Abstract
Elevated reactive oxygen species are implicated in pulmonary hypertension (PH). Superoxide dismutase (SOD) limits superoxide bioavailability, and decreased SOD activity is associated with PH. A decrease in SOD activity is expected to increase superoxide and reduce hydrogen peroxide levels. Such an imbalance of superoxide/hydrogen peroxide has been implicated as a mediator of nuclear factor of activated T cells (NFAT) activation in epidermal cells. We have shown that NFATc3 is required for chronic hypoxia-induced PH. However, it is unknown whether NFATc3 is activated in the pulmonary circulation in a mouse model of decreased SOD1 activity and whether this leads to PH. Therefore, we hypothesized that an elevated pulmonary arterial superoxide/hydrogen peroxide ratio activates NFATc3, leading to PH. We found that SOD1 knockout (KO) mice have elevated pulmonary arterial wall superoxide and decreased hydrogen peroxide levels compared with wild-type (WT) littermates. Right ventricular systolic pressure (RVSP) was elevated in SOD1 KO and was associated with pulmonary arterial remodeling. Vasoreactivity to endothelin-1 was also greater in SOD1 KO vs. WT mice. NFAT activity and NFATc3 nuclear localization were increased in pulmonary arteries from SOD1 KO vs. WT mice. Administration of A-285222 (selective NFAT inhibitor) decreased RVSP, arterial wall thickness, vasoreactivity, and NFAT activity in SOD1 KO mice to WT levels. The SOD mimetic, tempol, also reduced NFAT activity, NFATc3 nuclear localization, and RVSP to WT levels. These findings suggest that an elevated superoxide/hydrogen peroxide ratio activates NFAT in pulmonary arteries, which induces vascular remodeling and increases vascular reactivity leading to PH.
Keywords: pulmonary hypertension, superoxide, hydrogen peroxide, nuclear factor of activated T cells c3, vasoreactivity, endothelin-1, pulmonary arterial remodeling, chronic hypoxia
pulmonary hypertension (PH) is a progressive and often fatal disease (16). Reactive oxygen species (ROS) have been implicated in mediating PH (29); however, the mechanisms by which ROS contribute to this response are not fully understood. ROS include, but are not limited to, superoxide anion and hydrogen peroxide. Increases in lung superoxide levels have been demonstrated in several animal models of PH, including chronic hypoxia (CH) (11, 71), monocrotaline (74), and the transgenic Ren2 rat, which overexpresses the mouse renin gene (30). Additionally, our group has demonstrated that increased superoxide levels are implicated in CH-induced pulmonary vasoconstriction in rats (11, 49, 71). However, conflicting findings are reported regarding the levels of hydrogen peroxide in PH. Several reports suggest that elevated pulmonary arterial hydrogen peroxide levels contribute to the development of PH (38, 77, 98). On the contrary, a reduction in hydrogen peroxide has been implicated in hypoxic pulmonary vasoconstriction (67, 99), CH (84), experimental pulmonary arterial hypertension (PAH) (65), and pulmonary arterial hypertensive Fawn-Hooded rats (3, 88).
We have demonstrated that the Ca2+-regulated transcription factor nuclear factor of activated T cells isoform c3 (NFATc3) is required for CH-induced PH in mice (6, 28). NFATc3 activation leads to an immediate proliferative response followed by recovery of the contractile phenotype and hypertrophy of pulmonary arterial smooth muscle cells (PASMC) (6, 26, 28). Superoxide and hydrogen peroxide have opposite effects on crystalline silica-induced NFAT activation in epidermal cells (53). However, the molecular mechanism that mediates NFAT activation under those conditions is unknown. Increased superoxide and decreased hydrogen peroxide resulting from decreased antioxidant capacity and increased superoxide generation may also occur in several animal models of PH (15, 23, 29–31, 35, 37, 47, 57, 72, 73, 96) as well as in human PH (10, 62, 63); however, this superoxide/hydrogen peroxide imbalance is not well established.
Superoxide dismutases are the major antioxidant defense systems against superoxide. There are three superoxide dismutase (SOD) isoforms expressed in the vasculature (1): CuZnSOD (SOD1 and SOD3) and MnSOD (SOD2), with SOD3 being extracellular and SOD1 being the predominant cytosolic isoform (43, 52, 55). SOD2 deficiency initiates and sustains a heritable form of PAH by impairing redox signaling and creating a proliferative, apoptosis-resistant PASMC (3). However, the role of NFAT in this phenotype has not been explored. Dennis et al. (31) reported that the NADPH oxidase homolog family member 1 (NOX1) is increased, and expression and activity of SOD1 are diminished in pulmonary arteries of piglets exposed to 3 and 10 days of CH. This is associated with increased superoxide and decreased hydrogen peroxide. In addition, SOD1 expression and activity are decreased in rats with monocrotaline-induced PH, which is associated with increased markers of oxidative stress and decreased total antioxidant capacity despite enhanced SOD2 expression (103). Loss of function of SOD3 exacerbated monocrotaline-induced oxidative stress and PAH (103). Despite the availability of SOD1 knockout (KO) mice, no studies have examined whether these mice develop PH spontaneously or are more sensitive to hypoxia, as previously observed in SOD3 KO mice and SOD3 loss-of-function rats (103).
The goal of this study was to determine the role that an imbalance in superoxide/hydrogen peroxide plays in NFATc3 activation in PASMC and in PH pathogenesis.
On the basis of findings that ROS mediate NFAT activation in T cells (51), mouse epidermal (53), human lung bronchoepithelial (53), and cardiac cells (42), we hypothesized that SOD1 KO mice develop NFAT-dependent PH due to an imbalance of superoxide/hydrogen peroxide. Consistent with our hypothesis, we found that SOD1 KO mice display elevated pulmonary arterial superoxide and decreased hydrogen peroxide levels, increased PASMC NFATc3 activity and have signs of PH, which were all prevented by selective NFAT inhibition.
MATERIALS AND METHODS
All protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of New Mexico Health Sciences Center (Albuquerque, NM).
Animals.
All experiments used male and female SOD1 KO and wild-type (WT) mice that were backcrossed with NFAT-luciferase reporter (NFAT-luc) mice (at least 8 generations). SOD1 KO mice were obtained from Jackson Laboratory (Sod1 tm1Leb/J, no. 002972). NFAT-luc mice were provided by Dr. Jeffery D. Molkentin (Department of Pediatrics, Children's Hospital Medical Center, Cincinnati, OH). Heterozygous SOD1/NFAT-luciferase crossed mice were bred to obtain age-matched WT and KO mice. All animals were maintained on a 12-h:12-h light/dark cycle. SOD1 KO mice develop normally up to 6 mo of age when they show signs of motor axonopathy (83). No compensatory upregulation of SOD2 and/or SOD3 in the brain and kidney was found in this mouse model (13, 83). Ho et al. (46) reported that CuZn-SOD (SOD1 and SOD3) activity was absent in brain and liver of SOD1 KO and significantly reduced in SOD1+/− mice. However, a very low level of CuZn-SOD activity was present in lung. This activity presumably represents the activity of SOD3, as expression of this SOD isoform is relatively high in the lungs compared with other tissues. SOD1 KO mice had no differences in the activity of other cellular antioxidant enzymes such as SOD2, catalase, glutathione peroxidase, and the enzymes that participate in the recycling of oxidized glutathione including glutathione reductase and glucose-6-phosphate dehydrogenase in brain, liver, and lung compared with WT and SOD1+/− mice (46).
Animal treatments.
Animals were treated with vehicle [drinking water or 1:2,000 i.p. dimethyl sulfoxide (DMSO; Sigma, St. Louis, MO)], tempol [SOD mimetic (19, 30, 35, 79, 95) in drinking water (1 mmol/l, 20 mg/kg per day; 3 wk)], or A-285222 (0.16 mg/kg/day in 1:2,000 ip DMSO; 2 wk). A-285222 [NFAT selective inhibitor (7, 70, 94)] was kindly provided by Abbott Laboratories (Abbott Park, IL). Mice were used at ∼11 wk of age unless otherwise specified.
Chronic hypoxia exposure.
Animals designated for exposure to CH were housed in a hypobaric chamber with barometric pressure maintained at ∼380 Torr for 5 or 21 days. Control animals were housed at ambient barometric pressure (normoxia, N, ∼630 Torr). All animals were maintained on a 12-h:12-h light/dark cycle.
In vivo assessment of right ventricular systolic pressure and right ventricular hypertrophy.
Adult mice were anesthetized with 2% inhaled isofluorane (balance O2). A 23-gauge needle attached to a pressure transducer was inserted into the abdomen below the xiphoid process and directed into the thoracic cavity toward the right ventricle (RV) of the heart (64). Entry into the RV was confirmed by monitoring the pressure waveform. Peak RV systolic pressures (RVSP) and heart rate (HR) were obtained using Windaq data acquisition software (Dataq Instruments, Akron, OH).
After we collected hemodynamic data, the heart was isolated, and the atria and major vessels were removed. The RV was dissected from the left ventricle (LV) and septum (S). The degree of RV hypertrophy was expressed as the percentage ratio of RV to LV+S weight and RV to body weight (BW).
Mean arterial blood pressure measurement.
Telemeter catheters were implanted as previously described (24). Mice were given buprenex (0.05 mg/kg sc) 20 min before surgery and anesthetized using isofluorane (2%, balance O2). The catheter tip was inserted and secured in the carotid artery, and the transmitter body (PA-C20; Data Sciences International, New Brighton, MN) was secured subcutaneously above the right flank. Warmed sterile 0.9% NaCl solution (0.5 ml sc) was given after surgery, and mice recovered 5–7 days before recording was started. Mean arterial pressure (MAP), HR and activity were recorded daily for up to 4 days.
Vascular morphometry.
Animals were anesthetized with 2% isofluorane (balance O2) and perfused via the right ventricle with ∼5 ml of modified physiological saline solution (HEPES-PSS, 134 mM NaCl, 6 mM KCl, 1 mM MgCl, 10 mM HEPES, 2 mM CaCl2, 0.026 mM EDTA, and 10 mM glucose) containing heparin, 4% albumin (Sigma) and 10−4 M papaverine (Sigma), at 20 mmHg to maximally dilate and flush the circulation of blood. Mice were then perfused with 4% paraformaldehyde (Polyscience, Warrington, PA) in PBS at the same pressure. Following fixation, the lungs were inflated with fixative via the trachea to maximal capacity. The tissue was then dehydrated in increasing concentrations of ethanol, with a final dehydration in xylene, and then embedded in paraffin. Lung sections (5 μm) were stained with rabbit anti-smooth muscle α-actin (Abcam, Cambridge, MA) antibody followed by immunohistochemistry detection with anti-rabbit secondary antibody labeled with horseradish peroxidase and costained with hematoxylin. Vessels were examined with a ×40 objective with an Eclipse E400 scope, and images were acquired with DS-Fi1 camera using NIS-Elements F 3.0 software. Images were analyzed with Image J (NIH, Bethesda, MD). Vessels sectioned at oblique angles were excluded from analysis. Medial area was calculated and compared between groups using the following equation: external area − luminal area. The analysis was performed in arteries from three different diameter ranges <50, 50–100, and 100–300 μm. Approximately 10 arteries/animal were analyzed.
