Abstract
Inflammatory bowel disease is associated with increased reactive oxygen species (ROS) and decreased antioxidant response in the intestinal mucosa. Expression of the mitochondrial protein prohibitin (PHB) is also decreased during intestinal inflammation. Our previous study showed that genetic restoration of colonic epithelial PHB expression [villin-PHB transgenic (PHB Tg) mice] attenuated dextran sodium sulfate (DSS)-induced colitis/oxidative stress and sustained expression of colonic nuclear factor erythroid 2-related factor 2 (Nrf2), a cytoprotective transcription factor. This study investigated the role of Nrf2 in mediating PHB-induced protection against colitis and expression of the antioxidant response element (ARE)-regulated antioxidant genes heme oxygenase-1 (HO-1) and NAD(P)H quinone oxidoreductase-1 (NQO-1). PHB-transfected Caco-2-BBE human intestinal epithelial cells maintained increased ARE activation and decreased intracellular ROS levels compared with control vector-transfected cells during Nrf2 knockdown by small interfering RNA. Treatment with the ERK inhibitor PD-98059 decreased PHB-induced ARE activation, suggesting that ERK constitutes a significant portion of PHB-mediated ARE activation in Caco-2-BBE cells. PHB Tg, Nrf2−/−, and PHB Tg/Nrf2−/− mice were treated with DSS or 2,4,6-trinitrobenzene sulfonic acid (TNBS), and inflammation and expression of HO-1 and NQO-1 were assessed. PHB Tg/Nrf2−/− mice mimicked PHB Tg mice, with attenuated DSS- or TNBS-induced colitis and induction of colonic HO-1 and NQO-1 expression, despite deletion of Nrf2. PHB Tg/Nrf2−/− mice exhibited increased activation of ERK during colitis. Our results suggest that maintaining expression of intestinal epithelial cell PHB, which is decreased during colitis, reduces the severity of inflammation and increases colonic levels of the antioxidants HO-1 and NQO-1 via a mechanism independent of Nrf2.
Keywords: activator protein-1, ERK, inflammatory bowel disease, heme oxygenase-1, NAD(P)H quinone oxidoreductase-1, Nrf2, oxidative stress, prohibitin
the two common, but disparate, forms of inflammatory bowel disease (IBD), Crohn's disease and ulcerative colitis, are associated with increased reactive oxygen species (ROS) and decreased antioxidant enzymes in the intestinal mucosa (2, 34, 38, 41, 47). Exogenous ROS and the proinflammatory cytokine TNFα, both of which are increased during IBD, promote cellular injury via mitochondrial ROS production (12, 13, 44). Damaged mitochondria are a key source of increased intracellular ROS from respiratory chain dysfunction, disturbing cellular homeostasis, which can result in cell death (9).
Prohibitin 1 (PHB) belongs to a family of proteins that share an evolutionarily conserved stomatin/PHB/flotillin/HflK/C domain and serves diverse roles in cell function. PHB has been shown to regulate cell cycle progression, apoptosis, and transcription factor activity in multiple cell types (37, 54, 55). PHB is highly expressed in intestinal epithelial cells, with predominant subcellular localization to the mitochondria (49). Expression of PHB is decreased in mucosal biopsies from ulcerative colitis- and Crohn's disease-afflicted patients and in animal models of colitis (16, 49). TNFα decreases expression of intestinal epithelial PHB in vivo and in vitro (50). Restoration of colonic epithelial PHB expression by genetic manipulation [villin-PHB transgenic (Tg) mice] or therapeutic delivery to the colon via nanoparticle or adenovirus protects mice from experimental colitis and reduces oxidative stress (51, 52). These findings are in agreement with emerging data that suggest a role of PHB in alleviating oxidative stress in multiple cell types (14, 29, 35, 45, 53). Gene silencing of PHB in cultured intestinal epithelial cells induces mitochondrial membrane depolarization, increases intracellular ROS and mitophagy, and reduces cell viability (20), suggesting that PHB is involved in epithelial cell homeostasis.
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a basic leucine zipper, redox-sensitive transcription factor that plays a role in cellular defense against oxidative and electrophilic stress through the induction of antioxidant and phase II detoxification enzymes. These cytoprotective genes are regulated through an antioxidant response element (ARE) in their promoter region to which Nrf2 binds, thereby activating transcription (19). ARE-regulated genes include NAD(P)H quinone oxidoreductase-1 (NQO-1), heme oxygenase-1 (HO-1), peroxiredoxin 1, γ-glutamylcysteine ligase, glutathione peroxidase, and glutathione disulfide reductase (26). Nrf2 also attenuates early-phase tissue damage during inflammation in multiple organs through regulation of the innate immune response and repression of proinflammatory mediators (26). Nrf2 knockout mice show increased susceptibility to colitis and colitis-associated tumorigenesis (24, 25, 40). Our previous study showed that PHB-mediated protection from dextran sodium sulfate (DSS)-induced colitis in mice was associated with increased colonic Nrf2 expression and nuclear localization (52). This study investigates the role of Nrf2 in mediating PHB-induced protection against colitis and expression of the ARE-responsive antioxidant enzymes HO-1 and NQO-1.
METHODS AND MATERIALS
Cell culture.
The Caco-2-BBE human intestinal epithelial cell line (American Type Culture Collection, Manassas, VA) was used as an in vitro model of polarized intestinal epithelium. All cells were grown as a confluent monolayer in Dulbecco's modified Eagle's medium supplemented with 40 mg/l penicillin, 90 mg/l streptomycin, and 10% fetal calf serum. All experiments were performed on Caco-2-BBE cells between passages 30 and 40. Caco-2-BBE cells were plated onto permeable supports (0.4-μm pore size; Transwell-Clear polyester membranes, Costar Life Sciences, Acton, MA). Cells were transiently cotransfected with pEGFPN1 expression vector or pEGFPN1-PHB (20) and 20 μM Nrf2 small interfering RNA (siRNA) (Santa Cruz Biotechnology, Santa Cruz, CA) or 20 μM Stealth RNAi negative control medium GC (Invitrogen, Carlsbad, CA) by nucleofection (Amaxa transfection, cell line kit T; Lonza, Basel, Switzerland). At 48 h after transfection, cells were serum-deprived for 12 h and treated with 10 ng/ml recombinant human TNFα (R & D Systems, Minneapolis, MN) in the basolateral chamber. Subcellular fractions of nuclei and cytosol (which includes mitochondria) were isolated as previously described (50).
Animal models.
PHB Tg (C57BL/6) mice specifically overexpressing PHB in intestinal epithelial cells (52) and Nrf2−/− (C57BL/6) mice were crossed to generate PHB Tg/Nrf2−/− mice. Wild-type (WT), PHB Tg, Nrf2−/−, and PHB Tg/Nrf2−/− mice were 8 wk old at the beginning of the experimental protocol. Genotyping was performed using PCR on DNA extracted from the tail, as previously described (24, 52). All mice were group-housed in standard cages under a controlled temperature (25°C) and photoperiod (12:12-h light-dark cycle) and were allowed standard chow and tap water ad libitum. All experiments were approved by the Baylor Research Institute Institutional Animal Care and Use Committee.
Induction of colitis in mice.
DSS (50,000 mol wt; MP Biomedicals, Solon, OH) was administered orally at 2.5% (wt/vol) in tap water ad libitum for 7 days to age- and sex-matched male and female WT, PHB Tg, Nrf2−/−, and PHB Tg/Nrf2−/− mice. Normal tap water was administered to littermate controls of each genotype throughout the treatment period. Mean DSS water consumption, body weight, and clinical signs of inflammation were assessed daily during the treatment period.
As a second model of colitis, 2,4,6-trinitrobenzene sulfonic acid (TNBS; Sigma Aldrich, St. Louis, MO) dissolved in 50% ethanol was given by enema at 150 mg/kg body wt. Littermate controls of each genotype were given 50% ethanol by enema. Colonic inflammation was assessed 72 h after TNBS administration.
Clinical score assessment.
A clinical activity score was generated using body weight loss, stool consistency, and the presence of occult blood by a guaiac test (Hemoccult Sense, Beckman Coulter, Fullerton, CA), as described previously (52). The scores for each parameter were added to obtain a clinical activity score, with 12 being the maximal score.
Myeloperoxidase activity.
Myeloperoxidase (MPO) activity was measured as a marker of neutrophil infiltration. A portion of the colon was homogenized 1:20 (wt/vol) in 50 mmol/l phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide, sonicated for 10 s, subjected to three freeze-thaw cycles, and centrifuged at 14,000 rpm for 15 min. Supernatant was added to 1 mg/ml of o-dianisidine hydrochloride and 5 × 10−4% hydrogen peroxide, and the change in absorbance was measured at 460 nm.
Western blot analysis.
