Abstract
Activation of pancreatic stellate cells (PSCs) by transforming growth factor (TGF)-β is the key step in the development of pancreatic fibrosis, a common pathological feature of chronic pancreatitis (CP). Bone morphogenetic proteins (BMPs), members of the TGF-β superfamily, have anti-fibrogenic functions, in contrast to TGF-β, in the kidney, lung, and liver. However, it is not known whether BMPs have an anti-fibrogenic role in the pancreas. The current study was designed to investigate the potential anti-fibrogenic role of BMPs in the pancreas using an in vivo CP model and an in vitro PSC model. CP was induced by repetitive intraperitoneal injections of cerulein in adult Swiss Webster mice. The control mice received saline injections. Compared with the control, cerulein injections induced a time-dependent increase in acinar injury and progression of fibrosis and a steady increase in inflammation. Cerulein injections also induced increases of the extracellular matrix (ECM) protein fibronectin and of α-smooth muscle actin (α-SMA)-positive stellate cells (PSCs). The mice receiving cerulein injections showed increased BMP2 protein levels and phosphorylated Smad1 levels up to 4 wk and then declined at 8 wk to similar levels as the control. In vitro, the isolated mouse and human PSCs were cultured and pretreated with BMP2 followed by TGF-β treatment. BMP2 pretreatment inhibited TGF-β-induced α-SMA, fibronectin, and collagen type Ia expression. Knocking down Smad1 with small-interfering RNA reversed the inhibitory effect of BMP2 on TGF-β-induced α-SMA and fibronectin expression. Thus, BMP2 opposes the fibrogenic function of TGF-β in PSCs through the Smad1 signaling pathway.
Keywords: pancreas stellate cell, bone morphogenetic protein, transforming growth factor-β, pancreatic fibrosis
pancreatic fibrosis is the common pathological characteristic of chronic pancreatitis (CP), a major risk factor for pancreatic cancer (26). Accumulating evidence indicates that activation of pancreatic stellate cells (PSCs) plays a pivotal role in the development of pancreatic fibrosis (3, 17). In the normal pancreas, stellate cells comprise 4% of all pancreatic cells. They have a periacinar distribution and remain quiescent with the presence of vitamin A-containing lipid droplets in cytoplasm (1, 2). After pancreatic injury or inflammation, they can be activated by various proinflammatory and profibrogenic cytokines, including transforming growth factor (TGF)-β, interleukin (IL)-1β, and IL-6 (12, 13, 19) that are secreted by inflammatory cells, injured acinar cells, and platelets (12, 18, 22). The activated PSCs gradually lose cytoplasmic vitamin A-containing lipid droplets and differentiate into myofibroblast-like cells, with expression of α-smooth muscle actin (SMA). The activated PSCs proliferate, synthesize, and secrete excessive extracellular matrix (ECM) components, such as fibronectin (FN) and collagens, that accumulate in the pancreas and result in fibrosis.
TGF-β is a major profibrogenic cytokine in the pancreas. TGF-β is upregulated in CP tissues, promotes PSC activation, and stimulates ECM synthesis (11, 20, 24). Bone morphogenetic proteins (BMPs), members of the TGF-β superfamily, have anti-fibrogenic functions in multiple organs (10, 14, 28, 31). BMP2 is effective in the treatment of renal fibrosis in the rat unilateral urethral obstruction model and antagonizes TGF-β-induced renal fibrogenic signals in renal interstitial fibroblast cells (27, 28); BMP2 also prevents differentiation and cell migration of lung fibroblasts (21). BMP7 treatment reduces lung fibrosis in asbestos-exposed mice (14). Adenoviral gene transfer of BMP7 also reduces rat liver fibrosis induced by repetitive injections of thioacetamide and suppresses the expression of collagen Ia (ColIa) mRNA and α-SMA in rat hepatic stellate cells (10). However, whether BMPs have anti-fibrogenic function in the pancreas is unknown.
In the canonical BMP signaling pathway, BMPs bind to BMP receptors type I and type II on the cell surface and subsequently activate intracellular mediators, receptor-activated Smads, Smad1, Smad5, and Smad8, which form a complex with Smad4 and translocate to the nucleus to regulate transcription of target genes (25).
In this study, using a cerulein-induced CP model, we found that, as pancreatic fibrosis progressed, BMP2 and phosphorylated Smad1 (pSmad1) levels increased at earlier time points and then declined at a later time point in the pancreas during CP development, suggesting that the dynamic changes of BMP signaling relate to fibrosis progression. Using primary mouse and human PSCs pretreated with BMP2 followed by TGF-β treatment, we demonstrated that BMP2 inhibited TGF-β-induced PSCs activation and ECM formation. Therefore, we identify BMP signaling as an anti-fibrogenic pathway in the pancreas.
MATERIALS AND METHODS
Reagents.
Cerulein, the decapeptide analog of the potent pancreatic secretagogue cholecystokinin, was purchased from Bachem Americas (Torrance, CA); human recombinant TGF-β1 and BMP2 were purchased from R&D Systems (Minneapolis, MN) and were diluted in a vehicle solution (0.1% BSA, 4 mM HCl). BSA was purchased from Sigma-Aldrich (St. Louis, MO).
Animals.
Animal experiments were performed according to the protocols approved by the Animal Welfare Committee of the University of Texas Health Science Center at Houston. Adult (8–10 wk old) female Swiss Webster mice were purchased from Harlan Laboratories (Indianapolis, IN). The animals were housed in a climate-controlled room with an ambient temperature of 23°C and a 12:12-h light-dark cycle. Animals were fed standard laboratory chow and given water ad libitum.
In vivo model of CP.
Mice were randomized into two groups (n = 4/group). CP was induced by repetitive intraperitoneal injections of cerulein (50 μg/kg, 5 hourly injections/day, 3 days/wk) up to 8 wk. Saline was given with the same volume and frequency in control mice. The mice were killed at day 4 after completion of cerulein or saline injections. The pancreas was harvested for histological analysis and snap-frozen for protein preparation.
Morphological examination.
