Abstract
Physical activity-induced prevention of hepatic steatosis is maintained during short-term (7-day) transitions to an inactive state; however, whether these protective effects are present under a longer duration of physical inactivity is largely unknown. Here, we sought to determine whether previous physical activity had protective effects on hepatic steatosis and metabolic health following 4 wk of physical inactivity. Four-week old, hyperphagic, male Otsuka Long-Evans Tokushima fatty (OLETF) rats were randomly assigned to either a sedentary group for 16 wk (OLETF-SED), given access to running wheels for 16 wk with wheels locked 5 h (OLETF-WL5hr) or given access to running wheels for 12 wk with wheels locked 4 wk (OLETF-WL4wk) prior to death. Four weeks of physical inactivity caused hepatic steatosis development, but liver triglycerides remained 60% lower than OLETF-SED (P < 0.01), and this was associated with only a partial loss in activity-induced improvements in body composition, serum lipids, and glycemic control. Total hepatic mitochondrial palmitate oxidation, citrate synthase, and β-HAD activity returned to SED levels following 4 wk of inactivity, whereas markers of fatty acid uptake and lipogenesis remained relatively suppressed following 4 wk of inactivity. In addition, 4 wk of inactivity caused a complete loss of activity-induced increases in serum IL-6 and reductions in regulated upon activation, normal T-cell expressed, and secreted (RANTES), and a partial loss in reductions in leptin, monocyte chemoattractant protein-1, and TNF-α. In conclusion, 4 wk of physical inactivity does not result in a complete loss in physical activity-induced benefits but does cause deterioration in the liver phenotype and overall metabolic health in hyperphagic OLETF rats.
Keywords: mitochondrial function, physical inactivity, hepatic steatosis, nonalcoholic fatty liver disease
currently, more than 30% of the adult population of the United States is considered obese and more than 60% are overweight (18), and there is no denying that a significant contributing factor to this epidemic is the ease of access to unhealthy, calorically dense food choices. However, because of a plethora of circumstances, there also currently is little need for physical activity in our daily living. In fact, >95% of U.S. adults do not get the recommended amount of physical activity per week (52). Distressingly, the negative by-product of our modern civilization is an increased risk of chronic disease, such as heart disease, Type 2 diabetes, and nonalcoholic fatty liver disease (NAFLD).
NAFLD is a chronic, progressive liver disease characterized by increased hepatic triglyceride (TAG) accumulation (≥5% by weight for diagnosis) that occurs in the absence of excess alcohol consumption (>20 g/day) and encompasses a histological spectrum ranging from simple hepatic steatosis to nonalcoholic steatohepatitis, advanced fibrosis, and cirrhosis (41). NAFLD is considered the hepatic manifestation of the metabolic syndrome (16) and affects ∼30% of the U.S. adult population (8) and 75–100% of obese or morbidly obese individuals (3, 8).
Long-term physical inactivity is linked to virtually all disease outcomes, including insulin resistance and Type 2 diabetes (6) and is an actual known leading cause of death in the United States (29, 32). In addition, chronic habitual physical inactivity also is associated with increased incidence of NAFLD (21, 40). Furthermore, while it is well known that exercise cessation (or reduced daily ambulatory activity) and induction of acute, short-term physical inactivity leads to a rapid reduction in insulin sensitivity (5, 11, 23, 24, 30, 36, 51), increases in fat mass and reductions in lean body mass in both rodents and humans (25, 27, 36), the potential mechanistic links between short-term physical inactivity and NAFLD remain poorly understood.
