Abstract
Oxidative stress plays a major role in the pathogenesis of heart failure, where the contractile response to β-adrenergic stimulation is profoundly depressed. This condition involves L-type Ca2+ channels, but the mechanisms underlying their impaired adrenergic regulation are unclear. Thus the present study explored the basis for impaired adrenergic control of Ca2+ channels in a rat infarction model of heart failure. Patch-clamp recordings of L-type Ca2+ current (ICa,L) from ventricular myocytes isolated from infarcted hearts showed a blunted response to intracellular cAMP that was reversed by treatment with exogenous pyruvate. Biochemical studies showed that basal and cAMP-stimulated protein kinase A activities were similar in infarcted and sham-operated hearts, whereas molecular analysis also found that binding of protein kinase A to the α1C subunit of voltage-gated Ca2+ channel isoform 1.2 was not different between groups. By contrast, protein phosphatase 2A (PP2A) activity and binding to α1C were significantly less in infarcted hearts. The PP2A inhibitor okadaic acid markedly increased ICa,L in sham-operated myocytes, but this response was significantly less in myocytes from infarcted hearts. However, pyruvate normalized ICa,L stimulation by okadaic acid, and this effect was blocked by inhibitors of thioredoxin reductase, implicating a functional role for the redox-active thioredoxin system. Our data suggest that blunted β-adrenergic stimulation of ICaL in failing hearts results from hyperphosphorylation of Ca2+ channels secondary to oxidation-induced impairment of PP2A function. We propose that the redox state of Ca2+ channels or PP2A is controlled by the thioredoxin system which plays a key role in Ca2+ channel remodeling of the failing heart.
Keywords: L-type Ca2+ channel, pyruvate, PP2a, thioredoxin
ventricular dysfunction and failure elicited by chronic myocardial infarction (MI) involve a pathogenic process of electrical remodeling that is characterized by profound alterations in ion channel properties. This change in electrophysiological phenotype is proposed to contribute to arrhythmogenic abnormalities in repolarization (1) and to impaired contractile performance resulting from dysregulation of cellular Ca2+ (17). An essential regulator of cardiac contractility is the influx of Ca2+ through the L-type Ca2+ channel (LTCC), which triggers Ca2+ release from the sarcoplasmic reticulum (5, 6, 39, 44). It has been postulated therefore that abnormalities in LTCC function in the diseased heart are important determinants of depressed contraction. However, recent studies in hypertrophied and failing hearts have shown variable results for basal density of the L-type Ca2+ current (ICa,L), either being unchanged (30, 41) or decreased (9, 27) from control. A more consistent finding, relating to LTCC dysfunction, is a blunted response to β-adrenergic stimulation (5, 6), which is thought to arise from the chronic hyperactivation of sympathetic outflow during the progression of heart failure (45). Nevertheless, the reasons why LTCCs in myocytes from failing hearts are less responsive than normal to β-adrenergic receptor stimulation are not fully understood.
An important factor in the etiology of heart failure is oxidative stress, defined as an imbalance of reactive oxygen species (ROS) production and antioxidant defenses (28). Excess ROS dramatically alters cell redox state, which can underlie changes in cell function because of oxidation of lipids, proteins, and nucleic acids (3, 9). Typically, thiol (-SH) and thioether (-SCH3) side chains of cysteine and methionine residues, respectively, are targets for reversible redox modifications of proteins (3, 9, 44). It is proposed that oxidation of cysteine and/or methionine residues elicit changes in protein function that can be reversed by specific oxidoreductase systems whose primary biological role is to protect proteins from oxidative damage (3). The major cytosolic oxidoreductase network in heart is the thioredoxin system, which is composed of thioredoxin-1, thioredoxin reductase-1, and NADPH (3). The cytosolic glutaredoxin system is a second oxidoreductase network that includes glutaredoxin-1, reduced glutathione, glutathione reductase, and NADPH (3). We have shown that these oxidoreductase networks are significantly impaired in the failing rat heart and postulate that this condition partly underlies electrical remodeling via an oxidative shift in the redox state of cell proteins (31, 32, 41).
Experimental studies have shown that the redox state of cardiac myocytes is an important regulator of basal LTCC activity and its response to β-adrenergic stimulation (16, 35), but the underlying mechanisms are not clear. Thus the purpose of this study was to determine whether redox pathways control the blunted β-adrenergic stimulation of LTCC activity in a rat post-MI model of heart failure. We focused on the functional effects of exogenous pyruvate, which we and others have shown normalizes the redox state of cells affected by oxidative stress (2, 24, 31, 37). Our data suggest that the blunted response of ICa,L to β-adrenergic stimulation in myocytes from post-MI hearts is related to a hyperphosphorylation of LTCCs secondary to impairment of protein phosphatase 2A (PP2A) function. Moreover, exogenous pyruvate normalizes the adrenergic stimulation of ICa,L in myocytes from MI hearts through mechanisms involving the thioredoxin system.
MATERIALS AND METHODS
Post-MI model and isolation of ventricular myocytes.
All animal procedures were carried out in accordance with guidelines approved by University of Nebraska Medical Center Institutional Animal Care and Use Committee and conducted according to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996).
A chronic post-MI model of ventricular dysfunction was used in the present investigation as previously described (31, 32, 40, 41). Briefly, male Sprague-Dawley rats (180–200 g) were intubated and artificially ventilated under Brevital (methohexital sodium) anesthesia at 50 mg/kg ip. A left thoracotomy was performed, and the left coronary artery was ligated by a suture positioned between the pulmonary artery outflow tract and the left atrium. This ligation protocol produces infarcts of 30–40% of the left ventricular free wall and physiological signs of heart failure after several weeks (24, 41). Sham-operated animals that served as controls underwent the same surgical procedure but were not subjected to coronary artery ligation. Hemodynamic and echocardiographic parameters were measured in a subset of rats, at the time of the terminal experiment, to document left ventricular dysfunction in the MI group. For this purpose, rats from the MI and sham-operated groups were anesthetized with 2 to 3% isoflurane. Echocardiograms were performed using a Vevo 770 ultrasound system (Visualsonics,), whereas mean arterial and left ventricular end-diastolic pressures were measured by a Mikro-Tip catheter (Millar Instruments).
Six to eight weeks after MI or sham operation, rats were given an overdose of pentobarbital sodium (100 mg/kg ip), and the hearts were excised to obtain tissue samples or to isolate ventricular myocytes. For the latter purpose, myocytes were dissociated from Langendorff-perfused hearts by a collagenase digestion procedure previously described (31, 31, 32, 41). Dispersed myocytes from surviving regions of the left ventricle and septum were suspended in DMEM/F12 culture medium and stored in an incubator at 37°C until used, usually within 8 h of isolation.
Recording techniques.
