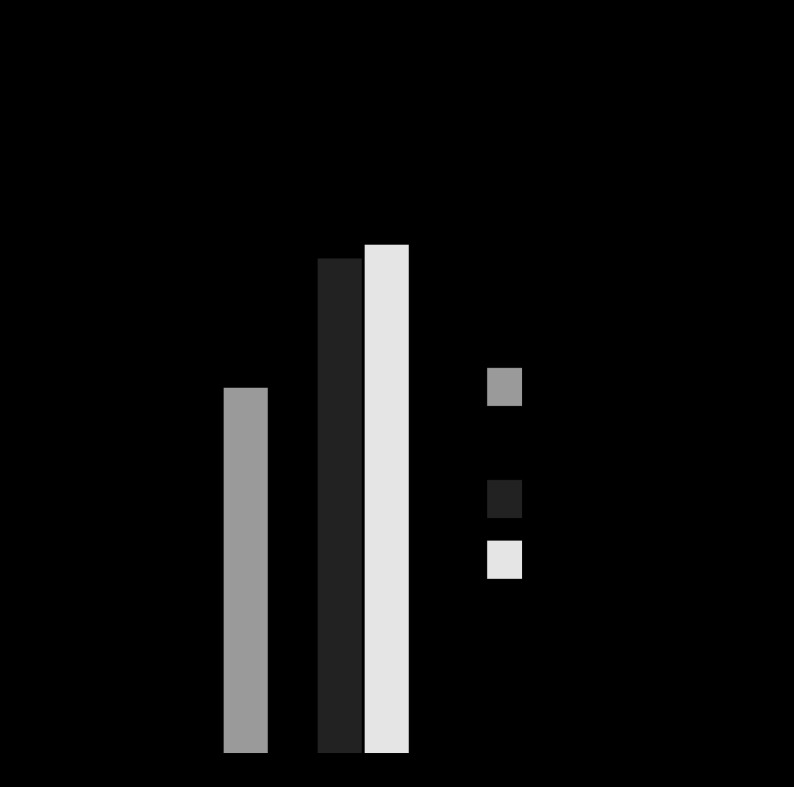Abstract
Chronic hypoxia attenuates soluble guanylate cyclase-induced vasorelaxation in serotonin (5-HT)-contracted ovine carotid arteries. Because protein kinase G (PKG) mediates many effects of soluble guanylate cyclase activation through phosphorylation of multiple kinase targets in vascular smooth muscle, we tested the hypothesis that chronic hypoxia reduces the ability of PKG to phosphorylate its target proteins, which attenuates the ability of PKG to induce vasorelaxation. We also tested the hypothesis that hypoxia attenuates PKG expression and/or activity. Arteries from normoxic and chronically hypoxic (altitude of 3,820 m for 110 days) fetal and adult sheep were denuded of endothelium and equilibrated with 95% O2-5% CO2 in the presence of nitro-l-arginine methyl ester (l-NAME) and NG-nitro-l-arginine (l-NNA) to inhibit residual endothelial nitric oxide synthase. Concentration-response relations for 5-HT were determined in the presence of prazosin to minimize activation of α-adrenergic receptors. The PKG activator 8-(p-chlorophenylthio)-guanosine 3′,5′-cyclic monophosphate (8-pCTP-cGMP) reduced agonist binding affinity of the 5-HT receptor in a concentration-dependent manner that was attenuated by hypoxia. Expression and activity of PKG-I was not significantly affected by chronic hypoxia in either fetal or adult arteries, although PKG-I abundance was greater in fetal arteries. Pretreatment with the large conductance calcium-sensitive potassium channel (BK) inhibitor iberiotoxin attenuated the vasorelaxation induced by 8-pCPT-cGMP in normoxic but not chronically hypoxic arteries. These results support the hypothesis that hypoxia attenuates the vasorelaxant effects of PKG through suppression of the ability of PKG to activate large conductance calcium-sensitive potassium channels in arterial smooth muscle. The results also reveal that this hypoxic effect is greater in fetal than adult arteries and that chronic maternal hypoxia can profoundly affect fetal vascular function.
Keywords: cGMP, chronic hypoxia, guanylate cyclase, iberiotoxin, postnatal maturation
chronic hypoxia is a common stressor in many clinical pathologies and is particularly devastating during pregnancy (17, 28). Maternal hypoxia induces a broad variety of effects in both mother and fetus, including numerous changes in vascular structure and function (26, 46). Prominent among these functional changes is a depressed capacity for vasorelaxation evident in both the pulmonary (50) and systemic (33) circulations. These effects are explained in part by hypoxic depression of endothelial nitric oxide (NO) release (35) and efficacy (45). In turn, chronic hypoxia also modestly depresses the ability of NO to activate soluble guanylate cyclase and stimulate the synthesis of cGMP (9, 46). More importantly, chronic hypoxia can attenuate the ability of cGMP to promote vasorelaxation in multiple vascular beds (36).
For vasorelaxation, the primary target of cGMP is cGMP-dependent protein kinase (PKG), a serine-threonine kinase expressed as two isoforms (Iα and Iβ) in mammalian vascular smooth muscle (47). When bound to cGMP both isoforms of PKG are enzymatically active as homodimers that phosphorylate serine-threonine residues in a broad variety of target proteins (2, 7, 39). At the plasmalemma, PKG can phosphorylate the α-subunit of the large conductance calcium-sensitive potassium (BK) channel and thereby increase its sensitivity to cytosolic Ca2+ and its opening probability (38, 40). PKG-mediated phosphorylation also activates plasma membrane Ca2+-ATPase (48) and inhibits the plasmalemmal L-type calcium channel (16); both of these effects reduce cytosolic Ca2+ concentration and promote vasorelaxation. Inside the smooth muscle cell, PKG-mediated phosphorylation can stimulate the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) calcium pump (8) and attenuate sarcoplasmic reticulum (SR) Ca2+ release through the inositol 1,4,5-trisphosphate (IP3) receptor (19). PKG-mediated phosphorylation of transcription factors such as cAMP response element binding protein (CREB) and serum response factor (SRF) influence transcription of multiple genes involved in regulation of smooth muscle phenotype (6, 37).
Our main hypothesis is that chronic hypoxia alters the capacity for PKG-mediated vasorelaxation through direct effects on PKG and/or the proteins it phosphorylates. To test this hypothesis, we examined the effects of chronic hypoxia on PKG abundance and activity in ovine carotid arteries (20). Because effects of chronic hypoxia differ in fetal and adult arteries (45), we examined both fetal and adult carotid arteries using a model designed to assess the effects of maternal hypoxia on fetal oxygenation. This model is clinically relevant to fetal hypoxemia resulting from placental insufficiency, maternal smoking, maternal pulmonary insufficiency, etc. (26). To minimize confounding effects of endothelial and extravascular stimulation of PKG, the vascular endothelium was mechanically removed, any residual endothelial NO synthase was inhibited, and adrenergic receptors were selectively antagonized so that the results uniquely represent the effects of PKG in smooth muscle pharmacomechanical coupling following hypoxic acclimatization. In an effort to identify the categories of protein targets whose phosphorylation by PKG may change with hypoxia, we compared multiple endpoints between normoxic and hypoxic arteries. These endpoints included the ability of PKG to attenuate 5-HT-induced contractile force, to alter agonist affinity, and to influence BK channel function. Serotonin was used as the contractile agonist in these experiments owing to its ubiquitous potency (43), well-characterized endothelium-independent 5-HT2A receptor-mediated mechanism of contraction (42), and the potential for regulation of the binding affinity of this receptor by phosphorylation (1). The BK channel was specifically examined because of its role in membrane potential homeostasis, high K+ currents, Ca2+ sensitivity, and known PKG phosphorylation sites. Together, these experiments provided a unique insight into the age-dependent effects of chronic hypoxia on PKG function in vascular smooth muscle.
MATERIALS AND METHODS
All procedures in this study were approved by the Animal Research Committee of Loma Linda University and adhere strictly to the policies and practices according to the National Institutes of Health Guide governing the care and use of laboratory animals.
Tissue harvest and preparation.