The Otsuka Long-Evans Tokushima fatty (OLETF) rat is a commonly studied animal model of obesity and Type 2 diabetes, in which animals are selectively bred for null expression of the cholecystokinin-1 receptor (33, 34). Thus, these animals exhibit hyperphagia, which leads to the progressive development of obesity, insulin resistance, Type 2 diabetes, and NAFLD (44). In fact, significant hepatic TAG accumulation occurred in as little as 4–5 wk postweaning in the sedentary, hyperphagic OLETF rats (witnessed at 8 wk of age) (44). In a series of studies, we have demonstrated that these pathological metabolic events are prevented when the OLETFs (4) are given daily access to voluntary running wheels and allowed to be physically active (42, 43, 45, 46). However, it is unclear how long these pathological events can be prevented after becoming inactive. It has been observed that the cessation of aerobic exercise in animals training for 6 wk resulted in greater hepatic TAG accumulation than in animals that were chronically sedentary (54), but it is unknown what metabolic mechanisms may contribute to these increases. In shorter-duration studies, we found that there were protective effects of daily physical activity on preventing NAFLD that persisted for 173 h (7 days of physical inactivity induced with wheel lock) after wheel running was stopped, despite an observed dramatic down-regulation of hepatic mitochondrial function and the rapid induction of several hepatic lipogenic proteins and intermediates during this 7-day period (42). Whether these protective effects of daily physical activity on NAFLD development persist for a longer duration is unknown and of potential mechanistic and clinical significance. Here, we sought to test our hypothesis that the physical activity-induced benefits on NAFLD would be lost during 4 wk of physical inactivity in an environment of overnutrition in the hyperphagic, OLETF rats. In addition, these metabolic maladaptations would be related to a worsening of several known systemic contributors to the disease progression (adiposity, insulin resistance, and systemic inflammation).
METHODS
Animal protocol.
The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Missouri. Four-week-old OLETF male rats were supplied by the Tokushima Research Institute, Otsuka Pharmaceutical (Tokushima, Japan). Animals were randomly assigned to one of the following groups (n = 8/group): sedentary group for 16 wk (OLETF-SED), group given access to running wheels for 16 wk with wheels locked 5 h (OLETF-WL5hr), or a group given access to running wheels for 12 wk with wheels locked 4 wk (OLETF-WL4wk) prior to death. The experimental design is shown in Fig. 1. Running wheels were outfitted with Sigma Sport BC 800 bicycle computers (Cherry Creek Cyclery, Foster Falls, VA) for measuring daily running activity. Nonhyperphagic, control Long-Evans Tokushima Otsuka rats remained in sedentary cage conditions (LETO-SED). Animals were individually housed with a 0600–1800-h light and 1800−0600-h dark cycle within temperature-controlled animal quarters (21°C). All animals were provided standard rodent chow (Formulab 5008, Purina Mills, St. Louis, MO) and were able to eat ad libitum. Body mass and food intake were measured weekly throughout the study. At 20 wk of age, rats were anesthetized with pentobarbital sodium (100 mg/kg) and then exsanguinated by removal of the heart. All animals were fasted for 5 h prior to death.
Fig. 1.
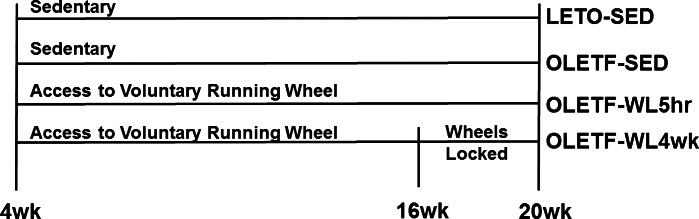
Overall experimental design. LETO, Long-Evans Tokushima (rats); SED, sedentary; OLETF, Otsuka Long-Evans Tokushima fatty (rats); WL, wheels locked.
Dual-energy X-ray absorptiometry.
Whole-body composition was measured using a Hologic QDR-1000W dual-energy, X-ray absorptiometry machine calibrated for rats, as previously described (26).
Serum assays.
Serum glucose (Sigma, St. Louis, MO), TAG (Sigma), free fatty acids (FFA; Wako Chemicals, Richmond, VA), and insulin (Linco Research, St. Charles, MO) were assessed using commercially available assays. Serum cytokine concentrations [leptin, monocyte chemoattractant protein-1 (MCP-1), TNF-α, IL-6, and regulated upon activation, normal T-cell expressed, and secreted (RANTES)] were determined using a Milliplex immunoassay kit (Millipore, Billercia, MA). All assays were completed according to the manufacturers' instructions.
