Abstract
Household air pollution (HAP) from indoor burning of biomass or coal is a leading global cause of morbidity and mortality, mostly due to its association with acute respiratory infection in children and chronic respiratory and cardiovascular diseases in adults. Interventions that have significantly reduced exposure to HAP improve health outcomes and may reduce mortality. However, we lack robust, specific, and field-ready biomarkers to identify populations at greatest risk and to monitor the effectiveness of interventions. New scientific approaches are urgently needed to develop biomarkers of human exposure that accurately reflect exposure or effect. In this Perspective, we describe the global need for such biomarkers, the aims of biomarker development, and the state of development of tests that have the potential for rapid transition from laboratory bench to field use.
Keywords: air pollution, exposure, global health, biomarkers
household air pollution (HAP) is a daily exposure for nearly half of the world's population and a leading environmental cause of death. HAP-related morbidity and mortality predominantly affects people in low and middle-income countries (LMIC), especially women and young children. Almost 4 million deaths per year due to childhood respiratory infection and chronic lung disease, lung cancer, and cardiovascular disease in adults are attributable to HAP (22, 32). HAP exposure in LMIC mostly results from the combustion of biomass fuels (wood, charcoal, dung and crop residues), coal, or kerosene within homes for cooking, heating, and lighting (49). Biomass fuels have also been implicated in the development of tuberculosis, asthma, cataracts, and low birth weight (13).
Even in high-income countries such as the US and Canada, HAP is an issue, especially for the rural poor (5). In Southwest Alaska, the Pacific Northwest, tribal lands, and Appalachia as much as 60% of the population burns solid fuels like wood, coal, and/or coke for seasonal heating. In these areas, HAP exceeds the World Health Organization air quality guidelines in up to 80% of homes (5). Similar exposures probably exist in other high-income countries, although this represents only a fraction of the global burden that occurs in LMIC (36).
Given the effects of exposure, and the large at-risk population, massive effort is being directed globally at HAP exposure reduction programs, mostly centered on the implementation of improved cookstoves. For example, a multinational public-private partnership between the US government and the United Nations Foundation launched the Global Alliance for Clean Cookstoves (http://www.cleancookstoves.org), which aims to have 100 million homes adopt clean and efficient stoves and fuels by 2020. Increasing the efficiency of combustion in cooking and heating appliances should provide multiple benefits, including reduced expenditure on fuel, local environmental protection leading to increased livelihood security, and potentially wider benefits on climate change. Reduction of health risks associated with HAP by any intervention remains challenging (25), and success is determined by the level of community and household adoption. Cook stove and other intervention programs urgently need to define and monitor personal exposure and directly evaluate the health benefits of exposure reduction, especially as intervention programs are scaled up. Effective biomarkers used in these studies will allow us to determine which interventions work for meaningful health outcomes and ensure that the significant resources now available are appropriately directed. This requires the scientific community to develop improved biomarkers of both exposure and health risk and to validate these in human studies to help assess the impact of millions of new stoves new types of fuel.
In this Perspective, we provide an overview of current approaches to HAP assessment, the aims of biomarker development, and the state of development of tests that have the potential for rapid transition from laboratory bench to field use. This is not a comprehensive review, but it should direct the reader to important and exciting areas of current research.
Current Approaches to Indoor Smoke Exposure Assessment
Current HAP assessment tools include direct quantitative measurement of products of incomplete combustion as well as qualitative methods including use of questionnaires or the categorization of HAP exposure by the type of cookstove or fuel.
Particulate matter.
Particulate matter [PM; measured as the concentration of particles less than 2.5 or 10 μm in diameter (PM2.5 and PM10)] is used to describe ambient outdoor and indoor air pollution, in tandem with measurements of ozone and oxides of nitrogen and sulfur. Ultrafine particles (less than 100-μm diameter) are also important because they have high toxicity per mass owing to their high surface area. PM is usually monitored at stationary sites and represents emissions from fossil fuel use for energy and transport, agricultural management and forest fires, and HAP. As a major constituent of HAP, black carbon (soot) represents a target for improving health (2). High levels of ambient PM exposure are associated with low birth weight (45), myocardial infarction (34), cardiovascular mortality, lung cancer with poorer lung cancer outcomes (50), reduced lung function (11), and total mortality (38).
Static particulate measurement has been the standard for assessment of outdoor air pollution. Gravimetric sampling is the accepted standard for quantification of airborne particulate mass and allows both cumulative mass exposure and chemical composition to be retrospectively determined. Photometric detection, by real-time monitoring of changes in light diffraction, generates more refined time-exposure data. Indoors, static measurement can be combined with positioning information to account for exposure variations due to time and proximity to the source, fuel type and condition (water content), burn conditions (completeness of oxidation), and personal behavior such as seasonal outdoor fire use and night time exposure from a partially extinguished source in the sleeping areas. These factors are also likely to determine the correlation between PM and other measures such as carbon monoxide, which can vary widely.
Direct exposure assessment of HAP by personal monitoring is commonly preferred to static monitoring of rooms because it takes into account proximity to the source and other behaviors that modify exposure. Difficulties arise due to the size, portability, and recording capacity of equipment and by acceptability to the user. New devices currently being field tested and scaled up for commercial use address many of these concerns.