Ionic currents were recorded using the whole cell configuration of the patch-clamp technique in myocytes bathed in a standard external solution containing (in mmol/l) 138 NaCl, 4 CsCl, 0.5 MgCl2, 5 HEPES, 1.8 CaCl2, and 10 glucose (pH 7.4). Recording glass pipettes were pulled (Sutter Instruments) to an internal tip diameter of ∼2 μm and filled with a solution containing (in mmol/l) 135 CsCl, 3 MgCl2, 10 EGTA, 5 Na2ATP, and 10 HEPES (pH 7.2). Filled pipettes with resistance from 2 to 3 MΩ were coupled to an Axopatch 200B amplifier (Molecular Devices). After correction of the liquid junction potential and creation of a giga-ohm seal, the membrane within the pipette was ruptured to achieve the whole cell recording configuration. A computer program (pClamp, Molecular Devices) controlled command potentials and acquired current signals that were filtered at 2 kHz. Currents were sampled at 4 kHz by a 12-bit resolution analog-to-digital converter and stored on the hard disk of a computer. All patch-clamp recordings were done at room temperature (22–24°C).
ICa,L was evoked by 500 ms depolarizing pulses to test potentials between −40 and +60 mV (0.2 Hz) from a holding potential of −50 mV, and current-voltage relationships were plotted after normalizing measured currents as current densities (in pA/pF). To assess the voltage-dependence of Ca2+ channel activation, whole cell conductance (G) was first calculated by dividing ICa,L density by the driving force (Vtest − Vrev) for Ca2+ ions, where Vtest is the test potential and Vrev is the apparent reversal potential for ICa,L. Activation curves were then generated by plotting normalized conductance (G/Gmax) as a function of Vtest and were fitted with a Boltzmann equation to derive the half-maximal activation voltage (V1/2) and slope factor (k). The inactivation time course of ICa,L was also fitted with a double exponential function to determine fast and slow time constants: y = A1e−t/τf + A2e−t/τs + A3, where τf and τs are the time constants of the fast and slow components of inactivation, respectively. Unless stated otherwise, all mean data from patch-clamp studies described in this report were derived from myocytes isolated from at least three rat hearts.
Protein kinase A and PP2A activity assays.
Protein kinase A (PKA) activity was determined in homogenized left ventricular tissue samples. Protein extracts were assayed with or without 10 μmol/l cAMP using a modification of previously described methods (19), namely using a reaction mixture consisting of 130 μmol/l PKA heptapeptide substrate (LRRASLG; Peninsula) in a buffer containing 20 mmol/l Tris·HCl (pH 7.5), 100 μmol/l IBMX, 20 mmol/l magnesium-acetate, and 200 μmol/l γ-[32P]ATP. Protein samples (20 μl) were added to 50 μl of the reaction mixture and incubated for 15 min at 30°C. The reaction was halted by spotting the assay mix (60 μl) onto P-81 phosphocellulose papers (Whatman). Papers were then washed five times for 5 min in 75 mmol/l phosphoric acid and washed once in ethanol. Dried papers were counted in nonaqueous scintillation fluid, and enzyme activity was expressed as picomoles per minute per milligram.
PP2A activity in homogenates from isolated rat ventricular myocytes was measured by a PP2A immunoprecipitation phosphatase assay kit (Millipore) according to the manufacturer's instructions. Briefly, cardiomyocytes were rinsed with TBS and lysed with 1% Nonidet P-40 lysis buffer on ice. A 400-μg aliquot of the supernatant was immunoprecipited with a monoclonal anti-PP2A antibody (Millipore) and protein A agarose at 4°C overnight with constant rocking. After TBS washing, the PP2A assay of the immunocomplex was initiated by addition of 60 μl of 1 mM phosphopeptide substrate and 20 μl of assay buffer and incubated at 30°C for 15 min. The reaction was terminated by addition of malachite green detection solution, and absorbance at 630 nm was recorded for each sample. PP2A activity was calculated using a phosphate standard curve and expressed as picomoles per minute per milligram protein.
Immunoprecipitation.
To assess the binding of PKA or PP2A with voltage-gated Ca2+ channel isoform 1.2 (Cav1.2), immunoprecipitation experiments were carried out. All homogenization and immunoprecipitation procedures were performed at 4°C. In these assays, equivalent amounts (400 μg) of left ventricle tissue homogenates from sham-operated and MI rats in lysis buffer consisting of 10 mM Tris, 1 mM EDTA, 1% SDS, and 0.1% Triton X-100 and containing complete protease inhibitor cocktail (BD BaculoGold) were subjected to preclearing with Roche Protein G beads (Roche) for 2 h with constant rocking. Cleared supernatants were gently rocked with rabbit antisera recognizing the α1C subunit of Cav1.2 (Millipore) or with the corresponding preimmune sera overnight in cold room. The immune complexes were precipitated after 4 h incubation with protein A/G agarose by centrifugation. The precipitates were washed twice with ice-cold lysis buffer. For Western blot analysis, samples were dissolved in 40 μl sample buffer, containing 200 mM Tris·HCl (pH 6.8), 6% SDS, 20% glycerol, 10% DTT, and 0.1 mg/ml bromophenol blue; separated by 7.5% SDS-PAGE; transferred to polyvinylidene difluoride membranes; and probed with anti-rat PKA (RIIα regulatory subunit; Abcam) or anti-mouse PP2A (Millipore) antibodies.
Statistical analysis.
All results are expressed as means ± SE. Statistical comparisons of two groups were made using a Student's t-test, whereas comparisons of more than two groups were made by ANOVA. When a significant difference between groups was indicated by the initial analysis, individual paired comparisons were made using a Student-Newman-Keuls t-test. Differences were considered significant at P < 0.05.
RESULTS
The hemodynamic characteristics of post-MI and sham-operated rats used in the present investigation are summarized in Table 1, which displays mean data from a subset of rats used in these studies. Briefly, the significant decrease in fractional shortening, ejection fraction, and intraventricular pressure kinetics [maximum rate of intraventricular pressure increase (+dP/dt) and decline(−dP/dt)], plus the marked increase in left ventricular end-diastolic pressure in the MI group, are consistent with clinical features of heart failure. In previous studies, we also documented the pathogenic condition of oxidative stress in this MI model. In particular, there is marked impairment of the thioredoxin and glutaredoxin systems in the surviving regions of the left ventricle (24, 31, 32), and isolated myocytes from post-MI hearts are characterized by significantly increased ROS levels and lower concentrations of reduced glutathione (GSH) (23, 42).
Table 1.
Characteristics of rat chronic myocardial infarction model
| Sham | MI | |
|---|---|---|
| Fractional shortening, % | 41 ± 1 | 19 ± 2* |
| Ejection fraction, % | 70 ± 1 | 37 ± 3* |
| LVEDP, mmHg | 2.0 ± 0.6 | 25.0 ± 4.0* |
| +dP/dt, mmHg/s | 7,786 ± 695 | 6,897 ± 584* |
| −dP/dt, mmHg/s | −8,431 ± 795 | −6,155 ± 790* |
| MAP, mmHg | 103 ± 9 | 102 ± 2 |
| Heart rate, beats/min | 342 ± 11 | 338 ± 8 |
Values are means ± SE from 5 sham-operated and 5 myocardial infarction (MI) rats.
LVEDP, left ventricular end-diastolic pressure; MAP, mean arterial pressure; +dP/dt, maximum rate of intraventricular pressure increase; −dP/dt, maximum rate of intraventricular pressure decline.
P < 0.05 compared with sham.
Effect of pyruvate on β-adrenergic stimulation of ICa,L.