Tissue collection and preparation procedure.
Livers were quickly removed from anesthetized rats and either flash frozen in liquid nitrogen, placed in 10% formalin, or placed in ice-cold isolation buffer (100 mM KCl, 40 mM Tris·HCl, 10 mM Tris-Base, 5 mM MgCl2·6 H2O, 1 mM EDTA, and 1 mM ATP; pH 7.4). Retroperitoneal and omental adipose tissue fat pads were excised from animals and weighed.
Fatty acid oxidation.
Fatty acid oxidation assays were performed in fresh hepatic tissue preparations using radiolabeled [1-14C] palmitate (American Radiolabeled Chemicals, St. Louis, MO), as previously described by our group (43). This assay represents the capacity for the oxidation of fatty acids, resulting in chain-shortened acyl-CoAs and acetyl CoA, which can potentially enter the TCA cycle. During complete oxidation of palmitate, radiolabeled CO2 is produced. In addition, a portion of lipids is incompletely oxidized, leading to the production of acid-soluble metabolites (ASMs; e.g., ketone bodies, acyl-CoAs, and acylcarnitines), which retain their radiolabel. Briefly, the oxidation rate of 14C palmitate was measured by collecting and counting the 14CO2 (representing complete fatty acid oxidation) and 14C-labeled acid-soluble metabolites (representing incomplete fatty acid oxidation) that were collected within a trapping device and counted with a liquid scintillation counter. Palmitate oxidation experiments were performed in the presence (100 μM) or absence of etomoxir (a specific inhibitor of mitochondrial carnitine palmitoyl-CoA transferease-1 and entry into the mitochondria) to examine the relative contribution of mitochondrial (-etomoxir) and extra-mitochondrial organelles (+etomoxir) in total fatty acid oxidation, as previously described (46).
Citrate synthase and β-hydroxyacyl-CoA dehydrogenase activity.
Citrate synthase and β-hydroxyacyl-CoA dehydrogenase (β-HAD) activities were determined using the methods of Srere (50) and Bass et al. (2), respectively, as previously described by our group (43).
Intrahepatic lipid content and liver morphology.
Intrahepatic TAG content was determined as previously described (43). To examine liver morphology, formalin-fixed paraffin-embedded sections of liver were stained with hematoxylin and eosin (H&E).
Western blot analysis.
Western blot analyses were used to determine protein content for CD36/fatty acid translocase (FAT), sterol regulatory element binding protein (SREBP)-1c, peroxisome proliferator-activated receptor (PPAR) γ, AMPK, AMPK Thr-172 phosphorylation-specific (pAMPK), acetyl coenzyme A carboxylase (ACC), ACC Ser-79 phosphorylation-specific (pACC), fatty acid synthase (FAS), and stearoyl-CoA desaturase-1 (SCD-1). Polyclonal antibodies for AMPK, AMPK Thr-172 phsophorylation-specific ACC, Ser-79 phosphorylation-specific ACC, and FAS were from Cell Signaling Technology (Beverly, MA). Polyclonal antibodies for CD36, PPARγ, and SREBP-1c were from Santa Cruz Biotechnology (Dallas, TX). SCD-1 polyclonal antibody was from Alpha Diagnostics International (San Antonio, TX). The content of phosphorylated proteins (using phosphorylated-specific antibodies) was calculated from the density of the band of the phosphorylated protein divided by the density (content) of the protein (total) using the appropriate antibody.