Particulate measurement alone also cannot differentiate the multiple sources of pollution (e.g., mixtures of HAP, tobacco smoke, and outdoor pollution).
Carbon monoxide monitoring.
Personal environmental CO monitoring is a helpful proxy of HAP, particularly when black carbon emission is low (e.g., charcoal burning); such situations are especially dangerous to users because the absence of visible smoke permits levels of CO to rise undetected. Electrochemical sensors record levels of CO with high temporal resolution but are expensive. Diffusion tubes represent a simpler method: by passive diffusion CO enters a short tube worn on the outer clothing and catalyzes the reduction of sodium pallado sulfite. The resulting palladium discolors a length of tube proportional to total CO exposure. These measurements are associated with important health effects, including the rate of respiratory infections (43) and neurodevelopmental outcomes in young children exposed in utero (10).
Qualitative measures.
Epidemiological studies continue to identify populations at risk from HAP and to define biological and behavioral correlates of risk. Questionnaire studies of exposure are commonly employed in intervention programs as an adjunct to quantitative data. Typically, self-report questionnaires are employed that detail information on exposure, often related to cooking times. Stove use monitoring systems can be used to objectively describe the pattern of exposures from cooking, and these measures can be compared with environmental monitoring of pollutant concentrations in household air. Time-activity surveys and questionnaires allow time-weighted exposures to be estimated (14). Modeling methods can refine these exposure estimates, and incorporating biomarker data might provide further improvements.
In summary, current HAP exposure measurement methods are expensive, technically challenging, and difficult to use with large population studies and have substantial limitations, making an urgent case for the development of biomarkers of both exposure and health effects.
Need for Improved Biomarkers of HAP
The National Institutes of Health defines a biomarker as a “characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacological responses to a therapeutic intervention.” In HAP, these may include biomarkers of exposure, susceptibility, or effect. Validation of biomarkers by comparison with direct measurement of specific HAP components is a key research need. We focus on biomarkers of exposure because these are most urgently needed and potentially most accessible. However, further development of biomarkers of susceptibility and effect could facilitate large-scale studies examining the impact of HAP on health and disease in human populations.
Effective biomarkers of HAP would 1) improve epidemiological accuracy in association studies with health effect, 2) reduce the cost and complexity of monitoring intervention studies, 3) provide data for education of the public and policymakers about risk, and 4) inform clinicians and the public health community about human environmental exposures that are not well characterized.
Biomarkers in public health interventions.
Public health interventions to reduce the health effects of HAP are well under way. These also address wider concerns about the environment (reducing fuel use), the empowerment of women (by reducing the time spent collecting wood), and community (improving fuel security). Although the stated intent is often to improve health, the complexity and expense of exposure and health measurements have limited good-quality data. The RESPIRE study in Guatemala demonstrated for the first time in a randomized controlled trial that improved cookstoves can significantly reduce exposures and reduce risk for severe pneumonia in children under the age of 5 (42). Adopting validated biomarkers within larger scale studies would be likely to improve the cost and ease of assessing health risks. Widespread adoption of such monitoring could enhance the effectiveness and geographical scope of large scale implementation programs, although this has yet to be tested.
Biomarkers for educational use.
Biomarker testing is a useful educational tool when results are communicated to the public and policymakers. Although the adoption of improved/advanced (nontraditional) cookstoves may by mostly driven by economics (30), biomarkers could demonstrate health benefits to policymakers as well as end users.
Biomarkers of clinical diagnosis and markers of disease progression.
Although biomarkers can be used for diagnostic testing, HAP biomarkers are unlikely to be useful in this context because 1) validation against disease outcomes is difficult because of the limited data available from health systems in developing countries; 2) biomass exposure is associated with common diseases such as lower respiratory tract infection and chronic obstructive pulmonary disease (COPD) that have multiple risk factors. Individual biological monitoring would allow more in depth mechanistic studies of harm, however, and would give useful data on inter- and intraindividual variation in exposure.
Biomarker development.
The Institute of Medicine in a recent report to the Food and Drug Administration suggested a three-stage framework for the development of biomarkers (28): 1) Analytical validation: to ensure reliability, reproducibility, sensitivity, and specificity; 2) Qualification: to confirm a strong association with the clinical outcome of concern; and 3) Utilization: contextual analysis to determine that the biomarker is appropriate for the proposed use. Specific issues relating to development and field use of HAP biomarkers are summarized in Table 1.
Table 1.
Characteristics of ideal HAP biomarkers
| Issue | Requirement |
|---|---|
| Field readiness | |
| Rural population | Stable to temperature, storage, and transport conditions |
| Lack of cold storage | |
| Limited laboratory equipment | Single point of care test is ideal(37), such as those sought in the Gates' Foundation “Grand Challenges in Global Health” |
| Marker of exposure | |
| Time lag from exposure to outcome | Chronic complications, such as COPD result from long exposures. Measuring exposure rather than health outcome is more immediately relevant for monitoring and intervention purposes |
| Heterogeneity of effect by age | Children most susceptible to infection, and adults to chronic respiratory complications. Biomarker of exposure more likely to be widely relevant |
| Rapid fluctuations in exposure levels | Reflect cumulative exposure over days rather than hours |
| Validity/applicability | |
| External validity: consistency with current measurements | Strong correlation between currently used metrics of exposure to ensure continuity within research and intervention programmes. Correlation with disease risk or outcome is ideal, but of secondary importance |
| Specificity | Discriminate from other particulate exposures (e.g., tobacco, traffic pollution) |
| Sensitivity | Be sensitive to multiple fuel sources (e.g., wood, charcoal, dung, paraffin) |
| Pretest likelihood of outcome unknown | Interpretable independent of background data. Unlike clinical biomarkers, there is no known “pretest probability” |
| Use in diverse populations | Consistent throughout the population at risk. Heterogeneity of response due to genetic polymorphisms [e.g., in metabolic pathways can be a problem (44)] |
To be useful and meaningfully implemented, household air pollution (HAP) biomarkers should pass tests of field use and applicability. This table summarizes key characteristics.
