Abstract
During ramp incremental cycling exercise increases in pulmonary O2 uptake (V̇o2p) are matched by a linear increase in systemic cardiac output (Q). However, it has been suggested that blood flow in the active muscle microvasculature does not display similar linearity in blood flow relative to metabolic demand. This study simultaneously examined both systemic and regional (microvascular) blood flow and O2 extraction during incremental cycling exercise. Ten young men (V̇o2 peak = 4.2 ± 0.5 l/min) and 10 young women (V̇o2 peak = 3.2 ± 0.5 l/min) were recruited to perform two maximal incremental cycling tests on separate days. The acetylene open-circuit technique and mass spectrometry and volume turbine were used to measure Q (every minute) and breath-by-breath V̇o2p, respectively; systemic arterio-venous O2 difference (a-vO2diff) was calculated as V̇o2p/Q on a minute-by-minute basis. Changes in near-infrared spectroscopy-derived muscle deoxygenation (Δ[HHb]) were used (in combination with V̇o2p data) to estimate the profiles of peripheral O2 extraction and blood flow of the active muscle microvasculature. The systemic Q-to-V̇o2p relationship was linear (∼5.8 l/min increase in Q for a 1 l/min increase in V̇o2p) with a-vO2diff displaying a hyperbolic response as exercise intensity increased toward V̇o2 peak. The peripheral blood flow response profile was described by an inverted sigmoid curve, indicating nonlinear responses relative to metabolic demand. The Δ[HHb] profile increased linearly with absolute V̇o2p until high-intensity exercise, thereafter displaying a “near-plateau”. Results indicate that systemic blood flow and thus O2 delivery does not reflect the profile of blood flow changes at the level of the microvasculature.
Keywords: near-infrared spectroscopy, a-vO2diff, cardiac output, V̇o2 max
several studies have demonstrated that increments in exercise intensity, and thus oxygen uptake (V̇o2), are accompanied by a linear increase in systemic blood flow [cardiac output (Q)] to match oxygen (O2) supply to the O2 demand within the active tissues (6, 16, 34, 38). According to the Fick equation [V̇o2 = Q × arterial-venous O2 difference (a-vO2diff)] , a linear relationship between whole body Q and V̇o2 (with a positive intercept), implies a hyperbolic response of the a-vO2diff and, indeed, such a hyperbolic relationship in systemic O2 extraction has been demonstrated during incremental maximal cycling exercise (10, 24, 36).
The profiles of systemic Q and a-vO2diff do not necessarily reflect the dynamic adjustments of blood flow to, and O2 extraction within, the active tissues. The mechanisms regulating peripheral skeletal muscle hyperemia during incremental exercise have been mainly attributed to the interplay of locally induced vasodilators and sympathetically mediated vasoconstrictors (4, 5, 11, 12, 42, 45, 46). Regional Q and a-vO2diff are also related to exercise intensity and active muscle mass recruited (11, 45). During incremental exercise, these changes occur to match local O2 delivery requirements with those of metabolism. To increase O2 delivery (and thus active muscle blood flow [Qm]), systemic increases in Q and arterial pressure and systemic reductions in blood flow to nonexercising tissue occur. As oxidative demands of the active tissue increase, Qm to the vascular beds is regulated by changes in perfusion pressure and vascular tone (14). However, measurements of microvascular blood flow and O2 extraction dynamics at the level of the active tissue are needed before the mechanisms regulating peripheral skeletal muscle hyperemia during incremental cycling exercise in humans can be further elucidated.
Much of what is known regarding peripheral hemodynamics (Qm) during maximal exercise in humans has been determined from thermodilution blood flow measurements during cycling (10, 12, 24, 28), knee-extension (1, 5, 35, 36), and skiing exercise (11). Assessments of femoral blood flow (FBF) and O2 extraction during cycling and knee-extension exercise have depicted a linear FBF to leg V̇o2 relationship (similar to the systemic Q-to-V̇o2 relationship) with fractional O2 extraction displaying asymptotic behaviors at ∼80– 85% of maximal O2 extraction (10, 24, 28, 36). Recently, the use of near-infrared spectroscopy (NIRS) has permitted the assessment of O2 extraction from the vastus lateralis (VL) muscle, as reflected by changes in the NIRS-derived deoxy-hemoglobin signal ([HHb]), within the exercising muscle microvasculature. Using this technique, two recent studies have estimated the dynamic response of O2 extraction within the area of NIRS interrogation and have suggested a nonlinear relationship between Qm and muscle V̇o2 (V̇o2m) (9, 17). However, no studies to date have simultaneously described the dynamic adjustment of estimates of systemic Q and a-vO2diff, and NIRS-derived peripheral (microvascular) blood flow and local muscle O2 extraction using the same protocol within the same individuals.
