Abstract
Vehicle exhaust is rich in polycyclic aromatic hydrocarbons (PAH) and can be a dominant contributor to ultrafine urban particulate matter (PM). Exposure to ultrafine PM is correlated with respiratory infections and asthmatic symptoms in young children. The lung undergoes substantial growth, alveolarization, and cellular maturation within the first years of life, which may be impacted by environmental pollutants such as PM. PAHs in PM can serve as ligands for the aryl hydrocarbon receptor (AhR) that induces expression of certain isozymes in the cytochrome P-450 superfamily, such as CYP1A1 and CYP1B1, localized in specific lung cell types. Although AhR activation and induction has been widely studied, its context within PM exposure and impact on the developing lung is poorly understood. In response, we have developed a replicable ultrafine premixed flame particle (PFP) generating system and used in vitro and in vivo models to define PM effects on AhR activation in the developing lung. We exposed 7-day neonatal and adult rats to a single 6-h PFP exposure and determined that PFPs cause significant parenchymal toxicity in neonates. PFPs contain weak AhR agonists that upregulate AhR-xenobiotic response element activity and expression and are capable inducers of CYP1A1 and CYP1B1 expression in both ages with different spatial and temporal patterns. Neonatal CYP1A1 expression was muted and delayed compared with adults, possibly because of differences in the enzyme maturation. We conclude that the inability of neonates to sufficiently adapt in response to PFP exposure may, in part, explain their susceptibility to PFP and urban ultrafine PM.
Keywords: polycyclic aromatic hydrocarbons, aryl hydrocarbon receptor, lung development, particulate matter
particulate matter (PM) air pollution is a complex aggregate mixture. These particles are emitted from both natural and anthropogenic sources and vary in size and composition. Exposures to PM have been correlated with the development and exacerbation of cardiopulmonary morbidities and mortalities (12, 13, 38) and are estimated to contribute to ∼800,000 premature deaths annually worldwide (8). PM is typically divided into three fractions. The coarse PM10 fraction contains particles with an aerodynamic diameter of <10 μm, the fine PM2.5 fraction consists of particles <2.5 μm in diameter, and the ultrafine PM0.1 fraction is comprised of particles of <0.1 μm in diameter. Of these fractions, exposure to PM2.5 and PM0.1 is thought to have the greatest health effects. Exposures to ambient PM2.5 have been linked to the development of respiratory infections, onset of asthma and exacerbation of preexisting asthmatic symptoms, and an increased risk of hospitalization in susceptible populations, such as young children and the elderly. Furthermore, exposure to PM0.1 has been correlated with decrements in lung development and function (22, 60).
The PM2.5 and PM0.1 fractions are easily respirable and can penetrate and deposit deep into the intrapulmonary regions of the lung, especially in the bronchioles and alveoli (31). PM0.1 is poorly cleared from the lung. Only a quarter of the deposited PM0.1 is cleared by mucociliary clearance within 24 h, and a significant fraction is still retained within the lung 48 h postexposure (33). This problem may be exacerbated in young susceptible children. The lung undergoes considerable growth and alveolarization within the first eight years of life, when 80% of the alveoli are developed (40). Furthermore, the lung epithelium undergoes continuous and extensive differentiation and maturation at the same time (25), including substantial shifts in metabolic and antioxidant protein expression in the early postnatal period (14, 15). Physiologically, children have a larger body surface area-to-volume ratio, higher minute ventilation and metabolic rate, and more oxygen consumption per body weight compared with adults (2). Taken together, these factors can enhance particle deposition and total dose (3), which may disrupt proper lung development, leading to reduced lung function.
Although PM originates from both natural and anthropogenic sources, polycyclic aromatic hydrocarbons (PAH) emitted from human activity are ubiquitous in the PM2.5 and PM0.1 fractions. Sources of PAH in the urban atmosphere include motor vehicles, power plants, solid fuel burning, and resuspended road dust. Gasoline and diesel fuels have a high PAH content (29) that is partitioned between particle and vapor PM phases depending on the PAH size and volatility. Vehicle combustion emits PAH-rich carbonaceous particles that contain over 15 mg PAH/g PM, composed of semivolatile PAHs, like naphthalenes, in the gas phase and nonvolatile PAHs, like benzo[a]pyrene, perylene, and benzo[b]chrycene, in the particle phase (27). These emissions can be a dominant contributor to PM2.5 and PM0.1 (39). It is estimated that vehicle exhaust contributes 50–70% of the PM2.5 mass in the urban environment (37, 39, 58).
PAHs from PM can be metabolized by enzymes within the cytochrome P-450 (CYP) superfamily. In the lung, CYPs have been detected in four pulmonary cell types: the nonciliated bronchiolar (Clara) cell, the type II epithelial cell, the endothelial cell, and the alveolar macrophage, which reside in different compartments within the lung (14). These enzymes metabolize PAHs through monooxygenation reactions that ultimately decrease the lipophilicity and enhance the water solubility of PAHs for excretion (11). However, some PAHs can be bioactivated by this process into electrophiles that can become carcinogenic and toxic compounds (9, 30, 51). These PAHs and their metabolites can also serve as ligands for the aryl hydrocarbon receptor (AhR), a ligand-dependent transcription factor that regulates the expression of genes containing xenobiotic response elements (XRE) (10). PAH-dependent activation of AhR and upregulation of XRE-containing genes have been widely studied, notably in relation to the induction of CYP1A1 and -1B1 isozymes. However, the literature on CYP1A1 and -1B1 regulation in the context of PM exposure has been conflicting. Studies have reported both increases (42, 43) and decreases (9) of CYP1A1 and -1B1 gene and protein expression after PM exposure. Furthermore, the impact of PM exposure on AhR activation in the developing lung is poorly understood.
To address this issue, we have developed and characterized a system to generate premixed flame particles (PFP) (26) that are abundant in PAHs to simulate the effects of inhaling vehicle exhaust in a developing neonatal rat model. We have previously shown that neonatal rat lungs are uniquely susceptible compared with adults (4). We have linked this susceptibility to alterations in levels of glutathione and an inability of neonates to upregulate antioxidant enzyme expression (5). Building upon our previous work, the current study was designed to address three questions: 1) do PFPs activate AhR, 2) does cytotoxicity resulting from exposure to PFP differ by lung compartments (conducting airways vs. surrounding parenchyma), and 3) is activation of AhR lung compartment specific, and how does that relate to age-specific toxicity? We hypothesized that PFPs are capable of activating AhR and subsequently upregulating CYP1A1 and -1B1. We further hypothesized that this would correlate with enhanced susceptibility. To test our hypothesis, we used two approaches. First, we analyzed AhR activity and downstream gene expression in an in vitro model. Second, we performed an in vivo time-course study, conducting whole body exposures of 7-day-old neonatal pups and young adult rats to a single acute 6-h exposure to PFPs and defined our results by exposure, age, and lung compartments.
METHODS
Flame and particle environment generation.
PFP are 70-nm ultrafine particles generated using a coannular premixed flame burner as detailed previously (4, 26). Briefly, an enclosed burner was fed a metered mixture of ethylene, oxygen, and argon. The burner effluent was mixed with clean high-efficiency particulate air and chemical/biological/radiological (CBR) filtered air (FA) added downstream and passed through a heated three-way catalyst to remove nitrite and nitrate and CO. Finally, PFPs were diluted and mixed with additional FA before entering the inhalation exposure chamber. Chamber and particle characteristics have been determined previously (4) and are listed below for reference. Chamber mass concentration was 22.40 ± 5.60 μg/m3 (mean ± SD), containing 9.37 × 104 ± 4.8 × 103 particles as determined by a condensation particle counter. Particles were high in organic carbon and had an elemental carbon/organic carbon ratio of 0.58. The total amounts of PAH were 54 ng/m3 attached to PFP and 405 ng/m3 in the gas phase.
Particle extraction.
Particle samples were collected on 47-mm quartz filters (Pallflex, Ann Arbor, MI) as previously described (4). The filter extraction protocol used in this study to remove the combustion-generated particles from the collection substrates before toxicological testing was designed to 1) maximize extraction efficiencies, 2) minimize compositional biases in the extracted PM, and 3) minimize extraction artifacts. This was accomplished using a combination of sonication, solvent extraction, filtration, and gravimetric analyses. Sonication provides the energy necessary to break the adhesive and cohesive forces holding the particles together and to the substrate. Solvent extraction, using several different solvents spanning the polarity scale, increases the amount of PM removed from the substrate and reduces volatilization losses. Filtration removes any potential contaminant filter material from the extracted PM, and gravimetric analysis provides quantitative estimates of extracted PM mass, as well as a means to assess mass closure. A detailed extraction protocol is presented below.
