Abstract
Recently platelet-derived growth factor-α-positive cells (PDGFRα+ cells), previously called “fibroblast-like” cells, have been described in the muscle layers of the gastrointestinal tract. These cells form networks and are involved in purinergic motor neurotransduction. Examination of colon from mice with enhanced green fluorescent protein (eGFP) driven from the endogenous Pdgfra (PDGFRα-eGFP mice) revealed a unique population of PDGFRα+ cells in the mucosal layer of colon. We investigated the phenotype and potential role of these cells, which have not been characterized previously. Expression of PDGFRα and several additional proteins was surveyed in human and murine colonic mucosae by immunolabeling; PDGFRα+ cells in colonic mucosa were isolated from PDGFRα-eGFP mice, and the gene expression profile was analyzed by quantitative polymerase chain reaction. We found for the first time that PDGFRα was expressed in subepithelial cells (subepithelial PDGFRα+ cells) forming a pericryptal sheath from the base to the tip of crypts. These cells were in close proximity to the basolateral surface of epithelial cells and distinct from subepithelial myofibroblasts, which were identified by expression of α-smooth muscle actin and smooth muscle myosin. PDGFRα+ cells also lay in close proximity to varicose processes of nerve fibers. Mouse subepithelial PDGFRα+ cells expressed Toll-like receptor genes, purinergic receptor genes, 5-hydroxytryptamine (5-HT) 4 receptor gene, and hedgehog signaling genes. Subepithelial PDGFRα+ cells occupy an important niche in the lamina propria and may function in transduction of sensory and immune signals and in the maintenance of mucosal homeostasis.
Keywords: pericryptal fibroblasts, Toll-like receptor, purinergic receptors, 5-hydroxytryptamine 4, hedgehog signaling
the lamina propria is a complex region lying beneath the epithelial cells of the gastrointestinal tract. This region is rich in blood vessels, lacteals of the lymphatic system, terminals of sensory neurons, immune cells, and myofibroblasts. Myofibroblasts have been identified in the intestine by the expression of various markers, including cytoskeletal proteins, α-smooth muscle actin (α-SMA), type 3 intermediate filaments (vimentin or desmin), and collagen type 1 maturation enzymes such as prolyl-4 hydroxylase (39). Anatomical location and α-SMA are the most common means of identifying myofibroblasts, and, according to recent reviews, these cells occupy the space immediately beneath the epithelial cells, with some more central projections into the lamina propria (34). Myofibroblasts are thought to be involved in growth and repair of the mucosa, tumorigenesis and progression of cancer, immune responses of the mucosa, and fibrosis. However, the precise definition of myofibroblasts and their functions has been obscured by the fact that α-SMA labels additional cells such as pericytes, bone marrow-derived stem cells, hematopoietic stem cells, muscularis mucosae, and organized smooth muscle cells of lymphatic lacteals (34). Thus, identification of more specific labels for these cells would be highly advantageous to further evaluate the functional role of these cells.
Platelet-derived growth factors (PDGF) are major mitogens for many cell types of mesenchymal origin, which include fibroblasts and smooth muscle cells (14). During embryogenesis, PDGF signaling is important in organogenesis (22). Adult cells in multiple organs expressing PDGF ligands and receptors often have important roles in pathophysiology. For example, PDGF receptor (PDGFR)-α and -β are found in fibroblasts in lung and mesangial cells in kidney, and PDGFRβ is found in hepatic stellate cells, which are involved in lung fibrosis, glomerulosclerosis, and liver cirrhosis (5, 11, 43).
Antibodies to PDGFRα label a distinct population of cells with the muscle layers of the gastrointestinal tract and bladder (7, 19, 24, 29, 31, 35). These cells are distinct from interstitial cells of Cajal but occupy similar anatomical niches in mice, nonhuman primates, and humans. PDGFRα-positive cells (PDGFRα+ cells) have an important role in enteric purinergic motor neurotransmission (32). Previously, PDGFRα+ cells were reported to have important roles in villus morphogenesis of mouse small intestinal mucosa (25, 44) and were also found to exist in the subepithelial layer of the adult guinea pig gastrointestinal tract (10). However, details of the distribution and functions of PDGFRα+ cells in the lamina propria of the adult mammalian gastrointestinal tract, especially mouse and human, have not been described.
We observed PDGFRα+ cells in the lamina propria in colons of mice expressing enhanced green fluorescent protein (eGFP) driven by the endogenous Pdgfra promoter [Pdgfratm11(EGFP)Sor/J mice; PDGFRα-eGFP mice] (21). The PDGFRα+ cells were in approximately the same locations attributed to α-SMA+ myofibroblasts (34). Therefore, immunolabeling experiments were performed on mucosal tissues of human and murine colon to determine the nature and basic phenotype of the PDGFRα+ cells. We also isolated PDGFRα+ cells from mucosal tissues of PDGFRα-eGFP mice by enzymatic dispersion and purified cells with fluorescence-activated cell sorting (FACS) to characterize gene transcripts in these cells.
MATERIALS AND METHODS
Human tissue preparation.
Segments of human colon were obtained as surgical margins of resected colon specimens from four patients who underwent transverse colon or sigmoidal colon resections for neoplasms at the Renown Regional Medical Center (Reno, NV). Tissues from regions outside areas affected by disease were used. The usage of human tissues was approved by Human Subjects Research Committees at the Renown Regional Medical Center and the Biomedical Institutional Review Board at the University of Nevada, Reno.
Mice tissue preparation.
Pdgfratm11(EGFP)Sor/J heterozygote mice (PDGFRα-eGFP mice), which have eGFP in nuclei of PDGFRα+ cells throughout the body (21, 32), and their wild-type siblings (C57BL/6) were obtained from Jackson Laboratory (Bar Harbor, ME). Animals (6–8 wk postpartum) were anesthetized by isoflurane inhalation (AErrane; Baxter, Deerfield, IL) and killed by cervical dislocation. Mice were maintained and the experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the Institutional Animal Use and Care Committee at the University of Nevada, Reno, NV, approved experimental protocols.
Immunohistochemistry.
Human colon mucosa and mice colons were fixed in 4% paraformaldehyde overnight at 4°C. Next, frozen tissue blocks were made with optimum cutting temperature compound (Sakura Finetek, Torrance, CA) by standard methods and were kept in a −80°C freezer.
Frozen tissue blocks were cut (5- to 20-μm sections) with a cryostat (Carl Zeiss, Thornwood, NY). Tissue sections were incubated in 1% bovine serum albumin (Sigma, St. Louis, MO) in PBS for 1 h at room temperature to block nonspecific antibody binding. After blocking, tissue sections were incubated in primary antibodies diluted in PBS with 0.5% Triton X-100 overnight at 4°C and then in secondary antibodies diluted in PBS for 1 h in the dark at room temperature. Primary and secondary antibodies used are shown in Table 1. Following antibody incubation, 4′,6-diamidino-2-phenylindol nuclear staining was applied on them.
Table 1.
The information of primary and secondary antibodies
| Species | Catalog No. | Dilution | Source | |
|---|---|---|---|---|
| Primary antibodies | ||||
| Human | ||||
| PDGFRα | Goat | AF-307-NA | 1:100 | R & D Systems |
| α-Smooth muscle actin | Mouse | A 2547 | 1:500 | Sigma |
| Smooth Muscle Myosin IgG | Rabbit | BT-562 | 1:200 | Biomedical Technologies |
| Desmin | Rabbit | 5332 | 1:100 | Cell Signaling Technology |
| Vimentin | Rabbit | 5741 | 1:100 | Cell Signaling Technology |
| Mouse | ||||
| PDGFRα | Goat | AF1062 | 1:100 | R & D Systems |
| α-Smooth muscle actin | Mouse | A 2547 | 1:500 | Sigma |
| Smooth muscle myosin IgG | Rabbit | BT-562 | 1:200 | Biomedical Technologies |
| Desmin | Rabbit | 5332 | 1:100 | Cell Signaling Technology |
| Vimentin | Rabbit | 5741 | 1:100 | Cell Signaling Technology |
| Secondary antibodies | ||||
| Alexa Fluor 488 donkey anti-goat IgG | A-11055 | 1:500 | Invitrogen | |
| Alexa Fluor 594 donkey anti-mouse IgG | A-21203 | 1:500 | Invitrogen | |
| Alexa Fluor 488 donkey anti-rabbit IgG | A-21206 | 1:500 | Invitrogen | |
| Alexa Fluor 594 donkey anti-rabbit IgG | A-21207 | 1:500 | Invitrogen | |
PDGFRα, platelet-derived growth factor receptor-α.