All common carotid arteries used in this study were harvested using sterile techniques from normoxic and chronically hypoxic fetal (139–142 days gestation) and young (18–24 mo old) nulliparous adult sheep. In normoxic animals maintained at sea level arterial oxygen tensions (PaO2) averaged 23 ± 1 and 102 ± 2 Torr in fetal and adult sheep, respectively (18). Corresponding arterial blood pressures averaged 44 ± 2 and 81 ± 3 mmHg, respectively. Chronically hypoxic sheep were maintained for ∼110 days at high altitude (altitude 3,820 m, Barcroft Laboratory, White Mountain Research Station, Bishop, CA). For pregnant ewes, the period at altitude corresponded to the final 110 days of gestation, where term averages 143 days. At altitude, fetal and adult PaO2 values averaged 19 ± 1 and 64 ± 2 Torr, respectively. Corresponding blood pressures in these animals averaged 52 ± 1 and 88 ± 4 mmHg, respectively.
Pregnant ewes were anesthetized with 30 mg/kg pentobarbital, intubated, and then placed on 1.5–2.0% halothane. The anesthetized fetus was then exteriorized through a midline vertical laparotomy and euthanized by rapid removal of the heart and exsanguination. Nonpregnant adult animals were euthanized by intravenous administration of 100 mg/kg pentobarbital. Harvested carotid arteries were placed in Krebs buffer solution containing (in mM) 122 NaCl, 25.6 NaHCO3, 5.17 KCl, 2.49 MgSO4, 1.60 CaCl2, 2.56 dextrose, 0.027 EGTA, and 0.114 ascorbic acid, bubbled with 95% O2-5% CO2. Arteries were debrided of loose extracellular and connective tissue and then denuded of endothelium by passage of a stainless steel rod through the lumen. The vascular endothelium was systematically removed to minimize the potentially confounding effects of endothelial NO release on cGMP synthesis and PKG activation.
Determination of concentration-response relations for 5-HT.
Denuded carotid arteries were cut into segments 1 mm to 2 mm in length, mounted on tungsten wire loops, and suspended from a force transducer in a sodium-replete Krebs buffer solution (pH 7.4) at 38°C (the ovine core temperature) and bubbled with 95% O2 and 5% CO2. To inhibit endogenous nitric oxide (NO) production, 10 μM nitro-l-arginine methyl ester (l-NAME) and 10 μM NG-nitro-l-arginine (l-NNA) were added to the Krebs buffer solution. To selectively inhibit activation of α1-adrenergic receptors by 5-HT, 1.0 μM prazosin and 0.2 μM cocaine were added. In preliminary experiments, both endothelium-intact and endothelium-denuded segments were studied. Because endothelium removal had no effect on average contractile efficacy, but reduced its variability, all further experiments were performed in endothelium-denuded segments. The denuded segments were equilibrated for 30 min and then stretched to a baseline tension of 1.5 g, which corresponds with an optimal stretch ratio of ∼1.8 times the unstressed diameter. Contractile force was measured directly using an isometric force transducer (Kent Scientific, Torrington, CT) and recorded directly by computer. Concentration-response curves were obtained by cumulative addition of half-log concentrations of 5-HT across a range of 10−10 to 10−4 M. In preliminary experiments, norepinephrine was also used as the contractile agonist, but it was significantly less potent and less efficacious than serotonin in producing contractions; all further experiments were performed using 5-HT. EC50 values (molar concentration at which the contractile response was half the maximal induced contraction) were expressed as pD2 values (−logEC50). All contractile responses were normalized to the maximum force produced by exposure to a potassium-Krebs solution containing (in mM) 5.17 NaCl, 25.6 NaHCO3, 122 KCl, 2.49 MgSO4, 1.60 CaCl2, 2.56 dextrose, and 0.027 EGTA.
To test the contribution of large conductance calcium-sensitive potassium (BK) channels to 5-HT contractions, concentration-response experiments were also carried out in the presence of the selective BK channel blocker iberiotoxin at a concentration of 100 nM (dissolved in Krebs), a concentration that previous work has shown to be optimal (41). Iberiotoxin was added to the baths, in both the presence and absence of 30 μM (4-chlorophenylthio)guanosine-3′,5′-cyclic monophosphate (8-pCPT-cGMP), 30 min before commencement of the concentration-response sequences.
Measurement of agonist binding affinity and receptor occupancy.
The 5-HT-receptor dissociation constants were determined via the Furchgott method of partial irreversible blockade (13) with the alkylating agent phenoxybenzamine (50–150 nM), as originally described in detail by our laboratory (15, 42). Briefly, pairs of artery segments were untreated or pretreated with the PKG activator 8-pCPT-cGMP across a range of concentrations from 0 to 30 μM to determine the influence of PKG. The final concentration of phenoxybenzamine used in each artery was chosen to achieve a 50% decrease in 5-HT efficacy with 30 min incubation, followed by a washout. Pairs of adjacent phenoxybenzamine-treated and -untreated artery segments were then assayed to obtain concentration-response curves across a range of 10−10 to 10−4 M 5-HT. Equiactive concentrations of 5-HT in treated [A′] and untreated [A] arteries were plotted as a double reciprocal plot of 1/[A] versus 1/[A′] from which the regression line was calculated; the dissociation constant was taken as the ratio Ka = slope − 1/intercept. Using the apparent Ka for the 5-HT2A receptor complex, fractional receptor occupancy was calculated from the equation: [RA]/[RT] = [A]/([A] + Ka), where [RA] was the concentration of the receptor-agonist complex, and [RT] was the total receptor concentration (31).
Western blot analysis of PKG abundance.
Common carotid artery segments were homogenized using glass-on-glass mortars and pestles in 50 mM HEPES (pH 7.4), 2 mM magnesium acetate, 2 mM DTT, 1 mM EDTA, 520 μM AEBSF, 7.5 μM pepstatin-A, 7 μM E-64, 20 μM bestatin, 100 μM leupeptin, and 0.4 μM aprotinin. Supernatants were collected after centrifugation at 100,000 g for 1 h at 4°C and analyzed for protein content using the Bradford assay, as described previously (4, 34), and then divided for separate analysis of PKG abundance by Western blot and PKG activity. Samples for PKG abundance were separated by SDS-PAGE and then transferred to nitrocellulose membranes for immunodetection. Primary antibodies for total PKG were obtained from Stressgen (1:1,000; catalog no. KAS-PK005). This antibody detected a PKG-I epitope common to both the α- and β-isoforms. Blots were visualized using the chemiluminescent substrate Supersignal West Dura (catalog no. 37071, Thermo Scientific), and images were then captured on an Alpha Innotech Fluorchem (Cell Biosciences, Santa Clara, CA). Relative abundances of total PKG were normalized to standards prepared from arteries harvested from adult normoxic nonpregnant ewes.
Measurement of PKG-I activity.
Samples of supernatants from the homogenates prepared for measurements of PKG abundance (described in Western blot analysis of PKG abundance) were cleared for 30 min at 30°C after which 5 mM NaF and 1 μM PKI (a PKA inhibitor, Sigma-Aldrich P6061) were added. After 30 min of incubation, sample aliquots were added to reaction buffer in 96-well plates to yield final concentrations of 200 μM ATP with ≈300–400 cpm/pmol [γ-32P]ATP (NEN), 200 μM PKG substrate BPDEtide (BML-P112-0001, Enzo Life Sciences), and 10 μM 8-pCPT-cGMP (Sigma-Aldrich, C5438) in homogenizing buffer. Timed reactions were terminated by addition of phosphoric acid. Phosphorylated BPDEtide and unreacted [γ-32P]ATP were separated through phosphocellulose paper using a Millipore 96-well filtration plate. Filters were washed with phosphoric acid, allowed to dry, added to vials with scintillation cocktail, and assayed using a β-scintillation counter. Counts were converted into moles 32P using a calibration curve counted in parallel with the samples and normalized to sample protein content to obtain activity in units of pmol 32P transferred per minute per milligram of protein. Each assay included blanks with: 1) no added 8-pCPT-cGMP; 2) no added BPDEtide substrate; and 3) neither 8-pCPT-cGMP nor BPDEtide to enable correction for total nonspecific background.
Data analysis and statistics.
Significant differences in contractile variables were determined using Behrens-Fischer tests for two-group comparisons and ANOVA followed by Duncan's post hoc simultaneous comparisons of more than two groups. Analyses using the SPSS software routinely confirmed homogeneity of variance and normal data distributions (SPSS v19). Determinations of pKa values were based on double reciprocal plots from which slopes were determined by linear regression as described by Furchgott (13, 31). Contractile forces were normalized relative to the contraction as measured upon exposure to isotonic 120 mM potassium-Krebs in the same artery segment. The relative influence of PKG stimulation on the BK potassium channel compared with all other downstream targets was estimated with an algebraic model where a = maximum response to 5-HT in control arteries (untreated), b = total maximum response to 5-HT in the presence of PKG stimulation by 8-pCPT-cGMP, c = maximum response to 5-HT in presence of iberiotoxin, and d = maximum response to 5-HT in the presence of iberiotoxin and with pretreatment using 8-pCPT-cGMP. The total effect of PKG activation was defined as (a − b), and the effect of PKG activation independent of BK channel activity was defined as (c − d). The BK channel-dependent effect of PKG activation was calculated as (a − b) − (c − d). Maximum efficacy of 5-HT was calculated using SPSS (version 19) with a nonlinear regression model using the four-parameter Hill equation y = (e + [(f − e)/{1 + 10∧[([agonist] − g) × h]}]), where “e” represents the calculated Emax.
