioral cross sensitization to a low dose of amphetamine. Neurosci. 2003a;122:17–20. doi: 10.1016/s0306-4522(03)00502-5. [DOI] [PubMed] [Google Scholar]
- Avena NM, Hoebel BG. Amphetamine-sensitized rats show sugar-induced hyperactivity (cross-sensitization) and sugar hyperphagia. Pharmacol Biochem Behav. 2003b;74:635–639. doi: 10.1016/s0091-3057(02)01050-x. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug seeking behavior. Behav Brain Res. 1996;77:23–43. doi: 10.1016/0166-4328(95)00203-0. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Ahmed SH, Koob GF, Ettenberg A. The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain Res. 2004;995:46–54. doi: 10.1016/j.brainres.2003.09.053. [DOI] [PubMed] [Google Scholar]
- Cain ME, Saucier DA, Bardo MT. Novelty seeking and drug use: contribution of an animal model. Exp Clin Psychopharm. 2005;13:367–375. doi: 10.1037/1064-1297.13.4.367. [DOI] [PubMed] [Google Scholar]
- Cain ME, Smith CM, Bardo MT. The effect of novelty on amphetamine self-administration in rats classified as high and low responders. Psychopharmacology. 2004;176:129–138. doi: 10.1007/s00213-004-1870-2. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anderson MM, Morgan AD. Regulation of i.v. cocaine self-administration in rats selectively bred for high and low saccharin intake. Psychopharmacology. 2007;190:331–341. doi: 10.1007/s00213-006-0600-3. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Tr Pharmacol Sci. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology (Berl) 2002;161(3):304–313. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Laviola G. Paradoxical effects of D-amphetamine in infant and adolescent mice: role of gender and environmental risk factors. Neurosci Biobehav Rev. 2000;24(1):73–84. doi: 10.1016/s0149-7634(99)00047-0. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, Hoebel BG. Evidence that Intermittent Excessive Sugar Intake Causes Endogenous Opioid Dependence. Obesity Research. 2002;10:478–488. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, Schwartz GJ, Moran TH, Hoebel BG. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. NeuroReport. 2001;12:3549–3552. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- Cools AR, Gingras MA. Nijmegen high and low responders to novelty: a new tool in the search after the neurobiology of drug abuse liability. Pharmacol Biochem Behav. 1998;60(1):151–159. doi: 10.1016/s0091-3057(97)00586-8. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Hajnal A. Too much of a good thing: Neurobiology of non-homeostatic eating and drug abuse. Physiol Behav. 2005;86:5–8. doi: 10.1016/j.physbeh.2005.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ESJ, Theobald DEH, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron J-C, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellu F, Piazza PV, Mayo W, Le Moal M, Simon H. Novelty-seeking in rats--biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology. 1996;34(3):136–145. doi: 10.1159/000119305. [DOI] [PubMed] [Google Scholar]
- Dess NK, Arnal J, Chapman CD, Siebel SI, VenderWeele DA, Green K. Exploring adaptations to famine: rats selectively bred for differential saccharin intake differ on deprivation-induced hyperactivity and emotionality. Int J Comp Psychol. 2000;13:34–52. [Google Scholar]
- Dess NK, Badia-Elder NE, Thiele TE, Kiefer SW, Blizard DA. Ethanol consumption in rats selectively bred for differential saccharin intake. Alcohol. 1998;16(4):275–278. doi: 10.1016/s0741-8329(98)00010-x. [DOI] [PubMed] [Google Scholar]
- Dess NK, Minor TR. Taste and emotionality in rats selectively bred for high versus low saccharin intake. An Learn Behav. 1996;24:105–115. [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, Mulder AH, Vanderschuren LJ. Drug-induced reinstatement of heroin- and cocaine-seeking behaviour following long-term extinction is associated with the expression of behavioural sensitization. Eur J Neurosci. 1998;10:3565–3571. doi: 10.1046/j.1460-9568.1998.00368.x. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, Raaso H, Vanderschuren LJ. Relapse to cocaine-and heroin-seeking behavior mediated by dopamine D2 receptors is time-dependent and associated with behavioral sensitization. Neuropsychopharm. 