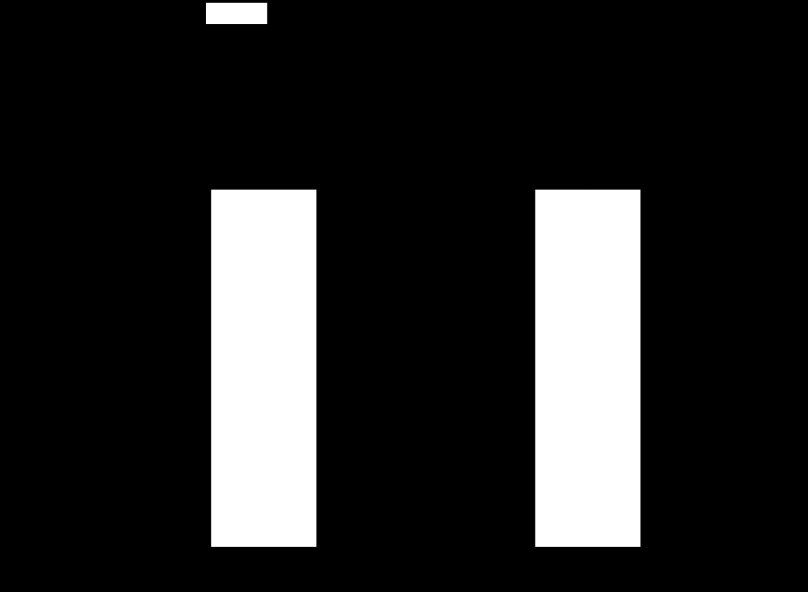Abstract
Fetal hypoxia causes protein kinase Cε (PKCε) gene repression in the heart resulting in heightened ischemic injury in male offspring in a sex-dependent manner. The present study tested the hypothesis that heightened methylation of the early growth response factor-1 (Egr-1) binding site at PKCε gene promoter contributes to sex dimorphism of hypoxia-induced programming of PKCε gene repression in the developing heart. Pregnant rats were divided into normoxic and hypoxic (10.5% O2 from day 15 to 21 of gestation) groups. Hypoxia selectively decreased PKCε mRNA and protein abundance in the heart of male, but not female, near-term (21 days) fetuses. Methylation of the CpG site at the Egr-1 binding site of PKCε promoter was significantly increased in the male hearts by hypoxia, resulting in decreased Egr-1 binding affinity and reduced Egr-1 binding to the PKCε promoter. Nuclear Egr-1 levels were not affected by hypoxia. There was significantly higher abundance of estrogen receptor α (ERα) and β (ERβ) isoforms in female than in male fetal hearts, which were not significantly altered by hypoxia. Both ERα and ERβ bind to the Egr-1 binding site with significant greater levels in the female fetal hearts. The increased methylation with reduced Egr-1 binding and PKCε gene repression persisted in 3-mo-old adult male hearts in a sex-dependent manner. The results indicate a key role for heightened methylation of the Egr-1 binding site in hypoxia-mediated programming of PKCε gene repression in the developing heart and suggest a novel protective mechanism of ER by binding to the Egr-1 binding site in epigenetic regulation of PKCε gene expression patterns in the early developmental stage.
Keywords: hypoxia, heart, PKCε, DNA methylation, Egr-1
large epidemiological studies have shown that adverse environment in utero causes increased risk of developing endocrine and cardiovascular disorders such as diabetes, hypertension, and ischemic heart disease in adult life (4, 5, 9, 11, 21, 32). Hypoxia during gestation is one of the most common insults to the fetal development and is thought to be associated with fetal intrauterine growth restriction and increased risk of cardiovascular dysfunction in offspring (3, 12, 22, 25, 35). Recent studies in rats have found that maternal hypoxia results in developmental programming of heightened heart vulnerability to ischemia and reperfusion injury in male offspring in a sex-dependent manner (3, 22). Among other mechanisms, it has been demonstrated that repression of protein kinase Cε (PKCε) gene in the developing heart is a congruent mechanism for programming of increased heart susceptibility to ischemia and reperfusion injury in offspring (2, 19, 24, 35).
DNA methylation is a chief mechanism for epigenetic modification of gene expression patterns that are essential for development and differentiation and allow an organism to respond to the environment. Our recent studies demonstrated that fetal hypoxia increased CpG methylation of the Sp1 binding sites at rat PKCε gene promoter, which contributed to PKCε gene repression in the developing heart (29). Subsequently, our studies also demonstrated a key role of the early growth response factor-1 (Egr-1) binding site in the regulation of PKCε promoter activity in fetal rat hearts (18). Yet, it remains elusive whether heightened methylation of the Egr-1 binding site contributes to sex dimorphism of hypoxia-induced programming of PKCε gene repression in the developing heart. Herein, we present evidence that fetal hypoxia causes an increase in methylation of the Egr-1 binding site at the proximal promoter region of the PKCε gene resulting in PKCε gene repression in the fetal and offspring rat hearts. Furthermore, we demonstrate a novel protective mechanism of estrogen receptors in sex-dependent epigenetic programming of PKCε gene expression patterns at early developmental stage by binding to the Egr-1 binding site.
MATERIALS AND METHODS
Experimental animals.
Time-dated pregnant Sprague-Dawley rats were purchased from Charles River Laboratories (Portage, MI) and were randomly divided into two groups: 1) normoxic control and 2) hypoxic treatment of 10.5% O2 from day 15 to 21 of gestation, as described previously (36). Hearts were isolated from near-term (21 days) fetuses and 3-mo-old offspring. To isolate hearts, rats were anesthetized with 75 mg/kg ketamine and 5 mg/kg xylazine injected intramuscularly. For a separate study, male fetal hearts were isolated and cultured in M199 media (Hyclone, Logan, UT) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (Gemini, Woodland, CA) at 37°C in 95% air-5% CO2. Hearts were given 24 h of recovery and then treated under normoxia or hypoxia (1% O2) in the absence or presence of 10 μM 5-aza-2′-deoxycytidine (5-Aza) (Sigma, St. Louis, MO) for 48 h, as previously described (23, 29). All procedures and protocols were approved by the Institutional Animal Care and Use Committee of Loma Linda University and followed the guidelines by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Western blot analysis.
Hearts were homogenized in a lysis buffer containing 150 mM NaCl, 50 mM Tris·HCl, 10 mM EDTA, 0.1% Tween-20, 0.1% β-mercaptoethanol, 0.1 mM phenylmethylsulfonyl fluoride, 5 μg/ml leupeptin, and 5 μg/ml aprotinin, pH 7.4, and allowed to incubate for 1 h on ice. Homogenates were then centrifuged at 4°C for 10 min at 10,000 g, and supernatants were collected. Nuclear extracts were prepared from hearts using NXTRACT CelLytic Nuclear Extraction Kit (Sigma). Protein concentrations were measured using a protein assay kit (Bio-Rad, Hercules, CA). Samples with equal amounts of protein were loaded onto 7.5% polyacrylamide gel with 0.1% SDS and separated by electrophoresis at 100 V for 90 min. Proteins were then transferred onto nitrocellulose membranes. Nonspecific binding sites were blocked for 1 h at room temperature in a Tris-buffered saline solution containing 5% dry milk. The membranes were then probed with primary antibodies against PKCε (sc-214), estrogen receptor α (ERα, sc-7207) and β (ERβ, sc-8974), and Egr-1 (sc-110) (Santa Cruz Biotechnology; Santa Cruz, CA). Actin antibody (A-4799) (Sigma) was used as a loading control. After washing was completed, membranes were incubated with secondary horseradish peroxidase-conjugated antibodies. Proteins were visualized with enhanced chemiluminescence reagents, and blots were exposed to Hyperfilm. The results were analyzed with the Kodak ID image analysis software.