COPD, chronic obstructive pulmonary disease.
Biomarkers of exposure to HAP.
Biomarkers for environmental particulate exposure are already in use: for example, cigarette smoke exposure can be estimated by concentrations of nicotine and its metabolites, tobacco-specific derivatives of nitrosamine, and metabolites of pyrene, acrolein, and butadiene, among other markers (40). This model underscores that biomarkers of inhaled substances may be assessed in multiple body compartments, each with different implications for chronicity of exposure. Biomass and coal smoke contain some of these and other constituents that may cause direct toxicity, generate reactive oxygen species, and enhance inflammatory pathways. Targets for HAP biomarker development therefore include the gaseous and particulate products of biomass combustion and less specific measures of inflammation, oxidative stress, carcinogenesis, and endothelial activation (23). Existing biomarkers and tracers of wood burning in the context of forest fires and in diesel exhaust and ambient air pollution might also be employed.
No single potential biomarker has been found to be sufficiently precise in both controlled and field conditions. In settings where HAP exposures are very high, single tests could be used in field trials even when their specificity is relatively low. In moderate and lower exposures, combining two or more measurements within a composite end point would increase costs but may be an effective approach.
Figure 1 shows in vivo metabolism and excretion of these potential biomarkers. Currently, urinary and plasma biomarkers seem most extensively studied. However, other methods provide significant advantages, including dried blood spots, exhaled breath, induced sputum, transcutaneous, and personal area monitoring.
Fig. 1.
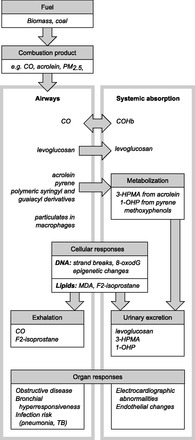
Potential biomarkers of biomass exposure, metabolism, and excretion. Biomarkers may be derived from primary products of combustion, from their metabolites or from products of their interaction with body proteins, DNA, and lipids. Biomarkers may measurable from one or more body compartment, as summarized here. PM2.5, particulate matter measured as the concentration of particles less than 2.5 μm in diameter; COHb, carboxyhemoglobin; MDA, malondialdehyde; 1-OHP, 1-hydroxypyrene; 3-HPMA, 3-hydroxypropylmercapturic acid.
Exhaled CO and COHb.
Invariably, CO is a by-product of incomplete combustion even with use of advanced stoves and “cleaner fuels” such as liquefied petroleum gas (LPG). Exhaled CO correlates with environmental CO in highly HAP exposed individuals (21) and can be measured in the field with relatively easy-to-use and inexpensive devices. Blood carboxyhemoglobin (COHb) can also be measured by spectrophotometry. In complex HAP reduction programs, COHb among 20 subjects fell from a preintervention range of 1.1 to 13.9% to a postintervention range of 0.7 to 1.3% (48). Transcutaneous measurement of COHb offers the potential for ease of use without the need for access to peripheral blood and correlates with blood samples, although field testing has shown considerable random error at low levels: the required accuracy is debatable even when used in clinical contexts (24). Further testing is needed to validate whether noninvasive assessment of COHb is accurate, reproducible, relevant to air quality monitoring, and practical in low-resource settings.
CO or COHb are already accepted clinical tests supported by a body of literature related to acute and chronic exposures. However, there is a further need for research to assess their validity as biomarkers in the context of HAP.
Methoxyphenols.
Methoxylated phenols contribute by weight around one-fifth of organic pollutants in urban air. Wood burning results in the pyrolysis of lignins to form polymeric syringyl or guaiacyl propane derivatives. Syringyl derivatives are found in higher proportions in hard wood combustion products (15), have lower background concentrations, and are detectable by gas chromatography-mass spectroscopy of urine. However, metabolism is variable: the usual elimination half-life for syringyls is around 2–3 h, but some individuals demonstrate late peaks around 30 h, which may represent enterohepatic recirculation (9). Dietary intake of smoked foods and some over-the-counter cold remedies increase levels of methoxyphenols (9), as might tobacco smoke.
Urinary syringyl methoxyphenols have been demonstrated to correlate with personal CO exposure in cookstove intervention studies (7) and with personal PM2.5 exposure to indoor wood fires (9).
Levoglucosan.
