Abstract
Prolonged hemodynamic load as a result of hypertension eventually leads to maladaptive cardiac adaptation and heart failure. The signaling pathways that underlie these changes are still poorly understood. The adaptive response to mechanical load is mediated by mechanosensors that convert the mechanical stimuli into a biological response. We examined the effect of cyclic mechanical stretch on myocyte adaptation using neonatal rat ventricular myocytes with 10% (adaptive) or 20% (maladaptive) maximum strain at 1 Hz for 48 h to mimic in vivo mechanical stress. Cells were also treated with and without nitro-l-arginine methyl ester (l-NAME), a general nitric oxide synthase (NOS) inhibitor to suppress NO production. Maladaptive 20% mechanical stretch led to a significant loss of intact sarcomeres that were rescued by l-NAME (P < 0.05; n ≥ 5 cultures). We hypothesized that the mechanism was through NO-induced alteration of myocyte gene expression. l-NAME upregulated the mechanosensing proteins muscle LIM protein (MLP; by 100%; P < 0.05; n = 5 cultures) and lipoma preferred partner (LPP), a novel cardiac protein (by 80%; P < 0.05; n = 4 cultures). l-NAME also significantly altered the subcellular localization of LPP and MLP in a manner that favored growth and adaptation. These findings suggest that NO participates in stretch-mediated adaptation. The use of isoform selective NOS inhibitors indicated a complex interaction between inducible NOS and neuronal NOS isoforms regulate gene expression. LPP knockdown by small intefering RNA led to formation of α-actinin aggregates and Z bodies showing that myofibrillogenesis was impaired. There was an upregulation of E3 ubiquitin ligase (MUL1) by 75% (P < 0.05; n = 5 cultures). This indicates that NO contributes to stretch-mediated adaptation via the upregulation of proteins associated with mechansensing and myofibrillogenesis, thereby presenting potential therapeutic targets during the progression of heart failure.
Keywords: mechanotransduction, heart failure, stretch, heart, hypertrophy
long-term hemodynamic overload results in maladaptive cardiac hypertrophy, which results in detrimental changes to myocardial gene expression, morphology, and function (1). Hypertrophy is initially beneficial to heart function but with time this deteriorates to heart failure. We, along with others, have hypothesized that the mechanosensing mechanisms within the heart become impaired following prolonged overload (5, 7). In addition to the well-documented changes in gene expression, alteration of the subcellular localization of proteins plays a key role in maintaining the adaptive process. Muscle LIM protein (MLP) is a nucleocytoplasmic shuttling mechanosensor (5) that localizes to the Z disc of sarcomeres where it plays a role in myocyte contraction and signal transduction (6, 18). In response to mechanical stretch we have shown that MLP relocalizes to the myocyte nucleolus where it stimulates ribosomal protein synthesis (5). The LIM domains of MLP enable it to interact with both proteins and nucleic acids, facilitating a function in the regulation of gene expression while in the nucleus (12). Through such mechanisms, MLP contributes to myocardial adaptation. In heart failure, MLP becomes mislocalized to the nucleus while the cytosol becomes depleted of the protein, thereby impairing the process of mechanosensation (5).
Recently, we have shown that a novel mechanosensitive cardiac protein, lipoma preferred partner (LPP), displays a fetal reexpression pattern typical of myocardial genes associated with adaptation and disease (16). LPP is downregulated in the adult heart but increases following pressure overload hypertrophy but not following myocardial infarction. LPP is a LIM domain-containing protein and a member of the zyxin family of adaptor proteins that includes thyroid hormone receptor interacting protein 6 (TRIP6) and zyxin (39). LPP is a nucleocytoplasmic shuttling protein located in the focal adhesion (27). It has been characterized as an oncogene, and mutations in the LPP gene have been associated with a number of tumors (29) where it permanently localizes to the nucleus. Mislocalization of LPP may contribute to disease progression. LPP also constitutes a structural role through interaction with cytoskeletal components such as actin stress fibers (28) and α-actinin (22) via its proline-rich domain. At the focal adhesion, LPP assists cell attachment through association with actin filaments (14). It also negatively mediates the rate of actin polymerization through modulation of vasodilator stimulated phosphoprotein (VASP) function (3). In knockout mice, loss of LPP reduced myofilament α-actinin (38).
Nitric oxide (NO) signaling plays an important role in the regulation of the cardiovascular system. NO regulates cardiac contraction (2) and gene expression (4, 33). NO is synthesized by nitric oxide synthase (NOS), for which there exists three isoforms [endothelial (eNOS), inducible (iNOS), and neuronal (nNOS)] (32, 34). These isoforms have a distinct subcellular localization (37). NO acts through cGMP-activated pathways to induce downstream signaling events or via direct interaction with target proteins to induce S-nitrosylation (20). NO production is increased in response to mechanical stress (9, 23, 25, 30) and may therefore play an important role in cardiac disease by altering calcium fluxes and myocyte apoptosis.
In this study, we studied the role of NO on myocyte adaptation following acute and chronic mechanical stress. We show for the first time that NO signaling can modulate myocyte remodeling during mechanical stress through the regulation of both the expression and subcellular localization of key mechanosensor proteins. Inhibition of NO production by nitro-l-arginine methyl ester (l-NAME) altered the subcellular localization of both MLP and LPP in a way that promoted myocyte remodeling, suggesting that NO signaling plays an important role in maladaptive remodeling following hemodynamic overload. LPP knockdown by small interfering (si)RNA resulted in α-actinin clusters and increased MUL1 ubiquitin ligase, indicating a role in myofribrillogenesis and protein degradation. These findings suggest that NO modulates cardiac adaptation by altering the expression of proteins associated with myofibrillogenesis. Selective inhibition of NOS isoforms suggests a complex interaction between iNOS and nNOS signaling, thereby presenting potential therapeutic targets during the progression of heart failure.
MATERIAL AND METHODS
Cell culture and treatments.
Animal experiments were performed according to the Institutional Animal Care of the University of Reading, United Kingdom and in accordance with Home Office Guidelines of the Animals (Scientific Procedures) Inspectorate, United Kingdom. Myocytes were isolated from the cardiac ventricles of 1- to 2-day-old Sprague-Dawley rats (killed by cervical dislocation) by sequential collagenase digestion, as previously described (5). Briefly, cells were plated (1 million cells/cm2) on fibronectin (25 μM/ml)-coated petri dishes in PC1 medium (BioWhittaker, Walkersville, MD) for 48 h and transferred to a DMEM:M199 serum free medium. Myocytes were cyclically stretched at 10 or 20% maximum strain at 1 Hz for 48 h using the Flexcell 4000, Flexcell International. The cyclic stretch was performed without the posts. Myocytes were treated with drugs before the cells being stretched and during stretch. Myocytes were treated with 5 mM l-NAME, 10 μM 1400W, 10 μM N-{(4S)-4-amino-5-[(2-aminoethyl)amino]pentyl}-N′-nitroguanidine tris(trifluoroacetate) salt, and 10 μM phenylephrine all for 48 h.
LPP knockdown.