Wall thickness was determined as outer − inner diameter (μm) in cannulated pressurized intrapulmonary arteries maximally dilated with Ca2+-free PSS [plus 10 μM ionomycin (Sigma), 3 μM cytochalasin B (Sigma), and 1 μM Myosin Light Chain Kinase Inhibitor Peptide (EMD Millipore, Billerica, MA)]. These arteries had an outer diameter that ranged from 120–150 μm.
Superoxide levels.
Lung cryostat sections were stained with the superoxide-sensitive fluorescent dye dihydroethidium (DHE) as previously described (20, 95, 104). Specific DHE fluorescence intensity was analyzed in the arterial wall using Image J software. Fluorescence intensity of four arteries per lung section per animal was averaged.
Hydrogen peroxide levels.
Hydrogen peroxide production was determined by the Amplex Red/Peroxidase Assay (Life Technologies, Gaithersburg, MD) (3) in one branch of intrapulmonary arteries (second and third order arteries, ∼4 mm in length), RV (1–2 mg), and LV (1–2 mg) isolated from WT and SOD1 KO mice incubated in HEPES-PSS for 30 min. The hydrogen peroxide concentration was calculated from a standard curve and normalized by total protein measured by Bradford Assay (Bio-Rad, Hercules, CA) and reported as the difference from the fluorescence signal obtained in tissues preincubated with polyethylene glycol (PEG)-catalase (250 U/ml) for 1 h. PEG-catalase was used to demonstrate the proportion of the signal that corresponds to hydrogen peroxide because it has been shown that one-electron oxidants such as radicals derived from ONOO− are able to cause oxidation of Amplex Red to resorufin, although at a considerably lower yield compared with peroxidase-mediated oxidation (105).
Luciferase activity.
Luciferase activity was measured in isolated intrapulmonary arteries, RV, and LV using a Luciferase Assay System kit (Promega, Madison, WI), and light detected with a luminometer (TD20/20, Turner, Promega). Protein content was determined by the Bradford method (Bio-Rad) and used to normalize luciferase activity (28).
Immunofluorescence confocal microscopy.
Lung cryostat sections (10 μm) were stained with primary rabbit polyclonal anti-NFATc3 or NFATc2 (Santa Cruz Biotechnology, Santa Cruz, CA) and mouse anti-α-actin (Sigma) followed by donkey anti-rabbit Cy5 and anti-mouse Cy3 (Jackson ImmunoResearch Laboratories, Bar Harbor, ME) as previously described (6, 28, 39, 45). Nuclei were stained using SYTOX green (Life Technologies). α-Actin staining was used to detect NFAT nuclear accumulation in PASMC. Sections were examined using a ×40 objective on a Zeiss 510 laser scanning confocal microscope. Specificity of immune staining was confirmed by the absence of fluorescence in tissues incubated with primary or secondary antibodies alone. For scoring of NFATc3 or c2-positive nuclei, multiple fields for each vessel were imaged and counted by two independent observers using MetaMorph software (Molecular Devices, Sunnyvale, CA). The software was programmed so that individual pixels would appear white instead of yellow if the green nucleic acid stain and red NFATc3 stain colocalized. Thus a cell was considered positive if colocalization (white) was uniformly distributed in the nucleus and negative if no colocalization (green only) or perinuclear colocalization was observed (6, 28, 39, 45).
Pulmonary arterial wall Ca2+ and vasoreactivity.
Second-order intrapulmonary arteries (120 – 150 μm) were carefully dissected from surrounding tissue and cannulated in a pressure myograph system (DMT-USA, Ann Arbor, MI). The endothelium was disrupted in all experiments by gentle rubbing of a piece of moose mane along the inner wall of the vessel and flushing the lumen with PSS. Arteries were incubated at room temperature with HEPES-PSS containing the cell-permeable radiometric Ca2+-sensitive fluorescent dye fura 2-AM (2 μM, Life Technologies) and 0.02% pluronic acid (Life Technologies) for 45 min. Arteries were then warmed to 37°C in PSS (containing in mM: 129.8 NaCl, 5.4 KCl, 0.83 MgSO4, 19 NaHCO3, 1.8 CaCl2, and 5.5 glucose) oxygenated with normoxic gas (10% O2-6% CO2-84% N2) for 20 min. Fura 2-AM-loaded vessels were alternately excited at 340 and 380 nm at a frequency of 10 Hz with an IonOptix Hyperswitch dual-excitation light source and the respective 510-nm emissions were collected with a photomultiplier tube (F340/F380). After subtracting background fluorescence, we calculated emissions ratios with Ion Wizard software (IonOptix) and recorded them continuously throughout the experiment. Inner diameter was continuously measured from bright-field images as described (69). A bolus of ET-1 (10−7 M) was added to the superfusate, and a steady-state diameter and F340/F380 were determined. Vasoreactivity and changes on F340/F380 in response to ET-1 were calculated from baseline.
ET-1 plasma, pre-pro ET-1, and NFAT isoform mRNA levels.
Lung, isolated intrapulmonary arteries, and RV were stored in RNAlater (Ambion, Austin, TX). Total RNA was isolated using the RNeasy Mini Kit (Qiagen, Valencia, CA) and reverse transcribed to cDNA using a High-Capacity Reverse Transcription kit (Life Technologies). For real-time detection of pre-pro ET-1 transcripts in lung, intrapulmonary arteries and RV, SYBR Green Master Mix (Life Technologies) was used with the following sets of primer pairs: for pre-pro ET-1, 5′-GACATCATCTGGGTCAACACTC-3′, 5′-CATCTAACTGCCTGGTCTGTG-3′, and for β-actin (endogenous control), 5′-ACCAACTGGGACGATATGGAGAAGA-3′, 5′-TACGACCAGAGGCATACAGGGACAA-3′ (25). NFATc1, c2, c3, and NFAT5 transcripts levels were measured in lung, and also NFATc2 and c3 mRNA levels were measured in intrapulmonary arteries using premade TaqMan Assays (Life Technologies). The normalized gene expression method (2−ΔΔCT) for relative quantification of gene expression was used (61). β-Actin was used as endogenous control. ET-1 plasma levels were measured using an ELISA kit following manufacturer's instructions (Enzo Life Biosciences, Exeter, UK).
Statistics.
Results are expressed as the means ± SE. Statistical significance was tested at the 95% (P < 0.05) confidence level using an unpaired t-test or two-way ANOVA followed by Bonferroni's posttest.
RESULTS
Superoxide levels are elevated, and hydrogen peroxide production is decreased in SOD1 KO mice.
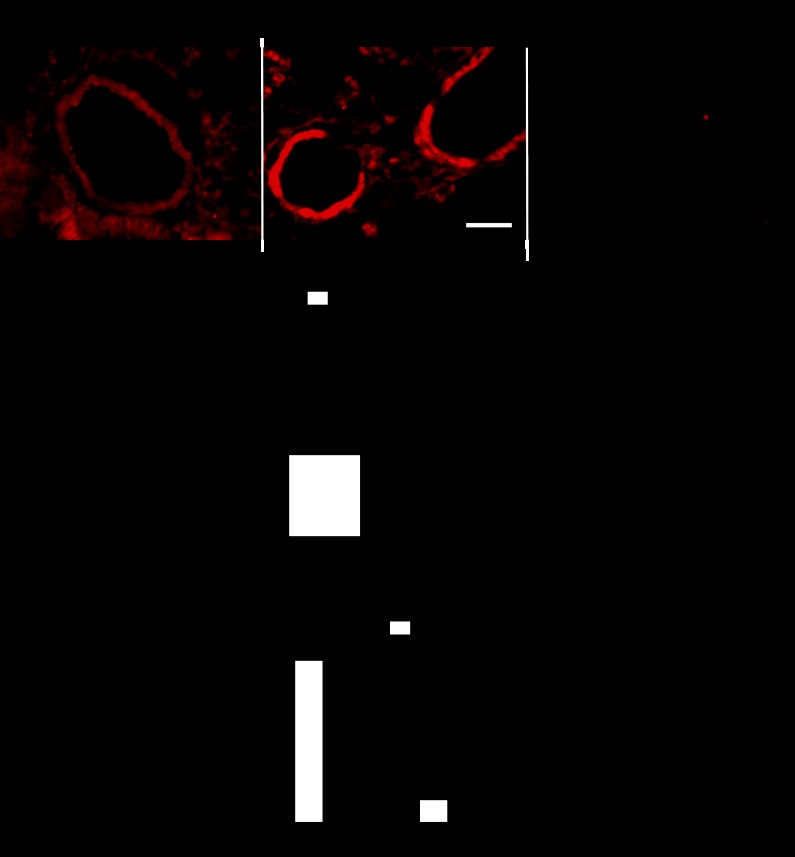
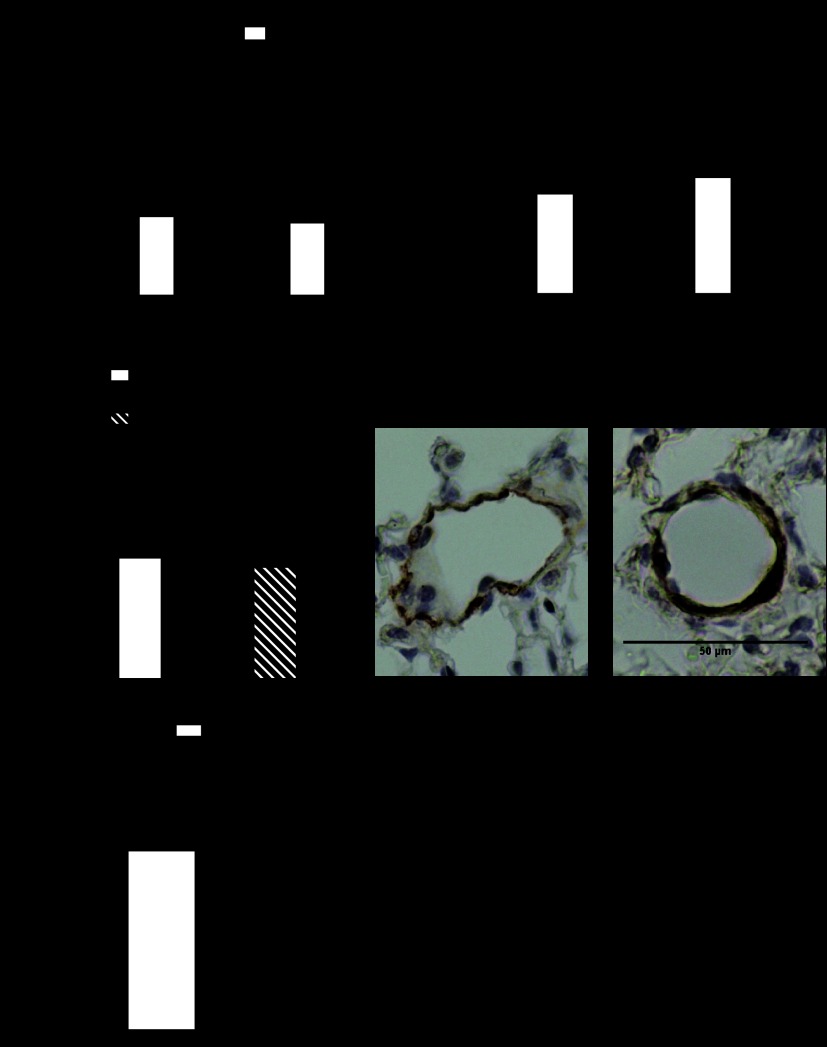
Figure 1, A and B, shows elevated DHE fluorescence intensity in pulmonary arteries of lung sections from SOD1 KO compared with WT mice. DHE fluorescence was reduced by incubation with PEG covalently linked to SOD (120 U/ml). Figure 1C shows that hydrogen peroxide production over a 30-min period was significantly lower in isolated pulmonary arteries from SOD1 KO vs. WT mice under normoxia. CH exposure (5 days) also significantly decreased hydrogen peroxide levels in pulmonary arteries from WT mice but did not further reduce the levels present in arteries from SOD1 KO mice (Fig. 1C). These results suggest that CH is as effective as SOD1 knockout in decreasing hydrogen peroxide production in pulmonary arteries.