Proteins were extracted as described previously (52), separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and electrotransferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). Membranes were probed with antibodies at 4°C overnight and subsequently incubated with corresponding horseradish peroxidase-conjugated secondary antibodies (Bio-Rad). Immunoreactive proteins were detected using Amersham ECL Plus reagent (GE Healthcare, Piscataway, NJ). Blots were reprobed with anti-β-actin (Sigma Aldrich) or anti-β-tubulin (Sigma Aldrich) antibodies. Antibodies were as follows: mouse monoclonal PHB antibody (Thermo Fisher, Fremont, CA), mouse monoclonal HO-1 (Abcam, Cambridge, MA), rabbit polyclonal histone H3 (Millipore, Billerica, MA), mouse monoclonal green fluorescent protein (GFP; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal Nrf2 (Santa Cruz Biotechnology), goat polyclonal NQO-1 (Santa Cruz Biotechnology), rabbit polyclonal c-Jun (Santa Cruz Biotechnology), goat polyclonal c-Fos (Santa Cruz Biotechnology), rabbit polyclonal ERK (Santa Cruz Biotechnology), and phosphorylated ERK (Cell Signaling, Danvers, MA).
Dual-luciferase reporter assay.
Caco-2-BBE cells were cotransfected using Amaxa nucleofection with 1.6 μg of ARE4-firefly luciferase reporter construct containing four tandem copies of an ARE sequence (36) and 5 ng of pRL-CMV Renilla luciferase construct (Promega) as an internal control. ARE4 binds activator protein-1 (AP-1) and Nrf2 (56). After 48 h, cells were treated with TNFα as described above and analyzed for firefly and Renilla luciferase activity using the Dual Luciferase Assay Kit (Promega) according to the manufacturer's protocol. For kinase inhibitor experiments, cells were incubated with the ERK1/2 inhibitor PD-08059 (20 μM; Cell Signaling), the JNK inhibitor SP-600125 (20 μM; Sigma Aldrich), or the p38 MAPK inhibitor SB-203580 (20 μM; Sigma Aldrich) for 16 h prior to TNFα treatment. Extracts were analyzed in triplicate across three independent experiments.
RNA isolation and quantitative RT-PCR analysis.
Total RNA was isolated from colon or Caco-2-BBE cells using the RNeasy kit (Qiagen, Valencia, CA). Two micrograms of reverse-transcribed cDNA (Optimaz First Strand cDNA Synthesis Kit, Biochain, Newark, CA) were amplified by quantitative real-time PCR (qRT-PCR) using 10 μM gene-specific primers and iQ SYBR Green Supermix (Bio-Rad). Expression level of 18S was used as an internal control (see Table 1 for primer sequences).
Table 1.
Primers for real-time PCR
| Primer | Oligonucleotide Sequence |
|---|---|
| HO-1 | |
| Sense | 5′-GTGATGGAGCGTCCACAGC-3′ |
| Antisense | 5′-TGGTGGCCTCCTTCAAGG-3′ |
| NQO-1 | |
| Sense | 5′-GGAAGCTGCAGACCTGGTGA-3′ |
| Antisense | 5′-CCTTTCAGAATGGCTGGCA-3′ |
| Human PHB | |
| Sense | 5′-GGGCACAGAGCTGTCATCTT-3′ |
| Antisense | 5′-TGACTGGCACATTACGTGGT-3′ |
| Mouse PHB | |
| Sense | 5′-GCATTGGCGAGGACTATGAT-3′ |
| Antisense | 5′-CTCTGTGAGGTCATCGCTCA-3′ |
| Nrf2 | |
| Sense | 5′-CTACTCGTGTGGGACAGCAA-3′ |
| Antisense | 5′-AGCAGACTCCAGGTCTTCCA-3′ |
| TNFα | |
| Sense | 5′-AGGCTGCCCCGACTACGT-3′ |
| Antisense | 5′-GACTTTCTCCTGGTATGAGATAGCAAA-3′ |
| IL-1β | |
| Sense | 5′-TCGCTCAGGGTCACAAGAAA-3′ |
| Antisense | 5′-CATCAGAGGCAAGGAGGAAAAC-3′ |
| 18S | |
| Sense | 5′-CCCCTCGATGCTCTTAGCTGAGTGT-3′ |
| Antisense | 5′-CGCCGGTCCAAGAATTTCACCTCT-3′ |
HO-1, heme oxygenase-1; NQO-1, NAD(P)H quinone oxidoreductase-1; PHB, prohibitin; Nrf2, nuclear factor erythroid 2-related factor 2.
2′,7′-Dichlorofluorescein assay.
As a measure of intracellular ROS generation, conversion of the nonionic, nonpolar 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; Invitrogen) to fluorescent 2′,7′-dichlorofluorescein (DCF) was measured. Caco-2-BBE cells stably overexpressing control vector or PHB were transfected with negative control siRNA or Nrf2 siRNA as described above and seeded in a 96-well plate. At 36 h after transfection, cells were serum-deprived for 12 h and treated with 10 ng/ml recombinant human TNFα for 8 h. Cells were then loaded with 10 μM H2DCFDA for 10 min and rinsed with 1× phosphate-buffered saline; after 10 min, fluorescence was quantitated using a plate reader following the manufacturer's protocol.
Statistical analysis.
Values are means ± SE. Statistical analysis was performed using two-way analysis of variance and subsequent pair-wise comparisons using Bonferroni's post hoc tests. P < 0.05 was considered statistically significant in all analyses.
RESULTS
Caco-2-BBE cells overexpressing PHB exhibit increased TNFα-induced Nrf2 nuclear localization, ARE activation, and HO-1 and NQO-1 expression.
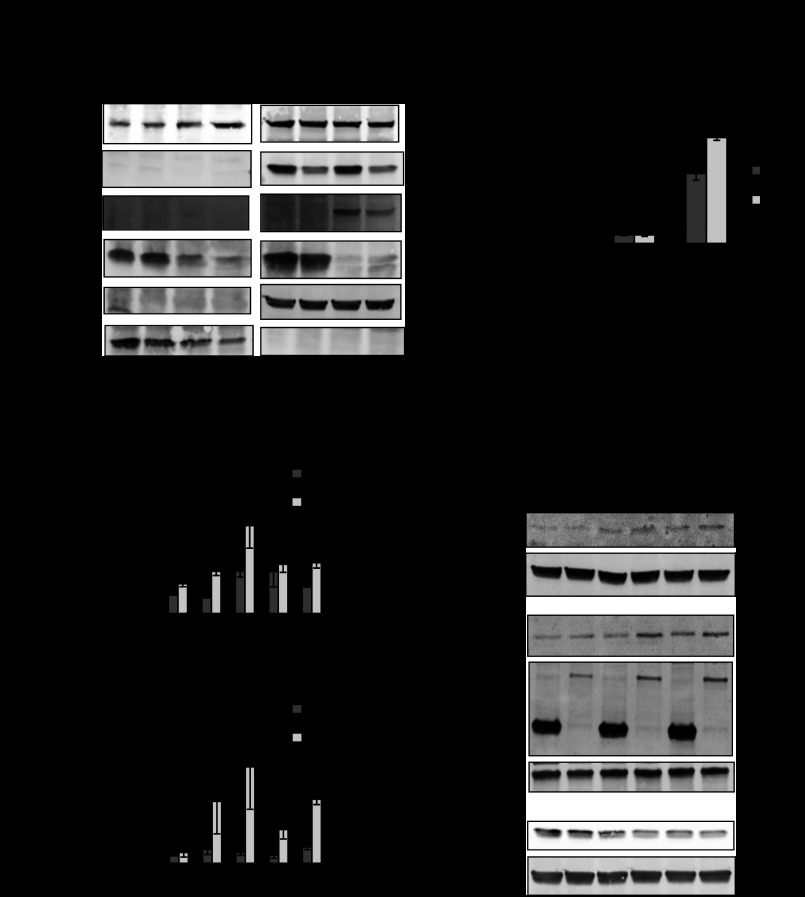
Our previous study suggested that PHB overexpression in intestinal epithelial cells protected against inflammation and oxidative stress in association with increased Nrf2 activation (52). It is widely accepted that TNFα is a central mediator of the proinflammatory response during intestinal inflammation (3). TNFα-induced ROS generation occurs primarily in the mitochondria, and signal transduction pathways that delineate with TNFα, including NF-κB activation and apoptosis, are reliant on mitochondrial ROS production (12, 13, 15). Furthermore, mitochondrial function and intracellular ROS levels are regulated by relative concentrations of PHB (20). To determine whether PHB overexpression induces Nrf2 during TNFα signaling, Caco-2-BBE cells were transfected with GFP-tagged PHB or GFP control vector and treated with TNFα. Protein expression of endogenous PHB, predominantly localized in the cytosolic fraction, is decreased by TNFα treatment (Fig. 1A). Caco-2-BBE cells overexpressing PHB show increased Nrf2 nuclear translocation 60 min after TNFα treatment compared with control vector-expressing cells. Blots were probed with an anti-GFP antibody to confirm expression of exogenous GFP-tagged PHB, which localized in the cytosol (Fig. 1A). PHB-overexpressing cells cotransfected with an ARE4-luciferase reporter construct exhibited more relative luciferase activity induced by TNFα than control vector-transfected cells (Fig. 1B). Although the mRNA (Fig. 1C) and protein (Fig. 1D) expression patterns of NQO-1 and HO-1 in vector-transfected cells are similar to those in PHB-overexpressing cells following TNFα treatment, the induction is significantly more robust and occurs more rapidly in cells overexpressing PHB. TNFα treatment decreases endogenous PHB protein expression to the same magnitude in control vector- and PHB-transfected cells (Fig. 1D). Blots were probed with an anti-GFP antibody to confirm expression of exogenous GFP-tagged PHB (Fig. 1D).