LPP was knocked down in myocytes by passive uptake of LPP siRNA (Thermo Scientific Dharmacon Accell LPP siRNA). Myocytes were cultured in Accell delivery media. Treatment of myocytes with 1 μM LPP siRNA Smartpool for 72 h led to LPP knockdown. The LPP siRNA Smartpool consists of four different sequences all specific for LPP mRNA. The four LPP-targeting siRNA sequences used were as follows: UGAUCGUGUUCAUGGAUUG, CCCUGCUACAUCAAUACGC, CCUGUAGAGUCAGUCGGUA, and UCUAGUAGUUCCAUUGCGU. As a control, a scrambled siRNA sequence was used: UGGUUUACAUGUCGACUAA.
Cellular composition and subcellular fractionation.
For subcellular fractionation of myocytes, the ProteoExtract Subcellular Proteome Kit from Calbiochem was used as described previously (6). This method uses a detergent-based protocol for cell fractionation. Cellular proteins were sequentially extracted into four compartments: cytosolic, membrane/organelles, nuclei, and cytoskeleton. After each fraction was removed, cells were observed by microscopy to ensure that they were still attached to the dish. Cell integrity is maintained throughout the fractionation process. The accuracy of the fractionation method was verified with antibodies to well-documented subcellular distributions markers (5).
Western blotting for analysis of protein expression.
Whole heart tissue and neonatal rat ventricular myocytes prepared for Western blotting as described previously (5). Blots were probed for anti-LPP (mouse, 1:2,000 dilution; Abcam), desmin (rabbit, 1:5,000 dilution; Abcam), S6 ribosomal protein (rabbit, 1:1,000 dilution; Cell Signaling), MLP (1:1,000 dilution, kindly donated by Dr. Jody Martin), actin (mouse, 1:1,000 dilution; Abcam), E3 ubiquitin-protein ligase MUL1 (rabbit, 1:1,000 dilution; Abcam), and TRIP6 (mouse, 1:1,000 dilution; Transduction Laboratories). Horseradish peroxidase-conjugated goat anti-mouse or goat anti-rabbit secondary antibodies (Research Diagnostics) were used to visualize proteins by enhanced chemiluminescence (ECL; Amersham). The bands corresponding to the various proteins were quantified by laser densitometry using Gel-Pro Analyzer 6.0 software. Protein bands were further standardized to total protein loading on the same membranes by using the Amido black-stained nitrocellulose membrane as described previously (6). After membranes were probed for the desired antibodies, they were incubated in stripping buffer [Tris pH 6.8 (1 M), 2% (wt/vol) SDS, and purified water] and 2-mercaptoethanol at 50°C for 30 min. Membranes were rinsed in water and stained for total protein using amido black stain (Sigma) for 5 min. The membranes were then destained in destain solution [45% (vol/vol) methanol, 45% ddH20, and 10% (vol/vol) acetic acid] for 1 h. Optical densities for individual protein bands were normalized against the total protein within the lane as determined by Amido black staining.
Immunochemistry and image analysis.
After the various experimental protocols, cells for immunocytochemical staining were fixed in 4% paraformaldehyde for 5 min and then 70% ethanol for storage at −20°C. Cells were rehydrated in PBS and then immunostained with antibodies as described previously (6). Cells were stained for α-actinin (rabbit, 1:1,000 dilution; Abcam), eNOS (mouse, 1:500 dilution; Abcam), iNOS (mouse, 1:500 dilution; Cell Signaling), and nNOS (mouse, 1:500 dilution; Cell Signaling). Alexa Fluor-conjugated secondary antibodies (Molecular Probes) were used to visualize the specific proteins. Fluorescently labeled cells were then viewed using a Leica DMIRE2 laser scanning confocal microscope. The number of intact sarcomeres was determined by counting the number of sarcomeres in a 10 × 10 μm box in five cells chosen at random per field of view. At least four fields of view were analyzed, and data are shown where n = field of view.
Statistics.
For the experiments described here, at least three separate primary cultures were averaged. Each culture used ∼30 neonatal hearts. All values are means ± SE. All values of significance were calculated using the appropriate comparisons: one-way ANOVA or the Students unpaired t-test. For data where the controls had been normalized to 100%, a nonparametric one-way ANOVA (Kruskal-Walis) was used instead. Differences among means were considered significant at P < 0.05. Data were analyzed using GraphPad, Minitab, and SigmaStat statistical software.
RESULTS
Reduced NO levels improve the sarcomere remodeling following mechanical stress.
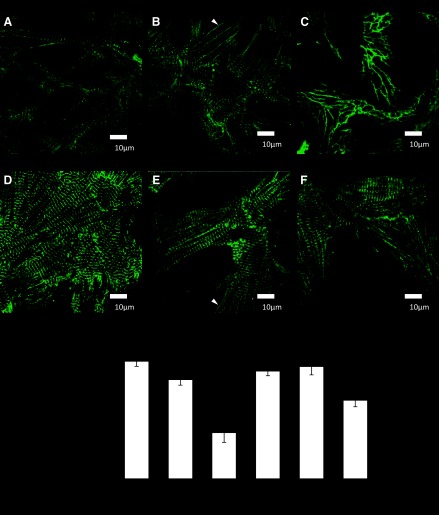
To examine the role of NO in mechanical stretch-induced remodeling of the myofilaments, we measured the number of intact sarcomeres in cultured neonatal myocytes with and without l-NAME treatment. Cells were treated with 5 mM l-NAME (a NOS inhibitor) for 48 h with either 10% cyclic stretch (which allows for a physiological adaptation) or maladaptive with 20% maximum strain. Figure 1, A–F, shows myocytes immunostained with the sarcomeric Z-disc protein α-actinin. The number of intact sarcomeres was determined in response to each treatment to determine the level of sarcomere damage. In response to 10% mechanical stretch, myocytes stained for α-actinin displayed the characteristic striated appearance (Fig. 1B). Upon 20% mechanical stretch sarcomere damage was seen throughout the cell (Fig. 1C). The number of intact sarcomeres within a 10 × 10 μm area on each cell was measured (Fig. 1G). There was sarcomere damage with 20% mechanical stretch, which was significantly improved with l-NAME treatment (Fig. 1G).
Fig. 1.
Immunostaining of cultured neonatal rat myocytes for α-actinin (green). A: control myocytes. B: myocytes following 10% mechanical stretch for 48 h. C: myocytes following 20% stretch. D: myocytes treated with nitro-l-arginine methyl ester (l-NAME) for 48 h. E: myocytes treated with l-NAME and 10% stretch F: myocytes treated with l-NAME and 20% stretch. White arrows show areas lacking in sarcomeres. G: quantitation of intact sarcomeres in cultured neonatal myocytes (P < 0.05, compared with control; n ≥ 5 cultures). Scale bars = 10 μm.
Regulation of myocyte protein expression by NO in response to physiological mechanical stress.