Liver samples were homogenized using lysis buffer. Protein (20–40 μg) was loaded into a SDS-PAGE gel and probed with primary antibodies. After washing, the membrane was incubated with horseradish peroxidase-conjugated secondary antibodies. Protein bands were quantified using a densitometer (Bio-Rad, Hercules, CA). To control for equal protein loading and transfer, the membranes were then stained with 0.1% amido-black (Sigma). The total protein staining for each lane was quantified by densitometry, and these values were used to correct for any differences in protein loading or transfer of all band densities. The intensities of the bands and total protein staining were quantified using Quantity One software (Bio-Rad).
Statistical analysis.
Each outcome measure was examined in 7 or 8 animals per group. For each outcome measure, a one-way ANOVA was performed (SPSS/19.0, SPSS, Chicago, IL), with significant interactions followed up using Fisher LSD post hoc comparisons. Values are reported as means ± SE, and statistical significance was determined as P < 0.05.
RESULTS
Animal characteristics.
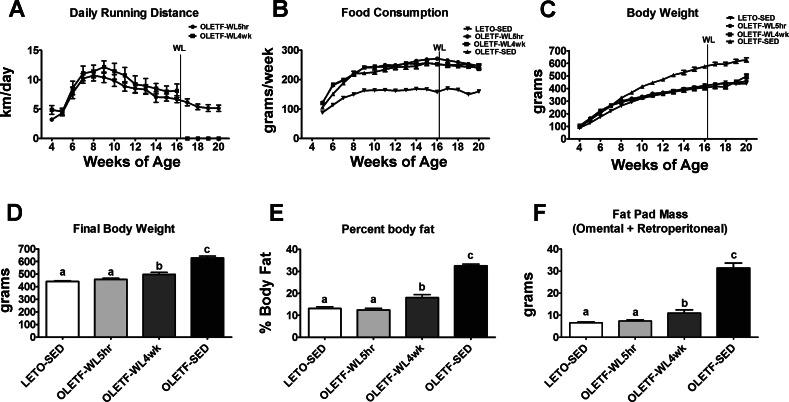
Average daily running did not differ between the OLETF-WL5hr and OLETF-WL4wk during the 12 wk in which both groups had access to unlocked running wheels (P > 0.05, Fig. 2A). Similar to our previous reports (26, 35), running animals displayed initial running distances of ∼4 km/day at 4 wk of age and 12 km/day at 9–10 wk of age and averaging 5–8 km/day for the remainder of the study (weeks 10–20). The health benefits of daily physical activity were lower body weight, body fat percentage, and fat pad mass (omental and retroperitoneal) in the OLETF-WL5hr (P < 0.05; see Fig. 2) compared with SED. These reductions were slightly lost with a transition to physical inactivity for 4 wk, with significant increases in body weight, percent body fat, and fat pad mass that remained significantly less than OLETF-SED (P < 0.01, Fig. 2C–F). The average weight gain during the 4-wk wheel-lock period was 80.6 g compared with 23.8 g in the OLETF-WL5hr rats (P < 0.05). Weekly food consumption was similar between all of the OLETF groups and significantly greater than LETO-SED (Fig. 2B). In addition, as shown in Fig. 2B, daily food consumption did not differ between the OLETF-WL5hr and OLETF-WL4wk during the 4-wk wheel-lock period, and food intake did not significantly change within the OLETF-WL4wk animals during the wheel lock. Finally, daily physical activity led to a significantly greater heart weight-to-body weight ratio in the WL5hr and WL4wk groups compared with OLETF-SED and LETO-SED (3.34 ± 0.10 g/kg, 3.05 ± 0.08, 2.40 ± 0.04, and 2.76 ± 0.08, respectively; P < 0.05), with 4 wk of physical inactivity resulting in a significantly lower ratio compared with OLETF-WL5hr (P < 0.05).
Fig. 2.
Average daily running distance and effects of 4 wk of physical inactivity on body weight and adiposity measures. Average daily running distance for the OLETF-WL5h and OLETF-WL4wk groups (A), weekly food consumption (B), weekly body weights (C), final body weight (D), percent body fat (E), and fat pad mass (omental + retroperitoneal; F). Values are expressed as means ± SE (n = 7–8 per group). a,b,cValues with different letter superscripts are significantly different, P < 0.05.