Fig. 1.
Caco-2-BBE cells overexpressing prohibitin (PHB) exhibit increased TNFα-induced nuclear factor erythroid 2-related factor 2 (Nrf2) nuclear localization, antioxidant response element (ARE) activation, and heme oxygenase-1 (HO-1) and NAD(P)H quinone oxidoreductase-1 (NQO-1) expression. A: Western blot of nuclear and cytosolic Nrf2 protein expression in Caco-2-BBE cells overexpressing pEGFPN1-PHB (PHB) or pEGFPN1-vector (V) and treated with 10 ng/ml TNFα for 60 min. Results are representative of 3 independent experiments. Histone H3 and β-actin were used as loading controls for nuclear and cytosolic extracts, respectively. B: relative ARE-driven luciferase activity in Caco-2-BBE cells. Values are means ± SE (n = 3 per treatment). ***P < 0.001. C: quantitative real-time (qRT)-PCR analysis of HO-1 and NQO-1 in total RNA isolated from Caco-2-BBE cells. Values are means ± SE (n = 3 per treatment). *P < 0.05 vs. vector. D: representative Western blots of HO-1, NQO-1, and endogenous PHB protein expression. Blots were subsequently probed with anti-green fluorescent protein (GFP) to ensure GFP-PHB expression.
PHB-induced ARE activation, NQO-1 and HO-1 protein expression, and decreased intracellular ROS levels during TNFα treatment are not exclusively mediated via Nrf2.
To assess the role of Nrf2 in PHB-induced antioxidant responses during proinflammatory signaling, Nrf2 expression in Caco-2-BBE cells was knocked down using siRNA. Basal ARE4-luciferase expression was unaffected by Nrf2 knockdown in vector- and PHB-overexpressing cells (Fig. 2A). Nrf2 knockdown caused a 71% reduction in TNFα-induced ARE4-luciferase activity in control vector-transfected cells (7.6 ± 0.5 and 2.0 ± 0.1 with TNFα and TNFα + Nrf2 siRNA, respectively, P < 0.01; Fig. 2A), suggesting that Nrf2 contributes to the majority of ARE activation during TNFα signaling. In PHB-overexpressing cells, Nrf2 knockdown caused only a 36% decrease in TNFα-induced ARE-luciferase activity (15.9 ± 1.7 and 10.1 ± 1.3 with TNFα and TNFα + Nrf2 siRNA, respectively, P < 0.05; Fig. 2A), suggesting that Nrf2 contributes to approximately one-third of PHB-induced ARE activation during PHB overexpression.
Fig. 2.
PHB-induced ARE activation, NQO-1 and HO-1 protein expression, and decreased intracellular reactive oxygen species (ROS) levels during TNFα treatment are not exclusively mediated via Nrf2. A: relative ARE-driven luciferase activity in Caco-2-BBE cells cotransfected with pEGFPN1-PHB (PHB) or pEGFPN1-vector (V) and Nrf2 small interfering RNA (siNrf2) or negative control small interfering RNA (siRNA, TNFα). Values are means ± SE (n = 6 per treatment). *P < 0.05, **P < 0.01. B: Western blot of NQO-1, HO-1, and endogenous PHB in cells transfected and treated as described in A. Duplicate blots were probed with anti-GFP or anti-Nrf2 to ensure GFP-PHB transfection efficiency and Nrf2 knockdown, respectively. β-Actin was used as a loading control. Blots are representative of 3 independent experiments. C: Western blot of Nrf2 protein expression in nuclear and cytosolic extracts. Histone H3 and β-actin were used as loading controls for nuclear or cytosolic extracts, respectively. Blots are representative of 3 independent experiments. D: 2′,7′-dichlorofluorescein (DCF) fluorescence in Caco-2-BBE cells. RFU, relative fluorescence units. Values are means ± SE (n = 8 per treatment). *P < 0.05, **P < 0.01.
Cells treated as described in Fig. 2A were collected for Western blotting and assayed for NQO-1, HO-1, and endogenous PHB protein expression (Fig. 2B). TNFα increased NQO-1 and HO-1 protein levels in vector- and PHB-transfected cells, but the magnitude was greater in cells overexpressing PHB, which is similar to results shown in Fig. 1D. Nrf2 knockdown did not affect TNFα-induced NQO-1 and HO-1 protein expression in PHB-transfected cells (Fig. 2B). Blots were probed for Nrf2 protein expression to ensure Nrf2 knockdown or GFP to confirm overexpression of GFP-PHB. Although Nrf2 knockdown did not affect endogenous PHB protein levels, TNFα treatment decreased endogenous PHB protein expression to the same magnitude in vector- and PHB-transfected cells, which is in agreement with endogenous PHB data in Fig. 1D. Nuclear and cytosolic extracts were also isolated from cells treated as described in Fig. 2B and assessed for Nrf2 protein expression by Western blotting. Nrf2 was predominantly localized in the cytosol, and protein levels were reduced during Nrf2 knockdown by siRNA (Fig. 2C). Translocation of Nrf2 to the nuclei was increased after TNFα treatment in vector- and PHB-overexpressing cells, but the translocation was greatest in cells overexpressing PHB. Nrf2 knockdown decreased TNFα-induced Nrf2 nuclear localization, regardless of PHB overexpression (Fig. 2C). Collectively, these results suggest that rescuing TNFα-mediated decreased endogenous PHB levels via forced overexpression increases HO-1 and NQO-1 protein expression via a non-Nrf2-dependent mechanism.