Specificity of immunolabeling for PDGFRα was checked by the methods described above but without inclusion of primary antibodies, preabsorption of PDGFRα antibodies, and preabsorption of donkey-raised goat IgG antibody (secondary antibody for PDGFRα labeling). In preabsorption tests of PDGFRα antibodies, the antibodies were mixed with proportional amounts of recombinant human PDGFRα (catalog no. 322-PR/CF; volume ratio; antibody-peptide = 1:2) and recombinant mouse PDGFRα (catalog no. 1062-PR-050; volume ratio; antibody-peptide = 1:2) (R & D Systems). In preabsorption tests of the secondary antibody, the same methods as used for PDGFRα antibodies were performed using nonimmune goat IgG (catalog no. sc-2028; Santa Cruz Biotechnology) (volume ratio; secondary antibody-goat IgG = 1:10).
After antibody labeling, tissue sections were examined with a confocal microscope (Olympus FluoView 1000; Olympus, Melville, NY).
Cell isolation.
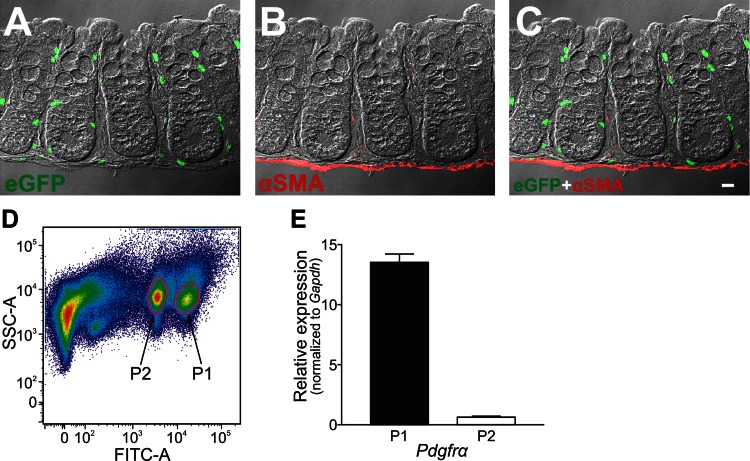
Colonic mucosa of PDGFRα-eGFP mice were dissected from the submucosal layer as shown in Fig. 11. Strips of mucosa were equilibrated in Ca2+-free Hanks' solution consisting of (in mM): 125 NaCl, 5.36 KCl, 15.5 NaHCO3, 0.336 Na2HPO4, 0.44 KH2PO4, 10 glucose, 2.9 sucrose, and 11 HEPES adjusted to pH 7.2 with NaOH, for 30 min. The buffer was replaced with an enzyme solution containing 2 mg/ml collagenase type 2 (Worthington Biochemical, Lakewood, NJ), 4 mg/ml bovine serum albumin (Sigma), and 4 mg/ml trypsin inhibitor (Sigma). The tissues were placed in a 37°C water bath for 15 min without agitation. After five washes with Ca2+-free Hanks' solution to remove the enzyme, the tissues were triturated through a series of two blunt pipettes of decreasing tip diameter to disperse cells. Green fluorescent protein-positive (GFP+) cells were sorted by FACS with a Becton-Dickinson FACSAria II instrument using a blue laser (488 nm) and the GFP/fluorescein isothiocyanate emission detector (530/30 nm BP and 505 nm LP). Sorting was performed using a 130-μm nozzle at a sheath pressure of 12 psi and sort rate of 1,000 to 3,000 events/s. Live cells, gated on exclusion of Hoechst 33258 viability indicator dye (data not shown), were subsequently gated on eGFP fluorescence intensity. Graphical plots were generated postanalysis using FloJo software version 8.8.7 (Treestar, San Carlos, CA).
Fig. 11.
A–C: immunolabeling of α-SMA in a profile section of the peeled colonic mucosa of PDGFRα-eGFP mouse. The mucosal layer of colon was peeled off from the submucosal layer and was confirmed to consist of epithelium, lamina propria, and muscularis mucosae (C). Almost all eGFP-positive nuclei appeared to be located in the subepithelial region (C). The scale bar represents 10 μm. D: purification of PDGFRα+ cells by fluorescence-activated cell sorter (FACS) analysis. Graphical plots of FACS of eGFP-positive cells as described in materials and methods are shown. Two populations of eGFP-positive cells, differing in fluorescence intensity, were sampled for further analysis. E: quantitative PCR (qPCR) analysis was performed on cell extracts from P1 and P2 populations. Relative expression of Pdgfra normalized to glyceraldehyde-3-phosphate dehydrogenase (Gapdh) in P1 and P2 are plotted. P1 expressed Pdgfra at a much higher level than P2 (fold change = 21.5), and P1 cells were used for subsequent qPCR studies.
RNA extraction, reverse transcription, and quantitative PCR.
Total RNA was isolated from collected PDGFRα+ cells and unsorted cells (total cell population from tissue dispersions before cell sorting), from six mice using an illustra RNAspin Mini RNA Isolation Kit (GE Healthcare), and First-strand cDNA was synthesized using SuperScript III (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions. RT-PCR was performed with specific primers (Table 2) using GoTaq DNA Polymerase (Promega, Madison, WI). PCR products were analyzed on 2% agarose gels and visualized by ethidium bromide. Quantitative PCR (qPCR) was performed with the same primers as PCR using Syber green chemistry on the 7500 HT Real-time PCR System (Applied Biosystems). Regression analysis of the mean values of eight multiplex qPCRs for the log10 diluted cDNA was used to generate standard curves. Unknown amounts of mRNA were plotted relative to the standard curve for each set of primers and graphically plotted using Microsoft Excel (Microsoft, Redmond, WA). This gave transcriptional quantitation of each gene relative to the endogenous glyceraldehyde-3-phosphate dehydrogenase (Gapdh) standard after log transformation of the corresponding raw data. A total of six mice were divided into two groups consisting of three mice each; qPCR analysis was run in duplicate in the two groups. There was high repeatability between the two groups, and the average was used for comparison between PDGFRα+ cells and unsorted cells.
Table 2.
The information of primers for qPCR
| Gene Name | Primer Sequence | Product Length, bp | Accession No. |
|---|---|---|---|
| Gapdh | F-GCCGATGCCCCCATGTTTGTGA | 178 | NM_008084 |
| R-GGGTGGCAGTGATGGCATGGAC | |||
| Pdgfra | F-ATGACAGCAGGCAGGGCTTCAACG | 195 | NM_011058 |
| R-CGGCACAGGTCACCACGATCGTTT | |||
| Acta2 (αSMA) | F-GATCAAGATCATTGCCCCTCCAGA | 176 | NM_007392 |
| R-GTGCTAGAGGCAGAGCAGGGG | |||
| Myh11 (smMHC) | F-CAGCTGGAAGAGGCAGAGGAGG | 198 | NM_013607 |
| R-AACAAATGAAGCCTCGTTTCCTCTC | |||
| Uchl1 (PGP9.5) | F-CGATGGAGATTAACCCCGAGATG | 169 | NM_011670 |
| R-TTTTCATGCTGGGCCGTGAG | |||
| Vim (Vimentin) | F-GCGAGAGAAATTGCAGGAGG | 112 | NM_011701 |
| R-CCGTTCAAGGTCAAGACGTG | |||
| Tlr1 | F-AGAGTGCCCAAGGACCTACCCT | 112 | NM_030682 |
| R-TCAGGACCCTCAGCTTGGACA | |||
| Tlr2 | F-GACCTCCGAGCGTGTGCGAA | 151 | NM_011905 |
| R-GAAAGGGGCCCGAACCAGGA | |||
| Tlr4 | F-AGAAATTCCTGCAGTGGGTCAAGGA | 170 | NM_021297 |
| R-CACAATCACACTGACCACTGACACA | |||
| Tlr5 | F-GGCTGGACGGTGGACATCACA | 187 | NM_016928 |
| R-AGCCGTGCGAAAGGTCCAGT | |||
| Tlr9 | F-GGCCTATACAGCCTGCGCGT | 160 | NM_031178 |
| R-GTTGGCGGGGCACCTTTGTG | |||
| P2ry1 | F-ACCGAGGTGCCTTGGTCGGT | 140 | NM_008772 |
| R-CCGGTCTTGGTCAGGGCACA | |||
| P2ry2 | F-AGCACCATCAATGGCACCTGGGA | 132 | NM_008773 |
| R-CACGACGTTCAGGCACAACCC | |||
| P2ry4 | F-GGCCCTCAATGCCCCAACCC | 169 | NM_020621 |
| R-GCCAGTGCCAAAGGGCCAGT | |||
| P2ry6 | F-ACCGCACTGTGTGCTACGAC | 136 | NM_183168 |
| R-CGGCGGGCCATGCGACAATA | |||
| P2ry12 | F-GGCTGACGTCACTGAACGCCTG | 162 | NM_027571 |
| R-TCTCTTCGCTTGGTTCGCCACC | |||
| P2ry13 | F-CAACACCACTGGGATGCAGGGCT | 157 | NM_028808 |
| R-GCTGGGGATGTGGACGAACACC | |||
| Htr3a | F-CGGCAGTACTGGACTGATGA | 128 | NM_013561 |
| R-CCCACGTCCACAAACTCATT | |||
| Htr3b | F-CACACCTCTGATTGGGGTCT | 127 | NM_020274 |
| R-CATAAGAGGCCGTTCTTGCC | |||
| Htr4 | F-GACAGGCAGCTCAGGAAAAT | 131 | NM_008313 |
| R-TCCCCATAAGCCCAGATGTC | |||
| Ptch1 | F-GTTTCACAAGCCCCTGTGTC | 133 | NM_008957 |
| R-GTGATATGGGTTTCGTGGGC | |||
| Gli1 | F-CCATTGGTACCATGAGCCCT | 134 | NM_010296 |
| R-CTGGGGGTGAAGCATCATTG |
F, forward; R, reverse.