1) Weigh filters to obtain a preweight for the filter extraction process; all weighing was performed using an A&D model HR-202i semimicro analytical balance (0.01 mg readability). 2) Sonicate filters for ∼1–2 h in a crystallization dish with ∼300 ml milli-Q H2O using a 5.5-gallon bath-style Branson model 8510 Bransonic tabletop ultrasonic cleaner. 3) Filter H2O sonication extract solution (H2O Ex) through an 8.0-μm Millipore membrane filter using a class M fritted glass disc Buchner funnel. 4) Transfer filtered H2O Ex to a 1-liter separatory funnel, add ∼100 ml of dichloromethane (DCM), shake vigorously for ∼5 min, and allow layers to separate overnight. 5) Drain the DCM layer from the bottom of the separatory funnel into a 500-ml beaker, partially evaporate DCM under N2 atmosphere, transfer to a 20-ml weighing beaker, evaporate the remaining DCM under N2 atmosphere, weigh to obtain the DCM soluble fraction (DCMW), and store in a freezer until reconstitution. 6) Repeat steps 4 and 5 using ∼100 ml of hexane (Hx) to obtain the Hx soluble fraction (HxW). 7) Transfer filtered and solvent-washed H2O Ex to a 1.2-liter lyophilization flask (Labconco Fast-Freeze flasks), freeze to −80°C, connect to a lyophilizer operating at ∼0.1 mbar pressure and −50°C (Labconco FreeZone 2.5-liter bench-top freeze dry system), and sublimate ice until almost gone. 8) Remove the flask from the lyophilizer, allow ice to partially melt, quantitatively transfer remaining H2O Ex to a 150-ml lyophilization flask, refreeze to −80°C, reconnect to the lyophilizer, and sublimate ice until almost gone. 9) Repeat step 8 for an 80-ml lyophilization flask, transfer remaining H2O Ex to final 10-ml storage vials, refreeze to −80°C, reconnect to the lyophilizer, sublimate remaining ice, seal vials under vacuum, and remove from the lyophilizer. 10) Weigh the 10-ml vials under vacuum and subtract vial preweights (also weighed under vacuum) to obtain the filtered and solvent-washed H2O sonication fraction; store vials under vacuum in a freezer until reconstitution. 11) Place the original filter on a drying rack in a relative humidity-controlled drying chamber, allow to dry for ∼24 h, and reweigh filters. 12) Sonicate filters for ∼1–2 h in a crystallization dish with ∼100 ml DCM. 13) Filter DCM sonication extract solution (DCM Ex) through a class F (4–5.5 μm porosity) fritted glass disc Buchner funnel, transfer DCM Ex to a 500-ml beaker, partially evaporate DCM under N2 atmosphere, transfer to a 20-ml weighing beaker, evaporate remaining DCM, weigh to obtain filtered DCM sonication fraction (DCMEx), and store in a freezer until reconstitution. 14) Repeat steps 11–13 using Hx to obtain the Hx sonication fraction (HxEx). 15) Reconstitute the PM sample: remove components from the freezer, break the vacuum on the 10-ml vials containing H2O Ex (step 10), dissolve organic fractions (DCMW, HxW, DCMEx, and HxEx) back into appropriate solvents and quantitatively transfer to the 10-ml vial (DCM and Hx are evaporated under N2 atmosphere as the fractions are successively added), put the 10-ml vial back under vacuum using a lyophilizer, and reweigh to obtain total extracted PM mass; crimp-seal with an aluminum seal, and store in a −20°C freezer until the toxicological studies.
Cell culture and transient transfection.
We obtained human U-937 monocytic cells from the American Tissue Culture Collection (Manassas, VA) and maintained them in RPMI 1640 medium containing 10% fetal bovine serum (Gemini, Woodland, CA), 100 units penicillin, and 100 μg/ml streptomycin supplemented with 4.5 g/l glucose and 1 mM sodium pyruvate. Cell culture was maintained at a cell concentration between 2 × 105 and 2 × 106 cells/ml. For differentiation into macrophages, U-937 cells were treated with 12-O-tetradecanoylphorbol-13-acetate (TPA, 3 μg/ml) and allowed to adhere for 48 h in a 5% CO2 tissue culture incubator at 37°C, after which they were fed with TPA-free medium.
For transient transfection of U-937 macrophages, the XRE luciferase reporter construct (kindly provided by T. Haarmann-Stemmann, University of Duesseldorf, Duesseldorf, Germany) was transfected via Nucleofector technology as described previously (55). Briefly, 106 U-937 macrophages were resuspended in 100 μl Nucleofector Solution V (Amaxa GmbH, Köln, Germany) and nucleofected with 1.0 μg plasmid DNA using program V-001, which is preprogrammed into the Nucleofector device (Amaxa). Following nucleofection, the cells were immediately mixed with 500 μl of prewarmed RPMI 1640 medium and transferred into six-well plates containing 1.5 ml RPMI 1640 medium/well. After 24 h transfection, macrophages were treated with PFP, urban dust particle (UDP)-standard reference material (SRM) 1649 [National Institute of Standards and Technology (NIST), Gaithersburg, MD], or 2,3,7,8-tetrachlorodibenzodioxin (TCDD, positive control) for 4 h. Luciferase activities were measured with the Luciferase Reporter Assay System (Promega) using a luminometer (Berthold Lumat LB 9501/16, Pittsburgh, PA). Relative light units are normalized to β-galactosidase activity and to protein concentration using Bradford dye assay (Bio-Rad, Hercules, CA).
Gel-mobility-shift assay.
Nuclear extracts were isolated from U-937 cells, as described previously (56). In brief, 5 × 106 cells were treated with PFP, UDP, or TCDD for 90 min and harvested in Dulbecco's PBS containing 1 mM phenylmethylsulfonyl fluoride (PMSF) and 0.05 μg/μl of aprotinin. After centrifugation, the cell pellets were gently resuspended in 1 ml of hypotonic buffer (20 mM HEPES, 20 mM NaF, 1 mM Na3VO4, 1 mM Na4P2O7, 1 mM EDTA, 1 mM EGTA, 0.5 mM PMSF, 0.13 μM okadaic acid, 1 mM dithiothreitol, pH 7.9, and 1 μg/ml each leupeptin, aprotinin, and pepstatin). The cells were allowed to swell on ice for 15 min and then homogenized by 25 strokes of a Dounce homogenizer. After centrifugation for 1 min at 16,000 g, nuclear pellets were resuspended in 300 μl ice-cold high-salt buffer (hypotonic buffer with 420 mM NaCl and 20% glycerol). The samples were passed through a 21-gauge needle and stirred for 30 min at 4°C. The nuclear lysates were microcentrifuged at 16,000 g for 20 min, separated into aliquots, and stored at −80°C. DNA-protein binding reactions were carried out in a total volume of 15 μl containing 10 μg nuclear protein, 60,000 counts/min of XRE oligonucleotide (5′-gcc ccg gag ttg cgt gag aag agc ctg g-3′), 25 mM Tris buffer (pH 7.5), 50 mM NaCl, 1 mM EDTA, 0.5 mM dithiothreitol, 5% glycerol, and 1 μg poly(dI-dC). The samples were incubated at room temperature for 20 min. Competition experiments were performed in the presence of a 100-fold molar excess of unlabeled DNA fragments. Protein-DNA complexes were resolved on a 4% nondenaturating polyacrylamide gel and visualized by exposure of the dehydrated gels to X-ray films. For quantitative analysis, respective bands were quantified using a ChemiImager 4400 (Alpha Innotech, San Leandro, CA).
Quantitative real-time reverse transcription-PCR.
Total RNA was isolated from U-937 cells using a Quick-RNA Mini prep isolation kit (Zymo Research, Irvine, CA), and cDNA synthesis was done as previously described (56). Quantitative detection of β-actin and differentially expressed genes was performed with a StepOnePlus Real-Time PCR System (Applied Biosystems) using the Fast SYBR Green Master Mix (Life Technologies, Grand Island, NY) according to the manufacturer's instructions. DNA-free total RNA (1.0 μg) was reverse-transcribed using 4 units Omniscript reverse transcriptase (RT; Qiagen) and 1 μg oligo(dT)15 in a final volume of 40 μl. The primers for each gene were designed on the basis of the respective cDNA or mRNA sequences using OLIGO primer analysis software provided by Steve Rozen and the Whitehead Institute/MIT Center for Genome Research (44) so that the targets were 100–200 bp in length. The following primer sequences were used: human β-actin (forward primer, 5′-GGACTTCGAGCAAGAGATGG-3′; reverse primer, 5′-AGCACTGTGTTGGCGTACAG-3′), human CYP1A1 (forward primer, 5′-TAGACACTGATCTGGCTGCAG-3′; reverse primer, 5′-GGGAAGGCTCCATCAGCATC-5′), and human AhR (forward primer, 5′-TCAACAGCAACAGTCCTTGG-3′; reverse primer, 5′-TCCAATTTTCAAACATGCCA-3′). PCR amplification was carried out in a total volume of 20 μl containing 2 μl cDNA, 10 μl 2× Fast SYBR Green Master Mix, and 0.2 μM of each primer. The PCR cycling conditions were 95°C for 30 s followed by 40 cycles of 94°C for 3 s, and 60°C for 30 s. Detection of the fluorescent product was performed at the end of the 72°C extension period. Negative controls were concomitantly run to confirm that the samples were not cross-contaminated. A sample with DNase- and RNase-free water instead of RNA was concomitantly examined for each of the reaction units described above. To confirm the amplification specificity, the PCR products were subjected to melting-curve analysis.
Animal exposure protocol.
Eight week reproductively capable adult male and newborn male Sprague Dawley rats with accompanying dams (Harlan Laboratories, Hayward, CA) were allowed to acclimate in CBR FA until newborns reached seven postnatal days of age. Adult rats were provided with Laboratory Rodent Diet (Purina Mills, St. Louis, MO) and water ad libitum. Animals were exposed to 22.40 ± 5.60 μg/m3 PFP or FA atmosphere for 6 h in two separate chambers as previously described (4, 26). All animal experiments are performed as described in protocols approved by the University of California Institutional Animal Care and Use Committee in accordance with guidelines set by the National Institutes of Health. Animals were necropsied at 2, 24, and 48 h following the single 6-h exposure and will be designated as PFP2, PFP24, and PFP48, respectively. All animals were killed by an intraperitoneal injection of an overdose of pentobarbital (150 mg/kg). At necropsy, tracheas were cannulated, the thorax was opened, and the lung was removed en bloc for processing.
Ethidium homodimer-1 cell viability assay.
Rat lungs were examined for the presence of membrane-permeable cells using 10 μM ethidium homodimer-1 as previously described (54). The lung was fixed under 30 cm hydrostatic pressure in 330 mosmol/l Karnovsky's fixative (24) for 1 h and stored at 4°C until dissection. The right accessory lobe was isolated, glued to a 22-mm2 cover slip (Corning Life Sciences, Lowell, MA) with Nexaband tissue adhesive (Abbott Animal Health, North Chicago, IL), and then dissected to expose bronchiolar airways. Distal airways were imaged using a Leica TCS LSI zoom confocal microscope (Leica Microsystems, Wetzlar, Germany) using a 488-nm excitation laser as previously described (49).