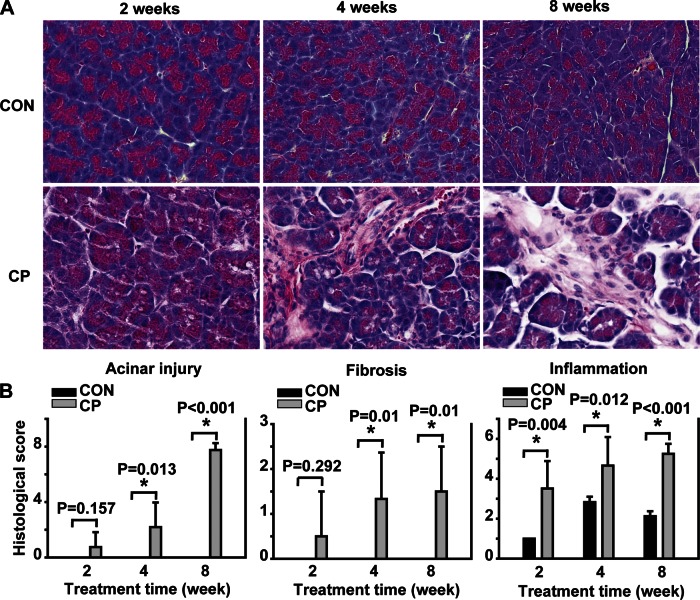
Pancreas tissues were fixed in 10% formalin and subsequently embedded in paraffin. Five-micrometer-thick sections were prepared for hematoxylin-eosin (H&E) staining (Vector Laboratories, Burlingame, CA). Histology scores were determined on H&E-stained sections by an experienced pathologist blinded to the sample identity. Histopathological changes of CP were scored according to the criteria proposed by Demols et al. (7) with minor modifications on the extent of acinar injury (including abnormal architecture, glandular atrophy, and pseudotubular complexes), fibrosis, and inflammation.
Immunohistochemistry analysis.
The pancreas sections (5 μm thick) were prepared for immunohistochemistry analysis of α-SMA with an ABC kit and DAB kit (Vector Laboratories) according to the manufacturer's instructions. For antigen retrieval, the sections were immersed in low-pH antigen unmasking solution (Vector Laboratories) diluted in distilled water (1:100) and heated in a steamer for 30 min after deparaffinization and hydration. To block endogenous peroxidase activity, the sections were treated with 0.3% hydrogen peroxide diluted in methanol. After being washed with PBS, the sections were treated for 30 min at room temperature with blocking serum provided in the ABC kit to block nonspecific protein binding sites. The sections were then incubated with a rabbit anti-human α-SMA monoclonal antibody (1:200 dilution in 3% BSA; Abcam, Cambridge, MA) overnight at 4°C followed by incubation with a biotinylated anti-rabbit antibody for 1 h at room temperature, and then with ABC regents for 30 min at room temperature. The sections were washed with 0.01% Tween 20 diluted in Tris-buffered saline (pH 7.4) between any two steps above. Finally, the sections were stained using the DAB kit followed by hemotoxylin nuclei counterstaining and dehydrated, mounted with a permanent mounting solution (Vector Laboratories), and covered with a cover glass.
Isolation and culture of mouse PSCs.
Mouse primary PSCs (mPSCs) were isolated from the pancreata of female Swiss Webster mice (8–10 wk old) by outgrowth after collagenase (Worthington Biochemical, Lakewood, NJ) digestion (32). The isolated mPSCs were seeded on six-well plates and cultured in Dulbecco's modified essential medium (DMEM; Mediatech, Manassas, VA) supplemented with 10% FBS and 1% penicillin/streptomycin (Life Technologies-Invitrogen, Grand Island, NY) at 37°C in a humidified incubator (containing 95% of O2 and 5% of CO2). Passages 1 to 3 were used.
Isolation and culture of human PSCs.
Following the guidelines of the University of Texas Medical Branch, the discarded human pancreatic tissue was obtained from surgical resection of a patient with CP undergoing pancreatectomy. Primary human PSCs (hPSCs) were isolated using an outgrowth method (2). Briefly, the human pancreatic tissue was rinsed three times in DMEM with 1% penicillin/streptomycin (Life Technologies-Invitrogen) in a 100-mm petri dish. The tissue was cut into blocks (1–2 mm3) and placed in 75-cm2 tissue culture flasks coated with 0.002% of collagen I (Sigma) and cultured in DMEM supplemented with 10% FBS, 1% insulin-transferrin-selenium-X (Life Technologies-GIBCO), 1% nonessential amino acid solution (Sigma), 50 μg/ml gentamycin solution (Life Technologies-GIBCO), and 1% penicillin/streptomycin at 37°C. The medium was changed every 3 days starting on day 3. As the colonies grew 80–90% confluent, the tissue clumps were removed, and cells were split using 0.025% trypsin/EDTA (Life Technologies-GIBCO). Passages 2 to 5 were used.
Oil red O staining.
hPSCs were cultured in eight-well glass slide chambers (BD Biosciences, Bedford, MA) for 24 h and then fixed with 4% paraformaldehyde for 15 min. Oil red O (Sigma) staining was performed to visualize lipid droplets in cells as described (9). Nuclei were counterstained with hematoxylin.
Immunofluorescence.
The PSCs were cultured in eight-well glass slide chambers for 24 h (for hPSC characterization) and further treated for 48 h (for the study of the effects of BMP2 and TGF-β). The cells were then fixed with 4% paraformaldehyde, permeabilized with 0.25% Triton X-100, and blocked with 3% BSA for 30 min at room temperature. The fixed cells were then incubated with the primary antibodies against α-SMA (Abcam), FN (Santa Cruz Biotechnology, Santa Cruz, CA), glial fibrillary acidic protein (GFAP), and vimentin (Sigma) at 4°C overnight followed by the fluorescence-labeled secondary antibodies Dylight 594 or Dylight 488 (Vector Laboratories) at room temperature for 1 h. Nuclei were counterstained with DAPI.
Western blot analysis.
Protein lysates were prepared from mouse pancreas tissue samples or cells in 1× lysis buffer (Cell Signaling Technology, Billerica, MA). Protein concentrations were measured using a protein assay dye (Bio-Rad Laboratories, Hercules, CA). Conditioned medium was collected after 48 h of treatment by BMP2 and TGF-β. Western blot analysis was performed as described previously (4, 16) using specific primary antibodies against BMP2 (R&D Systems), pSmad1 and Smad1 (Cell Signaling), FN and ColIa (Santa Cruz Biotechnology), and GAPDH (Sigma). Horseradish peroxidase-conjugated secondary antibody was from Bio-Rad Laboratories.
Smad1 gene silencing.
hPSCs were transiently transfected with human Smad1 small-interfering RNA (siRNA, 100 nM, L-012723-00; Dharmacon, Chicago, IL) using transfection reagent Dharma FECT 1 (Dharmacon) according to the manufacturer's instructions as previously described (4, 5). Scrambled siRNA (D-001206-13; Dharmacon) was used as a nonspecific siRNA control. After 48 h, cells were starved for 4 h followed by BMP2 pretreatment for 30 min and TGF-β treatment for 48 h.