Reliable measures of Q during ramp incremental exercise can be obtained noninvasively using the acetylene (C2H2) open circuit technique (23) and in combination with measures of pulmonary V̇o2 (V̇o2p), estimations of systemic a-vO2diff can be derived (29, 30). Thus, the goal of this study was to simultaneously compare the dynamic adjustment of systemic and peripheral (vastus lateralis microvascular) blood flow and O2 extraction (using open-circuit acetylene and NIRS, respectively) during ramp incremental exercise. We hypothesized that the linear relationship between Q and V̇o2 observed systemically would be distorted in the active tissue.
METHODS
Subjects.
Ten young men [24 ± 5 yr, 82 ± 10 kg, 181 ± 6 cm; body mass index (BMI) = 25.2 ± 2.6 kg/m2 (mean ± SD)] and 10 young women (22 ± 2 yr, 65 ± 7 kg, 171 ± 8 cm; BMI = 22.3 ± 1.6 kg/m2) gave written informed consent to participate in this study. All procedures were approved by The University of Western Ontario Research Ethics Board for Health Sciences Research Involving Human Subjects. All subjects were recreationally active, nonobese, and nonsmokers.
Protocol.
Subjects reported to the laboratory on two separate days separated by at least 48 h, but not more than 2 wk, to perform a ramp incremental test to exhaustion (4-min baseline at 20 W with 20 W/min increments) on a cycle ergometer (Lode Corival 400; Lode B.V., Groningen, Holland). Peak V̇o2 (V̇o2 peak) was determined as the highest 20-s average value from any of the tests. Measures of Q were taken at 2-min intervals during the ramp incremental exercise test, starting at minute 0 for test day 1 and minute 1 for test day 2, respectively. As such, minute-by-minute measures of Q were obtained for each subject after combining data from both days of testing.
Measurements.
Gas-exchange measurements were similar to those previously described (3). Briefly, inspired and expired flow rates were measured using a low dead space (90 ml) bidirectional turbine (Alpha Technologies VMM 110), which was calibrated before each test using a syringe of known volume. Inspired and expired gases were continuously sampled (50 Hz) at the mouth and analyzed for concentrations of O2, CO2, and N2 by mass spectrometry (Perkin Elmer MGA-1100) after calibration with precision-analyzed gas mixtures. Changes in gas concentrations were aligned with gas volumes by measuring the time delay for a square-wave bolus of gas passing the turbine to the resulting changes in fractional gas concentrations as measured by the mass spectrometer. Data were transferred to a computer, which aligned concentrations with volume information to build a profile of each breath. Breath-by-breath alveolar gas exchange was calculated using algorithms of Beaver et al. (7).
Q was measured using the acetylene (C2H2) open-circuit inert gas wash-in method and analyzed using custom data acquisition software. Although test-retest repeatability was not evaluated in the present study, data from previous studies in our laboratory have demonstrated highly reproducible values at both submaximal and peak intensities, so that measurements were consistent within ∼1 l/min with a mean of 14.6 ± 3.2 l/min on test 1 and 14.5 ± 3.2 l/min on test 2 (P = 0.43) and a reliability of r = 0.97 (29, 30). Further evidence for the high repeatability of this method comes from the fact that the Q measurements performed on two separate days in the present study (measured at odd- and even-minute intervals between test days) fell perfectly in line with each other within each individual (see results). Additionally, this technique was described and validated previously (23). Briefly, a pneumotachograph (Hans Rudolph model 3800; Kansas City, MO; transducer, Validyne MP45–871; Northridge, CA) was attached to a nonrebreathing Y valve (Hans Rudolph 7900), which was connected to a manual valve that allowed switching inspired gases between room air and a bag containing a mixture of C2H2 (0.7%), O2 (21%), He (9%), and balance N2. Changes in gas concentrations were aligned with gas volumes by measuring the time delay of the gases as described above. During measurements of Q, subjects were asked to avoid coughs, swallows, and partial breaths. At any given measurement, subjects were asked not to alter their breathing pattern when the source of inspired air was switched from room air to the bag containing the gas mixture. The concentration of C2H2 and He were displayed on a computer screen on a breath-by-breath basis, and after 10 breaths, the protocol was terminated. Data analysis for the calculation of Q was performed immediately after each maneuver using equations reported previously (23). The a-vO2diff was calculated from the Fick equation as a-vO2diff (ml O2/100 ml blood) = V̇o2 (l/min)/Q (l/min) × 100.