Intracellular ROS levels were significantly lower in cells overexpressing PHB as measured by DCF fluorescence (Fig. 2D). TNFα treatment increased intracellular ROS in control vector- and PHB-overexpressing cells, but levels were significantly lower in PHB-overexpressing than control vector-transfected cells. Basal ROS levels were unaffected by Nrf2 knockdown in vector- or PHB-transfected cells (Fig. 2D). TNFα increased ROS levels during Nrf2 knockdown in vector-transfected cells, but levels remained significantly lower in PHB-overexpressing cells, suggesting that PHB reduction in intracellular ROS is not mediated by Nrf2.
PHB Tg mice are less susceptible to DSS-induced colitis, regardless of Nrf2 knockout.
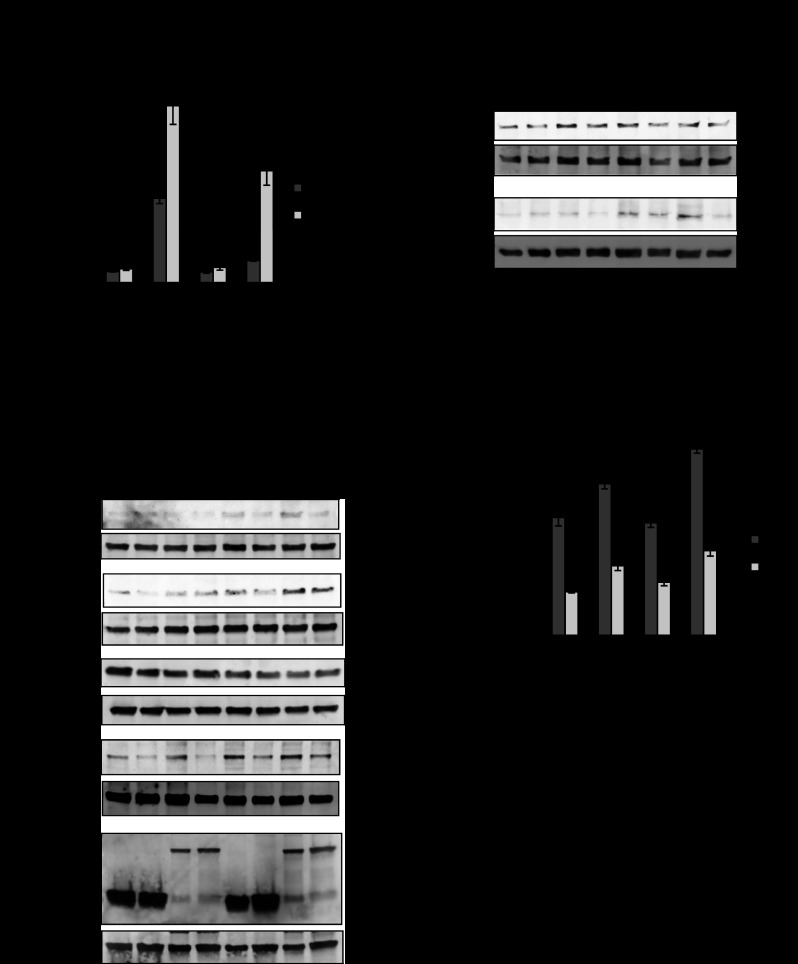
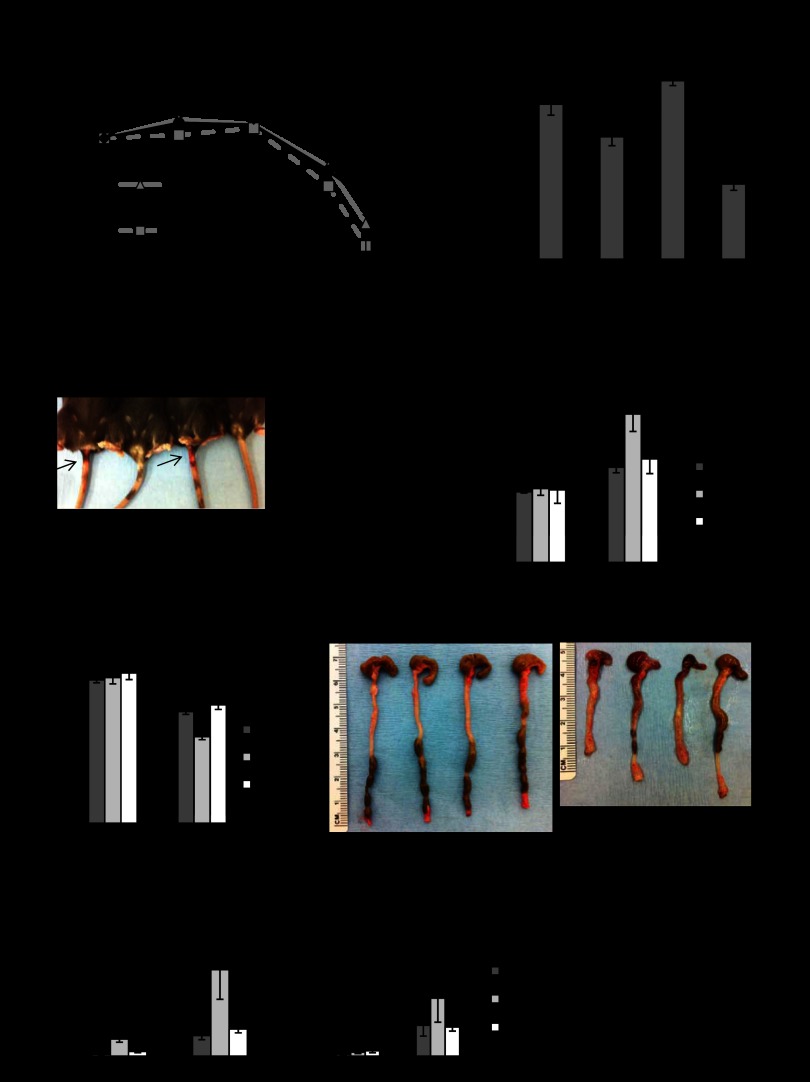
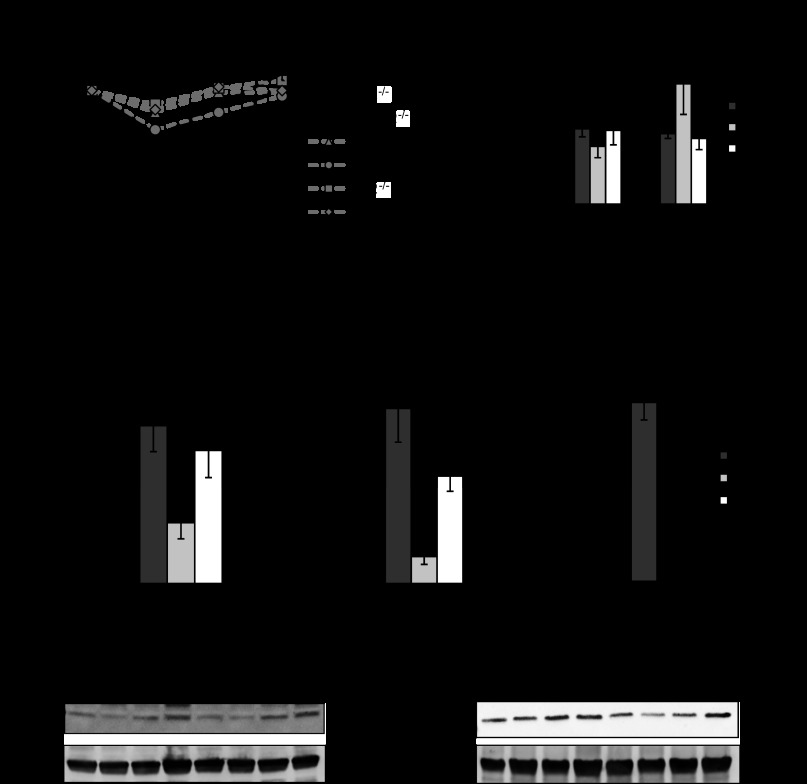
We previously generated PHB Tg mice that specifically overexpress PHB in intestinal epithelial cells (52). PHB Tg mice were less susceptible to DSS-induced colitis, which is associated with increased Nrf2 nuclear localization (52). To determine the role of Nrf2 in PHB-induced protection against colitis, PHB Tg mice were bred to Nrf2−/− mice to generate PHB-overexpressing Nrf2-deleted (PHB Tg/Nrf2−/−) mice. DSS-treated WT and Nrf2−/− mice showed significant weight loss starting on day 6 of DSS treatment (Fig. 3A). In contrast, PHB Tg and PHB Tg/Nrf2−/− mice lost less weight over the course of DSS treatment, with PHB Tg/Nrf2−/− mice maintaining their weight throughout the treatment period (Fig. 3A). The mice were assigned a clinical score consisting of severity of body weight loss, stool consistency, and presence of gross bleeding or blood in the stool on day 7 of DSS treatment before death. DSS-treated WT and Nrf2−/− mice showed a significantly higher clinical score than DSS-treated PHB Tg and PHB Tg/Nrf2−/− mice (Fig. 3B). Gross bleeding was evident only in WT and Nrf2−/− mice (Fig. 3C). All animals exhibited increased MPO activity, a marker of neutrophil infiltration, in the distal colon following DSS treatment compared with water-treated controls, but levels in DSS-treated PHB Tg and PHB Tg/Nrf2−/− mice were significantly less than in WT and Nrf2−/− mice (Fig. 3D). A reduction in colon length is a gross indicator of disease severity in the DSS model of colitis. All animals treated with DSS showed reduced colon length compared with water-treated controls; however, shrinkage was less severe in PHB Tg and PHB Tg/Nrf2−/− mice (Fig. 3E). mRNA expression of the proinflammatory cytokines IL-1β and TNFα was increased by DSS treatment across all groups of mice, but levels were significantly lower in PHB Tg and PHB Tg/Nrf2−/− than WT and Nrf2−/− mice. Collectively, these results suggest that epithelial PHB-modulated protection from colitis is not dependent on Nrf2 signaling.
Fig. 3.
Villin-PHB transgenic (PHB Tg) mice are less susceptible to dextran sodium sulfate (DSS)-induced colitis, regardless of Nrf2 knockout. Mice were treated with 2.5% DSS dissolved in water for 7 days. A: percent change in body weight. B: clinical score. C: gross bleeding as 1 parameter of clinical score was evident in wild-type (WT) and Nrf2−/− mice (arrows). D: neutrophil infiltration into the colon, quantified by myeloperoxidase (MPO) activity. E: colon length on day 7 of DSS treatment. Photos are representative colons from each genotype of mice. F: colonic cytokine mRNA levels quantified by qRT-PCR. Values are means ± SE (n = 15 per group). *P < 0.05, **P < 0.01.
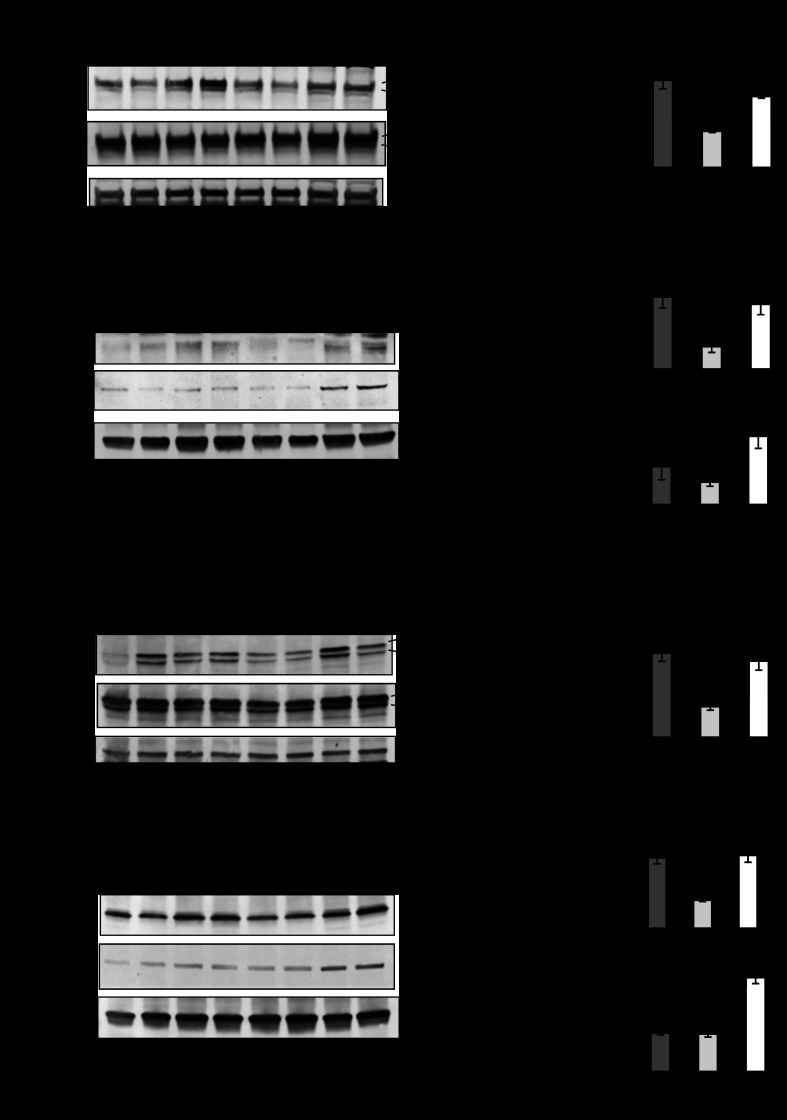
PHB Tg mice exhibit increased colonic HO-1 and NQO-1 expression during DSS-induced colitis, which is unaffected by Nrf2 deletion.