Levoglucosan (1,6-anhydro-β-d-glucopyranose) is formed by the burning of cellulose and starches. As the major single organic contributor to wood smoke, it is used as a tracer in ambient monitoring, but also forms in tobacco smoke. Relative quantities depend strongly on wood type, but increased urinary levels can be measured after wood smoke inhalation. By gas chromatography-mass spectrometry, 85% of an intranasal dose was excreted unmetabolized within 4 h (29). Diesel exhaust-exposed mice showed no increase, suggesting specificity of fuel type. Preliminary, uncontrolled human exposure experiments have not demonstrated a correlation between personal PM2.5 or home use of wood stove and urinary levoglucosan (4), perhaps because of dietary contribution from various sources (e.g., caramels). Among wild land firefighters, urinary levoglucosan does not consistently rise after exposure (17, 35).
Measures of oxidative stress.
MALONDIALDEHYDE/ISOPROSTANES.
Malondialdehyde (MDA, formed from the peroxidation of polyunsaturated lipid) and 8-isoprostane (derived from the interaction of arachidonic acid and free radicals) can be used to measure oxidative stress. Despite considerable evidence of increased levels in disease states, few studies have examined lipid peroxidation markers in HAP. 8-Isoprostane may be measured by liquid chromatography-mass spectroscopy or ELISA. Both MDA and 8-isoprostane may be measured in urine, serum, and exhaled breath condensate and may be increased after inhalation of various forms of PM, including that in cigarette smoke (51).
Controlled experimental exposure to wood smoke in humans increases MDA in exhaled breath and 8-isoprostane urinary excretion (3). A cohort study comparing highly biomass-exposed (rural) dwellers with an urban group found that serum levels of MDA in Turkish women were raised in the former group (18). A blinded randomized intervention used residential HEPA filtration as an intervention in a community shown to have high levels of residential wood combustion. Indoor PM2.5 was reduced by approximately two-thirds, but with low absolute decreases of 6.6 μg/m3. Both MDA and 8-isoprostane concentrations were unchanged (1). So far, there is insufficient evidence to use MDA and 8-isoprostane as biomarkers for clinical studies.
DNA DAMAGE/MUTAGENESIS.
DNA damage is a marker of oxidant-mediated injury from exposure to HAP and links such exposures mechanistically to increased rates of lung cancer (33). A simple screening assay for DNA damage is the comet assay, which measures DNA breaks by the differential movement of DNA subjected to electrophoresis on agarose gels. A more precise measure of DNA damage, 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG), is formed after oxidation of the guanine nucleotide in DNA. It may be detected in plasma or urine by HPLC, liquid chromatography or ELISA. 8-oxodG is increased in the urine of mice exposed to ultrafine particles (47), but the usefulness of this assay as a biomarker of HAP is extrapolated from the literature on atmospheric PM. Using more than one measure of DNA damage or combining with tests of oxidative stress should be considered to give a more accurate assessment of genotoxicity and carcinogenicity in humans.
A recent meta-analysis of the usefulness of these potential biomarkers of exposure to air pollution (31) suggested that 8-isoprostanes, MDA, and 8-oxodG show promise but require testing in large-scale prospective studies. It should be noted, however, that of more than 30 studies included, only one study (18) specifically related to HAP.
Polycyclic aromatic hydrocarbons and their metabolites.
Incomplete combustion of biomass or fossil fuels may result in the formation of polycyclic aromatic hydrocarbons (PAH, three or more fused benzene rings). Systemic absorption of pyrenes (4-ring structures) from traffic-related air pollution and occupational exposure can be measured by the metabolite urinary 1-hydroxypyrene (1-OHP) by HPLC. Urinary 1-OHP increases in those exposed to gas cookers compared with electric ones (12) and reflects environmental exposure even at low levels, although it is increased in passive cigarette smoke exposure and with some foods. In communities using wood as a fuel for cooking, 1-OHP is increased (39), but in only one of three Mexican communities examined did concentrations exceed those suggested as occupational exposure limits (1.4 μmol/mol creatinine). A study in Burundi compared urban and rural indoor environments: in the latter PAH mean concentrations were 43 μg/m3. In rural dwellers, urinary 1-OHP levels were 30 times higher than in urban dwellers (1.5 vs. 0.05 μmol/mol creatinine) and were independent of cigarette smoking (52). Similar results are reported from Afghanistan (2.1 vs. 0.75 μmol/mol creatinine in adults and children from rural vs. urban communities) (16). These studies were uncorrected for vehicle emissions.
In the context of clean stove interventions in Mexico, 1-OHP levels fell from 6.7 to 4.8 μmol/mol creatinine when associated with a fall in mean COHb from 4.9 to 1.0% (48). Similar results were obtained at a separate site, despite a threefold decrease in CO exposure, suggesting that overall exposures remained high. 1-OHP measurement is complicated by polymorphisms in the metabolic enzyme CYP1A1 that are associated with twofold higher urinary levels (53).
Aldehyde-protein compounds.
Acrolein, an unsaturated aldehyde, can be produced by burning almost any hydrocarbon and is both ingested and inhaled. It has been extensively investigated for its toxic effects that include lipid peroxidation and binding of cytoplasmic and nuclear nucleophiles. Downstream effects of acrolein include activation of proinflammatory signaling through NF-κB and AP-1 transcription factors. Specific metabolites such as 3-hydroxypropylmercapturic acid can be measured in the urine by liquid chromatography-tandem mass spectroscopy and gas chromatography. Levels are raised in cigarette smokers (6) but are unknown in HAP.
Epigenetic modifications.