In addition to oxidative stress, there is considerable experimental and clinical evidence showing that the failing myocardium is less responsive than normal to β-adrenergic stimulation (2, 5, 6, 9, 37). In the present study, ICa,L recorded in isolated myocytes from MI hearts typically showed a blunted response to isoproterenol (Iso). Specifically, the magnitude of increase in ICa,L (ΔICa,L) at 0 mV after 5–10 min of 1 μmol/l Iso in myocytes from MI hearts was only 20% of that observed in sham-operated controls (MI + Iso, 2.4 ± 0.5 pA/pF, n = 9; sham + Iso, 12.2 ± 2.1 pA/pF, n = 10; P < 0.05). In parallel experiments, which are summarized in Table 2, the response of ICa,L to 1 μmol/l forskolin (Fsk) was comparable to that of Iso. These data suggested therefore that abnormalities in adenylyl cyclase or downstream steps were involved in depressed adrenergic responsiveness in this animal model of chronic MI.
Table 2.
ICa,L parameters in myocytes from sham-operated and MI hearts treated with pyruvate
| Untreated |
Pyruvate |
|||||||
|---|---|---|---|---|---|---|---|---|
| n | Sham | n | MI | n | Sham | n | MI | |
| ICa,L, pA/pF (0 mV) | ||||||||
| Basal | 33 | −11.0 ± 0.4 | 10 | −11.7 ± 0.3 | 7 | −10.9 ± 0.6 | 10 | −11.2 ± 0.4 |
| Fsk, Δ | 9 | 10.4 ± 1.7 | 8 | 1.6 ± 1.7* | 6 | 10.7 ± 0.6 | 10 | 10.4 ± 0.5 |
| Activation | ||||||||
| V1/2, mV | 29 | −14.0 ± 1.1 | 8 | −12.6 ± 1.6 | 9 | −13.1 ± 1.2 | 5 | −10.4 ± 1.6 |
| k, mV | 29 | 6.9 ± 0.1 | 8 | 6.6 ± 0.1 | 9 | 6.6 ± 0.7 | 5 | 6.3 ± 0.2 |
| Inactivation kinetics | ||||||||
| Tf , ms | 29 | 7.3 ± 0.4 | 8 | 5.8 ± 0.4 | 9 | 6.0 ± 0.7 | 5 | 8.0 ± 0.9 |
| Ts, ms | 29 | 66.5 ± 3.4 | 8 | 65.3 ± 4.1 | 9 | 66.7 ± 2.6 | 5 | 73.2 ± 2.7 |
Values are means ± SE; n, number of myocytes. Myocytes were pretreated with 5 mmol/l pyruvate for 5–8 h before measuring L-type Ca2+ current (ICa,L) parameters. Δ, maximum change in current density, measured at 0 mV, in response to 1 μmol/l foskolin (Fsk); V1/2, voltage at 50% activation; k, slope factor; Tf and Ts, fast and slow components of inactivation, respectively. Activation and inactivation kinetic parameters were measured at 0 mV.
P < 0.05 compared with sham.
It has previously been shown that supraphysiological levels of the metabolite pyruvate improve β-adrenergic responsiveness of the stunned myocardium (37) and hemodynamic parameters in patients with heart failure (14, 33). Moreover, pyruvate has been shown to improve the redox state of cardiac myocytes in several experimental models of ventricular dysfunction (2, 26), including chronic MI (23). To examine whether pyruvate improves adrenergic sensitivity of hearts with chronic MI, myocytes from MI and sham-operated hearts were treated with 5 mmol/l pyruvate for 5–8 h before testing the response to Fsk. We chose this pretreatment duration because we previously reported that normalization of cell redox state by pyruvate in MI myocytes, as monitored by the recovery of intracellular GSH concentration, required 5 to 6 h to reach steady state (23). In the present study, the response of ICa,L to Fsk in MI myocytes after pyruvate treatment was essentially the same as for sham-operated myocytes (Table 2). Despite this marked increase in adrenergic responsiveness, however, the basal density of ICa,L (measured at 0 mV) was not significantly different between myocytes from sham-operated and MI hearts, and treatment with pyruvate was also without effect (Table 2). In addition, no significant differences in voltage-dependence of activation of ICa,L or its inactivation kinetics were observed between myocytes from MI and sham-operated hearts.
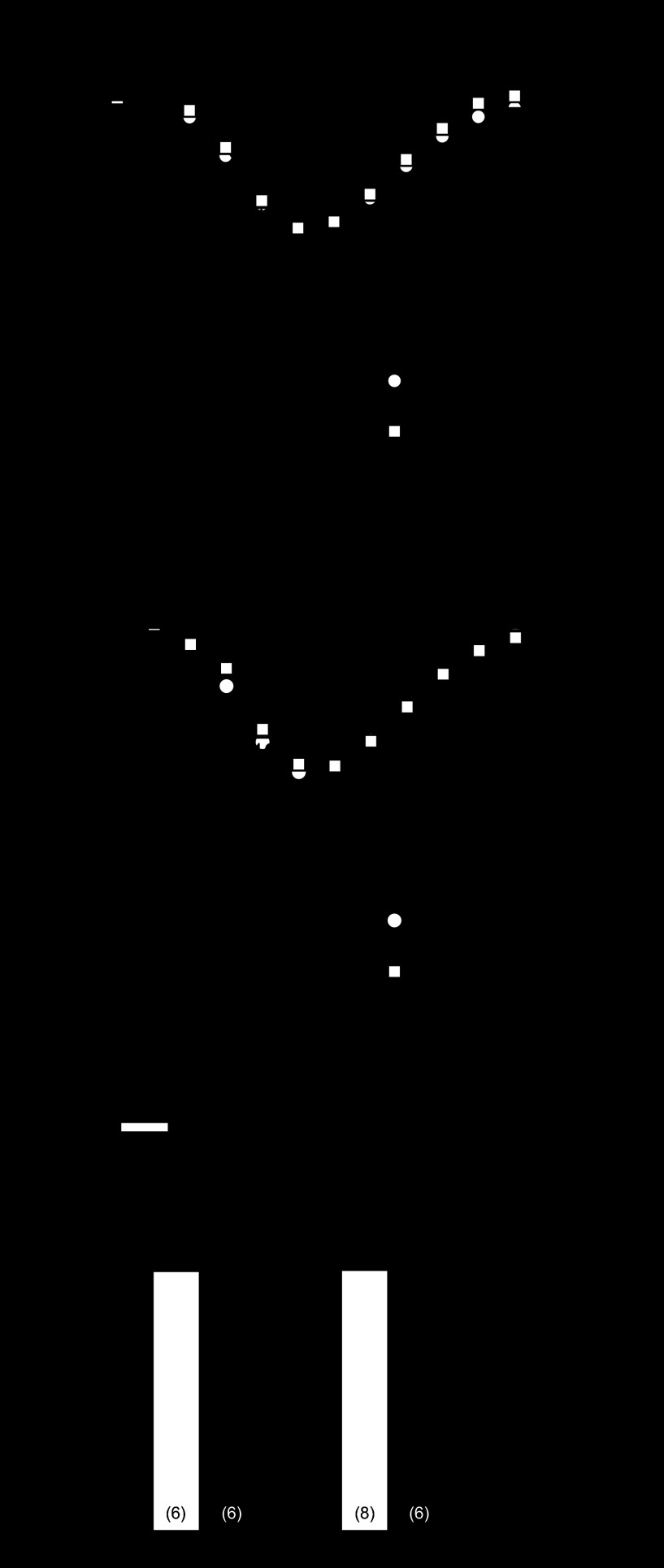
To further probe the basis of depressed β-adrenergic regulation in the MI heart, isolated myocytes were dialyzed with 10 μmol/l cAMP added to the pipette solution to maximally stimulate ICa,L. For these experiments, data were compared in the same cell shortly after obtaining whole cell recording conditions (i.e., before significant dialysis with cAMP) and at the peak effect of intracellular cAMP, which usually occurred after ∼10 min of dialysis. Figure 1A illustrates that cAMP dialysis elicited a similar increase in ICa,L as with Iso and Fsk in myocytes from sham-operated hearts and that pyruvate treatment had little effect on this response. By comparison, cAMP dialysis only slightly increased ICa,L in myocytes from MI hearts (Fig. 1B, black circles), and pyruvate pretreatment markedly increased the response to cAMP (black squares). These data are summarized in Fig. 1C, which plots the maximum change in ICa,L measured at 0 mV in response to cAMP in untreated and pyruvate-treated myocytes from sham-operated and MI hearts. Similar to the results from the Iso and Fsk experiments, the response of ICa,L to cAMP in MI myocytes was markedly less than in sham-operated controls, and pyruvate treatment restored this response to normal levels. These data suggested, therefore, that the dysfunction in β-adrenergic regulation of ICa,L in the MI heart was likely not due to depressed activity of adenylyl cyclase.