Statistical analysis.
Data were expressed as means ± SE. P < 0.05 was considered significant. In vitro experiments were repeated two to three times, and similar results were obtained. Differences between two groups were analyzed using the Student's t-test. Differences among groups were analyzed using ANOVA with the Tukey-Kramer multiple-comparison test.
RESULTS
Activation of BMP signaling in cerulein-induced CP.
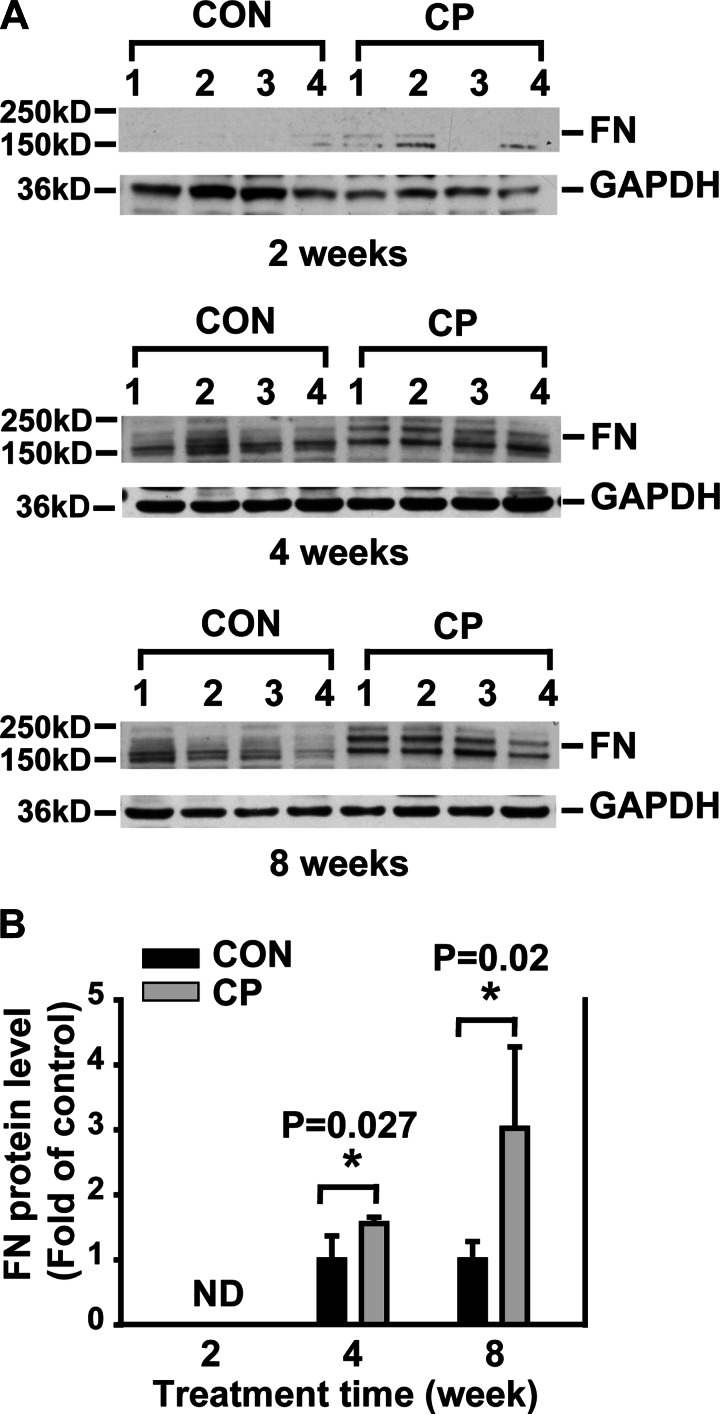
To begin to investigate the role of BMP signaling in CP, Swiss Webster mice were given cerulein by intraperitoneal injections up to 8 wk for the induction of CP, a well-established rodent CP model (15). The mouse pancreata were harvested at day 4 after 2-, 4-, and 8-wk cerulein injections for CP induction as described. The pancreata of the mice receiving cerulein revealed a morphological progression during CP induction with acinar injury, fibrosis, and inflammation on H&E staining compared with the control mouse pancreas (Fig. 1). The histopathological scores including all these changes were shown as a semiquantitative measurement of chronic pancreatic injury (Fig. 1B). To further evaluate ECM protein levels during fibrosis progression, protein lysates prepared from the mouse pancreata were subjected to Western blotting with a FN antibody. As shown in Fig. 2A, a time-dependent increase of FN (the band on top, 220 kDa) was observed in the pancreata from 2-, 4-, and 8-wk cerulein groups. To assess the activation of PSCs, immunohistochemistry analysis was carried out on the pancreas paraffin sections with an α-SMA antibody. α-SMA-positive cells were localized in the fibrotic areas around acini and vascular walls in cerulein groups and were significantly increased at 4 (P = 0.004) and 8 (P < 0.001) wk (Fig. 3). In control mice pancreata, a few α-SMA-positive cells were observed mostly in the vascular walls for all time points tested. Taken together, these results demonstrate that activated PSCs increased along with fibrosis progression.
Fig. 1.
Morphological changes in the mouse pancreas during chronic pancreatitis (CP) induction. A: representative hematoxylin and eosin (H&E)-stained pancreatic sections of control (CON) and CP mice after 2-, 4-, and 8-wk saline or cerulein injections. B: histological scores of acinar injury, fibrosis, and inflammation from H&E-stained pancreas sections. *P < 0.05 compared with control; n = 4 mice/group. Original magnification, ×400.
Fig. 2.
Time-dependent increase of fibronectin (FN) in CP mice. A: Western blotting of pancreas tissue lysates from CON and CP mice with anti-FN antibody. B: quantification of the Western blots. The protein levels were normalized against GAPDH and quantified as fold of control. ND, nondetectable. *P < 0.05 compared with control; n = 4 mice/group.
Fig. 3.