A recent report notes DNA methylation changes in newborns whose mothers were active tobacco smokers during pregnancy (19). Differential methylation across the genome occurs mostly in early life. It may explain HAP-associated adverse outcomes such as low birth weight, impaired lung function, childhood infections, and, potentially, the increased risk of noncommunicable diseases such as asthma and COPD. Postnatal cord blood specimen, together with exposure assessments during pregnancy, may provide insights into future disease risk of children exposed to HAP during vulnerable windows of organ development.
Biomarkers of Health Effect from HAP
Biomarkers of health effects may overlap with the anticipated physiological effects of exposure. A number of such associations have been noted, which are commonly early pathological events and subclinical effects. Although these tend to lack specificity, they may provide a useful proxy measure of exposure in well-defined populations. Further development could improve our current understanding of the physiological effects of HAP exposure, which is to date not well understood.
Pulmonary.
Cross-sectional surveys suggest a reduction in FEV1 in household members using biomass fuel compared with LPG. This correlates with PM10 concentrations and duration and intensity of exposure (8). Long lag times between exposure and disease, and considerable confounding, make these measurements alone unlikely to be useful as a HAP biomarker. Bronchial hyperresponsiveness is a feature of HAP-related lung disease but has not been examined in a HAP-exposed asymptomatic population.
As a marker of airway inflammation, the fraction of exhaled nitric oxide (FeNO) possibly increases after experimental and ambient wood smoke exposure, although the data are somewhat unclear (46, 51). FeNO has been expensive and complex, precluding its widespread use in field studies in LMIC, but continued improvements in the technology may allow its wider use. Experimental exposure to wood smoke causes systemic inflammation and increases a marker of alveolar epithelial permeability (CC16), which has relevance to cardiovascular effects.
Chest radiography is not sensitive enough to demonstrate effects of HAP. However, frequent interstitial changes in lungs of smokers suggest that it is plausible that similar changes may be present in those exposed to HAP.
Pulmonary macrophages may be isolated from spontaneously coughed or induced sputum, or more directly by bronchoalveolar lavage. Such measurements assess PM2.5 exposure, are increased with biomass exposure (20), and correlate inversely with lung function test results, but they require technical skill and resources that may be beyond the capabilities of many institutions in LMIC. Similarly, effects at the level of the immune system such as alveolar macrophage dysfunction are measurable but invasive and technologically demanding.
Cardiovascular.
Exposure to PM2.5 is associated with increased inflammatory markers (IL-6, IL-8, TNF-α, C-reactive protein), oxidative stress, reduced heart rate variability and increased arterial stiffness. HAP exposure reduction decreases blood pressure (27) and ECG abnormalities (26). None of these measures are specific to air pollutant exposures, although they are often sensitive enough to detect physiological and biological responses to the exposures.
Avenues for Developing the Potential Assays
Potential biomarkers for HAP exist but few if any have been validated for use in clinical studies. Without a gold standard test, external validation is challenging but should involve other accepted measures such as PM and CO concentrations. Disease pathogenesis that may be well understood in acute exposure is likely to be altered in chronic exposures (41). Animal models, especially for chronic exposures, could be useful for pharmacological and toxicological validation and for comparing effects across different fuel types and conditions.
Longitudinal studies of HAP health effects are likely to be confounded by multiple associations with poverty. They may be complicated by the effects of genetic predisposition, diet, recurrent infections, other exposures such as tobacco smoke, outdoor air pollution, etc. Developers of biomarkers must keep these in mind because the biomarker itself may be subject to alteration by unexpected confounding factors. When biomarkers are to be studied in human populations, these are ideally embedded within an intervention study. This allows an assessment of performance at “real world” concentrations and external validation. Additionally, physiological responses to chronic exposure are not well described but may induce protective phenotypes that alter the expected dose-response relationship. Because no single measurement describes HAP perfectly, multiple associations are required, and normal ranges are yet to be established. Some potential avenues of investigation, and their physiological basis, are given in Table 2.
Table 2.
Potential lines of investigation for HAP biomarkers — ambient and ex vivo measurement
| Investigation | Physiological Relevance |
|---|---|
| Exhaled CO/transcutaneous COHb: field test in HAP | High CO might explain atherosclerosis and foetal effects (through left shift in O2 dissociation curve and myoglobin binding) |
| Methoxyphenols, levoglucosan: field test and controlled use in HAP | Unmetabolized urinary product should reflect exposure — physiological determinants of this correlation are not well understood |
| 1-OHP: field tests for discrimination of pyrene metabolite levels at low concentration | Polyaromatic hydrocarbons known to be carcinogenic Unknown relevance of CYP enzyme polymorphisms in terms of biomarker |
| DNA methylation: case control or cohort studies of effect of HAP | Epigenetic effects may explain long term effects (e.g., ischaemic heart disease) Known effects of methylation on promoters of genes for inflammatory pathways (e.g., iNOS) |
| Generation of robust animal models for HAP, especially chronic exposures | Chronic exposure effects are likely to be different than experimental acute exposures due to physiological responses (e.g., antioxidant upregulation, negative and positive feedback within signalling pathways) |
| Malondialdehyde, 8-isoprostane, 8-oxo-7,8-dihydro-2′-deoxyguanosine: Investigation of urinary and plasma performance in controlled and field tests | HAP products are known to cause oxidative stress including lipid peroxidation and DNA strand breaks. Measurement within organisms is complicated by buffering and repair mechanisms. |
A summary of potential investigations and promising biomarkers: their physiological relevance.