RESULTS
A total of 57 sheep were used in this study of which 27 were term fetal lambs and 30 were adult sheep. From these animals we harvested a total of 233 carotid artery segments, including 113 fetal segments and 120 adult segments. Throughout the text, “n” represents the number of animals, and not the number of segments used in each experiment. Unless stated otherwise, statistical significance implies P < 0.05. All values are given as means ± SE.
Effects of hypoxia, age, and 8-pCPT-cGMP on 5-HT concentration-response relations.
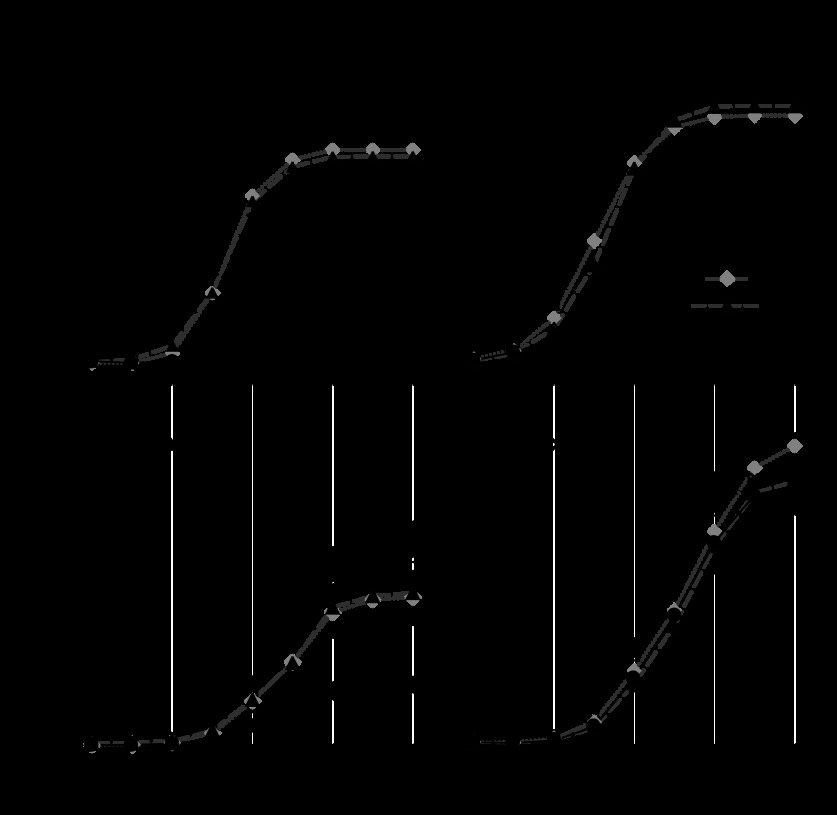
Maturation significantly reduced maximal responses to 5-HT (efficacy) in normoxic animals (Fig. 1, left). Chronic hypoxia increased maximal responses to 5-HT in both fetal and adult arteries and eliminated age-related differences in efficacy (Fig. 1, right). Pretreatment with 8-pCPT-cGMP, a cell-permeant and phosphodiesterase-resistant PKG activator, significantly attenuated 5-HT efficacy in normoxic fetal and adult arteries in a concentration-dependent manner. In hypoxic arteries, 8-pCPT-cGMP had no significant effects on 5-HT efficacy at any of the concentrations tested. The effects of 8-pCPT-cGMP on 5-HT efficacy were similar in endothelium-intact fetal (control: 131 ± 7; 8-pCPT-cGMP: 123 ± 5) and endothelium-denuded fetal (control: 138 ± 5; 8-pCPT-cGMP: 129 ± 5) artery pairs (n = 7). Similarly the effects of 8-pCPT-cGMP on 5-HT efficacy were the approximately the same in endothelium-intact adult (control: 126 ± 9; 8-pCPT-cGMP: 110 ± 16) and endothelium-denuded adult (control: 133 ± 4; 8-pCPT-cGMP: 124 ± 3) artery pairs (n = 5).
Fig. 1.
Effects of 8-(p-chlorophenylthio)-guanosine 3′,5′-cyclic monophosphate (8-pCPT-cGMP) and hypoxia on 5-HT concentration-response relations. Maximum contractile responses to graded concentrations of 5-HT did not vary significantly with either age or hypoxia in untreated arteries (0 μM 8-pCPT-cGMP). Pretreatment with 8-pCPT-cGMP attenuated 5-HT efficacy in a concentration-dependent manner but only in normoxic arteries. *Significantly (P < 0.05) less than corresponding untreated controls. In arteries pretreated with 30 μM 8-pCPT-cGMP, 5-HT efficacy was greater in hypoxic than normoxic arteries for both the fetus and adult. §Hypoxic values significantly different (P < 0.05) than equivalently treated normoxic values in corresponding age groups. Error bars indicate means ± SE for n ≥ 6 for all groups.
Effects of hypoxia, age, and 8-pCPT-cGMP on 5-HT agonist potency and binding affinity.
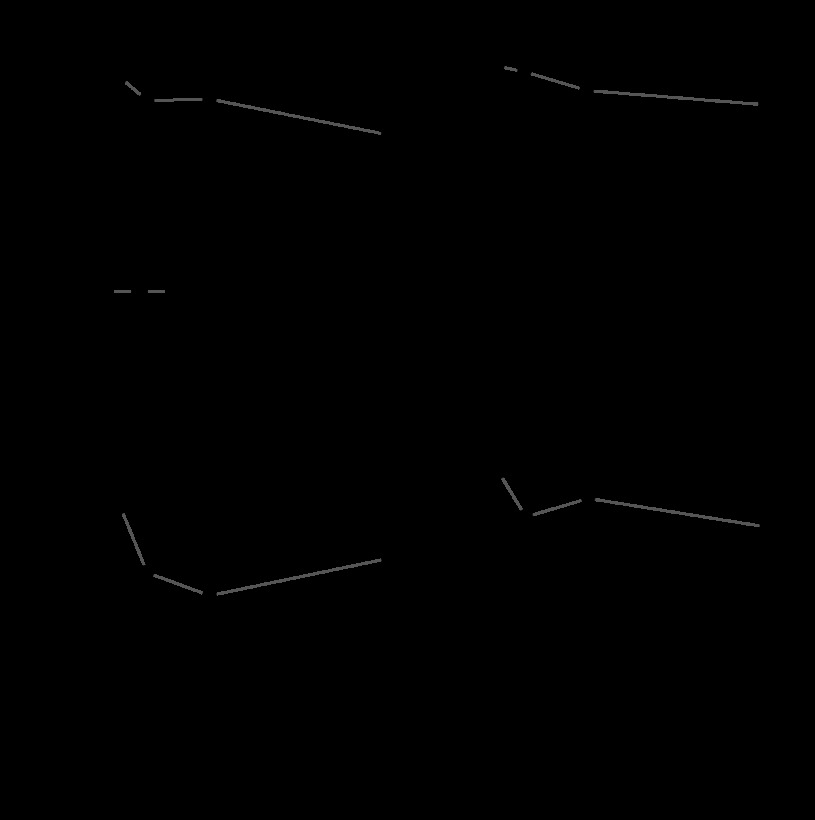
Values for 5-HT potency (pD2 = −log of EC50) were significantly greater in fetal (6.5 ± 0.2, n = 7) than adult (5.5 ± 0.3, n = 6) normoxic arteries (Fig. 2, top). Potency values were not significantly different in normoxic and hypoxic arteries for either age group. Pretreatment with 8-pCPT-cGMP attenuated 5-HT potency in normoxic fetal arteries only at 30 μM and was without effect in normoxic adult arteries at any concentration tested. Pretreatment with 8-pCPT-cGMP attenuated 5-HT potency at 10 and 30 μM in hypoxic fetal arteries and at 3, 10, and 30 μM in hypoxic adult arteries. Chronic hypoxia significantly enhanced the depressant effects of 8-pCPT-cGMP on 5-HT potency.