Fig. 1.
Effect of pyruvate on L-type Ca2+ current (ICa,L) response to cAMP. Myocytes from sham-operated and myocardial infarction (MI) hearts were dialyzed with 10 μmol/l cAMP with or without pyruvate pretreatment. A and B: mean current (I)-voltage relations of ICa,L in sham-operated (A) and MI (B) myocytes with (black symbols) or without (white symbols) cAMP stimulation. C: maximum increase in ICa,L in response to 10 μmol/l cAMP in myocytes untreated or pretreated with 5 mmol/l pyruvate for 5–8 h. *P < 0.05 compared with sham + cAMP; #P < 0.05 compared with untreated MI + cAMP. Numbers in parentheses indicate number of myocytes in each group. Vm, membrane voltage potential.
The diminished effect of cAMP in myocytes from MI hearts suggested that PKA activity was less compared with sham-operated controls. To address this possibility, PKA activity was compared in left ventricular tissue extracts from MI and sham-operated hearts under basal conditions and after stimulation with cAMP. Figure 2A illustrates that basal PKA activity (open bars) was not significantly different between the two groups of hearts and that added cAMP elicited a comparable increase in activity, which was ∼2.3-fold in both groups of hearts. We also assessed the association of PKA with LTCC by immunoprecipitation of left ventricular tissue homogenates with antibody recognizing the α1C-subunit of Cav1.2 and immunoblotting with antibody specific to the RIIα regulatory subunit of PKA. As summarized in Fig. 2B, there was no significant difference in the binding of PKA-RIIα with α1C-subunit of Cav1.2 between sham-operated and MI hearts.
Fig. 2.
Protein kinase A (PKA) activity in sham-operated and MI hearts. A: comparison of PKA activity in sham-operated (n = 3) and MI (n = 3) heart extracts with (black bars) or without (white bars) 10 μmol/l cAMP. *P < 0.05 compared with basal activity (−cAMP). B: Immunoprecipitation (IP) of PKA-RIIα from sham-operated and MI left ventricular homogenates using antibody recognizing the α1C-subunit of voltage-gated Ca2+ channel isoform 1.2 (Cav1.2): representative Western immunoblot (IB; top) and densitometry analyses of PKA-RIIα protein level normalized to sham operated (bottom). The position of the molecular weight standard is indicated at the left of the blot. Mean values were from 3 experiments.
Since PKA activity and its association with LTCC were not markedly altered in the MI heart, we next measured the activity of PP2A using an immunoprecipitation phosphatase assay kit. Mean data from these analyses, which are summarized in Fig. 3A, show that total PP2A activity was ∼26% less in myocyte homogenates from MI hearts compared with sham-operated controls (P < 0.05). However, recent data suggest that the function of PP2A is also dependent on direct binding to its target protein (11). Thus immunoprecipitation experiments were conducted in tissue extracts from sham-operated and MI hearts to compare the association of PP2A with the α1C-subunit of Cav1.2. Indeed, mean densitometry data in Fig. 3B show that the association of PP2A with Cav1.2 in MI hearts was ∼50% less than in sham-operated controls (P < 0.05).
Fig. 3.
Protein phosphatase 2A (PP2A) function in sham-operated and MI hearts. A: comparison of total PP2A activity in myocyte homogenates. *P < 0.05 compared with sham. Numbers in parentheses indicate number of hearts in each group. B: IP of PP2A from sham-operated and MI left ventricular homogenates using antibody recognizing the α1C-subunit of Cav1.2: representative Western IB (top) and densitometry analyses of PP2A protein level normalized to sham operated (bottom). The position of the molecular weight standard is indicated at the left of the blot. Mean values were from 4 experiments. *P < 0.05 compared with sham; #P < 0.05 compared with IP sham.
Pyruvate effects on the response to PP2A inhibition and the role of thioredoxin.