Fig. 1.
Superoxide is elevated, and hydrogen peroxide is decreased in superoxide dismutase 1 knockout (SOD1 KO) mice. A: representative images of dihydroethidium (DHE) fluorescence staining of pulmonary arteries in lung sections of wild-type (WT), SOD1 KO, and SOD1 KO mice incubated with polyethylene glycol (PEG)-SOD for 30 min (120 U/ml). Scale bar = 50 μm. B: summary of DHE fluorescence-integrated intensity. n = 4–7 mice. *P < 0.05 vs. WT. Arteries were traced; threshold and DHE fluorescence intensity was analyzed in at least 4 arteries per lung section per animal. C: hydrogen peroxide was determined using Amplex Red/peroxidase in isolated pulmonary arteries from WT and SOD1 KO mice exposed to normoxia or chronic hypoxia (CH) (5 days) after 30-min incubation in HEPES-physiological saline solution buffer. PEG-catalase (250 U/ml) was added to determine the proportion of the signal that corresponds to hydrogen peroxide; n = 5 arteries from 5 mice for normoxia and n = 4 for CH. *P < 0.05 vs. WT.
No significant difference in hydrogen peroxide production was found in RV and LV between genotypes (ΔH2O2 nmol/μg protein from PEG-catalase RV: WT = 0.0006 ± 0.0002 vs. KO = 0.0010 ± 0.0002, n = 6. LV: WT = 0.0015 ± 0.0004 vs. KO = 0.0005 ± 0.0001, n = 5).
SOD1 KO mice display signs of PH that are not exacerbated by CH exposure.
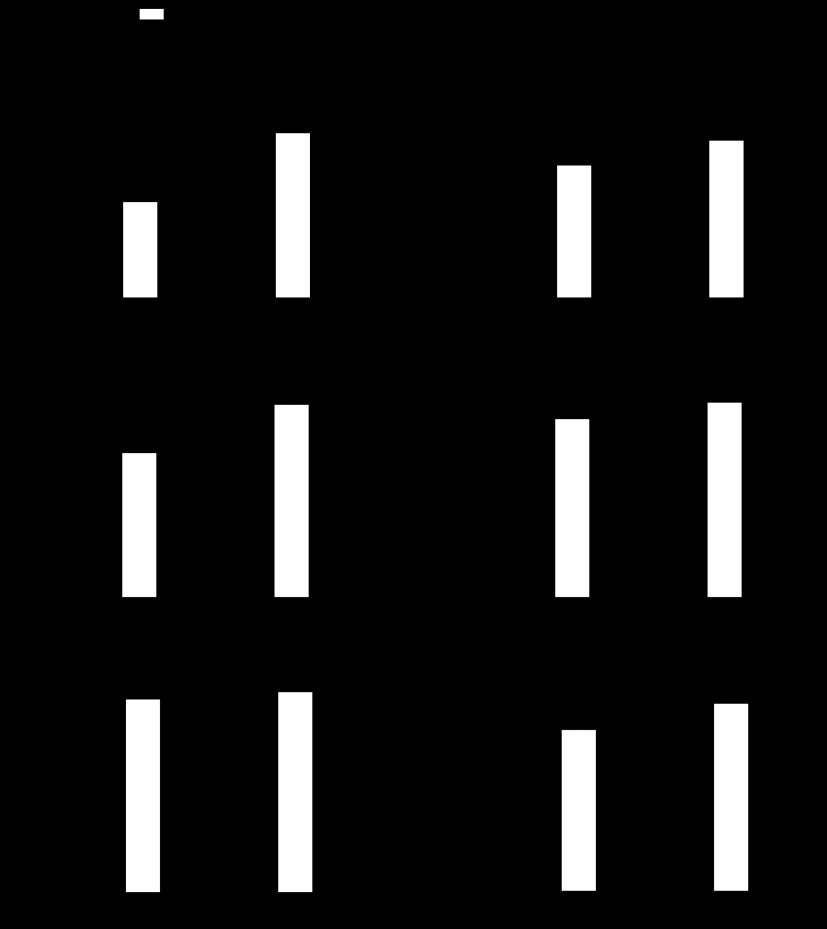
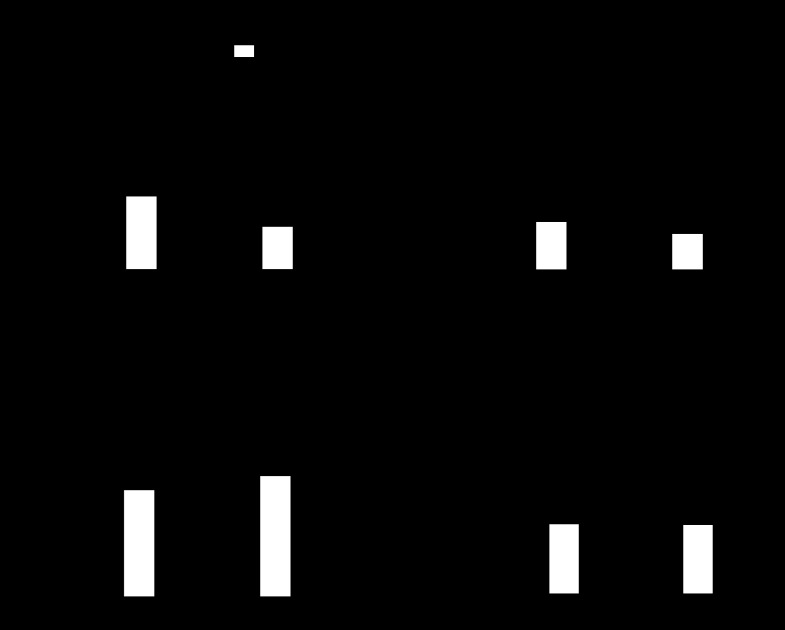
RVSP was significantly elevated in SOD1 KO compared with WT mice under normoxia (Fig. 2A). However, SOD1 KO mice showed no significant RV hypertrophy (Fig. 2, B and C, and Table 1). No significant sex-related effect was found. After exposure to CH, only WT mice responded with a significant increase in RVSP (Fig. 2A). Both genotypes display RV hypertrophy after exposure to CH (Fig. 2, B and C, and Table 1). RV/LV+S was significantly higher in SOD1 KO exposed to CH compared with WT mice but was to the expense of decreased LV+S weight (Fig. 2, B and D, Table. 1). LV/BW was not different between WT and SOD1 KO mice under normoxia (Fig. 2D and Table 1). Consistent with a lack of exacerbated CH-induced RV hypertrophy, RVSP was not different between normoxia- and CH-exposed SOD1 KO mice (Fig. 2A). Also, RVSP of CH-exposed WT was not different from normoxia-exposed SOD1 KO mice (Fig. 2A), suggesting that CH is as effective as knocking out SOD1 to increase RVSP. Both genotypes of mice display the same degree of polycythemia (Fig. 2F). No differences in HR were observed between groups (Fig. 2C).
Fig. 2.
SOD1 KO mice have signs of pulmonary hypertension (PH), which are not exacerbated by CH. A: right ventricular systolic pressure (RVSP) (n = 6–7). B: % RV/left ventricle and septum (LV+S) weight (n = 13–14 for normoxia, n = 6 for CH). C: RV/body weight (BW) (n = 13–14 for normoxia, n = 6 for CH). D: LV+S/BW (n = 13–14 for normoxia, n = 6 for CH). E: heart rate (HR) (n = 6–7). F: hematocrit (HCT) % (n = 6–7) of WT and SOD1 KO mice exposed to normoxia and 21 days of CH. *P < 0.05 vs. normoxia, #P < 0.05 vs. WT.
Table 1.
Heart and body weights in normoxic and CH WT and young and older SOD1 KO mice
| Genotype | Exposure | BW, g | RV, g | LV+S, g | TV/BW, g/kg | n = mice |
|---|---|---|---|---|---|---|
| SOD1 WT | Normoxia | 28.4 ± 2.0 | 0.028 ± 0.001 | 0.092 ± 0.004 | 3.29 ± 0.08 | 13 |
| SOD1 WT | CH | 26.7 ± 1.3 | 0.034 ± 0.003* | 0.094 ± 0.005 | 4.85 ± 0.23* | 6 |
| SOD1 KO | Normoxia | 26.6 ± 2.0 | 0.026 ± 0.001 | 0.082 ± 0.004 | 3.09 ± 0.09 | 14 |
| SOD1 KO | CH | 26.7 ± 1.8 | 0.033 ± 0.004* | 0.081 ± 0.0.07# | 4.27 ± 0.18*† | 6 |
| SOD1 WT 5 mo | Normoxia | 30.6 ± 2.9 | 0.92 ± 0.05 | 2.67 ± 0.08 | 3.59 ± 0.13 | 5 |
| SOD1 KO 5 mo | Normoxia | 28.1 ± 0.8 | 0.92 ± 0.01 | 2.61 ± 0.04 | 3.54 ± 0.05 | 7 |
Values are means ± SE. In the bottom 2 rows, right ventricle (RV) and left ventricle + septum weight (LV+S) values are per body weight (BW) and are in g/kg.
CH, chronic hypoxia; WT, wild-type; SOD1, superoxide dismutase 1; KO, knockout; TV, total ventricular weight.
P < 0.05 vs. normoxia.
P < 0.05 vs. WT.
Additionally, we found no differences in MAP (WT = 98 ± 1 vs. SOD1 KO = 98 ± 1 mmHg, n = 3) between genotypes under normoxia as previously reported (13). Total ventricle weights normalized by BW were not different between genotypes under normoxia (Table 1), contrary to what was previously reported (13). These results suggest that the elevated RVSP in the SOD1 KO mice is not a consequence of LV dysfunction.
To elucidate the reason for the lack of significant RV hypertrophy in ∼11-wk-old SOD1 KO mice under normoxia despite elevated RVSP, we determined RV/LV+S in 5-mo-old WT and SOD1 KO mice. The rationale for this study was that PH might have not been present for a sufficient duration to induce RV hypertrophy at 11 wk of age. However, we found no significant difference in RV/LV+S between WT (34.2 ± 1.2%, n = 5) and SOD1 KO mice (35.4 ± 0.7%, n = 7) at 5 mo of age. We also found no differences in BW, RV/BW, LV/BW, or total ventricular weight/BW between genotypes (Table 1).