Changes in glycemic control, serum TAGs, and FFAs.
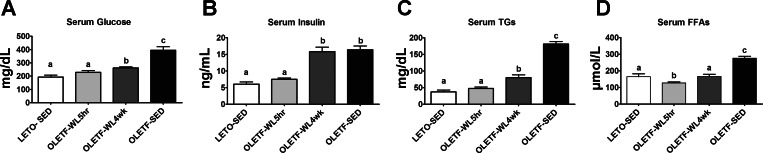
Daily physical activity-induced improvements in glycemic control (reduced serum glucose and insulin, Fig. 3, A and B) were partially lost with 4 wk of inactivity, with small but significant increases in serum glucose requiring much greater serum insulin levels (not different between OLETF-WL4wk and OLETF-SED rats; Fig. 3B). In addition, daily activity-induced reductions in serum TAGs and FFAs were partially lost with a transition to inactivity for 4 wk (P < 0.05, Fig. 3, C and D), but OLETF-WL4wk still exhibited 40–55% lower concentrations of these measures than the OLETF-SED group (Fig. 3).
Fig. 3.
Effects of 4 wk of physical inactivity on glycemic control, serum triglycerides (TAGs), and serum free fatty acids (FFAs). Serum glucose (A), serum insulin (B), serum TAG (C), and serum FFA (D) concentrations. All values are expressed as means ± SE (n = 7 or 8 per group). a,b,cValues with different letter superscripts are significantly different, P < 0.05.
Inactivity and intrahepatic TAG accumulation.
Daily activity-induced attenuation in hepatic steatosis was partially lost with 4 wk of physical inactivity [Fig. 4, A–D; note the return in lipid vacuolization seen in the representative hematoxylin-and-eosin images from randomly selected sections in the WL4wk (Fig. 4C) compared with WL5hr animals (Fig. 4B)]. These histological findings were confirmed with biochemical hepatic TAGs being significantly higher in WL4wk compared with WL5hr rats (Fig. 4E, P < 0.05). However, hepatic TAG content remained ∼60% lower than the levels witnessed in the OLETF-SED rats (P < 0.01, Fig. 4E).
Fig. 4.
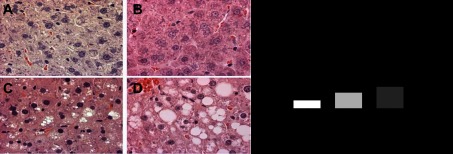
Effects of 4 wk of physical inactivity on liver histology and hepatic TAG content. Representative images of hematoxylin-and-eosin staining (×40 magnification, A–D), LETO-SED (A), OLETF-WL5h (B), OLETF-WL4wk (C), OLETF-SED (D), and quantification of hepatic triglyceride (E). All values are expressed as means ± SE (n = 7–8 per group). a,b,c,dValues with different superscripts are significantly different, P < 0.05.
Markers of hepatic mitochondrial function.
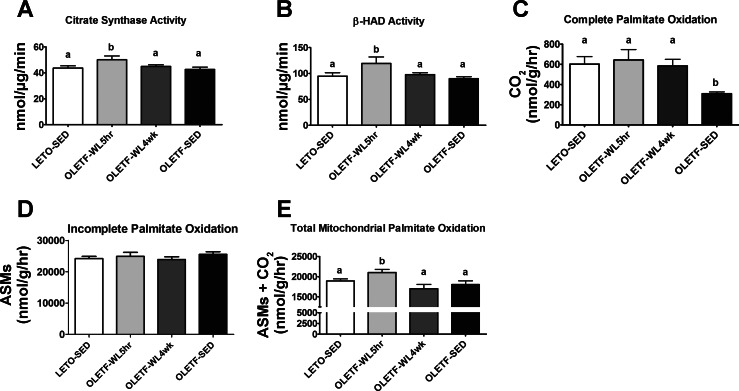
Hepatic mitochondrial function revealed a complete loss in the physical activity-induced increases in hepatic β-HAD and citrate synthase activities after the 4-wk transition to physical inactivity (P < 0.05; Fig. 5, A and B). In addition, while complete palmitate oxidation to CO2 was maintained following the 4-wk transition to inactivity (Fig. 5C), incomplete palmitate oxidation did not differ among groups (ASM production, Fig. 5D), but the daily physical activity-induced increases in the mitochondrial contribution (the etomoxir inhibitable portion) to total palmitate oxidation (CO2 + ASMs) were completely lost in the OLETF-WL4wk animals (P < 0.05, Fig. 5E).