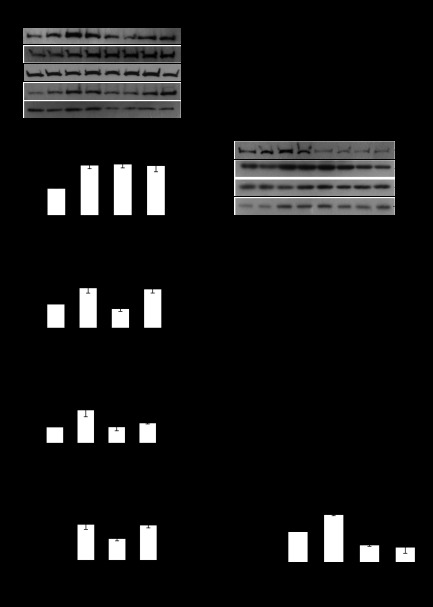
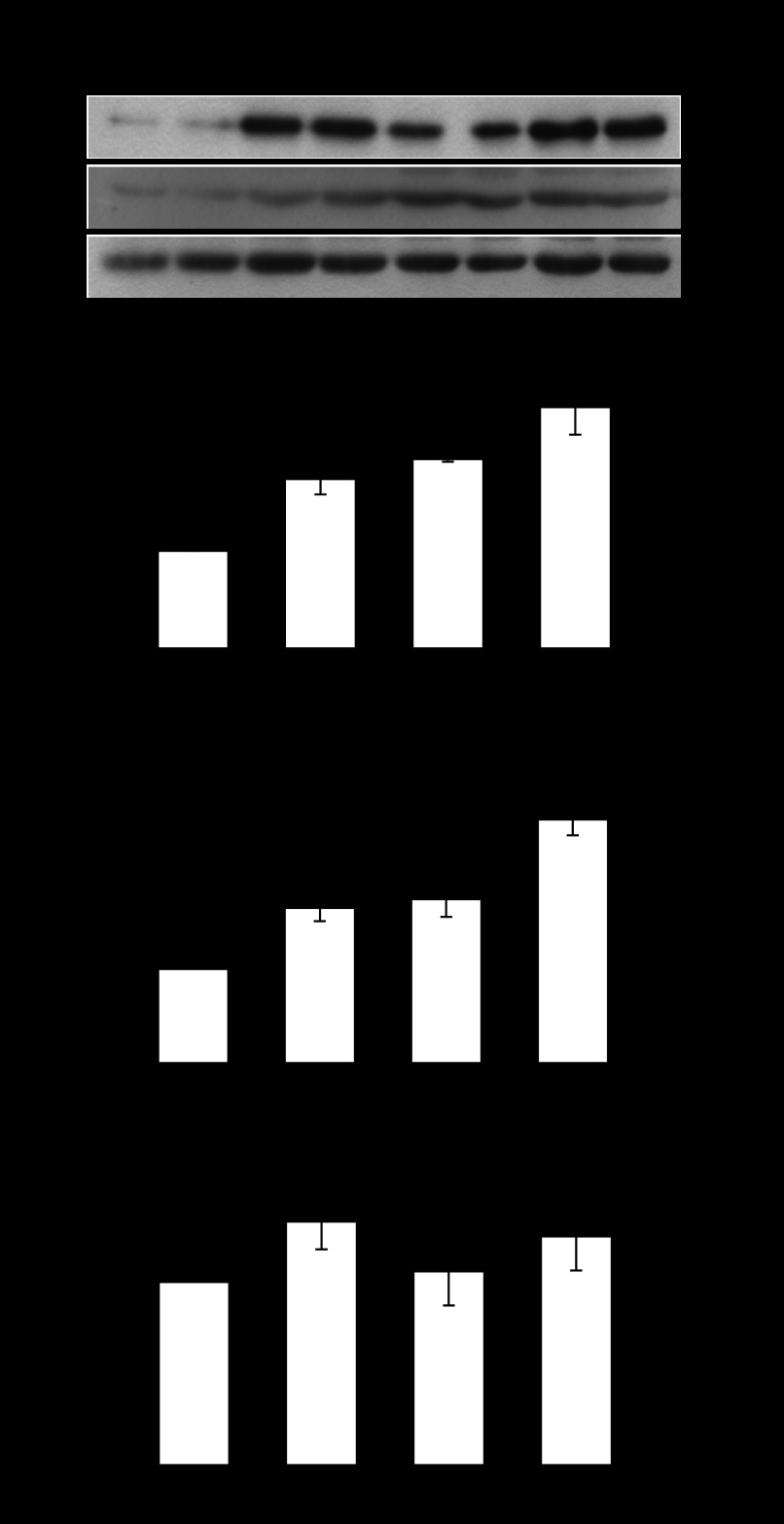
We hypothesized that the improved sarcomere remodeling following NO inhibition was due to increased expression of genes associated with myofibrillogenesis. To explore the mechanism we examined the protein expression of a number of genes associated with myocyte growth and myofibrillogenesis in the presence of l-NAME. Myocytes were stretched cyclically at a physiological 10% stretch for 48 h and cotreated with 5 mM l-NAME. Desmin is a component of the costamere and has been shown to respond to mechanical stimuli (24); S6 ribosomal protein is important for increased protein synthesis; and we have shown MLP to be essential for the adaptation of myocytes to mechanical stimuli (6). We also examined the expression of a novel myocyte focal adhesion protein called LPP. Following 10% cyclic mechanical stretch, only desmin expression increased (by 89%) following a 48-h treatment (Fig. 2B). However, l-NAME increased the expression of desmin, S6 ribosomal, MLP, and LPP protein expression by 86, 67, 105, and 85%, respectively (Fig. 2, B–E). l-NAME also increased the expression of desmin, S6 ribosomal protein, and LPP by 84, 63, 81%, respectively, in the presence of 10% stretch.
Fig. 2.
A: Western blots of lipoma preferred partner (LPP), desmin, S6 ribosomal protein, and muscle LIM protein (MLP) in response to 48-h 5-mM l-NAME treatment [a nonspecific nitric oxide synthase (NOS) inhibitor], alone and in combination with mechanical stretch at 10% maximum strain at 1 Hz. Desmin (B), actin, S6 ribosomal protein (C), MLP (D), and LPP protein (E) expression in response to l-NAME and 10% mechanical stretch. *P < 0.05, compared with control; n = 4 cultures. F: Western blots of LPP, desmin, and S6 ribosomal protein in response to 48-h 5-mM l-NAME treatment (a nonspecific NOS inhibitor), alone and in combination with mechanical stretch at 20% maximum strain at 1 Hz. Desmin (G), actin, S6 ribosomal protein (H), and LPP protein (I) expression in response to l-NAME and 20% mechanical stretch. *P < 0.05 compared with control; n = 3 cultures. Samples were run in duplicate and averaged for each n of 1.
Regulation of myocyte protein expression by NO in response to pathological mechanical stress.
To determine how NO influences myocytes when they are subjected to maladaptive levels of mechanical stress, cells were stretched at 20% maximal strain for 48 h with and without 5 mM l-NAME. Western blotting was used to measure desmin, ribosomal S6, and LPP expression. Desmin and S6 ribosomal protein expression increased in response to 20% mechanical stretch alone (by 84 and 64%, respectively) and in combination with l-NAME (by 74 and 52%, respectively, Fig. 2, G and H). In contrast, LPP expression declined by 43% after 20% mechanical stretch and remained reduced by 50% compared with controls when myocytes were cotreated with l-NAME (Fig. 2I).
LPP and MLP subcellular distribution are regulated by NO.