COHb, carboxyhemoglobin;1-OHP, 1-hydroxypyrene; iNOS, inducible nitric oxide synthase.
Current Challenges and Recommendations
There is a need to document worldwide exposures and health effects due to HAP, particularly in LMIC where billions of people continue to use solid fuels such as biomass or coal. The biology of exposure to fine particulate matter and the induction of oxidative stress have been well documented in other areas. However, few of the biomarkers discussed here have been used to assess the impact of HAP on these markers human health and disease. Virtually none meet usability criteria in the context of field use in low-resource settings (see Table 1). The grand challenge to the research community is to produce simple and validated tests to identify populations at risk from HAP and individual responses to exposure reduction strategies.
Development of biomarkers is time pressured. Large-scale implementation programs are already underway. Field researchers, public health officials, and advocacy groups need quick, simple, and robust HAP exposure biomarkers. Improvements in point-of-care screening and diagnostics are enhancing global healthcare delivery in hard-to-reach populations by using uncomplicated equipment that can be deployed at lower cost owing to economies of scale. The current global campaign to promote clean cooking solutions provides an opportunity to produce a biomarker of HAP exposure that could enhance intervention programs that are being applied to one of the most urgent and widespread public health issues of our time.
GRANTS
This manuscript was supported in part by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.R. and W.J.M. II conception and design of research; J.R., S.B.G., and W.J.M. II prepared figures; J.R., S.B.G., and W.J.M. II drafted manuscript; J.R., S.B.G., L.P.N., A.P., J.R.B., O.A., D.K.R., and W.J.M. II edited and revised manuscript; J.R., S.B.G., L.P.N., A.P., J.R.B., O.A., D.K.R., and W.J.M.I. approved final version of manuscript.
REFERENCES
- 1. Allen RW, Carlsten C, Karlen B, Leckie S, van Eeden S, Vedal S, Wong I, Brauer M. An air filter intervention study of endothelial function among healthy adults in a woodsmoke-impacted community. Am J Respir Crit Care Med 183: 1222–1230, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Anenberg SC, Schwartz J, Shindell D, Amann M, Faluvegi G, Klimont Z, Janssens-Maenhout G, Pozzoli L, Van Dingenen R, Vignati E, Emberson L, Muller NZ, West JJ, Williams M, Demkine V, Hicks WK, Kuylenstierna J, Raes F, Ramanathan V. Global air quality and health co-benefits of mitigating near-term climate change through methane and black carbon emission controls. Environ Health Perspect 120: 831–839, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barregard L, Sallsten G, Gustafson P, Andersson L, Johansson L, Basu S, Stigendal L. Experimental exposure to wood-smoke particles in healthy humans: effects on markers of inflammation, coagulation, and lipid peroxidation. Inhal Toxicol 18: 845–853, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Bergauff MA, Ward TJ, Noonan CW, Migliaccio CT, Simpson CD, Evanoski AR, Palmer CP. Urinary levoglucosan as a biomarker of wood smoke: results of human exposure studies. J Expo Sci Environ Epidemiol 20: 385–392, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bunnell JE, Garcia LV, Furst JM, Lerch H, Olea RA, Suitt SE, Kolker A. Navajo coal combustion and respiratory health near Shiprock, New Mexico. J Environ Public Health 2010: 260525, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carmella SG, Chen M, Zhang Y, Zhang S, Hatsukami DK, Hecht SS. Quantitation of acrolein-derived (3-hydroxypropyl)mercapturic acid in human urine by liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry: effects of cigarette smoking. Chem Res Toxicol 20: 986–990, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clark M, Paulsen M, Smith KR, Canuz E, Simpson CD. Urinary methoxyphenol biomarkers and woodsmoke exposure: comparisons in rural Guatemala with personal CO and kitchen CO, levoglucosan, and PM2.5. Environ Sci Technol 41: 3481–3487, 2007 [DOI] [PubMed] [Google Scholar]
- 8. da Silva LF, Saldiva SR, Saldiva PH, Dolhnikoff M. Impaired lung function in individuals chronically exposed to biomass combustion. Environ Res 112: 111–117, 2012 [DOI] [PubMed] [Google Scholar]
- 9. Dills RL, Paulsen M, Ahmad J, Kalman DA, Elias FN, Simpson CD. Evaluation of urinary methoxyphenols as biomarkers of woodsmoke exposure. Environ Sci Technol 40: 2163–2170, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Dix-Cooper L, Eskenazi B, Romero C, Balmes J, Smith KR. Neurodevelopmental performance among school age children in rural Guatemala is associated with prenatal and postnatal exposure to carbon monoxide, a marker for exposure to woodsmoke. Neurotoxicology 33: 246–254, 2012 [DOI] [PubMed] [Google Scholar]