Real-time RT-PCR.
RNA was extracted from hearts using TRIzol protocol (Invitrogen, Carlsbad, CA). PKCε mRNA abundance was determined by real-time RT-PCR using Icycler Thermal cycler (Bio-Rad) with the primers of 5′-GCGAAGCCCCTAAGACAAT-3′ (forward) and 5′-CACCCCAGATGAAATCCCTAC-3′ (reverse) (18). Real-time RT-PCR was performed in a final volume of 25 μl. Each PCR reaction mixture consisted of 600 nM of primers, 33 units of M-MLV reverse transcriptase (Promega, Madison, WI), and iQ SYBR Green Supermix (Bio-Rad) containing 0.625 unit Taq polymerase; 400 μM each of dATP, dCTP, dGTP, and dTTP; 100 mM KCl; 16.6 mM ammonium sulfate; 40 mM Tris·HCl; 6 mM MgSO4; SYBR Green I; 20 nM fluorescine; and stabilizers. We used the following RT-PCR protocol: 42°C for 30 min, 95°C for 15 min, followed by 40 cycles of 95°C for 20 s, 52°C for 1 min. GAPDH was used as an internal reference, and serial dilutions of the positive control were performed on each plate to create a standard curve. PCR was performed in triplicate and threshold cycle numbers were averaged.
Quantitative methylation-specific PCR.
DNA was isolated from hearts using a GenElute Mammalian Genomic DNA Mini-Prep kit (Sigma), denatured with 2 N NaOH at 42°C for 15 min, and treated with sodium bisulfite at 55°C for 16 h, as previously described (18). DNA was purified with a Wizard DNA clean up system (Promega) and resuspended in 120 μl of H2O. Bisulfite-treated DNA was used as a template for real-time fluorogenic methylation-specific PCR (MSP) using primers created to amplify promoter sequences containing Egr-1 binding site based on the previous sequencing of rat PKCε promoter (18). Real-time MSP was performed using the iQ SYBR Green Supermix with iCycler real-time PCR system (Bio-Rad).
Electrophoretic mobility shift assay.
Nuclear extracts were collected from hearts using NXTRACT CelLytic Nuclear Extraction Kit (Sigma). The oligonucleotide probes with CpG and mCpG of the Egr-1 binding site at rat PKCε promoter region were labeled and subjected to gel shift assays using the biotin 3′-end-labeling kit and LightShift Chemiluminescent EMSA Kit (Pierce Biotechnology, Rockford, IL), as previously described (21). Briefly, single-stranded oligos were incubated with terminal deoxynucleotidyl transferase (TdT) and biotin-11-dUTP in binding mixture for 30 min at 37°C. The TdT adds a biotin-labeled dUTP to the 3′-end of the oligonucleotides. The oligos were extracted using chloroform and isoamyl alcohol to remove the enzyme and unincorporated biotin-11-dUTP. Dot blots were performed to ensure the oligos were labeled equally. Combining sense and antisense oligos and exposing to 95°C for 5 min was done to anneal complementary oligos. The labeled oligonucleotides were then incubated with or without nuclear extracts in the binding buffer (from LightShift kit). Binding reactions were performed in 20 μl containing 50 fmol oligonucleotide probes, 1× binding buffer, 1 μg of poly (dI-dC), and 5 μg of nuclear extracts. For competition studies, increasing concentrations of nonlabeled oligonucleotides were added to binding reactions. The samples were then run on a native 5% polyacrylamide gel. The contents of the gel were then transferred to a nylon membrane (Pierce) and crosslinked to the membrane using a UV crosslinker (125 mJ/cm2). Membranes were blocked and then visualized using the reagents provided in the LightShift kit.
Chromatin immunoprecipitation.
Chromatin extracts were prepared from hearts. Chromatin immunoprecipitation (ChIP) assays were performed using the ChIP-IT kit (Active Motif), as previously described (18). Briefly, heart tissues were incubated with 1% formaldehyde for 10 min to crosslink and maintain DNA-protein interactions. After the reactions were stopped with glycine, tissues were washed, and chromatin was isolated and sheared into medium fragments (100–1,000 base pairs) using a sonicator. ChIP reactions were performed using an Egr-1 antibody or ERα and ERβ antibodies to precipitate the transcription factor/DNA complex. Crosslinking was then reversed using a salt solution, and the proteins were digested with proteinase K. Primers flanking the Egr-1 binding site were used for PCR: 5′-GATCCGAGGAGCACAGAC-3′ (forward) and 5′-GTGAGCCGAGCAGAAAAC-3′ (reverse). PCR amplification products were visualized on 2% agarose gel stained with ethidium bromide. To quantify PCR amplification, 45 cycles of real-time PCR were carried out with 3 min initial denaturation followed by 95°C for 30 s, 59°C for 30 s, and 72°C for 30 s, using the iQ SYBR Green Supermix with iCycler real-time PCR system (Bio-Rad).
Statistical analysis.
Data are expressed as means ± SE. Statistical significance (P < 0.05) was determined by analysis of variance (ANOVA) followed by Newman-Keuls post hoc testing or Student's t-test, where appropriate.
RESULTS
Hypoxia decreases PKCε expression in fetal hearts in a gender-dependent manner.
Figure 1 shows that maternal hypoxia resulted in significant decreases in both protein and mRNA abundance of PKCε in male, but not female, fetal rat hearts.
Fig. 1.
Effect of hypoxia on protein kinase Cε (PKCε) protein and mRNA abundance in fetal hearts. Hearts were isolated from 21-day fetuses from pregnant rats treated with normoxia control or hypoxia from day 15 to day 21 of gestation. PKCε protein and mRNA abundance was determined using Western blot analysis and quantitative real-time RT-PCR, respectively. Actin was used as a loading control. Data are presented as means ± SE; n = 5, analyzed by two-way ANOVA. *P < 0.05, hypoxia vs. control.