Local muscle deoxygenation (Δ[HHb]) profiles of the quadriceps vastus lateralis muscle were made with NIRS (Hamamatsu NIRO 300; Hamamatsu Photonics, Hamamatsu, Japan) throughout exercise. Briefly, optodes were placed on the belly of the muscle midway between the lateral epicondyle and greater trochanter of the femur. The system consisted of both an emission probe that carries NIR light from the laser diodes and a detector probe (interoptode spacing = 5 cm); optodes were housed in an optically dense plastic holder and secured on the skin surface with tape and then covered with an optically dense, black vinyl sheet, thus minimizing the intrusion of extraneous light. The thigh was wrapped with an elastic bandage to minimize movement of the optodes. Four laser diodes (λ = 775, 810, 850, and 910 nm) were pulsed in a rapid succession, and the light returning from the tissue was detected by the photodiode for online estimation and display of the concentration changes from the resting baseline of Δ[HHb]. Changes in light intensities were recorded continuously at 2 Hz and transferred to a computer for later analysis. The NIRS-derived signal was zero set with the subject sitting at rest on the cycle ergometer prior to the onset of baseline (i.e., 20 W) exercise. Given the uncertainty of the optical path length in the vastus lateralis at rest and during exercise, Δ[HHb] data are presented as normalized delta (%Δ; see below for normalization procedures) units.
Data analysis.
V̇o2p data were filtered by removing aberrant data points that lay outside 4 SD of the local mean and then linearly interpolated to 1-s intervals. The second-by-second V̇o2p data from tests 1 and 2 were time-aligned and ensemble-averaged to yield a single averaged response for each subject for the ramp incremental exercise protocol; subsequently, the min-by-min V̇o2p values were calculated using 10-s averages from the 5 s prior to and following the rounded minute. The second-by-second Δ[HHb] data were time-aligned and ensemble-averaged in the same manner. The ensemble-averaged Δ[HHb] responses were then normalized (%Δ[HHb]), such that 0% represented the steady-state value observed during 20 W cycling and 100% represented the highest average (i.e., Δ[HHb]peak) value observed in any continuous 20 s of exercise. As described by Boone et al. (9), %Δ[HHb] data were time-aligned with V̇o2p by left-shifting the V̇o2p signal by 20 s to account for the circulatory transit delay between muscle and lung; this was undertaken so that changes in “muscle V̇o2” (represented by V̇o2p) were aligned with changes in the %Δ[HHb] signal. Although this 20-s value may not precisely match the circulatory time lag in all individuals, our laboratory has recently described the limitations and challenges associated with its determination (31), and overall, this 20-s value represents a reasonable estimate for the group tested.
The whole body Q-to-V̇o2 relationship was determined using linear regression analyses. Data from baseline (20 W) to peak exercise were included to derive regressions for each individual. The whole body a-vO2diff-to-V̇o2 relationship was described by the following hyperbolic function: y = a·x / (B + x), where a represents the asymptotic value and B is the x value corresponding to 50% of the total amplitude. As with whole body Q-to-V̇o2, data spanned from baseline to peak exercise.
The profile of peripheral (vastus lateralis) blood flow (QVL) was determined, as described by Ferreira et al. (17). Briefly, peripheral a-vO2diff was estimated from the profile of %Δ[HHb] using published values for muscle a-vO2diff (24, 26, 37); baseline and peak exercise a-vO2diff were assumed to equal 10 ml O2/100 ml blood (26) and 18 ml O2/100 ml blood (24), respectively. To convert the normalized change in %Δ[HHb] to a-vO2diff (in ml O2/100 ml blood), we used the following formula: a-vO2diff = 10 + [%Δ[HHb]/100]·8, where 10 ml O2/100 ml blood corresponds to assumed a-vO2diff during baseline cycling and 8 ml O2/100 ml blood is the assumed change in a-vO2diff from baseline to peak exercise. Thus, second-by-second QVL was calculated (Fick equation) for each individual as the quotient of V̇o2 (measured) and peripheral a-vO2diff (as described immediately above).
We have previously reported that the profile of %Δ[HHb] (plotted as a function of V̇o2p) is better described by a piecewise equation that includes two linear segments [ hereafter referred to as “double-linear” as described by Spencer et al. (40)] compared with a symmetrical sigmoid function previously proposed. This has been confirmed in the present data set, and therefore, no data from the sigmoid functions are presented. The “double-linear” model characterizes the predominant increase in %Δ[HHb] observed throughout the middle portion of the exercise protocol (beginning at the point where the %Δ[HHb] signal began a systematic increase above baseline as determined by visual inspection) and the “plateau,” which follows (40): y = segment1(x) = [y1·(BP-x) + y2·(x-x1)] /(BP-x1); segment2(x) = [y2·(x2-x) + y3·(x-BP)] /(x2-BP), f = if [x ≤ BP, segment1(x); else, segment2(x)], where x1 and x2 represent the minimum and maximum x values, respectively; y1 and y3 represent the predicted %Δ[HHb] at x1 and x2, respectively; BP represents the x value at the break point between the two segments; and y2 represents the predicted %Δ[HHb] at BP (i.e., y2 = %Δ[HHb], where segments intersect). Thus, this “double-linear” analysis yields: y = m1·x + b1 for x < BP and y = m2·x + b2 for x > BP, where m represents the slope and b is the y-intercept value. The model parameters were estimated for each participant by least-squares linear and nonlinear (Q-to-V̇o2 and a-vO2diff-to-V̇o2, respectively; Origin, OriginLab, Northampton, MA); the “double-linear” function was fit using SigmaPlot 12.0 (Systat Software, Point Richmond, CA). For all regressions, the best fit was defined by minimization of the residual sum of squares and minimal variation of residuals around the y axis (y = 0) (40). All statistical analyses were performed using SPSS version 19.0 (SPSS, Chicago, IL). Descriptive data are presented as mean ± SD. Independent pairwise t-tests were used to detect differences between men and women. Statistical significance was accepted at a P value <0.05.