See text for other definitions.
RESULTS
Subepithelial PDGFRα+ cells form colonic pericryptal sheaths.
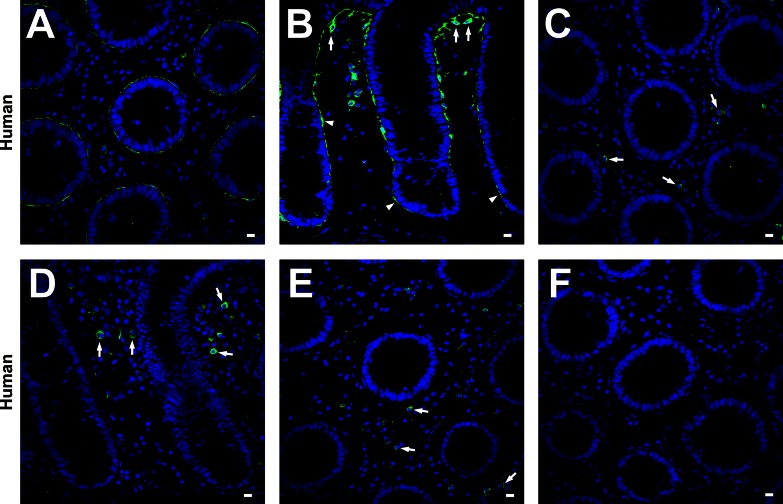
Immunolabeling of PDGFRα in the mucosa was performed on samples of humans, C57 mice, and PDGFRα-eGFP mice (Figs. 1 and 2). In human and mouse colons, PDGFRα+ cells (subepithelial PDGFRα+ cells) were located just beneath the colonic epithelium and formed pericryptal sheaths from the base to the top of crypts (Figs. 1B, 2B, and 2D). These cells were sparse at the bottom of the crypts and more densely distributed near the tops of crypts. The intensity of PDGFRα-like immunoreactivity was also less in cells at the bottom of crypts and increased in cells at the top of crypts. In transverse sections, only a few subepithelial PDGFRα+ cells, with long, thin cell bodies and processes, were needed to completely encircle a crypt (Figs. 1A, 2A, and 2C). These characteristics were similar to those of colonic pericryptal fibroblasts (26, 27, 36). Therefore, subepithelial PDGFRα+ cells may be a subpopulation of the cells identified previously as colonic pericryptal fibroblasts. Images of mice colonic mucosa showed a similar population of PDGFRα+ cells in the same anatomical locations as in human colonic mucosa (Figs. 1 and 2). The specificity of PDGFRα immunolabeling of pericryptal fibroblasts was verified by the experiments shown in Fig. 1, C and D. However, labeling of round-shaped cells in the lamina propria of human colon (Fig. 1, C and D) was due to nonspecific binding of the secondary antibody (Fig. 1, E and F). Mouse colons did not display nonspecific labeling with the secondary antibody (Fig. 2, E and F).
Fig. 1.
A and B: human transverse and sigmoid colon respectively, labeled with platelet-derived growth factor-α receptor (PDGFRα) antibody (green) and 4′,6-diamidino-2-phenylindol (DAPI, blue nuclei). Subepithelial PDGFRα-positive (PDGFRα+) cells were located just beneath the colonic epithelium and formed pericryptal sheaths from the base to the tip of crypts (A and B). Subepithelial PDGFRα+ cells had an octopus shape on the luminal surface of epithelium (arrows in B) and had a flattened morphology in the deeper regions of the crypt (arrowheads in B). The preabsorption test against PDGFRα antibody for humans was performed on transvers (C) and profile (D) sections of human transverse colon. Positive immunolabeling of the cells lining epithelium completely disappeared, but the round-shaped cells in lamina propria still had positive immunolabeling (arrows in C and D). The immune labeling without primary antibody was done on transvers sections of human transverse colon (E). The secondary antibody still bound to round-shaped cells in lamina propria without primary antibody (arrows in E). However, the preabsorption test against the secondary antibody completely blocked secondary antibody binding to round-shaped cells in lamina propria (F). Therefore, positive immunolabeling on those mesenchymal cells except subepithelial PDGFRα+ cells was concluded to be nonspecific binding of the secondary antibody. All scale bars represent 10 μm.
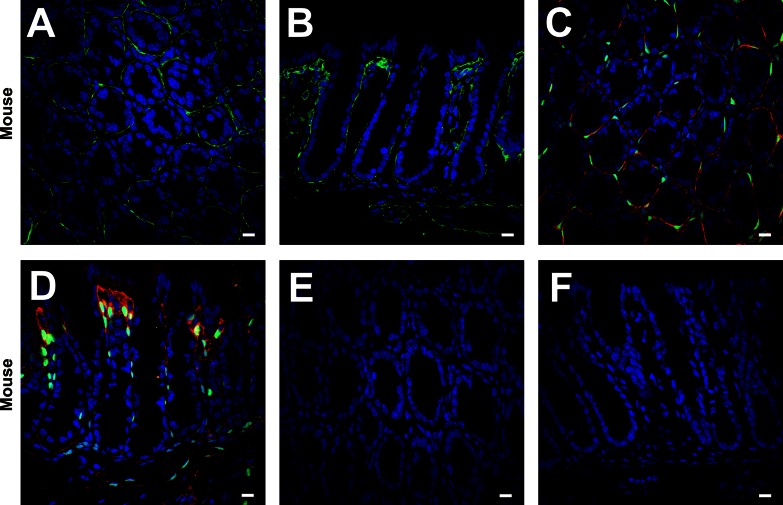
Fig. 2.
Immunolabeling of PDGFRα in the mucosal layer of C57 mice (A and B) labeled with PDGFRα antibody (green) and DAPI (blue nuclei) and PDGFRα-enhanced green fluorescent protein (eGFP) mice (C and D) labeled with PDGFRα antibody (red), DAPI (blue), and eGFP (green). Subepithelial PDGFRα+ cells were located just beneath the colonic epithelium and formed pericryptal sheaths from the base to the tip of crypts (A–D). All eGFP+ nuclei (green) were located in the cell bodies with positive immunolabeling (red) (C and D). The absorption test against PDGFRα antibody for mice was carried out on transverse (E) and profile (F) sections of C57 mouse colon. All PDGFRα immunolabeling was entirely eliminated. All scale bars represent 10 μm.
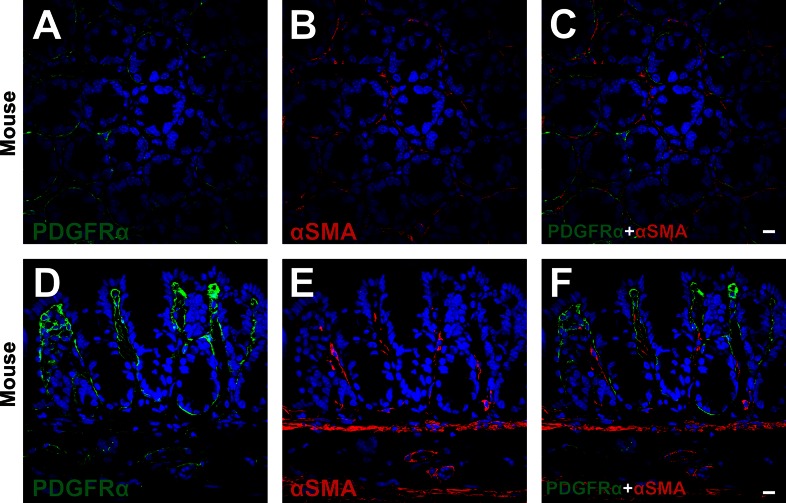
Subepithelial PDGFRα+ cells and subepithelial myofibroblasts are adjacent to one another, but distinct populations of cells.