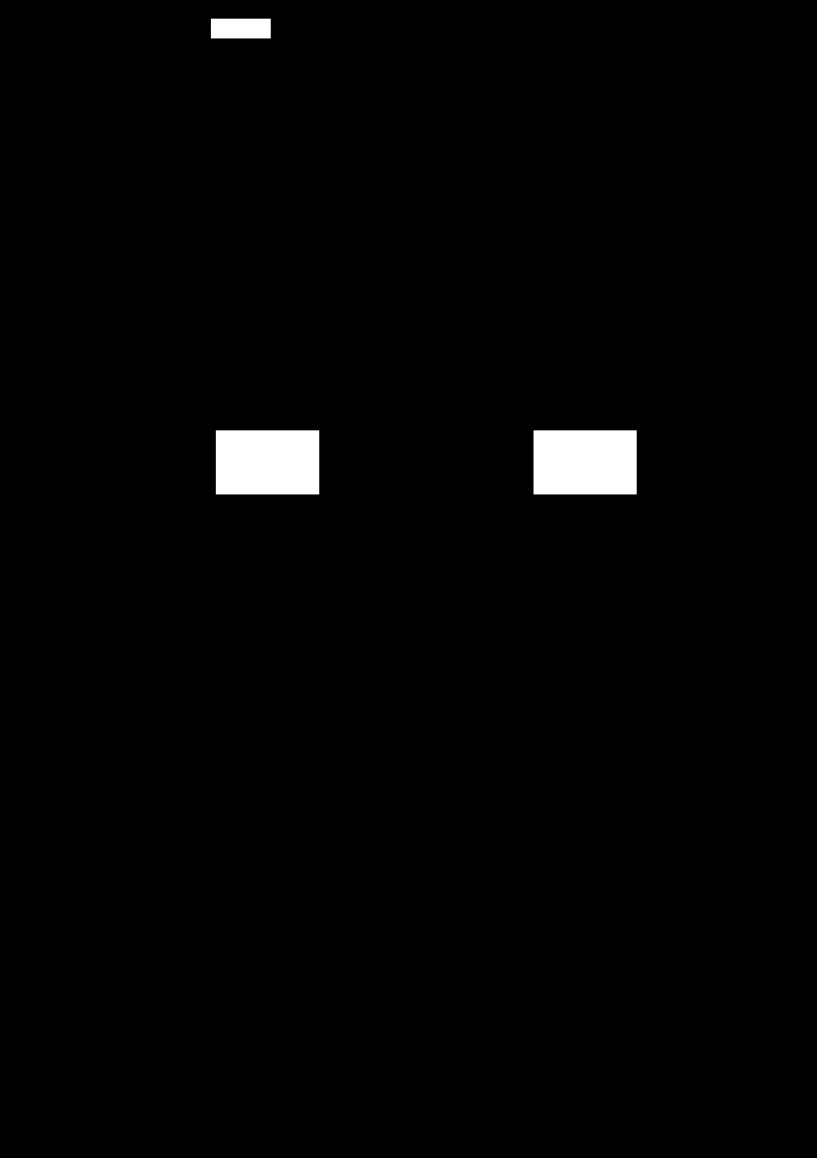
Hypoxia increases CpG methylation of the Egr-1 binding site.
Maternal hypoxic treatment caused a significant increase in CpG methylation of the Egr-1 binding site in male fetal hearts (Fig. 2A). In female hearts, the hypoxia-mediated increase of CpG methylation of the Egr-1 binding site was moderate, but also significant, which was significantly reduced compared with that seen in males (Fig. 2A).
Fig. 2.
Effect of hypoxia on CpG methylation of early growth response factor-1 (Egr-1) binding site at PKCε promoter and Egr-1 binding in fetal hearts. DNA and nuclear extracts were prepared from 21-day fetal hearts. A: bisulfite-treated DNA was used as a template for real-time methylation-specific PCR. B: competition binding was performed in pooled nuclear extracts with oligonucleotides containing the consensus Egr-1 motifs at −1008 with methylated (M oligo) and unmethylated (UM oligo) CpG. Data are presented as means ± SE; n = 5, analyzed by two-way ANOVA. aP < 0.05, hypoxia vs. control; bP < 0.05, female vs. male.
Methylation of the Egr-1 binding site decreases Egr-1 binding.
To determine whether methylation of the Egr-1 binding site at the PKCε promoter has any impact on transcription factor binding, we performed electrophoretic mobility shift assay with methylated and unmethylated oligonucleotide probes containing the Egr-1 binding site. As shown in Fig. 2B, competition studies performed in pooled nuclear extracts with increasing ratios of unlabeled unmethyled or methyled oligonucleotides indicated that methylation of the Egr-1 binding site significantly decreased the Egr-1 binding affinity in fetal hearts. To determine whether hypoxia-mediated heightened methylation of the Egr-1 binding site affects Egr-1 binding to the PKCε promoter in vivo in the context of intact chromatin in the hearts, we performed ChIP assays using an Egr-1 antibody. Quantitative real-time PCR revealed a marked decrease of Egr-1 binding to the PKCε promoter in hypoxia-treated male fetal hearts (Fig. 3). In contrast, the binding of Egr-1 to the PKCε promoter in female fetal hearts was not significantly altered (Fig. 3). Moreover, Egr-1 abundance in nuclear extracts of either male or female fetal hearts has no significant difference between hypoxia-treated and control animals (data not shown).
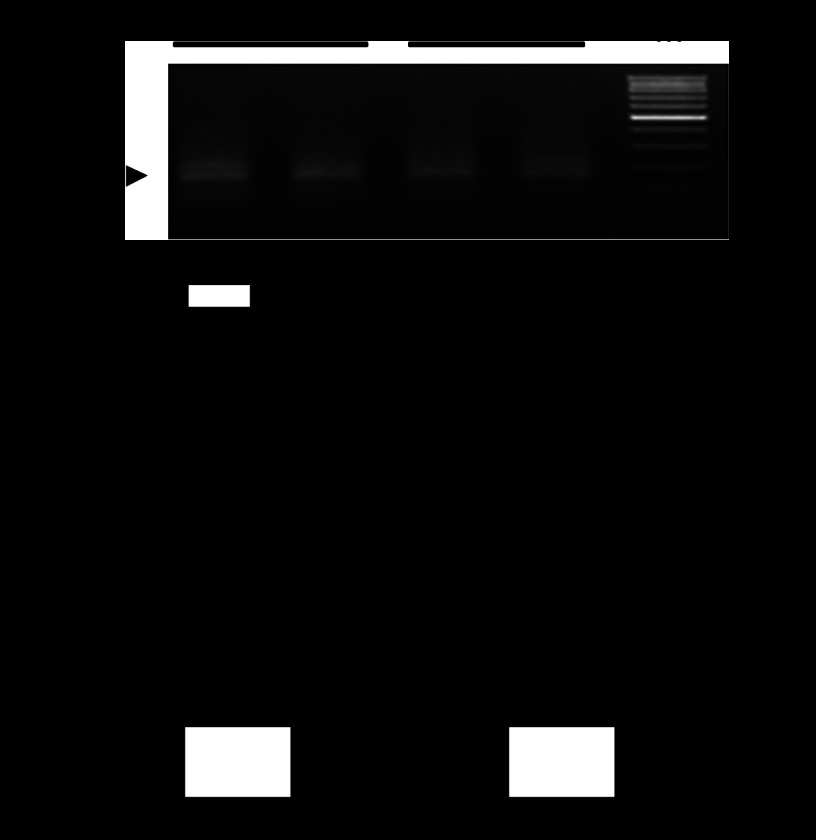
Fig. 3.