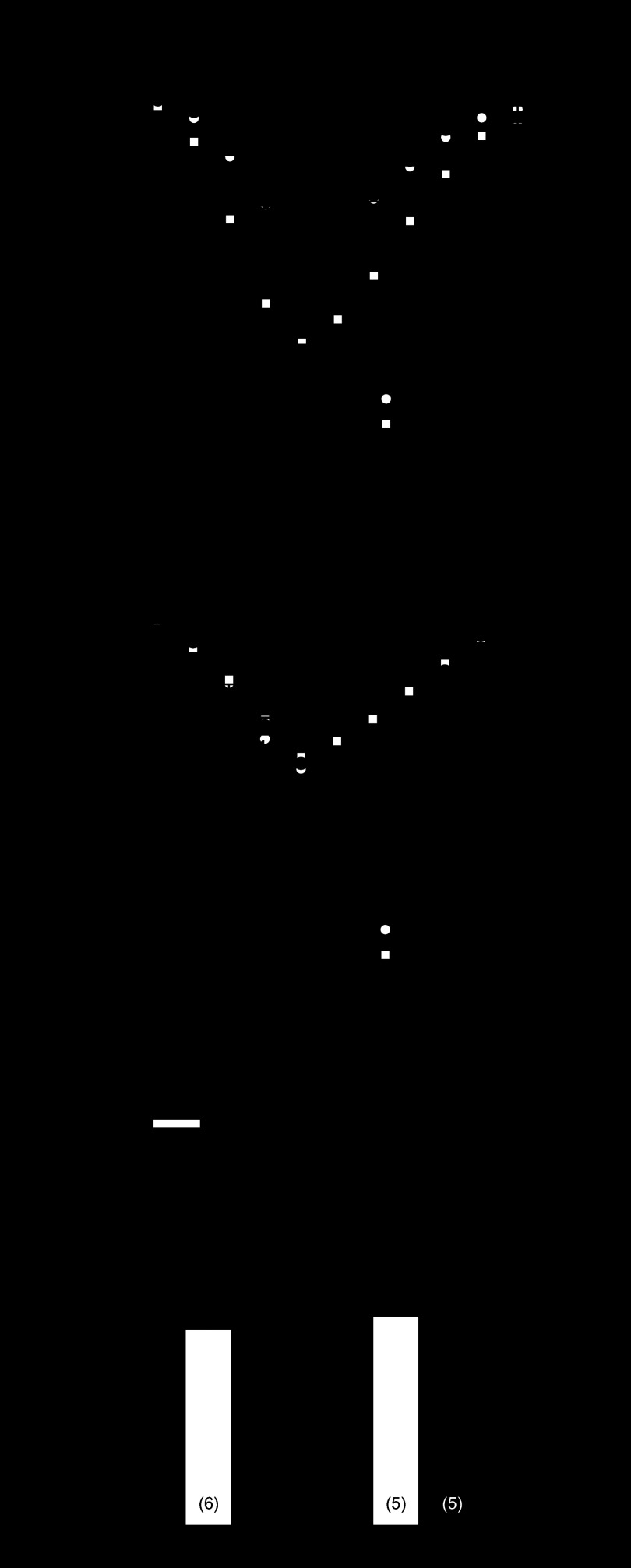
Mean data in Fig. 3 suggest that impaired PP2A function may play a central role in the depressed β-adrenergic regulation of ICa,L in the MI heart. To examine this further, isolated myocytes were dialyzed with the PP2A inhibitor okadaic acid (OA; 1 μmol/l) added to the pipette solution. Figure 4A shows that OA markedly increased ICa,L in myocytes from sham-operated hearts and that pyruvate pretreatment had no significant effect. This was in marked contrast to myocytes from MI hearts where OA had virtually no effect. Indeed, Fig. 4C illustrates that the maximum OA effect on ICa,L was only 4% of that observed in sham-operated myocytes. Nevertheless, pyruvate pretreatment (right-hand bars) significantly increased ICa,L in response to OA similar to that observed in sham-operated myocytes.
Fig. 4.
Effect of pyruvate on ICa,L response to okadaic acid (OA). Myocytes from sham-operated and MI hearts were dialyzed with 1 μmol/l OA with or without pyruvate pretreatment. A and B: mean current-voltage relations of ICa,L in sham-operated (A) and MI (B) myocytes with (black symbols) or without (white symbols) OA stimulation. C: maximum ΔICa,L in response to 1 μmol/l OA in myocytes untreated or pretreated with 5 mmol/l pyruvate for 5–8 h. *P < 0.05 compared with sham + OA; #P < 0.05 compared with untreated MI + OA. Numbers in parentheses indicate number of myocytes in each group.
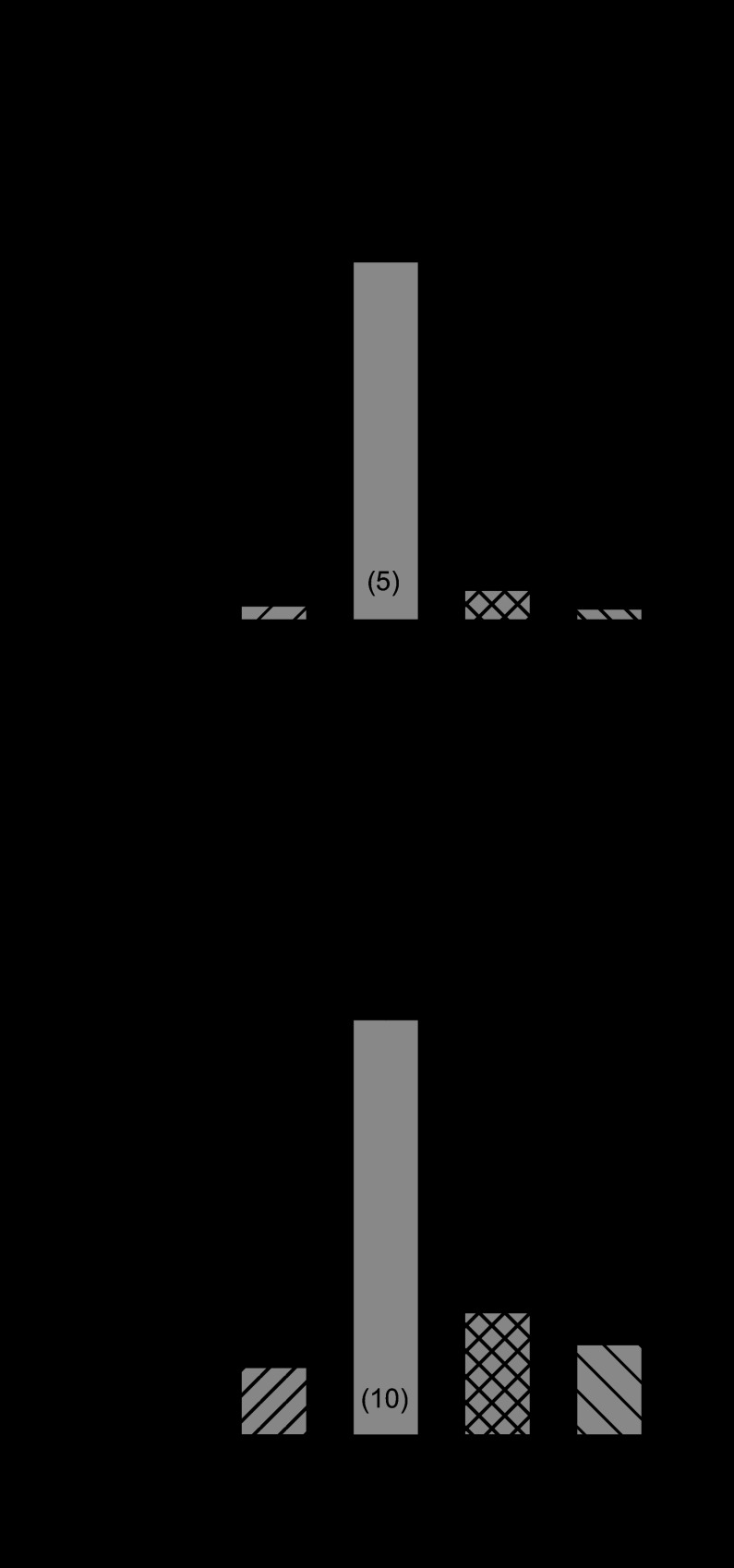
Previous studies from our laboratory reported that a major oxidoreductase network in heart, i.e., the thioredoxin system, plays an essential role in controlling K+ channel remodeling in rats with chronic MI (24, 41), which can be reversed by exogenous pyruvate (24). Thus, to determine if the thioredoxin system also mediates normalized responsiveness of ICa,L by pyruvate in MI hearts, the effects of two structurally different compounds with high selectivity profiles for thioredoxin reductase inhibition were examined: auranofin (10 nmol/l) and 13-cis-retinoic acid (1 μmol/l) (25). These compounds were previously determined to inhibit thioredoxin reductase in isolated myocytes by 60–70%, with minimal effects on glutathione reductase (25). Myocytes from MI hearts were pretreated with auranofin or 13-cis-retinoic acid for 30 min, followed by treatment of pyruvate (5 mmol/l) for an additional 5–8 h. Both compounds blocked the electrophysiological effects of pyruvate to restore responsiveness of ICa,L to 1 μmol/l OA (Fig. 5A) and to 1 μmol/l Fsk in post-MI rat myocytes (Fig. 5B). Neither blocker alone altered ICa,L density (at 0 mV) in MI myocytes treated for 4 h or in sham-operated control myocytes treated for the same duration (data not shown). These data confirm an essential electrophysiological role of the thioredoxin system and suggest a functional link between pyruvate metabolism and intracellular oxidoreductase activity in the control of ICa,L remodeling in failing hearts.
