Abstract
Serotonin [5-hydroxytryptamine (5-HT)] is released from enterochromaffin cells in the mucosa of the small intestine. We tested a hypothesis that elevation of 5-HT in the environment of enteric mast cells might degranulate the mast cells and release mediators that become paracrine signals to the enteric nervous system, spinal afferents, and secretory glands. Western blotting, immunofluorescence, ELISA, and pharmacological analysis were used to study expression of 5-HT receptors by mast cells in the small intestine and action of 5-HT to degranulate the mast cells and release histamine in guinea pig small intestine and segments of human jejunum discarded during Roux-en-Y gastric bypass surgeries. Mast cells in human and guinea pig preparations expressed the 5-HT1A receptor. ELISA detected spontaneous release of histamine in guinea pig and human preparations. The selective 5-HT1A receptor agonist 8-hydroxy-PIPAT evoked release of histamine. A selective 5-HT1A receptor antagonist, WAY-100135, suppressed stimulation of histamine release by 5-HT or 8-hydroxy-PIPAT. Mast cell-stabilizing drugs, doxantrazole and cromolyn sodium, suppressed the release of histamine evoked by 5-HT or 8-hydroxy-PIPAT in guinea pig and human preparations. Our results support the hypothesis that serotonergic degranulation of enteric mast cells and release of preformed mediators, including histamine, are mediated by the 5-HT1A serotonergic receptor. Association of 5-HT with the pathophysiology of functional gastrointestinal disorders (e.g., irritable bowel syndrome) underlies a question of whether selective 5-HT1A receptor antagonists might have therapeutic application in disorders of this nature.
Keywords: enteric mast cells, histamine, enteric nervous system, functional gastrointestinal disorders, irritable bowel syndrome
mast cells are present in continuously varying numbers in the intestinal mucosa, lamina propria, and smooth muscle coats. Mast cell numbers expand in parasitic nematode infections and are major factors associated with inflammation-related changes in Clostridium difficile and other bacterial infections (5, 52). Increased numbers of enteric mast cells are associated with inflammatory responses in general (e.g., radiation-induced inflammation) (36). In humans, numbers of enteric mast cells are elevated in diarrhea-predominant irritable bowel syndrome (D-IBS) relative to healthy controls (1, 9, 40, 41). Moreover, elevated mast cell numbers contribute to release of greater amounts of the preformed mast cell mediator histamine from mucosal biopsies removed from patients with postinfectious D-IBS (1). Increased concentrations of histamine in the incubation medium, in these cases, evoke higher frequencies of firing of secretomotor neurons when applied to preparations from the submucosal plexus of guinea pigs in vitro (6, 7).
Enteric mast cells release two kinds of mediators (44). Preformed mediators are stored in cytoplasmic granules and released when the mast cells discharge the granules into the extracellular environment. Examples of preformed mediators are histamine and serine proteases. Another kind of mediator is derived from enzymatic cleavage of membrane lipids. Examples of lipid mediators are prostaglandins and platelet-activating factor. Preformed and membrane-derived mediators are released when an antigen cross-links with its specific IgE antibodies bound to high-affinity FcεRI receptors at the surfaces of mast cells (44). Once released, the mediators diffuse in a paracrine manner into the extracellular milieu in the mast cell's neighborhood, where they become signals to receptors expressed by neurons in the enteric nervous system (ENS), spinal/vagal afferents, smooth muscle, and mucosal secretory glands.
Aside from mediator release evoked by antigen-antibody cross-linkages, neurotransmitters and paracrine signals from nonneural sources can similarly act at receptors on mast cells to evoke mediator release. The present study tested a hypothesis that enteric mast cells express receptors for 5-hydroxytryptamine (5-HT), which, when stimulated, evoke release of the preformed mediator histamine.
It has been estimated that >90% of the body's 5-HT is sequestered in the digestive tract. 5-HT is present in ENS neurons, mucosal enterochromaffin cells, mucosal enterocytes, and blood platelets leaving the gut in the splanchnic circulation. Signaling functions to enteric mast cells may involve release from any of these sources; nevertheless, ENS neurons and enterochromaffin cells are the most likely.
5-HT is recognized as an ENS neurotransmitter and as a paracrine signal released from enterochromaffin cells (39, 31, 46, 49, 54, 55). As a paracrine signal, 5-HT diffuses into the extracellular space to interact with its receptors on enteric neurons and sensory afferent nerves (2, 3, 32, 33, 51). Brushing of luminal contents past the mucosa is a mechanical stimulus for release from enterochromaffin cells, as implied by results from studies on human enterochromaffin cell-derived BON cells (29). Noxious stimulation by laxatives (e.g., senna), chemotherapeutic agents, or injurious ionizing radiation can evoke release (4, 12, 17). In view of the probability that enteric mast cells are exposed to 5-HT arriving from multiple sources, we aimed to investigate its actions on release of mast cell mediators and identify the receptors that might be involved.
MATERIALS AND METHODS
Male Hartley-strain guinea pigs (300–400 g; Charles River, Wilmington, MA) were used. The animal care and experimental protocols were approved by The Ohio State University Laboratory Animal Care and Use Committee and followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Preparations from the small intestine were obtained by microdissection for immunohistochemistry, Western blot analysis, and ELISA. Fresh preparations of healthy human small intestine were obtained from segments of jejunum discarded during Roux-en-Y gastric bypass surgeries, as described in previous reports from our laboratory (15, 49). The Institutional Review Board of The Ohio State University Office of Research Risks Protection approved the human protocols (protocol 02H0208).
Western Blotting
Proteins in guinea pig and human small intestinal preparations were extracted with lysis buffer containing 136.89 mM NaCl, 8.10 mM Na2HPO4, 2.68 mM KCl, 1.47 mM KH2PO4, 1% Triton X-100 (pH 7.4), a protease inhibitor cocktail (cOmplete, Mini Protease Inhibitor Cocktail Tablets, Roche Diagnostics, Indianapolis, IN), and 1 mM PMSF with homogenization for 30 s on ice followed by 1 h on an orbital shaker at 4°C and centrifugation at 1,000 rpm for 10 min at 4°C. The supernatant was collected for immunoblotting. Equivalents of 50 μg of extracted proteins were heated in microtubes at 100°C for 5 min and electrophoresed in 10% SDS-polyacrylamide gels and then transferred to nitrocellulose membranes (Amersham Pharmacia Biotech, Piscataway, NJ) at 4°C and 90 V for 60 min. The membranes were blocked with 5% nonfat milk in Tris·HCl-buffered saline containing 0.1% Tween 20 (TBST) and rotated for 60 min. Thereafter, the membranes were washed with TBST and incubated in solution containing the primary antibodies for the 5-HT1A receptor at a dilution of 1:200 (catalog no 24504, lot no. 136025, ImmunoStar, Hudson, WI) (Table 1) for 2–4 h at room temperature. The membranes were washed (3 times for 10 min each) with TBST and then reblocked with 10% nonfat dry milk for 10 min. The blot was incubated with the secondary antibody, which was anti-rabbit IgG, horseradish peroxidase-linked F(ab)2 fragment from donkey (catalog no. NA9340, ECL Western blotting reagents, Amersham Pharmacia Biotech), for 1–2 h at room temperature.
Table 1.