Fig. 5.
Effects of 4 wk of physical inactivity on markers of hepatic mitochondrial function. Citrate synthase activity (A), hepatic β-hydroxyacyl-CoA dehydrogenase activity (B), complete palmitate oxidation to CO2 (C), incomplete palmitate oxidation (ASMs; D), and total mitochondrial oxidation of palmitate (CO2 +ASMs; E). All values are expressed as means ± SE (n = 7 or 8 per group). a,bValues with different letter superscripts are significantly different, P < 0.05.
Hepatic markers of fatty acid uptake and de novo lipogenesis.
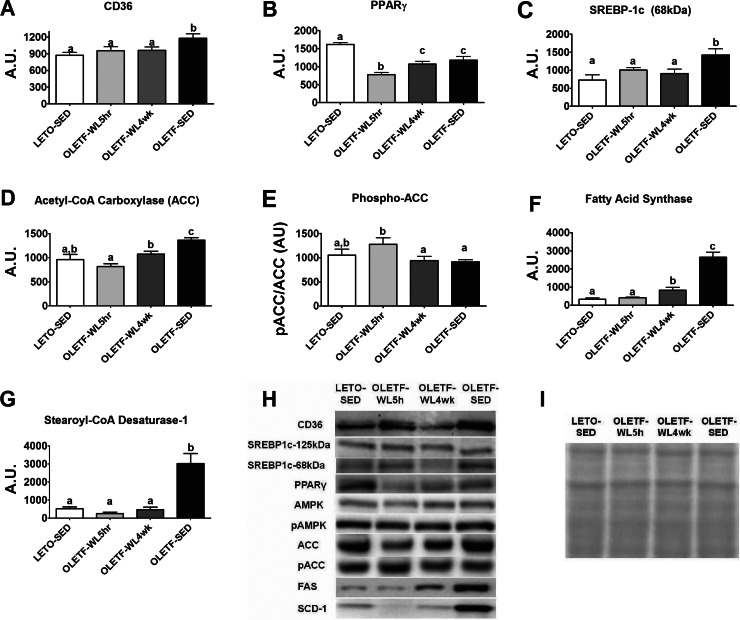
Physical activity-induced reductions in hepatic CD36/FAT, which is involved in fatty acid uptake, were maintained in the OLETF-WL4wk animals (P < 0.05, Fig. 6A); whereas, activity-induced reductions in PPARγ were lost in the WL4wk animals (P < 0.05, Fig. 6B). However, daily activity reduced the expression of the nuclear form of SREBP-1c (68 kDa, Fig. 6C), reductions that were maintained in the WL4wk rats. Similar to our previous reports (42, 43), daily physical activity also suppressed other hepatic markers of de novo fatty acid synthesis, including ACC, FAS, and SCD-1 and increased the phosphorylation and inactivation status of ACC (P < 0.01, Fig. 6, D–G). Here, we show that hepatic ACC protein content was significantly greater in OLETF-WL4wk than OLETF-WL5hr (P < 0.05) but remained ∼25% lower than the sedentary animals (P < 0.05, Fig. 6D). The 4 wk of physical inactivity also resulted in a significant decline in hepatic ACC Ser-79 phosphorylation (OLETF-WL5hr vs. OLETF-WL4wk, P < 0.05) to the level seen in the OLETF-SED rats (Fig. 6E). In addition, physical activity-induced reductions in FAS protein content was partially lost with 4 wk of physical inactivity (OLETF-WL5hr vs. OLETF-WL4wk, P < 0.05) but remained four-fold lower than OLETF-SED (P < 0.001, Fig. 6F), and SCD-1 protein content remained fully suppressed following the 4 wk of inactivity (P > 0.05, Fig. 6G). Protein content of AMPK and phospho-AMPK did not differ among groups (representative Western blots shown in Fig. 6H).