Since increased susceptibility to DSS-induced colitis in Nrf2−/− mice was previously shown to be associated with decreased expression of Nrf2-responsive genes, such as antioxidant/phase II detoxifying enzymes, including HO-1 and NQO-1 (24), we next assayed expression of HO-1 and NQO-1 in PHB Tg and PHB Tg/Nrf2−/− mice during DSS-induced colitis. PHB Tg and PHB Tg/Nrf2−/− mice exhibited increased colonic HO-1 and NQO-1 mRNA (Fig. 4A) and protein (Fig. 4, B–D) expression compared with WT and Nrf2−/− mice, suggesting that PHB protection from colitis is associated with increased expression of antioxidants but does not occur through Nrf2 signaling. Nrf2−/− mice exhibit decreased NQO-1 mRNA and protein expression compared with WT mice during DSS-induced colitis, whereas HO-1 mRNA remained unchanged, despite a significant decrease in HO-1 protein levels. Decreased HO-1 protein levels could be mediated by posttranslational modifications, leading to increased protein degradation (4). As shown previously (52), colonic mRNA expression of Nrf2 was increased in PHB Tg mice during DSS-induced colitis compared with WT mice; however, Nrf2 mRNA was undetectable in Nrf2−/− and PHB Tg/Nrf2−/− mice (Fig. 4A).
Fig. 4.
PHB Tg mice exhibit increased colonic HO-1 and NQO-1 expression during DSS colitis, which is unaffected by Nrf2 deletion. A: qRT-PCR analysis of HO-1, NQO-1, and Nrf2 in total RNA isolated from colon. Values are means ± SE (n = 8 per group). *P < 0.05, **P < 0.01. B and D: representative Western blots of colonic HO-1 and NQO-1 in DSS-treated mice. C and E: band densitometry for HO-1 and NQO-1 normalized to β-actin. Values are means ± SE (n = 6 per group). *P < 0.05, **P < 0.01.
PHB Tg mice are less susceptible to TNBS-induced colitis independently of Nrf2.
TNBS-induced colitis was used as a second well-known model of intestinal inflammation. On day 1 after administration of TNBS, all mice lost the same amount of body weight (Fig. 5A). PHB Tg and PHB Tg/Nrf2−/− mice recovered the lost weight by day 3 after TNBS administration, while WT and Nrf2−/− mice did not. Consistent with this observation, WT and Nrf2−/−, but not PHB Tg and PHB Tg/Nrf2−/−, mice exhibited increased MPO activity in the distal colon following TNBS treatment compared with vehicle controls (Fig. 5B). Increased expression of HO-1 and NQO-1 mRNA (Fig. 5C) and protein (Fig. 5D) was sustained in PHB Tg/Nrf2−/− mice during TNBS-induced colitis. These results corroborate our findings with the DSS model of colitis.
Fig. 5.
PHB Tg mice are less susceptible to 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis independently of Nrf2. A: percent change in body weight. B: neutrophil infiltration into the colon, quantified by MPO activity. C: qRT-PCR analysis of HO-1, NQO-1, and Nrf2 in total RNA isolated from colon. Values are means ± SE (n = 8 per group). *P < 0.05, **P < 0.01. D and E: representative Western blots of HO-1, NQO-1, and β-actin (loading control).
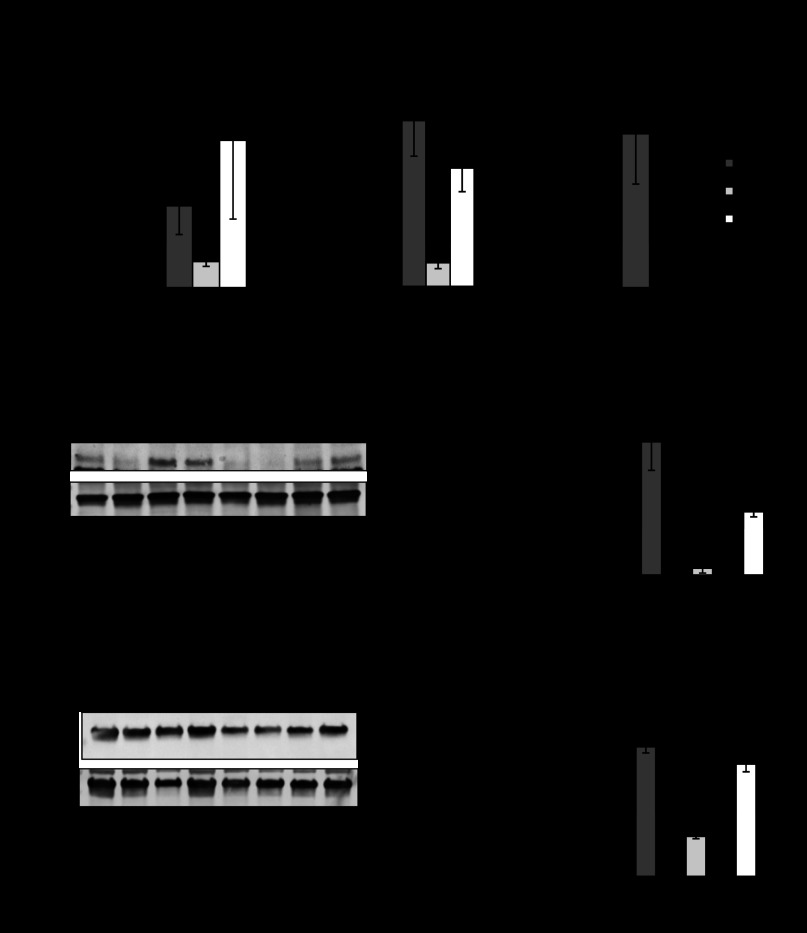
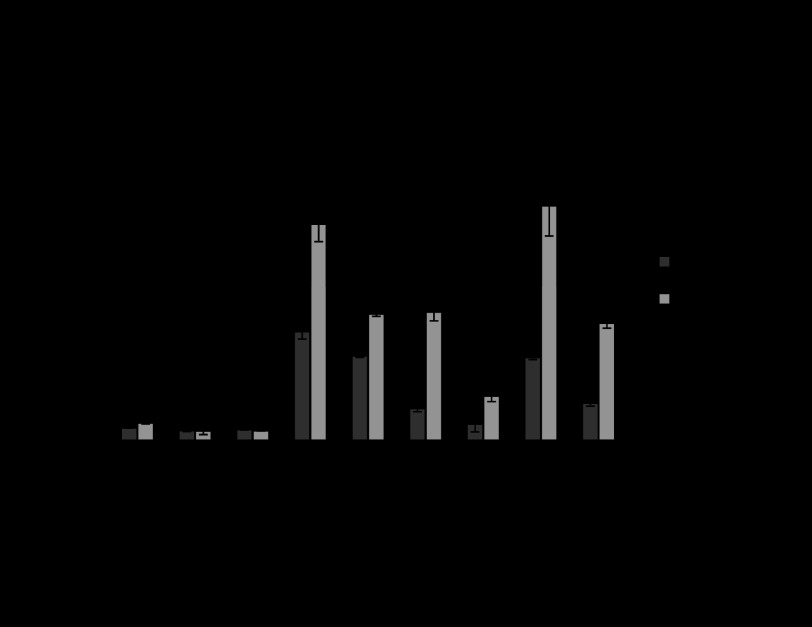
Colonic endogenous (mouse) and exogenous (human) PHB mRNA expression during DSS- and TNBS-induced colitis.
To determine colonic endogenous PHB or transgene expression, mouse PHB and human PHB mRNA expression, respectively, were assessed across all the genotypes of mice by qRT-PCR after DSS (Fig. 6A) or TNBS (Fig. 6B) treatment. There was no significant effect of Nrf2 knockout on endogenous colonic PHB mRNA expression. The induction of colitis by DSS or TNBS decreased endogenous PHB mRNA expression. These data are in agreement with our previous findings showing a decrease in endogenous PHB protein levels during colitis (49). Colonic PHB transgene expression is significantly increased in PHB Tg and PHB Tg/Nrf2−/− mice at baseline and during colitis.