NFATc3 is activated in PASMC of SOD1 KO mice.
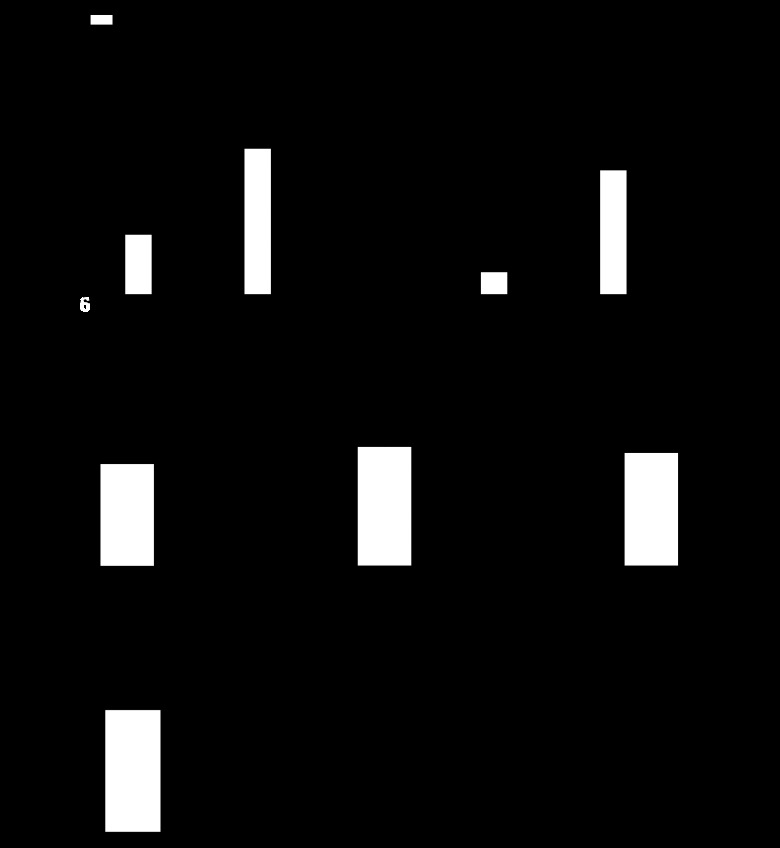
We have previously demonstrated that CH induces NFATc3 activation in mouse pulmonary arteries (28). To determine whether NFAT is activated in SOD1 KO compared with WT mice, luciferase activity was measured in isolated intrapulmonary arteries. Luciferase activity was significantly increased in pulmonary arteries from SOD1 KO under normoxic conditions (Fig. 3A) compared with WT mice. In this study, CH (21 days) again induced NFAT activation in pulmonary arteries from WT mice (Fig. 3A). However, consistent with the similar RVSP between normoxic and CH SOD1 KO mice (Fig. 2A), CH did not cause a further increase in NFAT activity in pulmonary arteries from SOD1 KO mice (Fig. 3A).
Fig. 3.
Nuclear factor of activated T cells (NFAT) is activated in pulmonary artery smooth muscle cells (PASMC) but not RV of SOD1 KO mice. A: NFAT activity (luciferase activity) in pulmonary arteries (n = 4–7). B: NFAT activity in RV (n = 4–5) of WT and SOD1 KO mice exposed to normoxia or 21 days of CH. C: pre-pro ET-1 mRNA levels in lung (n = 4–5), pulmonary arteries (n = 7–8), and RV (n = 5–6). D: ET-1 plasma levels (n = 15) of WT and SOD1 KO mice exposed to normoxia. *P < 0.05 vs. normoxia, #P < 0.05 vs. WT. RLU, relative light units.
It has been previously demonstrated that NFAT plays a major role in pathological cardiac hypertrophy (68, 100–102). Therefore, NFAT activity was measured in RV of WT and SOD1 KO ∼11-wk-old mice exposed to normoxia or CH. Contrary to our findings in pulmonary arteries, RV NFAT activity was not different between genotypes under normoxia but significantly increased upon exposure to 21 days of CH (Fig. 3B). This is consistent with the significant increase in RV/BW in response to CH in SOD1 KO mice (Fig. 2C). These findings suggest that increased RV afterload might not be sufficient to activate NFAT-mediated RV hypertrophy under normoxia in SOD1 KO mice.
NFAT activity was also measured in LV of normoxic WT and SOD1 KO mice, and no significant difference in luciferase activity was found between genotypes (NFAT activity: WT = 1.96 ± 0.21 vs. KO = 2.57 ± 0.59 relative light unit/μg protein, n = 4).
One of the biomarkers of PH is the elevated expression of ET-1 (2, 86). We have demonstrated that CH-induced NFATc3 activation in pulmonary arteries requires ET-1 upregulation and activation of endothelin A receptors (27). In addition, it has been shown that superoxide increases ET-1 levels in arteries and plasma (50, 56, 95). Therefore, we measured lung, RV, and pulmonary arterial pre-pro ET-1 transcripts and ET-1 plasma levels in normoxic WT and SOD1 KO mice. Contrary to our expectations, pre-pro ET mRNA levels (Fig. 3C) and ET-1 plasma (Fig. 3D) were not significantly different between genotypes.
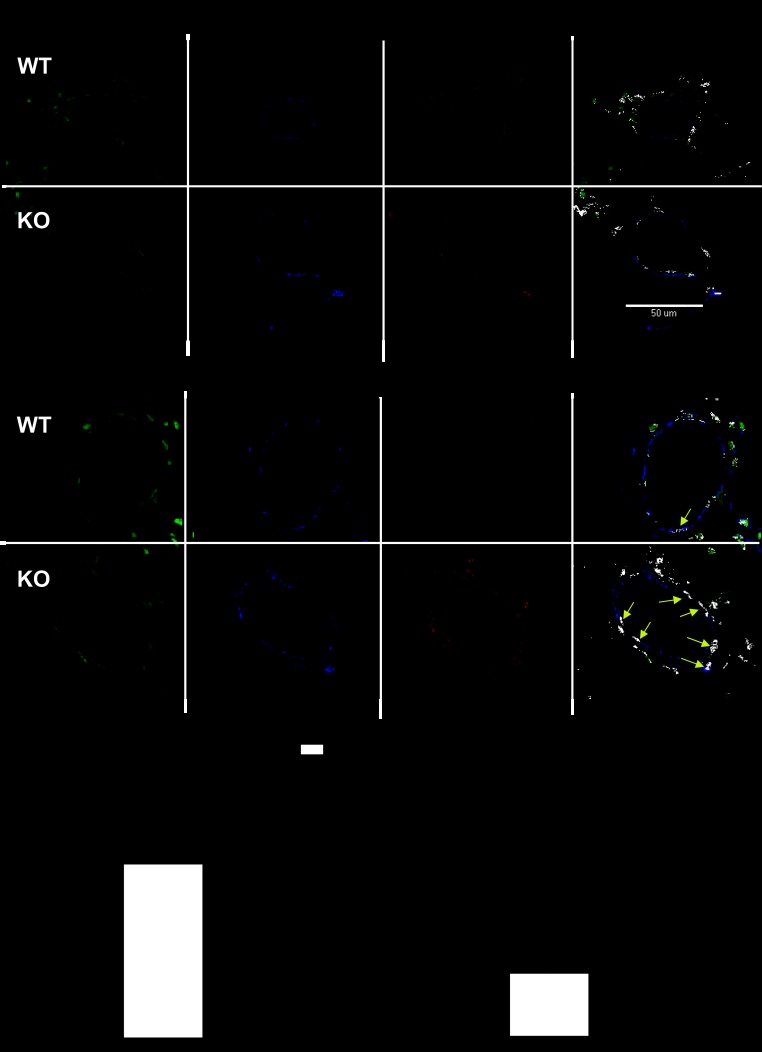
As mentioned above, the NFATc3 isoform has been implicated in the development of chronic hypoxic pulmonary hypertension in mice (6, 26–28). The contribution of other NFAT isoforms was ruled out in that study by crossing NFAT-luciferase reporter mice with NFATc3 KO mice and demonstrating no significant increase in luciferase activity in pulmonary arteries after these mice were exposed to CH (28). However, NFATc2 has been implicated in PAH. For example, it has been shown that NFATc2 nuclear localization is increased in PASMC from patients with PAH and from rats with monocrotaline-induced PH (9). In that study, NFATc3 nuclear localization was also found to be increased but in a lower proportion of cells. Therefore, to determine which isoform is activated in PASMC of SOD1 KO mice, NFATc2 and NFATc3 nuclear localization was assessed by immunofluorescence confocal microscopy. We found that NFATc3 but not NFATc2 nuclear localization is increased in PASMC of SOD1 KO mice (Fig. 4, A and B). Because NFATc2 is regulated not only by its subcellular localization but also by the transcription factor signal transducers and activators of transcription protein (STAT3), NFATc2 and NFATc3 transcript levels were determined in isolated intrapulmonary arteries. We found no differences in NFATc2 mRNA levels. However, a small but significant increase in NFATc3 mRNA in arteries from SOD1 KO was found compared with WT mice (2−ΔΔCT: NFATc2 = 0.99 ± 0.07 WT vs. 0.98 ± 0.03 KO, n = 7; NFATc3 = 1.14 ± 0.09 WT vs. 1.51 ± 0.09 KO, P < 0.01, n = 7). We also determined NFATc1, c2, c3, c4, and NFAT5 transcript levels in lung from WT and SOD1 KO mice. We found no significant differences in any of the isoforms between genotypes (2−ΔΔCT: NFATc1 = 0.85 ± 0.04 WT vs. 1.09 ± 0.19 KO, n = 5; NFATc2 = 0.75 ± 0.07 WT vs. 1.10 ± 0.23 KO, n = 5; NFATc3 = 0.91 ± 0.04 WT vs. 0.85 ± 0.10 KO, n = 5; NFATc4 = 0.89 ± 0.08 WT vs. 0.88 ± 0.19 KO, n = 5; and NFAT5 = 0.89 ± 0.06 WT vs. 1.14 ± 0.27 KO, n = 5).
Fig. 4.
NFATc3 but not NFATc2 is activated in PASMC from SOD1 KO mice. A: representative images of immunofluorescence confocal detection of NFATc2 and NFATc3 nuclear localization in PASMC of WT and SOD1 KO mice. Nuclei are shown in green, smooth muscle α-actin is shown in blue, and NFATc2 or NFATc3 in red. NFAT nuclear localization is shown in white in the overlay image. Yellow arrow depicts a PASMC nucleus with NFATc3 nuclear localization (positive). *P < 0.05 vs. WT. (n = 4 mice; average of 100–150 cells/mouse). Scale bar = 50 μm.
NFAT is required for the development of PH in SOD1 KO mice.