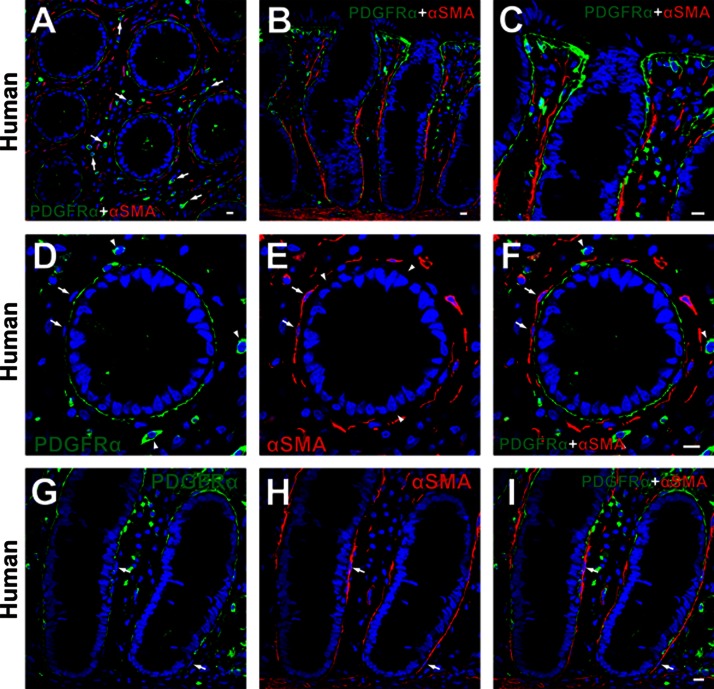
Double immunolabeling of PDGFRα and α-SMA was performed on human and C57 mouse mucosal tissues (Figs. 3 and 4). α-SMA+ cells were found in the subepithelial region (subepithelial α-SMA+ cells), surrounded colonic crypts at the lower two-thirds of crypt, and had a flattened profile with thin bodies and processes (Figs. 3 and 4). These characteristics of subepithelial α-SMA+ cells were consistent with subepithelial myofibroblasts described previously (4, 35, 38, 39). Subepithelial α-SMA+ cells (myofibroblasts) were adjacent to, but distinct from, subepithelial PDGFRα+ cells and were always located at the mesenchymal side of subepithelial PDGFRα+ cells (Figs. 3 and 4). Positive immunolabeling in the mesenchyme of human colons (Fig. 3, A, D, and F) was shown to be nonspecific binding of secondary antibody, as shown in Fig. 1. A few subepithelial PDGFRα+ cells expressed α-SMA very weakly (Fig. 3E).
Fig. 3.
Double immunolabeling of PDGFRα (green) and α-smooth muscle actin (α-SMA, red) in the mucosal layer of human. A and D–F: low and high-power image of transverse sections of human sigmoid colon. B, C, G, and H: low and high-power image of profile sections of human transverse colon. Subepithelial α-SMA+ cells (red) surrounded colonic crypts at the lower two-thirds of crypt and had a flattened profile with thin bodies (A–C). These cells (red) were adjacent to, but distinct from, subepithelial PDGFRα+ cells (green) (arrows in D–I). Positive immunolabeling in the mesenchyme (arrows in A and arrowheads in D and F) was considered to be nonspecific binding of secondary antibody as described in Fig. 1. Subepithelial PDGFRα+ cells expressed α-SMA weakly (arrowheads in E). All scale bars represent 10 μm.
Fig. 4.
Double immunolabeling of PDGFRα (green) and α-SMA (red) in a transverse (A–C) and a profile (D–F) section of C57 mouse. Subepithelial α-SMA+ cells (red) surrounded colonic crypts at the lower two-thirds of crypt and had a flattened profile with thin bodies (B and E). These cells (red) were adjacent to, but distinct from, subepithelial PDGFRα+ cells (green) (C and F). All scale bars represent 10 μm.
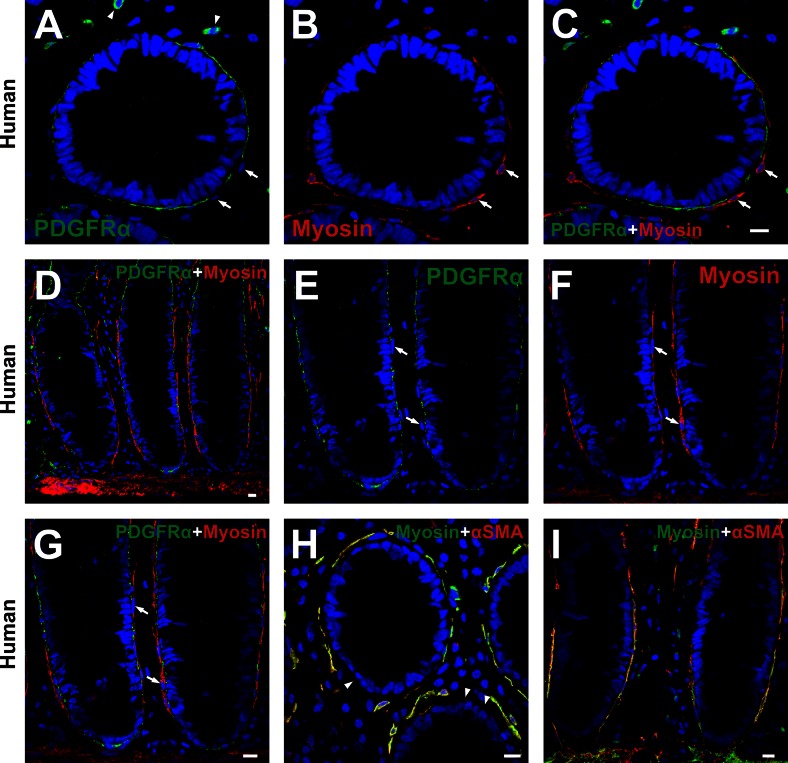
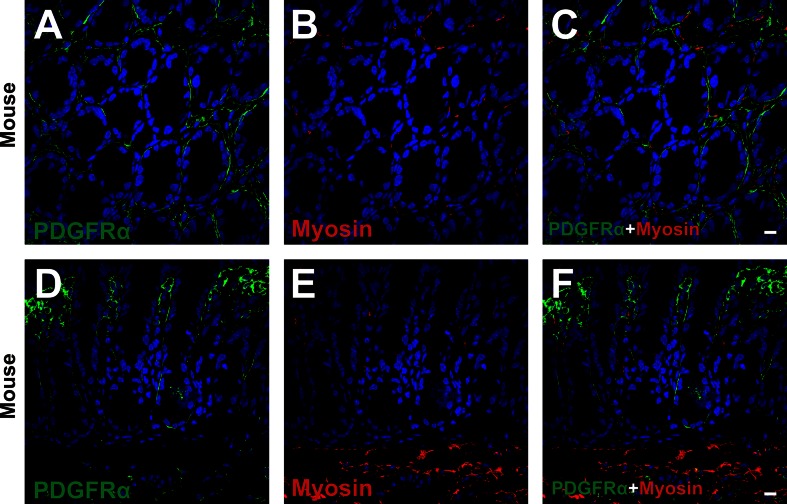
Subepithelial PDGFRα+ cells are distinct from smooth muscle myosin-positive cells.
Double immunolabeling of PDGFRα and myosin (Figs. 5, A–G, and 6) and α-SMA and myosin (Fig. 5, H and I) was also performed. In human colon, smooth muscle myosin-positive cells in the subepithelial region surrounded colonic crypts at the lower two-thirds of crypt and had morphologies similar to subepithelial myofibroblasts (Fig. 5, A–G). Subepithelial PDGFRα+ cells were distinct from smooth muscle myosin-positive cells (Fig. 5, A–C and E–G). On the other hand, subepithelial α-SMA+ cells (myofibroblasts) expressed smooth muscle myosin robustly (Fig. 5, H and I), as described previously (2). These data also support the conclusions that PDGFRα+ cells and myofibroblasts are distinct cell populations. Positive immunolabeling in the mesenchyme (Fig. 5A) was shown to be nonspecific binding of secondary antibody, as described in Fig. 1. Subepithelial PDGFRα+ cells and myosin-positive cells of C57 colons showed similar expression patterns as observed in human mucosal tissues (Fig. 6).
Fig. 5.
Double immunolabeling of PDGFRα (green) and myosin (red) (A–G) and α-SMA (red) and myosin (green) (H and I) in the mucosal layer of human colon. A–C: high-power image of transverse sections of human sigmoid colon. D and E–G: low- and high-power image of profile sections of human transverse colon, respectively. Subepithelial PDGFRα+ cells (green) were distinct from smooth muscle myosin-positive cells (red) (arrows in A–C and E–G). H and I: high-power image of transverse section of human sigmoid colon (H) and profile section of human transverse colon (I). Subepithelial α-SMA+ cells (red) (subepithelial myofibroblasts) displayed robust expression of smooth muscle myosin (green) (cellular colocalization of red and green results in yellow in H and I). Subepithelial PDGFRα+ cells were assumed to be in the area where α-SMA was weakly positive (arrowheads in H). Positive immunolabeling in the mesenchyme (arrowheads in A) was considered to be nonspecific binding of secondary antibody as described in Fig. 1. All scale bars represent 10 μm.