Stereology.
The amount of normal and cytotoxic airway and parenchymal cells was quantified on high-resolution images and was analyzed using stereology as previously discussed in detail (21) using 4-μm paraffin sections. A minimum of 40 fields/animal were sampled using overviews with a random number table and determined through systematic stratified sampling (20). The volume fractions (Vv) of damaged cells in airways (Vv airway) and parenchyma (Vv parenchyma) were defined by a point count of positive and negative epithelium and quantified (Image-Pro Plus; MediaCybernetics, Rockville, MD). Compartmental Vv were calculated by the formula: VV = Pp = Pn/Pt, where Pp is the point fraction of Pn, the number of test points hitting ethidium-positive cells, divided by Pt, the sum of the total points hitting the reference space (52).
Lung compartmental RNA isolation and RT-PCR.
Lung compartmental RNA was isolated from microdissected intrapulmonary airways and surrounding parenchymal tissue from RNAlater (Ambion, Austin, TX)-stabilized lung tissue using the Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA) as previously described (1). RNA purity was confirmed through spectrophotometric absorbance at 260/280 nm. Quantification of CYP1A1 (Rn01418021_g1), CYP1B1 (Rn00564055_m1), P-450 reductase (Rn00580820_m1), and hypoxanthine-guanine phosphoribosyltransferase (HPRT) (Rn01527840_m1) in the airway and parenchymal compartments was performed using inventoried Taqman probes and primers (Applied Biosystems, Foster City, CA) as previously described (1, 48). Results were calculated using the comparative Ct method (28, 45) using HPRT as the reference gene. HPRT was chosen as the reference gene because of consistency and low variance between exposure groups as previously assessed (4, 52). Results are expressed as a degree of change in gene expression relative to filtered animals of the same age, unless otherwise stated.
Immunofluorescence.
Lungs were inflated with 37% formaldehyde vapor bubbled under 30 cm hydrostatic pressure for 1 h as previously described (18, 61). Samples were stored in 1% paraformaldehyde for <24 h before tissue processing and paraffin embedment. Paraffin sections on poly-l-lysine-coated slides from all groups were stained simultaneously to minimize variability between runs and were immunostained for rabbit anti-AhR (Abcam, Cambridge, MA) at 1:500. The concentration of primary antibody was determined through a series of dilutions to optimize for staining density while minimizing background. This procedure was performed according to previously described methods (49). Signal was visualized using anti-rabbit Alexa 568 (Jackson ImmunoResearch, West Grove, PA) and subsequently stained with DAPI to visualize cell nuclei. Controls included substitution of primary antibody with phosphate-buffered saline to ensure specific positive staining.
Immunohistochemistry.
Paraffin sections of formaldehyde vapor-fixed lungs were immunostained for rabbit anti-CYP1A1 (Abcam) at 1:1,500, rabbit anti-CYP1B1 (Abcam) at 1:500, and rabbit anti-P-450 reductase (Abcam) at 1:2,000 according to previously described methods (52, 53) with several alterations. Following tissue hydration, endogenous peroxidase activity was quenched with a 10% solution of hydrogen peroxide. To eliminate nonspecific primary antibody binding, tissue sections were blocked with 5% albumin. Primary antibodies were allowed to incubate in a humidified chamber overnight at 4°C. Signal was amplified using the Vectastain IgG Avidin-Biotin-HRP Kit (Vector Labs, Burlingame, CA) and visualized using nickel chloride-enhanced 3,3′-diaminobenzidine tetrachloride (Sigma Chemical, St. Louis, MO) as the chromagen.
Statistics.
All data are reported as means ± SE unless otherwise stated. Statistical outliers were eliminated using the extreme studentized deviate method (Graphpad, La Jolla, CA). Undetected and samples observed below detection limit were treated as nondetects, and values were imputed using the natural-log regression on order statistics method (19, 46) using ProUCL [U.S. Environmental Protection Agency (EPA), Atlanta, GA]. Multivariate analysis of variance was applied against age, compartment, and exposure factors when appropriate. Multiple comparisons for factors containing more than two levels were performed using Fisher's protected least-significant difference (PLSD) method. Pairwise comparisons were performed individually using a one-way ANOVA followed by PLSD post hoc analysis using StatView (SAS, Cary, NC). P values of <0.05 were considered statistically significant.
RESULTS
PFPs activate the AhR receptor in vitro.
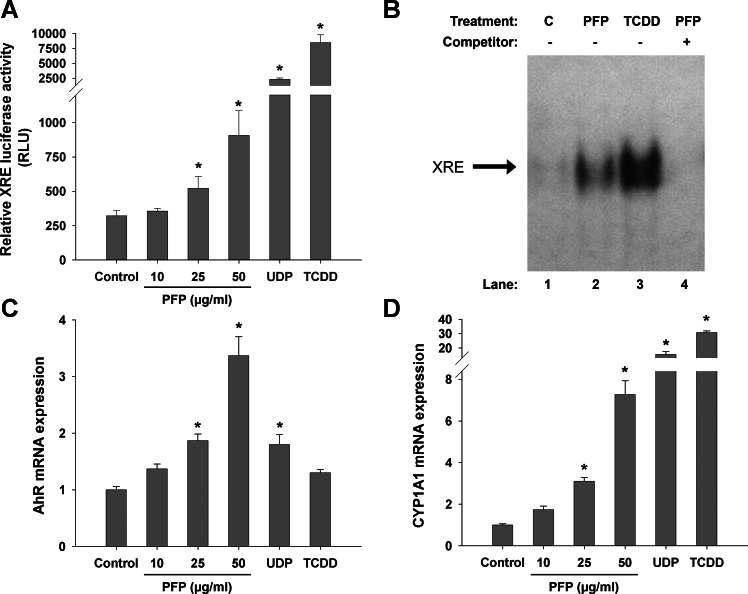
We have previously characterized PFPs and found that they are predominantly composed of biphenyls and naphthalenes in the vapor and particle phases, respectively (4). Although these two-ringed aromatic compounds are known to be unreactive with AhR, we also detected the presence of three-, four-, five-, and six- ringed PAHs present in nanogram per cubic meter quantities. The top 20 species and their concentrations are presented in both gas (Table 1) and particulate (Table 2) phases. We hypothesized that inhaling PFPs with these concentrations of larger multiringed PAH can activate AhR and upregulate XRE-containing genes. To determine whether PFP is capable of activating AhR, we used an in vitro reporter assay to measure XRE luciferase activity in response to particle application. As shown in Fig. 1A, PFP increases luciferase activity in a dose-dependent manner, with a 1.6-fold increase in the 25 μg/ml dose (P = 0.044) and a 2.5-fold enhancement in the 50 μg/ml dose (P = 0.025). Comparatively, UDP from NIST had an approximate sixfold induction (P = 0.005) at 10 μg/ml, and our positive control (1.0 nM TCDD) showed over 20-fold induction (P = 0.003), compared with untreated controls. Gel-mobility-shift assay studies (Fig. 1B) confirmed that AhR activation is associated with an increased binding activity of the XRE consensus element, which regulates the expression of CYP1A1. Next, we measured mRNA levels of AhR and found significant upregulation of about twofold at 25 μg/ml (P = 0.011) and threefold at 50 μg/ml (P < 0.0001) compared with untreated controls. UDP upregulated AhR similarly (P = 0.012) (Fig. 1C). PFP treatment induces CYP1A1 mRNA in a dose-dependent manner; levels were increased threefold at 25 μg/ml (P = 0.003) and sevenfold at 50 μg/ml (P < 0.0001). UDP and TCDD were more robust inducers of CYP1A1, increasing transcript levels by 15- and 30-fold, respectively (Fig. 1D).
Table 1.
Top 20 vapor-phase PAHs containing at least three rings
| Field Code | Compound | No. of Rings | Abundance, ng/m3 |
|---|---|---|---|
| FLUORE | Fluorene | 3 | 11.54 |
| PHENAN | Phenanthrene | 3 | 6.65 |
| M_3PHEN | 2-Methylphenanthrene | 3 | 4.28 |
| C_DMPH | C-Dimethylphenanthrene | 3 | 2.98 |
| ANTHRON | Anthrone | 3 | 1.99 |
| M_9PHEN | 9-Methylphenanthrene | 3 | 1.68 |
| FL9ONE | 9-Fluorenone | 3 | 1.38 |
| FLUORA | Fluoranthene | 3 | 1.30 |
| A_DMPH | A-dimethylphenanthrene | 3 | 1.07 |
| DBZFUR | Dibenzofuran | 3 | 0.99 |
| DM17PH | 1,7-Dimethylphenanthrene | 3 | 0.92 |
| DM36PH | 3,6-Dimethylphenanthrene | 3 | 0.76 |
| E_DMPH | E-dimethylphenanthrene | 3 | 0.61 |
| RETENE | Retene | 3 | 0.61 |
| MPHT_1 | 1-Methylphenanthrene | 3 | 0.38 |
| PYRENE | Pyrene | 4 | 2.14 |
| BAANTH | Benz(a)anthracene | 4 | 0.54 |
| M_45PHEN | 4,5-Methylenephenanthrene | 4 | 0.46 |
| M_4PYR | 4-Methylpyrene | 4 | 0.46 |
| ANTHAN | Anthanthrene | 6 | 0.38 |
PAH, polycystic aromatic hydrocarbalone. Adapted from Chan et al. (4).
Table 2.
