in a recent issue of this journal, Zemans and colleagues (13) reported a role for β-catenin and two of the CCN matricellular proteins in experimental inflammatory lung injury. Cell culture experiments and in vivo mouse models were used to demonstrate that signaling through the p300/β-catenin axis in lung epithelial cells promotes repair following neutrophil-dependent disruption of the epithelial barrier. The studies further delineated that this protective effect is mediated through two CCN matricellular proteins: cysteine-rich angiogenic inducer 61 (Cyr61) and Wnt-inducible signaling pathway protein 1 (WISP1). These studies, built upon previous findings by this group (12), provide a strong argument for the importance of the β-catenin pathway in lung epithelial cell repair processes.
β-Catenin has two prominent roles in the cell: one as a transcriptional regulator, the other as a component in the adherens junction. Under quiescent conditions, cytosolic β-catenin is phosphorylated and targeted for proteasome-mediated degradation. However, after Wnt binding to the cell surface receptor (Frizzled) or release of β-catenin from the adherens junction, β-catenin levels increase in the cytosol, allowing transfer to the nucleus. Nuclear β-catenin interacts with lymphoid enhancer factor-1/T-cell factor (LEF-1/TCF) and transcriptional coactivator proteins, including p300 or cAMP-response element binding protein (CBP), to regulate gene transcription (2, 11, 13). Pertinent to these studies, β-catenin-dependent effects can differ based on binding to p300 vs. CBP (2).
Previously recognized for its prominent role in lung development (9) and progenitor cell differentiation (10), the β-catenin pathway has been increasingly recognized for its roles in response to injury and the subsequent repair. As such, it has become a point of interest as to whether targeting the β-catenin pathway might be an effective strategy for enhancing lung repair following injury. To date, the results are mixed, which likely can be explained by several factors, including 1) the β-catenin pathway forms complex interactions with other signaling pathways, 2) different types of injury may lead to different outcomes, and 3) transcriptional regulatory complexes containing p300/β-catenin vs. CBP/β-catenin, the composition of which is based on cell type and activation state, mediate different patterns of gene expression.
In previous studies (12), Zemans and colleagues noted that neutrophil migration through a lung epithelial cell layer induced injury and resulted in epithelial cell nuclear translocation of β-catenin and upregulation of β-catenin-dependent transcription. Furthermore, knockdown of β-catenin by shRNA following neutrophil-mediated injury delayed epithelial cell repair. In vivo, the authors used the p300/β-catenin inhibitor IQ-1 and observed that this agent inhibited alveolar epithelial cell (AEC) proliferation following intratracheal keratinocyte chemokine (KC) administration but did not affect neutrophil influx into the alveolar space. In the recent article (13), they took a different approach by evaluating in vitro and in vivo models after promoting p300/β-catenin-dependent signaling with the compound ICG-001. ICG-001 is known to inhibit the CBP/β-catenin interaction, which then favors p300/β-catenin-dependent effects (2). In vitro, ICG-001 accelerated epithelial repair after neutrophil transmigration across an epithelial monolayer and in the scratch assay (13). Furthermore, ICG-001 reduced lung permeability in mice treated with intratracheal KC. Neutrophil elastase was found to cleave E-cadherin, which likely functions to release β-catenin into the cytosol and activate downstream events. Thus the primary injury itself appears to set into motion mechanisms to orchestrate repair (12, 13). Taken together, these results suggest that signaling through p300/β-catenin promotes epithelial repair processes.
Results from the present study complement other in vitro studies reporting that lung epithelial cell wound healing is dependent on the β-catenin axis (1, 11). In addition, recent investigations by our group demonstrated that deletion of β-catenin in the alveolar epithelium of the adult mouse increased AEC death and enhanced lung fibrosis following intratracheal bleomycin (11). In 2010, Henderson and colleagues (2) reported that ICG-001 diminished bleomycin induced lung fibrosis, an observation that likely reflects favorable effects of not only inhibiting CBP/β-catenin, but also of maintaining signaling through p300/β-catenin. Despite these complementary studies, Kim and colleagues (5) recently reported that knockdown of β-catenin in the lung using intratracheal administration of siRNA decreased bleomycin-induced lung fibrosis. Since β-catenin pathway activation may promote different effects in different cell populations, activation of β-catenin in nonepithelial cell types, including lung fibroblasts, could inhibit lung repair and worsen fibrosis (7). Thus β-catenin-dependent effects likely must strike a fine balance across different cell populations to help orchestrate normal lung repair following injury.