Fig. 2.
Effects of 8-pCPT-cGMP and hypoxia on agonist affinity and potency for 5-HT. In untreated control arteries (0 μM 8-pCPT-cGMP), agonist potencies (pD2 = −log EC50) were significantly greater in fetal than adult arteries for both normoxic and hypoxic groups. Normoxic (top left) and hypoxic (top right) potencies in untreated controls were not significantly different in either fetal or adult arteries. Treatment with 8-pCPT-cGMP significantly depressed pD2 in normoxic fetal arteries at 30 μM (top left), in hypoxic fetal arteries at 10 and 30 μM (top right), and in hypoxic adult arteries at all concentrations (top right). In untreated control arteries, agonist affinity (pKa) was significantly greater in fetal than adult arteries for both normoxic and hypoxic groups (bottom left and right). Normoxic (bottom left) and hypoxic (bottom right) affinities in untreated controls were not significantly different in either fetal or adult arteries. Treatment with 8-pCPT-cGMP significantly depressed pKa in normoxic fetal and adult arteries at 10 and 30 μM and also in hypoxic adult arteries at 30 μM. §Significant differences (P < 0.05) between untreated control fetal and adult arteries. *Values significantly different (P < 0.05) from corresponding untreated controls. Error bars indicate means ± SE for n ≥ 6 for all groups.
Values for 5-HT binding affinity (pKa) were significantly greater in fetal (6.14 ± 0.48, n = 7) than adult (5.49 ± 0.27, n = 6) normoxic arteries (Fig. 2, bottom). Binding affinity values were not significantly different in normoxic and hypoxia arteries for either age group. Pretreatment with 8-pCPT-cGMP attenuated pKa values at 10 and 30 μM in both fetal and adult normoxic arteries. Pretreatment with 8-pCPT-cGMP attenuated pKa values only at 30 μM in hypoxic adult arteries and was without effect in hypoxic fetal arteries. Chronic hypoxia significantly attenuated the depressant effects of 8-pCPT-cGMP on 5-HT binding affinity.
Effects of hypoxia, age, and 8-pCPT-cGMP on 5-HT occupancy-response relations.
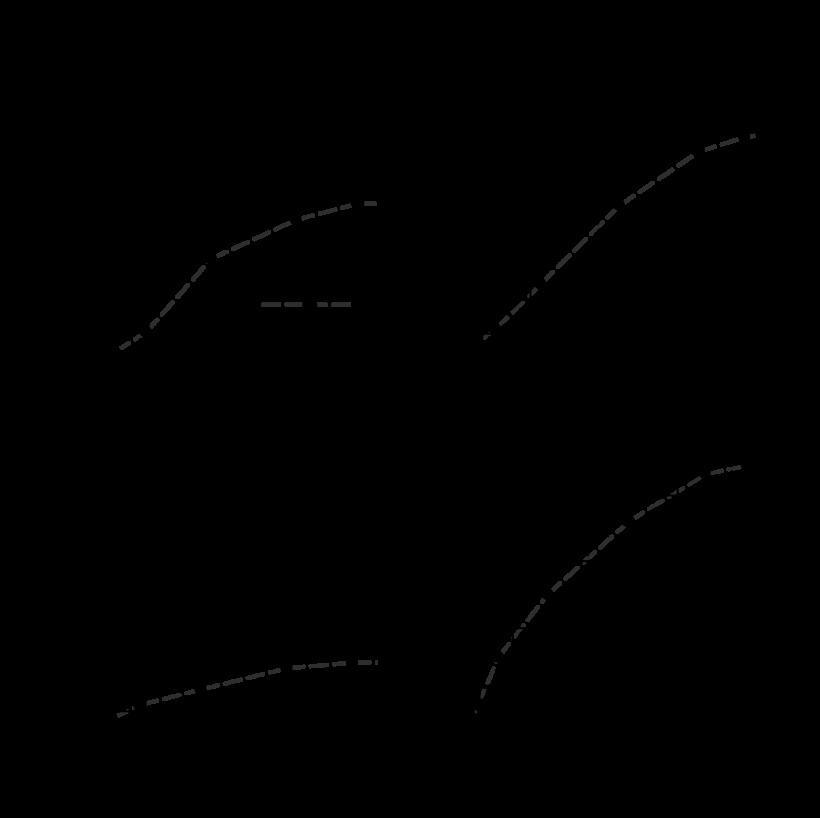
To correct for group differences in 5-HT binding affinity, the concentration-response relations were converted to occupancy-response relations using the Furchgott method (13). This correction altered the shapes of the concentration-response relations but still revealed a significant depressant effect of 8-pCPT-cGMP on occupancy-response relations in both fetal and adult normoxic arteries (Fig. 3, left). Equally important, this depressant effect of 8-pCPT-cGMP on occupancy-response relations was not evident in either fetal or adult hypoxic arteries (Fig. 3, right). These results revealed that 8-pCPT-cGMP attenuated 5-HT-induced contractility at a step downstream from ligand-receptor binding and that this effect of 8-pCPT-cGMP was absent in chronically hypoxic fetal and adult arteries.
Fig. 3.
Effects of 8-pCPT-cGMP and hypoxia on occupancy-response relations for 5-HT. The 5-HT concentration-response relations shown in Fig. 1 were converted to occupancy-response relations using values of affinity (pKa) shown in Fig. 2 to correct for differences in agonist binding affinity. The resulting occupancy-response relations revealed that compared with untreated control arteries, pretreatment with 30 μM 8-pCPT-cGMP significantly reduced maximum efficacy for 5-HT in normoxic (left) but not hypoxic (right) arteries from both age groups. *Significant differences (P < 0.05, repeated measures ANOVA) between untreated (0 μM 8-pCPT-cGMP) and treated (30 μM 8-pCPT-cGMP) arteries. Error bars indicate means ± SE for n ≥ 6 for all groups.
Effects of hypoxia, age, and 8-pCPT-cGMP on PKG abundance and specific activity.
When expressed relative to abundances in normoxic adult carotid arteries, the relative abundance of total PKG was significantly greater in fetal compared with adult arteries in both normoxic and hypoxic groups (Fig. 4).
Fig. 4.
Effects of hypoxia on the abundance of protein kinase G (PKG) isoforms. The abundance of total PKG was determined by Western blot analysis using an antibody against a PKG-I epitope common to both the α and β isoforms. All abundances were calculated relative to known amounts of a standard pool prepared from normoxic adult arteries. *Relative abundance of total PKG was significantly greater in fetal than adult arteries and was not affected by hypoxia. Error bars indicate means ± SE for n ≥ 6.
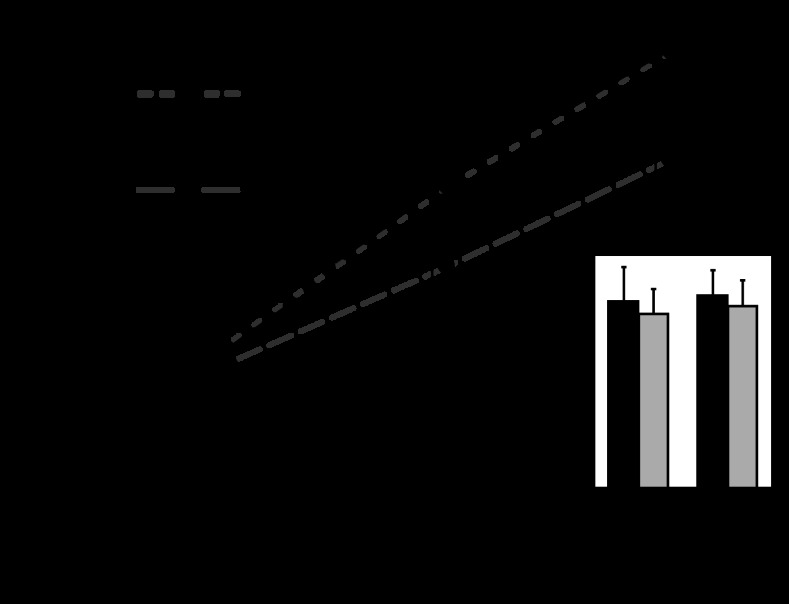
In tissue homogenates, total PKG activity was significantly greater in fetal than adult arteries at 5.5 and 8.0 min of reaction time (Fig. 5). Total PKG activity was not significantly different in normoxic than in hypoxic homogenates for either age group. When total PKG activity was normalized relative to total PKG abundance to estimate specific activity, the resulting values were similar in all groups (Fig. 5, inset).