Top 20 particulate-phase PAHs containing at least three rings
| Field Code | Compound | No. of Rings | Abundance, ng/m3 |
|---|---|---|---|
| M_9PHEN | 9-Methylphenanthrene | 3 | 1.91 |
| PHENAN | Phenanthrene | 3 | 0.46 |
| M_2PHEN | 3-Methylphenanthrene | 3 | 0.31 |
| FLUORE | Fluorene | 3 | 0.23 |
| RETENE | Retene | 3 | 0.23 |
| D_DMPH | d-Dimethylphenanthrene | 3 | 0.15 |
| DM17PH | 1,7-Dimethylphenanthrene | 3 | 0.15 |
| M_3PHEN | 2-Methylphenanthrene | 3 | 0.15 |
| A_MFLUO | A-methylfluorene | 3 | 0.08 |
| ANTHRON | Anthrone | 3 | 0.08 |
| DBZFUR | Dibenzofuran | 3 | 0.08 |
| MPHT_1 | 1-Methylphenanthrene | 3 | 0.08 |
| PYRENE | Pyrene | 4 | 0.15 |
| BAANTH | Benz(a)anthracene | 4 | 0.08 |
| BAFLUO | Benzo(a)fluorene | 4 | 0.08 |
| CHR_TR | Chrysene-triphenylene | 4 | 0.08 |
| DBAHACR | Dibenz(a,h)acridine | 5 | 0.15 |
| BPY910DIH | 9,10-Dihydrobenzo(a)pyrene-7(8H)-one | 5 | 0.08 |
| ANTHAN | Anthanthrene | 6 | 0.08 |
| CORONE | Coronene | 6 | 0.08 |
Adapted from Chan et al. (4).
Fig. 1.
In vitro xenobiotic response element (XRE) reporter assay. Human U-937 macrophages were transiently transfected with the XRE luciferase reporter construct and subsequently treated with premixed flame particles (PFP), urban dust particles (UDP), or 2,3,7,8-tetrachlorodibenzodioxin (TCDD, positive control). A: a dose-dependent increase in acyl hydrocarbon receptor (AhR)/XRE activity was observed after PFP treatment. Treatment with 25 μg/ml or more PFP yielded a significant response compared with controls. UDP (10 μg/ml) and TCDD (1.0 nM) provide strong AhR agonists and significantly induced XRE luciferase activity. B: PFP treatment increases binding activity of AhR at a consensus XRE-binding element using gel-mobility-shift assay with XRE consensus oligonucleotide after treatment of U-937 macrophages with PFP (25 μg/ml) and TCDD (1.0 nM) for 90 min. C: AhR mRNA was upregulated in a dose-dependent manner at 25 and 50 μg/ml PFP. UDP (10 μg/ml) induced AhR mRNA expression similarly to 25 μg/ml PFP. D: cytochrome P-450 (CYP) 1A1 mRNA level was significantly increased at 25 and 50 μg/ml PFP and strongly activated by UDP or TCDD treatment used as positive control. Data are presented as means + SE (n = 3 separate experiments). *Significantly different compared with controls.
Inhaling PFPs cause age- and compartmental-specific cytotoxicity.
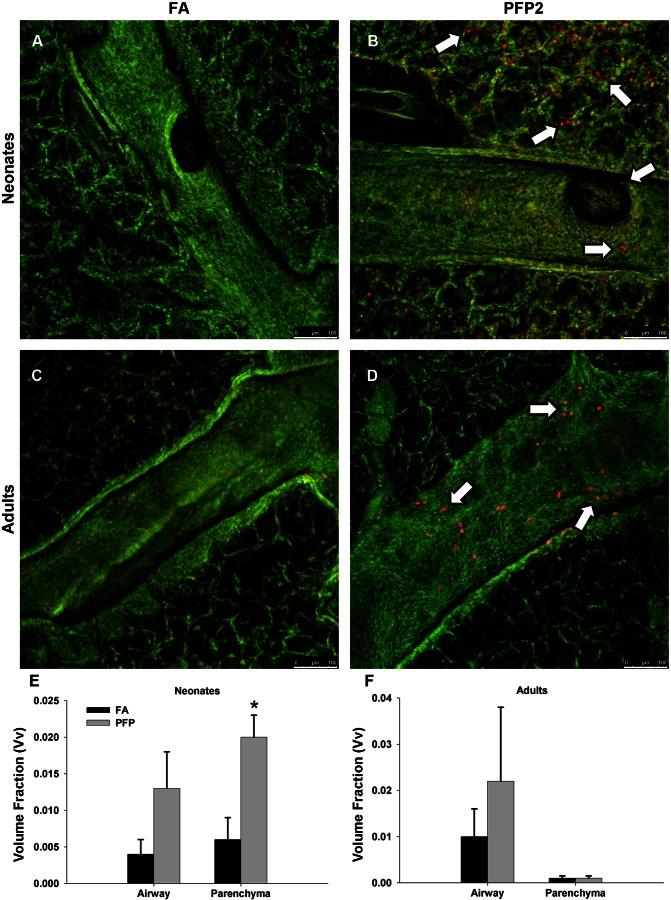
To determine biological effects in vivo, we exposed whole body neonatal and adult rats to PFPs and quantified lung cytotoxicity in two separate lung compartments. To correlate results from our in vitro studies, we also examined AhR-dependent enzyme expression. To evaluate lung cellular toxicity, we used ethidium homodimer-1 permeability as a marker for cellular cytotoxicity. We examined responses 2 h postexposure and analyzed the airways and parenchyma as separate lung compartments. Constitutively, ethidium-positive cells were rarely detected in either the neonatal (Fig. 2A) or the adult (Fig. 2C) lung. After PFP exposure, an abundance of permeable cells was observed in both lung compartments in neonates (Fig. 2B). In contrast, permeable cells were only observed localized in focal patches in the bronchiolar epithelium in adult animals; parenchyma were unaffected (Fig. 2D). Unbiased stereological approaches confirmed our assessments. Vv calculations revealed that neonates had approximately threefold more permeable cells in both airways (P = 0.462) and parenchyma (P = 0.001) after PFP exposure (Fig. 2E). Vv in adult airways were similarly increased by twofold (P = 0.371), but parenchyma was unaffected (P = 0.924) (Fig. 2F).
Fig. 2.
Airway cellular toxicity following PFP exposure. In situ ethidium homodimer-1 staining was analyzed using confocal microscopy in 7-day postnatal (A and B) and adult (C and D) rats exposed to either filtered air (FA, A and C) or PFP2 (B and D). Overall, ethidium staining was scarce in either 7-day-old neonates (A) or adults (C) reared in FA. Ethidium-positive cells (white arrows) were observed distributed in both bronchiolar epithelium and parenchyma in neonatal animals (B). Comparatively, the majority of cytotoxic cells was present in bronchiolar airways and was virtually absent in the parenchyma (D). Scale bars are 100 μm. Stereological quantification revealed an increased volume fraction of ethidium homodimer-1-positive cells after exposure. The volume fraction of ethidium-positive cells of neonates increased in both airway and parenchyma after exposure, but this increase was significant only in the parenchyma (E). Adult volume fractions also trended higher in the airways but were unaffected by PFPs in the parenchyma (F). Data are plotted as means ± SE (n = 4–5 rats/group, per compartment). *P < 0.05, significantly different from FA in the same compartment.
PFPs activate AhR in vivo.
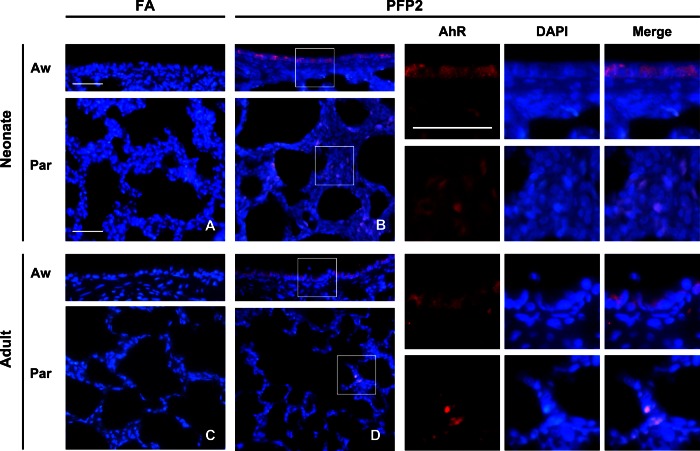
Next, we examined whether AhR is activated in vivo similar to in vitro experiments. We used immunofluoresence overlaid upon DAPI staining as a marker for AhR activation and measured AhR distribution at three time points (2, 24, and 48 h) postexposure, designated as PFP2, PFP24, and PFP48, respectively. The data presented in Fig. 3 show AhR overlaid upon DAPI and evaluated after FA or PFP exposure. AhR staining was rarely observed in FA controls of either age in both airway and parenchyma compartments. Postexposure, we observed robust AhR staining in both lung compartments in both ages. As seen in the unmerged images, AhR overlays directly over the DAPI nuclear marker and confirms our in vitro results. Furthermore, AhR activation was seen to be a very transient event. AhR staining was comparable to FA controls at PFP24 and PFP48 (data not shown).
Fig. 3.
AhR immunofluorescence. AhR immunofluorescence (red) was overlaid upon DAPI nuclear (blue) staining in neonate (A and B) and adult (C and D) lungs exposed to either FA (A and C) or PFP2 (B and D). AhR staining was virtually absent in FA-treated animals of either age. However, robust AhR staining was observed in both airway and parenchyma compartments in both ages. High-magnification insets are denoted by white squares and separate images showing AhR and DAPI colocalization, presented adjacent to B and D, respectively. Aw, airway; Par, parenchyma.