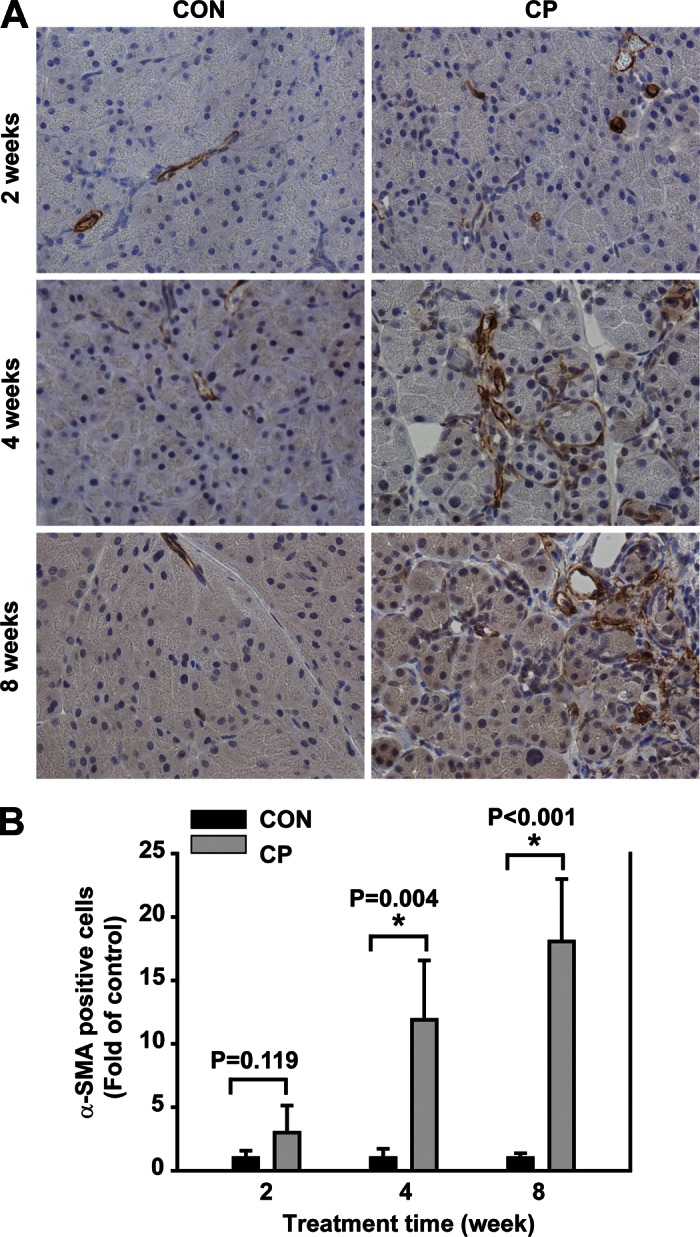
Time-dependent increase of α-smooth muscle actin (α-SMA) in CP mice. A: immunohistochemical staining of pancreas paraffin section from CON and CP mice using α-SMA antibody. α-SMA-positive cells [activated pancreatic stellate cells (PSCs)] are present in the periacinar fibrotic areas with the stellate morphology and vascular walls in CP specimens, but are present mostly in the vascular walls in CON specimens. B: α-SMA-positive cells in the periacinar areas were quantified based on 6–10 different fields/slide. *P < 0.05 compared with control; n = 4 mice/group. Original magnification, ×400.
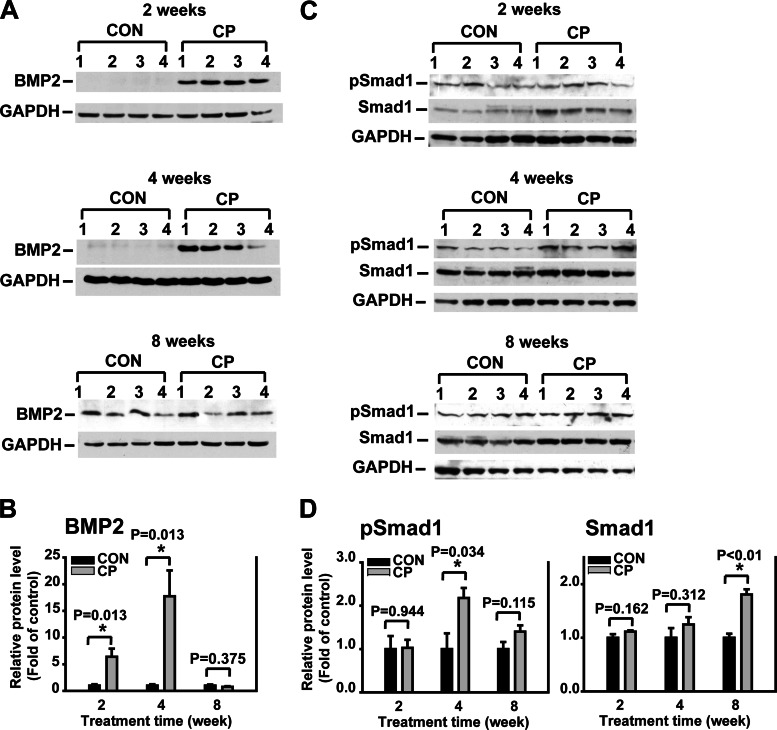
Furthermore, the protein levels of BMP2 and its intracellular signaling molecule Smad1 were assessed using Western blot analysis. BMP2 levels increased significantly in the pancreata of the CP mice at earlier time points of 2 and 4 wk after CP induction and declined at a later time point of 8 wk after CP induction to a similar level as the control. Similarly, pSmad1 levels increased significantly at 4 wk and declined at 8 wk after CP induction (Fig. 4). Thus, the dynamic changes of BMP signaling inversely correlate to the progression of pancreatic fibrosis during CP, suggesting a potential anti-fibrogenic role of BMP signaling in the pancreas. In addition, there was no difference of total Smad1 levels between CP and control at 2 and 4 wk, whereas there was a significant increase of total Smad1 levels in CP compared with control at 8 wk. The underlying mechanisms for this unexpected discrepancy between phospho-Smad1 and total Smad1 levels are not clear at this moment and deserve future investigation.
Fig. 4.
Bone morphogenetic protein (BMP) 2 and phosphorylated Smad1 (pSmad1) levels in the pancreas during progression of CP. A: Western blotting of pancreas tissue lysates from CON and CP mice using BMP2 antibodies. B: quantification of BMP2. The protein levels were normalized against GAPDH and quantified as fold of control. C: Western blotting of pancreas tissue lysates from CON and CP mice using pSmad1 and total Smad1 antibodies on the same blots. D: quantification of pSmad1 and total Smad1. The protein levels were normalized against GAPDH and quantified as fold of control. *P < 0.05 compared with control; n = 4 mice/group.
BMP2 inhibits TGF-β-induced mPSC activation and FN expression.