Fig. 5.
Effect of thioredoxin reductase inhibitors on ICa,L response to OA or forskolin (Fsk) after pyruvate (Pyr) treatment. A: mean maximum increase in ICa,L density by 1 μmol/l OA in post-MI myocytes pretreated for 30 min with the thioredoxin reductase inhibitors auranofin (AF; 10 nmol/l) or 13-cis-retinoic acid (RA; 1 μmol/l) before adding 5 mmol/l pyruvate for 5 h. * P < 0.05 compared with OA alone. B: maximum increase in ICa,L density elicited by 1 μmol/l Fsk in MI myocytes pretreated AF or RA before pyruvate. *P < 0.05 compared with Fsk alone. Numbers in parentheses indicate number of myocytes in each group.
DISCUSSION
Impaired β-adrenergic regulation of LTCCs.
It is well established that cardiac excitation-contraction coupling depends on the function of LTCCs, and thus it is logical to presume that these channels are involved in the etiology of heart failure. However, electrophysiological studies of hypertrophied and failing hearts have yielded inconsistent changes in basal ICa,L density (9, 27, 30, 41). What has been observed more consistently, however, is a blunted response of ICaL to β-adrenergic stimulation (5, 6), which is proposed to limit contractile reserve in vivo. Experimental evidence from different models of heart failure suggest that impaired β-adrenergic regulation of ICaL can be caused by dysfunction at the level of β-receptors, adenylyl cyclase, or downstream signaling steps (16, 29). Studies of ventricular myocytes from patients with heart failure have shown that basal LTCC availability and open probability are greater than normal, which is likely due to hyperphosphorylation of channel protein (34) that limits the extent to which ICaL can be stimulated by β-adrenergic agonists. Our data in rat myocytes from MI hearts are consistent with this mechanism because we found blunted responses of ICaL to intracellular cAMP and OA (Figs. 1 and 4) but preserved activity of PKA (Fig. 2A) and binding of PKA-RIIα with Cav1.2 (Fig. 2B).
Our experiments further support the hypothesis that hyperphosphorylation of LTCCs in post-MI heart results from impaired kinase/phosphatase balance, primarily at the level of PP2A. Previous studies of β-adrenergic regulation have shown that LTCCs operate within multiprotein complexes that include A-kinase anchoring protein, which targets PKA to the α1C-subunit (18) and phosphatases such as PP2A (11) and PP1 (38). It has been shown that Ser1928 in the pore forming α1C-subunit of rabbit LTCCs (Ser1898 in rat) is phosphorylated by PKA and dephosphorylated by PP2A that binds to the COOH-terminal region of α1C (11). It may be postulated therefore that disruption of PP2A-α1C binding favors increased basal phosphorylation of Ser1928 (or rat Ser1898) by endogenous PKA. In support of this mechanism, our coimmunoprecipitation studies showed that PP2A-α1C binding was 50% less in MI hearts compared with sham-operated controls (Fig. 3B). Moreover, we found total PP2A activity to be decreased from control by 26% in MI hearts (Fig. 3A). Thus the combination of decreased PP2A-α1C binding and decreased catalytic activity of PP2A could account for the small response of ICaL to the PP2A inhibitor OA in MI myocytes (Fig. 4).
Pyruvate effects on β-adrenergic responsiveness: redox mechanisms.
Impaired β-adrenergic regulation of cardiac performance has been documented in many different forms of heart failure, and in some instances this condition can be prevented or reversed. In particular, the glycolytic intermediate pyruvate has been shown to potentiate the β-adrenergic inotropic response of isolated ventricular muscle preparations obtained from explanted human hearts in end-stage failure (15) and stunned guinea pig hearts following ischemia-reperfusion (37). Moreover, intracoronary pyruvate improves hemodynamic parameters in patients with acute and chronic heart failure (14, 33). Pyruvate enters cells by a H+-monocarboxylate symporter in the plasma membrane and enters the tricarboxylic acid cycle by a monocarboxylate transporter located in the outer mitochondrial membrane (26). Thus part of the beneficial effect of pyruvate on cardiac performance is proposed to be mediated by increased cytosolic ATP phosphorylation potential and improved functioning of ATP-dependent Ca2+ handling proteins (26).
Additionally, pyruvate has been shown to affect cell redox state (23, 26), and it is this mechanism that most likely pertains to the present study. Specifically, exogenous pyruvate increases pyruvate carboxylation (26) and activates pyruvate dehydrogenase by inhibiting pyruvate dehydrogenase kinase (26). The impact of pyruvate metabolism on cytosolic oxidoreductase systems is proposed to be mediated by increased citrate formation that inhibits phosphofructokinase and diverts glucose-6-phosphate into the pentose pathway, thereby increasing NADPH bioavailability (23, 26). For the thioredoxin system, NADPH is used by thioredoxin reductase to convert oxidized thioredoxin (Trx-S2) to its active, reduced (Trx-SH2) form (3). In the glutaredoxin system, glutaredoxin is maintained in its reduced form (Grx-SH2) by GSH, which is generated by the reduction of glutathione disulfide, catalyzed by NADPH-dependent glutathione reductase (3, 23). Thus pyruvate promotes intracellular reducing conditions, which can reverse oxidative protein modifications, although a supraphysiological concentration appears to be required. Indeed, the pyruvate concentration used in the present study (5 mmol/l) was 25–50 times that measured in plasma of human subjects or experimental animals (26). Our culture medium also contained 0.5 mmol/l pyruvate that was insufficient to affect β-adrenergic stimulation of ICa in myocytes from MI hearts. Hence, the redox effects of exogenous pyruvate may be different from those mediated by endogenous metabolic sources.
Redox regulation of PP2A function.
Our experiments with thioredoxin reductase inhibitors (Fig. 5) implicate the thioredoxin system as a key factor regulating basal phosphorylation of LTCCs in ventricular myocytes from MI hearts. This hypothesis is consistent with previous data from our laboratory showing that thioredoxin reductase protein abundance and activity are significantly decreased in hearts with chronic MI (24). Moreover, our present results suggest that cell redox state is particularly important in regulating basal phosphorylation of LTCCs. Nevertheless, relatively little is known about the redox regulation of serine/threonine phosphatases, such as PP2A, in contrast to tyrosine phosphatases that are well known to be inhibited by oxidation of active site cysteine residues (36). Previous investigations have documented inhibition of PP2A activity by oxidative stress (22), and recent studies of brain PP2A have shown that specific cysteine residues in the catalytic subunit form intramolecular disulfide bonds upon oxidant treatment that are reversed by reducing agents (7). These latter findings suggest that PP2A contains a redox-sensitive motif that confers a regulatory role via a conformational change, since cysteine residues are not considered to be essential for catalysis (43). In the present study, we found that PP2A activity in MI hearts was significantly less than sham-operated controls, but it is not known whether this inhibition was the result of oxidative inactivation.