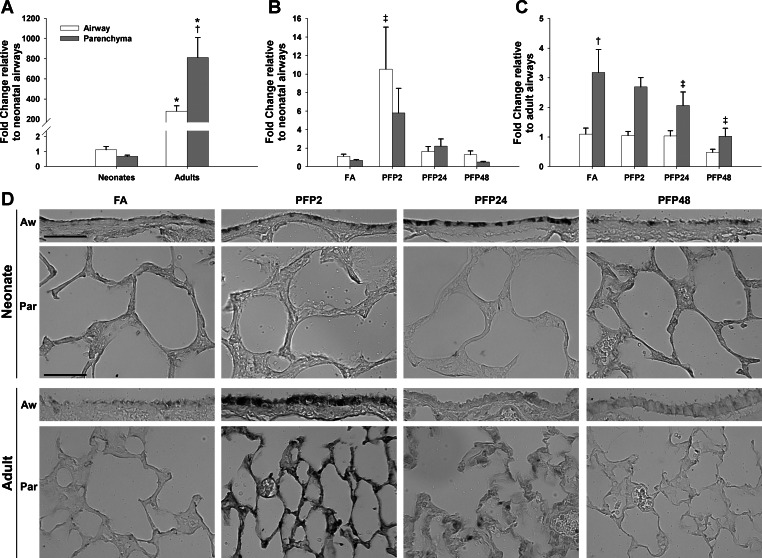
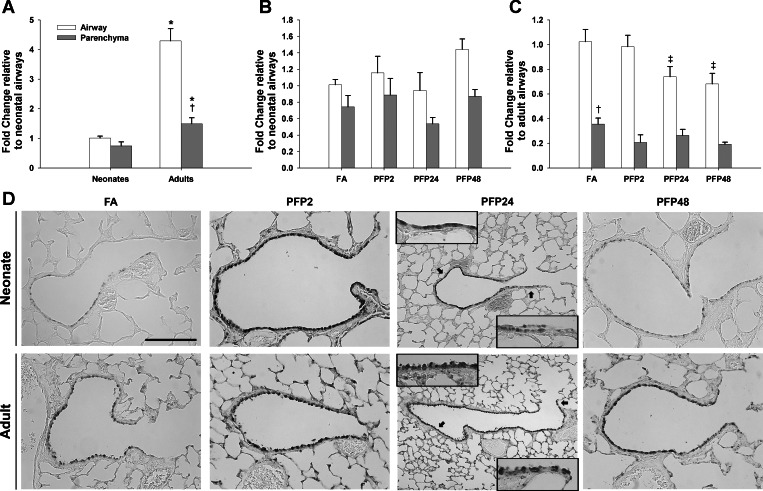
To confirm AhR activation, we measured mRNA levels and protein expression of the classical AhR-responsive and XRE-regulated gene CYP1A1. First, we measured mRNA levels using RT-PCR in microdissected airway and parenchyma separately in neonatal and adult rat lungs. Basal CYP1A1 mRNA levels were substantially different between lung compartments and ages. Compared with neonates, adult rat airways had over 250-fold more CYP1A1 than the neonatal counterpart. Furthermore, whereas neonates expressed CYP1A1 equally in both airway and parenchyma, adults had threefold more parenchymal mRNA compared with airways (Fig. 4A). After PFP exposure, we saw very different responses in the CYP1A1 mRNA between neonates and adults. As shown in Fig. 4B, CYP1A1 mRNA was acutely higher in airways (P = 0.001) and trended higher in the parenchyma (P = 0.064) as well, but was not statistically significant. CYP1A1 upregulation was a transient event. CYP1A1 levels returned to levels near those in the FA control by PFP24, and no further changes were observed. In contrast, adult CYP1A1 mRNA responded in an opposite manner. We found a downregulation of CYP1A1 mRNA, solely in the parenchyma, starting at PFP24 (P = 0.004) that extended further to PFP48 (P < 0.0001). Adult airway expression was unaffected by PFP exposure. Next, we compared protein responses using immunohistochemistry. Basal CYP1A1 protein was low in the lung and was intermittently expressed in the airways in both ages. However, CYP1A1 expression and localization were substantially different between the two ages after PFP exposure. Neonatal CYP1A1 protein followed a similar, but delayed, pattern as mRNA levels; bronchiolar epithelial CYP1A1 protein expression was transiently increased only at PFP24. We did not observe any differences in the parenchyma compartment. PFP caused a more acute response in adult CYP1A1. CYP1A1 was abundant in both airway and parenchymal compartments at PFP2. However, this upregulation was transient; CYP1A1 protein distribution and abundance returned to FA control levels by PFP24.
Fig. 4.
CYP1A1 compartmental mRNA and protein expression. RT-PCR expression in airway and parenchyma compartments in neonatal and adult rats exposed to PFPs. A: basal CYP1A1 mRNA levels were significantly higher in adults. Although no compartmental differences were observed in neonates, there was 4-fold more CYP1A1 in the parenchyma than in airways in adults. B: after PFP exposure, neonate CYP1A1 mRNA was transiently upregulated at PFP2 in the airways and reverted to FA levels by 24 h. C: a time-dependent decrease in CYP1A1 was observed in the adult parenchyma, reaching significance at 24 and 48 h postexposure. Data are presented as means + SE (n = 5–7 rats/group, in each compartment) *Significantly different compared with neonates in the same compartment. †Significantly different compared with airways in the same age. ‡Significantly different compared with FA in the same compartment. CYP1A1 immunohistochemical expression is presented in D. Although basal CYP1A1 was low in both ages across either lung compartment, CYP1A1 protein had different spatial and temporal patterns compared between neonates and adults after exposure. Neonatal CYP1A1 protein trailed mRNA levels, and CYP1A1 airway protein was enhanced at PFP24. CYP1A1 responded more acutely in adult animals, where staining was transiently observed in both lung compartments at PFP2. Scale bar is 50 μm.
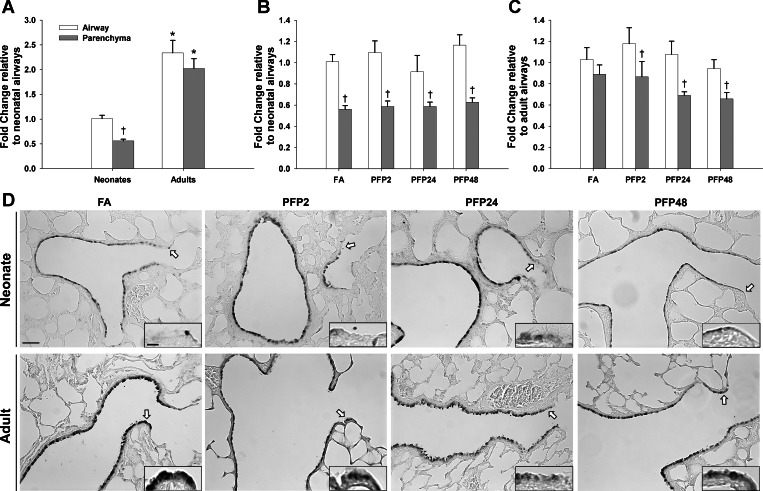
Next, we measured another CYP450 that had been shown to be affected by urban particulates, CYP1B1 (9). When we measured initial mRNA levels of CYP1B1, adult levels were significantly higher than neonates in both lung compartments. However, compared with CYP1A1 mRNA expression where expression levels were higher in the parenchyma than airways, with CYP1B1, transcript levels were significantly greater in airways than in the alveolar regions of the lung (Fig. 5A). After PFP exposure, the levels of CYP1B1 mRNA in neonates were unchanged from FA controls (Fig. 5B). In contrast, adult CYP1B1 was inhibited at PFP24 (P = 0.001) and PFP48 (P < 0.0001), similar to CYP1A1, but in the airways instead of the parenchyma (Fig. 5C). CYP1B1 protein was more abundant in adults compared with neonates, with dense localization in the bronchiolar epithelium (Fig. 5D). Two hours post-PFP exposure, robust airway and parenchyma staining was seen across both ages. By PFP24, neonatal CYP1B1 staining was diminished in the parenchyma and terminal bronchioles while adult staining remained consistently upregulated. By PFP48, neonatal CYP1B1 returned to FA conditions while adult airways remained elevated.
Fig. 5.
CYP1B1 compartmental mRNA and protein expression. RT-PCR: basal CYP1B1 mRNA was significantly higher in adults in both compartments, with the highest expression present in the airway (A), no exposure-related effects were observed in neonates (B), and adults had a time-dependent decrease in CYP1B1 expression in the airways at PFP days 24 (PFP24) and 48 (PFP48) (C). Data are presented as means + SE (n = 5–7 rats/group, in each compartment) *Significantly different compared with neonates in the same compartment. †Significantly different compared with airways in the same age. ‡Significantly different compared with FA in the same compartment. CYP1B1 immunohistochemistry showed a different spatial pattern. Whereas adults had more CYP1B1-positive cells basally, CYP1B1 was intensely upregulated in both ages. By PFP24, neonatal CYP1B1 began to fade from the most distal terminal bronchioles, whereas adult CYP1B1 expression remained elevated, as shown in the insets, as denoted by black arrows. At PFP48, neonatal CYP1B1 returned to FA control levels, whereas adults continue to remain slightly upregulated. Scale bar is 50 μm.
Because CYP1A1 and CYP1B1 require reduction by NADPH to function, we measured mRNA and protein expression of cytochrome P-450 reductase (POR). POR mRNA levels were significantly greater in the adult in both lung compartments (Fig. 6A). We did not observe any exposure-related effects in either neonates or adults on POR mRNA levels (Fig. 6, B and C). POR protein immunolocalization followed a similar trend. Although adult POR protein expression was abundant across the bronchiolar epithelium, and noticeably more abundant in the terminal bronchioles compared with neonates, POR protein remained consistent across both ages after exposure (Fig. 6D).
Fig. 6.
