Abstract
Myocardial metabolites such as adenosine mediate reactive hyperemia, in part, by activating ATP-dependent K+ (KATP) channels in coronary smooth muscle. In this study, we investigated the role of adenosine A2A and A2B receptors and their signaling mechanisms in reactive hyperemia. We hypothesized that coronary reactive hyperemia involves A2A receptors, hydrogen peroxide (H2O2), and KATP channels. We used A2A and A2B knockout (KO) and A2A/2B double KO (DKO) mouse hearts for Langendorff experiments. Flow debt for a 15-s occlusion was repaid 128 ± 8% in hearts from wild-type (WT) mice; this was reduced in hearts from A2A KO and A2A/2B DKO mice (98 ± 9 and 105 ± 6%; P < 0.05), but not A2B KO mice (123 ± 13%). Patch-clamp experiments demonstrated that adenosine activated glibenclamide-sensitive KATP current in smooth muscle cells from WT and A2B KO mice (90 ± 23% of WT) but not A2A KO or A2A/A2B DKO mice (30 ± 4 and 35 ± 8% of WT; P < 0.05). Additionally, H2O2 activated KATP current in smooth muscle cells (358 ± 99%; P < 0.05). Catalase, an enzyme that breaks down H2O2, attenuated adenosine-induced coronary vasodilation, reducing the percent increase in flow from 284 ± 53 to 89 ± 13% (P < 0.05). Catalase reduced the repayment of flow debt in hearts from WT mice (84 ± 9%; P < 0.05) but had no effect on the already diminished repayment in hearts from A2A KO mice (98 ± 7%). Our findings suggest that adenosine A2A receptors are coupled to smooth muscle KATP channels in reactive hyperemia via the production of H2O2 as a signaling intermediate.
Keywords: coronary circulation, ischemic vasodilatation, adenosine receptors, reactive oxygen species
the heart responds to acute ischemia by transiently increasing blood flow in a phenomenon called reactive hyperemia (8). This temporary reduction in coronary vascular resistance is mediated by chemical signals released into blood (38), including adenosine (35), which activates A1, A2A, A2B, and A3 receptors (29). Both A2A and A2B adenosine receptors (ARs) are expressed on endothelial and smooth muscle cells (2, 29, 33, 43). Pharmacological studies indicate that A2A and A2B receptors are the subtypes most likely involved in coronary reactive hyperemia (6, 10, 51); however, undesirable overlap in the pharmacological profiles of adenosine receptor antagonists obfuscates their relative contribution. This issue could be resolved with the gene-targeted approaches we have used previously to demonstrate pivotal roles for A2A receptors in the regulation of coronary vascular function (28, 39, 41). Thus, to specifically determine the roles of A2A and A2B ARs in coronary reactive hyperemia, in addition to distinguishing their role from A1 and A3 ARs, we used Langendorff-perfused hearts from wild-type (WT), A2A knockout (KO), A2B KO, and A2A/2B double knockout (DKO) mice, respectively.
Numerous mediators and end effectors of coronary reactive hyperemia have been proposed to function downstream of adenosine receptors, including H2O2 and ATP-dependent K+ (KATP) channels. The role of KATP channels in coronary reactive hyperemia is firmly established (3, 12), while the contribution of H2O2 and interactions with KATP channels are less clear. Some support for H2O2 in reactive hyperemia has been provided by studies of the reactive dilation of isolated coronary arterioles (18) and mesenteric reactive hyperemia (49). Additionally, it is reported that KATP channels mediate, to some degree, H2O2-induced dilation of arterioles from skeletal muscle and brain (24, 44). Thus we determined whether H2O2 couples adenosine receptor stimulation to KATP channel activity in coronary reactive hyperemia.
MATERIALS AND METHODS
Animals.
An Institutional Animal Care and Use Committee at West Virginia University School of Medicine approved all experimental protocols. We followed guidelines set forth by the American Physiological Society and National Institutes of Health regarding the care and use of laboratory animals. A2A and A2B KO mice, both backcrossed 12 generations to the WT C57BL/6 background (Jackson Laboratory; Bar Harbor, ME), were bred to generate A2A/A2B double heterozygotes. Double heterozygotes were intercrossed, 1/16 of the offspring were A2A/A2B DKO, and A2A/A2B knockout breeding pairs were established. Mice were caged in a 12:12-h light-dark cycle with free access to standard chow and water.
Langendorff-perfusion.
Mice (10–14 wk) were anesthetized with pentobarbital sodium (50 mg/kg ip) and hearts were excised into heparinized (5 U/ml) ice-cold Krebs-Henseleit buffer containing (in mM) 119 NaCl, 11 glucose, 22 NaHCO3, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 2.5 CaCl2, 2 pyruvate, and 0.5 EDTA. Hearts beat spontaneously when retrogradely perfused (80 mmHg) with 37°C buffer bubbled with 95% O2-5% CO2. The left atrium was removed and the left ventricle drained. A fluid-filled balloon was inserted into the left ventricle and connected to a transducer for pressure measurements. Left ventricular diastolic pressure was adjusted to 2–5 mmHg. Coronary flow was measured with a probe (Transonic Systems; Ithaca, NY) in the aortic perfusion line. Hearts were paced to 420 beats/min and function allowed to stabilize. Hearts with persistent arrhythmias or developed pressure less than 80 mmHg were excluded from the study. Coronary flow and cardiac function were measured using a Power Lab data acquisition system (AD Instruments; Colorado Springs, CO). Hearts were subjected to 15 s of total flow occlusion to elicit hyperemia. Catalase, glibenclamide, and Nω-nitro-l-arginine methyl ester (l-NAME) (Sigma Chemical; St. Louis, MO) were delivered into the aortic perfusion line using a microinjection pump (Harvard Apparatus; Holliston, MA) as 1% of coronary flow to achieve a final concentration of 1,250 U/ml (46, 49).
Patch clamp.
Single smooth muscle cells were enzymatically isolated from mouse aortas. For cell isolation, a HEPES-buffered saline was used and contained (in mM) 135 NaCl, 5 KCl, 1 MgCl2, 0.36 CaCl2, 10 glucose, 10 HEPES, and 5 Tris; pH 7.4. The aorta was placed in this solution plus (in mg/ml) 2 collagenase, 1 elastase, 2 bovine serum albumin, and 1 soybean trypsin inhibitor for 15 min at 37°C. The tissue was passed through the tip of a fire-polished Pasteur pipette to liberate single cells. Smooth muscle cells were resuspended in enzyme-free buffer, stored on ice, and used within 8 h. Cells were placed in a recording chamber atop an inverted microscope and perfused with a HEPES-buffered solution containing 140 mM K+. Patch pipettes (3–5 MΩ) were filled with 140 mM K+ solution supplemented with 5 mM Mg-ATP and 0.1 mM Na-GTP, pH 7.1. Inward KATP currents were recorded in the conventional whole cell mode (with Nernst equilibrium potential for K+ set to 0 mV). The membrane potential was held at −80 mV and ramped or stepped to potentials between −100 and +100 mV. Currents were low-pass filtered at 1 kHz and digitized at 5 kHz. Series resistance and whole cell capacitance were compensated as completely as possible through the amplifier (PC-505, Warner Instruments; Hamden, CT). This amplifier was interfaced to a computer with pClamp 9 software for data acquisition and analysis (Molecular Devices; Sunnyvale, CA). All currents are reported as glibenclamide-sensitive KATP conductance (and calculated as nS/pF); effects of drugs are represented relative to control.
Flow debt (equal to baseline flow rate × occlusion time) and repayment area [equal to integral of hyperemic area above baseline flow during the first minute of reactive hyperemia] were calculated as previously reported (6, 10, 51). Since absolute coronary flow rates change proportionally with heart mass and metabolic rate, the repayment area and debt area data are represented as milliliters per gram of wet heart weight (ml/g) and baseline and peak flow data are presented as milliliters per minute per gram of wet heart weight (ml·min−1·g−1). Each reactive hyperemia graph represents the average of all studies.