Zemans and colleagues (13) performed studies that indicated that two CCN proteins that are regulated by p300/β-catenin, WISP1 and Cyr61, are involved in epithelial cell repair. The CCN family consists of six homologous cysteine-rich proteins, with the acronym CCN derived from the first three members of the family: Cyr61, or CCN1; connective tissue growth factor (CTGF), or CCN2; and nephroblastoma overexpressed (NOV), or CCN3. WISP1, WISP2, and WISP3 comprise CCN4, CCN5, and CCN6, respectively (4). CCNs are involved in diverse functions, many of which are dependent on their interactions with integrins. Notably, the roles of CCN proteins in cell adhesion and spreading likely reflect the wound healing characteristics noted in this study. However, like β-catenin, many CCN-dependent effects are cell or situation specific, making generalizable predictions for the roles these proteins play as a class difficult.
In these investigations (13) and their prior study (12), Zemans and colleagues observed that WISP1 and Cyr61 were upregulated downstream of p300/β-catenin in their neutrophil mediated epithelial cell injury model. In fact, ICG-001 increased both WISP1 and Cyr61 expression in epithelial cells adjacent to the wounds. These facts, combined with the observation that recombinant WISP1 and Cyr61 also promoted epithelial wound healing, suggest that the beneficial effects of the p300/β-catenin axis are at least in part mediated through these two CCN proteins. Considering that ICG-001 also had favorable effects on lung injury in vivo combined with the observation that intratracheal LPS induces WISP1 expression in lung tissue, it is likely that this p300/β-catenin–CCN relationship is important to wound healing in vivo.
Taken together, these investigations provide a compelling argument for a protective role for CCN proteins, specifically Cyr61 and WISP1, in lung epithelial injury. However, as noted with the diverse reports concerning β-catenin and wound healing, similar discrepancies are noted with evaluations of the CCN proteins. In contrast to these present studies in which recombinant WISP1 improved epithelial wound healing following neutrophil migration injury (13), Li and colleagues (8) reported that recombinant WISP1 enhanced alveolar-capillary permeability in ventilator-induced lung injury in mice, whereas anti-WISP1 antibodies reduced permeability. Furthermore, Konigshoff and colleagues (6) demonstrated that administration of anti-WISP1 to mice attenuated bleomycin-induced lung fibrosis and improved survival. Although Cyr61 has not been analyzed as thoroughly in vivo, a study by Jin and colleagues (3) demonstrated a protective role in hyperoxia-mediated cell death in pulmonary epithelial cell lines.
In summary, this work by Zemans and colleagues provides several important insights into mechanisms of repair following injury to the lung epithelium, namely that 1) neutrophil elastase cleavage of E-cadherin is likely the initial step that sets into motion the subsequent β-catenin-dependent healing effects; 2) the p300/β-catenin axis appears to drive epithelial repair mechanisms; and 3) these repair mechanisms are likely mediated through the CCN proteins WISP1 and Cyr61 (Fig. 1). Taken together, their work provides an important contribution to the respiratory community's search for effective therapies to limit lung injury and enhance lung repair.
Fig. 1.
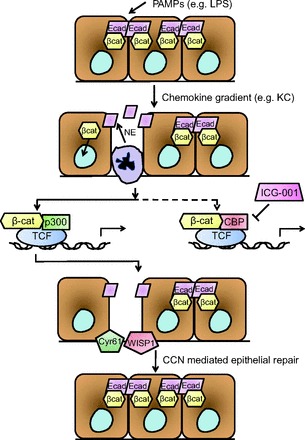
Proposed schematic for events involved in p300/β-catenin- and CCN-dependent alveolar epithelial repair. Pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharide (LPS) create a chemokine gradient, including keratinocyte chemokine (KC), that recruits neutrophils to migrate through the alveolar epithelial layer, leading to neutrophil elastase (NE)-dependent cleavage of E-cadherin (ECad) and release of β-catenin (βcat) into the cytoplasm. With nuclear translocation, β-catenin can then bind to lymphoid enhancer factor-1/T-cell factor (LEF-1/TCF) and other cofactors. p300/β-catenin-dependent transcription results in expression of targets promoting epithelial repair, a process which is promoted further when binding of β-catenin to cAMP-response element binding protein (CBP) is inhibited (for example, by the small molecule ICG-001). Among the many downstream targets of the p300/β-catenin axis, the CCN proteins Wnt-inducible signaling pathway protein 1 (WISP1) and cysteine-rich angiogenic inducer 61 (Cyr61) are expressed by epithelial cells in areas of injury, promoting repair of the alveolar epithelial cell layer.