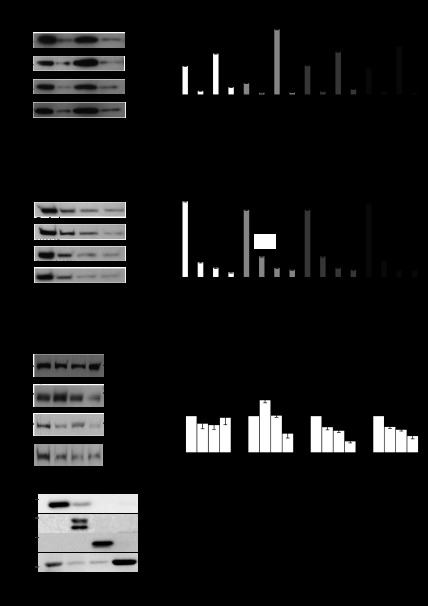
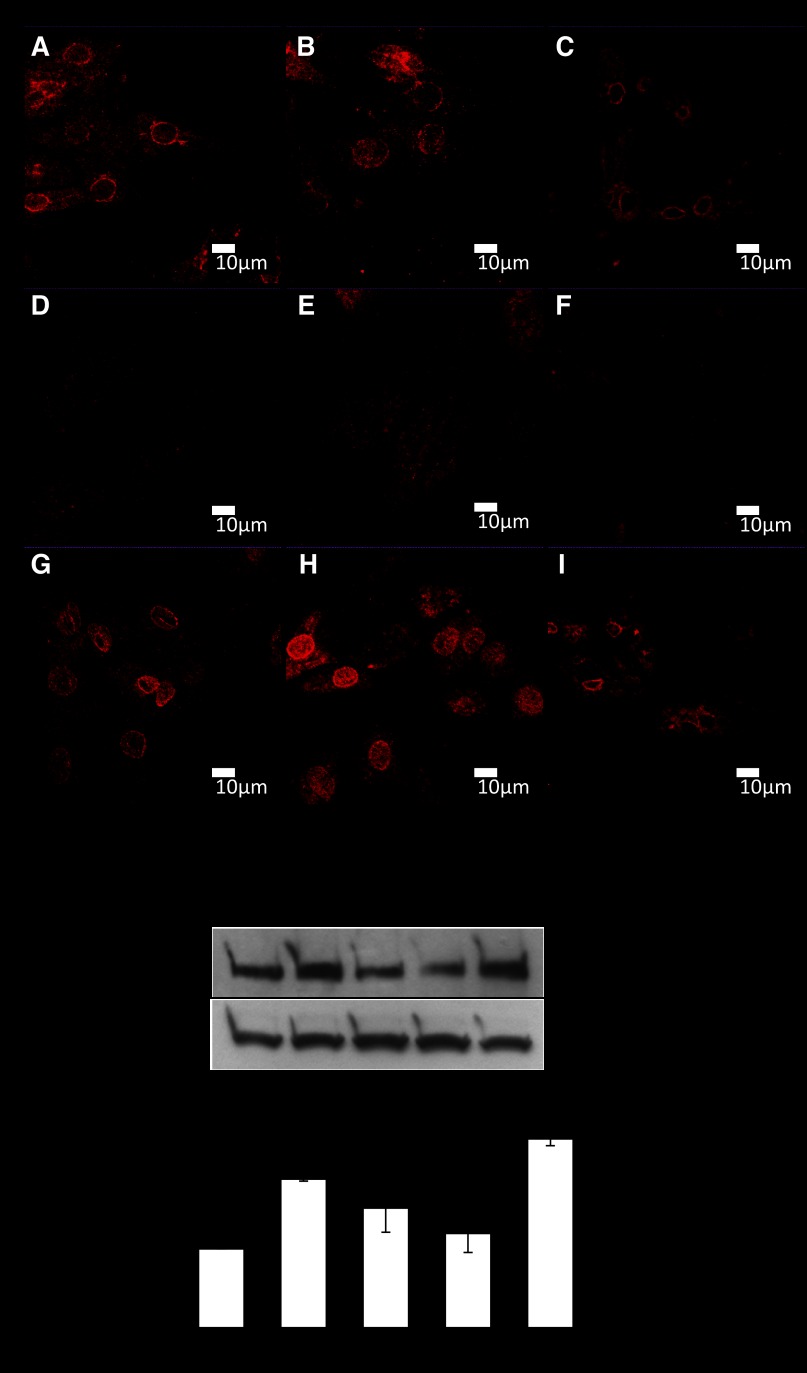
MLP and LPP are nucleocytoplasmic shuttling proteins; therefore, their location will influence their function. We determined their subcellular distribution in response to 48 h of a physiological 10% mechanical stretch with and without 5 mM l-NAME. With the use of a detergent-based method, myocytes were fractionated into cytosolic, membrane, nucleus, and cytoskeletal compartments for Western blot analysis. Surprisingly, the subcellular distribution of MLP was unaffected by 10% mechanical stretch (Fig. 3B). In contrast, l-NAME treatment alone led to a relocalization of MLP to the nucleus with decreased cytosolic expression. For LPP we used two approaches to determine its subcellular distribution. First, we measured the relative expression of the protein between the different compartments and this showed that l-NAME treatment led to a significant relocalization of the protein to the membrane fraction (Fig. 3D). The second approach was to measure the absolute amounts in each fraction following the various treatments. These two methods provide a fuller picture of the protein localization because the total amounts are also influenced by some of the treatments. When the absolute protein amounts were compared in each fraction, we found no change in the cytosolic fraction. However, as shown by the first approach l-NAME increased LPP by 43% in the membrane fraction but decreased by 47% when l-NAME was combined with stretch (Fig. 3F). Nuclear localization was significantly decreased by l-NAME, stretch, and stretch + l-NAME by 30, 40, and 69%, respectively. These three treatments also decreased expression in the cytoskeleton compartment. Control proteins were also run to verify the fractionation methodology (Fig. 3G).
Fig. 3.
LPP and MLP subcellular distribution in myocytes. Relative and absolute expressions of the proteins have been determined. Relative expression provides an indication of the shuttling of the protein between the subcellular compartments during a given treatment. The expression of the protein within each subcellular fraction is displayed as a percentage of the total amount of the protein within all 4 fractions, whereas the absolute values enable determination of the direct effects of a drug treatment on the expression of a protein within a distinct subcellular location. A: Western blot of the relative expression of MLP within the subcellular fractions. B: quantitation of relative MLP levels from subcellular fractions. *P < 0.05; n = 4. C: Western blot of the relative expression of LPP within the subcellular fractions. D: quantitation of relative LPP levels from subcellular fractions. *P < 0.05; n = 4. E: Western blot of absolute LPP expression within different subcellular fractions in response to l-NAME and 10% stretch. F: quantitation of absolute LPP levels within different subcellular fractions in response to l-NAME and 10% stretch. *P < 0.05, compared with control; n = 4. G: Western blot of controls for subcellular fractionation. The nitrocellulose blot was stripped and reprobed several times for proteins whose subcellular distribution is well documented. Aβ-crystallin was found in the cytosolic and membrane fractions. β1-Integrin was only found in the membrane fraction. Histone 2B was only found in the nuclear fraction and actin was found predominantly in the cytosolic and cytoskeletal fractions.
Localization of MLP and LPP in myocytes.