Statistical analysis.
Statistical analyses were made with t-test and one-way analysis of variance (ANOVA) as indicated. Results were considered significant when P < 0.05. Values are means ± SE from n number of animals.
RESULTS
A2A receptors are important in coronary reactive hyperemia.
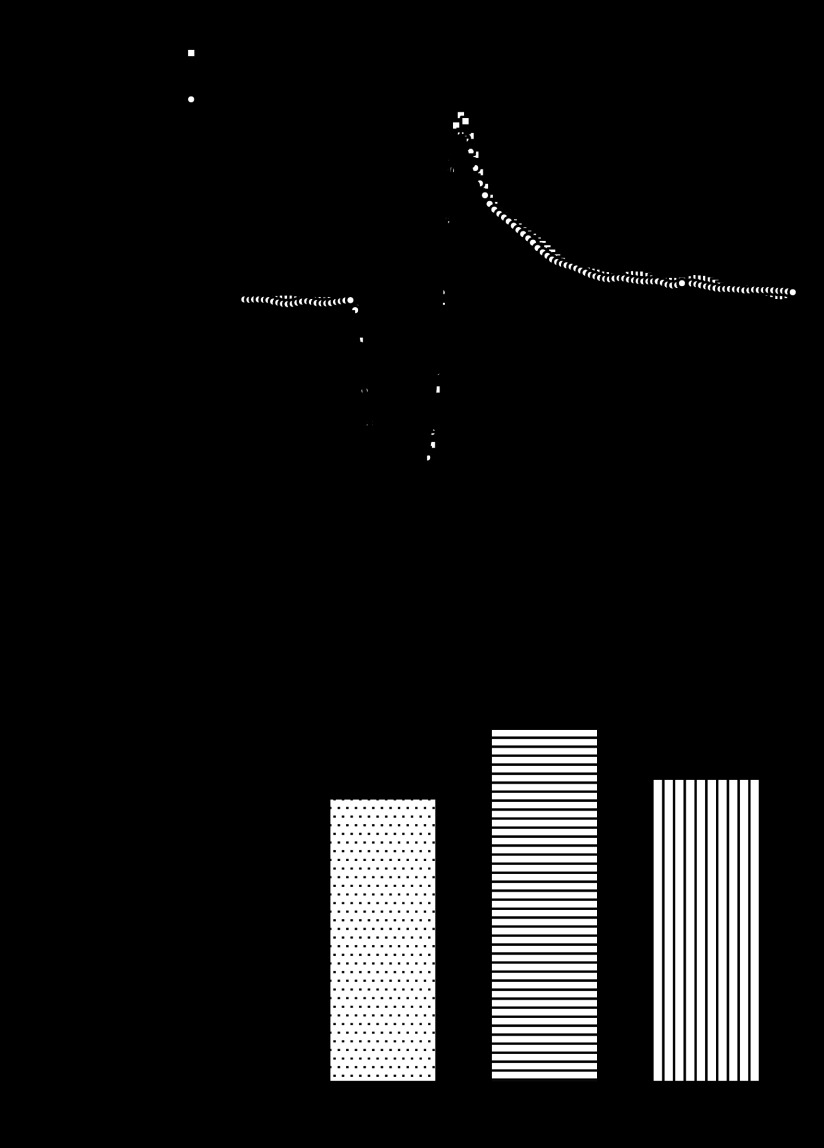
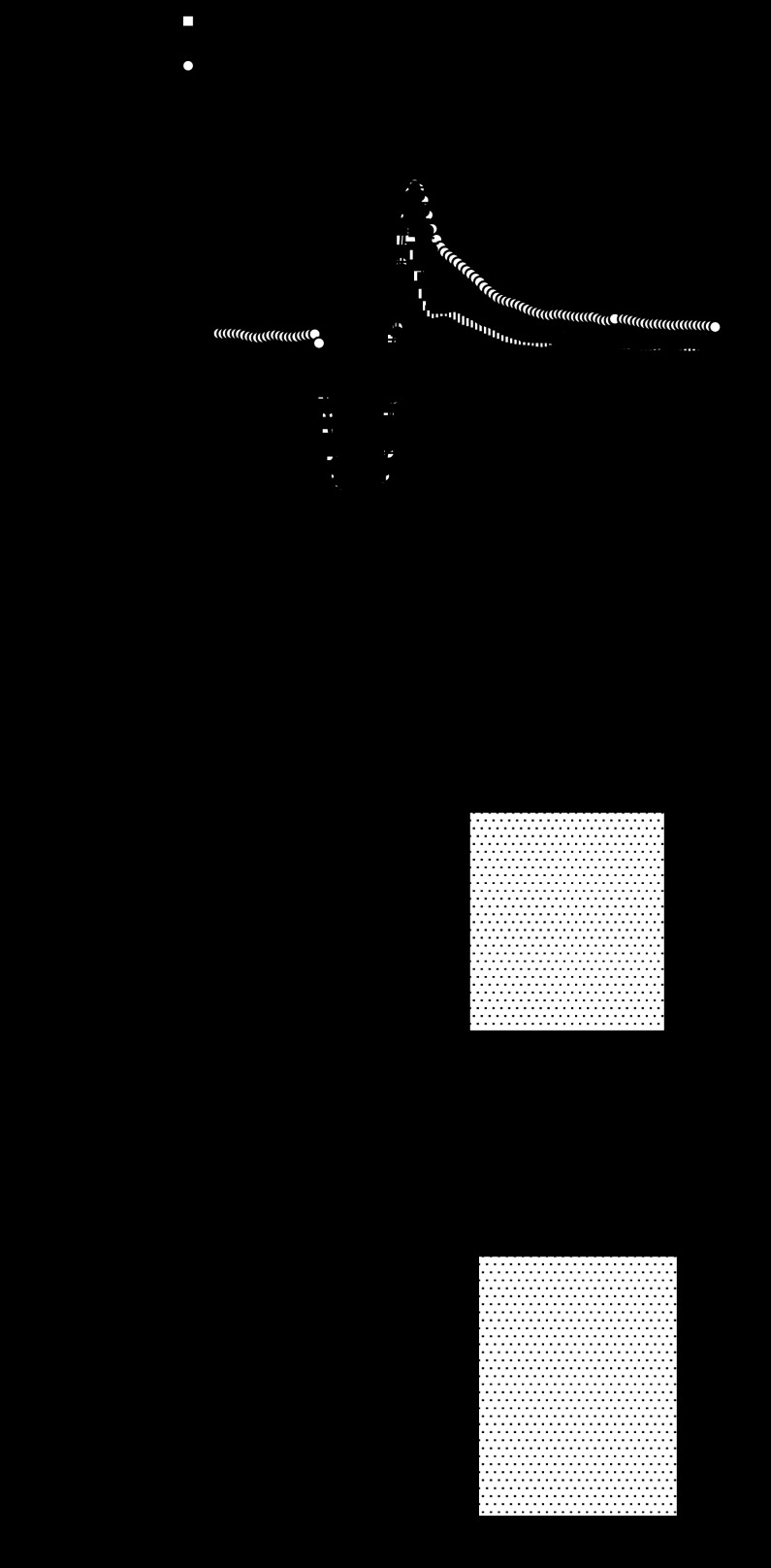
We used hearts from WT, A2A and A2B KO mice to test the involvement of adenosine receptor subtypes in coronary reactive hyperemia. Hearts from A2A/2B DKO mice were used to test the combined role of A2A and A2B ARs known to induce coronary vasodilation. Baseline flow in WT hearts (n = 6) was 19.8 ± 0.9 ml·min−1·g−1, and 4.9 ± 0.2 ml/g of flow debt was incurred during a 15-s occlusion. Peak hyperemic flow was 42.7 ± 2.0 ml·min−1·g−1, and the repayment volume was 6.3 ± 0.4 ml/g. Thus flow debt was repaid 128 ± 8% in hearts from WT mice (Fig. 1). Repayment volume was reduced in A2A KO mice (n = 8; Fig. 1). Specifically, while baseline flow (19.4 ± 1.6 ml·min−1·g−1), debt volume (4.9 ± 0.4 ml/g), and peak flow (42.9 ± 1.7 ml·min−1·g−1) were similar to WT, the repayment volume was only 4.5 ± 0.2 ml/g (P < 0.05 vs. WT). That is, flow debt was repaid only 98 ± 9% in hearts from A2A KO mice (just 77 ± 7% of the response seen in WT).