GRANTS
This work was supported by NIH NHBLI HL105479 (W. E. Lawson), HL85317 (T. S. Blackwell), HL92870 (T. S. Blackwell), and the Department of Veterans Affairs (T. S. Blackwell and W. E. Lawson).
DISCLOSURES
Both authors confirm that they have no competing interests regarding the content, investigations, and results outlined in this manuscript.
AUTHOR CONTRIBUTIONS
W.E.L. and T.S.B. conception and design of research; W.E.L. and T.S.B. prepared figures; W.E.L. and T.S.B. drafted manuscript; W.E.L. and T.S.B. edited and revised manuscript; W.E.L. and T.S.B. approved final version of manuscript.
REFERENCES
- 1. Flozak AS, Lam AP, Russell S, Jain M, Peled ON, Sheppard KA, Beri R, Mutlu GM, Budinger GR, Gottardi CJ. Beta-catenin/T-cell factor signaling is activated during lung injury and promotes the survival and migration of alveolar epithelial cells. J Biol Chem 285: 3157–3167, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Henderson WR, Jr, Chi EY, Ye X, Nguyen C, Tien YT, Zhou B, Borok Z, Knight DA Kahn M. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci USA 107: 14309–14314, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jin Y, Kim HP, Ifedigbo E, Lau LF, Choi AM. Cyr61 protects against hyperoxia-induced cell death via Akt pathway in pulmonary epithelial cells. Am J Respir Cell Mol Biol 33: 297–302, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov 10: 945–963, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim TH, Kim SH, Seo JY, Chung H, Kwak HJ, Lee SK, Yoon HJ, Shin DH, Park SS, Sohn JW. Blockade of the Wnt/beta-catenin pathway attenuates bleomycin-induced pulmonary fibrosis. Tohoku J Exp Med 223: 45–54, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Konigshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, Rose F, Fink L, Seeger W, Schaefer L, Gunther A, Eickelberg O. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest 119: 772–787, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lam AP, Flozak AS, Russell S, Wei J, Jain M, Mutlu GM, Budinger GR, Feghali-Bostwick CA, Varga J, Gottardi CJ. Nuclear beta-catenin is increased in systemic sclerosis pulmonary fibrosis and promotes lung fibroblast migration and proliferation. Am J Respir Cell Mol Biol 45: 915–922, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li HH, Li Q, Liu P, Liu Y, Li J, Wasserloos K, Chao W, You M, Oury TD, Chhinder S, Hackam DJ, Billiar TR, Leikauf GD, Pitt BR, Zhang LM. WNT1-inducible signaling pathway protein 1 contributes to ventilator-induced lung injury. Am J Respir Cell Mol Biol 47: 528–535, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mucenski ML, Nation JM, Thitoff AR, Besnard V, Xu Y, Wert SE, Harada N, Taketo MM, Stahlman MT, Whitsett JA. Beta-catenin regulates differentiation of respiratory epithelial cells in vivo. Am J Physiol Lung Cell Mol Physiol 289: L971–L979, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Smith MK, Koch PJ, Reynolds SD. Direct and indirect roles for β-catenin in facultative basal progenitor cell differentiation. Am J Physiol Lung Cell Mol Physiol 302: L580–L594, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tanjore H, Degryse AL, Crossno PF, Xu XC, McConaha ME, Jones BR, Polosukhin VV, Bryant AJ, Cheng DS, Newcomb DC, McMahon FB, Gleaves LA, Blackwell TS, Lawson WE. β-Catenin in the alveolar epithelium protects from lung fibrosis following intratracheal bleomycin. Am J Respir Crit Care Med 187: 630–637, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zemans RL, Briones N, Campbell M, McClendon J, Young SK, Suzuki T, Yang IV, De Langhe S, Reynolds SD, Mason RJ, Kahn M, Henson PM, Colgan SP, Downey GP. Neutrophil transmigration triggers repair of the lung epithelium via beta-catenin signaling. Proc Natl Acad Sci USA 108: 15990–15995, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zemans RL, McClendon J, Aschner Y, Briones N, Young SK, Lau LF, Kahn M, Downey GP. Role of β-catenin-regulated CCN matricellular proteins in epithelial repair after inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol 304: L415–L427, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]