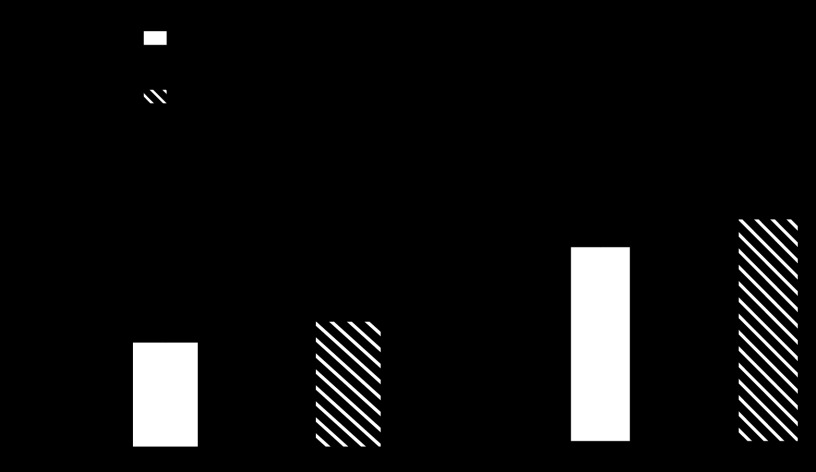
Because NFATc3 activity is increased in PASMC of SOD1 KO mice, we determined whether administration of the selective NFAT inhibitor A-285222 (33, 94) prevents the development of PH in these mice. Indeed, NFAT activation was prevented by A-285222 administration (Fig. 5A). More importantly, administration of A-285222 significantly prevented the increase in RVSP in SOD1 KO mice (Fig. 5B).
Fig. 5.
NFATc3 is required for the development of pulmonary hypertension in SOD1 KO mice. A: pulmonary arterial NFAT activity (n = 6–7). B: RVSP (n = 4–6). C: pulmonary arterial wall thickness (n = 4–6) in WT and SOD1 KO mice treated with vehicle or A-285222 for 2 wk. *P < 0.05 vs. WT vehicle, #P < 0.05 vs. SOD1 KO vehicle. D: representative images of pulmonary arteries from WT and SOD1 KO stained for smooth muscle α-actin by immunohistochemistry demonstrating increased media area. E: summary data of media area of arteries of <50 μm outer diameter, *P < 0.05 vs. WT. (n = 40 arteries, ∼10 arteries/mouse from 4 mice). Scale bar = 50 μm.
Additional hallmarks of PH are pulmonary arterial remodeling and increased vasoreactivity. Medial area and arterial wall thickness were determined in both SOD1 KO and WT mice by two different approaches (see materials and methods). SOD1 KO mice displayed elevated pulmonary arterial wall thickness (Fig. 5C; 120–150 μm outer diameter) and media area (Fig. 5, D and E; <50 μm outer diameter) compared with WT mice. The arterial wall Ca2+ (Fig. 6A) and associated constrictor (Fig. 6B) responses to ET-1 (10−7 M) were significantly greater in small pulmonary arteries from SOD1 KO vs. WT mice at 35 mmHg intraluminal pressure. Administration of A-285222 significantly prevented the increased arterial wall thickness, Ca2+, and associated constrictor response to ET-1 in SOD1 KO mice (Fig. 6, A and B). A-285222 had no effect on RVSP and NFAT activity in WT mice (Fig. 5, A and B); therefore, effects of this drug were not subsequently tested for the other parameters in WT mice.
Fig. 6.
NFATc3 is required for enhanced vasoreactivity to ET-1 in SOD1 KO mice. A: % arterial wall Ca2+ response to a bolus of ET-1 [10−7 M] (change from baseline) measured in isolated pulmonary arteries loaded with fura 2-AM (n = 4–7). B: % vasoconstriction (change in diameter from baseline) in response to ET-1 (n = 5–6). *P < 0.05 vs. WT, #P < 0.05 vs. SOD1 KO mice.
Normalization of superoxide/hydrogen peroxide imbalance with an SOD mimetic prevents NFATc3 activation and PH in SOD1 KO mice.
Administration of the SOD mimetic, tempol (19, 30, 35, 79, 95), to WT and SOD1 KO mice for 3 wk prevented the increased NFAT activity (Fig. 7A), NFATc3 nuclear localization (Fig. 7B), RVSP (Fig. 7C), and the elevated superoxide (Fig. 7D) in SOD1 KO mice.
Fig. 7.
Normalization of superoxide/hydrogen peroxide imbalance with a SOD mimetic prevents NFAT activation and PH in SOD1 KO mice. A: NFAT activity (n = 6–7). B: NFATc3 nuclear localization (n = 4–5). C: RVSP (n = 5–7) measured in tempol (drinking water)- and vehicle-treated WT and SOD1 KO mice. D: DHE staining. *P < 0.05 vs. WT vehicle, #P < 0.05 vs. SOD1 KO vehicle; n = 6 mice.
DISCUSSION
Our study generated major new findings related to the role of SOD1 and NFAT activity in the genesis of PH. The major new finding of this study is that PH developed in ∼11-wk-old SOD1 KO mice under normoxia. This response was not affected by exposure to CH. Second, SOD1 deficiency and CH caused a decrease in pulmonary arterial hydrogen peroxide levels. Third, SOD1 deficiency caused NFAT activation in pulmonary arteries and NFATc3 but not NFATc2 nuclear accumulation in PASMC, responses that were prevented by administration of the SOD mimetic, tempol. Finally, both NFAT inhibition and tempol prevented indices of PH present in SOD1 KO mice, demonstrating that NFAT contributes to the genesis of PH in this mouse model, which is associated with an imbalance in superoxide/hydrogen peroxide levels.
A reduction in SOD activity has been implicated in patients with PAH and animal models of PH (10, 15, 18, 23, 29–31, 35, 47, 57, 72, 73, 96). Mice deficient in SOD3 and rats that carry a loss-of-function mutation in SOD3 display exacerbated CH-induced PH (103). Furthermore, fawn-hooded rats, which exhibit decreased expression and activity of mitochondrial SOD2, develop spontaneous PAH (3, 88). SOD1 activity and expression are also diminished in pulmonary arteries of piglets exposed to CH (31). This decrease is accompanied by increased pulmonary arterial superoxide, increased NOX1, and decreased hydrogen peroxide. The authors concluded that ROS derived from NOX1, as well as from decreased SOD1, contribute to aberrant pulmonary resistance artery responses in newborn piglets with CH-induced pulmonary hypertension. In addition, SOD1 expression and activity are decreased in rats with monocrotaline-induced PH, a response associated with increased markers of oxidative stress and decreased antioxidant capacity, despite increased SOD2 expression (103). However, whether PH develops in response to SOD1 deficiency has not previously been studied. Our present data support the existence of PH in SOD1 KO mice, as demonstrated by elevated RVSP, pulmonary arterial remodeling, and increased vasoreactivity to ET-1 compared with WT mice.
It has been previously demonstrated that SOD1 KO mice have elevated superoxide levels determined by DHE staining in cerebral arterial wall (4), carotid arteries (32), and renal afferent arterioles (13). Superoxide is also increased in the aorta of these mice, as determined by the lucigenin assay (32). Consistent with these reported elevations in vascular superoxide, we found higher DHE intensity staining in the pulmonary arterial wall of SOD1 KO compared with WT mice. Despite the elevated superoxide levels and decreased SOD activity reported in these mice, there are no studies addressing whether hydrogen peroxide levels and/or production are decreased in this mouse model. In this study, we found that pulmonary arteries from SOD1 KO mice produce significantly less hydrogen peroxide than arteries from WT mice. We also found that CH is as effective as knocking out SOD1 in reducing hydrogen peroxide production in pulmonary arteries. This observation is consistent with the reported reduction in pulmonary arterial hydrogen peroxide levels in fawn-hooded rats, in which expression and activity of mitochondrial SOD2 are decreased (3). It is also consistent with the decreased pulmonary arterial hydrogen peroxide levels found in pulmonary arteries from CH-exposed piglets (31) in which SOD1 expression is decreased.
In this study, we also report the novel finding that increased superoxide and decreased hydrogen peroxide is associated with NFAT activation in PASMC of SOD1 KO mice. We (6, 26–28, 87) and others (8, 9, 22, 76, 92, 93) have previously demonstrated a role for NFAT in different PH animal models and in patients with PAH. Accordingly, the administration of a selective NFAT inhibitor (A-285222) to SOD1 KO mice completely prevented the increase in RVSP. In addition, this inhibitor significantly decreased the elevated pulmonary arterial wall thickness, NFAT activity, and vasoreactivity to ET-1 present in vehicle-treated SOD1 KO mice. A-285222 inhibits NFAT without affecting calcineurin activity and has no out-of-target side effects in either primates (7, 94) or rodents (70). These findings demonstrate that NFAT activation drives the development of PH in SOD1 KO mice. NFATc3 nuclear localization but not NFATc2 is increased in PASMC of SOD1 KO mice, strongly suggesting that the NFATc3 isoform is involved in the development of PH in these mice.
A causal association between the increased superoxide/hydrogen peroxide ratio and NFAT activation that we found in SOD1 KO mice is strongly supported by the fact that the SOD mimetic (19, 30, 35, 95), tempol, prevented the elevated NFAT activity, PASMC NFATc3 nuclear localization, and RVSP present in SOD1 KO mice. This association is also in agreement with a reported role for superoxide as an activator and hydrogen peroxide as an inhibitor of NFAT in epidermal cells (53) and also with reports showing that hydrogen peroxide inhibits both NFAT DNA binding (5, 40, 44, 75) and calcineurin activity (14, 36, 58, 60). NFAT activation requires Ca2+-dependent calcineurin-mediated dephosphorylation (reviewed in Ref. 81). Hydrogen peroxide-induced calcineurin inhibition involves multiple mechanisms. It has been demonstrated that hydrogen peroxide triggers proteolytic cleavage and inactivation of calcineurin in T cells (58). Also in T cells, hydrogen peroxide can directly oxidize the calmodulin-binding domain of calcineurin, thereby inactivating the enzyme (14). Finally, in neurons, Down Syndrome Candidate Region 1 (DSCR1, Adapt78, or RCAN1) protein inactivates calcineurin in response to elevations in hydrogen peroxide or large and acute increases in intracellular Ca2+ (36, 60). DSCR1 also inhibits the calcineurin/NFAT pathway in systemic vascular smooth muscle cells (59); however, its expression in PASMC has not yet been reported. In contrast, others have shown that NFAT nuclear import is enhanced by hydrogen peroxide-induced increases in intracellular Ca2+ in a leukemia cell line, neuroblastoma cells, and cardiac myocytes (41, 54, 97). Beside Ca2+ and calcineurin, additional mechanisms are implicated in the regulation of NFAT activity. We have recently demonstrated that RhoA/Rho kinase-dependent actin cytoskeleton remodeling is required for ET-1-mediated NFATc3 activation in PASMC from chronically hypoxic PH mice (27). Both ET-1 (12, 17) and CH (10, 82) decrease SOD activity, and ROS regulate RhoA/Rho kinase (11, 49) activity and actin cytoskeleton polymerization (66). In the present study, we found that plasma ET-1 and lung RV and pulmonary arterial pre-pro ET-1 transcript levels were not different between genotypes, suggesting that ET-1 might not be involved in the elevated NFAT activity and RVSP observed in SOD1 KO mice under normoxia. However, further studies are needed to determine whether the RhoA/Rho kinase/actin polymerization pathway participates in NFAT activation under conditions in which superoxide is increased and hydrogen peroxide is decreased.