RESULTS
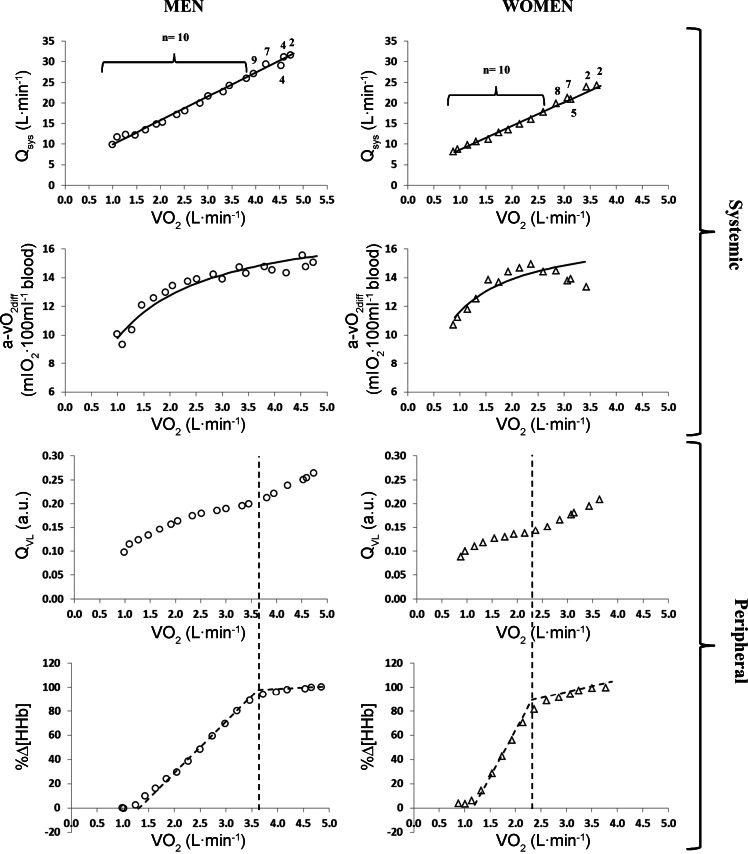
Absolute (men, 4.2 ± 0.5 l/min; women, 3.2 ± 0.5 l/min; P < 0.05) but not relative (men, 52.0 ± 7.5 ml·kg−1·min−1; women, 49.0 ± 7.9 ml·kg−1·min−1; P > 0.05) V̇o2 peak was greater in men compared with women. Figure 1 displays the dynamic adjustment of systemic blood flow and O2 extraction (a-vO2diff) and the profile of the adjustment of peripheral blood flow response (QVL) and exercising muscle O2 extraction (Δ%[HHb]) as a function of V̇o2 during the ramp incremental test. Table 1 provides the parameter estimates of the model fits to the data. Systemic Q displayed the characteristic linear relationship with V̇o2 that was similar in men (Q = 5.8 × V̇o2 + 4.2) and women (Q = 5.7 × V̇o2 + 2.9). Similarly, the hyperbolic response for a-vO2diff was the same in men and women. The derived QVL was depicted as an inverted sigmoid response, whereas the %Δ[HHb] was best described with two linear segments. The slope and y-intercept of the %Δ[HHb] signal before BP (hereafter referred to as Δ[HHb] − BP) (i.e., m1 and b1) were different between men and women, showing a greater rate of increase in women.
Fig. 1.
Profiles of systemic and peripheral blood flow (Qsys and QVL, respectively) and O2 extraction (a-vO2diff and %Δ[HHb], respectively) as a function of V̇o2. Left: Qsys, a-V̇o2diff, QVL, and %Δ profiles for men (open circles). Right: Qsys, a-V̇o2diff, QVL, and %Δ profiles for women (open triangles). Data points are the mean across all subjects at the given V̇o2p; model fits are the fit parameters from the mean of the fits to data in each individual. Dashed line indicates location of Δ[HHb] -BP. Numbers displayed above individual data points indicate the number of subjects from which the specific point was derived.
Table 1.