Fig. 6.
Colonic endogenous (mouse) and exogenous (human) PHB mRNA expression during DSS- and TNBS-induced colitis. A and B: qRT-PCR assay of colonic endogenous PHB or transgene expression in mouse PHB and human PHB mRNA expression, respectively, across all genotypes of mice after DSS or TNBS treatment. Values are means ± SE (n = 5 per group). *P < 0.05, **P < 0.10, ***P < 0.005, ****P < 0.001 vs. WT H2O (for DSS experiment) or WT EtOH (for TNBS experiment).
PHB Tg/Nrf2−/− mice show increased ERK1/2 activation and AP-1 expression during colitis.
In addition to Nrf2, the transcription factor AP-1 binds to some ARE sites and activates transcription of antioxidant/phase II detoxifying enzymes, including HO-1 and NQO-1 (18, 33). Furthermore, ERK signaling has been identified as an upstream mediator involved in AP-1 activation (17). AP-1 is a dimeric transcription factor composed of c-Jun, c-Fos, or activating transcription factor subunits (28). Since increased expression of HO-1 and NQO-1 was sustained in PHB Tg mice, even with Nrf2 deletion, we next assessed activation of ERK, as well as protein levels of c-Fos and c-Jun. PHB Tg and PHB Tg/Nrf2−/− mice exhibited increased colonic phosphorylated ERK protein levels during colitis induced by DSS (Fig. 7A) and TNBS (Fig. 7C) compared with WT and Nrf2−/− mice. Colonic c-Jun protein levels were increased in PHB Tg and PHB Tg/Nrf2−/− mice treated with DSS (Fig. 7B) or TNBS (Fig. 7D), while c-Fos protein levels were increased predominantly in PHB Tg/Nrf2−/− mice during colitis.
Fig. 7.
PHB Tg/Nrf2−/− mice show increased ERK1/2 activation and activator protein 1 (AP-1) expression during colitis. A and C: colonic phosphorylated ERK1/2 (pERK1/2) expression quantified by Western blot in DSS- and TNBS-treated mice. B and D: representative Western blots of c-Jun and c-Fos protein expression in DSS- and TNBS-treated mice. Values are means ± SE (n = 6 per group). *P < 0.05.
ERK signaling contributes to PHB-induced ARE activation during TNFα treatment.
To assess the role of ERK in PHB-induced ARE activation during proinflammatory signaling and Nrf2 knockdown, ERK signaling was inhibited using PD-98059 in Caco-2-BBE cells. As shown in Fig. 8, addition of the ERK inhibitor PD-98059 in cells overexpressing PHB decreased TNFα-induced ARE4 activation by 41% (16.80 ± 3.3 and 9.83 ± 0.3 with TNFα and TNFα + PD-98059, respectively, P < 0.01) compared with only 21% in vector-transfected cells (8.46 ± 1.4 and 6.60 ± 0.2 with TNFα and TNFα + PD-98059, respectively, P < 0.05). Vector-transfected cells with Nrf2 knockdown showed a 70% reduction in TNFα-induced ARE4-luciferase expression (8.46 ± 1.4 and 2.50 ± 0.6 with TNFα and TNFα + Nrf2 siRNA, respectively, P < 0.01; Fig. 8), whereas PHB-overexpressing cells showed only a reduction of 41% (16.80 ± 3.3 and 9.98 ± 1.7 with TNFα and TNFα + Nrf2 siRNA, respectively, P < 0.01), which are similar to results shown in Fig. 2A. Addition of PD-98059 during Nrf2 knockdown decreased TNFα-induced ARE4 activation by an additional 38% compared with Nrf2 knockdown alone in cells overexpressing PHB (9.98 ± 1.7 and 3.47 ± 1.9 with TNFα + Nrf2 siRNA and TNFα + Nrf2 siRNA + PD-98059, respectively, P < 0.01) vs. an additional 15% in cells overexpressing control vector (2.50 ± 0.6 and 1.3 ± 0.5 with TNFα + Nrf2 siRNA and TNFα + Nrf2 siRNA + PD-98059, respectively, P < 0.05; Fig. 8). Addition of SP-600125 (a JNK inhibitor) or SB-203580 (a p38 MAP kinase inhibitor) did not further reduce ARE4-luciferase expression.
Fig. 8.
Contribution of ERK signaling to PHB-induced ARE activation during TNFα treatment shown as relative ARE4-driven luciferase activity in Caco-2-BBE cells. Values are means ± SE (n = 6 per treatment). *P < 0.05, **P < 0.01, ***P < 0.001.
DISCUSSION
In IBD, increased ROS levels have been demonstrated to play a pathophysiological role in tissue damage, barrier dysfunction, apoptosis, and wound healing (22, 39). In addition to excessive production of ROS, inadequate antioxidant responses in the mucosa of IBD patients are thought to contribute to the pathogenesis and progression of the inflammatory process (23, 30, 31). We and others have shown that the mitochondrial protein PHB is decreased in mucosal biopsies during active and inactive IBD and in animal models of colitis (16, 49). We show here that intestinal epithelial cell-specific PHB overexpression protects from DSS- and TNBS-induced colitis in association with upregulation of the antioxidants HO-1 and NQO-1 independent of Nrf2 signaling.
Under quiescent conditions, Kelch-like ECH-associating protein (Keap1) sequesters Nrf2 in the cytoplasm and promotes its constitutive ubiquitination and proteasomal degradation. During high levels of ROS generation, oxidative modification of cysteine residues on Keap1 and phosphorylation of serine or threonine residues on Nrf2 result in the release of Nrf2 from Keap1, thereby escaping proteasomal degradation, allowing accumulation and translocation into the nucleus and binding to cis-acting ARE sites in promoters of antioxidant and phase II detoxifying genes (19). Although data of Nrf2 expression in the mucosa of IBD patients during active inflammation are not readily available, multiple rodent models of colitis have shown decreased colonic Nrf2 expression following induction of inflammation that persists after inflammation has subsided (7, 52, 57). Nrf2−/− mice show increased susceptibility to intestinal, liver, lung, kidney, and brain inflammation (8, 24, 25, 40). Loss of Nrf2 allows oxidative stress to persist, since many antioxidant systems are not readily upregulated. In addition to HO-1 and NQO-1, deletion of Nrf2 diminishes upregulation of MnSOD, catalase, glutathione S-transferases, and γ-glutamylcysteine synthetase regulatory subunit, all of which are involved in the cellular response to oxidative/xenobiotic stress (32). Therefore, Nrf2 induces expression of antioxidant enzyme systems, many of which increase levels of glutathione synthesis and regeneration and stimulate NADPH synthesis. Contrary to our expectation, this study showed that PHB Tg/Nrf2−/− mice mimicked PHB Tg mice in response to DSS or TNBS treatment, with attenuated severity of colitis and induction of colonic HO-1 and NQO-1 expression, despite deletion of Nrf2. Therefore, Nrf2 is not required for epithelial PHB-dependent attenuation of experimental colitis.
TNFα decreases expression of intestinal epithelial PHB in vivo and in vitro (50). We show here that Caco-2-BBE cells overexpressing PHB exhibit increased TNFα-induced Nrf2 nuclear localization, ARE4 activation, and HO-1 and NQO-1 expression compared with control vector-transfected cells. Using Nrf2 knockdown by siRNA, we show that rescuing TNFα-mediated-decreased endogenous PHB levels via forced overexpression increases HO-1 and NQO-1 protein expression and ARE4 activation via a mechanism not dependent on Nrf2. TNFα stimulates intracellular ROS predominantly generated from the mitochondria, which can initiate signaling cascades modulating cell survival and apoptosis (12, 13, 15). Interestingly, basal intracellular ROS levels were significantly decreased in cells overexpressing PHB (Fig. 2D), despite no concurrent increase in ARE4-luciferase (Figs. 1B and 2A), suggesting that basal reduction of intracellular oxidative stress by PHB is not through induction of ARE-responsive genes. Since it has been shown that PHB interacts with and regulates complex I and subunits of cytochrome c oxidase of the respiratory chain (45, 53), it is possible that decreased ROS levels during PHB overexpression could be due to less oxidant leak from mitochondria during normal mitochondrial respiratory function.