Fig. 5.
Effects of hypoxia on PKG activity. Using a peptide substrate derived from bovine phosphodiesterase (abbreviated as BPDE in the figure), whole artery PKG activity (line graph, nmol 32P-labeled BPDE/relative unit PKG) was not significantly affected by hypoxia in either fetal or adult homogenates. Total PKG activity was greater in fetal compared with adult artery tissue homogenates, which reflects the modestly greater total PKG abundance in fetal arteries. When whole artery PKG activity was normalized relative to PKG abundance (Fig. 4), estimates of specific activity (inset, nmol 32P-labeled BPDE/min/relative unit PKG) did not vary significantly with hypoxia. Error bars indicate means ± SE for n ≥ 5.
Interactive effects of 8-pCPT-cGMP and iberiotoxin on 5-HT-induced contraction.
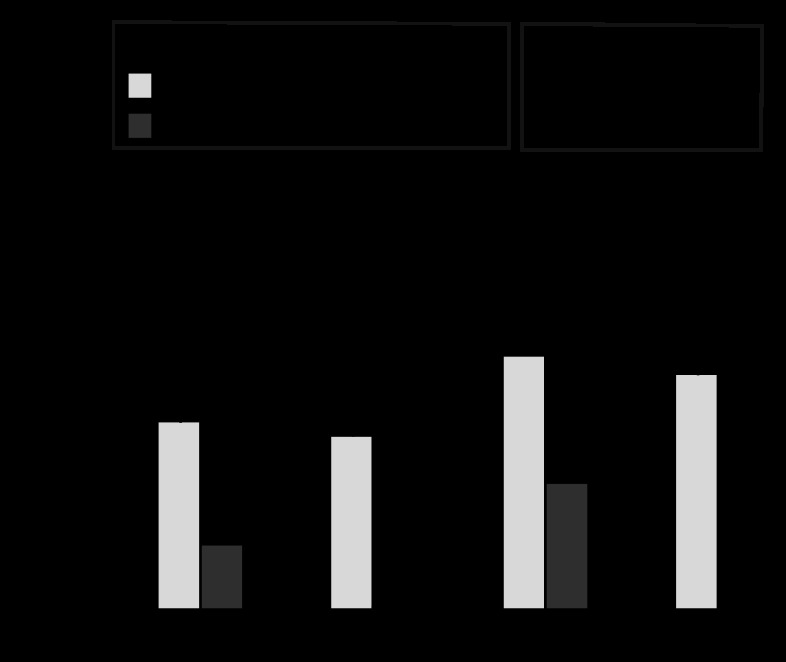
In all arteries, the magnitude of decrease in 5-HT efficacy produced by 8-pCPT-cGMP was significant in both the presence and absence of iberiotoxin, although the magnitudes of these effects varied considerably with both age (Fig. 6, top vs. bottom) and hypoxia (Fig. 6, left vs. right). Iberiotoxin attenuated the magnitude of 5-HT-induced contraction in all arteries, indicating a strong BK-channel influence on vasorelaxation. Analysis of these results with our algebraic model quantified the relative contributions of BK-dependent and BK-independent components of PKG action on 5-HT efficacy. In both fetal and adult arteries the total magnitude of PKG-mediated inhibition of 5-HT efficacy was reduced by hypoxia, and the BK-dependent component was virtually eliminated (Fig. 7). In contrast, the BK-independent component of PKG-mediated inhibition of 5-HT efficacy was unchanged by hypoxia in both age groups.
Fig. 6.
Interactive effects of 8-pCPT-cGMP and iberiotoxin on 5-HT concentration-response relations. The relative contributions of PKG and BK channels to contractile responses to 5-HT were identified by addition of 30 μM 8-pCPT-cGMP, a PKG activator (▼), 100 nM iberiotoxin, a BK channel blocker (●), or both (◆), respectively, in matched sets of adjacent segments from the same artery. §Total effect of PKG activation on 5-HT contraction was defined as the difference in maximum response between control and 8-pCPT-cGMP-treated arteries. *Effect of PKG activation independent of BK channels was defined as the difference in maximum response between arteries treated with iberiotoxin alone and both iberiotoxin and 8-pCPT-cGMP. Error bars indicate means ± SE for n ≥ 8.
Fig. 7.
Estimates of the relative magnitudes of BK-dependent and BK-independent components of PKG activation on 5-HT-induced contractions. The total effects of PKG activation on the maximum contractile response to 5-HT were partitioned into BK-independent and BK-dependent components via calculation of differences in efficacy as defined in Fig. 6. Hypoxia virtually eliminated the BK-dependent component of PKG-mediated inhibition of 5-HT efficacy in both fetal and adult arteries. *Hypoxia attenuated total magnitude of effect of PKG on contractility in both fetal and adult arteries but had no significant effect on the BK-independent component. §All components of the PKG effect were significantly greater than zero except for the BK-dependent component in hypoxic arteries. Error bars indicate means ± SE for n ≥ 6.
DISCUSSION
This study of the effects of chronic hypoxia on the vasorelaxant efficacy of PKG in endothelium-denuded and NO synthase-inhibited arteries offers five main observations: 1) the ability of PKG to attenuate 5-HT-induced contractions was dramatically reduced in hypoxic compared with normoxic arteries, and this effect was greater in adult than fetal carotids; 2) PKG activation attenuated ligand binding affinity for 5-HT, and this effect was significantly reduced by chronic hypoxia in both fetal and adult arteries; 3) receptor occupancy-response relations, which corrected for differences in binding affinity, also revealed that the ability of PKG to attenuate 5-HT-induced contractions was virtually eliminated by chronic hypoxia in both fetal and adult arteries; 4) the catalytic activity of total PKG was similar in fetal and adult arteries and was not affected by chronic hypoxia; and 5) the ability of PKG activation to reduce 5-HT-induced contractions was attenuated by pretreatment with the BK channel blocker iberiotoxin in normoxic but not hypoxic arteries from both fetus and adult. Given that these observations were made in endothelium-denuded arteries treated with l-NAME to inhibit NO synthase activity and with prazosin to inhibit activation of adrenergic receptors, this study focused on the ability of smooth muscle PKG to influence serotonergic contraction. Specifically, these observations support the hypothesis that chronic hypoxia acts directly on vascular smooth muscle to attenuate cGMP-induced vasorelaxation through reduced ability of PKG to activate BK channels. Equally important, the results demonstrate that these effects of hypoxia on PKG-mediated inhibition of serotonergic contractions are markedly different in fetal and adult arteries.
Effects of hypoxia and 8-pCPT-cGMP on 5-HT efficacy and pKa.
Chronic hypoxia is well established as a major modulator of vascular structure, contractility, and vasorelaxant efficacy in many artery types (3, 25, 42). A key finding among previous studies is that chronic hypoxia can attenuate endothelial NO release through reductions in endothelial NO synthase-specific activity (35). In parallel, chronic hypoxia can also reduce relaxation responses to NO in part through reduced soluble guanylate cyclase activity, and in part through attenuation of the ability of 8-pCPT-cGMP, a PKG activator, to promote vasorelaxation (46). These results support the hypothesis that chronic hypoxia depresses cGMP-dependent protein kinase activity. Consistent with these results, chronic hypoxia markedly attenuated the ability of 8-pCPT-cGMP to inhibit contractile responses to 5-HT (Fig. 1). As previously reported, this effect of hypoxia was greater in adult than in fetal arteries (46).
For a majority of G protein-coupled receptors, including those that mediate contractile responses to 5-HT, agonist binding affinity is subject to regulation through phosphorylation by multiple serine-threonine kinases including PKC, PKA, and specific GPCR receptor kinases (1, 5). This evidence suggests that PKG could attenuate 5-HT-induced contraction through desensitization of the 5-HT-2a receptors that mediate contractile responses to 5-HT in ovine common carotids (42). To explore this hypothesis and its corollary that hypoxia attenuates this effect of PKG, our experimental approach examined the effects of the PKG activator 8-pCPT-cGMP on 5-HT binding affinity (pKa) and potency (pD2) in normoxic and hypoxic arteries (Fig. 2). Treatment with 8-pCPT-cGMP significantly depressed pKa at multiple concentrations in normoxic fetal and adult arteries, but at only 30 μM in hypoxic adult arteries, and was without effect in hypoxic fetal arteries. Conversely, in normoxic arteries 8-pCPT-cGMP significantly attenuated pD2 only at 30 μM in fetal carotids, but in hypoxic arteries it significantly attenuated pD2 at multiple concentrations in both fetal and adult arteries. Together, these results demonstrate that 8-pCPT-cGMP significantly modulates interaction between 5-HT and its receptor through mechanisms that are sensitive to hypoxia. Given that pKa is determined primarily by interactions between a ligand and its receptor, but pD2 also includes influences of receptor density, the results are consistent with previous reports that hypoxia alters 5-HT receptor density (3). The small sizes of these effects, however, suggest that influences on binding affinity and potency alone cannot explain the ability of hypoxia to ablate PKG-mediated attenuation of serotonergic contractions (Fig. 1).