Fig. 1.
Reactive hyperemia is reduced in hearts lacking the adenosine A2A receptor. A: group data show baseline flow and the hyperemic response following a 15-s total occlusion of coronary flow. Responses were similar in hearts from wild-type (WT) (n = 6) and A2B knockout (KO) (n = 5) mice, but were significantly reduced in hearts from A2A KO (n = 6) and A2A/A2B double knockout (DKO) (n = 8) mice. B: repayment of flow debt was reduced 22–29% in A2A KO and A2A/A2B DKO mice (*P < 0.05 vs. WT by one-way ANOVA; detailed in text) but not in A2B KO (n = 5).
In contrast, hyperemic responses in hearts from A2B KO mice (n = 5) were indistinguishable from those in WT (Fig. 1). There were no differences in baseline flow (20.3 ± 1.1 ml·min−1·g−1), debt volume (5.1 ± 0.3 ml/g), peak flow (44.2 ± 0.5 ml·min−1·g−1), or repayment volume (6.1 ± 0.1 ml/g). Flow debt in hearts from A2B KO mice was repaid (123 ± 13%), representing 95 ± 9% of the response in hearts from WT mice. Responses in hearts from A2A/2B DKO mice (n = 8) were similar to A2A KO mice (Fig. 1). That is, repayment volume (4.9 ± 0.2 ml/g) and the repayment of flow debt (105 ± 6%) were reduced (P < 0.05 vs. WT). Resting flow (18.6 ± 0.6 ml·min−1·g−1), debt volume (4.7 ± 0.1 ml/g), and peak flow (.6 ± 1.2 ml·min−1·g−1) were not different from WT, but the repayment of flow debt in hearts from A2A/2B DKO mice was only 82 ± 5% of the response seen in WT (Table 1).
Table 1.
Resting flow, debt area, peak flow, and flow repayment area
| Resting Flow. ml·min−1·g−1 | Debt Area, ml/g | Peak Flow, ml·min−1·g−1 | Repayment Area, ml/g | |
|---|---|---|---|---|
| WT | 19.8 ± 0.9 | 4.9 ± 0.2 | 42.7 ± 2 | 6.3 ± 0.4 |
| A2A KO | 19.4 ± 1.6 | 4.9 ± 0.4 | 42.9 ± 1.7 | 4.5 ± 0.2* |
| A2B KO | 20.3 ± 1.1 | 5.1 ± 0.3 | 44.2 ± 0.5 | 6.1 ± 0.1 |
| A2A/2B KO | 18.6 ± 0.6 | 4.7 ± 0.1 | 41.6 ± 1.2 | 4.9 ± 0.2* |
| WT + Cat | 21.4 ± 2.1 | 5.3 ± 0.5 | 38.6 ± 2.4* | 4.4 ± 0.4* |
| A2A KO + Cat | 16.8 ± 0.9* | 4.2 ± 0.2* | 36.3 ± 1.4* | 4.1 ± 0.4* |
| WT + l-NAME | 11.7 ± 0.8* | 2.9 ± 0.24* | 31.9 ± 0.9* | 2.7 ± 0.3* |
| WT + l-NAME + Cat | 11.2 ± 1.3* | 2.8 ± 0.30* | 26.8 ± 1.6* | 1.9 ± 1.2* |
All values are means ± SE.
WT, wild type; KO, knockout; Cat, catalase; l-NAME, Nω-nitro-l-arginine methyl ester.
P < 0.05 compared with WT (n = 5–7).
Adenosine activates KATP channels through A2A receptors.
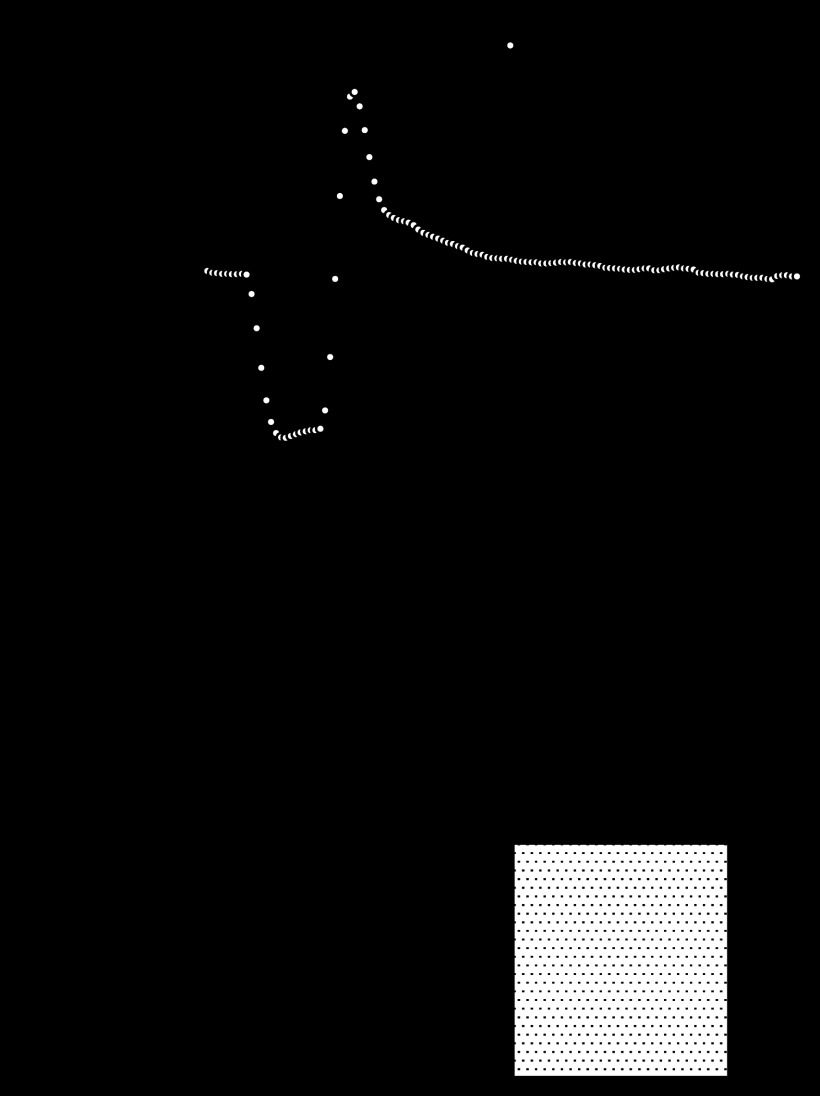
We tested the effect of KATP channel blockade on coronary reactive hyperemia response of WT isolated hearts. Glibenclamide (10 μM) significantly reduced WT (n = 5) baseline flow from 17.3 ± 0.5 to 12.8 ± 0.5 ml·min−1·g−1 and flow debt from 4.3 ± 0.1 to 3.2 ± 0.1 ml/g. Peak hyperemic flow and the repayment volume were reduced by glibenclamide from 35.9 ± 0.8 to 29.3 ± 1.1 ml·min−1·g−1 and 6.9 ± 0.4 to 2.7 ± 0.4 ml/g, respectively. Thus flow debt was repaid 161 ± 12% in hearts from WT mice while this was reduced to 87.5 ± 11.2% in the presence of glibenclamide (Fig. 2).
Fig. 2.