Parameter estimates for linear (Q), hyperbolic (a-vO2diff), and double-linear (%Δ[HHb]) models as a function of V̇o2p displayed for men and women
|
Q |
a-vO2diff |
%Δ[HHb] |
||||||
|---|---|---|---|---|---|---|---|---|
| m | b | a | B | m1 | b1 | m2 | b2 | |
| Men | 5.8 ± 1.1 | 4.2 ± 2.4 | 18.3 ± 3.5 | 0.9 ± 0.5 | 42.4 ± 6.4 | −55.7 ± 20.6 | 2.0 ± 9.7 | 90.7 ± 38.8 |
| Women | 5.7 ± 1.0 | 2.9 ± 1.6 | 17.2 ± 3.4 | 0.5 ± 0.3 | 76.8 ± 23.4* | −89.3 ± 39.0* | 9.8 ± 10.1 | 66.6 ± 31.2 |
Values are expressed as means ± SD, derived from each individual. m, slope of the linear regression; b, y-intercept of the linear regression; a, asymptotic value; B, x value corresponding to 50% of the total amplitude; m1 and m2 slope of linear regression before and after Δ[HHb] -BP, respectively; b1 and b2 y-intercept of linear regression before and after Δ[HHb] -BP, respectively.
Significant difference from men, P < 0.05; n = 10 for men and women.
DISCUSSION
This study examined the responses of systemic and peripheral (vastus lateralis muscle) blood flow and O2 extraction during ramp incremental exercise. The novelty of the study design resided in obtaining simultaneous measures and estimates of central and regional (microvascular) responses in the same subjects during cycling exercise throughout the full range of exercise intensities. The main finding was that as V̇o2 progressively increased, the dynamic adjustments of systemic blood flow and O2 extraction were different compared with that in the periphery in that there was a nonlinear increase in QVL concomitant with a rapid increase of muscle deoxygenation throughout “moderate”-intensity exercise followed by a plateauing of local deoxygenation in heavy-intensity to maximal exercise.
Despite certain reports suggesting otherwise (28, 41), there is compelling information indicating that Q adjustments to incremental exercise occur in a linear fashion and with a positive intercept when evaluated as a function of metabolic rate (V̇o2) (1, 6, 16, 34, 36). This implies that the adjustment of a-vO2diff during incremental exercise displays a hyperbolic response and, indeed, this response has been demonstrated previously (10, 24, 36). Data from the present investigation support that contention and are in line with past reports indicating ∼5–6 l/min increase in Q for a 1 l/min increase in V̇o2 (2, 22, 27, 34, 39). Importantly, the regression lines obtained in this study were derived from many data points during the ramp incremental test (i.e., minute by minute), which increases confidence in the quality of the fit and Q-to-V̇o2 linearity.
Recently, several studies have characterized the dynamic response of the NIRS-derived [HHb] signal during ramp incremental testing (9, 17, 19) to characterize the profile of O2 extraction within some of the active tissues (vastus lateralis muscle) and, as a consequence, make inferences with regard to the blood flow response within the microcirculation. Similar to the results from the present investigation, the implication from those studies was that there is a nonlinear muscle blood flow-to-V̇o2 relationship in the area of NIRS interrogation. Several factors may play a role for the loss of this linearity within the measured muscle. For instance, a rapid increase in blood flow during the exercise on-transient has been proposed to be connected to the mechanical effects of the muscle pump and rapid vasodilation of unknown origin (43, 44). Following this rapid increase, there is a period during exercise (until ∼60–70% of V̇o2 peak) in which peripheral (i.e., vastus lateralis) blood flow increases modestly, despite a comparatively larger increase in systemic blood flow. This larger reliance on peripheral O2 extraction for a given increase in V̇o2 might reflect an initial sympathetically mediated vasoconstrictor effect (controlling blood pressure). Thereafter, peripheral O2 extraction reaches a “plateau-like” response and further increases in blood flow in the active tissues are necessary to support a further increase in V̇o2 by ∼1 l/min. The idea that adequate redistribution of blood flow is required to support an increase in V̇o2 at higher intensities of exercise has previously been proposed (33). Such a redistribution of blood flow to the active tissues might be supported by increased sympathetic vasoconstriction of peripheral nonactive tissues and the splanchnic and renal regions (38). However, this increase of blood flow may also be supported by an intensity-dependent functional sympatholysis (45), such that sympathetic vasoconstriction in active muscles is greatly attenuated (by metabolic events in contracting skeletal muscle) to optimize muscle perfusion (21, 42). In support of this hypothesis, Tschakovsky et al. (45) reported a diminished reduction of forearm vascular conductance (while evoking high endogenous norepinephrine release) during rhythmic handgrip exercise, as the intensity of exercise increased from rest to heavy exercise. Additionally, VanTeeffelen and Segal (46) demonstrated that functional hyperemia increased in response to intensity of contraction in the presence of prevailing vasoconstriction in the feed and proximal arterioles and vasodilation in distal arterioles. These “arteriole level”-dependent vasomotor response differences implicate the necessity for blood flow and O2 extraction measurement at the level of the microvasculature when examining the relationship of blood flow/O2 delivery to muscle O2 utilization. Recently, the Δ[HHb] -BP has been shown to be coincident to the intensity associated with the maximal lactate steady state (8). It is possible that beyond this marker of exercise intensity, metabolite accumulation within the interstitial fluid (e.g., K+, lactate) may contribute to vasomotor relaxation and thus improved exercise hyperemia.