To correct for any effects of 8-pCPT-cGMP and hypoxia on 5-HT binding affinity and potency, the concentration-response relations shown in Fig. 1 were converted into occupancy-response relations using the Furchgott transformation (13). This conversion eliminated the influence of changes in binding affinity and revealed changes in coupling between activated receptors and the contractile apparatus. As shown in Fig. 3, 8-pCPT-cGMP still significantly attenuated 5-HT-induced contractility in normoxic, but not hypoxic, arteries. These results demonstrate that 8-pCPT-cGMP significantly depressed coupling between the 5-HT-2a receptor and the contractile apparatus in both fetal and adult arteries through mechanisms that were highly sensitive to the effects of chronic hypoxia.
Effects of hypoxia on PKG abundance and specific activity.
Downstream from receptor activation, a key determinant of the ability of 8-pCPT-cGMP to alter 5-HT-induced contraction is the abundance of PKG. In turn, it is possible that hypoxic reductions in PKG abundance could explain hypoxic ablation of the ability of 8-pCPT-cGMP to reduce 5-HT-induced contractions. To test this hypothesis, the experimental approach included abundance measurements of total PKG, which included contributions from both the Iα and Iβ isoforms (11). These measurements indicated that neither postnatal age nor hypoxia had any significant effect on the abundance of total PKG (Fig. 4). These findings strongly demonstrated that hypoxic reductions in PKG abundance could not explain hypoxic ablation of the ability of 8-pCPT-cGMP to reduce 5-HT-induced contractions in adult arteries; another mechanism must be involved. Another key determinant of the ability of 8-pCPT-cGMP to alter 5-HT-induced contraction is the kinase activity of the PKG enzyme. Correspondingly, inhibition of PKG kinase activity could potentially explain hypoxic ablation of the ability of 8-pCPT-cGMP to reduce 5-HT-induced contractions. To test this hypothesis, our experimental approach included measurements of PKG-dependent kinase activity in artery homogenates. Consistent with the observed age-related differences in PKG abundance (Fig. 4), total tissue activity was greater in fetal than adult arteries. However, hypoxia had no significant effect on total PKG activity in either fetal or adult artery homogenates (Fig. 5). Normalization of total tissue activities relative to PKG abundance further revealed that specific kinase activity for PKG varied with neither postnatal age nor hypoxia (Fig. 5, inset). Together, these findings demonstrated that changes in neither PKG abundance nor specific kinase activity could explain hypoxic inhibition of 8-pCPT-cGMP-induced reductions in 5-HT-induced contractions. Equally important, these results suggest that effects of hypoxia on protein targets of PKG may explain hypoxic inhibition of PKG-induced attenuation of 5-HT-induced contractions.
Interactive effects of hypoxia, 8-pCPT-cGMP, and iberiotoxin on 5-HT efficacy.
Among the many protein targets of PKG, one of the most prominent in regards to smooth muscle contractility is the BK channel (11, 29). Phosphorylation of S1072 by PKG directly promotes activation of the BK channel (12) and thereby hyperpolarizes the plasmalemma and promotes vasorelaxation (24, 47). These characteristics raise the possibility that PKG may attenuate 5-HT-induced contractions by activating the BK channel. In addition, it is possible that hypoxia in some way interferes with the ability of PKG to promote activation of the BK channel and thereby ablates the effects of PKG activation of 5-HT-induced contractility. To test this hypothesis, the experimental approach evaluated the effects of BK channel blockade on the ability of 8-pCPT-cGMP to inhibit 5-HT-induced contractions. Blockade of BK channel currents relied on the effects of iberiotoxin, a well-defined and highly selective blocker of the BK channel (14). Previous studies from our laboratory have shown that 5-HT increases BK channel activity in sheep arteries, and that iberiotoxin at 100 nM can block this contribution (41).
To quantify contributions of BK channel activation to PKG-mediated inhibition of 5-HT efficacy, we repeated the 5-HT-concentration-response experiments as shown in Fig. 1, in the presence of iberiotoxin. With this approach, the total effect of PKG was defined by the difference in 5-HT efficacy measured in the presence and absence of 30 μM 8-pCPT-cGMP (Fig. 6). Similarly, the BK-independent effect of PKG was defined by the difference in 5-HT efficacy measured in the presence of iberiotoxin either with or without 30 μM 8-pCPT-cGMP. In turn, the BK-dependent component of PKG-mediated inhibition of 5-HT efficacy was the simple paired difference between the total component and the BK-dependent component of PKG-mediated inhibition. To simplify comparisons among these components, their average values were plotted in Fig. 7.
As shown in Figs. 1, 6, and 7, hypoxia reduced total PKG-mediated inhibition of 5-HT efficacy in both fetal and adult arteries. The BK-independent component of PKG-mediated inhibition (Fig. 7, light gray) was significantly greater than the BK-dependent component in both fetal and adult arteries (Fig. 7, dark gray). More importantly, BK-independent component was not significantly different in normoxic and hypoxic arteries of either age group. This finding suggests that BK-independent mechanisms of PKG-mediated vasorelaxation, such as inhibition of calcium sequestration and release (8, 19), calcium influx (16), calcium extrusion (23), and myofilament calcium desensitization (30) are resistant to chronic hypoxia. In contrast, the BK-dependent component of PKG-mediated vasorelaxation was greater in fetal than adult arteries and was virtually ablated by chronic hypoxia in both age groups. These results extend previous findings in cultured pulmonary artery smooth muscle (36) and strongly suggest that hypoxic attenuation of PKG-mediated inhibition of 5-HT efficacy is attributable to suppression of the BK-dependent component of PKG-mediated vasorelaxation.
Overview.
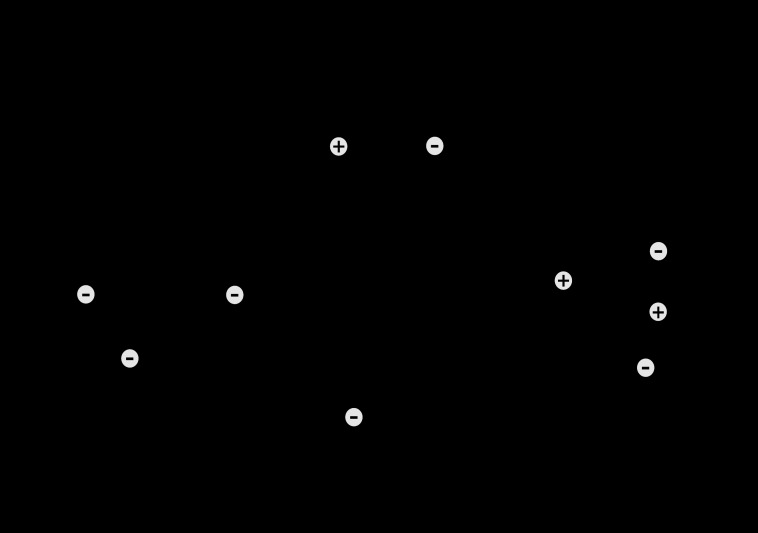
The mechanisms whereby hypoxia suppresses PKG-mediated activation of BK channels remain uncertain. These effects could be explained by hypoxic inhibition of BK channel expression, but support for this mechanism is not evident in the literature. Hypoxia has been shown to upregulate expression of the β regulatory subunit of the BK channel (49), but how this effect might help mediate hypoxic suppression of PKG-mediated activation of BK channels remains to be demonstrated. Similarly, the effects of chronic hypoxia on expression of the BK α-subunit also remain unreported. An alternate mechanism could involve a generalized hypoxic inhibition of BK activation (32), perhaps through depression of calcium sensitivity of the channel (10, 22). Hypoxic upregulation of generalized phosphatase activity could explain such an effect, but support for this mechanism is lacking. Another interesting possibility is that hypoxia alters the organization and distribution of PKG within the smooth muscle cell and thereby limits access of the kinase to its primary targets. Certainly, the mechanisms that mediate hypoxic inhibition of PKG-mediated activation of BK channels are worthy of future investigation (Fig. 8). Another worthy topic is how PKG inhibits agonist-binding affinity, and how this effect can be modulated by hypoxia; none of these mechanisms are known. What the present study does show is that hypoxia has little effect on PKG abundance or catalytic activity. Instead, hypoxia influences vascular smooth muscle to modulate the targets of PKG, including most prominently the BK channel. This finding raises new questions regarding possible effects of hypoxia on other PKG targets, what duration and severity of hypoxia are necessary to elicit these effects, and what other pathophysiological perturbations might produce similar effects.