Reactive hyperemia is attenuated in the presence of glibenclamide. A: group data present baseline flow and the hyperemic response following a 15-s total occlusion of coronary flow. Responses were significantly reduced in hearts with KATP channel blockade by glibenclamide (10 μM, n = 5). B: repayment of flow debt was reduced by 54.4% in WT mice treated with glibenclamide (*P < 0.05 vs. WT by unpaired t-test) (n = 5).
We isolated smooth muscle cells from the aortas of WT, A2A and A2B KO, and A2A/2B DKO mice and used whole cell patch-clamp to determine whether A2A receptors couple to KATP channels. Recordings were made in symmetrical 140 mM K+ so that inward linear KATP current was more easily resolved. Effects of adenosine (10 μM) were compared with pinacidil (10 μM) as a positive control. Further, adenosine-activated currents were blocked with glibenclamide (10 μM) to confirm that KATP channels mediated them. Adenosine activated current in smooth muscle cells from WT mice (Fig. 3A), but not A2A KO mice (Fig. 3B). Responses in smooth muscle cells from A2B KO mice (Fig. 3C) were similar to WT, while responses in cells from A2A/2B DKO mice (Fig. 3D) were like those from A2A KO mice. Group data (cells from n = 4–5 mice in each group; Fig. 3E) show that adenosine-induced KATP current was diminished in smooth muscle cells from mice lacking the adenosine A2A receptor (P < 0.05 vs. WT by one-way ANOVA). Importantly, however, functional KATP channel expression was not different as pinacidil-activated KATP current was similar in smooth muscle cells from all four strains of mice.
Fig. 3.
Adenosine-induced KATP current is reduced in smooth muscle cells lacking the adenosine A2A receptor. Representative traces show that adenosine (10 μM) activated glibenclamide-sensitive KATP current in smooth muscle cells from WT (A) and A2B KO (C) mice, but not A2A KO (B) or A2A/A2B (D) DKO mice. For group data (E), KATP conductance (nS/pF) was normalized to control for cells from n = 4–5 mice in each group. *P < 0.05 vs. WT by one-way ANOVA.
H2O2 activates KATP current in smooth muscle.
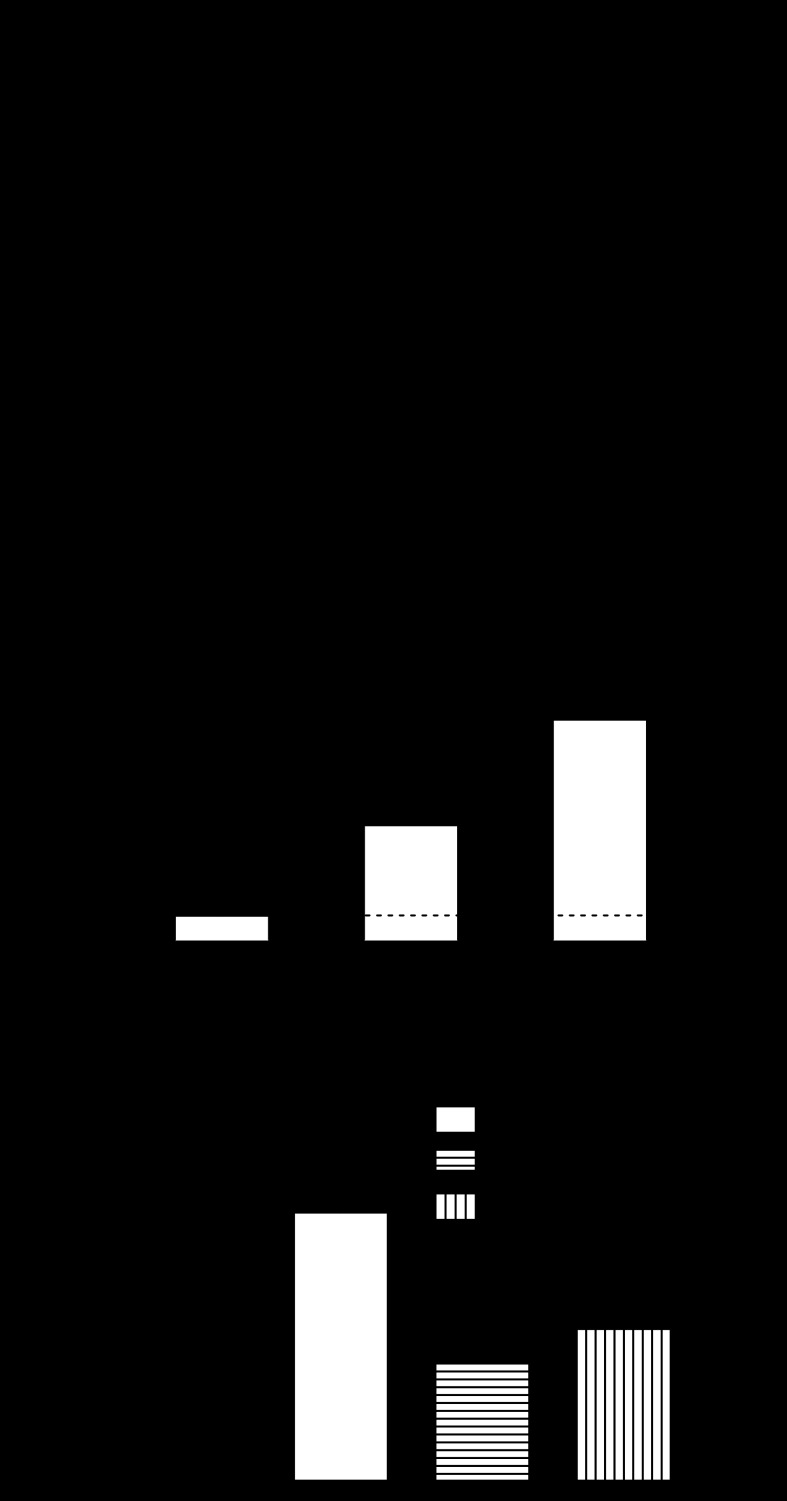
We isolated smooth muscle cells from the aortas of WT mice and used whole cell patch clamp to determine whether H2O2 activates KATP current. Current was recorded in the symmetrical 140 mM K+ solutions described above. Figure 4A shows current sensitive to glibenclamide (10 μM) under control conditions and when activated by H2O2 (1 mM) and pinacidil (10 μM). Group data (cells from n = 5 mice) are shown in Fig. 4B; KATP conductance (nS/pF) was 0.018 ± 0.006 under control conditions and significantly increased to 0.070 ± 0.026 and 0.142 ± 0.033 with 1 mM H2O2 and with 10 μM pinacidil, respectively (both P < 0.05 vs. control by one-way ANOVA). To determine the role of KATP channels in H2O2-mediated coronary vasodilation, we examined the effect of glibenclamide (10 μM) on changes in flow induced by H2O2 (10 μM). In our isolated heart system, glibenclamide significantly decreased the baseline coronary flow (79 ± 6%) and consistent with the patch-clamp data, our data showed that glibenclamide significantly reduced the H2O2-induced increase in coronary flow from 164 ± 16 to 79 ± 6% (n = 4, Fig. 4C).
Fig. 4.
H2O2 activates KATP channels in smooth muscle cells. A: representative trace showing effect of H2O2 (1 mM) to increase KATP current. B: group data (cells from n = 5 mice) illustrate effect of H2O2 on KATP current relative to pinacidil (10 μM). C: group data (n = 4) demonstrate the effect of glibenclamide (10 μM) on H2O2-mediated increase in coronary flow of WT hearts. * and # indicate P < 0.05 vs. WT and H2O2 effect, respectively.
Adenosine-induced coronary dilation involves H2O2.