Effect of hypoxia on in vivo Egr-1 binding to PKCε promoter in fetal hearts. Hearts were isolated from 21-day fetuses from pregnant rats treated with normoxia control or hypoxia from day 15 to day 21 of gestation. Chromatin extracts prepared from fetal hearts were sonicated to produce DNA fragments between 100 and 500 bp in length. Chromatin immunoprecipitation (ChIP) assays were performed using an Egr-1 antibody. Antibody-pulled chromatin extracts were used as templates for quantitative real-time PCR using primers flanking the element of Egr-1 binding site at PKCε promoter. Data are presented as means ± SE; n = 5, analyzed by two-way ANOVA. *P < 0.05, hypoxia vs. control.
Inhibition of DNA methylation restores PKCε gene expression.
To determine the causal role of Egr-1 binding site methylation in hypoxia-mediated PKCε gene repression, male fetal hearts were exposed ex vivo to hypoxic treatment in the absence or presence of the DNA methylation inhibitor 5-Aza. As shown in Fig. 4, 5-Aza blocked hypoxia-induced methylation of the Egr-1 binding site (Fig. 4A) and restored the expression of PKCε mRNA (Fig. 4B) and protein (Fig. 4C) in fetal hearts.
Fig. 4.
5-Aza-2′-deoxycytidine (5-Aza) abrogates hypoxia-induced Egr-1 CpG methylation and PKCε gene repression in male fetal hearts. Hearts isolated from 20-day male fetuses were treated ex vivo with 21% O2 (control) or 1% O2 (hypoxia) for 48 h in the absence or presence of 10 μM 5-Aza. Egr-l CpG methylation was determined by real-time methylation-specific PCR (A). PKCε mRNA (B) and protein (C) abundance was determined by Western blot and quantitative real-time RT-PCR, respectively. Data are presented as means ± SE; n = 5, analyzed by two-way ANOVA. *P < 0.05, hypoxia vs. control.
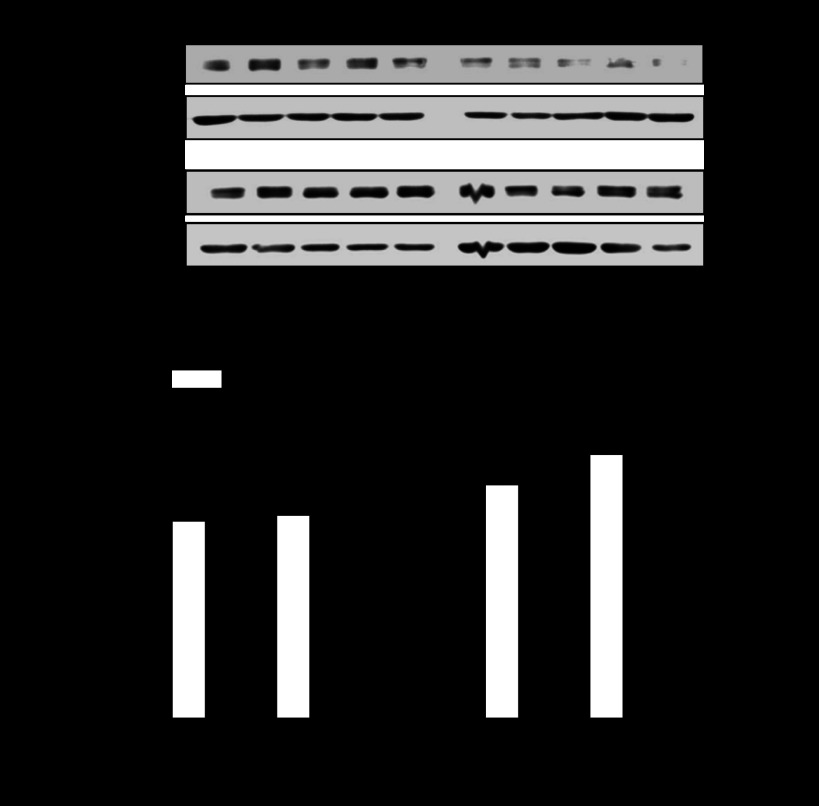
ERα and -β bind to the Egr-1 binding site in rat hearts.
The sex difference of hypoxia-mediated heightened methylation of the Egr-1 binding site and PKCε gene repression observed in fetal hearts was intriguing given that both male and female fetuses were likely exposed to the similar concentrations of steroid hormones in utero. ChIP assays demonstrated the PCR products of the Egr-1 binding site in the DNA sequences pulled down by both ERα and ERβ antibodies in fetal hearts (Fig. 5), indicating a binding of both estrogen receptors to the Egr-1 binding sites at the PKCε promoter. The binding of ER with the Egr-1 binding site was significantly greater in the female hearts compared with that in the male hearts (Fig. 5). Our previous study demonstrated that the expression of both ERα and ERβ protein was significantly higher in female fetal hearts than in male fetal hearts (29). In the present study, the expression of ERα and ERβ were determined in fetal hearts exposed to prenatal hypoxia treatment. As shown in Fig. 6, ERα protein abundance maintained significantly higher in female than male fetal hearts, although ERβ abundance was not significantly different between female and male hearts in hypoxia-treated animals.
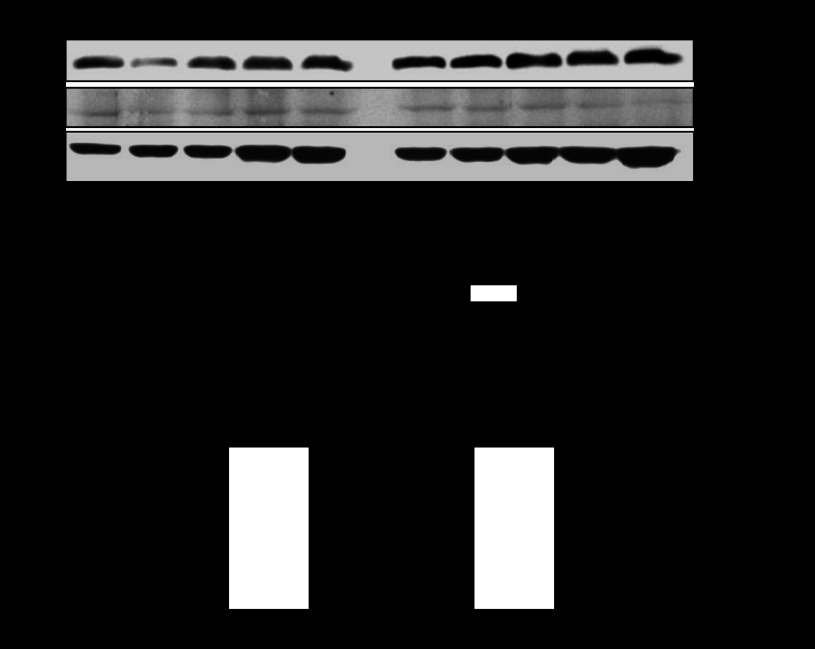
Fig. 5.