Fig. 6.
Double immunolabeling of PDGFRα (green) and smooth muscle myosin (red) in a transverse (A–C) and a profile (D–F) section of C57 mouse. Subepithelial PDGFRα+ cells (green) in human colon were located adjacent to but distinct from smooth muscle myosin-positive cells in the subepithelial region (red) (arrows in A–F). All scale bars represent 10 μm.
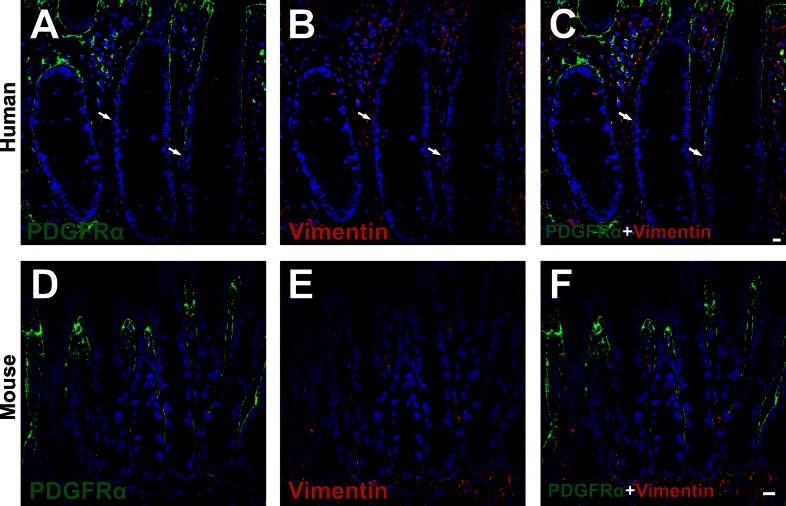
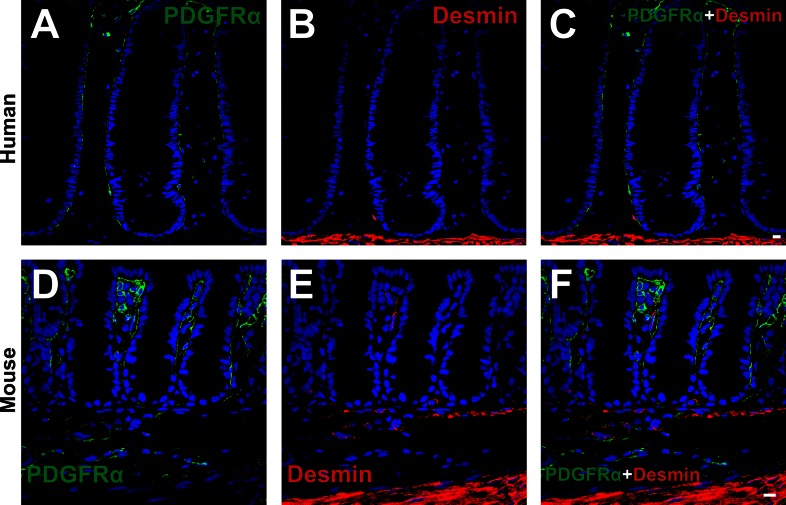
Subepithelial PDGFRα+ cells express vimentin weakly, but not desmin.
Double immunolabeling for PDGFRα and vimentin (Fig. 7) and PDGFRα and desmin (Fig. 8) was performed. Vimentin was expressed diffusely in the lamina propria of human and mouse colonic mucosa (Fig. 7). Weak vimentin immunoreactivity was found in some subepithelial PDGFRα+ cells, but cells adjacent to subepithelial PDGFRα+ cells in human, identified as subepithelial myofibroblasts on the basis of α-SMA expression, displayed relatively stronger vimentin immunoreactivity than PDGFRα+ cells (Fig. 7, A–C). Vimentin expression was sparse in the subepithelial region, unlike either PDGFRα or α-SMA (Fig. 3). Therefore, vimentin labeling is not useful as a means to distinguish between subepithelial PDGFRα+ cells and subepithelial myofibroblast. Mice displayed similar vimentin immunoreactivity as in human colon (Fig. 7, D–F). Desmin immunoreactivity was also sparse in the lamina propria of colon (Fig. 8). Desmin immunoreactivity was not resolved in PDGFRα+ cells in colonic lamina propria of human or mouse colon (Fig. 8).
Fig. 7.
Double immunolabeling of PDGFRα (green) and vimentin (red) in the mucosal layer of human transverse colon (A–C) and in profile sections of C57 mice colon (D–F). Vimentin was expressed diffusely in the lamina propria of human and mice colonic mucosa. A few subepithelial PDGFRα+ cells displayed weak expression of vimentin. The cells adjacent to subepithelial PDGFRα+ cells in human mucosa appeared to have more robust expression of vimentin immunoreactivity than PDGFRα+ cells (arrows in A–C). All scale bars represent 10 μm.
Fig. 8.
Double immunolabeling of PDGFRα (green) and desmin (red) in the mucosal layer of human transverse colon (A–C) and in profile sections of C57 mice colon (D–F). In both humans and mice, desmin appeared to be hardly expressed in the subepithelial region, and subepithelial PDGFRα+ cells did not express desmin. All scale bars represent 10 μm.
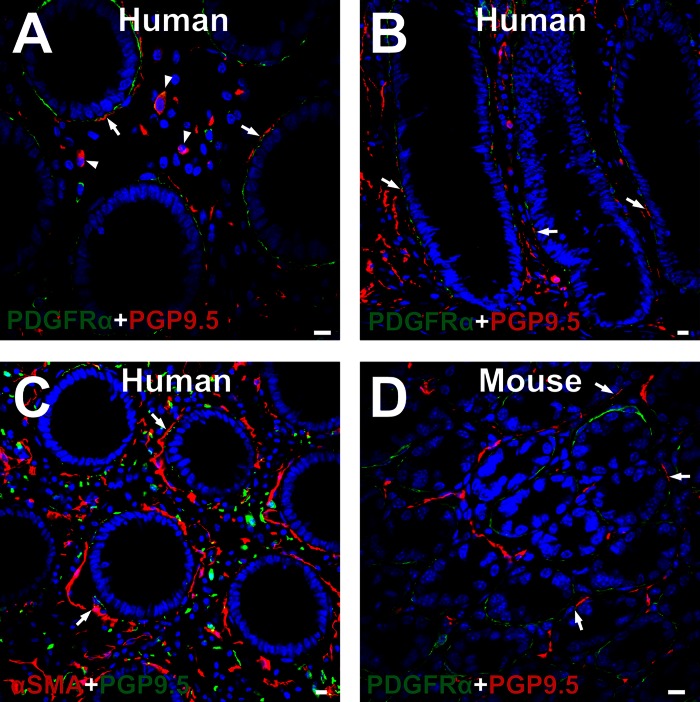
Subepithelial PDGFRα+ cells are closely associated with nerve endings in colonic lamina propria.
Double labeling of PDGFRα and PGP9.5 (Fig. 9, A and B) α-SMA and PGP9.5 (Fig. 9C) in human colon and PDGFRα and PGP9.5 in C57 mouse colon (Fig. 9D) was performed. PGP9.5 immunoreactive nerve fibers innervated the lamina propria in human and mouse colons. Some nerve fibers were closely associated with subepithelial PDGFRα+ cells and subepithelial myofibroblasts (α-SMA+ cells) (Fig. 9). PGP9.5 immunoreactive fibers were found at the mesenchymal surface of subepithelial PDGFRα+ cells (Fig. 9, A, B, and D), and some fibers were found at the epithelial side of the myofibroblasts (Fig. 9C). Similar distributions of PGP9.5 immunoreactive nerve fibers were observed in the murine colon (Fig. 9D). Cells in the lamina propria of human colon also expressed PGP9.5-like immunoreactivity (Fig. 9A). This may have been due to nonspecific labeling; however, intramucosal ganglion cells have been described previously (41).
Fig. 9.
Double immunolabeling of PDGFRα (green) and PGP9.5 (red) in human transverse colon (A and B), α-SMA (red) and PGP9.5 (green) in human transverse colon (C), and PDGFRα (green) and PGP9.5 (red) in C57 mouse colon (D). All PGP9.5 immunoreactive fibers were located at the mesenchymal surface of PDGFRα+ cells (arrows in A, B, and D), while some fibers were located at the epithelial side of α-SMA+ cells (arrows in C). A few cells in the lamina propria of human colon expressed PGP9.5 (arrowheads in A). The identity of these cells was not further investigated. All scale bars represent 10 μm.