2002;26:18–26. doi: 10.1016/S0893-133X(01)00293-7. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Dopamine in disturbances of food and drug motivated behavior: A case of homology? Physiol Behav. 2005;86:9–11. doi: 10.1016/j.physbeh.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Eiler WJ, II, Woods JE, II, Masters J, McKay PF, Hardy L, III, Goergen JJ, Mensah-Zoe B, Cook JB, Johnson NJ, Nune HL. Brain stimulation reward performance and sucrose maintained behaviors in alcohol preferring and -nonpreferring rats. Alcohol Clin Exp Res. 2005;29:571–583. doi: 10.1097/01.alc.0000158934.50534.b7. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psy. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Festa ED, Quinones-Jenab V. Gonadal hormones provide the biological basis for sex differences in behavioral responses to cocaine. Horm Behav. 2004;46:509–519. doi: 10.1016/j.yhbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Gahtan E, LaBounty LP, Wyvell C, Carroll ME. The relationship between saccharin consumption, oral ethanol and IV cocaine self-administration. Pharm Biochem Behav. 1996;53:919–925. doi: 10.1016/0091-3057(95)02148-5. [DOI] [PubMed] [Google Scholar]
- George FR. Cocaine toxicity: Genetic evidence suggest different mechanisms for cocacine-induced seizures and lethality. Psychopharmacology. 1991;104:307–311. doi: 10.1007/BF02246028. [DOI] [PubMed] [Google Scholar]
- George FR, Goldberg SR. Genetic approaches to the analysis of addiction processes. Trends Pharmacol Sci. 1989;10(2):78–83. doi: 10.1016/0165-6147(89)90083-7. [DOI] [PubMed] [Google Scholar]
- George FR, Goldberg SR. Genetic factors in response to cocaine. In: Clouet D, Asghar K, Brown R, editors. Mechanisms of CocaineAbuse and Toxicity. NIDA Research Monograph 88; Rockville: 1988. pp. 239–249. [PubMed] [Google Scholar]
- Goeders NE. Stress and cocaine addiction. J Pharmacol Exp Ther. 2002;301(3):785–789. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- Gosnell BA. Sucrose intake enhances behavioral sensitization produced by cocaine. Brain Research. 2005;1031:194–201. doi: 10.1016/j.brainres.2004.10.037. [DOI] [PubMed] [Google Scholar]
- Gosnell BA. Sucrose intake predicts rate of acquisition of cocaine self-adminisration. Psychopharmacology. 2000;149:286–292. doi: 10.1007/s002130000375. [DOI] [PubMed] [Google Scholar]
- Gosnell BA, Krahn DD. The relationship between saccharin and alcohol intake in rats. Alcohol. 1992;9:203–206. doi: 10.1016/0741-8329(92)90054-e. [DOI] [PubMed] [Google Scholar]
- Gosnell BA, Lane KE, Bell SM, Krahn DD. Intravenous self-administration by rats with low vs. high saccharine preferences. Psychopharmacology. 1995;117:248–252. doi: 10.1007/BF02245194. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Hoover BR, Larson GA, Zahniser NR. Individual differences in cocaine-induced locomotor activity in rats: behavioral characteristics, cocaine pharmacokinetics, and the dopamine transporter. Neuropsychopharmacology. 2003;28(12):2089–2101. doi: 10.1038/sj.npp.1300279. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Rada PV, Mark GP, Pothos EN. Neural systems for reinforcement and inhibition of behavior: relevance to eating, addiction, and depression. In: Kahneman D, Diener E, Schwarz N, editors. Well Being: The Foundations of Hedonic Psychology. The Russell Sage Foundation; New York, NY: 1999. pp. 558–574. 1999. [Google Scholar]
- Homberg JR, Akker MVD, Raasø HS, Wardeh G, Binnekade R, Schoffelmeer ANM, de Vries TJ. Enhanced motivation to self-administer cocaine is predicted by self-grooming behaviour and relates to dopamine release in the rat medial prefrontal cortex and amygdala. Euro J Neurosci. 2002;15:1542–1550. doi: 10.1046/j.1460-9568.2002.01976.x. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Durry P, Striplin C, Kalivas PW. Behavioral and neurochemical sensitization fowwowing cocaine self-administration. Psychopharmacology (Berl.) 1994;115:265–272. doi: 10.1007/BF02244782. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr Individual differences in locomotor activity and sensitization. Pharmacol Biochem Behav. 1991a;38(2):467–470. doi: 10.1016/0091-3057(91)90308-o. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB. Response to novelty predicts the locomotor and nucleus accumbens dopamine response to cocaine. Synapse. 1991b;9(2):121–128. doi: 10.1002/syn.890090206. [DOI] [PubMed] [Google Scholar]