On the other hand, Bonnet and colleagues (9, 21, 76) have demonstrated a distinct activation pathway for NFATc2 in PASMC from patients with PAH and animal models. Increased circulating levels of growth factors, agonists, and cytokines trigger activation of the transcription factor STAT3, resulting in enhanced NFATc2 transcription and increased Pim1 activation. Once activated, Pim1 triggers NFATc2 activation (nuclear translocation) (9, 21, 76). In our study, we did not find differences in lung and pulmonary arterial NFATc2 expression between WT and SOD1 KO, suggesting that this pathway might not be involved in PH resulting from SOD1 deficiency. However, this pathway could be implicated in the small but significant increase in pulmonary arterial NFATc3 transcripts found in SOD1 KO mice, but its contribution remains to be determined.
Although we measured an increase in RVSP, a very close approximation of pulmonary artery systolic pressure, in 11-wk-old SOD1 KO mice, we did not observe significant RV hypertrophy at this age. RV hypertrophy was also absent in 5-mo-old SOD1 KO mice, suggesting that the duration of elevated RVSP might not be a contributing factor for this phenotype. We did not study older mice because it has been reported that SOD1 KO mice develop motor axonopathy at about 6 mo of age (90). Interestingly, this is not the only animal model in which PH develops without a concomitant increase in RV weight. Nitric oxide synthase 3-deficient (NOS3 KO) mice (91) and young transgenic Ren2 rats (30) also display increased RVSP without RV hypertrophy. The reason for the dissociation between elevated RVSP and RV hypertrophy was not determined in the study of NOS3 KO mice (91). In the case of Ren2 rats, aging might be a contributing factor because RV hypertrophy is not evident in young rats but develops in 13–14-wk-old rats (78). Nonetheless, it is intriguing that increased RVSP and pulmonary vascular remodeling occur in the absence of RV hypertrophy in Ren2 rats, NOS2 KO, and SOD1 KO mice. This is in contrast with models of CH- and monocrotaline-induced PH, in which RV hypertrophy parallels the rise in pulmonary arterial pressure. Interestingly, in these two models, ET-1 levels are elevated in pulmonary arteries and/or RV (27, 48, 80), whereas ET-1 levels are not different between WT and SOD1 KO mice under normoxia. Therefore, our study suggests that increased RV afterload might not be sufficient to induce RV hypertrophy, and activation of the ET-1 system might also be required for the hypertrophic response. This possibility is supported by the increased NFAT activity in the RV in response to CH in both genotypes but absence of NFAT activation under normoxia. It is also consistent with the important role that NFAT plays in pathological cardiac hypertrophy (68, 100–102). This possibility is further supported by the fact that LV hypertrophy is ET-1 dependent in both older (12–16 wk old) and younger Ren2 rats (6–9 wk old) on a high-salt diet but not in those younger rats on a normal-salt diet (27, 34, 85, 89). An alternative explanation is that a decrease in hydrogen peroxide might be required for NFAT activation in the RV. Because RV hydrogen peroxide levels are not different between genotypes then NFAT activation could not occur in response to increased RV afterload. The reason for a lack of decreased hydrogen peroxide in the RV of SOD1 KO mice remains to be determined. Further studies are also needed to identify the precise mechanism of the dissociation between elevated pulmonary pressure and RV hypertrophy in young Ren2 rats, NOS3 KO, and SOD1 KO mice.
In conclusion, our study demonstrates that NFATc3 is required for the development of PH in SOD1 KO mice, likely due to an elevation in pulmonary arterial superoxide and a reduction in hydrogen peroxide. The NFATc3-regulated genes that cause PH in this and other animal models in which ROS levels are altered remain to be determined.
GRANTS
This work was supported by NIH Grants R01-HL-088151 (and a supplement from the American Recovery and Reinvestment Act of 2009 to R01-HL-088151), R01-HL-088192, T32-HL-007736, and a CTSC Pilot Award from the University of New Mexico Health Sciences Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.R.-D., C.H.N., T.C.R., and L.V.G.B. conception and design of research; J.R.-D., C.H.N., L.D.M., S.J.C., and W.G. performed experiments; J.R.-D., C.H.N., and L.V.G.B. analyzed data; J.R.-D., C.H.N., T.C.R., and L.V.G.B. interpreted results of experiments; J.R.-D., C.H.N., T.C.R., and L.V.G.B. prepared figures; J.R.-D., C.H.N., L.D.M., T.C.R., and L.V.G.B. edited and revised manuscript; J.R.-D., C.H.N., L.D.M., S.J.C., W.G., T.C.R., and L.V.G.B. approved final version of manuscript; T.C.R. and L.V.G.B. drafted manuscript.
ACKNOWLEDGMENTS
We thank Mary Walsh and Dr. Mary K. Walker (School of Pharmacy, University of New Mexico) for assisting on systemic blood pressure measurements by telemetry. Images in this paper were generated in the University of New Mexico & Cancer Center Fluorescence Microscopy Shared Resource, funded as detailed on http://hsc.unm.edu/crtc/microscopy/acknowledgement.shtml.
REFERENCES
- 1. Ahmed MN, Zhang Y, Codipilly C, Zaghloul N, Patel D, Wolin M, Miller EJ. Extracellular superoxide dismutase overexpression can reverse the course of hypoxia-induced pulmonary hypertension. Mol Med 18: 38–46, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aoshima D, Murata T, Hori M, Ozaki H. Time-dependent phenotypic and contractile changes of pulmonary artery in chronic hypoxia-induced pulmonary hypertension. J Pharm Sci 110: 182–190, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, Dyck JRB, Gomberg-Maitland M, Thébaud B, Husain AN, Cipriani N, Rehman J. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension. Circulation 121: 2661–2671, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baumbach GL, Didion SP, FARACIFM Hypertrophy of cerebral arterioles in mice deficient in expression of the gene for CuZn superoxide dismutase. Stroke 37: 1850–1855, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Beiqing L, Chen M, Whisler RL. Sublethal levels of oxidative stress stimulate transcriptional activation of c-jun and suppress IL-2 promoter activation in Jurkat T cells. J Immunol 157: 160–169, 1996 [PubMed] [Google Scholar]
- 6. Bierer R, Nitta CH, Friedman JK, Codianni SJ, De Frutos S, Dominguez-Bautista JA, Howard TA, Resta TC, Gonzalez Bosc LV. NFATc3 is required for chronic hypoxia-induced pulmonary hypertension in adult and neonatal mice. Am J Physiol Lung Cell Mol Physiol 301: L872–L880, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bîrsan T, Dambrin C, Marsh KC, Jacobsen W, Djuric SW, Mollison KW, Christians U, Carter GW, Morris RE. Preliminary in vivo pharmacokinetic and pharmacodynamic evaluation of a novel calcineurin-independent inhibitor of NFAT. Transpl Int 17: 145–150, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Bonnet S, Paulin R, Sutendra G, Dromparis P, Roy M, Watson KO, Nagendran J, Haromy A, Dyck JR, Michelakis ED. Dehydroepiandrosterone reverses systemic vascular remodeling through the inhibition of the Akt/GSK3-beta/NFAT axis. Circulation 120: 1231–1240, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Bonnet S, Rochefort G, Sutendra G, Archer SL, Haromy A, Webster L, Hashimoto K, Bonnet SN, Michelakis ED. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Pro Natl Acad Sci USA 104: 11418–11423, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bowers R, Cool C, Murphy RC, Tuder RM, Hopken MW, Flores SC, Voelkel NF. Oxidative stress in severe pulmonary hypertension. Am J Respir Crit Care Med 169: 764–769, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Broughton BRS, Jernigan NL, Norton CE, Walker BR, Resta TC. Chronic hypoxia augments depolarization-induced Ca2+ sensitization in pulmonary vascular smooth muscle through superoxide-dependent stimulation of RhoA. Am J Physiol Lung Cell Mol Physiol 298: L232–L242, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Callera GE, Tostes RC, Yogi A, Montezano AC, Touyz RM. Endothelin-1-induced oxidative stress in DOCA-salt hypertension involves NADPH-oxidase-independent mechanisms. Clin Sci (Lond) 110: 243–253, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Carlström M, Lai EY, Ma Z, Steege A, Patzak A, Eriksson UJ, Lundberg JO, Wilcox CS, Persson AE. Superoxide dismutase 1 limits renal microvascular remodeling and attenuates arteriole and blood pressure responses to angiotensin II via modulation of nitric oxide bioavailability. Hypertension 56: 907–913, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carruthers NJ, Stemmer PM. Methionine oxidation in the calmodulin-binding domain of calcineurin disrupts calmodulin binding and calcineurin activation. Biochemistry 47: 3085–3095, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chandrasekar I, Eis A, Konduri GG. Betamethasone attenuates oxidant stress in endothelial cells from fetal lambs with persistent pulmonary hypertension. Pediatr Res 63: 67–72, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Cheever KH. An overview of pulmonary arterial hypertension: risks, pathogenesis, clinical manifestations, and management. J Cardiovasc Nurs 20: 108–116, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Chen DD, Dong YG, Yuan H, Chen AF. Endothelin 1 activation of endothelin A receptor/NADPH oxidase pathway and diminished antioxidants critically contribute to endothelial progenitor cell reduction and dysfunction in salt-sensitive hypertension. Hypertension 59: 1037–1043, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen MJ, Chiang LY, Lai YL. Reactive oxygen species and substance P in monocrotaline-induced pulmonary hypertension. Toxicol Appl Pharmacol 171: 165–173, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Chen YF, Cowley AW, Jr, Zou AP. Increased H2O2 counteracts the vasodilator and natriuretic effects of superoxide dismutation by tempol in renal medulla. Am J Physiol Regul Integr Comp Physiol 285: R827–R833, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Cherng TW, Paffett ML, Jackson-Weaver O, Campen MJ, Walker BR, Kanagy NL. Mechanisms of diesel-induced endothelial nitric oxide synthase dysfunction in coronary arterioles. Environ Health Perspect 119: 98–103, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Courboulin A, Barrier M, Perreault T, Bonnet P, Tremblay VL, Paulin R, Tremblay VL, Lambert C, Jacob MH, Bonnet SN, Provencher S, Bonnet S. Plumbagin reverses proliferation and resistance to apoptosis in experimental PAH. Eur Respir J 40: 618–629, 2012 [DOI] [PubMed] [Google Scholar]