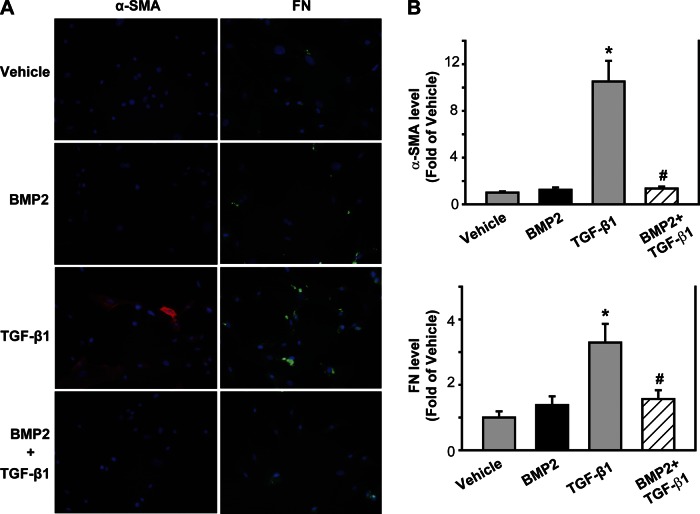
To evaluate the direct effect of BMP on PSC activation and ECM formation in vitro, mPSCs were isolated and pretreated with BMP2 (250 ng/ml) for 30 min followed by TGF-β treatment (1 ng/ml) for 48 h. Using immunofluorescence analysis with α-SMA and FN antibodies, we found that, compared with the vehicle control, TGF-β increased α-SMA and FN expression (P < 0.05); BMP2 alone had no effect on α-SMA and FN expression. However, BMP pretreatment inhibited TGF-β-induced α-SMA and FN expression compared with TGF-β alone (P < 0.05, Fig. 5).
Fig. 5.
BMP2 inhibits transforming growth factor (TGF)-β-induced mouse PSC (mPSC) activation and FN formation. mPSCs were pretreated with 250 ng/ml of BMP2 for 30 min followed by 1 ng/ml TGF-β for 48 h. A: immunofluorescence was performed using antibodies against α-SMA (red) and FN (green), and the cell nucleus was stained with DAPI (blue). B: quantification of fluorescence intensity from 5–10 different fields/sample. *P < 0.05 compared with vehicle group. #P < 0.05 compared with TGF-β group. Original magnification, ×200.
Characterization of hPSCs.
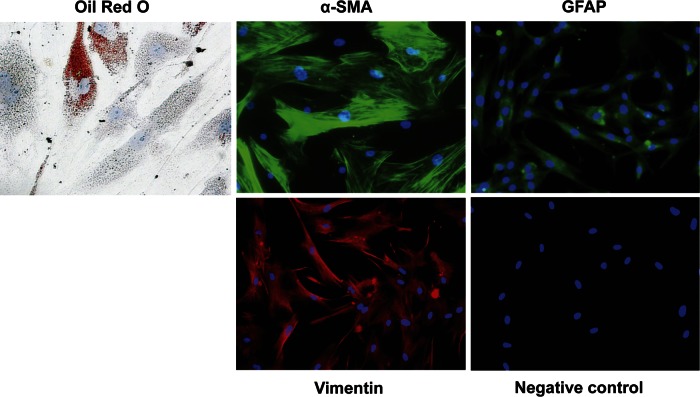
Considering hPSCs are more clinically relevant, primary hPSCs isolated from a CP patient were used to validate the findings in mouse PSCs. To characterize the hPSCs of passage 5, oil red O staining was used to identify quiescent PSCs, and immunofluorescence analysis was applied with antibodies against α-SMA and several other PSC markers, including GFAP and vimentin. These cells were 20.3 ± 1.1% oil red O positive, 87.1 ± 1.5% α-SMA positive, and 100% GFAP and vimentin positive (Fig. 6). These results indicate a high purity of hPSCs obtained.
Fig. 6.
Characterization of human PSCs (hPSCs). Lipid droplets were visualized with oil red O staining (original magnifications, ×400). Immunofluorescence was performed using antibodies against α-SMA, glial fibrillary acidic protein (GFAP), and vimentin (original magnification, ×200), and the cell nucleus was stained with DAPI (blue). Negative control, without primary antibodies.
BMP2 inhibits TGF-β-induced hPSC activation and ECM formation.
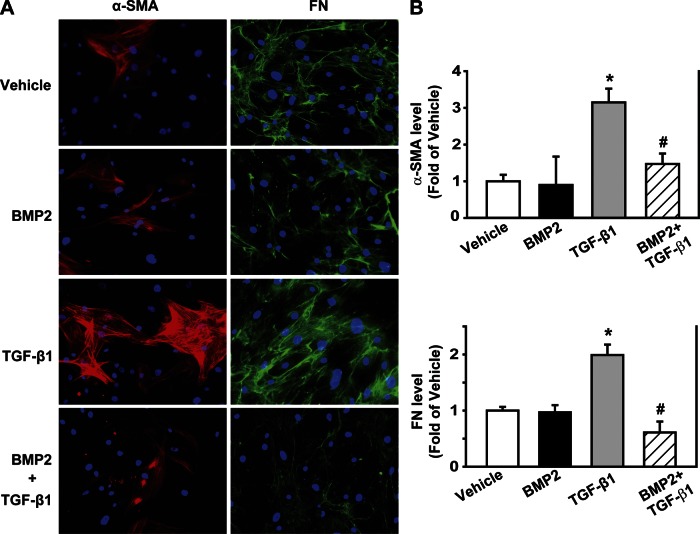
To validate the findings of the effect of BMP2 on mPSC activation and ECM formation induced by TGF-β, the isolated hPSCs were pretreated with BMP2 (250 ng/ml) for 30 min followed by TGF-β treatment (1 ng/ml) for 48 h. With the use of immunofluorescence analysis with α-SMA and FN antibodies, similar findings were observed as in mPSCs. Compared with the vehicle control, TGF-β increased α-SMA and FN expression (P < 0.05); BMP2 alone had no effect on α-SMA and FN expression. However, BMP2 pretreatment suppressed TGF-β-induced α-SMA and FN expression compared with TGF-β alone (P < 0.05, Fig. 7).
Fig. 7.
BMP2 inhibits TGF-β-induced α-SMA and FN expression in hPSCs. hPSCs were pretreated with BMP2 (250 ng/ml) for 30 min followed by TGF-β (1 ng/ml) for 48 h. A: immunofluorescence was performed using antibodies against α-SMA (red) and FN (green), and the cell nucleus was stained with DAPI (blue). B: quantification of fluorescent intensity from 5–10 different fields/sample. *P < 0.05 compared with vehicle group. #P < 0.05 compared with TGF-β group. Original magnifications, ×200.