Codes and sources of primary antibodies
| Antigen | Host | Code | Dilution | Source |
|---|---|---|---|---|
| 5-HT1A receptor | Rabbit | 24504 | 1:100 | ImmunoStar |
| 5-HT1A receptor | Guinea pig | 550469 | 1:200 | BD Bioscience |
| Anti-Hu | Mouse | A21271 | 1:200 | Molecular Probes |
| Anti-S100 | Mouse | S2532 | 1:500 | Molecular Probes |
| Tryptase | Mouse | VP4IV433 | 1:100 | Vector Laboratories |
| Tryptase | Mouse | MAb 1222 | 1:100 | Chemicon |
| Chymase | Mouse | MAb 1254 | 1:100 | Chemicon |
Anti-Hu, anti-human neuronal protein HuC/HuD; anti-S-100, low-molecular-weight protein.
ELISA
Segments of ileum 10–20 cm proximal to the ileocecal junction were removed from guinea pigs, and the lumen was flushed with ice-cold Krebs solution and then divided into ∼1-cm segments. The full-thickness segments were placed separately into 1.5-ml microfuge tubes and incubated in 1–2 ml of Dulbecco's modified Eagle's medium, which was changed at 10-min intervals during the first 40 min. Full-thickness human jejunal segments (0.5–1 cm long, 0.5 cm wide) were placed separately into 5-ml microfuge tubes (because of larger mass than guinea pig preparations) and handled in the same manner. Protocols for study of mast cell degranulation after 40 min of equilibrium were as follows: 1) 10 min were allowed for basal release, and three samples were withdrawn for analysis of histamine content; 2) the segments were exposed to a serotonergic receptor agonist alone for 10 min, and three samples were withdrawn for analysis of histamine content; and 3) a serotonergic receptor antagonist or mast cell-stabilizing drug was applied for 10 min, the preparation was exposed to a serotonergic receptor agonist for 10 min, and three samples were withdrawn for analysis. The samples were centrifuged at 200 rpm for 5 min, and the supernatants were stored at −20°C until they were assayed. Amounts of the mast cell mediator histamine in the supernatants were determined with ELISA kits designed for this purpose (SPI-BIO, Massy Cedex, France; MP Biomedicals, Orangeburg, NY). Each experiment was terminated by removal of the segments from the bathing medium; the segments were blotted on filter paper, and their weight was recorded. Data are expressed as nanograms or picograms per gram of tissue weight.
Immunohistochemistry
Whole-mount immunohistochemistry was done essentially as we described elsewhere (15, 35). Therefore, the current presentation of methods summarizes and quotes from these papers. Whole-mount preparations were obtained by microdissection from segments of guinea pig and human small intestine and transferred to a disposable chamber filled with fixative solution containing 4% formaldehyde and 2% saturated picric acid solution in 0.1 mol/l PBS. Nonspecific immunological binding was blocked with 10% normal donkey serum in 0.01 M PBS (pH 7.4) for 1 h at room temperature. The tissues were incubated with the primary antibodies (Table 1) diluted in 0.01 mol/l PBS containing 10% normal donkey serum, 0.3% Triton X-100, and 0.05% sodium azide for 18 h at room temperature. The tissues were then washed (3 times for 10 min each) in PBS (pH 7.4) and transferred to an incubation medium that contained a single secondary antibody or a mixture of secondary antibodies (Table 2). Combination of primary antibodies for the 5-HT1A receptor, tryptase, and chymase was done at the same time to achieve double immunolabeling. After incubation with the antibodies, the tissues incubated with appropriate secondary antibodies were conjugated with FITC or indocarbocyanin (Cy3) diluted in 0.01 mol/l PBS. The tissues were then rinsed in PBS, and coverslips were applied using Vectorshield (Vector Laboratories, Burlingame, CA). Preabsorption of the two 5-HT1A receptor antibodies with 10 μg of 5-HT1A receptor protein (catalog no. ab152462, Abcam, Cambridge, MA) or 10 μg of 5-HT1A receptor peptide (catalog no. ab133019, Abcam) was done as a control (Fig. 1). Specificity of immunostaining was also checked by omission of the primary or secondary antibody.
Table 2.
Codes and sources of secondary antibodies
| Antigen | Host | Code | Dilution | Source |
|---|---|---|---|---|
| Rabbit IgG | Donkey FITC | 711-095-152 | 1:100 | Jackson Laboratories |
| Rabbit IgG | Donkey Cy3 | 715-165-152 | 1:500 | Jackson Laboratories |
| Rabbit IgG | Donkey HRP | A21271 | 1:5,000 | Amersham ECL |
| Mouse IgG | Donkey FITC | 715-095-150 | 1:100 | Jackson Laboratories |
| Mouse IgG | Donkey Cy3 | 715-165-150 | 1:500 | Jackson Laboratories |
| Guinea pig IgG | Donkey FITC | AP193F | 1:100 | Chemicon |
| Guinea pig IgG | Goat Texas red | MI-7000 | 1:500 | Vector Laboratories |
Cy3, indocarbocyanin; HRP, horseradish peroxidase.
Fig. 1.
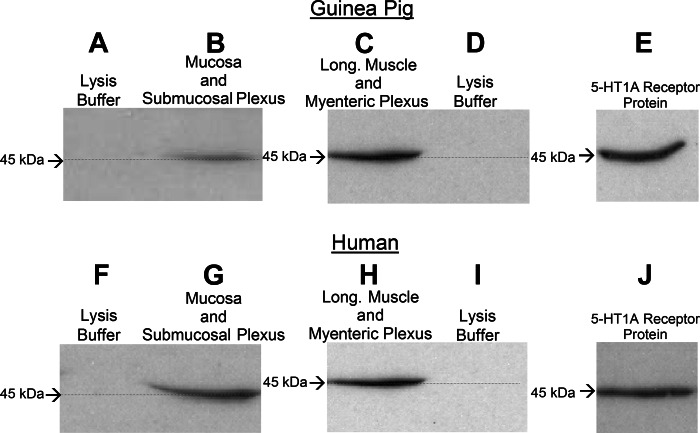
A purified polyclonal 5-HT1A receptor antibody recognized a 45-kDa band on Western blots, indicative of 5-HT1A receptor expression, in 50-μg protein extracts from guinea pig and human mucosa with attached submucosal plexus and from guinea pig and human myenteric plexus-longitudinal muscle preparations. The same antibody recognized 5-HT1A receptor protein on Western blots. A: absence of protein recognition by the primary antibody for the lysis buffer used for guinea pig mucosal-submucosal plexus preparations. B: antibody recognition of a 45-kDa band in extract obtained from mucosal-submucosal plexus preparations from guinea pig small intestine. C: antibody recognition of a 45-kDa band in extract obtained from myenteric plexus-longitudinal (Long) muscle preparations from guinea pig small intestine. D: absence of protein recognition by the primary antibody for the lysis buffer used for guinea pig myenteric plexus-longitudinal muscle preparations. E: recognition of 5-HT1A receptor protein by the antibody used for guinea pig preparations. F: absence of protein recognition by the primary antibody for the lysis buffer used for human mucosal-submucosal plexus preparations. G: antibody recognition of a 45-kDa band in extract obtained from mucosal-submucosal plexus preparations from human small intestine. H: antibody recognition of a 45-kDa band in extract obtained from myenteric plexus-longitudinal muscle preparations from human small intestine. I: absence of protein recognition by the primary antibody for the lysis buffer used for human myenteric plexus-longitudinal muscle preparations. J: recognition of 5-HT1A receptor protein by the antibody used for human preparations. Primary antibody (5 ml, 1:200 dilution) was obtained from ImmunoStar (catalog no. 24504); secondary antibody was donkey horseradish peroxidase (10 ml, 1:5,000 dilution) 5-HT1A receptor protein (catalog no. ab152462, Abcam).