Cytochrome P-450 oxidoreductase (POR) compartmental mRNA and protein expression. RT-PCR: basal POR expression was higher in adults compared with neonates and POR mRNA levels were lowest in the neonatal parenchyma (A), POR expression was unchanged post-PFP exposure in neonates (B), and, in adults, compartmental differences were observed after exposure (C). Parenchyma mRNA was significantly less than airways in all exposure time points. Data are presented as means + SE (n = 5–7 rats/group, in each compartment) *Significantly different compared with neonates in the same compartment. †Significantly different compared with airways in the same age. POR immunohistochemical protein expression revealed a similar trend. POR expression was highest in the airways. The only differences observed were terminal bronchiole staining, where POR were absent in neonates, vs. heavy staining in adults, as denoted by white arrows and high-magnification insets. Scale bars are 50 μm.
DISCUSSION
In the current study, we used in vitro and in vivo models to interrogate the influence of PFP exposure on the AhR pathway in an attempt to identify the underlying cause of neonatal susceptibility to PM pollution. We found that PFPs are capable inducers of AhR and its downstream effector genes and causes age-dependent lung cytotoxicity. We showed that the neonatal lung is more susceptible to inhaled PFPs, and has cytotoxicity that is especially severe in the parenchyma, which contains developing alveoli, compared with the mature lung.
PFP contain numerous PAHs, and we hypothesized that some of these PAHs may play a role in AhR activation. It is known that PAHs can serve as ligands for the AhR transcription factor, which subsequently binds to XRE to upregulate CYP gene transcription (10). Using previously established U-937 human macrophages (57), we systematically showed that PFPs are capable of activating AhR, enhancing binding to XRE, and upregulating CYP1A1 downstream gene expression in a dose-dependent manner. AhR is activated upon at least 25 μg/ml PFP treatment in vitro, but activation is not as robust as that observed to NIST UDP particles and the classic AhR agonist TCDD. Our data correlate well with the literature; combustion-derived particles are known to activate AhR and increase binding to XRE, upregulating CYP1A1 expression (30, 43, 59). However, in contrast to TCDD, PFP treatment led to an increased expression of AhR mRNA. Upregulation of AhR mRNA and protein level may present a critical event in increasing the susceptibility to PM pollution. Many components within PM pollution are capable of activating AhR, including those found in PFPs used in the current study, which could consequently lead to an enhanced sensitivity toward additional PAH exposure. This is expected because of the composition of PFP. Various naphthalene species, which are not agonists for AhR, comprise ∼88% (48/54 ng PAH) of the PAHs detected. PFPs are scarce in PAHs larger than anthracene/phenanthrene compared with UDP (62). Common three-ringed species in PFP, including fluorene and phenanthrene, fail to stimulate activity in the AhR reporter assay or ethoxyresorufin-O-deethylase (EROD) activity (35). Although PFP contains several AhR agonists like anthanthrene and coronene, they are present in nanogram concentrations (Table 2). Other agonists found in UDP, like TCDD, dibenzofurans, benzo[a]pyrene, and benzo[ghi]perylene (7, 35, 62), were absent in PFPs and may explain the relatively low activity of PFPs in the XRE reporter assay compared with UDP. In contrast to TCDD, PFP and UDP treatment led to an increased AhR mRNA expression. Activation of AhR by TCDD can lead to an increase or decrease of AhR mRNA level (16, 47, 63). In the current study we did not identify the mechanism of the PFP-induced increase of AhR expression. However, the activation of AhR has been shown to mediate the increase of other signaling pathways such as NF-κB, responsible for activation of inflammatory enzymes such as cyclooxygenase-2 or cytokines IL-1b and IL-8, which may serve as critical mediators of pathological effects. Alternatively, changes in AhR mRNA and protein stability from PFP exposure should also be considered. Table 3 shows a comparison of selected PAH concentrations between UDP and PFP normalized to PM mass. Taken together, we conclude that PFPs contain weak AhR agonists in relatively low abundance.
Table 3.
Comparison of selected PAH concentrations between PFP and UDP (SRM 1649)
| PFP, ng/mg | UDP, ng/mg | |
|---|---|---|
| Phenanthrene | 22.4 | 4.5 |
| Fluoranthene | 3.7 | 7.1 |
| Pyrene | 7.5 | 6.3–7.2 |
| Benz(a)anthracene | 3.7 | 2.6 |
| Chrysene | 3.7† | 3.5 |
| Triphenylene | 1.7 | |
| Benzo[b]fluoranthene | ND‡ | 6.2 |
| Benzo[k]fluoranthene | 2 | |
| Benzo[e]pyrene | 3.7 | 3.3 |
| Benzo[a]pyrene | ND | 2.9 |
| Perylene | ND | 0.65–0.84 |
| Benzo[ghi]perylene | ND | 4.5 |
| Indeno[1,2,3-cd]pyrene | ND | 3.3 |
| Dibenz[a,h]anthracene | ND | 0.41 |
PFP, premixed flame particle; UDP, urban dust particle; SRM, standard reference material. Adapted from Wise et al. (62).
Chrysene and triphenylene were detected as one PAH species.
Benzo[b]fluoranthene and benzo[k]fluoranthene were detected as one PAH species. ND, not detected.
We confirmed our in vitro results with a time-course, whole animal study, and using whole body exposures of 7-day-old neonatal and adult rats to 22.4 μg/m3 PFP. This dose is well below 35 μg/m3, the 2006 EPA-revised 24-h standard for PM2.5. This particle number concentration is also comparable to values reported 30 meters from a major Los Angeles, CA, highway for the same size fraction (64). We have previously reported that the neonatal lung is susceptible to PFPs based on increased LDH leakage and the presence of ethidium homodimer-1-positive alveolar macrophages and type II cells (4). Additionally, we showed that PFPs induce oxidative stress and deplete glutathione in neonatal animals because of an inability to upregulate key antioxidant enzymes in the lung (5) and perturbs lung airway development (26). To follow up on our previous work, we first visualized ethidium homodimer-1, a marker for permeable cells, using confocal microscopy and stereological approaches to determine the spatial localization and abundance of toxicity within the lung.
Using confocal microscopy, we were able to image the entire depth of a specific airway and the surrounding parenchyma that allowed us to sample a much deeper cross-sectional area of the lung compared with paraffin sections that we have relied on previously. As a result, we were able to both confirm and expand upon our previous findings. We found ethidium-positive cells pervasive in the neonatal lung after PFP exposure and also focal patches of dead cells in the adult airway that we were unable to visualize previously. There are multiple factors that may contribute to the disparate age- and compartment-specific response to PFP. Physiological factors can play a role. Young animals have higher ventilation and oxygen consumption rates compared with adult rats normalized to body weight (34). Lung volume increases proportionally to their body weight within the first 10 days of life (41). Furthermore, alveolar proliferation and expansion also occur between the first 4–14 and 14–28 days of life, respectively (40). Higher respiratory rates and increased air exchanges enhance particle retention and deposition (3), which may cause preferential deposition in the alveoli to cause cellular toxicity. We have previously shown that inhaling PFPs subchronically during this critical period of growth and differentiation perturbs lung growth and development (26). These effects may be because of the combined influences of delivered dose and the differential susceptibility of a still differentiating and growing lung.
The CYP1 family includes three genes, CYP1A1, CYP1A2, and CYP1B1, that are all inducible by AhR agonists. Although these enzymes can metabolize a variety of environmental toxicants and xenobiotics, CYP1A1 and CYP1B1 are especially effective at metabolizing PAHs and contain XREs within their promoter regions (36). CYP maturation varies during pre- and postnatal development by isozyme, and CYPs are localized in four pulmonary cell types: the nonciliated bronchiolar (Clara) cell, the type II epithelial cell, the endothelial cell, and the alveolar macrophage (14). The location of these unique cell types may result in differential susceptibility and cytotoxicity. Differences in cellular development and maturation may also play a role in susceptibility to PFPs. Although CYP1A1 is expressed at very low levels constitutively, CYP1A1 is most abundant in Clara cells and is preferentially induced upon TCDD treatment (6). In the developing lung, we were able to detect CYP1A1 sparsely in the bronchiolar epithelium, which is in good agreement with detection of CYP1A1 localized in Clara cell apices in the 7-day-old rat (23). Contrastingly, CYP1B1 has much higher basal levels, but is generally less inducible compared with CYP1A1 (50). Our results correlated well with this observation; CYP1B1 protein was readily observed in the unexposed adult lung and to a lesser degree in the neonatal lung. Furthermore, POR must also be present for CYP enzymatic activity. Although POR maturation precedes CYP development and has been experimentally shown to be most efficient upon a 1:1 association with CYP1A1 (32), we were unable to detect POR protein expression in the terminal bronchioles and the alveoli in the neonatal lung, implying that CYP maturation is incomplete at 7 days postnatal age.
To tease apart how the bronchiolar epithelium and alveoli responded to PFP exposure, we microdissected bronchiolar airways separate from alveolar parenchyma and analyzed gene expression of each compartment separately. Our in vivo results correlated well with the in vitro data. We observed modest AhR activation in both airway and parenchymal compartments of both ages. However, downstream CYP1A1 and CYP1B1 gene and protein expression had differing spatial and temporal patterns between the two ages. Neonatal CYP1A1 gene and protein expression was transiently upregulated in neonatal airways at PFP2 and PFP24, respectively, whereas CYP1B1 protein was abundant in the bronchiolar epithelium at PFP2 and PFP24. Our results correlated well with the literature. Although CYP1A1 can be detectable in 7-day-old rats, CYP1A1 is typically enzymatically inactive until 14 days postnatal age (17). Exposure to PAH-rich environmental pollutants like tobacco smoke particulates has been shown to stimulate CYP1A1 expression, and EROD enzymatic activity can be detected as early as 7 days postnatal age (17, 23), possibly because of accelerated lung cell maturation. Additionally, an increase of CYP1A1 may also be responsible for increased susceptibility through bioactivation of PM components into more toxic intermediates that may increase cellular oxidative stress.