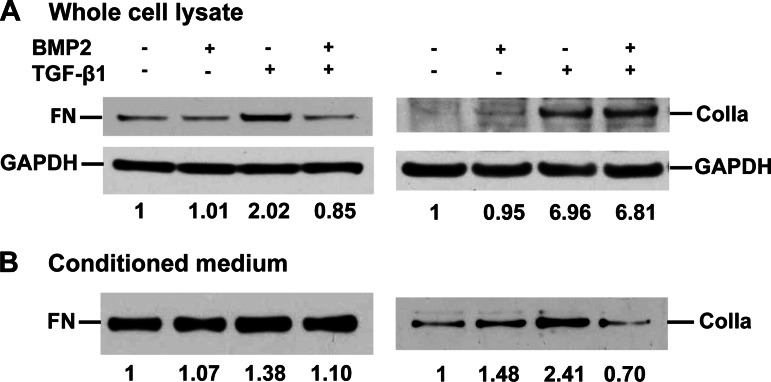
Furthermore, Western blot analysis from the whole cell lysates revealed a 2.02-fold increase of FN induced by TGF-β treatment, no change of FN with BMP2 alone, and a complete suppression of TGF-β-induced FN by BMP2 pretreatment (0.85-fold, Fig. 8A) compared with the vehicle control. Similar effects were observed in FN levels from the conditioned medium (Fig. 8B), although not as brisk as in whole cell lysates. However, a different pattern was observed for ColIa levels. BMP2 did not suppress TGF-β-induced ColIa expression in whole cell lysates (Fig. 8A) but abolished TGF-β-induced ColIa secretion in the conditioned medium (Fig. 8B). As reported in the literature, TGF-β, a key profibrogenic factor in the pancreas, activates PSCs and induces collagen synthesis and secretion and other ECM formation, including FN (1, 23). This is also observed in our current study shown in Fig. 8. However, in whole cell lysates, BMP2 inhibited FN expression, but not collagen expression (Fig. 8A). Hence, the data of FN and collagen levels in conditioned medium (Fig. 8B) demonstrate that BMP2 inhibited both FN and collagen secretion. Our results suggest that BMP2 inhibits TGF-β-induced FN synthesis and secretion but predominantly inhibits TGF-β-induced collagen secretion and may not affect its synthesis. Future experimentation is warranted to investigate how BMP2 regulates TGF-β-induced ECM formation and its biochemical processes. Taken together, we have confirmed the findings from mouse PSCs that BMP2 suppressed TGF-β-induced PSC activation and ECM formation in human PSCs.
Fig. 8.
BMP2 inhibits TGF-β-induced ECM expression and secretion by hPSCs. hPSCs were pretreated with BMP2 (250 ng/ml) for 30 min followed by TGF-β (1 ng/ml) for 48 h, and then both medium and whole cell lysates were harvested. A: Western blotting of whole cell lysate using anti-FN or collagen type Ia (ColIa) antibody. B: Western blotting of equal volume of the conditioned medium using FN or ColIa antibodies. Specific bands were normalized to total cell protein and quantified as fold of vehicle.
Smad1 signaling pathway mediates the anti-fibrogenic function of BMP2 in hPSCs.
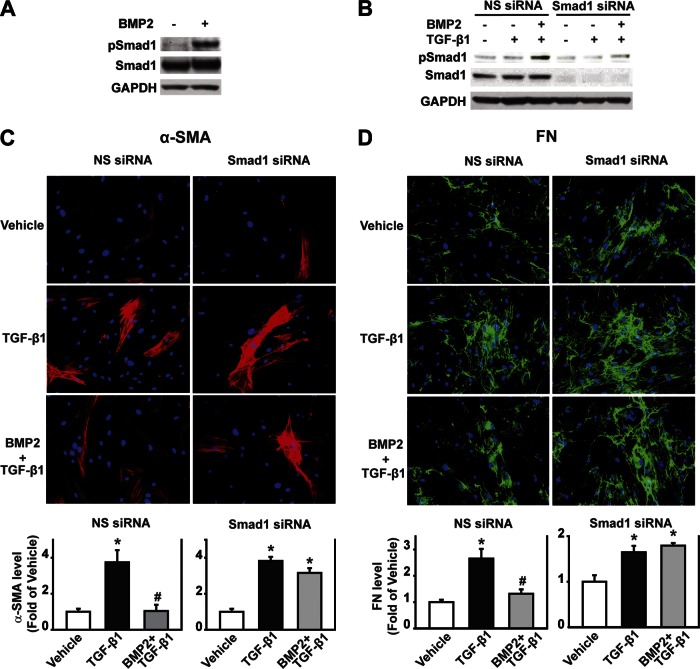
To assess Smad1 signaling in the anti-fibrogenic function of BMP2, hPSCs were treated with BMP2 for 30 min, and pSmad1 and total Smad1 levels were measured by Western blot analysis. In the presence of BMP2, the pSmad1 level increased 5.0-fold compared with the vehicle control, whereas the total Smad1 level did not change (Fig. 9A), demonstrating an activation of BMP signaling in hPSCs upon BMP2 stimulation. Furthermore, Smad1 was silenced transiently by transfecting Smad1 siRNA into hPSCs. At 48 h after transfection, the cells were starved for 4 h and then pretreated with BMP2 (250 ng/ml) at 30 min followed by TGF-β treatment (1 ng/ml) for 30 min for Western blot analysis of Smad1 signaling (Fig. 9B), and for 48 h for immunofluorescence analysis of ECM expression (Fig. 9, C and D). As expected, Smad1 siRNA transfection knocked down Smad1 expression by 86% and decreased BMP2-induced phosphorylation of Smad1 by 62%. This demonstrates the specific gene knockdown effect on Smad1 expression by Smad1 siRNA transfection, which resulted in a decreased phosphorylation of Smad1 (Fig. 9B). Furthermore, immunofluorescence analysis demonstrates that Smad1 siRNA transfection did not affect TGF-β-induced α-SMA and FN expression but reversed the inhibitory effect of BMP2 on TGF-β-induced α-SMA and FN expression (Fig. 9, C and D). These results demonstrate that the anti-fibrogenic function of BMP2 is mediated by the Smad1 signaling pathway.
Fig. 9.