Immunohistochemistry was done also with cryostat sections obtained from guinea pig and human intestine. The guinea pigs were anesthetized with 20% urethane and infused transcardially with chilled 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Segments of human jejunum were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer. The fixed tissues were washed in 0.2 mol/l PBS and then dehydrated in 30% sucrose overnight at 4°C. Then the tissues were frozen and embedded in Tissue-Tek optimal cutting temperature compound (OCT, Ted Pella, Redding, CA). Sequential 4-μm sections were cut on a cryostat microtome. Mast cells were visualized in sections stained with 0.5% toluidine blue (pH 1) for 30 min or with 0.1% Alcian blue in 0.5 N HCl for 30 min and counterstained with fast red for 2 min. Endogenous peroxidase activity was blocked by treatment of the sections with 0.3% hydrogen peroxide solution for 30 min and then with normal horse and goat serum at room temperature for 2 h to block nonspecific protein binding. Pretreated sections were incubated with one or the other of the primary antibodies for the 5-HT1A receptor (Table 1) overnight at 4°C. After incubation, the sections were washed with 0.01 mol/l PBS (pH 7.4) and incubated with biotin-labeled secondary antibody for 60 min. At the end of secondary antibody incubation, the sections were rinsed with PBS and incubated in VECTASTAIN Elite ABC system reagents (Vector Laboratories) for 30 min. Peroxidase activity was detected by exposure to diaminobenzidine with nickel (Vector Laboratories) for 5–10 min and then counterstained with eosin or Mayer's hematoxylin solution (Sigma-Aldrich, St. Louis, MO). All experiments were done in humidified chambers. Parallel sections incubated with nonimmune serum were used as negative controls.
Whole-mount preparations were examined with an epifluorescence microscope (Eclipse 1000, Nikon, Melville, NY) and analyzed with filter combinations that enabled separate visualization of multiple fluorophores. Tissue sections were examined with general light microscopy. Digital images were obtained with a SPOT RT cooled charge-coupled device digital camera (Diagnostic Instruments, Sterling Heights, MI) and analyzed with SPOT III software.
Reagents and Antibodies
SB-269970, 8-hydroxy-PIPAT oxalate, WAY-100135, LY-53857, Y-25130, GR-113808, and 5-HT were purchased from Tocris (Ellisville, MO). TTX, doxantrazole, and cromolyn sodium were purchased from Sigma-Aldrich. Antibodies and antisera are listed in Tables 1 and 2. Histamine ELISA kits were purchased from SPI-BIO and MP Biomedicals.
Data Analysis
Values are means ± SE. Student's t-test and paired t-test were used for statistical analysis of significance. P < 0.05 was accepted as significant.
RESULTS
Western Blotting
Expression of 5-HT1A receptor protein in extracts from guinea pig and human whole-mount myenteric and submucosal preparations appeared on Western blots (Fig. 1). A purified rabbit polyclonal 5-HT1A receptor antibody (catalog no. 20079, ImmunoStar) and a guinea pig polyclonal 5-HT1A receptor antibody (catalog no. 550469, BD Bioscience, Franklin Lakes, NJ), which labeled 5-HT1A receptor protein on Western blots, recognized a corresponding 45-kDa band in protein extracts from whole-mount myenteric and submucosal plexuses and from intact intestinal segments obtained from three guinea pigs and three human Roux-en-Y gastric bypass surgeries (Fig. 1).
Immunohistochemistry
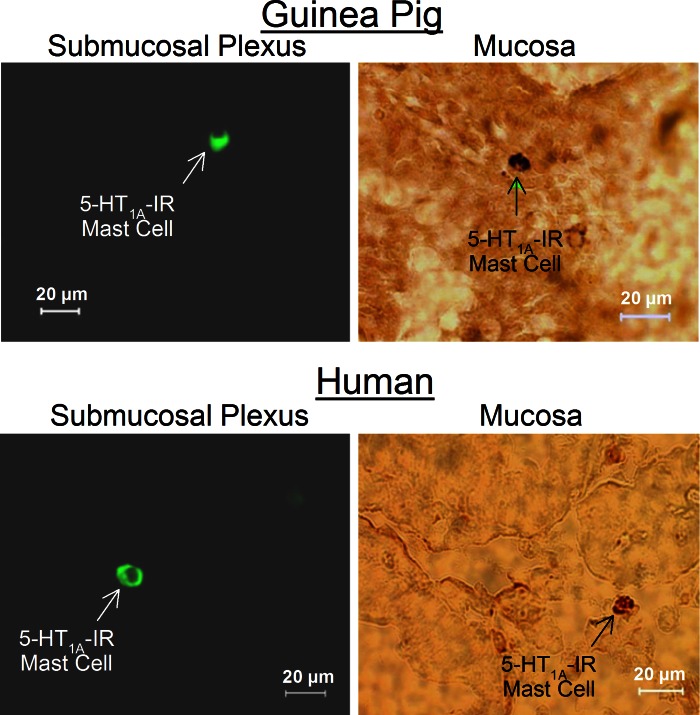
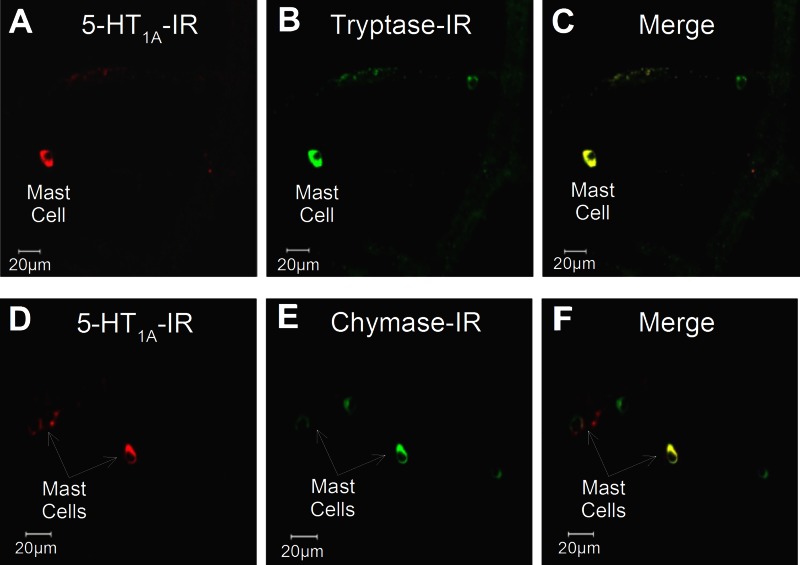
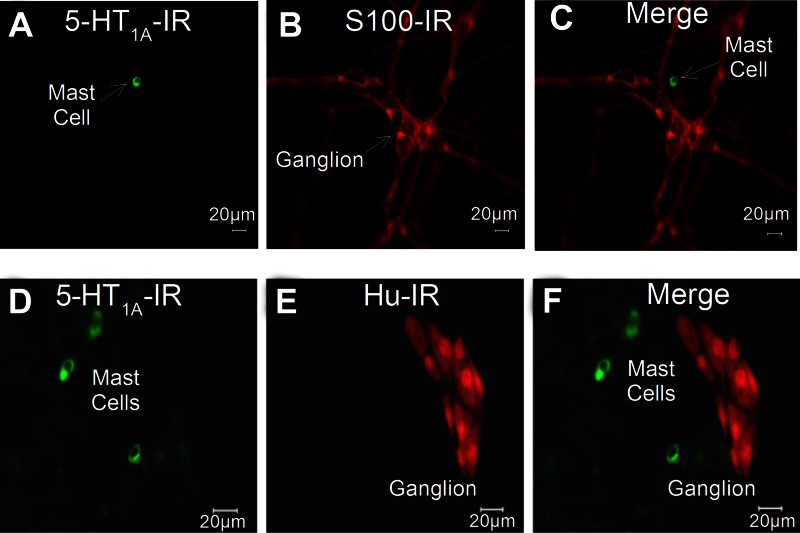
Immunoreactivity (IR) for the 5-HT1A receptor was localized to small (<10-μm) round- or elliptical-shaped cells distributed in the mucosa and lamina propria in small intestinal preparations obtained from 17 guinea pigs and 5 human Roux-en-Y gastric bypass surgeries (Fig. 2). Preabsorption of the 5-HT1A receptor antibodies with 10 μg of 5-HT1A receptor protein or 10 μg of 5-HT1A receptor peptide abolished the immunostaining and supported confidence in the specificity of the antibodies. Immunostaining was not changed when the primary antibody was incubated with a noncorresponding peptide. IR for tryptase and chymase identified