In addition to catalytic activity, the function of PP2A is critically determined by its subcellular location, and in this regard PP2A binding to its target protein(s) is an important factor controlling dephosphorylation (11). We found that PP2A-α1C binding was markedly less in MI hearts compared with sham-operated controls (Fig. 3B) and hypothesize that the redox state of the α1C-subunit regulates binding of PP2A. In the putative PP2A binding region of rat α1C, downstream of the phosphorylation site (Ser1898), there are two cysteine residues (Cys1903 and Cys1992) that potentially may be involved in the redox regulation of PP2A binding. Certainly, further experimentation is necessary to establish whether these or other cysteines are involved in redox sensitivity of LTCC adrenergic regulation. Moreover, it is possible that oxidation of cysteine residues in PP2A impairs its ability to bind to its target protein(s). These residues may be related to those implicated in the redox sensitivity of PP2A activity discussed above (7). Nevertheless, in light of what little is known about the redox regulation of PP2A, our study provides new evidence suggesting that the thioredoxin system controls the binding of PP2A to Ca2+ channels and, to a lesser extent, its catalytic activity.
Limitations.
Although our studies focus on role of PP2A in impaired β-adrenergic regulation of LTCCs, we cannot rule out the possible impact of PP1 in MI hearts. This phosphatase has been postulated to regulate the availability of LTCCs in ventricular myocytes by PKA-mediated phosphorylation at a site distinct from Ser1928 (38). Moreover, recent data in a mouse model of Iso-induced heart failure suggest that PP1 and PP2A may differentially affect subcellular populations of LTCCs in ventricular myocytes (21). Second, we did not directly assess the phosphorylation status of Cav1.2 channels in post-MI and sham-operated hearts. This has proven to be technically problematic and there are many unresolved issues. For example, Ca2+ channel phosphorylation in heart failure could be mediated by PKA or Ca2+/calmodulin-dependent protein kinase II, which is increased in MI hearts (12). Moreover, the β-subunit of LTCCs has putative PKA phosphorylation sites (8), and there are multiple splice variants of the β-isoform (4). Finally, although our experiments with pharmacological inhibitors of thioredoxin reductase implicate the thioredoxin system in regulating β-adrenergic control of LTCCs, off-target effects of these agents are still possible. Nevertheless, we previously reported (25) that auranofin and 13-cis-retinoic acid at the concentrations used in the present study predominantly inhibit thioredoxin reductase and not glutathione reductase, suggesting that the glutaredoxin system is not likely involved in LTCC adrenergic control in this rat model.
In summary, our data suggest that PP2A function in the ventricle of post-MI hearts is impaired in a redox-sensitive manner, which contributes to basal hyperphosphorylation of LTCCs and blunting of the response of ICa to β-adrenergic stimulation. We propose that the thioredoxin system regulates the redox state of LTCCs or PP2A, which in turn sets the adrenergic sensitivity of ICa. Under conditions of oxidative stress, such as with chronic MI, metabolic agonists like pyruvate can indirectly stimulate the thioredoxin system to restore the redox state of functional proteins impaired by excess ROS. Thus these findings suggest that endogenous oxidoreductase networks, such as the thioredoxin system, may be a therapeutic target to prevent or reverse electrical remodeling of the failing heart.
GRANTS
This work was supported by National Institutes of Health Grants HL-066446 (to G. J. Rozanski), HL-062222 (to K. P. Patel), and AA-017993 (to T. A. Wyatt).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.-Q.Z., X.L., T.A.W., K.P.P., K.R.B., and G.J.R. conception and design of research; M.-Q.Z., X.L., K.T., N.M.S., T.A.W., and L.G. performed experiments; M.-Q.Z., X.L., K.T., N.M.S., L.G., and G.J.R. analyzed data; M.-Q.Z., X.L., K.P.P., and G.J.R. interpreted results of experiments; M.-Q.Z., X.L., K.T., N.M.S., T.A.W., K.P.P., L.G., K.R.B., and G.J.R. approved final version of manuscript; N.M.S., K.P.P., and G.J.R. edited and revised manuscript; G.J.R. prepared figures; G.J.R. drafted manuscript.
REFERENCES
- 1. Armoundas AA, Wu R, Juang G, Marban E, Tomaselli GF. Electrical and structural remodeling of the failing ventricle. Pharmacol Ther 92: 213–230, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Bassenge E, Sommer O, Schwemmer M, Bunger R. Antioxidant pyruvate inhibits cardiac formation of reactive oxygen species through changes in redox state. Am J Physiol Heart Circ Physiol 279: H2431–H2438, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Berndt C, Lillig CH, Holmgren A. Thiol-based mechanisms of the thioredoxin and glutaredoxin systems: implications for diseases in the cardiovascular system. Am J Physiol Heart Circ Physiol 292: H1227–H1236, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Carrillo ED, Escobar Y, Gonzalez A, Galindo JM, Garcia MC, Sanchez JA. Posttranscriptional regulation of the β2-subunit of cardiac L-type Ca2+ channels by MicroRNAs during long-term exposure to isoproterenol in rats. J Cardiovasc Pharmacol 58: 470–478, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Chen X, Piacentino V, Furukawa S, Goldman B, Margulies KB, Houser SR. L-type Ca2+ channel density and regulation are altered in failing human ventricular myocytes and recover after support with mechanical assist devices. Circ Res 91: 517–524, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Chen X, Zhang X, Harris DM, Piaccentino V, Berretta RM, Margulies KB, Houser SR. Reduced effects of BAY K 8644 on L-type Ca2+ current in failing human cardiac myocytes are related to abnormal adrenergic regulation. Am J Physiol Heart Circ Physiol 294: H2257–H2267, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Foley TD, Melideo SL, Healey AE, Lucas EJ, Koval JA. Phenylarsine oxide binding reveals redox-active and potential regulatory vicinal thiols on the catalytic subunit of protein phosphatase 2A. Neurochem Res 36: 232–240, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gerhardstein BL, Puri TS, Chien AJ, Hosey MM. Identification of the sites phosphorylated by cyclic AMP-dependent protein kinase on the beta 2 subunit of L-type voltage-dependent calcium channels. Biochemistry 38: 10361–10370, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Gilbert HF. Molecular and cellular aspects of thiol-disulfide exchange. Adv Enzymol Relat Areas Mol Biol 63: 69–172, 1990 [DOI] [PubMed] [Google Scholar]