In vitro studies were used to assess the role of PHB-mediated ERK signaling in ARE4 activation in Caco-2-BBE cells with Nrf2 knockdown by siRNA to mimic our in vivo model. The majority (70%) of ARE4 activation in control cells is mediated by Nrf2, with a smaller portion (20%) contributed by ERK. PHB overexpression resulted in an approximately twofold higher TNFα-induced ARE4-luciferase expression than in vector control cells. ERK inhibition in combination with Nrf2 knockdown almost completely inhibited PHB-induced ARE4 activation during TNFα treatment, with each pathway contributing ∼40% of ARE4 activation, suggesting that these pathways act separately in an additive manner. TNFα treatment downregulated PHB expression and upregulated Nrf2, in which case the majority (70%) of ARE4 activation is Nrf2-dependent, with only a fraction (20%) dependent on PHB. Exogenous expression of PHB negated the downregulation of PHB by TNFα and further activated ARE4 beyond that which is Nrf2-dependent through the ERK signaling pathway. These results, coupled with our in vivo results showing that PHB Tg and PHB Tg/Nrf2−/− mice exhibited increased colonic ERK activation during DSS- or TNBS-induced colitis compared with WT and Nrf2−/− mice, suggest that PHB overexpression induces activation of ERK, which has been shown to contribute to ARE activation through activation of AP-1 (18, 27, 33). Similar to Nrf2, transcriptional activity mediated by AP-1 can be regulated by a redox mechanism. Reduction of a conserved cysteine residue in the DNA-binding domains of c-Fos and c-Jun is required for AP-1/DNA-binding activity (1). Previous studies have indicated plasma membrane-associated PHB in modulating ERK activation (42, 43, 46, 58). PHB is thought to interact with CRAF kinase in the plasma membrane, thereby stimulating phosphorylation of MEK1/2, which in turn phosphorylates ERK1/2, leading to cell proliferation. Recently, ERK and its upstream kinase MEK1/2 have been shown to be present in the mitochondria and shuttle to the cytosol or nuclei (10). Given the mitochondrial localization of PHB in our studies, PHB could activate ERK in the mitochondria, leading to downstream signaling beyond the organelle.
Nrf2 signaling is considered a double-edged sword in regard to single-cell protection from oxidative and electrophilic stresses vs. cancer cell survival. It is thought that Nrf2 suppresses cancer initiation through elimination of ROS and protection of DNA from oxidative damage. Many potential therapeutic compounds, such as pterostilbene, resveratol, and peracetylated (−)-epigallocatechin-3-gallate, that possess anti-inflammatory, antioxidant, and/or anticarcinogenic properties induce Nrf2 as their mechanism of action (5, 6). However, after initiation of cancer, dysregulation of the Nrf2/Keap1 pathway promotes tumorigenicity and chemoresistance through upregulation of detoxification pathways in cancer cells (48). The link between chronic inflammation and increased risk of cancer in multiple organs is well established. Patients with IBD are at an increased relative risk of developing colorectal cancer compared with the general public (11). A valid concern regarding PHB overexpression is the potential to increase tumorigenicity due to upregulation of detoxification pathways, rendering cells resistant to death. However, our recent study showed that PHB Tg mice are protected from colitis-associated tumorigenesis induced by azoxymethane-DSS treatment (21), suggesting that PHB overexpression suppresses initiation of inflammation-induced cancer.
Collectively, our results suggest that maintaining expression of intestinal epithelial cell PHB, which is decreased during colitis, reduces the severity of inflammation and increases colonic levels of the antioxidants HO-1 and NQO-1, regardless of Nrf2 deletion. PHB utilizes the ERK pathway to increase ARE activation, and during situations of Nrf2 loss, significant ARE induction remains if PHB is exogenously expressed. Loss of PHB expression during intestinal inflammation may contribute to decreased mucosal antioxidant expression, perpetuating tissue damage.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant K01-DK-085222 (A. L. Theiss) and funds from the Baylor Research Institute (A. L. Theiss).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.S.K., M.A.B., and A.L.T. performed the experiments; A.S.K. and A.L.T. analyzed the data; A.S.K. and A.L.T. prepared the figures; A.S.K. and A.L.T. drafted the manuscript; M.A.B., J.M.H., and A.L.T. edited and revised the manuscript; M.A.B., J.M.H., and A.L.T. approved the final version of the manuscript; J.M.H. and A.L.T. interpreted the results of the experiments; A.L.T. is responsible for conception and design of the research.
ACKNOWLEDGMENTS
The authors thank Dr. Shanthi V. Sitaraman, who was a brilliant scientist and mentor, for dedication to this project. The authors are grateful to Dr. Rhonda F. Souza (Veterans Affairs North Texas Health Care System, University of Texas Southwestern Medical Center, Dallas, TX) for helpful scientific discussions.
REFERENCES
- 1. Abate C, Patel L, Rauscher FJ, 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science 249: 1157–1161, 1990 [DOI] [PubMed] [Google Scholar]
- 2. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med 361: 2066–2078, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bantel H, Schulze-Osthoff K. TNF antagonists in IBD: novel anti-inflammatory mechanisms beyond cytokine inhibition. Inflamm Bowel Dis 19: E51–E52, 2013 [DOI] [PubMed] [Google Scholar]
- 4. Barone E, Di Domenico F, Sultana R, Coccia R, Mancuso C, Perluigi M, Butterfield DA. Heme oxygenase-1 posttranslational modifications in the brain of subjects with Alzheimer disease and mild cognitive impairment. Free Radic Biol Med 52: 2292–2301, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiou YS, Ma NJ, Sang S, Ho CT, Wang YJ, Pan MH. Peracetylated (−)-epigallocatechin-3-gallate (AcEGCG) potently suppresses dextran sulfate sodium-induced colitis and colon tumorigenesis in mice. J Agric Food Chem 60: 3441–3451, 2012 [DOI] [PubMed] [Google Scholar]
- 6. Chiou YS, Tsai ML, Nagabhushanam K, Wang YJ, Wu CH, Ho CT, Pan MH. Pterostilbene is more potent than resveratrol in preventing azoxymethane (AOM)-induced colon tumorigenesis via activation of the NF-E2-related factor 2 (Nrf2)-mediated antioxidant signaling pathway. J Agric Food Chem 59: 2725–2733, 2011 [DOI] [PubMed] [Google Scholar]
- 7. Choi K, Chen J, Mitra S, Sarna SK. Impaired integrity of DNA after recovery from inflammation causes persistent dysfunction of colonic smooth muscle. Gastroenterology 141: 1293–1301, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Copple IM, Goldring CE, Kitteringham NR, Park BK. The Nrf2-Keap1 defence pathway: role in protection against drug-induced toxicity. Toxicology 246: 24–33, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Du G, Mouithys-Mickalad A, Sluse FE. Generation of superoxide anion by mitochondria and impairment of their functions during anoxia and reoxygenation in vitro. Free Radic Biol Med 25: 1066–1074, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Galli S, Antico Arciuch VG, Poderoso C, Converso DP, Zhou Q, Bal de Kier Joffe E, Cadenas E, Boczkowski J, Carreras MC, Poderoso JJ. Tumor cell phenotype is sustained by selective MAPK oxidation in mitochondria. PLos One 3: e2379, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gillen CD, Walmsley RS, Prior P, Andrews HA, Allan RN. Ulcerative colitis and Crohn's disease: a comparison of the colorectal cancer risk in extensive colitis. Gut 35: 1590–1592, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goossens V, Grooten J, De Vos K, Fiers W. Direct evidence for tumor necrosis factor-induced mitochondrial reactive oxygen intermediates and their involvement in cytotoxicity. Proc Natl Acad Sci USA 92: 8115–8119, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hansen JM, Zhang H, Jones DP. Mitochondrial thioredoxin-2 has a key role in determining tumor necrosis factor-α-induced reactive oxygen species generation, NF-κB activation, and apoptosis. Toxicol Sci 91: 643–650, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Henschke P, Vorum H, Honore B, Rice GE. Protein profiling the effects of in vitro hyperoxic exposure on fetal rabbit lung. Proteomics 6: 1957–1962, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Higuchi M, Proske RJ, Yeh ET. Inhibition of mitochondrial respiratory chain complex I by TNF results in cytochrome c release, membrane permeability transition, and apoptosis. Oncogene 17: 2515–2524, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Hsieh SY, Shih TC, Yeh CY, Lin CJ, Chou YY, Lee YS. Comparative proteomic studies on the pathogenesis of human ulcerative colitis. Proteomics 6: 5322–5331, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Iles KE, Dickinson DA, Wigley AF, Welty NE, Blank V, Forman HJ. HNE increases HO-1 through activation of the ERK pathway in pulmonary epithelial cells. Free Radic Biol Med 39: 355–364, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Inamdar NM, Ahn YI, Alam J. The heme-responsive element of the mouse heme oxygenase-1 gene is an extended AP-1 binding site that resembles the recognition sequences for MAF and NF-E2 transcription factors. Biochem Biophys Res Commun 221: 570–576, 1996 [DOI] [PubMed] [Google Scholar]