Smad1 signaling pathway mediates the inhibitory effect of BMP2 on TGF-β-induced hPSC activation and FN production. A: hPSCs were treated with BMP2 for 30 min. Whole cell lysates were subject to Western blotting using pSmad1 and total Smad1 antibody on the same blot. B: Smad1 small-interfering RNA (siRNA) transfection efficiency. hPSCs were transfected with human Smad1 siRNA for 48 h, starved for 4 h, and then pretreated with BMP2 (250 ng/ml) for 30 min followed by TGF-β (1 ng/ml) for 30 min. Whole cell lysates were subject to Western blotting using Smad1 and pSmad1 antibodies on the same blot. C and D: hPSCs were transfected with human Smad1 siRNA for 48 h, starved for 4 h, and then pretreated with BMP2 (250 ng/ml) for 30 min followed by TGF-β (1 ng/ml) for 48 h. Immunofluorescence was performed using α-SMA (red) antibody and the respective quantification (C) or using FN (green) antibody and the respective quantification (D). Cell nucleus was stained with DAPI (blue). NS siRNA, nonspecific siRNA. *P < 0.05 compared with vehicle group. #P < 0.05 compared with TGF-β group. Original magnification, ×200.
DISCUSSION
Pancreatic fibrosis is the common pathological change of CP. Our results from a mouse experimental model show that repetitive administration of cerulein induced CP with gradually aggravated acinar injury, fibrosis lesion, and increased FN levels. Along with fibrosis progression, activated PSCs increased and appeared in the periacinar fibrosis area. Interestingly, we observed dynamic changes of BMP2 and pSmad1 levels that were inversely associated with fibrosis development, suggesting an inhibitory role of BMP signaling in fibrosis progression. Using isolated mouse and human PSCs, we showed that BMP2 inhibited TGF-β-induced PSC activation and ECM formation. Furthermore, knocking down of Smad1 reversed the effect of BMP2, indicating that Smad1 mediates the inhibitory effect of BMP2 on TGF-β-induced PSC activation and ECM formation. Thus, our study reveals the anti-fibrogenic role of BMP signaling in the pancreas.
BMPs play important roles during pancreas development by controlling cellular differentiation, proliferation, and apoptosis (29), and in pancreatic endocrine functions (8). Most recently, we show that BMP signaling is activated in acute pancreatitis, and noggin (a BMP antagonist) attenuates cerulein-induced acute pancreatitis, suggesting a promoting role of BMP signaling in acute pancreatitis induction (6). However, the role of BMPs in the course of pancreatic fibrosis development during CP is largely unknown although BMPs are reported to be anti-fibrogenic in the kidney, lung, and liver (10, 14, 28, 31). To explore the potential anti-fibrogenic role of BMPs in the pancreas, we examined BMP signaling during cerulein-induced CP. We observed dynamic changes of BMP signaling. BMP2 levels increased at 2- and 4-wk cerulein injections along with increased pSmad1 levels at 4-wk cerulein injections. Both BMP2 and pSmad1 levels then declined back to the similar levels as saline control (Fig. 4). Thus BMP signaling changes inversely correlate to the fibrosis development (Fig. 1) and PSC activation (Fig. 3), suggesting a potential inhibitory role of BMP signaling in fibrosis development. Based on these in vivo findings, we focused on PSCs, the key cells for ECM formation in the development of pancreatic fibrosis, and directly tested the effect of BMP on TGF-β-induced PSC activation and ECM formation in vitro. We found that BMP2 pretreatment caused a reduction of α-SMA and FN expression with TGF-β treatment in mouse PSCs (Fig. 5), as well as in human PSCs (Fig. 7). The current study, to our knowledge, provides the first evidence on the anti-fibrogenic role of BMPs in the pancreas by examining BMP signaling during CP induction in vivo and the effect of BMP on TGF-β-induced PSC activation and ECM formation in vitro. It is known that TGF-β and BMP signaling pathways interact to involve in multiple biological processes and diseases and are subject to complex levels of regulation. Therefore, future experimentation using more sophisticated models is necessary for delineating the anti-fibrogenic role of BMPs in the pancreas.
TGF-β is known as a profibrogenic factor in the pancreas by activation of PSCs, the key cell type in the development of pancreatic fibrosis (20, 24, 30). Systemic blockade of TGF-β may induce unexpected results, since TGF-β has a broad spectrum of biological functions. Therefore, exploring approaches to desensitize the cellular responses to TGF-β or to antagonize the TGF-β signal transduction pathway has become a heavily investigated field (28). Thus, further exploring BMP signaling in vivo as native approaches to selectively antagonize TGF-β signaling becomes critically important in facilitating the development of therapeutic strategies to pancreatic fibrosis.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant P01-DK-035608 and National Science Foundation Grants NSFC30871189 and NSFC81171841.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: X.G., Y.C., W.Y., C.D., J.F.A., C.R., C.C., M.R.H., and T.C.K. conception and design of research; X.G., Y.C., W.Y., C.D., J.F.A., C.R., C.C., M.R.H., and T.C.K. interpreted results of experiments; X.G. and Y.C. drafted manuscript; Y.C., W.Y., C.D., and J.F.A. edited and revised manuscript; Y.C. and T.C.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank K. L. Ives in the Dept. of Surgery, UTMB for technical support on human PSC culture, G. He in the Dept. of Surgery, UTHSC-H for technical support on animal study, Dr. X. Deng in the Dept. of Surgery, UTHSC-H for critical reading of the manuscript, D. A. Staloch for the manuscript editing, E. Figueroa and S. Schuenke in the Dept. of Surgery, UTMB, for the manuscript and figure preparation and submission.