- 10. Hajjar RJ, Muller FU, Schmitz W, Schnabel P, Bohm M. Molecular aspects of adrenergic signal transduction in cardiac failure. J Mol Med 76: 747–755, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Hall DD, Feekes JA, Arachchige Don AS, Shi M, Hamid J, Chen L, Strack S, Zamponi GW, Horne MC, Hell JW. Binding of protein phosphatase 2A to the L-type calcium channel Cav1.2 next to Ser1928, its main PKA site, is critical for Ser1928 dephosphorylation. Biochemistry 45: 3448–3459, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Hashambhoy Y, Winslow RL, Greenstein JL. CaMKII-induced shift in modal gating explains L-type Ca2+ current facilitation: a model study. Biophys J 96: 1770–1785, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. He J, Conklin MW, Foell JD, Wolff MR, Haworth RA, Coronado R, Kamp TJ. Reduction in density of transverse tubules and L-type Ca2+ channels in canine tachycardia-induced heart failure. Cardiovasc Res 49: 298–307, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Hermann HP, Pieske B, Schwartzmuller E, Keul J, Just H, Hasenfuss G. Haemodynamic effects of intracoronary pyruvate in patients with congestive heart failure: an open study. Lancet 353: 1321–1323, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Hermann HP, Zeitz O, Lehnart SE, Keweloh B, Datz N, Hasenfuss G, Janssen PML. Potentiation of beta-adrenergic inotropic response by pyruvate in failing human myocardium. Cardiovasc Res 53: 116–123, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Hool L. Hypoxia increases the sensitivity of L-type Ca2+ current to β-adrenergic receptor stimulation via a C2 region-containing protein kinase C isoform. Circ Res 87: 1164–1171, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Houser SR, Piacentino V, Weisser J. Abnormalities of calcium cycling in the hypertrophied and failing heart. J Mol Cell Cardiol 32: 1595–1607, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Hulme JT, Scheuer T, Catterall WA. Regulation of cardiac ion channels by signaling complexes: role of modified leucine zipper motifs. J Mol Cell Cardiol 37: 625–631, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Jiang H, Colbran JL, Francis SH, Corbin JD. Direct evidence for cross-activation of cGMP-dependent protein kinase by cAMP in pig coronary arteries. J Biol Chem 267: 1015–1019, 1992 [PubMed] [Google Scholar]
- 20. Kamp TJ, Hell JW. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res 87: 1095–1102, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Kashihara T, Nakada T, Shimojo H, Horiuchi-Hirose M, Gomi S, Shibazaki T, Sheng X, Hirose M, Hongo M, Yamada M. Chronic receptor-mediated activation of Gi/o proteins alters basal t-tubular and sarcolemmal L-type Ca2+ channel activity through phosphatases in heart failure. Am J Physiol Heart Circ Physiol 302: H1645–H1654, 2012 [DOI] [PubMed] [Google Scholar]
- 22. Levinthal DJ, Defranco DB. Reversible oxidation of ERK-directed protein phosphatases drives oxidative toxicity in neurons. J Biol Chem 280: 5875–5883, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Li S, Zheng MQ, Rozanski GJ. Glutathione homeostasis in ventricular myocytes from rats with chronic myocardial infarction. Exp Physiol 94: 815–824, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Li X, Tang K, Xie B, Li S, Rozanski GJ. Regulation of Kv4 channel expression in failing rat heart by the thioredoxin system. Am J Physiol Heart Circ Physiol 295: H416–H424, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liang H, Li X, Li S, Zheng MQ, Rozanski GJ. Oxidoreductase regulation of Kv currents in rat ventricle. J Mol Cell Cardiol 44: 1062–1071, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mallet RT, Sun J, Knott EM, Sharma AB, Olivencia-Yurvatt AH. Metabolic cardioprotection by pyruvate: recent progress. Exp Biol Med (Maywood) 230: 435–443, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Mukherjee R, Spinale FG. L-type calcium channel abundance and function with cardiac hypertrophy and failure: a review. J Mol Cell Cardiol 30: 1899–1916, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Annu Rev Immunol 15: 351–369, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Post SR, Hammond HK, Insel PA. Beta-adrenergic receptors and receptor signaling in heart failure. Annu Rev Pharmacol Toxicol 39: 343–360, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Qin D, Zhang ZH, Caref EB, Boutjdir M, Jain P, el-Sherif N. Cellular and ionic basis of arrhythmias in postinfarction remodeled ventricular myocardium. Circ Res 79: 461–473, 1996 [DOI] [PubMed] [Google Scholar]
- 31. Rozanski GJ, Xu Z, Zhang K, Patel KP. Altered K+ current of ventricular myocytes in rats with chronic myocardial infarction. Am J Physiol Heart Circ Physiol 274: H259–H265, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Rozanski GJ, Xu Z. Glutathione and K+ channel remodeling in postinfarction rat heart. Am J Physiol Heart Circ Physiol 282: H2346–H2355, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Schillinger W, Hunlich M, Sossalla S, Hermann HP, Hasenfuss G. Intracoronary pyruvate in cardiogenic shock as an adjunctive therapy to catecholamines and intra-aortic balloon pump shows beneficial effects on hemodynamics. Clin Res Cardiol 100: 433–438, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schroder F, Handrock R, Beuckelmann DJ, Hirt S, Hullin R, Priebe L, Schwinger RH, Weil J, Herzig S. Increased availability and open probability of single L-type calcium channels from failing compared with nonfailing human ventricle. Circulation 98: 969–976, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Sims C, Harvey RD. Redox modulation of basal and beta-adrenergically stimulated cardiac L-type Ca2+ channel activity by phenylarsine oxide. Br J Pharmacol 142: 797–807, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tanner JJ, Parsons ZD, Cummings AH, Zhou H, Gates KS. Redox regulation of protein tyrosine phosphatases: structural and chemical aspects. Antioxid Redox Signal 15: 77–97, 2011 [DOI] [PubMed] [Google Scholar]
- 37. Tejero-Taldo MI, Caffrey JL, Sun J, Mallet RT. Antioxidant properties of pyruvate mediate its potentiation of beta-adrenergic inotropism in stunned myocardium. J Mol Cell Cardiol 31: 1863–1872, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Wiechen K, Yue DT, Herzig S. Two distinct functional effects of phosphatase inhibitors on guinea-pig cardiac L-type Ca2+ channels. J Physiol 484: 583–592, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yano M, Ikeda Y, Matsuzaki M. Altered intracellular Ca2+ handling in heart failure. J Clin Invest 115: 556–564, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yao JA, Jiang M, Fan JS, Zhou YY, Tseng GN. Heterogeneous changes in K currents in rat ventricles three days after myocardial infarction. Cardiovasc Res 44: 132–145, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Zhang XQ, Moore RL, Tillotson DL, Cheung JY. Calcium currents in postinfarction rat cardiac myocytes. Am J Physiol Cell Physiol 269: C1464–C1473, 1995 [DOI] [PubMed] [Google Scholar]
- 42. Zheng MQ, Tang K, Zimmerman MC, Liu L, Xie B, Rozanski GJ. Role of γ-glutamyl transpeptidase in redox regulation of K+ channel remodeling in post-myocardial infarction rat hearts. Am J Physiol Cell Physiol 297: C253–C262, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhuo S, Clemens JC, Stone RL, Dixon JE. Mutational analysis of a Ser/Thr phosphatase. Identification of residues important in phosphoesterase substrate binding and catalysis. J Biol Chem 269: 26234–26238, 1994 [PubMed] [Google Scholar]
- 44. Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res 71: 310–321, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Zucker IH, Wang W, Brändle M, Schultz HD, Patel KP. Neural regulation of sympathetic nerve activity in heart failure. Prog Cardiovasc Dis 37: 397–414, 1995 [DOI] [PubMed] [Google Scholar]