- 22. Courboulin A, Paulin R, Giguere NJ, Saksouk N, Perreault T, Meloche J, Paquet ER, Biardel S, Provencher S, Cote J, Simard MJ, Bonnet S. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med 208: 535–548, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Csiszar A, Labinskyy N, Olson S, Pinto JT, Gupte S, Wu JM, Hu F, Ballabh P, Podlutsky A, Losonczy G, de Cabo R, Mathew R, Wolin MS, Ungvari Z. Resveratrol prevents monocrotaline-induced pulmonary hypertension in rats. Hypertension 54: 668–675, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Frutos S, Duling L, Alo D, Berry T, Jackson-Weaver O, Walker M, Kanagy N, Gonzalez BL. NFATc3 is required for intermittent hypoxia-induced hypertension. Am J Physiol Heart Circ Physiol 294: H2382–H2390, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Frutos S, Duling L, Alo D, Berry T, Jackson-Weaver O, Walker M, Kanagy N, Gonzalez Bosc L. NFATc3 is required for intermittent hypoxia-induced hypertension. Am J Physiol Heart Circ Physiol 294: H2382–H2390, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Frutos S, Nitta CH, Caldwell E, Friedman J, Gonzalez Bosc LV. Regulation of soluble guanylyl cyclase-α1 expression in chronic hypoxia-induced pulmonary hypertension: role of NFATc3 and HuR. Am J Physiol Lung Cell Mol Physiol 297: L475–L486, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. De Frutos S, Ramiro-Diaz JM, Nitta CH, Sherpa ML, Gonzalez Bosc LV. Endothelin-1 contributes to increased NFATc3 activation by chronic hypoxia in pulmonary arteries. Am J Physiol Cell Physiol 301: C441–C450, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Frutos S, Spangler R, Alo D, Gonzalez Bosc LV. NFATc3 mediates chronic hypoxia-induced pulmonary arterial remodeling with alpha-actin up-regulation. J Biol Chem 282: 15081–15089, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Demarco VG, Whaley-Connell AT, Sowers JR, Habibi J, Dellsperger KC. Contribution of oxidative stress to pulmonary arterial hypertension. World J Cardiol 2: 316–324, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. DeMarco VG, Habibi J, Whaley-Connell AT, Schneider RI, Heller RL, Bosanquet JP, Hayden MR, Delcour K, Cooper SA, Andresen BT, Sowers JR, Dellsperger KC. Oxidative stress contributes to pulmonary hypertension in the transgenic (mRen2)27 rat. Am J Physiol Heart Circ Physiol 294: H2659–H2668, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Dennis KE, Aschner JL, Milatovic D, Schmidt JW, Aschner M, Kaplowitz MR, Zhang Y, Fike CD. NADPH oxidases and reactive oxygen species at different stages of chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol 297: L596–L607, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Didion SP, Ryan MJ, Didion LA, Fegan PE, Sigmund CD, FARACIFM Increased superoxide and vascular dysfunction in cuznsod-deficient mice. Circ Res 91: 938–944, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Djuric SW, BaMaung NY, Basha A, Liu H, Luly JR, Madar DJ, Sciotti RJ, Tu NP, Wagenaar FL, Wiedeman PE, Zhou X, Ballaron S, Bauch J, Chen YW, Chiou XG, Fey T, Gauvin D, Gubbins E, Hsieh GC, Marsh KC, Mollison KW, Pong M, Shaughnessy TK, Sheets MP, Smith M, Trevillyan JM, Warrior U, Wegner CD, Carter GW. 3,5-Bis(trifluoromethyl)pyrazoles: a novel class of NFAT transcription factor regulator. J Med Chem 43: 2975–2981, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Dvorak P, Kramer HJ, Backer A, Maly J, Kopkan L, Vaneckova I, Vernerova Z, Opocensky M, Tesar V, Bader M, Ganten D, Janda J, Cervenka L. Blockade of endothelin receptors attenuates end-organ damage in homozygous hypertensive ren-2 transgenic rats. Kidney Blood Press Res 27: 248–258, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Elmedal B, de Dam MY, Mulvany MJ, Simonsen U. The superoxide dismutase mimetic, tempol, blunts right ventricular hypertrophy in chronic hypoxic rats. Br J Pharmacol 141: 105–113, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ermak G, Harris CD, Davies KJ. The DSCR1 (Adapt78) isoform 1 protein calcipressin 1 inhibits calcineurin and protects against acute calcium-mediated stress damage, including transient oxidative stress. FASEB J 16: 814–824, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Fike CD, Dikalova A, Slaughter JC, Kaplowitz MR, Zhang Y, Aschner JL. Reactive oxygen species-reducing strategies improve pulmonary arterial responses to nitric oxide in piglets with chronic hypoxia-induced pulmonary hypertension. Antioxid Redox Signal. 2013. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fike CD, Slaughter JC, Kaplowitz MR, Zhang Y, Aschner JL. Reactive oxygen species from NADPH oxidase contribute to altered pulmonary vascular responses in piglets with chronic hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 295: L881–L888, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Filosa JA, Nelson MT, Gonzalez Bosc LV. Activity-dependent NFAT3 nuclear accumulation in pericytes from cortical parenchymal microvessel. Am J Physiol Cell Physiol 293: C1797–C1805, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Flescher E, Tripoli H, Salnikow K, Burns FJ. Oxidative stress suppresses transcription factor activities in stimulated lymphocytes. Clin Exp Immunol 112: 242–247, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Frossi B, Rivera J, Hirsch E, Pucillo C. Selective activation of Fyn/PI3K and p38 MAPK regulates IL-4 production in BMMC under nontoxic stress condition. J Immunol 178: 2549–2555, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Fujii T, Onohara N, Maruyama Y, Tanabe S, Kobayashi H, Fukutomi M, Nagamatsu Y, Nishihara N, Inoue R, Sumimoto H, Shibasaki F, Nagao T, Nishida M, Kurose H. Galpha12/13-mediated production of reactive oxygen species is critical for angiotensin receptor-induced NFAT activation in cardiac fibroblasts. J Biol Chem 280: 23041–23047, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 15: 1583–1606, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Furuke K, Shiraishi M, Mostowski HS, Bloom ET. Fas ligand induction in human NK cells is regulated by redox through a calcineurin-nuclear factors of activated T cell-dependent pathway. J Immunol 162: 1988–1993, 1999 [PubMed] [Google Scholar]
- 45. Gonzalez Bosc LV, Wilkerson MK, Bradley KN, Eckman DM, Hill-Eubanks DC, Nelson MT. Intraluminal pressure is a stimulus for NFATc3 nuclear accumulation—Role of calcium, endothelium-derived nitric oxide, and cGMP-dependent protein kinase. J Biol Chem 279: 10702–10709, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Ho YS, Gargano M, Cao J, Bronson RT, Heimler I, Hutz RJ. Reduced fertility in female mice lacking copper-zinc superoxide dismutase. J Biol Chem 273: 7765–7769, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Jankov RP, Kantores C, Pan J, Belik J. Contribution of xanthine oxidase-derived superoxide to chronic hypoxic pulmonary hypertension in neonatal rats. Am J Physiol Lung Cell Mol Physiol 294: L233–L245, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Jasmin JF, Cernacek P, Dupuis J. Activation of the right ventricular endothelin (ET) system in the monocrotaline model of pulmonary hypertension: response to chronic ETA receptor blockade. Clin Sci (Lond) 105: 647–653, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Jernigan NL, Walker BR, Resta TC. Reactive oxygen species mediate RhoA/Rho kinase-induced Ca2+ sensitization in pulmonary vascular smooth muscle following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 295: L515–L529, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kahler J, Ewert A, Weckmuller J, Stobbe S, Mittmann C, Koster R, Paul M, Meinertz T, Munzel T. Oxidative stress increases endothelin-1 synthesis in human coronary artery smooth muscle cells. J Cardiovasc Pharmacol 38: 49–57, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Kalivendi SV, Konorev EA, Cunningham S, Vanamala SK, Kaji EH, Joseph J, Kalyanaraman B. Doxorubicin activates nuclear factor of activated T-lymphocytes and Fas ligand transcription: role of mitochondrial reactive oxygen species and calcium. Biochem J 389: 527–539, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kawamata H, Manfredi G. Import, maturation, and function of SOD1 and its copper chaperone CCS in the mitochondrial intermembrane space. Antioxid Redox Signal 13: 1375–1384, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ke Q, Li J, Ding J, Ding M, Wang L, Liu B, Costa M, Huang C. Essential role of ROS-mediated NFAT activation in TNF-α induction by crystalline silica exposure. Am J Physiol Lung Cell Mol Physiol 291: L257–L264, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Kim SY, Seo M, Kim Y, Lee YI, Oh JM, Cho EA, Kang JS, Juhnn YS. Stimulatory heterotrimeric GTP-binding protein inhibits hydrogen peroxide-induced apoptosis by repressing BAK induction in SH-SY5Y human neuroblastoma cells. J Biol Chem 283: 1350–1361, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Kinnula VL, Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med 167: 1600–1619, 2003 [DOI] [PubMed] [Google Scholar]
- 56. Knappe D, Sill B, Tharun B, Koester R, Baldus S, Muenzel T, Meinertz T, Kahler J. Endothelin-1 in humans is increased by oxygen-derived radicals ex vivo and in vivo. J Investig Med 55: 306–314, 2007 [DOI] [PubMed] [Google Scholar]
- 57. Konduri GG, Bakhutashvili I, Eis A, Pritchard K. Oxidant stress from uncoupled nitric oxide synthase impairs vasodilation in fetal lambs with persistent pulmonary hypertension. Am J Physiol Heart Circ Physiol 292: H1812–H1820, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Lee JE, Kim H, Jang H, Cho EJ, Youn HD. Hydrogen peroxide triggers the proteolytic cleavage and the inactivation of calcineurin. J Neurochem 100: 1703–1712, 2007 [DOI] [PubMed] [Google Scholar]
- 59. Lee MY, Garvey SM, Baras AS, Lemmon JA, Gomez MF, Schoppee Bortz PD, Daum G, LeBoeuf RC, Wamhoff BR. Integrative genomics identifies DSCR1 (RCAN1) as a novel NFAT-dependent mediator of phenotypic modulation in vascular smooth muscle cells. Hum Mol Genet 19: 468–479, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lin HY, Michtalik HJ, Zhang S, Andersen TT, Van Riper DA, Davies KK, Ermak G, Petti LM, Nachod S, Narayan AV, Bhatt N, Crawford DR. Oxidative and calcium stress regulate DSCR1 (Adapt78/MCIP1) protein. Free Radic Biol Med 35: 528–539, 2003 [DOI] [PubMed] [Google Scholar]
- 61. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-[Delta][Delta]CT method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 62. Masri FA, Comhair SA, Tanic-Larson I, Kaneko FT, Dweik RA, Arroliga AC, Erzurum SC. Deficiency of lung antioxidants in idiopathic pulmonary arterial hypertension. Clin Transl Sci 1: 99–106, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mata M, Sarrion I, Milian L, Juan G, Ramon M, Naufal D, Gil J, Ridocci F, Fabregat-Andres O, Cortijo J. PGC-1alpha induction in pulmonary arterial hypertension. Oxid Med Cell Longev 2012: 236572, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Maxey TS, Fernandez LG, Reece TB, Keeling WB, Kron IL, Laubach VE. Endothelial nitric oxide synthase is essential for postpneumonectomy compensatory vasodilation. Ann Thorac Surg 81: 1234–1238, 2006 [DOI] [PubMed] [Google Scholar]