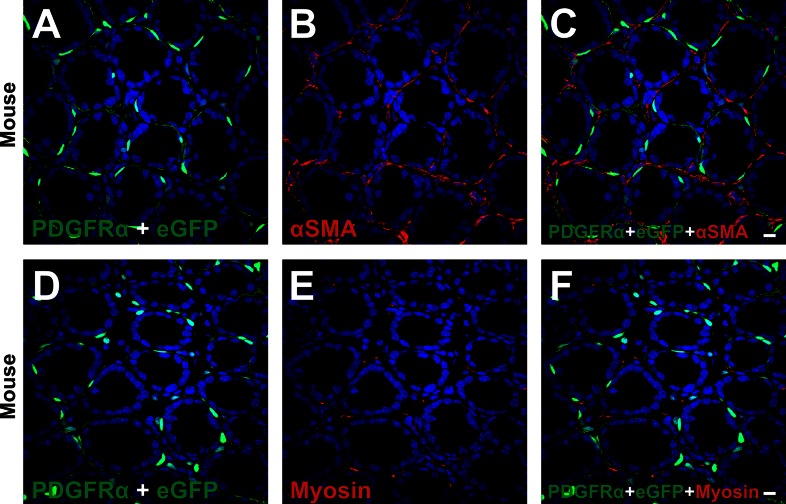
Pdgfra promoter-driven expression of eGFP was evident in subepithelial PDGFRα+ cells but not in myofibroblasts.
Double immunolabeling for PDGFRα and α-SMA (Fig. 10, A–C) and PDGFRα and smooth muscle myosin (Fig. 10, D–F) was performed on colonic mucosa of PDGFRα-eGFP mice. As shown in Fig. 10, all subepithelial PDGFRα+ cells expressed eGFP in nuclei, but eGFP was not expressed in nuclei of subepithelial myofibroblasts (α-SMA+ cells) or cells with myosin immunoreactivity. These data show that the promoter driving Pdgfra expression is active in subepithelial PDGFRα+ cells but not in subepithelial myofibroblasts. Thus, subepithelial PDGFRα+ cells are a class of cells distinct from subepithelial myofibroblasts.
Fig. 10.
Double immunolabeling of PDGFRα (green) and α-SMA (red) (A–C) and PDGFRα (green) and smooth muscle myosin (red) (D–F) in the mucosal layer of PDGFRα-eGFP mice. The color of eGFP-positive nuclei was brighter and whiter by DAPI nuclei staining than the color of PDGFRα immunolabeling, so we could distinguish eGFP and PDGFRα immunolabeling. All scale bars represent 10 μm.
Mouse subepithelial PDGFRα+ cells express transcripts that suggest participation in innate immunity, sensory responses, and maintenance of mucosal homeostasis.
Having a reporter specific to subepithelial PDGFRα+ cells made it possible to isolate these cells from mixed cell populations resulting from enzymatic digestion of the mucosae. The mucosal layer of PDGFRα-eGFP mice was removed as shown in Fig. 11, A–C. Mucosal strips consisted of epithelium, lamina propria, and muscularis mucosae (Fig. 11, A–C). PDGFRα+ cells within these strips (identified by eGFP+ nuclei) originated from the subepithelial region. After dispersing tissues, FACS was performed to purify subepithelial PDGFRα+ cells (Fig. 11D). Two populations of eGFP+ cells were observed that differed in fluorescence intensity (P1 and P2 in Fig. 11D). For this study, we used P1 cells for analysis of gene expression, as shown in Fig. 12. P1 cells (PDGFRα+ cells) accounted for about 6% of cells in mucosa based on cell counting by FACS.
Fig. 12.
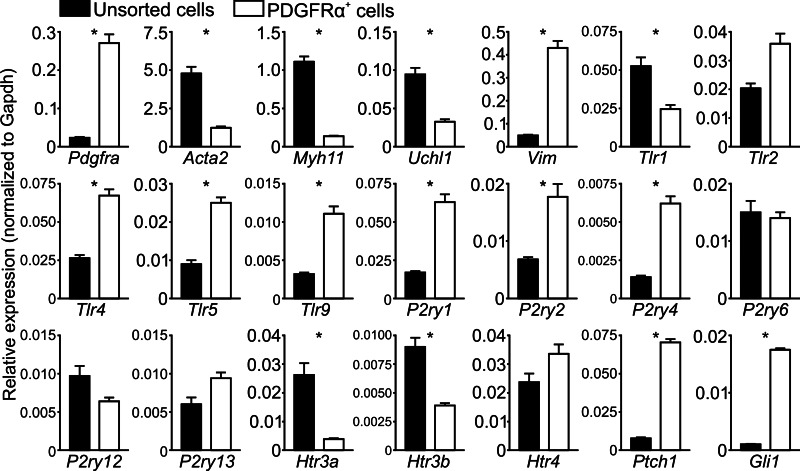
The comparison of relative expression of 21 genes between PDGFRα+ cells and unsorted cellular dispersions (unsorted cells). The relative expression level of each gene was normalized to that of Gapdh. Normalized expression levels were then converted to fold changes (PDGFRα+ cells vs. unsorted cells). *There are 2-fold or more between PDGFRα+ cells and unsorted cells.
qPCR was performed on cDNAs obtained from sorted PDGFRα+ cells and unsorted cells representing all cell types dispersed from the mucosa. Relative expression (normalized to Gapdh) of 21 gene transcripts was compared in these two populations of cells (Table 2 and Fig. 12). Purity resulting from FACS sorting of PDGFRα+ cells was evaluated by comparing expression of Pdgfra, Acta2 (α-SMA), Myh11 (smooth muscle myosin heavy chain), and Uch11 (PGP9.5) (Fig. 12, 4 graphs on top left). These data showed that transcripts of Pdgfra were significantly enriched in PDGFRα+ cells, and transcripts of Acta2, Myh11, and Uch11 were minimal or not resolved in sorted PDGFRα+ cells. These results recapitulate the immunodetection of proteins expressed in subepithelial PDGFRα+ cells in situ.
We also checked for vimentin (Vim) expression, which has been previously shown to be expressed in myofibroblasts (39). Vim transcripts were expressed in PDGFRα+ cells, but PDGFRα+ cells were weakly labeled with vimentin. These findings suggest that vimentin is not a good marker to discriminate PDGFRα+ cells from myofibroblasts.
We also probed transcripts of Toll-like receptors (Trl1, -2, -4, -5, and -9), which are considered to be important mediators in the innate immune response against bacteria (1, 3, 33). These studies revealed that subepithelial PDGFRα+ cells express Tlr4, -5, and -9 more strongly compared with the other cell types in mucosa. PDGFRα+ cells shared expression of Trl2 with other cell types (Fig. 12).
5-Hydroxytryptamine (5-HT) and ATP are released from enterochromaffin (EC) cells in the mucosa, and these mediators activate sensory nerve terminals in the lamina propria. PDGFRα+ cells are very closely associated with the basolateral surface of mucosal cells and juxtaposed between EC cells and terminals of sensory neurons as described above. Thus, PDGFRα+ cells must experience some of the highest concentrations of mediators achieved during sensory transduction or secretomotor input. Therefore, we also probed purine receptors (P2ry1, -2, -4, -6, -12, and -13) and 5-HT receptors (Htr3a, Htr3b, and Htr4) that have functional roles in mechanosensory and secretomotor neurotransduction (6, 13, 17, 18, 20, 23, 45). Results from these tests showed that colonic subepithelial PDGFRα+ cells expressed P2ry1, -2, and -4 more robustly than other cell types and shared expression of Htr4 with other cell types in the mucosa (Fig. 12). Htr3a and Htr3b expressions were much lower in PDGFRα+ cells than in other mucosal cells.
Recently, mesenchymal PDGFRα+ cells in embryonic small intestine were reported to express hedgehog signaling molecules, Patched1 and the glioblastoma transcription factor 1 (Gli1) (44) and hedgehog signaling is expressed in colonic mesenchymal cells and contribute to maintenance of mucosal homeostasis (42, 8). Therefore we probed the expression of two hedgehog signaling molecules (Ptch1 and Gli1). Interestingly, qPCR analysis data revealed that subepithelial PDGFRα+ cells expressed Ptch1 and Gli1 at much greater levels than other cell types (fold changes = 9.01 and 16.72, respectively) (Fig. 12).