Although we were unable to detect CYP1A1 and CYP1B1 gene induction in adults, it is possible that we may have missed the mRNA upregulation, since our first time point occurred 8 h from the beginning of exposure. PFPs induced CYP1A1 expression and XRE-binding activity at 4- and 1.5-h treatments, respectively. Also, we have also shown robust CYP1A1 and CYP1B1 protein upregulation in the PFP2 group. These data imply that AhR activation and CYP gene upregulation are rapid, but transient, events. Some data in the literature support this assertion. A study by Rouse et al. (43) reported substantially upregulated CYP1A1 and CYP1B1 mRNA levels in mice directly after a 4-day exposure to butadiene soot, whereas Courter et al. (9) showed CYP1A1 and CYP1B1 protein inhibition 24 h after a 1-mg dose of UDP in mice. When we compared expression patterns between neonates and adults, we saw substantially different regional and temporal patterns within the respiratory tract. Adults appeared to have a more immediate and prolonged response to PFP. CYP1A1 protein was significantly upregulated within the adult lung, whereas its upregulation was limited to the bronchiolar epithelium in neonates. CYP1B1 protein in the adult lung was upregulated even at PFP48 compared with levels in comparable FA controls in the neonates at the same time point. Furthermore, lack of CYP1A1 and POR expression in the lung parenchyma correlated with enhanced parenchymal toxicity. From these data, it appears that AhR activation and downstream upregulation of CYP1A1 and CYP1B1 may have a protective effect in the lung by decreasing the PAH burden from inhaling PFPs. However, the long-term effects of PFP inhalation are unknown, depending on the persistence and burden of PFP and its downstream metabolites. Sustained elevated levels of CYP1A1 or inflammatory markers may eventually lead to pathophysiological effects that may detrimentally affect lung cellular integrity and function.
In summary, the present study establishes that laboratory-generated PFP is a capable, but weak, AhR agonist. We determined that PFP caused lung cytotoxicity, and neonatal animals are more susceptible, especially in the gas-exchange alveolar region. CYP1A1 and CYP1B1 gene and enzyme expression were variably altered after PFP exposure, depending on age, lung compartment, and postexposure recovery time. We showed that maturation of these isozymes is incomplete in the 7-day-old neonatal lung, and neonates have a delayed and incomplete response to PFPs compared with adults. The inability to upregulate these metabolic enzymes could ultimately increase the PAH burden in the developing lung because of physiological and cellular-based differences that result in increased toxicity and altered lung development.
GRANTS
Support for the University of California (UC) at Davis core facilities used in this work was from the Cellular and Molecular Imaging Core Facility, the confocal microscope (S10-RR-026422), and the inhalation exposure facility at the California National Primate Research Center (P51OD011107). The project was also supported in part by the Superfund Basic Sciences Research Program at UC Davis (P42-ES-004699). J. K. W. Chan's effort was supported by a training program in Environmental Health Sciences (T32-ES-007059). C. F. Vogel's effort was supported by a grant from the National Institute of Environmental Health Sciences (1R01-ES-019898).
DISCLOSURES
One Author, Dr. Laura Van Winkle, has identified a potential apparent competing financial interest with the American Petroleum Institute (API). Dr. Van Winkle has received research grants from API to study the kinetics of naphthalene bioactivation and cytotoxicity in the respiratory system and has received honoraria from API for speaking at research conferences sponsored by API on naphthalene. API did not fund the work presented in the attached study and the research grant funded by API has complete freedom to publish the results regardless of whether they are in API's interest, and without input from API, in keeping with University of California policy. The remaining authors declare they have no actual or potential competing financial interests. Although the research described in the article has been funded primarily by the United States Environmental Protection Agency (EPA) through grant RD-83241401–0 to the University of California, Davis, it has not been subject to the Agency's required peer and policy review and, therefore, does not necessarily reflect the views of the Agency and no official endorsement should be inferred. The views expressed in this publication are solely those of the authors, and EPA does not endorse any products or commercial services mentioned in this publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
AUTHOR CONTRIBUTIONS
Author contributions: J.K.C., D.S.A., and L.S.V.W. conception and design of research; J.K.C., C.F.V., J.B., S.D.K., R.S.U., K.J.B., and D.S.A. performed experiments; J.K.C., C.F.V., S.D.K., R.S.U., and K.J.B. analyzed data; J.K.C., C.F.V., and L.S.V.W. interpreted results of experiments; J.K.C., S.D.K., and R.S.U. prepared figures; J.K.C. drafted manuscript; J.K.C., C.F.V., K.J.B., and L.S.V.W. edited and revised manuscript; J.K.C., C.F.V., J.B., S.D.K., R.S.U., K.J.B., D.S.A., and L.S.V.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to the following people for their skilled technical assistance during exposures, sample collection, and processing: Brian Tarkington, Ashley Cooper, Louise Olson, Patricia Edwards, and Christopher Wallis. We thank Dr. Alan Buckpitt and Dr. Anthony Wexler for reading and editing the manuscript.
REFERENCES
- 1. Baker GL, Shultz MA, Fanucchi MV, Morin DM, Buckpitt AR, Plopper CG. Assessing gene expression in lung subcompartments utilizing in situ RNA preservation. Toxicol Sci 77: 135–141, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Bearer CF. How are children different from adults. Environ Health Perspect 103: 7–12, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Branis M, Safranek J, Hytychova A. Exposure of children to airborne particulate matter of different size fractions during indoor physical education at school. Build Environ 44: 1246–1252, 2009 [Google Scholar]
- 4. Chan JK, Fanucchi MV, Anderson DS, Abid AD, Wallis CD, Dickinson DA, Kumfer BM, Kennedy IM, Wexler AS, Van Winkle LS. Susceptibility to inhaled flame-generated ultrafine soot in neonatal and adult rat lungs. Toxicol Sci 124: 472–486, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan JK, Kodani SD, Charrier JG, Morin D, Edwards PC, Anderson DS, Anastasio C, Van Winkle LS. Age-specific effects on rat lung glutathione and antioxidant enzymes after inhaling ultrafine soot. Am J Respir Cell Mol Biol 48: 114–124, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chang H, Chang LW, Cheng YH, Tsai WT, Tsai MX, Lin P. Preferential induction of CYP1A1 and CYP1B1 in CCSP-positive cells. Toxicol Sci 89: 205–213, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Cherng SH, Lin PP, Yang JL, Hsu SL, Lee H. Benzo[g,h,i]perylene synergistically transactivates benzo[a]pyrene-induced CYP1A1 gene expression by aryl hydrocarbon receptor pathway. Toxicol Appl Pharm 170: 63–68, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Cohen AJ, Anderson HR, Ostro B, Pandey KD, Krzyzanowski M, Kunzli N, Gutschmidt K, Pope A, Romieu I, Samet JM, Smith K. The global burden of disease due to outdoor air pollution. J Toxicol Env Heal A 68: 1301–1307, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Courter LA, Musafia-Jeknic T, Fischer K, Bildfell R, Giovanini J, Pereira C, Baird WM. Urban dust particulate matter alters PAH-induced carcinogenesis by inhibition of CYP1A1 and CYP1B1. Toxicol Sci 95: 63–73, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Denison MS, Pandini A, Nagy SR, Baldwin EP, Bonati L. Ligand binding and activation of the Ah receptor. Chem Biol Interact 141: 3–24, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Denison MS, Whitlock JP., Jr Xenobiotic-inducible transcription of cytochrome P450 genes. J Biol Chem 270: 18175–18178, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Dockery DW. Health effects of particulate air pollution. Ann Epidemiol 19: 257–263, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dockery DW, Pope CA, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG, Speizer FE. An association between air pollution and mortality in six U.S. Cities. N Engl J Med 329: 1753–1759, 1993 [DOI] [PubMed] [Google Scholar]
- 14. Fanucchi M. Development of Antioxidant and Xenobiotic Metabolizing Enzyme Systems. San Diego, CA: Elsevier, 2004 [Google Scholar]
- 15. Fanucchi MV, Buckpitt AR, Murphy ME, Storms DH, Hammock BD, Plopper CG. Development of phase II xenobiotic metabolizing enzymes in differentiating murine clara cells. Toxicol Appl Pharmacol 168: 253–267, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Fitzgerald CT, Nebert DW, Puga A. Regulation of mouse Ah receptor (Ahr) gene basal expression by members of the Sp family of transcription factors. DNA Cell Biol 17: 811–822, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Gebremichael A, Chang AM, Buckpitt AR, Plopper CG, Pinkerton KE. Postnatal development of cytochrome P4501A1 and 2B1 in rat lung and liver: effect of aged and diluted sidestream cigarette smoke. Toxicol Appl Pharmacol 135: 246–253, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Hammond TG, Mobbs M. Lung oedema–microscopic detection. J Appl Toxicol 4: 219–221, 1984 [DOI] [PubMed] [Google Scholar]