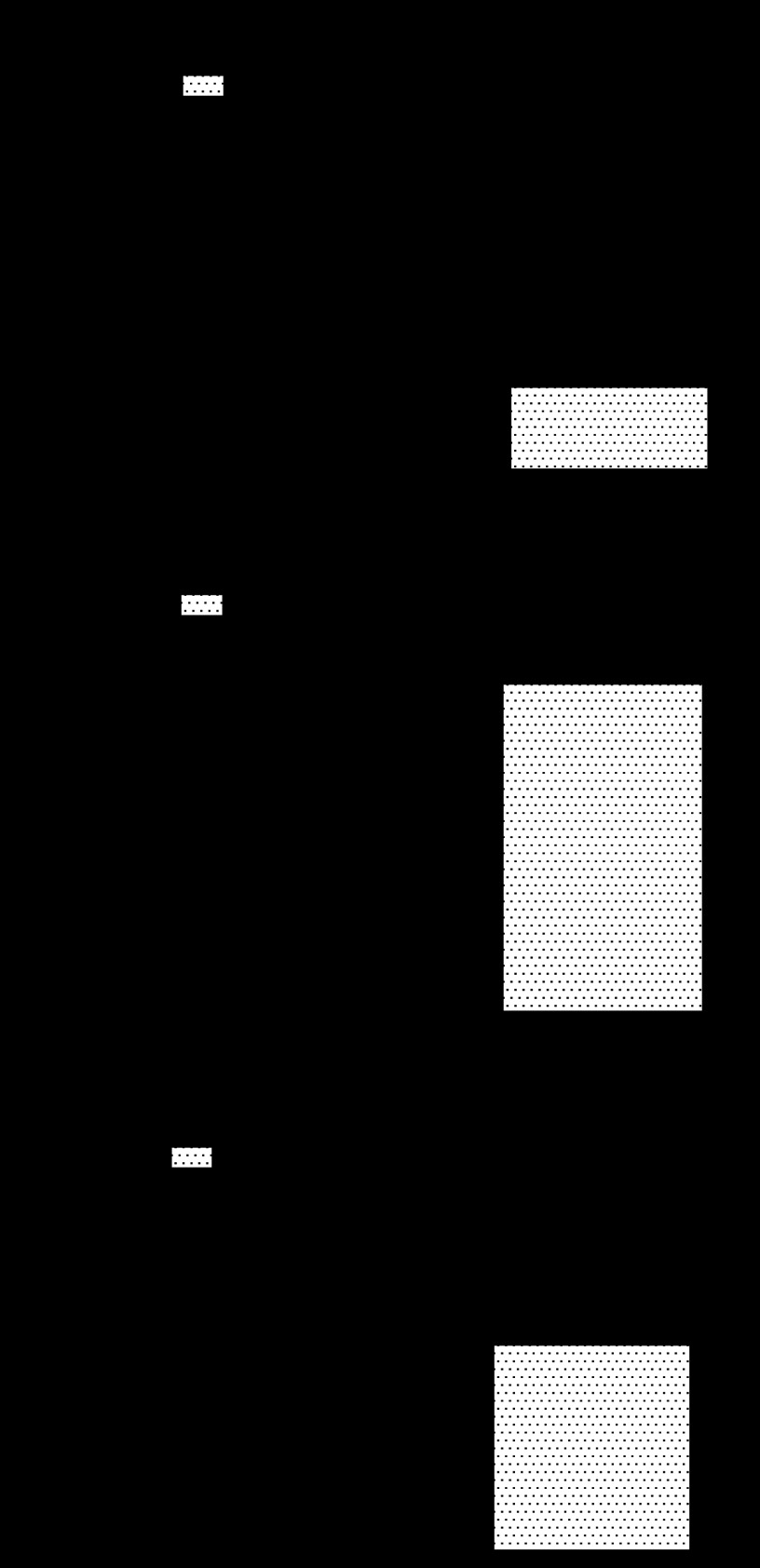
We used catalase to determine the role of endogenous H2O2 production in adenosine-induced coronary vasodilation. Adenosine-induced coronary vasodilation is catalase-sensitive (Fig. 5A), indicating a role for endogenous H2O2 production. Specifically, adenosine (1 μM) increased coronary flow (284 ± 53%) and catalase (1,250 U/ml) reduced the baseline flow to 86 ± 2%; however, catalase reduced the adenosine response to 89 ± 13% (P < 0.05 vs. control by paired t-test). Catalase reduced left ventricular developed pressure (Fig. 5C), but had no effect on heart rate (Fig. 5B). These data suggest that 1) H2O2 is involved in adenosine-mediated effects on cardiac contractility or 2) cardiac function decreased secondary to a reduction in coronary flow. Since we have previously shown that adenosine does not increase the coronary flow in A2A/2B KO mice (39), this experiment was not performed on double knockout mice.
Fig. 5.
H2O2 dilates the coronary circulation and adenosine-induced vasodilation is catalase-sensitive. A–C show group data (n = 4) illustrating the catalase sensitivity of adenosine-induced coronary dilation (A), contractility (C), and heart rate (B) in WT mice. LVDP, left ventricular developed pressure. *P < 0.05 vs. adenosine alone.
H2O2 contributes to coronary reactive hyperemia.
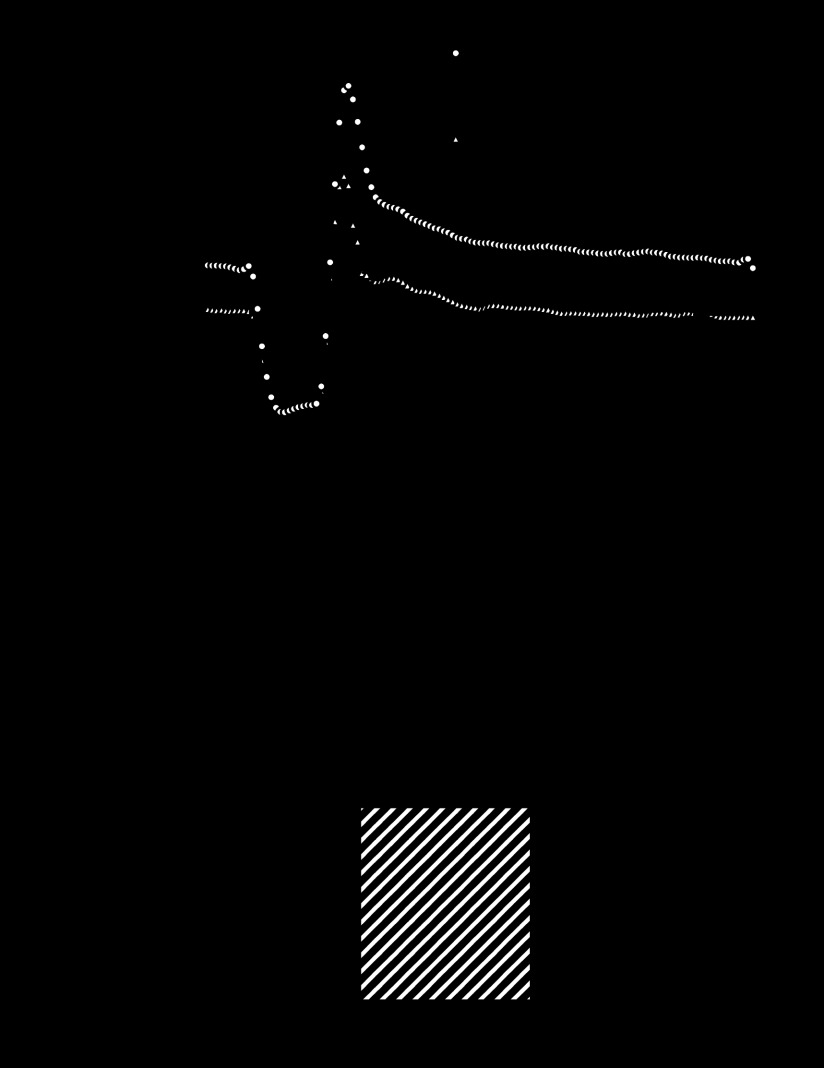
We used hearts from WT and A2A KO mice to determine whether H2O2 links A2A receptor activation to coronary flow during reactive hyperemia. To do so, reactive hyperemia in hearts from both strains was compared with and without catalase (1,250 U/ml) to degrade H2O2 (Fig. 6). In hearts from WT mice, catalase significantly decreased the repayment of flow debt (from 128 ± 8 to 84 ± 9%; P < 0.05; n = 6). In hearts from A2A KO mice, catalase had no effect on the already diminished repayment of flow debt (98 ± 9 vs. 98 ± 7%; n = 6, Table 1).