these cells as mast cells (20, 21). Double staining showed that the small cells expressing IR for the 5-HT1A receptor also expressed IR for the mast cell markers tryptase and chymase in 5 human and 17 guinea preparations (Fig. 3). We found that 92.4% (2,585 of 2,798) of tryptase-IR cells and 92.4% (1,521 of 1,647) of chymase-IR cells expressed IR for 5-HT1A receptors in preparations from 12 guinea pigs. The same analysis for human preparations found that 87.4% (1,779 of 2,035) of tryptase-IR cells and 91.6% (1,632 of 1,781) of chymase-IR cells expressed IR for the 5-HT1A receptor. Colabeling of the 5-HT1A receptor with IR for the S-100 protein marker for enteric glia and the anti-Hu marker for enteric ganglion cells showed minimal evidence of expression of IR for the 5-HT1A receptor by neurons or glia in 24 preparations (Fig. 4).
Fig. 2.
Immunoreactivity (IR) for the 5-HT1A receptor was expressed by enteric mast cells in whole mounts of submucosal plexus and mucosa of guinea pig and human small intestine.
Fig. 3.
IR for 5-HT1A receptor was coexpressed with IR for tryptase and chymase, both of which are markers for mast cells. A–C: coexpression of IR for the 5-HT1A receptor with IR for tryptase by a mast cell in guinea pig submucosal plexus. D–F: coexpression of IR for the 5-HT1A receptor with IR for chymase by a mast cell in human submucosal plexus.
Fig. 4.
IR for the 5-HT1A receptor was strongly expressed by mast cells and expressed minimally, if at all, by glial cells or ganglion cells in guinea pig submucosal plexus. A: expression of IR for 5-HT1A receptor by a mast cell. B: IR for S100 protein, a glial cell marker, in preparation in A; no IR for 5-HT1A receptor was expressed by the ganglion or interganglionic fiber tract. C: merge of A and B confirming expression of IR for 5-HT1A receptor by the mast cell and rarity of expression of IR for 5-HT1A receptor by glia or ENS neurons. D: expression of IR for 5-HT1A receptor by mast cells in a different preparation from that in A–C. E: IR for the pan-neuronal marker HuC/HuD (anti-Hu) was not expressed by mast cells but labeled all neurons in the ganglion. F: merge of D and E illustrating expression of IR for 5-HT1A receptor by mast cells and rarity of expression of IR for 5-HT1A receptor by neuronal cell bodies in the ganglion.
ELISA
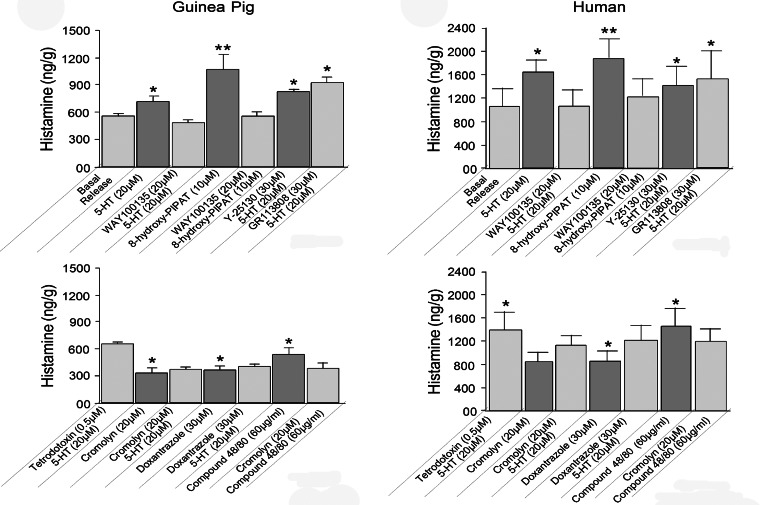
ELISA effectively measured histamine concentrations in incubation medium containing guinea pig or human intestinal preparations. For six guinea pigs, application of 20 μM 5-HT increased the concentration of histamine from a basal level of 557.1 ± 23.8 to 725.6 ± 59.0 ng/g (P < 0.05; Fig. 5). For preparations from three human Roux-en-Y gastric bypass surgeries, 20 μM 5-HT increased histamine release from a basal level of 1,050 ± 331.51 to 1,641 ± 222.8 ng/g (P < 0.05; Fig. 5).
Fig. 5.
Pharmacodynamics for 5-HT-evoked release of histamine from guinea pig and human small intestinal preparations. A selective 5-HT1A serotonergic receptor antagonist, WAY-100135, suppressed 5-HT-evoked release of histamine. A selective 5-HT1A serotonergic receptor agonist, 8-hydroxy-PIPAT, mimicked action of 5-HT to stimulate release of histamine. WAY-100135 suppressed 8-hydroxy-PIPAT-evoked release of histamine. A selective 5-HT3 serotonergic receptor antagonist, Y-25130, did not suppress 5-HT-evoked release of histamine. GR-113808, a selective 5-HT4 receptor antagonist, did not suppress 5-HT-evoked release of histamine. Enteric neuronal blockade by TTX suppressed 5-HT-evoked histamine release in human (P < 0.05), but not guinea pig (P > 0.05), samples. Mast cell-stabilizing drug cromolyn sodium suppressed basal histamine release and suppressed 5-HT-evoked release. Mast cell-stabilizing drug doxantrazole suppressed basal histamine-, as well as 5-HT-evoked, release. Compound 48/80, which acts to degranulate mast cells, stimulated release of histamine to levels greater than basal release. Action of compound 48/80 was suppressed by mast cell-stabilizing drug cromolyn sodium. Values are means ± SE for 24 samples from 6 guinea pigs each done in triplicate and 12 samples from 3 Roux-en-Y gastric bypass surgeries each done in triplicate. *P < 0.05; **P < 0.01.