Although it was expected that the overall profiles of the systemic and peripheral responses would not be affected by sex (18), we have displayed these profiles in men and women separately in Fig. 1. V̇o2 peak was smaller in women compared with men (3.2 l/min vs. 4.2 l/min); therefore, by including both sexes when displaying systemic and peripheral responses, the profiles would be distorted by the reduction in subject number, especially at higher V̇o2 values. For example, at 3.0 l/min, 17 subjects would contribute to the profile; however, the number would be reduced to 13 at 3.5 l/min, seven at 4.0 l/min, and two at 4.5 l/min. However, it should be noted that the lines of best fit (presented in all panels in Fig. 1) are derived from individual response profiles and not the mean response profile. Nevertheless, the general responses were similar in men and women; although women displayed a steeper slope (m1) for %Δ[HHb], these differences were to be expected as the absolute V̇o2 peak was smaller in women [O2 extraction is associated with relative (rather than absolute) intensity, such that peak O2 extraction will be reached when approaching maximal intensities, independent of absolute V̇o2 peak].
Limitations.
A limitation of this study was the use of the NIRS-derived Δ[HHb] signal as a measure of O2 extraction as a replacement for a-vO2diff in the Fick equation to derive an estimate of QVL. Our recent study has criticized this approach to calculate the profile of peripheral blood flow during exercise transitions from baseline to moderate intensity (32). The major problem presented in that study was related to the uncertainties with the use of micromolar units as a valid replacement for a-vO2diff in the equation. The normalization of the Δ[HHb] used in the present study overcomes this limitation. This normalization requires assumed “usual” values of the muscle a-vO2diff. However, the range of values for resting and peak exercise a-vO2diff would certainly vary among subjects and differ from those assumed in this paper. Accepting this limitation, the real profile of QVL should remain similar to those presented here, unless large deviations occurred between the actual a-vO2diff and those previously reported (24, 26, 37), which were used to normalize the Δ[HHb] data and derive QVL.
Another factor to consider is that the number of subjects composing the overall response was progressively reduced as exercise intensity increased. However, two facts should be noted: 1) despite the reduced number of subjects at higher-exercise intensities, the values obtained for Q in the “reduced” data set (with Q used as an example due to its well-established relationship with metabolic demand) fell directly within what would be predicted from its linear relationship with V̇o2; and 2) the fits displayed in Fig. 1 were modeled from the mean values derived from the individual responses from each subject. As such, the fits presented in this study were not affected by the loss of subjects at higher-exercise intensities.
Finally, it is acknowledged that choosing to evaluate the vastus lateralis muscle only during ramp incremental exercise does not provide a complete picture of the skeletal muscle blood flow response to exercise. It has previously been reported that the profile of rectus femoris deoxygenation during ramp incremental exercise differed compared with either the vastus lateralis and vastus medialis muscle groups (13), and it has been shown that muscle group activation during cycling changes nonuniformly with increasing relative intensity, and there is a considerable heterogeneity with respect to individual muscle group activation (e.g., gluteus maximus has been reported to increase ∼40-fold at maximal exercise) (20). Although these factors may have contributed to some of the differences observed between central and peripheral components, it is important to note that different studies have shown no differences (15) or minimal differences in spatial heterogeneities in muscle deoxygenation within the quadriceps (25).
Perspectives and Significance
This study demonstrated that the linear relationship that exists between blood flow and metabolic demand during ramp incremental cycling exercise does not occur at the level of the active muscle vasculature. The inverse sigmoidal-like response of QVL indicates that blood flow and, thus, O2 delivery are regulated by different mechanisms in the vascular beds compared with those observed systemically as exercise intensity increases. Active muscle microvascular perfusion and O2 extraction differ considerably from the responses observed systemically during maximal incremental exercise and, therefore, caution should be used when applying systemic circulatory responses to interpret those of the periphery.
GRANTS
This study was supported by Natural Sciences and Engineering Research Council of Canada research and equipment grants.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.M.M., M.D.S., and D.H.P. conception and design of research; J.M.M. and M.D.S. performed experiments; J.M.M., M.D.S., and D.A.K. analyzed data; J.M.M., M.D.S., D.A.K., and D.H.P. interpreted results of experiments; J.M.M. and M.D.S. prepared figures; J.M.M. and D.A.K. drafted manuscript; J.M.M., M.D.S., D.A.K., and D.H.P. edited and revised manuscript; J.M.M., M.D.S., D.A.K., and D.H.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We would like to express our gratitude to the subjects in this study and to acknowledge the assistance provided by Brad Hansen.