- 65. McMurtry MS, Bonnet S, Wu X, Dyck JRB, Haromy A, Hashimoto K, Michelakis ED. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ Res 95: 830–840, 2004 [DOI] [PubMed] [Google Scholar]
- 66. Moldovan L, Moldovan NI, Sohn RH, Parikh SA, Goldschmidt-Clermont PJ. Redox changes of cultured endothelial cells and actin dynamics. Circ Res 86: 549–557, 2000 [DOI] [PubMed] [Google Scholar]
- 67. Moudgil R, Michelakis ED, Archer SL. Hypoxic pulmonary vasoconstriction. J Appl Physiol 98: 390–403, 2005 [DOI] [PubMed] [Google Scholar]
- 68. Nagendran J, Gurtu V, Fu DZ, Dyck JRB, Haromy A, Ross DB, Rebeyka IM, Michelakis ED. A dynamic and chamber-specific mitochondrial remodeling in right ventricular hypertrophy can be therapeutically targeted. J Thorac Cardiovasc Surg 136: 168–178, 2008 [DOI] [PubMed] [Google Scholar]
- 69. Naik JS, Earley S, Resta TC, Walker BR. Pressure-induced smooth muscle cell depolarization in pulmonary arteries from control and chronically hypoxic rats does not cause myogenic vasoconstriction. J Appl Physiol 98: 1119–1124, 2005 [DOI] [PubMed] [Google Scholar]
- 70. Nilsson-Berglund LM, Zetterqvist AV, Nilsson-Ohman J, Sigvardsson M, Gonzalez Bosc LV, Smith ML, Salehi A, Agardh E, Fredrikson GN, Agardh CD, Nilsson J, Wamhoff BR, Hultgardh-Nilsson A, Gomez MF. Nuclear factor of activated T cells regulates osteopontin expression in arterial smooth muscle in response to diabetes-induced hyperglycemia. Arterioscler Thromb Vasc Biol 30: 218–224, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Norton CE, Broughton BR, Jernigan NL, Walker BR, Resta TC. Enhanced depolarization-induced pulmonary vasoconstriction following chronic hypoxia requires EGFR-dependent activation of NAD(P)H oxidase 2. Antioxid Redox Signal. 2012. Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nozik-Grayck E, Stenmark KR. Role of reactive oxygen species in chronic hypoxia-induced pulmonary hypertension and vascular remodeling. Adv Exp Med Biol 618: 101–112, 2007 [DOI] [PubMed] [Google Scholar]
- 73. Nozik-Grayck E, Suliman HB, Majka S, Albietz J, Van RZ, Roush K, Stenmark KR. Lung EC-SOD overexpression attenuates hypoxic induction of Egr-1 and chronic hypoxic pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol 295: L422–L430, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ou ZJ, Wei W, Huang DD, Luo W, Luo D, Wang ZP, Zhang X, Ou JS. l-Arginine restores endothelial nitric oxide synthase-coupled activity and attenuates monocrotaline-induced pulmonary artery hypertension in rats. Am J Physiol Endocrinol Metab 298: E1131–E1139, 2010 [DOI] [PubMed] [Google Scholar]
- 75. Pahlavani M, Harris M. Effect of in vitro generation of oxygen free radicals on T cell function in young and old rats. Free Radic Biol Med 25: 903–913, 1998 [DOI] [PubMed] [Google Scholar]
- 76. Paulin R, Courboulin A, Meloche J, Mainguy V, Dumas de la RE, Saksouk N, Cote J, Provencher S, Sussman MA, Bonnet S. Signal transducers and activators of transcription-3/pim1 axis plays a critical role in the pathogenesis of human pulmonary arterial hypertension. Circulation 123: 1205–1215, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Perez-Vizcaino F, Cogolludo A, Moreno L. Reactive oxygen species signaling in pulmonary vascular smooth muscle. Respir Physiol Neurobiol 174: 212–220, 2010 [DOI] [PubMed] [Google Scholar]
- 78. Pinto YM, Buikema H, van Gilst WH, Scholtens E, van Geel PP, de Graeff PA, Wagner J, Paul M. Cardiovascular end-organ damage in Ren-2 transgenic rats compared to spontaneously hypertensive rats. J Mol Med 75: 371–377, 1997 [DOI] [PubMed] [Google Scholar]
- 79. Rak R, Chao DL, Pluta RM, Mitchell JB, Oldfield EH, Watson JC. Neuroprotection by the stable nitroxide Tempol during reperfusion in a rat model of transient focal ischemia. J Neurosurg 92: 646–651, 2000 [DOI] [PubMed] [Google Scholar]
- 80. Ran P, Xu J, Chen S. [Expression of endothelin-1 mRNA, endothelin receptor-A and nitric oxide synthase mRNA in pulmonary artery and right ventriculus cordis of rats exposed to hypoxia]. Zhonghua Yi Xue Za Zhi 75: 479–481, 511, 1995 [PubMed] [Google Scholar]
- 81. Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol 15: 707–747, 1997 [DOI] [PubMed] [Google Scholar]
- 82. Rashid M, Fahim M, Kotwani A. Efficacy of tadalafil in chronic hypobaric hypoxia-induced pulmonary hypertension: possible mechanisms. Fundam Clin Pharmacol. 2012. Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 83. Reaume AG, Elliott JL, Hoffman EK, Kowall NW, Ferrante RJ, Siwek DF, Wilcox HM, Flood DG, Beal MF, Brown RH, Jr, Scott RW, Snider WD. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat Genet 13: 43–47, 1996 [DOI] [PubMed] [Google Scholar]
- 84. Reeve HL, Michelakis E, Nelson DP, Weir EK, Archer SL. Alterations in a redox oxygen sensing mechanism in chronic hypoxia. J Appl Physiol 90: 2249–2256, 2001 [DOI] [PubMed] [Google Scholar]
- 85. Rossi GP, Sacchetto A, Rizzoni D, Bova S, Porteri E, Mazzocchi G, Belloni AS, Bahcelioglu M, Nussdorfer GG, Pessina AC. Blockade of angiotensin II type 1 receptor and not of endothelin receptor prevents hypertension and cardiovascular disease in transgenic (mREN2)27 rats via adrenocortical steroid-independent mechanisms. Arterioscler Thromb Vasc Biol 20: 949–956, 2000 [DOI] [PubMed] [Google Scholar]
- 86. Rubens C, Ewert R, Halank M, Wensel R, Orzechowski HD, Schultheiss HP, Hoeffken G. Big endothelin-1 and endothelin-1 plasma levels are correlated with the severity of primary pulmonary hypertension. Chest 120: 1562–1569, 2001 [DOI] [PubMed] [Google Scholar]
- 87. Said SI, Hamidi SA, Gonzalez Bosc L. Asthma and pulmonary arterial hypertension: do they share a key mechanism of pathogenesis? Eur Respir J 35: 730–734, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sato K, Webb S, Tucker A, Rabinovitch M, O'Brien RF, McMurtry IF, Stelzner TJ. Factors influencing the idiopathic development of pulmonary hypertension in the fawn hooded rat. Am J Respir Crit Care Med 145: 793–797, 1992 [DOI] [PubMed] [Google Scholar]
- 89. Seccia TM, Belloni AS, Kreutz R, Paul M, Nussdorfer GG, Pessina AC, Rossi GP. Cardiac fibrosis occurs early and involves endothelin and AT-1 receptors in hypertension due to endogenous angiotensin II. J Am Coll Cardiol 41: 666–673, 2003 [DOI] [PubMed] [Google Scholar]
- 90. Shefner JM, Reaume AG, Flood DG, Scott RW, Kowall NW, Ferrante RJ, Siwek DF, Upton-Rice M, Brown RH., Jr Mice lacking cytosolic copper/zinc superoxide dismutase display a distinctive motor axonopathy. Neurology 53: 1239–1246, 1999 [DOI] [PubMed] [Google Scholar]
- 91. Steudel W, Scherrer-Crosbie M, Bloch KD, Weimann J, Huang PL, Jones RC, Picard MH, Zapol WM. Sustained pulmonary hypertension and right ventricular hypertrophy after chronic hypoxia in mice with congenital deficiency of nitric oxide synthase 3. J Clin Invest 101: 2468–2477, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sutendra G, Bonnet S, Rochefort G, Haromy A, Folmes KD, Lopaschuk GD, Dyck JR, Michelakis ED. Fatty acid oxidation and malonyl-CoA decarboxylase in the vascular remodeling of pulmonary hypertension. Sci Transl Med 2: 44ra58, 2010 [DOI] [PubMed] [Google Scholar]
- 93. Sutendra G, Dromparis P, Bonnet S, Haromy A, McMurtry MS, Bleackley RC, Michelakis ED. Pyruvate dehydrogenase inhibition by the inflammatory cytokine TNFalpha contributes to the pathogenesis of pulmonary arterial hypertension. J Mol Med 89: 771–783, 2011 [DOI] [PubMed] [Google Scholar]
- 94. Trevillyan JM, Chiou XG, Chen YW, Ballaron SJ, Sheets MP, Smith ML, Wiedeman PE, Warrior U, Wilkins J, Gubbins EJ, Gagne GD, Fagerland J, Carter GW, Luly JR, Mollison KW, Djuric SW. Potent inhibition of NFAT activation and T cell cytokine production by novel low molecular weight pyrazole compounds. J Biol Chem 276: 48118–48126, 2001 [DOI] [PubMed] [Google Scholar]
- 95. Troncoso Brindeiro CM, da Silva AQ, Allahdadi KJ, Youngblood V, Kanagy NL. Reactive oxygen species contribute to sleep apnea-induced hypertension in rats. Am J Physiol Heart Circ Physiol 293: H2971–H2976, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Van RZ, Fattman C, Domarski S, Majka S, Klemm D, Stenmark KR, Nozik-Grayck E. Lung extracellular superoxide dismutase overexpression lessens bleomycin-induced pulmonary hypertension and vascular remodeling. Am J Respir Cell Mol Biol 44: 500–508, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Walther S, Edwards JN, Blatter LA. Effects of reactive oxygen species on NFAT activation and translocation in adult rabbit ventricular myocytes. Biophys J 104: 302a, 2013 [Google Scholar]
- 98. Wedgwood S, Steinhorn RH, Bunderson M, Wilham J, Lakshminrusimha S, Brennan LA, Black SM. Increased hydrogen peroxide downregulates soluble guanylate cyclase in the lungs of lambs with persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 289: L660–L666, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Weir EK, Archer SL. The mechanism of acute hypoxic pulmonary vasoconstriction: the tale of two channels. FASEB J 9: 183–189, 1995 [DOI] [PubMed] [Google Scholar]
- 100. Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res 94: 110–118, 2004 [DOI] [PubMed] [Google Scholar]
- 101. Wilkins BJ, De Windt LJ, Bueno OF, Braz JC, Glascock BJ, Kimball TF, Molkentin JD. Targeted disruption of NFATc3, but not NFATc4, reveals an intrinsic defect in calcineurin-mediated cardiac hypertrophic growth. Mol Cell Biol 22: 7603–7613, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wilkins BJ, Molkentin JD. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochem Biophys Res Commun 322: 1178–1191, 2004 [DOI] [PubMed] [Google Scholar]
- 103. Xu D, Guo H, Xu X, Lu Z, Fassett J, Hu X, Xu Y, Tang Q, Hu D, Somani A, Geurts AM, Ostertag E, Bache RJ, Weir EK, Chen Y. Exacerbated pulmonary arterial hypertension and right ventricular hypertrophy in animals with loss of function of extracellular superoxide dismutase. Hypertension 58: 303–309, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med 34: 1359–1368, 2003 [DOI] [PubMed] [Google Scholar]
- 105. Zielonka J, Zielonka M, Sikora A, Adamus J, Joseph J, Hardy M, Ouari O, Dranka BP, Kalyanaraman B. Global profiling of reactive oxygen and nitrogen species in biological systems: high-throughput real-time analyses. J Biol Chem 287: 2984–2995, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]