Fig. 6.
Catalase (Cat)-sensitive portion of reactive hyperemia is absent in hearts lacking the adenosine A2A receptor. A: group data show baseline flow and hyperemic responses in hearts from WT and A2A KO mice in the absence or presence of catalase (1,250 U/ml). Hyperemic responses were reduced by catalase in hearts from WT (n = 7) but not A2A KO (n = 6) mice. Catalase reduced the repayment of flow debt 30% in hearts from WT (B) mice (*P < 0.05 by unpaired t-test), but had no effect in hearts from A2A KO mice (C).
H2O2 contributes to coronary reactive hyperemia independent of nitric oxide (NO).
Adenosine A2A and A2B receptors can also signal through NO (32). NO is a well-known vasodilator involved in regulating baseline coronary flow and reactive hyperemia. Thus, to separate the role of H2O2 from NO, we performed reactive hyperemia experiments on hearts from WT mice with l-NAME (50 μM, a NO synthase inhibitor) and l-NAME plus catalase (Fig. 7). l-NAME significantly decreased the repayment of flow debt (73 ± 8% of control; n = 5, P < 0.05). Importantly, however, addition of catalase to l-NAME further reduced the repayment of flow debt (57 ± 9%; P < 0.05). These data indicate that the effect of catalase is on H2O2 signaling, not NO signaling.
Fig. 7.
Catalase-sensitive portion of reactive hyperemia is independent of nitric oxide. A (n = 4–9) shows group data of baseline flow and hyperemic responses in hearts from WT mice in the presence and absence of Nω-nitro-l-arginine methyl ester (l-NAME) (5 × 10−5 M, nitric oxide synthase inhibitor) or l-NAME + catalase (1,250 U/ml). B shows the further reduced hyperemic response in the presence of both l-NAME and catalase (68.5%) relative to l-NAME alone (53.1%) compared with WT. *P < 0.05 and #P < 0.05 vs. control and l-NAME effect, respectively.
DISCUSSION
Our study provides several new substantial lines of evidence regarding mechanisms of coronary reactive hyperemia. First, using gene-targeted mice, we offer a definitive comparison of the roles of A2A and A2B receptors in coronary reactive hyperemia. Our finding that A2A receptor plays a dominant role in adenosine-mediated coronary reactive hyperemia substantially advances knowledge gained from studies using adenosine receptor antagonists (6, 10, 51). Second, with our catalase experiments, we provide the first evidence of a role for H2O2 in reactive hyperemia in the intact heart. These data add significantly to previous observations of roles for H2O2 in mesenteric reactive hyperemia (49) and reactive dilation in isolated coronary arterioles (18). Third, through a combination of experiments, we offer the original observation that H2O2 couples A2A receptor stimulation to KATP channel activation in coronary reactive hyperemia.
Previously, two general strategies have been employed to determine the role of adenosine and its receptors in coronary reactive hyperemia: 1) enzymatic degradation of adenosine (e.g., infusing adenosine deaminase) or 2) pharmacological antagonism of adenosine receptors (e.g., administering aminophylline). With these tools, it had been demonstrated previously that adenosine plays a small, but significant role, in coronary reactive hyperemia in dogs, lambs, and pigs (4, 6, 10, 23). Because two of the four known adenosine receptor subtypes, A2A and A2B, are linked to coronary vasodilation, a logical question centers on which receptor subtype(s) is/are involved. Gene deleted knockout mice have been an important tool to dissect physiological signaling pathways, e.g., elucidating the role of receptor through its absence. Using PCR, we confirmed the absence of A2A and A2B receptors in knockout mouse tail, aorta and coronary arteries (data not shown). Therefore, the present results obtained using adenosine receptor knockout mice support the view that adenosine and the A2A receptor mediate a portion of coronary reactive hyperemia.
A2A and A2B receptors both function in signaling in coronary smooth muscle and endothelium (31, 37, 40), but the relative role of these two receptor subtypes in coronary reactive hyperemia has remained, until now, obscure. Using an antagonist of A2A receptors (SCH58261), it was suggested that 30–50% of flow repayment could be attributed to A2A receptor activation (6, 51). Our results with hearts from A2A KO and A2A/2B DKO mice indicate that the A2A receptor is responsible for 22–29% of flow repayment, which is toward the lower end of the previous pharmacological estimates. Our results from A2B KO and A2A/2B DKO mice did not suggest a significant role for A2B receptors in reactive hyperemia, similar to previous pharmacological studies (6, 51). Due to its low affinity for adenosine, A2B receptors are thought to be activated under more severely ischemic conditions, such as during ischemia/reperfusion and preconditioning (13, 15).
We tested the involvement of H2O2 in reactive hyperemia and adenosine-induced coronary vasodilation. We demonstrate that catalase, which breaks down H2O2 into water and oxygen, significantly attenuates adenosine-induced coronary vasodilation. This suggests that H2O2 plays a critical role in adenosine-mediated signaling pathways. We also demonstrate that the repayment of flow debt was reduced by catalase, showing the contribution of endogenous H2O2 production to coronary reactive hyperemia. Furthermore, we suggest that H2O2 released during coronary reactive hyperemia is, at least partly, mediated through activation of A2A adenosine receptors since catalase did not further decrease the flow repayment in A2A KO hearts. This is not likely due to changes in H2O2 degradation in A2A KO mice, as the concentration-response curve to exogenous H2O2 (100 nM to 80 μM) is unchanged compared with WT control or A2B KO (data not shown). In the vasculature, NAD(P)H oxidases (Nox) are responsible for the majority of ROS production (21). Nox4 is shown to be the dominant isoform producing H2O2 and also expressed in vascular smooth muscle cells (45). Therefore, expression level of factors involved in vascular H2O2 production such as NOX4 in A2A KO remain to be examined in future studies. Nevertheless, the idea that H2O2 is a mediator of coronary metabolic (36, 48) and endothelium-dependent (22, 27) vasodilation is gaining traction; however, no previous study had indicated a role for H2O2 in intact coronary reactive hyperemia. Two related observations had, however, been made in other vascular beds. Video microscopy of the cremasteric microcirculation indicated striking similarities in the pharmacological sensitivity of reactive hyperemia and exogenous H2O2 (46). A study using microspheres in the mouse mesenteric circulation indicated that ∼40% of hyperemic flow was catalase-sensitive, i.e., attributable to endogenous H2O2 production (49). Our data are in agreement and indicate that ∼35% of hyperemic flow in the mouse heart is catalase-sensitive.
We demonstrated previously that A2A-mediated coronary vasodilation depends upon KATP channels, as it is blocked by glibenclamide (39). Here we show that 1) adenosine-induced coronary vasodilation depends upon H2O2, 2) H2O2 increases KATP channel current, and 3) increase in coronary flow by H2O2 is mediated through KATP channels. The latter finding extends the knowledge that KATP channels mediate, at least in part, H2O2-induced dilation of arterioles from skeletal muscle and brain (20, 24, 44). Further, it has been shown that H2O2 activates KATP channels in pancreatic beta cells and cardiac myocytes (16, 30). Nevertheless, the evidence of this mechanism in smooth muscle and the role of adenosine were lacking. We show for the first time that KATP current in smooth muscle cells is activated by H2O2. This adds to previous literature indicating that H2O2 activates K+ channels in coronary vascular smooth muscle cells including Ca2+-activated K+ channels (5, 42, 52) and voltage-dependent K+ channels (34). Additionally, glibenclamide and catalase both decreased the baseline coronary flow, with glibenclamide effect being about 7% more than catalase, suggesting that KATP channels may have additional pathways of activation in addition to adenosine-induced-H2O2 although H2O2 may be the major mediator in this activation.