- 11. Forbes LJ, Kapetanakis V, Rudnicka AR, Cook DG, Bush T, Stedman JR, Whincup PH, Strachan DP, Anderson HR. Chronic exposure to outdoor air pollution and lung function in adults. Thorax 64: 657–663, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Freire C, Abril A, Fernandez MF, Ramos R, Estarlich M, Manrique A, Aguirre A, Ibarluzea J, Olea N. Urinary 1-hydroxypyrene and PAH exposure in 4-year-old Spanish children. Sci Total Environ 407: 1562–1569, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Fullerton DG, Bruce N, Gordon SB. Indoor air pollution from biomass fuel smoke is a major health concern in the developing world. Trans R Soc Trop Med Hyg 102: 843–851, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fullerton DG, Semple S, Kalambo F, Suseno A, Malamba R, Henderson G, Ayres JG, Gordon SB. Biomass fuel use and indoor air pollution in homes in Malawi. Occup Environ Med 66: 777–783, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hawthorne SB, Krieger MS, Miller DJ, Mathiason MB. Collection and quantitation of methoxylated phenol tracers for atmospheric pollution from residential wood stoves. Environ Sci Technol 23: 470–475, 1989 [Google Scholar]
- 16. Hemat H, Wittsiepe J, Wilhelm M, Muller J, Goen T. High levels of 1-hydroxypyrene and hydroxyphenanthrenes in urine of children and adults from Afghanistan. J Expo Sci Environ Epidemiol 22: 46–51, 2012 [DOI] [PubMed] [Google Scholar]
- 17. Hinwood AL, Trout M, Murby J, Barton C, Symons B. Assessing urinary levoglucosan and methoxyphenols as biomarkers for use in woodsmoke exposure studies. Sci Total Environ 402: 139–146, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Isik B, Isik RS, Akyildiz L, Topcu F. Does biomass exposure affect serum MDA levels in women? Inhal Toxicol 17: 695–697, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Joubert BR, Haberg SE, Nilsen RM, Wang X, Vollset SE, Murphy SK, Huang Z, Hoyo C, Midttun O, Cupul-Uicab LA, Ueland PM, Wu MC, Nystad W, Bell DA, Peddada SD, London SJ. 450K epigenome-wide scan identifies differential DNA methylation in newborns related to maternal smoking during pregnancy. Environ Health Perspect 120: 1425–1431, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kulkarni NS, Prudon B, Panditi SL, Abebe Y, Grigg J. Carbon loading of alveolar macrophages in adults and children exposed to biomass smoke particles. Sci Total Environ 345: 23–30, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Lam N, Nicas M, Ruiz-Mercado I, Thompson LM, Romero C, Smith KR. Non-invasive measurement of carbon monoxide burden in Guatemalan children and adults following wood-fired temazcal (sauna-bath) use. J Environ Monit 13: 2172–2181, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Hanafiah KM, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, 3rd, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2224–2260, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu J, Liang Q, Frost-Pineda K, Muhammad-Kah R, Rimmer L, Roethig H, Mendes P, Sarkar M. Relationship between biomarkers of cigarette smoke exposure and biomarkers of inflammation, oxidative stress, and platelet activation in adult cigarette smokers. Cancer Epidemiol Biomarkers Prev 20: 1760–1769, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Maisel WH, Lewis RJ. Noninvasive measurement of carboxyhemoglobin: how accurate is accurate enough? Ann Emerg Med 56: 389–391, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Martin WJ, 2nd, Glass RI, Balbus JM, Collins FS. A major environmental cause of death. Science 334: 180–181, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCracken J, Smith KR, Stone P, Diaz A, Arana B, Schwartz J. Intervention to lower household wood smoke exposure in Guatemala reduces ST-segment depression on electrocardiograms. Environ Health Perspect 119: 1562–1568, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McCracken JP, Smith KR, Diaz A, Mittleman MA, Schwartz J. Chimney stove intervention to reduce long-term wood smoke exposure lowers blood pressure among Guatemalan women. Environ Health Perspect 115: 996–1001, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Micheel C, Ball J, Institute of Medicine (U. S.); Committee on Qualification of Biomarkers and Surrogate Endpoints in Chronic Disease Evaluation of Biomarkers and Surrogate Endpoints in Chronic Disease. Washington, DC: National Academies Press, 2010, p. xx, 314 p [PubMed] [Google Scholar]
- 29. Migliaccio CT, Bergauff MA, Palmer CP, Jessop F, Noonan CW, Ward TJ. Urinary levoglucosan as a biomarker of wood smoke exposure: observations in a mouse model and in children. Environ Health Perspect 117: 74–79, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mobarak AM, Dwivedi P, Bailis R, Hildemann L, Miller G. Low demand for nontraditional cookstove technologies. Proc Natl Acad Sci USA 109: 10815–10820, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moller P, Loft S. Oxidative damage to DNA and lipids as biomarkers of exposure to air pollution. Environ Health Perspect 118: 1126–1136, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mortimer K, Gordon SB, Jindal SK, Accinelli RA, Balmes J, Martin WJ. Household air pollution is a major avoidable risk factor for cardiorespiratory disease. Chest 142: 1308–1315, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mumford JL, He XZ, Chapman RS, Cao SR, Harris DB, Li XM, Xian YL, Jiang WZ, Xu CW, Chuang JC, Wilson WE, Cooke M. Lung cancer and indoor air pollution in Xuan Wei, China. Science 235: 217–220, 1987. [DOI] [PubMed] [Google Scholar]