The high-affinity 5-HT1A receptor agonist 8-hydroxy-PIPAT (56) stimulated histamine release with potency equal to or greater than 20 μM 5-HT when applied at 20 μM to guinea pig and human preparations (Fig. 5). For six guinea pigs, application of 20 μM 8-hydroxy-PIPAT increased the concentration of histamine over 5-HT-evoked release: from 725.6 ± 59.0 ng/g with 5-HT to 1,068.3 ± 168.8 ng/g with 8-hydroxy-PIPAT (P < 0.01). For preparations from three human Roux-en-Y gastric bypass surgeries, histamine release evoked by 20 μM 8-hydroxy-PIPAT was 1,887.7 ± 289.1 ng/g vs. 1,641 ± 222.8 ng/g with 20 μM 5-HT (P < 0.05; Fig. 5).
Preapplication of WAY-100135 (1–20 μM), a commonly used 5-HT1A receptor antagonist (16, 38), had no effect on basal histamine release for human or guinea pig small intestine. However, the action of 5-HT and 8-hydroxy-PIPAT to evoke the release of histamine was suppressed by preapplication of 10–20 μM WAY-100135. For six guinea pigs, preapplication of 20 μM WAY-100135 suppressed responses to 20 μM 5-HT from 725.6 ± 59.0 to 503.5 ± 41.9 ng/g (P < 0.01; Fig. 5). For preparations from three human Roux-en-Y gastric bypass surgeries, preapplication of 20 μM WAY-100135 suppressed responses to 20 μM 8-hydroxy-PIPAT from 1,887.7 ± 289.1 to 1,245.6 ± 273.2 ng/g (P < 0.01; Fig. 5).
Histamine release evoked by 5-HT from six guinea pig preparations was not suppressed by preapplication of 1) 5–30 μM LY-53857, a selective 5-HT2 receptor antagonist (8); 2) 5–30 μM Y-25130, a selective 5-HT3 receptor antagonist (39); or 3) 5–30 μM GR-113808, a selective 5-HT4 receptor antagonist (25) (Fig. 5). Similarly, histamine release evoked by 5-HT, in the same manner, from three human Roux-en-Y gastric bypass surgeries was unchanged by the same 5-HT2, 5-HT3, or 5-HT4 receptor antagonists (Fig. 5).
Mast Cell-Stabilizing Agents
Doxantrazole and cromolyn sodium, mast cell-stabilizing drugs, have been used for many years to treat asthma and other allergies. They act to prevent opening of Ca2+ channels necessary for degranulation and release of histamine (10, 42, 48).
Cromolyn sodium.
For preparations from six guinea pigs, application of cromolyn sodium (5–20 μM) suppressed basal histamine release: 557.2 ± 23.8 vs. 336.6 ± 52.4 ng/g with 20 μM cromolyn sodium (P < 0.01; Fig. 5). For preparations from three human Roux-en-Y gastric bypass surgeries, application of cromolyn sodium (5–20 μM) suppressed basal histamine release: 1,050.6 ± 331.5 vs. 875.2 ± 163.4 ng/g with 20 μM cromolyn sodium (P < 0.01; Fig. 5). For six guinea pigs, the action of 5-HT to evoke release of histamine was suppressed by a 20-min preapplication of 20 μM cromolyn: 725.6 ± 59.6 vs. 378.8 ± 30.5 ng/g with cromolyn sodium (P < 0.01; Fig. 5). For preparations from three human Roux-en-Y gastric bypass surgeries, the action of 5-HT to evoke release of histamine was suppressed by preapplication (20 min) of 20 μM cromolyn sodium: 1,641.7 ± 222.8 vs. 1,146.4 ± 169.4 ng/g with cromolyn sodium (P < 0.01; Fig. 5).
Doxantrazole.
For preparations from six guinea pigs, pretreatment with doxantrazole (30 μM) in the bathing medium for 20 min suppressed basal histamine release: 557.2 ± 23.8 vs. 376.48 ± 47.3 ng/g with doxantrazole (P < 0.0.01; Fig. 5). For preparations from three human Roux-en-Y gastric bypass surgeries, application of doxantrazole (30 μM) suppressed basal histamine release: 1,050.6 ± 331.5 vs. 869.4 ± 184.4 ng/g with doxantrazole (P < 0.01; Fig. 5). For six guinea pigs, the action of 5-HT to evoke release of histamine was suppressed by a 20-min preapplication of 30 μM doxantrazole: 725.6 ± 59.6 vs. 417.5 ± 31.4 ng/g with doxantrazole (P < 0.01; Fig. 5). For preparations from three human Roux-en-Y gastric bypass surgeries, the action of 5-HT to evoke release of histamine was suppressed by preapplication (20 min) of 30 μM doxantrazole: 1,641.7 ± 222.8 vs. 1,235.7 ± 254.2 ng/g with doxantrazole (P < 0.01; Fig. 5).
Compound 48/80
Compound 48/80 is an agent commonly used experimentally to evoke mast cell degranulation and release of histamine (47). Application of 20 μg/ml compound 48/80 did not stimulate release of histamine beyond basal release for preparations from six guinea pigs: 557.2 ± 23.8 vs. 553.6 ± 71.5 ng/g with compound 48/80 (P > 0.05). Nevertheless, compound 48/80, when increased to 60 μg/ml, did stimulate histamine release (Fig. 5). Exposure of preparations from three human Roux-en-Y gastric bypass surgeries to 60 μg/ml compound 48/80 evoked increases in histamine release above basal: 1,050.6 ± 331.6 vs. 1,476.8 ± 309.4 ng/g with compound 48/80 (P < 0.01; Fig. 5). For preparations from three human Roux-en-Y gastric bypass surgeries, the action of compound 48/80 to evoke histamine release was suppressed by preapplication of 20 μM cromolyn: 1,476.8 ± 309.4 vs. 1,203.2 ± 236.3 ng/g with cromolyn sodium (P < 0.05; Fig. 5).
Ketotifen
Ketotifen is a pharmacological agent that has been reported to suppress IgE- or compound 48/80-evoked release of histamine from mast cells, but it also acts as an antagonist at the histamine H1 receptor (11, 22, 45). Presence of 100 μM ketotifen in the incubation medium did not suppress 5-HT-evoked release of histamine from guinea pig or human preparations (data not shown).
TTX.
TTX is a neurotoxin widely used experimentally to block action potential generation in ENS neurons and, thereby, to distinguish neurally evoked effects from direct actions of an agent on effector cells, such as smooth muscle, secretory glands, and mast cells (19, 53). In samples from six guinea pigs, histamine release evoked by 20 μM 5-HT was unchanged in the presence of 0.5 μM TTX: 725.6 ± 59.6 vs. 656.7 ± 21.0 ng/g with TTX (P > 0.05; Fig. 5). On the other hand, in samples from three human Roux-en-Y gastric bypass surgeries, histamine release evoked by 20 μM 5-HT was decreased in the presence of 0.5 μM TTX: 1,641.7 ± 222.8 vs. 1,405.3 ± 324.5 ng/g with TTX (P < 0.05; Fig. 5).