- Jonas JM. Do substance-abuse, including alcoholism, and bulimia covary? In: Reid LD, editor. Opioids, Bulimia, and Alcoholism. Springer Verlag; New York: 1990. pp. 247–258. [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16(3):223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Schiltz CA, Landry CF. Neural systems recruited by drug- and food-related cues: Studies of gene activation in corticolimbic regions. Physiol Behav. 2005;86:11–14. doi: 10.1016/j.physbeh.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Kim JH, Vezina P. The mGlu2/3 receptor agonist LY379268 blocks the expression of locomotor sensitization by amphetamine. Pharmacol Biochem Behav. 2002;73(2):333–337. doi: 10.1016/s0091-3057(02)00827-4. [DOI] [PubMed] [Google Scholar]
- Klebaur JE, Bardo MT. Individual differences in novelty seeking on the playground maze predict amphetamine conditioned place preference. Pharmacol Biochem Behav. 1999;63:131–136. doi: 10.1016/s0091-3057(98)00258-5. [DOI] [PubMed] [Google Scholar]
- Klebaur JE, Phillips SB, Kelly TH, Bardo MT. Exposure to novel environmental stimuli decreases amphetamine self-administration in rats. Exp Clin Psychopharm. 2001;9:372–379. [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJD, Chi S, Nestler EJ. Fischer and Lewis rat strains show differential cocaine effects in conditioned place preference and behavioral sensitization but not in locomotor activity or conditioned taste aversion. J Pharmacol Exp Ther. 1994;269:137–144. [PubMed] [Google Scholar]
- Krahn DD, Gosnell BA. Fat-preferring rats consume more alcohol than carbohydrate-preferring rats. Alcohol. 1991;8:313–316. doi: 10.1016/0741-8329(91)90465-9. [DOI] [PubMed] [Google Scholar]
- Larson EB, Anker JJ, Gliddon LA, Carroll ME. Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharm. 2007 doi: 10.1037/1064-1297.15.5.461. Submitted. [DOI] [PubMed] [Google Scholar]
- Larson EB, Carroll ME. Wheel-running as a predictor of cocaine self-administration and reinstatement in female rats. Pharm Biochem Behav. 2005;82:590–600. doi: 10.1016/j.pbb.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Lattanzio SB, Eikelboom R. Wheel access duration in rats: I. Effects on feeding and running. Behav Neurosci. 2003;117(3):496–504. doi: 10.1037/0735-7044.117.3.496. [DOI] [PubMed] [Google Scholar]
- Laviola G, Wood RD, Kuhn C, Francis R, Spear LP. Cocaine sensitization in periadolescent and adult rats. J Pharmacol Exp Ther. 1995;275:345–357. [PubMed] [Google Scholar]
- Le AD, Li Z, Funk D, Shram M, Li TK, Shaham Y. Increased vulnerability to nicotine self-administration and relapse in alcohol-naïve offspring of rats selectively bred for high alcohol intake. J Neurosci. 2006;26(6):1872–1879. doi: 10.1523/JNEUROSCI.4895-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer J, Stephens DN. Food-induced behavioral sensitization, its cross-sensitization to cocaine and morphine, pharmacological blockade, and effect on food intake. J Neurosci. 2006;26(27):7163–7171. doi: 10.1523/JNEUROSCI.5345-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Ahmed SH. Heroin-induced reinstatement is specific to compulsive heroin use and dissociable from heroin reward and sensitization. Neuropsychopharmacology. 2007;32:616–624. doi: 10.1038/sj.npp.1301083. [DOI] [PubMed] [Google Scholar]
- Levine AS, Kotz CM, Gosnell BA. Sugars and fats: the neurobiology of preference. J Nutr. 2003;133:831S–834S. doi: 10.1093/jn/133.3.831S. [DOI] [PubMed] [Google Scholar]
- Li TK, Lumeng L, Doolittle DP. Selective breeding for alcohol preference and associated responses. Behav Genet. 1993;23:163–170. doi: 10.1007/BF01067421. [DOI] [PubMed] [Google Scholar]