REFERENCES
- 1. Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut 43: 128–133, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grunert A, Adler G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 115: 421–432, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Bachem MG, Schunemann M, Ramadani M, Siech M, Beger H, Buck A, Zhou S, Schmid-Kotsas A, Adler G. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology 128: 907–921, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Cao Y, Chen L, Zhang W, Liu Y, Papaconstantinou HT, Bush CR, Townsend CM, Jr, Thompson EA, Ko TC. Identification of apoptotic genes mediating TGF-beta/Smad3-induced cell death in intestinal epithelial cells using a genomic approach. Am J Physiol Gastrointest Liver Physiol 292: G28–G38, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Cao Y, Liu X, Zhang W, Deng X, Zhang H, Liu Y, Chen L, Thompson EA, Townsend CM, Jr, Ko TC. TGF-beta repression of Id2 induces apoptosis in gut epithelial cells. Oncogene 28: 1089–1098, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cao Y, Yang W, Tyler MA, Gao X, Duan CJ, Kim SO, Aronson JF, Popov V, Takahashi H, Saito H, Evers BM, Chao C, Hellmich MR, Ko TC. Noggin attenuates cerulein-induced acute pancreatitis and the impaired autophagy. Pancreas In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Demols A, Van Laethem JL, Quertinmont E, Degraef C, Delhaye M, Geerts A, Deviere J. Endogenous interleukin-10 modulates fibrosis and regeneration in experimental chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol 282: G1105–G1112, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Goulley J, Dahl U, Baeza N, Mishina Y, Edlund H. BMP4-BMPR1A signaling in beta cells is required for and augments glucose-stimulated insulin secretion. Cell Metab 5: 207–219, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Kim N, Yoo W, Lee J, Kim H, Lee H, Kim YS, Kim DU, Oh J. Formation of vitamin A lipid droplets in pancreatic stellate cells requires albumin. Gut 58: 1382–1390, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Kinoshita K, Iimuro Y, Otogawa K, Saika S, Inagaki Y, Nakajima Y, Kawada N, Fujimoto J, Friedman SL, Ikeda K. Adenovirus-mediated expression of BMP-7 suppresses the development of liver fibrosis in rats. Gut 56: 706–714, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kordes C, Brookmann S, Haussinger D, Klonowski-Stumpe H. Differential and synergistic effects of platelet-derived growth factor-BB and transforming growth factor-beta1 on activated pancreatic stellate cells. Pancreas 31: 156–167, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Luttenberger T, Schmid-Kotsas A, Menke A, Siech M, Beger H, Adler G, Grunert A, Bachem MG. Platelet-derived growth factors stimulate proliferation and extracellular matrix synthesis of pancreatic stellate cells: implications in pathogenesis of pancreas fibrosis. Lab Invest 80: 47–55, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Mews P, Phillips P, Fahmy R, Korsten M, Pirola R, Wilson J, Apte M. Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut 50: 535–541, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Myllarniemi M, Lindholm P, Ryynanen MJ, Kliment CR, Salmenkivi K, Keski-Oja J, Kinnula VL, Oury TD, Koli K. Gremlin-mediated decrease in bone morphogenetic protein signaling promotes pulmonary fibrosis. Am J Respir Crit Care Med 177: 321–329, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Neuschwander-Tetri BA, Bridle KR, Wells LD, Marcu M, Ramm GA. Repetitive acute pancreatic injury in the mouse induces procollagen alpha1(I) expression colocalized to pancreatic stellate cells. Lab Invest 80: 143–150, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Nguyen KA, Cao Y, Chen JR, Townsend CM, Jr, Ko TC. Dietary fiber enhances a tumor suppressor signaling pathway in the gut. Ann Surg 243: 619–627, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest 117: 50–59, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schmid-Kotsas A, Gross HJ, Menke A, Weidenbach H, Adler G, Siech M, Beger H, Grunert A, Bachem MG. Lipopolysaccharide-activated macrophages stimulate the synthesis of collagen type I and C-fibronectin in cultured pancreatic stellate cells. Am J Pathol 155: 1749–1758, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schneider E, Schmid-Kotsas A, Zhao J, Weidenbach H, Schmid RM, Menke A, Adler G, Waltenberger J, Grunert A, Bachem MG. Identification of mediators stimulating proliferation and matrix synthesis of rat pancreatic stellate cells. Am J Physiol Cell Physiol 281: C532–C543, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Shek FW, Benyon RC, Walker FM, McCrudden PR, Pender SL, Williams EJ, Johnson PA, Johnson CD, Bateman AC, Fine DR, Iredale JP. Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am J Pathol 160: 1787–1798, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shlyonsky V, Soussia IB, Naeije R, Mies F. Opposing effects of bone morphogenetic protein-2 and endothelin-1 on lung fibroblast chloride currents. Am J Respir Cell Mol Biol 45: 1154–1160, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Siech M, Zhou Z, Zhou S, Bair B, Alt A, Hamm S, Gross H, Mayer J, Beger HG, Tian X, Kornmann M, Bachem MG. Stimulation of stellate cells by injured acinar cells: a model of acute pancreatitis induced by alcohol and fat (VLDL). Am J Physiol Gastrointest Liver Physiol 297: G1163–G1171, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Sparmann G, Hohenadl C, Tornoe J, Jaster R, Fitzner B, Koczan D, Thiesen HJ, Glass A, Winder D, Liebe S, Emmrich J. Generation and characterization of immortalized rat pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol 287: G211–G219, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Vogelmann R, Ruf D, Wagner M, Adler G, Menke A. Effects of fibrogenic mediators on the development of pancreatic fibrosis in a TGF-beta1 transgenic mouse model. Am J Physiol Gastrointest Liver Physiol 280: G164–G172, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Wagner DO, Sieber C, Bhushan R, Borgermann JH, Graf D, Knaus P. BMPs: from bone to body morphogenetic proteins. Sci Signal 3: mr1, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Whitcomb DC. Inflammation and Cancer V. Chronic pancreatitis and pancreatic cancer. Am J Physiol Gastrointest Liver Physiol 287: G315–G319, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Yang YL, Ju HZ, Liu SF, Lee TC, Shih YW, Chuang LY, Guh JY, Yang YY, Liao TN, Hung TJ, Hung MY. BMP-2 suppresses renal interstitial fibrosis by regulating epithelial-mesenchymal transition. J Cell Biochem 112: 2558–2565, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Yang YL, Liu YS, Chuang LY, Guh JY, Lee TC, Liao TN, Hung MY, Chiang TA. Bone morphogenetic protein-2 antagonizes renal interstitial fibrosis by promoting catabolism of type I transforming growth factor-beta receptors. Endocrinology 150: 727–740, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Ye L, Lewis-Russell JM, Kyanaston HG, Jiang WG. Bone morphogenetic proteins and their receptor signaling in prostate cancer. Histol Histopathol 22: 1129–1147, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Yoo BM, Yeo M, Oh TY, Choi JH, Kim WW, Kim JH, Cho SW, Kim SJ, Hahm KB. Amelioration of pancreatic fibrosis in mice with defective TGF-beta signaling. Pancreas 30: e71–e79, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 9: 964–968, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Zion O, Genin O, Kawada N, Yoshizato K, Roffe S, Nagler A, Iovanna JL, Halevy O, Pines M. Inhibition of transforming growth factor beta signaling by halofuginone as a modality for pancreas fibrosis prevention. Pancreas 38: 427–435, 2009 [DOI] [PubMed] [Google Scholar]