- 19. Helsel DR. More than obvious: better methods for interpreting nondetect data. Environ Sci Technol 39: 419a–423a, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Howard CV. Unbiased Stereology: Three-Dimensional Measurement in Microscopy. Webster Groves, MO: BIOS Scientific Publishers, 1998 [Google Scholar]
- 21. Hyde DM, Plopper CG, George JA, Harkema JR. Morphometric cell biology of air space epithelium. In: Electron Microscopy of the Lung, edited by Schraufnagel DE. New York, NY: Dekker, 1990, p. 71–120 [Google Scholar]
- 22. Ibald-Mulli A, Wichmann HE, Kreyling W, Peters A. Epidemiological evidence on health effects of ultrafine particles. J Aerosol Med 15: 189–201, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Ji CM, Plopper CG, Witschi HP, Pinkerton KE. Exposure to sidestream cigarette smoke alters bronchiolar epithelial cell differentiation in the postnatal rat lung. Am J Respir Cell Mol Biol 11: 312–320, 1994 [DOI] [PubMed] [Google Scholar]
- 24. Karnovsky MJ. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 27: 137–138A, 1965 [Google Scholar]
- 25. Langston C. Normal and abnormal structural development of the human lung. Prog Clin Biol Res 140: 75–91, 1983 [PubMed] [Google Scholar]
- 26. Lee D, Wallis C, Wexler AS, Schelegle ES, Van Winkle LS, Plopper CG, Fanucchi MV, Kumfer B, Kennedy IM, Chan JK. Small particles disrupt postnatal airway development. J Appl Physiol 109: 1115–1124, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee WJ, Wang YF, Lin TC, Chen YY, Lin WC, Ku CC, Cheng JT. Pah characteristics in the ambient air of traffic-source. Sci Total Environ 159: 185–200, 1995 [Google Scholar]
- 28. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Marr LC, Kirchstetter TW, Harley RA, Miguel AH, Hering SV, Hammond SK. Characterization of polycyclic aromatic hydrocarbons in motor vehicle fuels and exhaust emissions. Environ Sci Technol 33: 3091–3099, 1999 [Google Scholar]
- 30. Matsumoto Y, Ide F, Kishi R, Akutagawa T, Sakai S, Nakamura M, Ishikawa T, Fujii-Kuriyama Y, Nakatsuru Y. Aryl hydrocarbon receptor plays a significant role in mediating airborne particulate-induced carcinogenesis in mice. Environ Sci Technol 41: 3775–3780, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Mitsakou C, Housiadas C, Eleftheriadis K, Vratolis S, Helmis C, Asimakopoulos D. Lung deposition of fine and ultrafine particles outdoors and indoors during a cooking event and a no activity period. Indoor Air 17: 143–152, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Miwa GT, West SB, Huang MT, Lu AY. Studies on the association of cytochrome P-450 and NADPH-cytochrome c reductase during catalysis in a reconstituted hydroxylating system. J Biol Chem 254: 5695–5700, 1979 [PubMed] [Google Scholar]
- 33. Moller W, Felten K, Sommerer K, Scheuch G, Meyer G, Meyer P, Haussinger K, Kreyling WG. Deposition, retention, and translocation of ultrafine particles from the central airways and lung periphery. Am J Respir Crit Care Med 177: 426–432, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Mortola JP. Hamsters versus rats: ventilatory responses in adults and newborns. Respir Physiol 85: 305–317, 1991 [DOI] [PubMed] [Google Scholar]
- 35. Murahashi T, Watanabe T, Kanayama M, Kubo T, Hirayama T. Human aryl hydrocarbon receptor ligand activity of 31 non-substituted polycyclic aromatic hydrocarbons as soil contaminants. J Health Sci 53: 715–721, 2007 [Google Scholar]
- 36. Nebert DW, Puga A, Vasiliou V. Role of the Ah receptor and the dioxin-inducible [ah] gene battery in toxicity, cancer, and signal-transduction. Ann NY Acad Sci 685: 624–640, 1993 [DOI] [PubMed] [Google Scholar]
- 37. Omar NYMJ, Bin Abas MR, Ketuly KA, Tahir NM. Concentrations of PAHs in atmospheric particles (PM-10) and roadside soil particles collected in Kuala Lumpur, Malaysia. Atmospher Environ 36: 247–254, 2002 [Google Scholar]
- 38. Peters A, Dockery DW, Heinrich J, Wichmann HE. Short-term effects of particulate air pollution on respiratory morbidity in asthmatic children. Eur Respir J 10: 872–879, 1997 [PubMed] [Google Scholar]
- 39. Pey J, Querol X, Alastuey A, Rodriguez S, Putaud JP, Van Dingenen R. Source apportionment of urban fine and ultra-fine particle number concentration in a Western Mediterranean city. Atmospher Environ 43: 4407–4415, 2009 [Google Scholar]
- 40. Pinkerton KE, Joad JP. Influence of air pollution on respiratory health during perinatal development. Clin Exp Pharmac Physiol 33: 269–272, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Powell JT, Whitney PL. Postnatal development of rat lung. Changes in lung lectin, elastin, acetylcholinesterase and other enzymes. Biochem J 188: 1–8, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rengasamy A, Barger MW, Kane E, Ma JK, Castranova V, Ma JY. Diesel exhaust particle-induced alterations of pulmonary phase I and phase II enzymes of rats. J Toxicol Environ Health A 66: 153–167, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Rouse RL, Murphy G, Boudreaux MJ, Paulsen DB, Penn AL. Soot nanoparticles promote biotransformation, oxidative stress, and inflammation in murine lungs. Am J Respir Cell Mol Biol 39: 198–207, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132: 365–386, 2000 [DOI] [PubMed] [Google Scholar]
- 45. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protocols 3: 1101–1108, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Shumway RH, Azari RS, Kayhanian M. Statistical approaches to estimating mean water quality concentrations with detection limits. Environ Sci Technol 36: 3345–3353, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Song Z, Pollenz RS. Ligand-dependent and independent modulation of aryl hydrocarbon receptor localization, degradation, and gene regulation. Mol Pharmacol 62: 806–816, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Stelck RL, Baker GL, Sutherland KM, Van Winkle LS. Estrous cycle alters naphthalene metabolism in female mouse airways. Drug Metab Dispos 33: 1597–1602, 2005 [DOI] [PubMed] [Google Scholar]
- 49. Sutherland KM, Edwards PC, Combs TJ, Van Winkle LS. Sex differences in the development of airway epithelial tolerance to naphthalene. Am J Physiol Lung Cell Mol Physiol 302: L68–L81, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tang WM, Wo YYP, Stewart J, Hawkins AL, Griffin CA, Sutter TR, Greenlee WF. Isolation and characterization of the human cytochrome p450 CYP1B1 gene. J Biol Chem 271: 28324–28330, 1996 [DOI] [PubMed] [Google Scholar]
- 51. Uppstad H, Ovrebo S, Haugen A, Mollerup S. Importance of CYP1A1 and CYP1B1 in bioactivation of benzo[a]pyrene in human lung cell lines. Toxicol Lett 192: 221–228, 2010 [DOI] [PubMed] [Google Scholar]
- 52. Van Winkle LS, Chan JK, Anderson DS, Kumfer BM, Kennedy IM, Wexler AS, Wallis C, Abid AD, Sutherland KM, Fanucchi MV. Age specific responses to acute inhalation of diffusion flame soot particles: cellular injury and the airway antioxidant response. Inhal Toxicol 22, Suppl 2: 70–83, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Van Winkle LS, Isaac JM, Plopper CG. Repair of naphthalene-injured microdissected airways in vitro. Am J Respir Cell Mol Biol 15: 1–8, 1996 [DOI] [PubMed] [Google Scholar]
- 54. Van Winkle LS, Johnson ZA, Nishio SJ, Brown CD, Plopper CG. Early events in naphthalene-induced acute Clara cell toxicity: comparison of membrane permeability and ultrastructure. Am J Respir Cell Mol Biol 21: 44–53, 1999 [DOI] [PubMed] [Google Scholar]
- 55. Vogel CF, Sciullo E, Li W, Wong P, Lazennec G, Matsumura F. RelB, a new partner of aryl hydrocarbon receptor-mediated transcription. Mol Endocrinol 21: 2941–2955, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vogel CF, Sciullo E, Park S, Liedtke C, Trautwein C, Matsumura F. Dioxin increases C/EBPbeta transcription by activating cAMP/protein kinase A. J Biol Chem 279: 8886–8894, 2004 [DOI] [PubMed] [Google Scholar]
- 57. Vogel CF, Sciullo E, Wong P, Kuzmicky P, Kado N, Matsumura F. Induction of proinflammatory cytokines and C-reactive protein in human macrophage cell line U937 exposed to air pollution particulates. Environ Health Perspect 113: 1536–1541, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang WC, Chen KS, Chen SJ, Lin CC, Tsai JH, Lai CH, Wang SK. Characteristics and receptor modeling of atmospheric PM2.5 at urban and rural sites in Pingtung, Taiwan. Aerosol Air Qual Res 8: 112–129, 2008 [Google Scholar]
- 59. Wenger D, Gerecke AC, Heeb NV, Hueglin C, Seiler C, Haag R, Naegeli H, Zenobi R. Aryl hydrocarbon receptor-mediated activity of atmospheric particulate matter from an urban and a rural site in Switzerland. Atmospher Environ 43: 3556–3562, 2009 [Google Scholar]
- 60. Wichmann HE, Peters A. Epidemiological evidence of the effects of ultrafine particle exposure. Philos T Roy Soc A 358: 2751–2768, 2000 [Google Scholar]
- 61. Wilson HH, Chauhan J, Kerry PJ, Evans JG. Ethanol vapour-fixation of rat lung for immunocytochemistry investigations. J Immunol Methods 247: 187–190, 2001 [DOI] [PubMed] [Google Scholar]
- 62. Wise SA, Sander LC, Schantz MM, Hays MJ, Benner BA. Recertification of standard reference material (SRM) 1649, urban dust, for the determination of polycyclic aromatic hydrocarbons (PAHs). Polycycl Aromat Comp 13: 419–456, 1999 [Google Scholar]
- 63. Xing XR, Bi HL, Chang AK, Zang MX, Wang M, Ao X, Li S, Pan HM, Guo QP, Wu HJ. SUMOylation of AhR modulates its activity and stability through inhibiting its ubiquitination. J Cell Physiol 227: 3812–3819, 2012 [DOI] [PubMed] [Google Scholar]
- 64. Zhu Y, Hinds WC, Kim S, Sioutas C. Concentration and size distribution of ultrafine particles near a major highway. J Air Waste Manag Assoc 52: 1032–1042, 2002 [DOI] [PubMed] [Google Scholar]