REFERENCES
- 1. Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol 366: 233–249, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Astrand PO, Cuddy TE, Saltin B, Stenberg J. Cardiac output during sub-maximal and maximal work. J Appl Physiol 19: 268–274, 1964 [DOI] [PubMed] [Google Scholar]
- 3. Babcock MA, Paterson DH, Cunningham DA, Dickinson JR. Exercise on-transient gas exchange kinetics are slowed as a function of age. Med Sci Sports Exerc 26: 440–446, 1994 [PubMed] [Google Scholar]
- 4. Bada AA, Svendsen JH, Secher NH, Saltin B, Mortensen SP. Peripheral vasodilatation determines cardiac output in exercising humans: insight from atrial pacing. J Physiol 590: 2051–2060, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barden J, Lawrenson L, Poole JG, Kim J, Wray DW, Bailey DM, Richardson RS. Limitations to vasodilatory capacity and V̇o2 max in trained human skeletal muscle. Am J Physiol Heart Circ Physiol 292: H2491–H2497, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Barker RC, Hopkins SR, Kellogg N, Olfert IM, Brutsaert TD, Gavin TP, Entin PL, Rice AJ, Wagner PD. Measurement of cardiac output during exercise by open-circuit acetylene uptake. J Appl Physiol 87: 1506–1512, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Beaver WL, Lamarra N, Wasserman K. Breath-by-breath measurement of true alveolar gas exchange. J Appl Physiol 51: 1662–1675, 1981 [DOI] [PubMed] [Google Scholar]
- 8. Bellotti C, Calabria E, Capelli C, Pogliaghi S. Determination of maximal lactate steady state in healthy adults: can NIRS help? Med Sci Sports Exerc.In press [DOI] [PubMed] [Google Scholar]
- 9. Boone J, Koppo K, Barstow TJ, Bouckaert J. Pattern of deoxy [Hb+Mb] during ramp cycle exercise: influence of aerobic fitness status. Eur J Appl Physiol 105: 851–859, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Calbet JA, Gonzalez-Alonso J, Helge JW, Sondergaard H, Munch-Andersen T, Boushel R, Saltin B. Cardiac output and leg and arm blood flow during incremental exercise to exhaustion on the cycle ergometer. J Appl Physiol 103: 969–978, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Calbet JA, Jensen-Urstad M, van Hall G, Holmberg HC, Rosdahl H, Saltin B. Maximal muscular vascular conductances during whole body upright exercise in humans. J Physiol 558: 319–331, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Calbet JA, Lundby C, Sander M, Robach P, Saltin B, Boushel R. Effects of ATP-induced leg vasodilation on V̇o2 peak and leg O2 extraction during maximal exercise in humans. Am J Physiol Regul Integr Comp Physiol 291: R447–R453, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Chin LM, Kowalchuk JM, Barstow TJ, Kondo N, Amano T, Shiojiri T, Koga S. The relationship between muscle deoxygenation and activation in different muscles of the quadriceps during cycle ramp exercise. J Appl Physiol 111: 1259–1265, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol 97: 393–403, 2004 [DOI] [PubMed] [Google Scholar]
- 15. duManoir GR, DeLorey DS, Kowalchuk JM, Paterson DH. Kinetics of V̇o2 limb blood flow and regional muscle deoxygenation in young adults during moderate intensity, knee-extension exercise. Eur J Appl Physiol 108: 607–617, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Faulkner JA, Heigenhauser GJ, Schork MA. The cardiac output–oxygen uptake relationship of men during graded bicycle ergometry. Med Sci Sports 9: 148–154, 1977 [PubMed] [Google Scholar]
- 17. Ferreira LF, Koga S, Barstow TJ. Dynamics of noninvasively estimated microvascular O2 extraction during ramp exercise. J Appl Physiol 103: 1999–2004, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Fu Q, Levine BD. Cardiovascular response to exercise in women. Med Sci Sports Exerc 37: 1433–1435, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Gravelle BM, Murias JM, Spencer MD, Paterson DH, Kowalchuk JM. Adjustments of O2 uptake and muscle deoxygenation during ramp incremental exercise and constant-load moderate-intensity exercise in young and older adults. J Appl Physiol 304: R238–R247, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Green HJ, Patla AE. Maximal aerobic power: neuromuscular and metabolic considerations. Med Sci Sports Exerc 24: 38–46, 1992 [PubMed] [Google Scholar]
- 21. Hansen J, Thomas GD, Harris SA, Parsons WJ, Victor RG. Differential sympathetic neural control of oxygenation in resting and exercising human skeletal muscle. J Clin Invest 98: 584–596, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hossack KF, Bruce RA. Maximal cardiac function in sedentary normal men and women: comparison of age-related changes. J Appl Physiol 53: 799–804, 1982 [DOI] [PubMed] [Google Scholar]