DISCUSSION
Previously, the receptor tyrosine kinase PDGFRα was considered mainly a developmental growth factor receptor in gastrointestinal tissues. However, the discovery of PDGFRα expression in cells previously known as “fibroblast-like” cells in gastrointestinal muscles (24) has led to a broader concept of the role of these cells (31, 32). In this study we report robust expression of PDGFRα in a population of subepithelial cells in colons of mice and humans. These cells lie immediately beneath the epithelial cells and express transcripts that suggest involvement of PDGFRα+ cells in a variety of physiological functions. We have also characterized a reporter strain that can be used to obtain large numbers of PDGFRα+ cells that can be used to characterize the phenotype and physiology of these cells and to determine disease-dependent changes in gene expression and function.
Subepithelial PDGFRα+ cells displayed long, thin cell bodies and processes in transverse and profile sections. These cells connected to each other and appeared to cover the subepithelial surface of crypts, from the base to the luminal surface of the epithelium. Nevertheless, there were morphological differences between the cells at the bottom and the upper regions of crypts: 1) cells at the bottom of crypts were more sparse and became more dense in distribution near the tops of crypts, and 2) cells at the bottom of the crypts had lower PDGFRα-like immunoreactivity than cells at the tops of crypts.
Electron microscopic studies described the details of colonic pericryptal fibroblasts, which were shown to form a sheath with “overlapping or shingling of several layers of fibroblasts” around the deep one-third of crypts (26). This overlapping organization might be equivalent to the double layers of subepithelial PDGFRα+ cells and subepithelial myofibroblasts we noted in human and murine colonic mucosae. The distribution of subepithelial PDGFRα+ cells is somewhat similar to the distribution of fibroblast-like cells reported in a previous ultrastructural study of rat colonic mucosa (30). We found that subepithelial myofibroblasts merged into the muscularis mucosa, and their distribution appeared to be less organized at the bottoms of crypts and did not extend to the luminal surface of the epithelium (Figs. 3–6). These observations suggested that subepithelial PDGFRα+ cells have a closer relationship with epithelial cells, sensory and secretomotor signaling, the epithelial cell life cycle (i.e., proliferation from the stem cell niche, differentiation, and apoptosis), and the epithelial cellular pathology (e.g., inflammatory responses, tumorigenesis, and wound healing) than subepithelial myofibroblasts. Recognition of a specific immunolabel for these cells and identification of a reporter strain of mice will facilitate future detailed studies of this potentially important, but heretofore neglected, class of interstitial cells.
Many studies have been based on hypotheses proposed for myofibroblasts in a 1999 review (38), but our data suggest that cells usually considered to be subepithelial myofibroblasts may be a mixed cell population consisting of myofibroblasts and subepithelial PDGFRα+ cells. Subepithelial PDGFRα+ cells appear to have low expression of α-SMA, but our preliminary cell culture experiments with these cells suggest that α-SMA expression can increase in PDGFRα+ cells under some circumstances (data not shown). Thus, it is possible that cells from the lamina propria might remodel or change their phenotype under cell culture conditions. Therefore, conclusions about the characteristics, phenotype, and roles of subepithelial myofibroblasts, particularly from studies of cultured cells, may need reconsideration. The ability to identify and purify freshly dispersed subepithelial PDGFRα+ cells unequivocally may make it possible to develop more specific concepts about the cell biology and integrative role of these cells in the near future.
Isolation and purification of colonic subepithelial PDGFRα+ cells was accomplished by enzymatic dispersion of mucosa from PDGFRα-GFP mice (21, 32) and cell sorting by FACS. From immunolabeling, we showed that only rare cells in addition to subepithelial PDGFRα+ cells in colonic mucosa were PDGFRα immunopositive (Figs. 10 and 11, A–C). After sorting, we verified that gene transcripts in the population of sorted PDGFRα+ cells were consistent with findings from immunolabeling: Pdgfra transcripts were much more enriched, and Acta2 (α-SMA), Myh11 (smooth muscle myosin heavy chain), and Uch11 (PGP9.5) were much less enriched compared with unsorted cells. Finding that gene transcripts, such as Pdgfra, were greatly enriched and other cell-specific marker genes were depressed in PDGFRα+ cells sorted from mouse colon cells tends to validate conclusions about the enrichment of other gene transcripts in PDGFRα+ cells. At present, we have not developed a method to isolate these cells from human colon efficiently. In the future it might be possible to obtain cells from mucosal biopsies, label with PDGFRα antibodies directed at extracellular epitopes, and purify cells by FACS.
We used the P1 population of cells (Fig. 11) as the purified subepithelial PDGFRα+ cells because of the robust expression of Pdgfra in this population. P2 cells might represent damaged cells or the cells at the bottom of crypts where the immunoreactivity of cells was far less than in cells along and at the top of crypts. It will be interesting to compare expression of important genes in these two populations of PDGFRα+ cells in the future.
The initial gene expression analysis performed yielded interesting and novel findings about mouse subepithelial PDGFRα+ cells (Fig. 12). There was enrichment in transcripts of several Toll-like receptors (Tlr2, -4, -5, and -9), which are known to be important in mediating initial innate immune responses (33). Ligands for these receptors are lipoprotein, peptidoglycan, etc. for TLR2, lipopolysaccharide, fusion protein, etc. for TLR4, flagellin for TLR5, and CpG-containing DNA for TLR9 (3). Expression of these receptors by subepithelial PDGFRα+ cells seems appropriate because these cells lie just below the epithelium and may provide first-line immunological responses if invasive bacteria surmount the epithelial barrier.
PDGFRα+ cells also express purinergic receptors P2ry1, -2, and -4. Well-known ligands for these receptors are ATP for P2Y1 and ATP and UTP for P2Y2 and P2Y4 (9). Previous studies have shown that ATP may be a neuromodulatory agent in sensory transduction, and possibly in nociception (6, 13, 20, 28, 45). In a previous study it was noted that cells referred to as subepithelial fibroblasts in small intestines of rat expressed P2Y1 receptors and released ATP in response to mechanical stimulation (16). What parallel or modulatory effects subepithelial PDGFRα+ cells contribute to sensory signaling will be the subject of future investigations. It is interesting to note that a similar population of cells lies in close association to Aδ- and C-fibers in the urinary bladder and is thought to have a neuromodulatory role in afferent signaling in that organ (12, 15, 40).
PDGFRα+ cells from the mouse were also found to express Htr4. We have shown that subepithelial PDGFRα+ cells are more closely associated with epithelial cells (which would include EC cells) than nerve fibers. Therefore, PDGFRα+ cells may respond to and play a neuromodulatory role in mechanosensory inputs from EC cells. A recent report showed that 5-HT4 receptors were expressed in the epithelial layer, muscularis mucosa, and by neurons (23). This study does not contradict our findings, however, because the GFP immunolabeling in sections of bacterial artificial chromosome-GFP mouse tissues might not have been able to precisely localize this immunolabeling.
Last, we found that murine subepithelial PDGFRα+ cells expressed hedgehog signaling molecules, Ptch1 and Gli1, almost exclusively. These data suggest that subepithelial PDGFRα+ cells in murine colonic mucosa may be targets for Indian hedgehog, which is released from the colonic epithelium (42). Hedgehog signaling has an important role in maintaining homeostasis in the intestinal mucosa, but questions about its role remain unanswered (8). Finding a new target for hedgehog signaling might provide greater insight into the role of this pathway.
It should also be noted that, while experiments were focused on colonic tissues in this study, we also tested mucosal tissues of the esophagus, stomach, and small intestine. PDGFRα+ cells were found in the same anatomical niche within the lamina propria in each organ (data not shown).
PDGFRα is a unique immunolabel for a population of cells that might correspond to the cells identified by electron microscopy as pericryptal fibroblasts. Subepithelial PDGFRα+ cells form a sheath just beneath the epithelium and cover crypts from their base to the luminal surface of epithelium. The roles of these cells in physiological and pathophysiological processes are unknown, but the labeling techniques and the use of a reporter strain we have characterized will make comprehensive studies of these cells possible for the first time. Several key transcripts are expressed by these cells in mouse, suggesting these cells may have modulatory functions in immune and sensory responses and in the maintenance of mucosal homeostasis.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant P01-DK-41315.
DISCLOSURES
The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
Author contributions: M.K. conception and design of research; M.K., Y.N., L.E.P., and J.B.T. performed experiments; M.K., L.E.P., and J.B.T. analyzed data; M.K., L.E.P., and J.B.T. interpreted results of experiments; M.K. prepared figures; M.K. drafted manuscript; M.K., S.M.W., and K.M.S. edited and revised manuscript; M.K., S.M.W., and K.M.S. approved final version of manuscript.