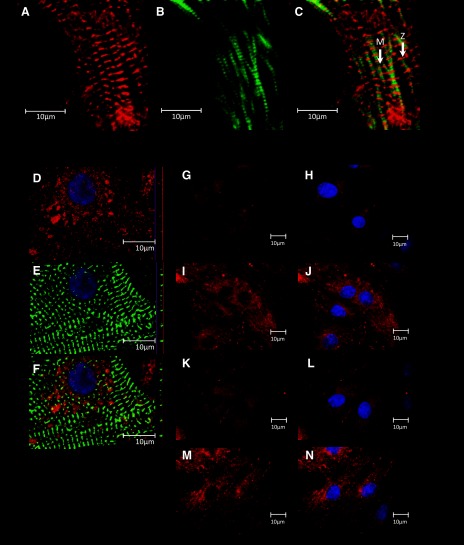
The localization of MLP has been controversial over the last few years, and we have previously shown the protein to be located in cytosol, membrane, nucleus, and cytoskeleton (5). However, it is this cytoskeletal localization that is most disputed. We utilized our detergent based subcellular fraction methodology to sequentially remove the cytosolic and membrane fractions from cultured neonatal myocytes and stained for MLP, and actin with phalloidin. With high-resolution confocal microscopy MLP can be seen in the Z disks with two clear actin bands being seen in between the Z disks (Fig. 4, A–C). The gap between the actin bands represent the M band. Next, we stained for LPP and actin (Fig. 4, D–F). A confocal Z stack shows that LPP colocalizes with actin at times and is found throughout the cell cytoplasm and nucleus. Immunostaining for LPP alone shows l-NAME or in combination with stretch increased the filamentous structure of the protein within the cytoplasm of myocytes (Fig. 4, G–N).
Fig. 4.
Localizing MLP and LPP proteins in cardiac myocytes. A: immunostaining for cytoskeletal MLP (in red) visualized by confocal microscopy. Immunostaining for cytoskeletal MLP was achieved following sequential removal of the cytosol and membrane by a detergent-based method. B: staining for actin with phalloidin (green). C: combination of actin and MLP. Arrows point to Z and M bands. D: immunostaining for LPP (in red). E: staining for actin with phalloidin (green). F: combination of LPP with actin with confocal Z stack. Scale bars represent 10 μm. G–N: LPP staining in cardiac myocytes (red) with DAPI (blue). G and H: untreated myocytes. I and J: l-NAME-treated myocytes. K and L: 10% stretch for 48 h. M and N: l-NAME + stretch for 48 h.
l-NAME augments hypertrophic gene expression.
The focus of this study has been to examine the role of NO following mechanical stress. However, we wondered whether NOS inhibition would also influence cells treated with phenylephrine, an α-adrenergic agonist that increases myocyte contractility and induces the prohypertrophic phenotype within these cells. We have already shown that MLP is upregulated by phenylephrine treatment (6). Myocytes were treated with 5 mM l-NAME or 10 μM phenylephrine and in combination for 48 h (Fig. 5A). Following phenylephrine treatment, both desmin protein (Fig. 5B) and LPP (Fig. 5C) increased by 73 and 65%, respectively. This expression was further augmented in combination with l-NAME by 147% for desmin and 159% for LPP. None of the treatments significantly altered actin protein expression (Fig. 5D).
Fig. 5.
A: Western blots of LPP, desmin and actin protein in response to 48 h of 10μM phenylephrine (to induce hypertrophy); alone and in combination with 5 mM l-NAME (a nonspecific NOS inhibitor). Desmin (B), LPP (C), and actin (D) protein expression in response to phenylephrine and l-NAME. *P < 0.05, compared with control; n = 3. Samples were run in duplicate and averaged for each n of 1.
NO synthase distribution in myocytes.
To determine which NO producing enzyme may be influencing gene expression, we determined the distribution of the three isoforms of NOS in cardiac myocytes with and without mechanical strain using immunocytochemistry. Figure 6, A–I, shows that eNOS and iNOS have a strong cytoplasmic and perinuclear distribution, while nNOS is mostly cytoplasmic. This distribution was maintained following 10% mechanical stretch; however, the distribution of all three isoforms seemed disrupted following 20% stretch.
Fig. 6.
Immunostaining of neonatal myocytes to show the subcellular localization of the three nitric oxide synthase isoforms in cardiac myocytes. A, D, and G: myocytes immunostained for endothelial (eNOS), neuronal (nNOS), and inducible (iNOS) forms, respectively, in control cells. B, E, and H: cells after 10% stretch for 48 h at 1 Hz. C, F, and I: images following 20% stretch for 48 h at 1 Hz. Scale bars = 10 μm. J: Western blot of LPP protein following 48-h treatments with different NOS inhibitors: 5 mM l-NAME (a nonspecific NOS inhibitor), 10 μM of the iNOS inhibitor 1400W, and 10 μM of the nNOS inhibitor N-{(4S)-4-amino-5-[(2-aminoethyl)amino]pentyl}-N′-nitroguanidine tris(trifluoroacetate) salt and a combination of iNOS and nNOS inhibitors. K: LPP protein expression in response to l-NAME and to separate inhibition of iNOS or nNOS. *P < 0.05, compared with control; n = 3 cultures.
Interplay between different NOS isoforms regulates gene expression.
l-NAME is a broad spectrum NOS inhibitor, so to identify which isoform may be responsible for the changes we observed, myocytes were treated with selective inhibitors for iNOS (10 μM 1400W) and nNOS [10 μM N-{(4S)-4-amino-5-[(2-aminoethyl)amino]pentyl}-N′-nitroguanidine tris(trifluoroacetate) salt] for 48 h. Inhibitors of eNOS were not used in this study since most of these are not thought to be specific for the enzyme. Western blotting indicated that LPP protein expression remained unchanged after treatment with either the iNOS or nNOS inhibitors alone. Cotreatment of myocytes with the iNOS and nNOS inhibitors augmented LPP expression by 139%, similar to l-NAME treatment (Fig. 6K).
LPP knockdown leads to the accumulation of myofilament Z bodies.