Fig. 6.
Effects of 4 wk of physical inactivity on markers of hepatic fatty acid uptake and lipogenesis. CD36/FAT (A), peroxisome proliferator-activated receptor γ (PPARγ; B), sterol regulatory element binding protein-1c (SREBP-1c; 68 kDa) (C), acetyl CoA carboxylase (ACC; D), phosphorylated acetyl CoA carboxylase (phospho-ACC; E), fatty acid synthase (F), steroyl-CoA desaturase-1 (SCD; G), representative Western blot images (H), and representation of protein loaded from amido-black staining (I). AU, arbitrary units. Quantifications of protein content were corrected for total protein loaded. All values are expressed as means ± SE (n = 7 or 8 per group). a,b,cValues with different superscripts are significantly different, P < 0.05.
Systemic markers of inflammation.
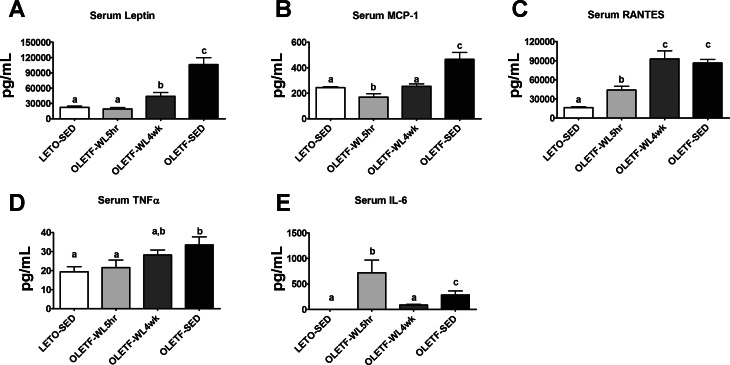
Physical activity-induced reductions in serum leptin and MCP-1 were partially lost with 4 wk of inactivity (P < 0.05, Fig. 7, A and B), but concentrations remained 50–75% lower than those seen in the OLETF-SED animals. In addition, findings for TNF-α tended to be similar (Fig. 7D). Serum concentrations of RANTES completely returned to OLETF-SED levels with 4 wk of physical inactivity (Fig. 7C). Moreover, physical activity-induced increases in serum IL-6 were completely lost with 4 wk of physical inactivity, with OLETF-WL4wk being significantly lower than OLETF-SED (P < 0.05, Fig. 7E).
Fig. 7.
Effects of 4 wk of physical inactivity on serum markers of systemic inflammation. Leptin (A), monocyte chemoattractant protein-1 (B), RANTES (C), tumor necrosis factor alpha (D), and interleukin-6 (E). All values are expressed as means ± SE (n = 7 or 8/group). a,b,cValues with different superscripts are significantly different, P < 0.05.