Estrogen receptors (ERα and ERβ) bind to Egr-1 binding site in fetal hearts. Chromatin extracts prepared from 21-day fetal hearts were sonicated to produce DNA fragments between 100 and 500 bp in length. ERα and ERβ antibody-pulled chromatin extracts were used as templates for quantitative real-time PCR using primers flanking the element of Egr-1 binding site at PKCε promoter. M, markers. Data are presented as means ± SE; n = 5, analyzed by two-way ANOVA. *P < 0.05, female vs. male.
Fig. 6.
ERα and ERβ protein abundance in fetal hearts. Hearts were isolated from 21-day fetuses from pregnant rats treated with hypoxia from day 15 to day 21 of gestation. ERα and ERβ protein abundance in fetal hearts were determined by Western blot using specific antibodies. Data are presented as means ± SE; n = 5, analyzed by two-way ANOVA. *P < 0.05, female vs. male.
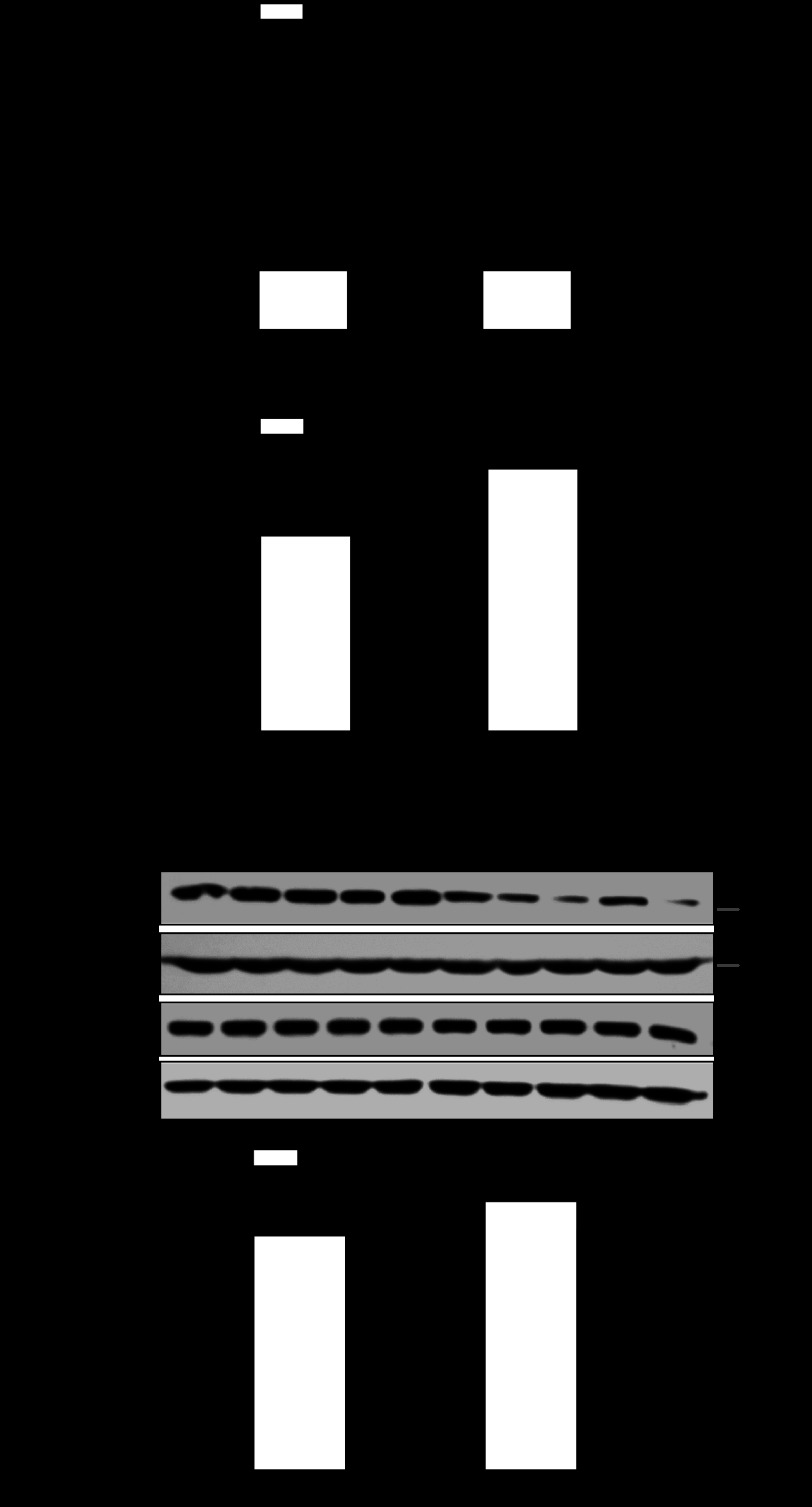
Hypoxia-mediated heightened methylation of the Egr-1 binding site and PKCε gene repression persisted in adult male offspring.
Figure 7 shows that hypoxia-induced sex dimorphism of heightened methylation of the Egr-1 binding site and PKCε gene repression in the developing heart persisted in adult offspring, showing increased methylation of the Egr-1 binding site, reduced Egr-1 binding to the PKCε promoter, and decreased PKCε mRNA and protein abundance in the hearts of 3-mo-old male offspring that had been exposed to hypoxia before birth. In contrast, the hearts of female offspring were not significantly affected (Fig. 7).