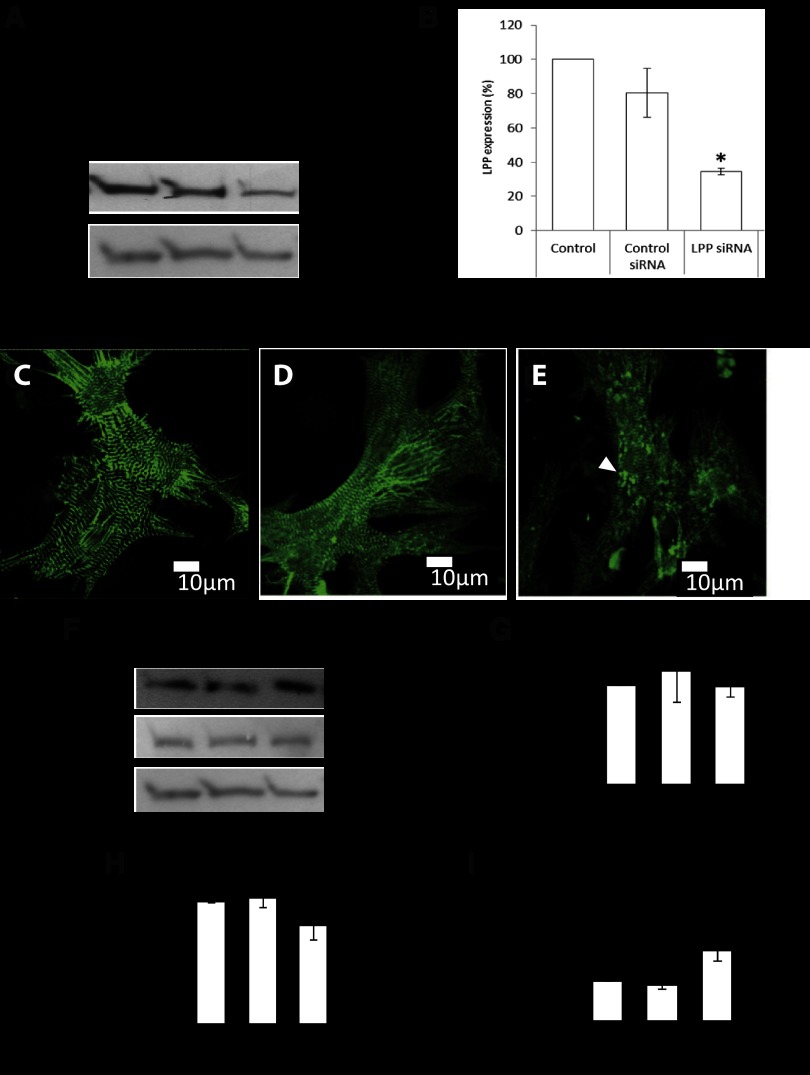
We have shown LPP to be upregulated following inhibition of NOS, phenylephrine treatment, and heart failure suggesting that the protein may have a role in hypertrophic growth and remodeling. To explore the potential functions of LPP in myocytes we knocked down LPP with siRNA. Western blotting demonstrated that following siRNA treatment, LPP protein expression decreased by 70% compared with controls (Fig. 7B). Myocytes were immunostained for the sarcomeric Z-disc protein α-actinin to determine possible morphological effects of LPP knockdown. When LPP was knocked down, the myocytes showed an accumulation of α-actinin aggregates throughout the cells suggesting an accumulation of immature myofilament Z bodies (Fig. 7E).
Fig. 7.
A: Western blot of LPP protein following knockdown by small interfering (si)RNA. B: quantitation of LPP protein after knockdown (*P < 0.05 compared with control, n = 5 cultures). C–E: immunostaining of neonatal myocytes with α-actinin of untreated myocytes, control siRNA, and LPP siRNA, respectively. Arrow shows α-actinin aggregates. Scale bars = 10 μm. F: Western blots of E3 ubiquitin ligase, TRIP6 and actin protein following LPP knockdown TRIP6 (G), actin (H), and E3 ubiquitin ligase (I; MUL1) protein expression. *P < 0.05, compared with control siRNA; n = 5 cultures.
A role for LPP in protein degradation.
To further examine the role of LPP in myocytes the expression of a number of proteins was determined following LPP knockdown. TRIP6 remained unchanged following LPP knockdown (Fig. 7G). Next, we measured actin after LPP knockdown, and this too remained unchanged (Fig. 7H). E3 ubiquitin ligase has been shown to interact with LPP using yeast two-hybrid method and may provide a link with protein degradation (36). We measured E3 ligase (MUL1) protein expression following LPP knockdown, which resulted in a 74% increase in protein expression (Fig. 7I).
DISCUSSION
The factors that regulate remodeling in response to mechanical stimuli are still poorly understood; however, NO levels are known to increase with mechanical stress (9) and in the failing heart (26, 40). Although NO has been shown to be antihypertrophic, its role in mechanical stress-induced remodeling is unknown. In this study we show for the first time that inhibition of NO production improves myocyte remodeling by increasing the expression of proteins associated with myofibrillogenesis. This suggests that NO is intricately involved in myocyte remodeling and possibly mechanosensing. Our work examined the effects of adaptive and maladaptive mechanical stretch on myocytes to determine whether NO is important in either forms of adaptation. We have previously shown that 10% cyclic stretch produces myocyte hypertrophy (6). This hypertrophy is in part due to the translocation of MLP to the myocyte nucleus/nucleolus that increases ribosomal protein synthesis. However, with 20% strain the rate of damage exceeds the rate of repair and presents a pathological model of adaptation in vitro. Inhibition of NO may increase the rate of myofilament repair allowing the cells to adapt more adequately to the mechanical stress. Our findings are supported by data from skeletal muscle where NOS mediates cellular responses to mechanical stress (19). The upregulation of MLP by NO inhibition is also consistent with a previous finding that showed MLP mRNA is downregulated by NO (15).
This is the first time that NOS inhibition alone has been shown to alter ribosomal S6 and desmin protein levels in the absence of additional hypertrophic stimuli. Inhibition of endogenous NOS augmented phenylephrine induced gene expression suggesting that on balance NO serves to inhibit cellular growth. It is unclear at present whether inhibition of NO production would be beneficial in vivo since this would be complicated by effects on the vasculature.
Our most novel finding is that NOS inhibition alters the subcellular distribution of mechanosensor proteins in a way that favors myocyte growth. Inhibition of NO increases nuclear MLP, which we have previously shown to be required for hypertrophic growth in response to mechanical stress and phenylephrine treatment (6). NOS inhibition also decreases nuclear LPP, which would increase growth through reduced repression of transcription factors associated with growth (13). l-NAME specifically increases MLP protein expression and increases its nuclear localization, a process that would increase myocyte remodeling and growth. LPP is a novel cardiac nucleocytoplasmic shuttling mechanosensing protein that we have recently shown to increase in heart failure induced by hemodynamic overload (16). We now show that LPP knockdown impairs myofibrillogenesis and increases MUL1 ubiquitin ligase expression. l-NAME also decreased nuclear and cytoskeletal LPP. Decreased nuclear LPP increases cellular growth through reduced repression of transcription factors associated with growth (13). Decreased LPP in the cytoskeleton also allows greater actin polymerization through increased VASP activity. This implies that NO is able to coordinate changes in gene expression and the subcellular localization to regulate growth.
Knockdown of LPP resulted in α-actinin aggregates suggesting that the protein is important for myofibrillogenesis. Formation of the mature myofibril begins with the association of α-actinin, titin, and membrane-associated actin filaments. This complex forms at the membrane and is organized into punctate aggregates called Z bodies (10, 11). Titin then governs their fusion to adjacent Z bodies, which leads to formation of myofibrils (10). LPP is able to bind directly to α-actinin so the increased formation of aggregates in the absence of the protein suggests that LPP is required for the transition from the Z-body stage to the mature myofibril. LPP has been shown to influence cytoskeletal organization in other cell types (14, 28) as shown in Fig. 8. Clearly, the upregulation of LPP by l-NAME would be seen as beneficial for myofibrillar remodeling and organization. Moreover, LPP may provide a link between environmental sensing and protein degradation since knockdown of the protein resulted in increased E3 ligase. LPP has been shown to interact directly with E3 ligase and may provide a means of targeting the protein to sites of protein turnover (36). In addition to structural roles, LPP has been shown to regulate cell growth and gene expression in various cancers (29). All these data suggest that LPP may play a multifunctional role as adaptor protein that coordinates signaling events, cytoskeletal and focal adhesion stability, and the regulation of gene expression through interaction with transcription factors (see Fig. 8). The regulation of LPP appears to be quite complex since pathological stretch at 20% decreased the protein levels. However, we have shown previously that with aortic banding LPP increased but remained unchanged following myocardial infarction (16). These data indicate that LPP may be regulated by a complex combination of mechanical and neurohumoral cues.