Perhaps a better approach would be to study smooth muscle cells isolated from mouse coronary arteries or arterioles. This is because differences between large- and small-caliber vessels exist (e.g., effects on intravascular pressure and relative responses to metabolic, neurohumoral, myogenic, and flow-mediated mechanisms) (7, 11). Moreover, it has been demonstrated that differences in K+ channel expression may explain some segmental differences in vascular reactivity (1, 25). Importantly, however, there is no a priori reason to assume that, in this context, KATP channels in smooth muscle cells from the aorta and coronary arterioles would respond differently to adenosine and H2O2 signaling. Specifically, both conduit and resistance arteriolar smooth muscle cells express KATP channels (17, 19), adenosine A2 receptors (9, 47), and relax or dilate to H2O2 (26, 27). In fact, we demonstrate here that KATP electrophysiology from aortic smooth muscle cells (Figs. 3 and 4) matches very well with functional responses from coronary resistance vessels (Fig. 4C). Thus, until demonstrated otherwise, it is justifiable to conclude that aortic smooth muscle cells serve as an excellent electrophysiological model for coronary arteriolar dilation, at least with regard to adenosine signaling, reactive oxygen species, and KATP channel regulation.
With regard to the limitations of our study, since we have used isolated heart system, the contribution of flow/shear stress- or pressure-dependent coronary mechanisms in the development of reactive hyperemia could not be clearly elucidated or isolated from the effect of local metabolic factors. Additionally, Krebs buffer used in crystalloid-perfused mouse heart, unlike blood, does not have O2-carrying capacity and hence some may suggest that the heart would be ischemic (by definition) or hypoxic compared with circulating blood. Regardless, constantly oxygenated ex vivo isolated heart model is a well-established method that has been used to answer mechanistic questions. Data gathered from isolated heart reflect the overall end effect of shear/pressure stress- and metabolites-induced effects via eliminating the complexity of the role of neuronal and hormonal effects during coronary reactive hyperemia, thus allowing a better understanding of the coronary flow regulation.
Our study provides novel data to further understand mechanisms of coronary reactive hyperemia and adenosine-induced vasodilation. Using adenosine receptor KO mice, we show that A2A receptors, but not A2B receptors, are critical to coronary reactive hyperemia. This finding supports and extends previous studies using pharmacological tools. Additionally, we demonstrate a role for H2O2 in reactive hyperemia in the intact heart. This finding supports observations made in the microcirculation of skeletal muscle and brain and represents the first such report for the coronary circulation. Finally, our data suggest that H2O2 is a signaling molecule coupling the stimulation of A2A receptors to the opening of KATP channels. This finding is entirely novel and will require additional research to unravel molecular mechanisms linking A2A receptor activation to H2O2 production and finally to KATP channel opening. At present, a list of likely mechanisms might include A2A-induced activation of NADPH oxidases (14), bioconversion of superoxide to H2O2, and redox regulation of the KATP channel or proteins that regulate it (50).
GRANTS
National Heart, Lung, and Blood Institute Grants HL-027339, HL-094447, HL-071802, and T32-HL-090610 supported this project.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.S.S. conception and design of research; M.S.S., X.Z., S.A., and G.M.D. performed experiments; M.S.S., X.Z., S.A., and G.M.D. analyzed data; M.S.S., X.Z., S.A., B.T., G.M.D., and S.J.M. interpreted results of experiments; M.S.S., X.Z., and G.M.D. prepared figures; M.S.S. and G.M.D. drafted manuscript; M.S.S., X.Z., B.T., G.M.D., and S.J.M. edited and revised manuscript; M.S.S., X.Z., S.A., S.L.T., C.L., B.T., G.M.D., and S.J.M. approved final version of manuscript.
REFERENCES
- 1. Archer SL, Huang JM, Reeve HL, Hampl V, Tolarova S, Michelakis E, Weir EK. Differential distribution of electrophysiologically distinct myocytes in conduit and resistance arteries determines their response to nitric oxide and hypoxia. Circ Res 78: 431–442, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Arif Hasan AZ, Abebe W, Mustafa SJ. Antagonism of coronary artery relaxation by adenosine A2A-receptor antagonist ZM241385. J Cardiovasc Pharmacol 35: 322–325, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Aversano T, Ouyang P, Silverman H. Blockade of the ATP-sensitive potassium channel modulates reactive hyperemia in the canine coronary circulation. Circ Res 69: 618–622, 1991 [DOI] [PubMed] [Google Scholar]
- 4. Bache RJ, Dai XZ, Schwartz JS, Homans DC. Role of adenosine in coronary vasodilation during exercise. Circ Res 62: 846–853, 1988 [DOI] [PubMed] [Google Scholar]
- 5. Barlow RS, White RE. Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. Am J Physiol Heart Circ Physiol 275: H1283–H1289, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Berwick ZC, Payne GA, Lynch B, Dick GM, Sturek M, Tune JD. Contribution of adenosine A2A and A2B receptors to ischemic coronary dilation: role of KV and KATP channels. Microcirculation 17: 600–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chilian WM, Eastham CL, Marcus ML. Microvascular distribution of coronary vascular resistance in beating left ventricle. Am J Physiol Heart Circ Physiol 251: H779–H788, 1986 [DOI] [PubMed] [Google Scholar]
- 8. Coffman JD, Gregg DE. Reactive hyperemia characteristics of the myocardium. Am J Physiol 199: 1143–1149, 1960 [DOI] [PubMed] [Google Scholar]
- 9. Collis MG, Brown CM. Adenosine relaxes the aorta by interacting with an A2 receptor and an intracellular site. Eur J Pharmacol 96: 61–69, 1983 [DOI] [PubMed] [Google Scholar]
- 10. Dick GM, Bratz IN, Borbouse L, Payne GA, Dincer UD, Knudson JD, Rogers PA, Tune JD. Voltage-dependent K+ channels regulate the duration of reactive hyperemia in the canine coronary circulation. Am J Physiol Heart Circ Physiol 294: H2371–H2381, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev 88: 1009–1086, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Duncker DJ, Van Zon NS, Altman JD, Pavek TJ, Bache RJ. Role of K+ATP channels in coronary vasodilation during exercise. Circulation 88: 1245–1253, 1993 [DOI] [PubMed] [Google Scholar]
- 13. Eckle T, Kohler D, Lehmann R, El Kasmi K, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation 118: 166–175, 2008 [DOI] [PubMed] [Google Scholar]
- 14. El-Awady MS, Ansari HR, Fil D, Tilley SL, Mustafa SJ. NADPH oxidase pathway is involved in aortic contraction induced by A3 adenosine receptor in mice. J Pharmacol Exp Ther 338: 711–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ 14: 1315–1323, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Ichinari K, Kakei M, Matsuoka T, Nakashima H, Tanaka H. Direct activation of the ATP-sensitive potassium channel by oxygen free radicals in guinea-pig ventricular cells: its potentiation by MgADP. J Mol Cell Cardiol 28: 1867–1877, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Klieber HG, Daut J. A glibenclamide sensitive potassium conductance in terminal arterioles isolated from guinea pig heart. Cardiovasc Res 28: 823–830, 1994 [DOI] [PubMed] [Google Scholar]
- 18. Koller A, Bagi Z. Nitric oxide and H2O2 contribute to reactive dilation of isolated coronary arterioles. Am J Physiol Heart Circ Physiol 287: H2461–H2467, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Kovacs RJ, Nelson MT. ATP-sensitive K+ channels from aortic smooth muscle incorporated into planar lipid bilayers. Am J Physiol Heart Circ Physiol 261: H604–H609, 1991 [DOI] [PubMed] [Google Scholar]