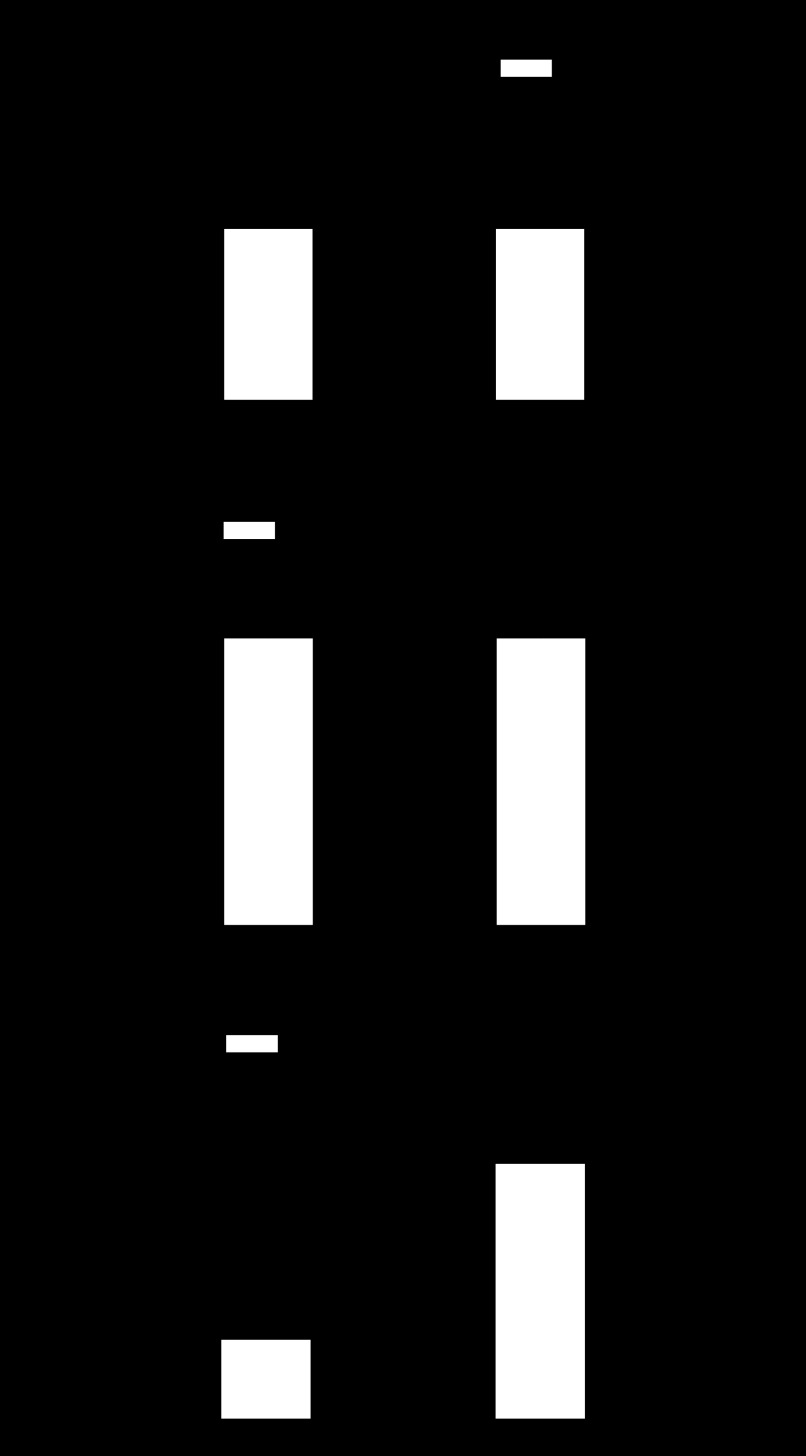
Fig. 7.
Effect of fetal hypoxia on Egr-1 binding site methylation, Egr-1 binding, and PKCε expression in adult hearts. Hearts were isolated from 3-mo-old male and female offspring of control and prenatally hypoxic animals. Methylation of Egr-1 binding site was determined by methylation-specific PCR (A). Egr-1 binding was determined by ChIP assays (B). PKCε protein and mRNA abundance was determined by Western blot and quantitative real-time RT-PCR, respectively (C). Data are presented as means ± SE; n = 5, analyzed by two-way ANOVA. *P < 0.05, hypoxia vs. control.
DISCUSSION
Our previous studies demonstrated that prenatal hypoxia caused an increase in heart susceptibility to ischemia and reperfusion injury in a sex-dependent manner, which was due to fetal programming of PKCε gene repression resulting in downregulation of PKCε function in the heart of adult male offspring (36). Subsequent studies demonstrated that increased methylation of both Sp1 and Egr-1 binding sites at PKCε promoter played an important role in intrauterine programming of PKCε gene repression in the heart, leading to the enhanced susceptibility to ischemia and reperfusion injury in adult offspring (18, 29). Deletion of either Sp1 or Egr-1 binding site caused a significant decrease of PKCε promoter activity, indicating that both Sp1 and Egr-1 binding sites play an important role in modulating PKCε gene expression. The previous study demonstrated that both ERα and ERβ bound to the Sp1 binding site at the PKCε promoter in fetal hearts, suggesting a possible mechanism for the increased protection of Sp1 binding sites and PKCε transcription in the female hearts in response to hypoxic stress (29). In the present study, we demonstrated further that, in addition to the Sp1 binding site, ERα and ERβ also bound to the Egr-1 binding site at PKCε promoter in fetal hearts, which was significantly greater in female hearts than male hearts. Whereas the interaction of ER and Sp1 binding sites has been well documented in the previous studies (28), the binding of ER to Egr-1 binding sites has not been previously reported. Thus the present study provides a novel finding and suggests a new mechanism of ER in protecting promoter methylation in female hearts, which may contribute to the sex dimorphism of hypoxia-induced programming of PKCε gene expression patterns in the developing heart. The finding that the sex dichotomy of Egr-1 site methylation and transcription factor binding observed in fetal hearts were sustained in the hearts of 3-mo-old offspring suggests the long-term effect of ER-mediated protection of PKCε gene expression in the developing heart. Future studies are needed to determine whether ER binds to the Egr-1 binding site directly or through a protein-protein interaction with the Egr-1 transcription factor.
Egr-1 is a nuclear protein with sequence-specific DNA binding activity, and it is suggested as an intracellular “third messenger” by regulating expression of multiple downstream target genes (16). It has been reported that Egr-1 is over expressed in the heart after ischemia and reperfusion challenge (1, 8). Downregulation of Egr-1 expression has been shown to have a potential protective effect on heart ischemia and reperfusion injury (6, 13, 38). Accordingly, methylation of the Egr-1 binding site at PKCε gene promoter in the heart could be a self-protection mechanism against enhanced susceptibility to ischemia and reperfusion-mediated injury induced by hypoxia. Therefore, the present study proceeded to determine the potential epigenetic-regulating roles of Egr-1 binding site in prenatal hypoxia-induced sex-dependent downregulation of PKCε gene in fetal and adult offspring hearts. In the present study, we found that prenatal chronic hypoxia significantly increased the methylation level of Egr-1 binding site at PKCε promoter in male fetal and adult offspring hearts, whereas little or no increasing of Egr-1 binding site methylation at PKCε promoter was found in female fetal or adult hearts.
Although the previous study showed that an extended 5′-deletions of the PKCε promoter from −1163 to −444, which contains the Egr-1 binding site at −1008, as well as multiple other binding sites, had no significant effect on the promoter activity (37), the more confined deletion from −1163 to −826 in another study revealed a significant decrease in the PKCε promoter activity (18). Furthermore, the functional significance of the Egr-1 binding site in regulating PKCε gene activity was demonstrated by a site-specific deletion of the Egr-1 element at −1008 (18). Our previous electrophoretic mobility shift assays confirmed the true interaction of Egr-1 binding site with its transcription factors by causing supershifting of the DNA-protein complex with an Egr-1 antibody (18). To determine whether methylation of the Egr-1 binding site at the PKCε promoter inhibits Egr-1 binding, we performed competitive electrophoretic mobility shift assays using pooled nuclear extracts with increasing ratios of unlabeled unmethyled and methyled oligonucleotides and demonstrated that methylation of Egr-1 binding site decreased its binding with Egr-1 in both male and female hearts. These findings indicate that CpG methylation of sequence-specific Egr-1 binding site may directly inhibit DNA binding of Egr-1 complexes, resulting in downregulation of PKCε gene expression in the heart. This is consistent with the previous findings that methylation of the Egr-1 binding site in muscle p57kip2 promoter repressed its expression by directly interfering methyl groups with the binding of the transcription factor (10). To determine whether the hypoxia-mediated increase in methylation status of the Egr-1 binding site may inhibit Egr-1 binding to PKCε promoter in vivo in the context of intact chromatin, ChIP assays were performed using the Egr-1 antibody. It was demonstrated that prenatal hypoxic exposure significantly decreased the recruitment of Egr-1 to the PKCε promoter in the hearts of male fetuses and adult offspring. The finding that Egr-1 abundance was not changed by hypoxia in both male and female fetal hearts excluded the possible effect of altered Egr-1 abundance on Egr-1 binding to PKCε promoter. In addition, the finding that methylation inhibitor 5-Aza blocked hypoxia-induced methylation of Egr-1 binding site and restored the expression of PKCε gene in male fetal hearts further supports the notion that CpG methylation of transcription factor binding sites at PKCε promoter plays a pivotal role in hypoxia-mediated PKCε gene repression in the developing heart.