DISCUSSION
Four lines of evidence, obtained in the present study, suggest that enteric mast cells express a 5-HT1A serotonergic receptor that is linked to degranulation in guinea pigs and humans: 1) IR for the 5-HT1A receptor is coexpressed with mast cell markers but weakly, if not at all, with markers for enteric neurons or for enteric glia; 2) a selective 5-HT1A receptor agonist stimulates release of the preformed mast cell mediator histamine; 3) a selective 5-HT1A receptor antagonist suppresses 5-HT-evoked histamine release; and 4) activation of 5-HT2, 5-HT3, or 5-HT4 serotonergic receptors did not stimulate mast cell degranulation. The Western blot data support the expression of 5-HT1A receptor protein in the guinea pig and human small bowel but are not direct evidence for expression by the enteric mast cells.
Mast Cell-Stabilizing Agents and TTX
Our finding that the mast cell stabilizers cromolyn sodium and doxantrazole suppressed 5-HT-evoked histamine release was a degree of assurance that the action of 5-HT was on mast cells directly. TTX is commonly used to block action potential generation in intramural neurons in the digestive tract and to distinguish neurally evoked effects from direct actions of an agent on effector cells, which in the present study was histamine release from mast cells. Our finding that suppression of 5-HT-evoked histamine release in human preparations, when TTX was in the bathing medium, suggests that 5-HT stimulation of unidentified intramural neurons accounts for a small fraction (14%) of 5-HT-evoked histamine release in human intestine. In this case, the kinds of neurons that might be activated to firing threshold by 5-HT are ENS neurons, spinal or vagal afferents, and sympathetic postganglionic axons. The most likely are ENS neurons and spinal or vagal afferent terminals, because both are known to be fired by application of 5-HT (31–33, 37). TTX did not significantly suppress 5-HT-evoked release of histamine in guinea pig preparations. This suggests that application of 5-HT did not activate any intramural neuronal stimulation of mast cell degranulation in this species.
Ketotifen
Lack of effect of ketotifen on 5-HT-evoked histamine release was unexpected in view of reports that it effectively stabilizes mucosal mast cells in animal models for mucosal inflammation induced by C. difficile toxin A, trinitrobenzene sulfonic acid, or acetic acid (14, 43). Moreover, ketotifen suppresses histamine release evoked by allergins in food-sensitized animal models (34) and has been documented, in a pilot study, to have applicability for treatment of ulcerative colitis in children (24). An explanation for the absence of any ketotifen effect on 5-HT stimulation of degranulation is uncertain, other than to invoke the often-recognized variability in properties of the various types of mast cells.
Enteric Neurons and Glia
We were astonished not to find distinct expression of IR for the 5-HT1A receptor by enteric neurons, in contrast to strong staining for mast cells, by the antibodies we used, because the antibodies were from two different sources that provided convincing documentation of specificity for the 5-HT1A receptor in the brain and spinal cord. The absence of strongly expressed IR for the 5-HT1A receptor by anti-Hu-labeled ENS neurons was unexpected in light of the literature, which reports histological and functional evidence implicating expression of 5-HT1A receptors by ENS neurons.
Kirchgessner et al. (30) reported results of a histological study designed to localize sites of expression of the 5-HT1A receptor in the ENS. Their results, obtained with in situ hybridization, showed that expression of mRNA transcripts for the 5-HT1A receptor was associated with most ganglion cells in the myenteric and submucosal plexuses. With immunohistochemistry, they found that most ganglion cell bodies in both plexuses were encircled by diffuse rings of IR for the 5-HT1A receptor, with minimal IR expressed in the cytoplasm. The in situ hybridization and immunohistochemical observations of Kirchgessner et al. are reminiscent of expression in the brain, where 5-HT1A receptor expression is mainly localized to presynaptic axonal terminals (26–28). The distribution of IR for the 5-HT1A receptor in rings at the somal surfaces in the ENS is consistent with expression at presynaptic axonal terminals. Localization to presynaptic terminals is reminiscent of electrophysiological results, obtained with intracellular microelectrodes in ENS neurons, showing that 5-HT1A receptors are mainly involved in presynaptic inhibition of neurotransmitter release (18, 39). The manner in which IR for 5-HT1A receptor protein is distributed in the ENS might account for the differential between strong staining at the surfaces of mast cells and weaker staining or lack of staining of neurons in our protocol.
The scarcity of expression of IR for the 5-HT1A receptor by ENS glia in the present study is reminiscent of the rarity of expression by glia in the brain (26–28). Rarity of expression by ENS glia might be expected, because ENS glial cells have properties in common with astrocytes in the brain (23).
Functional evidence for expression of 5-HT1A receptors in the ENS derives from pharmacological analysis of intestinal motility and electrophysiological behavior of ENS neurons during exposure to 5-HT1A receptor agonists and antagonists. Neal and Bornstein (39) reviewed the sparse literature on involvement of the 5-HT1A receptor in the cellular neurobiology of ENS neurons and concluded that it might mediate a hyperpolarizing action in the cell bodies and, in addition, act presynaptically to suppress nicotinic neurotransmission. Galligan and North (18) reported that application of 5-HT1A receptor agonists hyperpolarizes the membrane potential of ENS neurons that have AH-type electrophysiological behavior and Dogiel type II morphology. On the other hand, Kirchgessner et al. (30) did not find expression of IR for the 5-HT1A receptor by calbindin-positive AH-type neurons. Fast nicotinic synaptic input to neurons with S-type electrophysiological behavior and single axonal morphology in guinea pigs was reported to be mimicked by the 5-HT1A receptor agonist 8-OH-DPAT, and its action was suppressed by the 5-HT1A receptor antagonist spiperone without effects on the postsynaptic action of applied acetylcholine (18). Although the selectivity of these drugs is questionable, their actions support a conclusion that activation of presynaptic 5-HT1A receptors suppresses release of acetylcholine from axonal nerve terminals. Evidence also suggests that prejunctional inhibitory 5-HT1A receptors are expressed by the noncholinergic-nonadrenergic ENS inhibitory motor innervation of the intestinal circular musculature (50). The evidence consists of suppression of purinergic inhibitory junction potentials by the selective 5-HT1A receptor agonist BP-554 and exclusive suppression of BP-554 action by the selective 5-HT1A receptor antagonist spiroxatrine (50). Relative to ENS-controlled motility, Dickson, Heredia, and Smith (13) reported suppression of putative ENS-mediated migrating contractile complexes by 5-HT1A receptor antagonists in mouse large intestine.
Translational Implications
Aside from a role as an ENS neurotransmitter, 5-HT is a recognized paracrine signal released in large amounts from enterochromaffin cells that reside in the intestinal mucosa (4, 12, 17, 46, 55). As a paracrine signal, it spreads in diffuse fashion to bind with its receptors on enteric neurons and intramural sensory afferents. Our results suggest that 5-HT from enterochromaffin cells could similarly be a paracrine signal that binds with the 5-HT1A receptor to degranulate enteric mast cells and release histamine, as well as multiple other preformed mast cell mediators.
The association of cramping abdominal pain, fecal urgency, and acute watery diarrhea as common mast cell-associated hallmarks of D-IBS, infectious enteritis, radiation-induced enteritis, and food allergy raises a question of whether there might be therapeutic application for selective 5-HT1A receptor antagonists in conditions of this nature.
Conclusion
Serotonergic degranulation of enteric mast cells and release of preformed mediators, including histamine, are mediated by stimulation of the 5-HT1A serotonergic receptor subtype, which is expressed by enteric mast cells.