- 20. Lacza Z, Puskar M, Kis B, Perciaccante JV, Miller AW, Busija DW. Hydrogen peroxide acts as an EDHF in the piglet pial vasculature in response to bradykinin. Am J Physiol Heart Circ Physiol 283: H406–H411, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 285: R277–R297, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res 93: 573–580, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Mainwaring RD, Ely SW, Mentzer RM., Jr Myocardial reactive hyperemia in the newborn. J Surg Res 44: 603–608, 1988 [DOI] [PubMed] [Google Scholar]
- 24. Marvar PJ, Hammer LW, Boegehold MA. Hydrogen peroxide-dependent arteriolar dilation in contracting muscle of rats fed normal and high salt diets. Microcirculation 14: 779–791, 2007 [DOI] [PubMed] [Google Scholar]
- 25. McCulloch KM, Kempsill FE, Buchanan KJ, Gurney AM. Regional distribution of potassium currents in the rabbit pulmonary arterial circulation. Exp Physiol 85: 487–496, 2000 [PubMed] [Google Scholar]
- 26. Mian KB, Martin W. The inhibitory effect of 3-amino-1,2,4-triazole on relaxation induced by hydroxylamine and sodium azide but not hydrogen peroxide or glyceryl trinitrate in rat aorta. Br J Pharmacol 116: 3302–3308, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res 92: e31–e40, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Morrison RR, Talukder MA, Ledent C, Mustafa SJ. Cardiac effects of adenosine in A2A receptor knockout hearts: uncovering A2B receptors. Am J Physiol Heart Circ Physiol 282: H437–H444, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Mustafa SJ, Morrison RR, Teng B, Pelleg A. Adenosine receptors and the heart: role in regulation of coronary blood flow and cardiac electrophysiology. Handb Exp Pharmacol: 161–188, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nakazaki M, Kakei M, Koriyama N, Tanaka H. Involvement of ATP-sensitive K+ channels in free radical-mediated inhibition of insulin secretion in rat pancreatic beta-cells. Diabetes 44: 878–883, 1995 [DOI] [PubMed] [Google Scholar]
- 31. Olanrewaju HA, Gafurov BS, Lieberman EM. Involvement of K+ channels in adenosine A2A and A2B receptor-mediated hyperpolarization of porcine coronary artery endothelial cells. J Cardiovasc Pharmacol 40: 43–49, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Olanrewaju HA, Mustafa SJ. Adenosine A2A and A2B receptors mediated nitric oxide production in coronary artery endothelial cells. Gen Pharmacol 35: 171–177, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Olanrewaju HA, Qin W, Feoktistov I, Scemama JL, Mustafa SJ. Adenosine A2A and A2B receptors in cultured human and porcine coronary artery endothelial cells. Am J Physiol Heart Circ Physiol 279: H650–H656, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Rogers PA, Chilian WM, Bratz IN, Bryan RM, Jr, Dick GM. H2O2 activates redox- and 4-aminopyridine-sensitive Kv channels in coronary vascular smooth muscle. Am J Physiol Heart Circ Physiol 292: H1404–H1411, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Rubio R, Berne RM, Katori M. Release of adenosine in reactive hyperemia of the dog heart. Am J Physiol 216: 56–62, 1969 [DOI] [PubMed] [Google Scholar]
- 36. Saitoh S, Zhang C, Tune JD, Potter B, Kiyooka T, Rogers PA, Knudson JD, Dick GM, Swafford A, Chilian WM. Hydrogen peroxide: a feed-forward dilator that couples myocardial metabolism to coronary blood flow. Arterioscler Thromb Vasc Biol 26: 2614–2621, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Sato A, Terata K, Miura H, Toyama K, Loberiza FR, Jr, Hatoum OA, Saito T, Sakuma I, Gutterman DD. Mechanism of vasodilation to adenosine in coronary arterioles from patients with heart disease. Am J Physiol Heart Circ Physiol 288: H1633–H1640, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Scott JB, Daugherty RM, Jr, Dabney JM, Haddy FJ. Role of chemical factors in regulation of flow through kidney, hindlimb, and heart. Am J Physiol 208: 813–824, 1965 [DOI] [PubMed] [Google Scholar]
- 39. Sharifi Sanjani M, Teng B, Krahn T, Tilley SL, Ledent C, Mustafa SJ. Contributions of A2A and A2B adenosine receptors in coronary flow responses in relation to KATP channel using A2B and A2A/2B double knockout mice. Am J Physiol Heart Circ Physiol 301: H2322–H2333, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Talukder MA, Morrison RR, Ledent C, Mustafa SJ. Endogenous adenosine increases coronary flow by activation of both A2A and A2B receptors in mice. J Cardiovasc Pharmacol 41: 562–570, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Teng B, Ledent C, Mustafa SJ. Up-regulation of A2B adenosine receptor in A2A adenosine receptor knockout mouse coronary artery. J Mol Cell Cardiol 44: 905–914, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thengchaisri N, Kuo L. Hydrogen peroxide induces endothelium-dependent and -independent coronary arteriolar dilation: role of cyclooxygenase and potassium channels. Am J Physiol Heart Circ Physiol 285: H2255–H2263, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Wang J, Whitt SP, Rubin LJ, Huxley VH. Differential coronary microvascular exchange responses to adenosine: roles of receptor and microvessel subtypes. Microcirculation 12: 313–326, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wei EP, Kontos HA, Beckman JS. Mechanisms of cerebral vasodilation by superoxide, hydrogen peroxide, and peroxynitrite. Am J Physiol Heart Circ Physiol 271: H1262–H1266, 1996 [DOI] [PubMed] [Google Scholar]
- 45. Wingler K, Wunsch S, Kreutz R, Rothermund L, Paul M, Schmidt HH. Upregulation of the vascular NAD(P)H-oxidase isoforms Nox1 and Nox4 by the renin-angiotensin system in vitro and in vivo. Free Radic Biol Med 31: 1456–1464, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Wolin MS, Rodenburg JM, Messina EJ, Kaley G. Similarities in the pharmacological modulation of reactive hyperemia and vasodilation to hydrogen peroxide in rat skeletal muscle arterioles: effects of probes for endothelium-derived mediators. J Pharmacol Exp Ther 253: 508–512, 1990 [PubMed] [Google Scholar]
- 47. Yada T, Hiramatsu O, Kimura A, Tachibana H, Chiba Y, Lu S, Goto M, Ogasawara Y, Tsujioka K, Kajiya F. Direct in vivo observation of subendocardial arteriolar response during reactive hyperemia. Circ Res 77: 622–631, 1995 [DOI] [PubMed] [Google Scholar]
- 48. Yada T, Shimokawa H, Hiramatsu O, Shinozaki Y, Mori H, Goto M, Ogasawara Y, Kajiya F. Important role of endogenous hydrogen peroxide in pacing-induced metabolic coronary vasodilation in dogs in vivo. J Am Coll Cardiol 50: 1272–1278, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Yada T, Shimokawa H, Morikawa K, Takaki A, Shinozaki Y, Mori H, Goto M, Ogasawara Y, Kajiya F. Role of Cu,Zn-SOD in the synthesis of endogenous vasodilator hydrogen peroxide during reactive hyperemia in mouse mesenteric microcirculation in vivo. Am J Physiol Heart Circ Physiol 294: H441–H448, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Yang Y, Shi W, Chen X, Cui N, Konduru AS, Shi Y, Trower TC, Zhang S, Jiang C. Molecular basis and structural insight of vascular KATP channel gating by S-glutathionylation. J Biol Chem 286: 9298–9307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zatta AJ, Headrick JP. Mediators of coronary reactive hyperaemia in isolated mouse heart. Br J Pharmacol 144: 576–587, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang DX, Borbouse L, Gebremedhin D, Mendoza SA, Zinkevich NS, Li R, Gutterman DD. H2O2-induced dilation in human coronary arterioles: role of protein kinase G dimerization and large-conductance Ca2+-activated K+ channel activation. Circ Res 110: 471–480 [DOI] [PMC free article] [PubMed] [Google Scholar]