In the present study, the extent of decreased Egr-1 binding in intact chromatin was comparable to that of increased methylation at the Egr-1 binding site in male fetal and adult offspring hearts. However, no difference was observed in both female fetal and adult offspring hearts. The sex-different changes of PKCε mRNA and protein abundance were consistent with alterations in the methylation pattern of Egr-1 binding site and Egr-1 binding to PKCε promoter in both fetal and adult offspring hearts, suggesting that changes in CpG methylation and Egr-1 binding to the Egr-1 binding site play a key role in the sex-dependent epigenetic modification of PKCε expression in the heart. It has been reported that gender differences exist in susceptibility to and mortality from a variety of cardiovascular diseases (20). Recent studies in animal models and cardiomyocytes further showed that female hearts had greater resistance to ischemia and reperfusion-mediated injury (30, 33). Besides the possible roles of sex steroid hormones, sex-specific molecular cell death pathways, sex-specific myocardial inflammatory response, and differences between genetically male (XX) and female (XY) cells (14, 30, 33), the underlying mechanisms of the sex-different susceptibility to cardiovascular diseases is far less clear. Indirect evidence including the reduced risk of cardiovascular diseases in premenopausal women and in postmenopausal women with estrogen replacement therapy pointed to the protective effect of estrogen in the heart (27). In a recent publication, Lancaster and colleague (17) provide a strong rationale for the efficacy of acute PKCε activation to improve ischemic tolerance with postmenopausal estrogen deficiency, which indicated the possible cardioprotective role of PKCε through an estrogen/ER pathway.
Although it is plausible to speculate a primary role of sex hormones developed postnatally for the sex dichotomy seen in fetal programming of adult disease, our previous studies demonstrated that fetal hypoxia and cocaine exposure caused sex-dependent changes in PKCε gene expression patterns in hearts of fetuses and neonates before the sexual maturity (29, 37). The finding of greater expression of ERα and ERβ in the hearts of female fetuses is intriguing and may suggest a mechanism for the sex difference in the early developmental stage of programming. Although the mechanisms of differential ER expression in male and female fetuses are not clear at present, it is possible that ER expression may be based upon X chromosomal activity (31). Additionally, maternal estrogen may be handled differently by the placenta of female and male fetuses. Although ER acts as ligand-gated receptor to alter gene expression by binding to consensus estrogen response element (ERE) sites on the promoter, ER may also function in a ligand-independent manner to alter gene transcription. ER can be phosphorylated allowing it to bind to ERE or bind to DNA indirectly via another transcriptional factor, which is referred to as “transcriptional cross-talk” (26). It has been reported that ER can bind to DNA via transcriptional factors such as Ap1 and Sp1 (7, 15). Our previous study also demonstrated that both ERα and ERβ bound to the Sp1 binding site at the PKCε promoter in intact chromatin in the fetal heart (29), suggesting a possible protective role of ER by increasing Sp1 binding and PKCε transcription in the female hearts in response to hypoxic stress. The present finding that ER bound to the Egr-1 binding site provides a new target of transcription factor binding site in which ER may interact and regulate promoter activity and gene expression.
Perspectives and Significance
The sex difference is often observed in developmental programming of adult disease from large epidemiological as well as animal studies. Although female sex hormone estrogen produced postnatally is often thought to be a primary mechanism, recent findings of sex-dependent programming in early developmental stages before the sexual maturity suggest much earlier effects of sex hormones. The present study provides a novel finding and suggests a new mechanism of ER in protecting promoter methylation by binding to the Egr-1 binding site in fetal hearts, contributing to the sex dimorphism of fetal hypoxia-induced programming of PKCε gene expression patterns in the developing heart. Although the caution should be observed in extrapolating the findings of animal studies directly to humans, several lines of evidence suggest a possible clinical significance of these studies. Thus hypoxia is one of the most important and clinically relevant stresses to the fetus. The previous study has demonstrated that fetal hypoxia has distinct effects on programming of cardiovascular function, which is independent from the maternal nutrient status (34). Although gross adverse effect to the fetus may not be observed for pregnant women traveling to altitude,the notion that hypoxia may cause subtle epigenetic modifications of gene expression patterns in the fetus leading to abnormal function later in life cannot be excluded. Given that repression of PKCε gene in the developing heart is a congruent mechanism for fetal stress-mediated programming of increased heart susceptibility to ischemia and reperfusion injury in offspring (2, 19, 24, 36), the present study provides a new mechanism in our understanding of the sex dichotomy in epigenetic modifications of gene expression patterns in developmental programming of cardiac function in response to adverse intrauterine environment.
GRANTS
This work was supported by National Institutes of Health Grants HL-82779 (to L. Zhang), HD-31226 (to L. Zhang), HL-89012 (to L. Zhang), HL-83966 (to L. Zhang), and HL-110125 (to L. Zhang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.C. and F.X. performed experiments; M.C., F.X., and L.Z. analyzed data; M.C., F.X., and L.Z. interpreted results of experiments; M.C. prepared figures; M.C. drafted manuscript; M.C., F.X., and L.Z. edited and revised manuscript; L.Z. conception and design of research; L.Z. approved final version of manuscript.