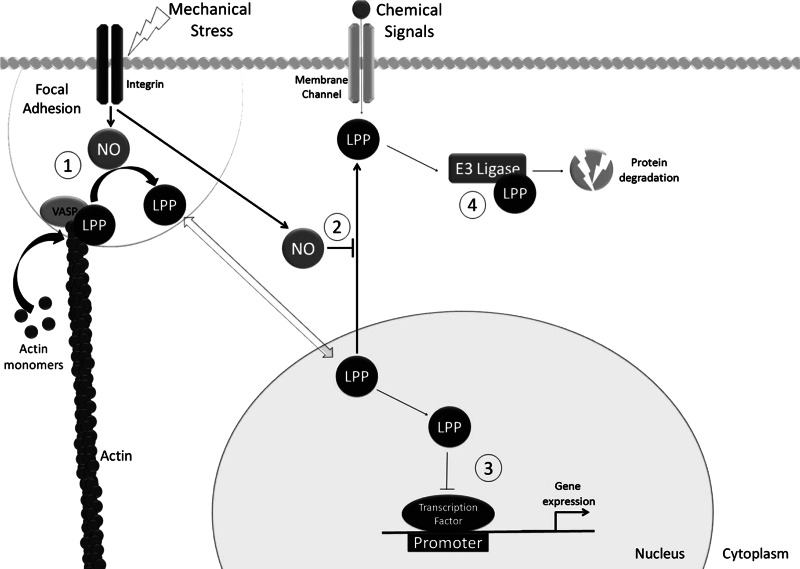
Fig. 8.
Diagram showing the mechanism by which NO and LPP may regulate cellular function in combination with mechanical stress. 1) LPP forms links with the actin cytoskeleton where it acts to inhibit actin polymerization. Mechanical stress increases NO, which leads to a loss of cytoskeletal LPP. A loss of cytoskeletal LPP releases VASP leading to enhanced actin polymerization and cell growth. 2) A loss of NO reduces nuclear LPP levels, suggesting that NO may inhibit LPP nuclear export. 3) In the nucleus the proline-rich NH2-terminal domain of LPP acts as an inhibitory domain to suppress gene expression. Thus the loss of nuclear LPP seen in the absence of NO is likely to promote (growth associated) gene expression. 4) LPP may recruit proteins for degradation through association with MUL1 ubiquitin ligase.
Previous experiments have suggested a contrasting role for NO in the regulation of sarcomere remodeling. It has been shown that nNOS-derived NO promotes sarcomere addition during remobilization in skeletal muscle (19). In skeletal muscle NO may have conflicting functions. For example, it has been shown that NO is necessary for hypertrophic growth in overloaded skeletal muscle (35), whereas in myocytes the action of NO is antihypertrophic (31). NO influences gene expression through its participation in various signaling pathways, including ERK and JNK signaling (8). In addition, NO can directly activate gene expression through its ability to interact with the zinc-finger component of transcription factors (21). We found that inhibition of both nNOS and iNOS was required to achieve the same effect seen with l-NAME. These findings highlight the complex interplay between the different NOS isoforms in regulating myocyte function (17, 41).
Mechanosensing linked to myofibrillogenesis is essential in the process of cardiac adaptation to mechanical stress and we have previously shown that this process becomes impaired in the failing heart (5). Whether changes in NO production are involved in this transition from adaptive to maladaptive remodeling in vivo is still unclear, but it remains a distinct possibility. In conclusion we show that inhibition of NO production improves myocyte remodeling during mechanical stress suggesting that NO may be involved in the complex process of mechanosensing in cardiac myocytes. Inhibition of NO production alters the subcellular distribution of mechanosensor proteins in a way that favors growth and upregulates proteins associated with myofibrillogenesis. The use of NOS isoform specific inhibitors suggests a complex interplay between iNOS and nNOS may mediate the effect in cardiac myocytes. Further studies are needed to determine whether NOS isoforms could present a therapeutic target to improve cardiac remodeling in hypertrophy and failure.
GRANTS
This research was supported by American Heart Association Grant 0630307N, British Heart Foundation, European Commission, and the Royal Society.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.L.H. and A.P. performed experiments; C.L.H. and A.P. analyzed data; C.L.H. and A.P. prepared figures; C.L.H. drafted manuscript; C.L.H., A.P., P.R.D., and S.Y.B. approved final version of manuscript; A.P., P.R.D., and S.Y.B. edited and revised manuscript; S.Y.B. conception and design of research; S.Y.B. interpreted results of experiments.
REFERENCES
- 1. Anversa P, Olivetti G, Loud AV. Morphometric study of early postnatal development in the left and right ventricualr myocardium of the rat. I. Hypertrophy, hyperplasia, and binucleation of myocytes. Circ Res 46: 495–502, 1980 [DOI] [PubMed] [Google Scholar]
- 2. Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O'Rourke B, Rodriguez ER, Huang PL, Lima JA, Berkowitz DE, Hare JM. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature 416: 337–340, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Bear JE, Svitkina TM, Krause M, Schafer DA, Loureiro JJ, Strasser GA, Maly IV, Chaga OY, Cooper JA, Borisy GG, Gertler FB. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell 109: 509–521, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Berendji D, Kolb-Bachofen V, Zipfel PF, Skerka C, Carlberg C, Kröncke KD. Zinc finger transcription factors as molecular targets for nitric oxide-mediated immunosuppression: inhibition of IL-2 gene expression in murine lymphocytes. Mol Med 5: 721–730, 1999 [PMC free article] [PubMed] [Google Scholar]
- 5. Boateng SY, Belin RJ, Geenen DL, Margulies KB, Martin JL, Hoshijima M, de Tombe PP, Russell B. Cardiac dysfunction and heart failure are associated with abnormalities in the subcellular distribution and amounts of oligomeric muscle LIM protein. Am J Physiol Heart Circ Physiol 292: H259–H269, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Boateng SY, Senyo SE, Qi L, Goldspink PH, Russell B. Myocyte remodeling in response to hypertrophic stimuli requires nucleocytoplasmic shuttling of muscle LIM protein. J Mol Cell Cardiol 47: 426–435, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buyandelger B, Ng KE, Miocic S, Gunkel S, Piotrowska I, Ku CH, Knöll R. Genetics of mechanosensation in the heart. J Cardiovasc Trans Res 4: 238–244, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cappola TP, Cope L, Cernetich A, Barouch LA, Minhas K, Irizarry RA, Parmigiani G, Durrani S, Lavoie T, Hoffman EP, Ye SQ, Garcia JG, Hare JM. Deficiency of different nitric oxide synthase isoforms activates divergent transcriptional programs in cardiac hypertrophy. Physiol Genomics 14: 25–34, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Cheng TH, Chen JJ, Shih NL, Lin JW, Liu JC, Chen YL, Chen CH. Mechanical stretch induces endothelial nitric oxide synthase gene expression in neonatal rat cardiomyocytes. Clin Exp Pharmacol Physiol 36: 559–566, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Dabiri GA, Turnacioglu KK, Sanger JM, Sanger JW. Myofibrillogenesis visualized in living embryonic cardiomyocytes. Proc Natl Acad Sci USA 94: 9493–9498, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ehler E, Rothen BM, Hammerle SP, Komiyama M, Perriard JC. Myofibrillogenesis in the developing chick heart: assembly of Z-disk, M-line and the thick filaments. J Cell Sci 112: 1529–1539, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Gardiwal A, Klein G, Kraemer K, Durgac T, Koenig T, Niehaus M, Heineke J, Mohammadi B, Krampfl K, Schaefer A, Wollert KC, Korte T. Reduced delayed rectifier K+ current, altered electrophysiology, and increased ventricular vulnerability in MLP-deficient mice. J Card Fail 13: 687–693, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Guo B, Sallis R, Greenall A, Petit M, Jansen E, Young L, Van de Ven W, Sharrocks A. The LIM domain protein LPP is a coactivator for the ETS domain transcription factor PEA3. Mol Cell Biol 26: 