Fig. 8.
Schematic representation of the interactive effects of PKG and chronic hypoxia on 5-HT induced contractions. Serotonin binds the 5-HT2A receptor to mobilize intracellular calcium and promote depolarization, which facilitates calcium influx and promotes contraction. Left side indicates large conductance calcium-sensitive potassium channel (BK)-independent effects of PKG, which include inhibition of agonist binding affinity. Right side indicates BK-dependent effects of PKG, which include direct or indirect activation of BK channels, with subsequent membrane hyperpolarization, attenuation of calcium influx, and reduced contraction. Chronic hypoxia inhibits both the effects of PKG on agonist binding affinity and the ability of PKG to activate BK channels. Hypoxia may also have other PKG-independent effects including stimulation of myofilament calcium sensitivity and inhibition of BK activation.
The model used in the present study was designed to assess the effects of maternal hypoxia on fetal oxygenation. Even though basal oxygen tensions under both normoxic and hypoxic conditions were markedly different in fetuses compared with nonpregnant adults, it is striking that the ability of hypoxic acclimatization to ablate PKG-mediated vasodilation of serotonergic contractions was similar in both adult and fetal sheep. This finding strongly suggests that a common mechanism of response to chronic hypoxia was operating in both adults and fetuses despite major differences in their ambient oxygen tensions. Although it is not certain that chronic hypoxia can ablate PKG-mediated inhibition of contractions induced by agonists other than 5-HT, it is conceivable that other calcium-dependent (27) and calcium-independent (21) signal transduction pathways might have a similar effect on BK channel function. Many contractile agonists utilize common intracellular IP3-dependent and calcium-dependent signal transduction pathways with potential to activate BK channels (44); it seems probable that other contractile agonists might reveal similar effects of chronic hypoxia (32). Conversely, preliminary results from our laboratory suggest that the effects of chronic hypoxia on the ability of PKG to attenuate tone may be specific for the agonist used to initiate contraction, particularly in fetal arteries. To fully evaluate how chronic hypoxia differentially influences the PKG-sensitive and agonist-specific components of contraction will require many additional studies.
Whether the observed loss of PKG function represents a pathophysiological consequence or a biologically advantageous adaptation remains an open question. What does seem clear is that chronic hypoxia depresses multiple components of the NO-cGMP-PKG vasorelaxation pathway including endothelium-dependent vasodilation (45), inhibition of endothelial NO release (35), and inhibition of smooth muscle soluble guanylate cyclase activity (46). Because the patterns of PKG-dependent effects were similar in endothelium-intact and endothelium-denuded arteries, the present results further demonstrate that chronic hypoxia also depresses the ability of smooth muscle PKG to promote vasodilatation. The consequences of this pattern of effects remain uncertain but seem likely to enhance vasoconstrictor responses, which could help overcome typical hypoxic depression of contractility (32) but might also reduce the efficiency of flow-metabolism coupling and blood flow autoregulation. Such changes could increase vulnerability to, and could compromise recovery from, ischemic insults. For example, this pattern of increased vulnerability could be particularly important for the neurovascular unit, where the fine balance between metabolism and perfusion determines cell fate following a major insult. Alternatively, increased contractility may help maintain peripheral vascular resistance and arterial pressure at altitude. Overall, these effects of chronic hypoxia on the NO-cGMP-PKG vasorelaxation pathway seem likely to be compensatory given that pregnant sheep and their fetuses thrive at high altitude.
Perspectives and Significance
From a clinical perspective, these results predict that vascular reactivity to both endogenous and exogenous NO should be significantly depressed in both neonates and adults adapted to chronic hypoxia due to attenuation of the ability of PKG to activate BK channels. The results also suggest, however, that a BK-independent component of PKG-mediated vasorelaxation persists following hypoxic acclimatization, in which case increased activation of soluble guanylate cyclase or inhibition of phosphodiesterase might yield increased inhibition of vasoconstriction through BK-independent pathways (Figs. 7 and 8). Alternatively, hypoxia might also alter BK function, perhaps through PKC-mediated phosphorylation of the BK channel, which can block the effects of subsequent phosphorylation by PKG (51, 52). Therapeutically, such an effect of PKC might be reversed by PKC inhibitors. Another alternate approach might be to drive the BK channels more directly through caffeine-induced activation of ryanodine receptors with subsequent release of subsarcolemmal calcium and BK channel activation (44). Identification of which of these approaches might be most efficacious in clinical settings awaits future experimentation.
GRANTS
The work reported in this manuscript was supported by National Institutes of Health Grants HL-54120, HD-31266, HL-64867 and by the Loma Linda University School of Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.B.T., S.L.S., and J.M.W. performed experiments; R.B.T., S.L.S., J.M.W., and W.J.P. analyzed data; R.B.T., S.L.S., J.M.W., T.M.L., and W.J.P. interpreted results of experiments; R.B.T. and J.M.W. prepared figures; R.B.T. and J.M.W. drafted manuscript; R.B.T., T.M.L., and W.J.P. edited and revised manuscript; R.B.T. and W.J.P. approved final version of manuscript; J.M.W., T.M.L., and W.J.P. conception and design of research.
REFERENCES
- 1. Albert PR, Tiberi M. Receptor signaling and structure: insights from serotonin-1 receptors. Trends Endocrinol Metab 12: 453–460, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Alioua A, Tanaka Y, Wallner M, Hofmann F, Ruth P, Meera P, Toro L. The large conductance, voltage-dependent, and calcium-sensitive K+ channel, Hslo, is a target of cGMP-dependent protein kinase phosphorylation in vivo. J Biol Chem 273: 32950–32956, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Angeles DM, Williams J, Zhang L, Pearce WJ. Acute hypoxia modulates 5-HT receptor density and agonist affinity in fetal and adult ovine carotid arteries. Am J Physiol Heart Circ Physiol 279: H502–H510, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 5. Brinks HL, Eckhart AD. Regulation of GPCR signaling in hypertension. Biochim Biophys Acta 1802: 1268–1275, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choi C, Sellak H, Brown FM, Lincoln TM. cGMP-dependent protein kinase and the regulation of vascular smooth muscle cell gene expression: possible involvement of Elk-1 sumoylation. Am J Physiol Heart Circ Physiol 299: H1660–H1670, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colyer J. Phosphorylation states of phospholamban. Ann NY Acad Sci 853: 79–91, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Cornwell TL, Pryzwansky KB, Wyatt TA, Lincoln TM. Regulation of sarcoplasmic reticulum protein phosphorylation by localized cyclic GMP-dependent protein kinase in vascular smooth muscle cells. Mol Pharmacol 40: 923–931, 1991 [PubMed] [Google Scholar]
- 9. Crawley DE, Zhao L, Giembycz MA, Liu S, Barnes PJ, Winter RJ, Evans TW. Chronic hypoxia impairs soluble guanylyl cyclase-mediated pulmonary arterial relaxation in the rat. Am J Physiol Lung Cell Mol Physiol 263: L325–L332, 1992 [DOI] [PubMed] [Google Scholar]
- 10. Cui J, Yang H, Lee US. Molecular mechanisms of BK channel activation. Cell Mol Life Sci 66: 852–875, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Francis SH, Busch JL, Corbin JD, Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev 62: 525–563, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fukao M, Mason HS, Britton FC, Kenyon JL, Horowitz B, Keef KD. Cyclic GMP-dependent protein kinase activates cloned BKCa channels expressed in mammalian cells by direct phosphorylation at serine 1072. J Biol Chem 274: 10927–10935, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Furchgott RF. The pharmacological differentiation of adrenergic receptors. Ann NY Acad Sci 139: 553–570, 1967 [DOI] [PubMed] [Google Scholar]