REFERENCES
- 1. Aebert H, Cornelius T, Ehr T, Holmer SR, Birnbaum DE, Riegger GA, Schunkert H. Expression of immediate early genes after cardioplegic arrest and reperfusion. Ann Thorac Surg 63: 1669–1675, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Bae S, Xiao Y, Li G, Casiano CA, Zhang L. Effect of maternal chronic hypoxic exposure during gestation on apoptosis in fetal rat heart. Am J Physiol Heart Circ Physiol 285: H983–H990, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Bae S, Zhang L. Gender differences in cardioprotection against ischemia/reperfusion injury in adult rat hearts: focus on Akt and protein kinase C signaling. J Pharmacol Exp Ther 315: 1125–1135, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet 341: 938–941, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 298: 564–567, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhindi R, Fahmy RG, McMahon AC, Khachigian LM, Lowe HC. Intracoronary delivery of DNAzymes targeting human EGR-1 reduces infarct size following myocardial ischaemia reperfusion. J Pathol 227: 157–164, 2012 [DOI] [PubMed] [Google Scholar]
- 7. Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol 19: 833–842, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Brand T, Sharma HS, Fleischmann KE, Duncker DJ, McFalls EO, Verdouw PD, Schaper W. Proto-oncogene expression in porcine myocardium subjected to ischemia and reperfusion. Circ Res 71: 1351–1360, 1992 [DOI] [PubMed] [Google Scholar]
- 9. Eriksson JG, Forsen T, Tuomilehto J, Winter PD, Osmond C, Barker DJ. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ 318: 427–431, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Figliola R, Busanello A, Vaccarello G, Maione R. Regulation of p57(KIP2) during muscle differentiation: role of Egr1, Sp1 and DNA hypomethylation. J Mol Biol 380: 265–277, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Forsen T, Eriksson JG, Tuomilehto J, Osmond C, Barker DJ. Growth in utero and during childhood among women who develop coronary heart disease: longitudinal study. BMJ 319: 1403–1407, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Giussani DA, Phillips PS, Anstee S, Barker DJ. Effects of altitude versus economic status on birth weight and body shape at birth. Pediatr Res 49: 490–494, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Huang Z, Li H, Guo F, Jia Q, Zhang Y, Liu X, Shi G. Egr-1, the potential target of calcium channel blockers in cardioprotection with ischemia/reperfusion injury in rats. Cell Physiol Biochem 24: 17–24, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Hurn PD, Vannucci SJ, Hagberg H. Adult or perinatal brain injury: does sex matter? Stroke 36: 193–195, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Jakacka M, Ito M, Weiss J, Chien PY, Gehm BD, Jameson JL. Estrogen receptor binding to DNA is not required for its activity through the nonclassical AP1 pathway. J Biol Chem 276: 13615–13621, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Kaufmann K, Thiel G. Epidermal growth factor and thrombin induced proliferation of immortalized human keratinocytes is coupled to the synthesis of Egr-1, a zinc finger transcriptional regulator. J Cell Biochem 85: 381–391, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Lancaster TS, Jefferson SJ, Korzick DH. Local delivery of a PKCε-activating peptide limits ischemia reperfusion injury in the aged female rat heart. Am J Physiol Regul Integr Comp Physiol 301: R1242–R1249, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lawrence J, Chen M, Xiong F, Xiao D, Zhang H, Buchholz JN, Zhang L. Foetal nicotine exposure causes PKCepsilon gene repression by promoter methylation in rat hearts. Cardiovasc Res 89: 89–97, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lawrence J, Xiao D, Xue Q, Rejali M, Yang S, Zhang L. Prenatal nicotine exposure increases heart susceptibility to ischemia/reperfusion injury in adult offspring. J Pharmacol Exp Ther 324: 331–341, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leinwand LA. Sex is a potent modifier of the cardiovascular system. J Clin Invest 112: 302–307, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leon DA, Lithell HO, Vagero D, Koupilova I, Mohsen R, Berglund L, Lithell UB, McKeigue PM. Reduced fetal growth rate and increased risk of death from ischaemic heart disease: cohort study of 15,000 Swedish men and women born 1915–29. BMJ 317: 241–245, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li G, Xiao Y, Estrella JL, Ducsay CA, Gilbert RD, Zhang L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J Soc Gynecol Investig 10: 265–274, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Meyer K, Zhang H, Zhang L. Direct effect of cocaine on epigenetic regulation of PKCepsilon gene repression in the fetal rat heart. J Mol Cell Cardiol 47: 504–511, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meyer KD, Zhang H, Zhang L. Prenatal cocaine exposure abolished ischemic preconditioning-induced protection in adult male rat hearts: role of PKCε. Am J Physiol Heart Circ Physiol 296: H1566–H1576, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moore LG, Brodeur P, Chumbe O, D'Brot J, Hofmeister S, Monge C. Maternal hypoxic ventilatory response, ventilation, and infant birth weight at 4,300 m. J Appl Physiol 60: 1401–1406, 1986 [DOI] [PubMed] [Google Scholar]
- 26. Murphy E. Estrogen signaling and cardiovascular disease. Circ Res 109: 687–696, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nabulsi AA, Folsom AR, White A, Patsch W, Heiss G, Wu KK, Szklo M. Association of hormone-replacement therapy with various cardiovascular risk factors in postmenopausal women. The Atherosclerosis Risk in Communities Study Investigators. N Engl J Med 328: 1069–1075, 1993 [DOI] [PubMed] [Google Scholar]
- 28. Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev 81: 1535–1565, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Patterson AJ, Chen M, Xue Q, Xiao D, Zhang L. Chronic prenatal hypoxia induces epigenetic programming of PKCε gene repression in rat hearts. Circ Res 107: 365–373, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ranki HJ, Budas GR, Crawford RM, Jovanovic A. Gender-specific difference in cardiac ATP-sensitive K(+) channels. J Am Coll Cardiol 38: 906–915, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Smethurst M, Bishun NP, Williams DC. Relationship between X chromosome activation, Barr body frequency and oestrogen receptor status in human breast cancer: a hypothesis. Oncology 37: 30–32, 1980 [DOI] [PubMed] [Google Scholar]
- 32. Stein CE, Fall CH, Kumaran K, Osmond C, Cox V, Barker DJ. Fetal growth and coronary heart disease in south India. Lancet 348: 1269–1273, 1996 [DOI] [PubMed] [Google Scholar]
- 33. Wang M, Baker L, Tsai BM, Meldrum KK, Meldrum DR. Sex differences in the myocardial inflammatory response to ischemia-reperfusion injury. Am J Physiol Endocrinol Metab 288: E321–E326, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Williams SJ, Campbell ME, McMillen IC, Davidge ST. Differential effects of maternal hypoxia or nutrient restriction on carotid and femoral vascular function in neonatal rats. Am J Physiol Regul Integr Comp Physiol 288: R360–R367, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Xiao D, Ducsay CA, Zhang L. Chronic hypoxia and developmental regulation of cytochrome c expression in rats. J Soc Gynecol Investig 7: 279–283, 2000 [PubMed] [Google Scholar]
- 36. Xue Q, Zhang L. Prenatal hypoxia causes a sex-dependent increase in heart susceptibility to ischemia and reperfusion injury in adult male offspring: role of protein kinase C epsilon. J Pharmacol Exp Ther 330: 624–632, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang H, Meyer KD, Zhang L. Fetal exposure to cocaine causes programming of Prkce gene repression in the left ventricle of adult rat offspring. Biol Reprod 80: 440–448, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhou Y, Shi G, Zheng J, Huang Z, Gao F, Zhang Y, Guo F, Jia Q, Zheng Y. The protective effects of Egr-1 antisense oligodeoxyribonucleotide on cardiac microvascular endothelial injury induced by hypoxia-reoxygenation. Biochem Cell Biol 88: 687–695, 2010 [DOI] [PubMed] [Google Scholar]