- Li Y, Acerbo MJ, Robinson TE. Cocaine-induced dendritic plasticity in the core (but not the shell) of the nucleus accumbens is associated with the induction of psychomotor sensitization. Eur J Neurosci. 2004;20:1647–1654. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Kolb B, Robinson TE. The location of persistent amphetamine-induced changes in the density of dendritic spines on medium spiny neurons in the nucleus accumbens and caudate-putamen. Neuropsychopharmacology. 2003;28:1082–1085. doi: 10.1038/sj.npp.1300115. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology. 1999a;148:196–200. doi: 10.1007/s002130050042. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 1999b;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Ho A, Schlussman SD, Kreek MJ. Predictable individual differences in the initiation of cocaine self-administration by rats under extended-access conditions are dose-dependent. Psychopharmacology (Berl) 2001;157(1):31–39. doi: 10.1007/s002130100744. [DOI] [PubMed] [Google Scholar]
- Marks-Kaufman R, Lipeles BJ. Patterns of nutrient selection in rats orally self-administering morphine. Nutr Behav. 1982;1:33–46. [Google Scholar]
- Mitchell JM, Cunningham CL, Mark GP. Locomotor activity preducts acquisition of self- administration behavior but not cocaine intake. Behav Neurosci. 2005;119:464–472. doi: 10.1037/0735-7044.119.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . Guidelines for the care and use of mammals in Neuroscience and Behavioral Research. The National Academies Press; Washington D.C.: 2003. p. 209. [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Increased motivation for self-administered cocaine after escalated cocaine intake. NeuroReport. 2003;14:2229–2232. doi: 10.1097/00001756-200312020-00019. [DOI] [PubMed] [Google Scholar]
- Perry JL, Anderson M, Nelson SE, Carroll ME. Acquisition of i.v. cocaine self-administration in adolescent and adult male rats selectively bred for high and low saccharin intake. Physiology and Behavior. 2007a doi: 10.1016/j.physbeh.2007.02.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of i.v. cocaine self-administration in female rats. Psychopharmacology. 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Perry JL, Morgan AD, Anker JJ, Dess NK, Carroll ME. Escalation of i.v. cocaine self-administration and reinstatement of cocaine-seeking behavior in rats bred for high and low saccharin intake. Psychopharmacology. 2006;186:235–245. doi: 10.1007/s00213-006-0371-x. 2006. [DOI] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Anderson MM, Morgan AD, Carroll ME. Impulsivity (delay discounting) for food and cocaine in male and female rats selectively bred for high and low saccharin intake. Pharmacol, Biochem and Behav. 2007b doi: 10.1016/j.pbb.2007.03.012. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Carroll ME. Impulsive choice as a predictor of acquisition of i.v. cocaine self-administration and reinstatement of cocaine-seeking behavior in male and female rats. Psychopharmacology. 2007c doi: 10.1037/1064-1297.16.2.165. under review. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Di Ciano P. Behavioral sensitization is induced by intravenous self-administration of cocaine by rats. Psychophramacology (Berl) 1996;124:279–281. doi: 10.1007/BF02246669. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245(4925):1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Maccari S, Mormede P, Le Moal M, Simon H. Individual reactivity to novelty predicts probability of amphetamine self-administration. Behav Pharmacol. 1990;1(4):339–345. doi: 10.1097/00008877-199000140-00007. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonet V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Le Moal ME. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol. 1996;36:359–78. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- Quinones-Jenab V, Ho A, Schlussman SD, Franck J, Kreek MJ. Estrous cycle differences in cocaine-induced stereotypic and locomotor behaviors in Fischer rats. Behav Brain Res. 1999;101(1):15–20. doi: 10.1016/s0166-4328(98)00073-4. [DOI] [PubMed] [Google Scholar]