4529–4538, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hansen MD, Beckerle MC. Opposing roles of zyxin/LPP ACTA repeats and the LIM domain region in cell-cell adhesion. J Biol Chem 281: 16178–16188, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Heineke J, Kempf T, Kraft T, Hilfiker A, Morawietz H, Scheubel RJ, Caroni P, Lohmann SM, Drexler H, Wollert KC. Downregulation of cytoskeletal muscle LIM protein by nitric oxide: impact on cardiac myocyte hypertrophy. Circulation 107: 1424–1432, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Hooper CL, Dash PR, Boateng SY. Lipoma preferred partner is a mechanosensitive protein regulated by nitric oxide in the heart. FEBS Open Bio 2: 135–144, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Janssens S, Pokreisz P, Schoonjans L, Pellens M, Vermeersch P, Tjwa M, Jans P, Scherrer-Crosbie M, Picard MH, Szelid Z, Gillijns H, Van de Werf F, Collen D, Bloch KD. Cardiomyocyte-specific overexpression of nitric oxide synthase 3 improves left ventricular performance and reduces compensatory hypertrophy after myocardial infarction. Circ Res 94: 1256–1262, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Knöll R, Hoshijima M, Hoffman HM, Person V, Lorenzen-Schmidt I, Bang ML, Hayashi T, Shiga N, Yasukawa H, Schaper W, McKenna W, Yokoyama M, Schork NJ, Omens JH, McCulloch AD, Kimura A, Gregorio CC, Poller W, Schaper J, Schultheiss HP, Chien KR. The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell 111: 943–955, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Koh TJ, Tidball JG. Nitric oxide synthase inhibitors reduce sarcomere addition in rat skeletal muscle. J Physiol 519: 189–196, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kohr MJ, Aponte AM, Sun J, Wang G, Murphy E, Gucek M, Steenbergen C. Characterization of potential S-nitrosylation sites in the myocardium. Am J Physiol Heart Circ Physiol 300: H1327–H1335, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kröncke KD, Carlberg C. Inactivation of zinc finger transcription factors provides a mechanism for a gene regulatory role of nitric oxide. FASEB J 13: 166–173, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Li B, Zhuang L, Reinhard M, Trueb B. The lipoma preferred partner LPP interacts with alpha-actinin. J Cell Sci 116: 1359–1366, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Liao X, Liu JM, Du L, Tang A, Shang Y, Wang SQ, Chen LY, Chen Q. Nitric oxide signaling in stretch-induced apoptosis of neonatal rat cardiomyocytes. FASEB J 20: 1883–1885, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Monreal G, Nicholson LM, Han B, Joshi MS, Philips AB, Wold LE, Bauer JA, Gerhardt MA. Cytoskeletal remodeling of desmin is a more accurate measure of cardiac dysfunction than fibrosis or myocyte hypertrophy. Life Sci 83: 786–794, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Morad M, Javaheri A, Risius T, Belmonte S. Multimodality of Ca2+ signaling in rat atrial myocytes. Ann NY Acad Sci 1047: 112–121, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Patten RD, Denofrio D, El-Zaru M, Kakkar R, Saunders J, Celestin F, Warner K, Rastegar H, Khabbaz KR, Udelson JE, Konstam MA, Karas RH. Ventricular assist device therapy normalizes inducible nitric oxide synthase expression and reduces cardiomyocyte apoptosis in the failing human heart. J Am Coll Cardiol 45: 1419–1424, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Petit MM, Fradelizi J, Golsteyn RM, Ayoubi TA, Menichi B, Louvard D, Van de Ven WJ, Friederich E. LPP, an actin cytoskeleton protein related to zyxin harbors a nuclear export signal and transcriptional activation capacity. Mol Biol Cell 11: 117–129, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Petit MM, Meulemans SM, Van de Ven WJ. The focal adhesion and nuclear targeting capacity of the LIM-containing lipoma-preferred partner (LPP) protein. J Biol Chem 278: 2157–2168, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Petit MM, Swarts S, Bridge JA, Van de Ven WJ. Expression of reciprocal fusion transcripts of the HMGIC and LPP genes in parosteal lipoma. Cancer Genet Cytogenet 106: 18–23, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Petroff MG, Kim SH, Pepe S, Dessy C, Marban E, Balligand JL, Sollott SJ. Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+ release in cardiomyocytes. Nat Cell Biol 3: 867–873, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Prabhu SD. Nitric oxide protects against pathological ventrciular remodeling: reconsideration of the role of NO in the failing heart. Circ Res 94: 1155–1157, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Rees DD, Palmer RM, Schulz R, Hodson HF, Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol 101: 746–752, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rupprecht HD, Akagi Y, Keil A, Hofer G. Nitric oxide inhibits growth of glomerular mesangial cells: role of the transcription factor EGR-1. Kidney Int 57: 70–82, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Sartoretto JL, Kalwa H, Pluth MD, Lippard SJ, Michel T. Hydrogen peroxide differentially modulates cardiac myocyte nitric oxide synthesis. Proc Natl Acad Sci USA 108: 15792–15797, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smith LW, Smith JD, Criswell DS. Involvement of nitric oxide synthase in skeletal muscle adaptation of chronic overload. J Appl Physiol 92: 2005–2011, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Stelzl U, Worm U, Lalowski M, Haenlg C, Brembeck FH, Goehler H, Strodicke M, Zenkner M, Schoenherr A, Koeppen S, Timm J, Mintzlaff S, Abraham C, Bock N, Kletzmann S, Goedde A, Toksöz E, Droege A, Krobitsch S, Korn B, Birchmeler W, Lehrach H, Wanker EE. A human protein-protein interaction network: A resource for annotating the proteome. Cell 122: 957–968, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Umar S, van der Laarse A. Nitric oxide and nitric oxide synthase isoforms in the normal, hypertrophic, and failing heart. Mol Cell Biochem 333: 191–201, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Vervenne HB, Crombez KR, Delvaux EL, Janssens V, Van de Ven WJ, Petit MM. Targeted disruption of the mouse lipoma preferred partner gene. Biochem Biophys Res Commun 379: 368–373, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Wang Y, Gilmore TD. LIM domain protein Trip6 has a conserved nuclear export signal, nuclear targeting sequences, and multiple transactivation domains. Biochim Biophys Acta 1538: 260–272, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Winlaw DS, Smythe GA, Keogh AM, Schyvens CG, Spratt PM, Macdonald PS. Increased nitric oxide production in heart failure. Lancet 344: 373–374, 1994 [DOI] [PubMed] [Google Scholar]
- 41. Xu KY, Huso DL, Dawson TM, Bredt DS, Becker LC. Nitric oxide synthase in cardiac sarcoplasmic reticulum. Proc Natl Acad Sci USA 96: 657–662, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]