- 34. Mustafic H, Jabre P, Caussin C, Murad MH, Escolano S, Tafflet M, Perier MC, Marijon E, Vernerey D, Empana JP, Jouven X. Main air pollutants and myocardial infarction: a systematic review and meta-analysis. JAMA 307: 713–721, 2012 [DOI] [PubMed] [Google Scholar]
- 35. Neitzel R, Naeher LP, Paulsen M, Dunn K, Stock A, Simpson CD. Biological monitoring of smoke exposure among wildland firefighters: a pilot study comparing urinary methoxyphenols with personal exposures to carbon monoxide, particular matter, and levoglucosan. J Expo Sci Environ Epidemiol 19: 349–358, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Orozco-Levi M, Garcia-Aymerich J, Villar J, Ramirez-Sarmiento A, Anto JM, Gea J. Wood smoke exposure and risk of chronic obstructive pulmonary disease. Eur Respir J 27: 542–546, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Peeling RW, Mabey D. Point-of-care tests for diagnosing infections in the developing world. Clin Microbiol Infect 16: 1062–1069, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Pope CA, 3rd, Ezzati M, Dockery DW. Fine-particulate air pollution and life expectancy in the United States. N Engl J Med 360: 376–386, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pruneda-Alvarez LG, Perez-Vazquez FJ, Salgado-Bustamante M, Martinez-Salinas RI, Pelallo-Martinez NA, Perez-Maldonado IN. Exposure to indoor air pollutants (polycyclic aromatic hydrocarbons, toluene, benzene) in Mexican indigenous women. Indoor Air 22: 140–147, 2012 [DOI] [PubMed] [Google Scholar]
- 40. Shields PG. Tobacco smoking, harm reduction, biomarkers. J Natl Cancer Inst 94: 1435–1444, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Shimazu T, Ikeuchi H, Sugimoto H, Goodwin CW, Mason AD, Jr, Pruitt BA., Jr Half-life of blood carboxyhemoglobin after short-term and long-term exposure to carbon monoxide. J Trauma 49: 126–131, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Smith KR, McCracken JP, Thompson L, Edwards R, Shields KN, Canuz E, Bruce N. Personal child and mother carbon monoxide exposures and kitchen levels: methods and results from a randomized trial of woodfired chimney cookstoves in Guatemala (RESPIRE). J Expo Sci Environ Epidemiol 20: 406–416, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smith KR, McCracken JP, Weber MW, Hubbard A, Jenny A, Thompson LM, Balmes J, Diaz A, Arana B, Bruce N. Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomised controlled trial. Lancet 378: 1717–1726, 2011 [DOI] [PubMed] [Google Scholar]
- 44. Sram RJ. Effect of glutathione S-transferase M1 polymorphisms on biomarkers of exposure and effects. Environ Health Perspect 106, Suppl 1: 231–239, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res 117: 100–111, 2012 [DOI] [PubMed] [Google Scholar]
- 46. Stockfelt L, Sallsten G, Olin AC, Almerud P, Samuelsson L, Johannesson S, Molnar P, Strandberg B, Almstrand AC, Bergemalm-Rynell K, Barregard L. Effects on airways of short-term exposure to two kinds of wood smoke in a chamber study of healthy humans. Inhal Toxicol 24: 47–59, 2012 [DOI] [PubMed] [Google Scholar]
- 47. Tellabati A, Fernandes VE, Teichert F, Singh R, Rylance J, Gordon S, Andrew PW, Grigg J. Acute exposure of mice to high-dose ultrafine carbon black decreases susceptibility to pneumococcal pneumonia. Part Fibre Toxicol 7: 30, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Torres-Dosal A, Perez-Maldonado IN, Jasso-Pineda Y, Martinez Salinas RI, Alegria-Torres JA, Diaz-Barriga F. Indoor air pollution in a Mexican indigenous community: evaluation of risk reduction program using biomarkers of exposure and effect. Sci Total Environ 390: 362–368, 2008 [DOI] [PubMed] [Google Scholar]
- 49. Torres-Duque C, Maldonado D, Perez-Padilla R, Ezzati M, Viegi G. Biomass fuels and respiratory diseases: a review of the evidence. Proc Am Thorac Soc 5: 577–590, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Turner MC, Krewski D, Pope CA, 3rd, Chen Y, Gapstur SM, Thun MJ. Long-term ambient fine particulate matter air pollution and lung cancer in a large cohort of never-smokers. Am J Respir Crit Care Med 184: 1374–1381, 2011 [DOI] [PubMed] [Google Scholar]
- 51. Van Miert E, Sardella A, Nickmilder M, Bernard A. Respiratory effects associated with wood fuel use: a cross-sectional biomarker study among adolescents. Pediatr Pulmonol 47: 358–366, 2012 [DOI] [PubMed] [Google Scholar]
- 52. Viau C, Hakizimana G, Bouchard M. Indoor exposure to polycyclic aromatic hydrocarbons and carbon monoxide in traditional houses in Burundi. Int Arch Occup Environ Health 73: 331–338, 2000 [DOI] [PubMed] [Google Scholar]
- 53. Zhang J, Ichiba M, Hara K, Zhang S, Hanaoka T, Pan G, Yamano Y, Takahashi K, Tomokuni K. Urinary 1-hydroxypyrene in coke oven workers relative to exposure, alcohol consumption, and metabolic enzymes. Occup Environ Med 58: 716–721, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]