GRANTS
This work was supported by National Institutes of Health Grants R01 DK-37238, R01 DK-57075, R01 DK-068258, and K08-060468.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.-D.W., S.L., Y.X., B.J.N., D.J.M., and J.D.W. are responsible for conception and design of the research; G.-D.W., X.-Y.W., F.Z., M.Q., S.L., G.F., and J.D.W. performed the experiments; G.-D.W., X.-Y.W., F.Z., M.Q., S.L., G.F., Y.X., B.J.N., D.J.M., and J.D.W. analyzed the data; G.-D.W., M.Q., S.L., G.F., Y.X., B.J.N., D.J.M., and J.D.W. interpreted the results of the experiments; G.-D.W., X.-Y.W., F.Z., M.Q., S.L., G.F., Y.X., B.J.N., D.J.M., and J.D.W. approved the final version of the manuscript; S.L. and J.D.W. edited and revised the manuscript; J.D.W. prepared the figures; J.D.W. drafted the manuscript.
ACKNOWLEDGMENTS
Some of the results presented here have been published in abstract form (51).
Present addresses: G. Fei, Div. of Gastroenterology, Peking Union Medical College Hospital, Beijing, China; F. Zou, Dept. of Physiology, Medical College, China Three Gorges University, Hubei, China; M. Qu, College of Pharmacy, Weifang Medical University, Sandong, China; S. Liu, Dept. of Biology, University of Wisconsin, La Crosse, Lacrosse, WI 54601.
REFERENCES
- 1. Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, Collins SM, Corinaldesi R. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 126: 693–702, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Bertrand PP. Real-time measurement of serotonin release and motility in guinea pig ileum. J Physiol 577: 689–704, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bertrand PP, Bertrand RL. Serotonin release and uptake in the gastrointestinal tract. Auton Neurosci 153: 47–57, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Beubler E, Schirgi-Degen A. Serotonin antagonists inhibit sennoside-induced fluid secretion and diarrhea. Pharmacogenetics 1: 64–69, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Broaddus RR, Castro GA. Mast cell-mediated colonic immune function and its inhibition by dietary aspirin in mice infected with Trichinella spiralis. Int Arch Allergy Immunol 105: 135–142, 1994 [DOI] [PubMed] [Google Scholar]
- 6. Buhner S, Li Q, Berger T, Vignali S, Barbara G, De Giorgio R, Stanghellini V, Schemann M. Submucous rather than myenteric neurons are activated by mucosal biopsy supernatants from irritable bowel syndrome patients. Neurogastroenterol Motil 24: 1134–e572, 2012 [DOI] [PubMed] [Google Scholar]
- 7. Buhner S, Li Q, Vignali S, Barbara G, De Giorgio R, Stanghellini V, Cremon C, Zeller F, Langer R, Daniel H, Michel K, Schemann M. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology 137: 1425–1434, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Cohen ML, Fuller RW, Kurz KD. LY53857, a selective and potent serotonergic (5-HT2) receptor antagonist, does not lower blood pressure in the spontaneously hypertensive rat. J Pharmacol Exp Ther 227: 327–332, 1983 [PubMed] [Google Scholar]
- 9. Collins SM, Barbara G. East meets West: infection, nerves, and mast cells in the irritable bowel syndrome. Gut 53: 1068–1069, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cook EB, Stahl JL, Barney NP, Graziano FM. Mechanisms of antihistamines and mast cell stabilizers in ocular allergic inflammation. Curr Drug Targets Inflamm Allergy 1: 167–180, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Craps LP, Ney UM. Ketotifen: current views on its mechanism of action and their therapeutic implications. Respiration 45: 411–421, 1984 [DOI] [PubMed] [Google Scholar]
- 12. Cubeddu LX, Hoffmann IS, Fuenmayor NT, Malave JJ. Changes in serotonin metabolism in cancer patients: its relationship to nausea and vomiting induced by chemotherapeutic drugs. Br J Cancer 66: 198–203, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dickson EJ, Heredia DJ, Smith TK. Critical role of 5-HT1A, 5-HT3, and 5-HT7 receptor subtypes in the initiation, generation, and propagation of the murine colonic migrating motor complex. Am J Physiol Gastrointest Liver Physiol 299: G144–G157, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eliakim R, Karmeli F, Okon E, Rachmilewitz D. Ketotifen effectively prevents mucosal damage in experimental colitis. Gut 33: 1498–1503, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fang X, Liu S, Wang XY, Gao N, Hu HZ, Wang GD, Cook CH, Needleman BJ, Mikami DJ, Xia Y, Fei GJ, Hicks GA, Wood JD. Neurogastroenterology of tegaserod (HTF 919) in the submucosal division of the guinea-pig and human enteric nervous system. Neurogastroenterol Motil 20: 80–93, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Fletcher A, Bill DJ, Bill SJ, Cliffe IA, Dover GM, Forster EA, Haskins JT, Jones D, Mansell HL, Reilly Y. WAY100135: a novel, selective antagonist at presynaptic and postsynaptic 5-HT1A receptors. Eur J Pharmacol 237: 283–291, 1993 [DOI] [PubMed] [Google Scholar]
- 17. Fukui H, Yamamoto M, Ando T, Sasaki S, Sato S. Increase in serotonin levels in the dog ileum and blood by cisplatin as measured by microdialysis. Neuropharmacology 32: 959–968, 1993 [DOI] [PubMed] [Google Scholar]
- 18. Galligan JJ, North RA. Opioid, 5-HT1A and α2 receptors localized to subsets of guinea-pig myenteric neurons. J Auton Nerv Syst 32: 1–11, 1991 [DOI] [PubMed] [Google Scholar]
- 19. Gershon MD. Effects of tetrodotoxin on innervated smooth muscle preparations. Br J Pharmacol Chemother 29: 259–279, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gibson S, Mackeller A, Newlands GF, Miller HR. Phenotypic expression of mast cell granule proteinases. Distribution of mast cell proteinases I and II in the rat digestive system. Immunology 62: 621–627, 1987 [PMC free article] [PubMed] [Google Scholar]
- 21. Huang RY, Blom T, Hellman L. Cloning and structural analysis of MMCP-1, MMCP-4 and MMCP-5, three mouse mast cell-specific serine proteases. Eur J Immunol 21: 1611–1621, 1991 [DOI] [PubMed] [Google Scholar]
- 22. Huston DP, Bressler RB, Kaliner M, Sowell LK, Baylor MW. Prevention of mast-cell degranulation by ketotifen in patients with physical urticarias. Ann Intern Med 104: 507–510, 1986 [DOI] [PubMed] [Google Scholar]
- 23. Jessen KR, Mirsky R. Glial fibrillary acidic polypeptides in peripheral glia. Molecular weight, heterogeneity and distribution. J Neuroimmunol 8: 377–393, 1985 [DOI] [PubMed] [Google Scholar]
- 24. Jones NL, Roifman CM, Griffiths AM, Sherman P. Ketotifen therapy for acute ulcerative colitis in children: a pilot study. Dig Dis Sci 43: 609–615, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Kaumann AJ. Blockade of human atrial 5-HT4 receptors by GR 113808. Br J Pharmacol 110: 1172–1174, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kia HK, Brisorgueil MJ, Daval G, Langlois X, Hamon M, Verge D. Serotonin1A receptors are expressed by a subpopulation of cholinergic neurons in the rat medial septum and diagonal band of Broca—a double immunocytochemical study. Neuroscience 74: 143–154, 1996 [DOI] [PubMed] [Google Scholar]