- 19. Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236: 313–322, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Kathiria AS, Butcher LD, Feagins LA, Souza RF, Boland CR, Theiss AL. Prohibitin 1 modulates mitochondrial stress-related autophagy in human colonic epithelial cells. PLos One 7: e31231, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kathiria AS, Neumann WL, Rhees J, Hotchkiss E, Cheng Y, Genta RM, Meltzer SJ, Souza RF, Theiss AL. Prohibitin attenuates colitis-associated tumorigenesis in mice by modulating p53 and STAT3 apoptotic responses. Cancer Res 72: 5778–5779, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keshavarzian A, Morgan G, Sedghi S, Gordon JH, Doria M. Role of reactive oxygen metabolites in experimental colitis. Gut 31: 786–790, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Keshavarzian A, Sedghi S, Kanofsky J, List T, Robinson C, Ibrahim C, Winship D. Excessive production of reactive oxygen metabolites by inflamed colon: analysis by chemiluminescence probe. Gastroenterology 103: 177–185, 1992 [DOI] [PubMed] [Google Scholar]
- 24. Khor TO, Huang MT, Kwon KH, Chan JY, Reddy BS, Kong AN. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res 66: 11580–11584, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Khor TO, Huang MT, Prawan A, Liu Y, Hao X, Yu S, Cheung WK, Chan JY, Reddy BS, Yang CS, Kong AN. Increased susceptibility of Nrf2 knockout mice to colitis-associated colorectal cancer. Cancer Prev Res (Phila) 1: 187–191, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim J, Cha YN, Surh YJ. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res 690: 12–23, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Kim KC, Kang KA, Zhang R, Piao MJ, Kim GY, Kang MY, Lee SJ, Lee NH, Surh YJ, Hyun JW. Up-regulation of Nrf2-mediated heme oxygenase-1 expression by eckol, a phlorotannin compound, through activation of Erk and PI3K/Akt. Int J Biochem Cell Biol 42: 297–305, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Klaunig JE, Kamendulis LM, Hocevar BA. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol 38: 96–109, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Ko KS, Tomasi ML, Iglesias-Ara A, French BA, French SW, Ramani K, Lozano JJ, Oh P, He L, Stiles BL, Li TW, Yang H, Martinez-Chantar ML, Mato JM, Lu SC. Liver-specific deletion of prohibitin 1 results in spontaneous liver injury, fibrosis, and hepatocellular carcinoma in mice. Hepatology 52: 2096–2108, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kruidenier L, Kuiper I, Lamers CB, Verspaget HW. Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants. J Pathol 201: 28–36, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Kruidenier L, Kuiper I, Van Duijn W, Mieremet-Ooms MA, van Hogezand RA, Lamers CB, Verspaget HW. Imbalanced secondary mucosal antioxidant response in inflammatory bowel disease. J Pathol 201: 17–27, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Kwak MK, Itoh K, Yamamoto M, Sutter TR, Kensler TW. Role of transcription factor Nrf2 in the induction of hepatic phase 2 and antioxidative enzymes in vivo by the cancer chemoprotective agent, 3H-1,2-dimethiole-3-thione. Mol Med 7: 135–145, 2001 [PMC free article] [PubMed] [Google Scholar]
- 33. Li Y, Jaiswal AK. Regulation of human NAD(P)H:quinone oxidoreductase gene. Role of AP1 binding site contained within human antioxidant response element. J Biol Chem 267: 15097–15104, 1992 [PubMed] [Google Scholar]
- 34. Lih-Brody L, Powell SR, Collier KP, Reddy GM, Cerchia R, Kahn E, Weissman GS, Katz S, Floyd RA, McKinley MJ, Fisher SE, Mullin GE. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig Dis Sci 41: 2078–2086, 1996 [DOI] [PubMed] [Google Scholar]
- 35. Liu X, Ren Z, Zhan R, Wang X, Wang X, Zhang Z, Leng X, Yang Z, Qian L. Prohibitin protects against oxidative stress-induced cell injury in cultured neonatal cardiomyocyte. Cell Stress Chaperones 14: 311–319, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lu ML, Schneider MC, Zheng Y, Zhang X, Richie JP. Caveolin-1 interacts with androgen receptor. A positive modulator of androgen receptor mediated transactivation. J Biol Chem 276: 13442–13451, 2001 [DOI] [PubMed] [Google Scholar]
- 37. McClung JK, Jupe ER, Liu XT, Dell'Orco RT. Prohibitin: potential role in senescence, development, and tumor suppression. Exp Gerontol 30: 99–124, 1995 [DOI] [PubMed] [Google Scholar]
- 38. McKenzie SJ, Baker MS, Buffinton GD, Doe WF. Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J Clin Invest 98: 136–141, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Musch MW, Walsh-Reitz MM, Chang EB. Roles of ZO-1, occludin, and actin in oxidant-induced barrier disruption. Am J Physiol Gastrointest Liver Physiol 290: G222–G231, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Osburn WO, Karim B, Dolan PM, Liu G, Yamamoto M, Huso DL, Kensler TW. Increased colonic inflammatory injury and formation of aberrant crypt foci in Nrf2-deficient mice upon dextran sulfate treatment. Int J Cancer 121: 1883–1891, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Rachmilewitz D, Stamler JS, Bachwich D, Karmeli F, Ackerman Z, Podolsky DK. Enhanced colonic nitric oxide generation and nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Gut 36: 718–723, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rajalingam K, Rudel T. Ras-Raf signaling needs prohibitin. Cell Cycle 4: 1503–1505, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Rajalingam K, Wunder C, Brinkmann V, Churin Y, Hekman M, Sievers C, Rapp UR, Rudel T. Prohibitin is required for Ras-induced Raf-MEK-ERK activation and epithelial cell migration. Nat Cell Biol 7: 837–843, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci 36: 30–38, 2011 [DOI] [PubMed] [Google Scholar]
- 45. Schleicher M, Shepherd BR, Suarez Y, Fernandez-Hernando C, Yu J, Pan Y, Acevedo LM, Shadel GS, Sessa WC. Prohibitin-1 maintains the angiogenic capacity of endothelial cells by regulating mitochondrial function and senescence. J Cell Biol 180: 101–112, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sharma A, Qadri A. Vi polysaccharide of Salmonella typhi targets the prohibitin family of molecules in intestinal epithelial cells and suppresses early inflammatory responses. Proc Natl Acad Sci USA 101: 17492–17497, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shiratora Y, Aoki S, Takada H, Kiriyama H, Ohto K, Hai K, Teraoka H, Matano S, Matsumoto K, Kamii K. Oxygen-derived free radical generating capacity of polymorphonuclear cells in patients with ulcerative colitis. Digestion 44: 163–171, 1989 [DOI] [PubMed] [Google Scholar]
- 48. Singh A, Boldin-Adamsky S, Thimmulappa RK, Rath SK, Ashush H, Coulter J, Blackford A, Goodman SN, Bunz F, Watson WH, Gabrielson E, Feinstein E, Biswal S. RNAi-mediated silencing of nuclear factor erythroid-2-related factor 2 gene expression in non-small cell lung cancer inhibits tumor growth and increases efficacy of chemotherapy. Cancer Res 68: 7975–7984, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Theiss AL, Idell RD, Srinivasan S, Klapproth JM, Jones DP, Merlin D, Sitaraman SV. Prohibitin protects against oxidative stress in intestinal epithelial cells. FASEB J 21: 197–206, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Theiss AL, Jenkins AK, Okoro NI, Klapproth JM, Merlin D, Sitaraman SV. Prohibitin inhibits tumor necrosis factor α-induced nuclear factor-κB nuclear translocation via the novel mechanism of decreasing importin-α3 expression. Mol Biol Cell 20: 4412–4423, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Theiss AL, Laroui H, Obertone TS, Chowdhury I, Thompson WE, Merlin D, Sitaraman SV. Nanoparticle-based therapeutic delivery of prohibitin to the colonic epithelial cells ameliorates acute murine colitis. Inflamm Bowel Dis 17: 1163–1176, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Theiss AL, Vijay-Kumar M, Obertone TS, Jones DP, Hansen JM, Gewirtz AT, Merlin D, Sitaraman SV. Prohibitin is a novel regulator of antioxidant response that attenuates colonic inflammation in mice. Gastroenterology 137: 199–208, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tsutsumi T, Matsuda M, Aizaki H, Moriya K, Miyoshi H, Fujie H, Shintani Y, Yotsuyanagi H, Miyamura T, Suzuki T, Koike K. Proteomics analysis of mitochondrial proteins reveals overexpression of a mitochondrial protein chaperon, prohibitin, in cells expressing hepatitis C virus core protein. Hepatology 50: 378–386, 2009 [DOI] [PubMed] [Google Scholar]
- 54. Wang S, Fusaro G, Padmanabhan J, Chellappan SP. Prohibitin co-localizes with Rb in the nucleus and recruits N-CoR and HDAC1 for transcriptional repression. Oncogene 21: 8388–8396, 2002 [DOI] [PubMed] [Google Scholar]
- 55. Wang S, Zhang B, Faller DV. Prohibitin requires Brg-1 and Brm for the repression of E2F and cell growth. EMBO J 21: 3019–3028, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wild AC, Gipp JJ, Mulcahy T. Overlapping antioxidant response element and PMA response element sequences mediate basal and β-naphthoflavone-induced expression of the human γ-glutamylcysteine synthetase catalytic subunit gene. Biochem J 332: 373–381, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yalniz M, Demirel U, Orhan C, Bahcecioglu IH, Ozercan IH, Aygun C, Tuzcu M, Sahin K. Nadroparin sodium activates Nrf2/HO-1 pathway in acetic acid-induced colitis in rats. Inflammation 35: 1213–1221, 2012 [DOI] [PubMed] [Google Scholar]
- 58. Yurugi H, Tanida S, Ishida A, Akita K, Toda M, Inoue M, Nakada H. Expression of prohibitins on the surface of activated T cells. Biochem Biophys Res Commun 420: 275–280, 2012 [DOI] [PubMed] [Google Scholar]