REFERENCES
- 1. Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 10: 131–144, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Adeqboyega PA, Mifflin RC, DiMari JF, Saada JI, Powell DW. Immunohistochemical study of myofibroblasts in normal colonic mucosa, hyperplastic polyps, and adenomatous colorectal polyps. Arch Pathol Lab Med 126: 829–836, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol 4: 499–511, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Andoh A, Bamba S, Brittan M, Fujiyama Y, Wright NA. Role of intestinal subepithelial myofibroblasts in inflammation and regenerative response in the gut. Pharmacol Ther 114: 94–106, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev 22: 1276–1312, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bertrand PP. ATP and sensory transduction in the enteric nervous system. Neuroscientist 9: 243–260, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Blair PJ, Bayguinov Y, Sanders KM, Ward SM. Relationship between enteric neurons and interstitial cells in the primate gastrointestinal tract. Neurogastroenterol Motil 24: 437–449, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buller NV, Rosekrans SL, Westerlund J, van den Brink GR. Hedgehog signaling and maintenance of homeostasis in the intestinal epithelium. Physiology (Bethesda) 27: 148–155, 2012 [DOI] [PubMed] [Google Scholar]
- 9. Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci 64: 1471–1483, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chan F, Liu Y, Sun H, Li X, Shang H, Fan D, An J, Zhou D. Distribution and possible role of PDGF-AA and PDGFR-alpha in the gastrointestinal tract of adult guinea pigs. Virchows Arch 457: 381–388, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Chen SW, Chen YX, Zhang XR, Qian H, Chen WZ, Xie WF. Targeted inhibition of platelet-derived growth factor receptor-beta subunit in hepatic stellate cells ameliorates. Gene Ther 15: 1424–1435, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Chopra B, Gever J, Barrick SR, Hanna-Mitchell AT, Beckel JM, Ford AP, Birder LA. Expression and function of rat urothelial P2Y receptors. Am J Physiol Renal Physiol 294: F821–F829, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cooke HJ, Wunderlich J, Christofi FL. “The force be with you”: ATP in gut mechanosensory transduction. News Physiol Sci 18: 43–49, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Frendriksson L, Li H, Eriksson U. The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev 15: 197–204, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Fry CH, Sui GP, Kanai AJ, Wu C. The function of suburothelial myofibroblasts in bladder. Neurourol Urodyn 26: 914–919, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Furuya K, Sokabe M, Furuya S. Characteristics of subepithelial fibroblasts as a mechano-sensor in the intestine: cell-chape-dependent ATP release and P2Y1 signaling. J Cell Sci 118: 3289–3304, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Gershon MD, Tack J. The serotonin singaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132: 397–414, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Grider JR, Foxx-Orenstein AE, Jin JG. 5-Hydroxytryptamine4 receptor agonists initiate the peristaltic reflex in human, rat, and quinea pig intestine. Gastroenterology 115: 370–380, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Grover M, Bernard CE, Pasricha PJ, Parkman HP, Abell TL, Nguyen LA, Snape W, Shen KR, Sarr M, Swain J, Kendrick M, Gibbons S, Ordog T, Farrugia G. Platelet-derived growth factor receptor α (PDGFRα)-expressing “fibroblast-like cells” in diabetic and idiopathic gastroparesis of humans. Neurogastroenterol Motil 24: 844–852, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gryqorczyk R, Hanrahan JW. CFTR-independent ATP release from epithelial cells triggered by mechanical stimuli. Am J Physiol Cell Physiol 272: C1058–C1066, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Hamilton TG, Klinghoffer RA, Corrin PD, Soriano P. Evolutionary divergence of platelet-derived growth factor alpha receptor signaling mechanisms. Mol Cell Biol 23: 4013–4025, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoch RV, Soriano P. Roles of PDGF in animal development. Development 130: 4769–4784, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Hoffman JM, Tyler K, MacEachern SJ, Balemba OB, Johnson AC, Brooks EM, Zhao H, Swain GM, Moses PL, Galligan JJ, Sharkey KA, Greenwood-Van Meerveld B, Mawe GM. Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology 142: 844–854, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iino S, Horiguchi K, Horiguchi S, Nojyo Y. c-Kit-negative fibroblast-like cells express platelet-derived growth factor receptor alpha in the murine gastrointestinal musculature. Histochem Cell Biol 131: 691–702, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Karlsson L, Lindahl P, Heath JK, Betsholtz C. Abnormal gastrointestinal development in PDGF-A and PDGFR-(alpha) deficient mice implicates a novel mesenchymal structure with putative instructive properties in villus morphogenesis. Development 127: 3457–3466, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Kaye GI, Lane N, Pascal RR. Colonic pericryptal fibroblast sheath: replication, migration, and cytodifferentiation of a mesenchmal cell system in adult tissue. II. Fine structural aspects of normal rabbit and human colon. Gastroenterology 54: 852–865, 1968 [PubMed] [Google Scholar]
- 27. Kaye GI, Pascal RR, Lane N. The colonic pericryptal fibroblast sheath: replication, migration, and cytodifferentiation of a mesenchmal cell system in adult tissue. 3. Replication and differentiation in human hyperplastic and adenomatous polyps. Gastroenterology 60: 515–536, 1971 [PubMed] [Google Scholar]
- 28. Kirkup AJ, Booth CE, Chessell IP, Humphrey PP, Grundy D. Excitatory effect of P2X receptor activation on mesenteric afferent nerves in the anaesthetized rat. J Physiol 520: 551–563, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koh BH, Roy R, Hollywood MA, Thornbury KD, McHale NG, Sergeant GP, Hatton WJ, Ward SM, Sanders KM, Koh SD. Platelet-derived growth factor receptor-α cells in mouse urinary bladder: a new class of interstitial cells. J Cell Mol Med 16: 691–700, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Komuro T, Hashimoto Y. Three-dementional structure of the rat intestinal wall (mucosa and submucosa). Arch Histol Cytol 53: 1–21, 1990 [DOI] [PubMed] [Google Scholar]
- 31. Kurahashi M, Nakano Y, Hennig GW, Ward SM, Sanders KM. Platelet-derived growth factor receptor α-positive cells in the tunica muscularis of human colon. J Cell Mol Med 16: 1397–1404, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kurahashi M, Zheng H, Dwyer L, Ward SM, Don Koh S, Sanders KM. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J Physiol 589: 697–710, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol 1: 135–145, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Mifflin RC, Pinchuk IV, Saada JI, Powell DW. Intestinal myofibroblasts: targets for stem cell therapy. Am J Physiol Gastrointest Liver Physiol 300: G684–G696, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Monaghan KP, Johnston L, McCloskey KD. Identification of PDGFRα positive populations of interstitial cells in human and guinea pig bladders. J Urol 188: 639–647, 2012 [DOI] [PubMed] [Google Scholar]
- 36. Pascal RR, Kaye GI, Lane N. Colonic pericryptal fibroblast sheath: replication, migration, and cytodifferentiation of a mesenchmal cell system in adult tissue. I. Autoradiographic studies of normal rabbit colon Gastroenterology 54: 835–851, 1968 [PubMed] [Google Scholar]
- 37. Powell DW, Adeqboyega PA, Di Mari JF, Mifflin RC. Epithelial cells and their neighbors I. Role of intestinal myofibroblasts in development, repair, and cancer. Am J Physiol Gastrointest Liver Physiol 289: G2–G7, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol Cell Physiol 277: C183–C201, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Powell DW, Pinchuk IV, Saada JI, Mifflin RC. Mesenchymal cells of the intestinal lamina propria. Annu Rev Physiol 73: 213–237, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sui GP, Wu C, Roosen A, Ikeda Y, Kanai AJ, Fry CH. Modulation of bladder myofibroblast activity: implications for bladder function. Am J Physiol Renal Physiol 295: F688–F697, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tunru-Dinh V, Wu ML. Intramucosal ganglion cells in normal adult colorectal mucosa. Int J Surg Pathol 15: 31–37, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Van Dop WA, Uhmann A, Wijgerde M, Sleddens-Linkels E, Heijmans J, Offerhaus GJ, van den Bergh Weerman MA, Boeckxstaens GE, Hommes DW, Hardwick JC, Hahn H, van den Brink GR. Depletion of the colonic epithelial precursor cell compartment upon conditional activation of the hedgehog pathway. Gastroenterology 136: 2195–2203, 2009 [DOI] [PubMed] [Google Scholar]
- 43. van Roeven CR, Ostendorf T, Denecke B, Bokemeyer D, Behrmann I, Strutz F, Lichenstein HS, LaRochelle WJ, Pena CE, Chaudhuri A, Floege J. Biological responses to PDGF-BB versus PDGF-DD in human mesangial cells. Kidney Int 69: 1393–1402, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Walton KD, Kolterud A, Czerwinski MJ, Bell MJ, Prakash A, Kushwaha J, Grosse AS, Schnell S, Gumucio DL. Hedgehog-responsive mesenchymal clusters direct patterning and emergence of intestinal villi. Proc Natl Acad Sci USA 109: 15817–15822, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wood JD. The enteric purinergic P2Y1 receptor. Curr Opin Pharmacol 6: 564–570, 2006 [DOI] [PubMed] [Google Scholar]