- 14. Gao YD, Garcia ML. Interaction of agitoxin2, charybdotoxin, and iberiotoxin with potassium channels: selectivity between voltage-gated and Maxi-K channels. Proteins 52: 146–154, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Hu XQ, Yang S, Pearce WJ, Longo LD, Zhang L. Effect of chronic hypoxia on alpha-1 adrenoceptor-mediated inositol 1,4,5-trisphosphate signaling in ovine uterine artery. J Pharmacol Exp Ther 288: 977–983, 1999 [PubMed] [Google Scholar]
- 16. Ishikawa T, Hume JR, Keef KD. Regulation of Ca2+ channels by cAMP and cGMP in vascular smooth muscle cells. Circ Res 73: 1128–1137, 1993 [DOI] [PubMed] [Google Scholar]
- 17. Julian CG. High altitude during pregnancy. Clin Chest Med 32: 21–31, vii, 2011 [DOI] [PubMed] [Google Scholar]
- 18. Kamitomo M, Longo LD, Gilbert RD. Right and left ventricular function in fetal sheep exposed to long-term high-altitude hypoxemia. Am J Physiol Heart Circ Physiol 262: H399–H405, 1992 [DOI] [PubMed] [Google Scholar]
- 19. Komalavilas P, Lincoln TM. Phosphorylation of the inositol 1,4,5-trisphosphate receptor by cyclic GMP-dependent protein kinase. J Biol Chem 269: 8701–8707, 1994 [PubMed] [Google Scholar]
- 20. Kumar R, Joyner RW, Komalavilas P, Lincoln TM. Analysis of expression of cGMP-dependent protein kinase in rabbit heart cells. J Pharmacol Exp Ther 291: 967–975, 1999 [PubMed] [Google Scholar]
- 21. Li D, Wang Z, Sun P, Jin Y, Lin DH, Hebert SC, Giebisch G, Wang WH. Inhibition of MAPK stimulates the Ca2+ -dependent big-conductance K channels in cortical collecting duct. Proc Natl Acad Sci USA 103: 19569–19574, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin MT, Hessinger DA, Pearce WJ, Longo LD. Modulation of BK channel calcium affinity by differential phosphorylation in developing ovine basilar artery myocytes. Am J Physiol Heart Circ Physiol 291: H732–H740, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Lincoln TM, Cornwell TL, Rashatwar SS, Johnson RM. Mechanism of cyclic-GMP-dependent relaxation in vascular smooth muscle. Biochem Soc Trans 16: 497–499, 1988 [DOI] [PubMed] [Google Scholar]
- 24. Lincoln TM, Dey N, Sellak H. Invited review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: from the regulation of tone to gene expression. J Appl Physiol 91: 1421–1430, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Longo LD, Hull AD, Long DM, Pearce WJ. Cerebrovascular adaptations to high-altitude hypoxemia in fetal and adult sheep. Am J Physiol Regul Integr Comp Physiol 264: R65–R72, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Longo LD, Pearce WJ. Fetal cerebrovascular acclimatization responses to high-altitude, long-term hypoxia: a model for prenatal programming of adult disease? Am J Physiol Regul Integr Comp Physiol 288: R16–R24, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Miwa S, Kawanabe Y, Okamoto Y, Masaki T. Ca2+ entry channels involved in endothelin-1-induced contractions of vascular smooth muscle cells. J Smooth Muscle Res 41: 61–75, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Moore LG, Charles SM, Julian CG. Humans at high altitude: hypoxia and fetal growth. Respir Physiol Neurobiol 178: 181–190, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morgado M, Cairrao E, Santos-Silva AJ, Verde I. Cyclic nucleotide-dependent relaxation pathways in vascular smooth muscle. Cell Mol Life Sci 69: 247–266, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nauli SM, Zhang L, Pearce WJ. Maturation depresses cGMP-mediated decreases in [Ca2+]i and Ca2+ sensitivity in ovine cranial arteries. Am J Physiol Heart Circ Physiol 280: H1019–H1028, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Parker RB, Waud DR. Pharmacological estimation of drug-receptor dissociation constants. Statistical evaluation I Agonists. J Pharmacol Exp Ther 177: 1–12, 1971 [PubMed] [Google Scholar]
- 32. Pearce W. Hypoxic regulation of the fetal cerebral circulation. J Appl Physiol 100: 731–738, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Pearce WJ, Butler SM, Abrassart JM, Williams JM. Fetal cerebral oxygenation: the homeostatic role of vascular adaptations to hypoxic stress. Adv Exp Med Biol 915: 225–232, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pearce WJ, Hull AD, Long DM, Longo LD. Developmental changes in ovine cerebral artery composition and reactivity. Am J Physiol Regul Integr Comp Physiol 261: R458–R465, 1991 [DOI] [PubMed] [Google Scholar]
- 35. Pearce WJ, Williams JM, Hamade MW, Chang MM, White CR. Chronic hypoxia modulates endothelium-dependent vasorelaxation through multiple independent mechanisms in ovine cranial arteries. Adv Exp Med Biol 578: 87–92, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Peng W, Hoidal JR, Karwande SV, Farrukh IS. Effect of chronic hypoxia on K+ channels: regulation in human pulmonary vascular smooth muscle cells. Am J Physiol Cell Physiol 272: C1271–C1278, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Pilz RB, Casteel DE. Regulation of gene expression by cyclic GMP. Circ Res 93: 1034–1046, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Robertson BE, Schubert R, Hescheler J, Nelson MT. cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol 265: C299–C303, 1993 [DOI] [PubMed] [Google Scholar]
- 39. Schlossmann J, Desch M. cGK substrates. Handb Exp Pharmacol 163–193, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Standen NB, Quayle JM. K+ channel modulation in arterial smooth muscle. Acta Physiol Scand 164: 549–557, 1998 [DOI] [PubMed] [Google Scholar]
- 41. Teng GQ, Nauli SM, Brayden JE, Pearce WJ. Maturation alters the contribution of potassium channels to resting and 5HT-induced tone in small cerebral arteries of the sheep. Brain Res Dev 133: 81–91, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Teng GQ, Williams J, Zhang L, Purdy R, Pearce WJ. Effects of maturation, artery size, and chronic hypoxia on 5-HT receptor type in ovine cranial arteries. Am J Physiol Regul Integr Comp Physiol 275: R742–R753, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Vanhoutte PM. Cardiovascular effects of serotonin. J Cardiovasc Pharmacol 10, Suppl 3: S8–S11, 1987 [PubMed] [Google Scholar]
- 44. Wellman GC, Nelson MT. Signaling between SR and plasmalemma in smooth muscle: sparks and the activation of Ca2+-sensitive ion channels. Cell Calcium 34: 211–229, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Williams JM, Pearce WJ. Age-dependent modulation of endothelium-dependent vasodilatation by chronic hypoxia in ovine cranial arteries. J Appl Physiol 100: 225–232, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Williams JM, White CR, Chang MM, Injeti ER, Zhang L, Pearce WJ. Chronic hypoxic decreases in soluble guanylate cyclase protein and enzyme activity are age dependent in fetal and adult ovine carotid arteries. J Appl Physiol 100: 1857–1866, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Wu RS, Marx SO. The BK potassium channel in the vascular smooth muscle and kidney: alpha- and beta-subunits. Kidney Int 78: 963–974, 2010 [DOI] [PubMed] [Google Scholar]
- 48. Yoshida Y, Sun HT, Cai JQ, Imai S. Cyclic GMP-dependent protein kinase stimulates the plasma membrane Ca2+ pump ATPase of vascular smooth muscle via phosphorylation of a 240-kDa protein. J Biol Chem 266: 19819–19825, 1991 [PubMed] [Google Scholar]
- 49. Zhang R, Sun H, Liao C, Yang H, Zhao B, Tian J, Dong S, Zhang Z, Jiao J. Chronic hypoxia in cultured human podocytes inhibits BKCa channels by upregulating its beta4-subunit. Biochem Biophys Res Commun 420: 505–510, 2012 [DOI] [PubMed] [Google Scholar]
- 50. Zhou W, Dasgupta C, Negash S, Raj JU. Modulation of pulmonary vascular smooth muscle cell phenotype in hypoxia: role of cGMP-dependent protein kinase. Am J Physiol Lung Cell Mol Physiol 292: L1459–L1466, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Zhou XB, Wulfsen I, Utku E, Sausbier U, Sausbier M, Wieland T, Ruth P, Korth M. Dual role of protein kinase C on BK channel regulation. Proc Natl Acad Sci USA 107: 8005–8010, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhu S, Browning DD, White RE, Fulton D, Barman SA. Mutation of protein kinase C phosphorylation site S1076 on alpha-subunits affects BK(Ca) channel activity in HEK-293 cells. Am J Physiol Lung Cell Mol Physiol 297: L758–L766, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]