- 27. Kia HK, Brisorgueil MJ, Hamon M, Calas A, Verge D. Ultrastructural localization of 5-hydroxytryptamine1A receptors in the rat brain. J Neurosci Res 46: 697–708, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Kia HK, Miquel MC, Brisorgueil MJ, Daval G, Riad M, El Mestikawy S, Hamon M, Verge D. Immunocytochemical localization of serotonin1A receptors in the rat central nervous system. J Comp Neurol 365: 289–305, 1996 [DOI] [PubMed] [Google Scholar]
- 29. Kim M, Christofi FL, Xue J, Robinson JM, Cooke HJ. Mechanically evoked 5-hydroxytryptamine release is mediated by caveolin-associated cholesterol rich membrane domains. Neurogastroenterol Motil 19: 309–317, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Kirchgessner AL, Liu MT, Raymond JR, Gershon MD. Identification of cells that express 5-hydroxytryptamine1A receptors in the nervous systems of the bowel and pancreas. J Comp Neurol 364: 439–455, 1996 [DOI] [PubMed] [Google Scholar]
- 31. Kirchgessner AL, Tamir H, Gershon MD. Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity. J Neurosci 12: 235–248, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kirkup AJ, Brunsden AM, Grundy D. Receptors and transmission in the brain-gut axis: potential for novel therapies. I. Receptors on visceral afferents. Am J Physiol Gastrointest Liver Physiol 280: G787–G794, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Kreis ME, Jiang W, Kirkup AJ, Grundy D. Cosensitivity of vagal mucosal afferents to histamine and 5-HT in the rat jejunum. Am J Physiol Gastrointest Liver Physiol 283: G612–G617, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Liu S, Hu HZ, Gao N, Gao C, Wang G, Wang X, Peck OC, Kim G, Gao X, Xia Y, Wood JD. Neuroimmune interactions in guinea pig stomach and small intestine. Am J Physiol Gastrointest Liver Physiol 284: G154–G164, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Liu S, Ren W, Qu MH, Bishop GA, Wang GD, Wang XY, Xia Y, Wood JD. Differential actions of urocortins on neurons of the myenteric division of the enteric nervous system in guinea pig distal colon. Br J Pharmacol 159: 222–236, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. MacNaughton WK, Leach KE, Prud'homme-Lalonde L, Harding RK. Exposure to ionizing radiation increases responsiveness to neural secretory stimuli in the ferret jejunum in vitro. Int J Radiat Biol 72: 219–226, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Mawe GM, Branchek TA, Gershon MD. Peripheral neural serotonin receptors: identification and characterization with specific antagonists and agonists. Proc Natl Acad Sci USA 83: 9799–9803, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mickle A, Kannampalli P, Bruckert M, Miranda A, Banerjee B, Sengupta JN. Pronociceptive effect of 5-HT1A receptor agonist on visceral pain involves spinal N-methyl-d-aspartate (NMDA) receptor. Neuroscience 219: 243–254, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Neal KB, Bornstein JC. Serotonergic receptors in therapeutic approaches to gastrointestinal disorders. Curr Opin Pharmacol 6: 547–552, 2006 [DOI] [PubMed] [Google Scholar]
- 40. O'Sullivan M, Clayton N, Breslin NP, Harman I, Bountra C, McLaren A, O'Morain CA. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil 12: 449–457, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Park CH, Joo YE, Choi SK, Rew JS, Kim SJ, Lee MC. Activated mast cells infiltrate in close proximity to enteric nerves in diarrhea-predominant irritable bowel syndrome. J Korean Med Sci 18: 204–210, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pauwels R, Lamont H, van der Straeten M. The effect of oral doxantrazole on allergen-induced bronchospasm. Acta Allergol 31: 239–244, 1976 [DOI] [PubMed] [Google Scholar]
- 43. Pothoulakis C, Karmeli F, Kelly CP, Eliakim R, Joshi MA, O'Keane CJ, Castagliuolo I, LaMont JT, Rachmilewitz D. Ketotifen inhibits Clostridium difficile toxin A-induced enteritis in rat ileum. Gastroenterology 105: 701–707, 1993 [DOI] [PubMed] [Google Scholar]
- 44. Prussin C, Metcalfe DD. IgE, mast cells, basophils, eosinophils. J Allergy Clin Immunol 117: S450–S456, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Radermecker M. Inhibition of allergen-mediated histamine release from human cells by ketotifen and oxatomide. Comparison with other H1 antihistamines. Respiration 41: 45–55, 1981 [DOI] [PubMed] [Google Scholar]
- 46. Raybould HE, Cooke HJ, Christofi FL. Sensory mechanisms: transmitters, modulators and reflexes. Neurogastroenterol Motil 16 Suppl 1: 60–63, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Rothschild AM. Mechanisms of histamine release by compound 48–80. Br J Pharmacol 38: 253–262, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shapiro GG, Konig P. Cromolyn sodium: a review. Pharmacotherapy 5: 156–170, 1985 [DOI] [PubMed] [Google Scholar]
- 49. Sun X, Wang X, Wang GD, Xia Y, Liu S, Qu M, Needleman BJ, Mikami DJ, Melvin WS, Bohn LM, Ueno R, Wood JD. Lubiprostone reverses the inhibitory action of morphine on mucosal secretion in human small intestine. Dig Dis Sci 56: 330–338, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang GD, Wang XY, Hu HZ, Liu S, Gao N, Fang X, Xia Y, Wood JD. Serotonin acts at pre-junctional 5-HT1A receptors to suppress purinergic inhibitory neurotransmission to the circular muscle in guinea-pig small intestine (Abstract). Gastroenterology 128: A363, 2005 [Google Scholar]
- 51. Wang XY, Wang GD, Xia Y, Liu S, Gao N, Fei GJ, Hu HZ, Wood JD. Characteristics of the serotonergic1A receptor expressed by mast cells in guinea-pig intestine (Abstract). Gastroenterology 130: A379, 2006 [Google Scholar]
- 52. Wershil BK, Castagliuolo I, Pothoulakis C. Direct evidence of mast cell involvement in Clostridium difficile toxin A-induced enteritis in mice. Gastroenterology 114: 956–964, 1998 [DOI] [PubMed] [Google Scholar]
- 53. Wood JD. Excitation of intestinal muscle by atropine, tetrodotoxin, and xylocaine. Am J Physiol 222: 118–125, 1972 [DOI] [PubMed] [Google Scholar]
- 54. Wood JD, Mayer CJ. Slow synaptic excitation mediated by serotonin in Auerbach's plexus. Nature 276: 836–837, 1978 [DOI] [PubMed] [Google Scholar]
- 55. Xue J, Askwith C, Javed NH, Cooke HJ. Autonomic nervous system and secretion across the intestinal mucosal surface. Auton Neurosci 133: 55–63, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhuang ZP, Kung MP, Kung HF. Synthesis of (R,S)-trans-8-hydroxy-2-[N-n-propyl-N-(3′-iodo-2′-propenyl)amino]tetral in (trans-8-OH-PIPAT): a new 5-HT1A receptor ligand. J Med Chem 36: 3161–3165, 1993 [DOI] [PubMed] [Google Scholar]