- Ramos A, Mormede P. Stress and emotionality: a multidimensional and genetic approach. Neurosci Biobehav Rev. 1998;22:33–57. doi: 10.1016/s0149-7634(97)00001-8. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Bauco P, McCormick S, Cools AR, Wise RA. Equal sensitivity to cocaine reward in addiction-prone and addiction-resistant rat genotypes. Behav Pharmacol. 2001;12:527–34. doi: 10.1097/00008877-200111000-00014. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396(2):157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Ann Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: A review of preclinical studies. Neurosci Biobehav Rev. 2004;28:533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Spanagel R. Modulation of drug-induced sensitization processes by endogenous opioid systems. Behav Brain Res. 1995;70(1):37–49. doi: 10.1016/0166-4328(94)00176-g. [DOI] [PubMed] [Google Scholar]
- Stead JDH, Clinton S, Neal C, Schneider J, Jama A, Miller S, Vazquez DM, Watson SJ, Akil H. Selective breeding for divergence in novelty-seking trait: heritability and enrichment in spontaneous anxiety-related behaviors. Behavior Genetics. 2006;36:697–712. doi: 10.1007/s10519-006-9058-7. [DOI] [PubMed] [Google Scholar]
- Stewart RB, Russell RN, Lumen L, Li TK, Murphy JM. Consumption of sweet, salty, sour, and bitter solutions by selectively bred alcohol-preferring and alcohol-nonpreferring lines of rats. Alcohol Clin Exp Res. 1994;18:375–381. doi: 10.1111/j.1530-0277.1994.tb00028.x. [DOI] [PubMed] [Google Scholar]
- Stoffel EC, Craft RM. Ovarian hormone withdrawal-induced “depression” in female rats. Physiol Behav. 2004;15:505–513. doi: 10.1016/j.physbeh.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Karanian DA, Self DW. Factors that determine a propensity for cocaine-seeking behavior during abstinence in rats. Neuropsychopharm. 2000;22(6):626–641. doi: 10.1016/S0893-133X(99)00160-8. [DOI] [PubMed] [Google Scholar]
- Suzuki T, George FR, Meisch RA. Differential establishment and maintenance of oral ethanol reinforced behavior in Lewis and Fischer 344 inbred rat strains. J Parmacol Exp Ther. 1988a;245:164–170. [PubMed] [Google Scholar]
- Suzuki T, George FR, Meisch RA. Etonitazene delivered orally serves as a reinforcer for Lewis but not Fischer 344 rats. Pharmacol Biochem Behav. 1992;42:579–586. doi: 10.1016/0091-3057(92)90002-w. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Koike Y, Yanaura S, George FR, Meisch RA. Genetic differences in the development of physical dependence on pentobarbital in four inbred rat strains. Jpn J Pharmacol. 1987;45:479–486. doi: 10.1254/jjp.45.479. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Otani K, Koike Y, Misawa M. Genetic differences in preferences for morphine and codeine in Lewis and Fischer 344 inbred rat strains. Jpn J Pharmacol. 1988b;47:425–431. doi: 10.1254/jjp.47.425. [DOI] [PubMed] [Google Scholar]
- Uhl G. Premature poking: impulsivity, cocaine and dopamine. Nature Medicine. 2007;13:413–414. doi: 10.1038/nm0407-413. [DOI] [PubMed] [Google Scholar]
- VanderWeele DA, Dess NK, Castonguay TW. Ingestional responses to metabolic challenges in rats selectively bred for high and low saccharin intake. Physiol Behav. 2002;75(1-2):97–104. doi: 10.1016/s0031-9384(01)00641-2. [DOI] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27(8):827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Vitale MA, Chen D, Kanarek RB. Chronic access to a sucrose solution enhances the development of conditioned place preferences for fentanyl and amphetamine in male Long-Evans rats. Pharmacol Biochem Behav. 2003;74:529–539. doi: 10.1016/s0091-3057(02)01034-1. [DOI] [PubMed] [Google Scholar]
- Volkow N, Li TK. The neuroscience of addiction. Nat Neurosci. 2005;11:1429–1430. doi: 10.1038/nn1105-1429. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Werme M, Lindholm S, Thoren P, Pranck J, Brene S. Running increases ethanol preference. Behavior Brain Res. 2002;133:301–308. doi: 10.1016/s0166-4328(02)00027-x. [DOI] [PubMed] [Google Scholar]
- Werme M, Thoren P, Olson L, Brene S. Addiction-prone Lewis but not Fischer rats develop compulsive running that coincides with downregulation of nerve growth factor inducible-B and neuron-derived orphan receptor 1. Journal of Neuroscience. 1999;19:6169–6174. doi: 10.1523/JNEUROSCI.19-14-06169.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. Addiction: making the connection between behavioral changes and neuronal plasticity in specific pathways. Mol Interv. 2002;2:146–157. doi: 10.1124/mi.2.3.146. [DOI] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]