- 23. Johnson BD, Beck KC, Proctor DN, Miller J, Dietz NM, Joyner MJ. Cardiac output during exercise by the open circuit acetylene washin method: comparison with direct Fick. J Appl Physiol 88: 1650–1658, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Knight DR, Poole DC, Schaffartzik W, Guy HJ, Prediletto R, Hogan MC, Wagner PD. Relationship between body and leg V̇o2 during maximal cycle ergometry. J Appl Physiol 73: 1114–1121, 1992 [DOI] [PubMed] [Google Scholar]
- 25. Koga S, Poole DC, Ferreira LF, Whipp BJ, Kondo N, Saitoh T, Ohmae E, Barstow TJ. Spatial heterogeneity of quadriceps muscle deoxygenation kinetics during cycle exercise. J Appl Physiol 103: 2049–2056, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Koga S, Poole DC, Shiojiri T, Kondo N, Fukuba Y, Miura A, Barstow TJ. Comparison of oxygen uptake kinetics during knee extension and cycle exercise. Am J Physiol Regul Integr Comp Physiol 288: R212–R220, 2005 [DOI] [PubMed] [Google Scholar]
- 27. McDonough JR, Danielson RA. Variability in cardiac output during exercise. J Appl Physiol 37: 579–583, 1974 [DOI] [PubMed] [Google Scholar]
- 28. Mortensen SP, Dawson EA, Yoshiga CC, Dalsgaard MK, Damsgaard R, Secher NH, Gonzalez-Alonso J. Limitations to systemic and locomotor limb muscle oxygen delivery and uptake during maximal exercise in humans. J Physiol 566: 273–285, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Murias JM, Kowalchuk JM, Paterson DH. Mechanisms for increases in V̇o2 max with endurance training in older and young women. Med Sci Sports Exerc 42: 1891–1898, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Murias JM, Kowalchuk JM, Paterson DH. Time course and mechanisms of adaptations in cardiorespiratory fitness with endurance training in older and young men. J Appl Physiol 108: 621–627, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Murias JM, Spencer MD, Kowalchuk JM, Paterson DH. Influence of phase I duration on phase II V̇o2 kinetics parameter estimates in older and young adults. Am J Physiol Regul Integr Comp Physiol 301: R218–R224, 2011 [DOI] [PubMed] [Google Scholar]
- 32. Murias JM, Spencer MD, Pogliaghi S, Paterson DH. Non-invasive estimation of microvascular O2 provision during exercise on-transients in healthy young males. Am J Physiol Regul Integr Comp Physiol 303: R815–R823, 2012 [DOI] [PubMed] [Google Scholar]
- 33. Paterson ND, Kowalchuk JM, Paterson DH. Kinetics of V̇o2 and femoral artery blood flow during heavy-intensity, knee-extension exercise. J Appl Physiol 99: 683–690, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Proctor DN, Beck KC, Shen PH, Eickhoff TJ, Halliwill JR, Joyner MJ. Influence of age and gender on cardiac output-V̇o2 relationships during submaximal cycle ergometry. J Appl Physiol 84: 599–605, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Richardson RS, Grassi B, Gavin TP, Haseler LJ, Tagore K, Roca J, Wagner PD. Evidence of O2 supply-dependent V̇o2 max in the exercise-trained human quadriceps. J Appl Physiol 86: 1048–1053, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick KF, Erickson BK, Wagner PD. High muscle blood flow in man: is maximal O2 extraction compromised? J Appl Physiol 75: 1911–1916, 1993 [DOI] [PubMed] [Google Scholar]
- 37. Roach RC, Koskolou MD, Calbet JA, Saltin B. Arterial O2 content and tension in regulation of cardiac output and leg blood flow during exercise in humans. Am J Physiol Heart Circ Physiol 276: H438–H445, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev 54: 75–159, 1974 [DOI] [PubMed] [Google Scholar]
- 39. Smyth RJ, Gledhill N, Froese AB, Jamnik VK. Validation of noninvasive maximal cardiac output measurement. Med Sci Sports Exerc 16: 512–515, 1984 [DOI] [PubMed] [Google Scholar]
- 40. Spencer MD, Murias JM, Paterson DH. Characterizing the profile of muscle deoxygenation during ramp incremental exercise in young men. Eur J Appl Physiol 112: 3349–3360, 2012 [DOI] [PubMed] [Google Scholar]
- 41. Stringer WW, Whipp BJ, Wasserman K, Porszasz J, Christenson P, French WJ. Non-linear cardiac output dynamics during ramp-incremental cycle ergometry. Eur J Appl Physiol 93: 634–639, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Thomas GD, Victor RG. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol 506: 817–826, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tschakovsky ME, Rogers AM, Pyke KE, Saunders NR, Glenn N, Lee SJ, Weissgerber T, Dwyer EM. Immediate exercise hyperemia in humans is contraction intensity dependent: evidence for rapid vasodilation. J Appl Physiol 96: 639–644, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Tschakovsky ME, Sheriff DD. Immediate exercise hyperemia: contributions of the muscle pump vs. rapid vasodilation. J Appl Physiol 97: 739–747, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol 541: 623–635, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. VanTeeffelen JW, Segal SS. Interaction between sympathetic nerve activation and muscle fibre contraction in resistance vessels of hamster retractor muscle. J Physiol 550: 563–574, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]