DISCUSSION
Increasing daily physical activity can be beneficial in the prevention and likely in the treatment of NAFLD (reviewed in Ref. 41). However, it is less clear what hepatic alterations occur in response to physical inactivity that may lead to NAFLD development and progression. We have previously reported that an acute transition to physical inactivity for 7 days following 16 wk of voluntary wheel running in the OLETF rat resulted in the rapid loss of many of the beneficial effects of daily physical activity on hepatic lipid metabolism, but these animals remained protected against NAFLD development despite their hypercaloric environment (42). Here, we report that longer-duration physical inactivity of 4 wk in the presence of persistent hyperphagia resulted in several peripheral (increased body mass, fat pad mass, serum glucose, TAGs, FFAs, and insulin) and hepatic changes associated with NAFLD development and progression. Namely, significant hepatic TAG accumulation was seen after 4 wk of inactivity, yet hepatic TAGs still remained markedly less compared with chronically sedentary animals. In addition, some markers of hepatic mitochondrial content and function returned to levels of chronically sedentary animals, and many of the activity-induced changes in systemic inflammation and cytokine levels were lost after 4 wk of inactivity. Collectively, the consequences of the interaction between hyperphagic and a physically inactive state appear to promote the development and likely the future progression of NAFLD. However, there appears to be a prolonged resistance to a more complete hepatic dysfunction as seen in the chronically sedentary OLETF-SED group, in part, due to a continued suppression of hepatic fatty acid uptake and de novo lipogenesis markers.
The wheel-lock model that our research group utilizes is a model that results in a reduction in physical activity by preventing voluntary wheel running. As reviewed by Roberts et al. (47), this model is less drastic than other more traditional models of inactivity (i.e., hindlimb unloading or immobilization) and is effective in eliciting metabolic alterations in an attempt to understand early alterations in hepatic lipid metabolism caused by a transition to physical inactivity after chronic access to daily running. Our findings are in support of previous work showing that it may take up to 6 wk of inactivity to see increases in hepatic TAGs in previously trained rats fed normal rat chow (54). We have expanded upon these previous findings and report that significant peripheral adaptations were observed with 4 wk of inactivity, including increased body weight and adiposity, as well as increases in serum TAG, FFAs, glucose, insulin, and leptin, changes not seen with 7 days of inactivity (42). However, all of the aforementioned increases, except insulin, were a small percentage of the differences between OLETF-WL5hr and OLETF-SED; serum insulin reached the high value of OLETF-SED. A number of these factors, including excess FFAs (9) and decrements in glycemic control, may result in increased risk for the development of NAFLD. Increases in FFAs being shunted to the liver can promote an environment for lipid infiltration (19), while peripheral insulin resistance may also be essential for hepatic lipid accumulation (7). Although these changes in peripheral precursors may promote hepatic lipid accumulation, many of these peripheral adaptations remained lower than values observed in sedentary animals, suggesting that previous physical activity may have some protective effects even after 4 wk of being sedentary.
Our plasma inflammatory cytokine data revealed a number of findings relevant to the physical inactivity and overnutrition-induced development of NAFLD. Systemic inflammation may contribute to the progression of NAFLD, in part, through activation of stellate cells and promotion of collagen formation once steatosis is present (12). In addition, chemokines, such as MCP-1 and RANTES, may contribute to the inflammatory response by recruiting macrophages and other immune cells that can produce proinflammatory cytokines (49). The protective effects of daily activity on plasma markers of systemic inflammation that have solid links to the progression of NAFLD (12, 28) were either partially (leptin, MCP-1, and TNF-α) or completely (RANTES) abrogated by a transition to inactivity. These findings were linked to changes in adiposity and serum lipids in the WL4wk rats, consistent with the known interrelationships among physical inactivity, adipose tissue expansion, and inflammation (37). These findings warrant future investigation of tissue-specific changes in both adipose tissue and liver.
Our plasma IL-6 data, however, revealed complex influences of obesity, regular physical activity, and a transition to inactivity on this controversial cytokine. The finding of higher IL-6 concentrations in OLETF-SED compared with LETO-SED animals is consistent with the well-documented mild elevations in IL-6 associated with obesity (48). IL-6 has been traditionally thought to have negative health effects and is associated with insulin resistance (reviewed in Ref. 15). Therefore, it might be regarded as somewhat surprising that our WL5hr-OLETF rats had substantially greater plasma IL-6 levels compared with all other groups. This elevation was likely the result of physical activity-induced IL-6 production from the last bout of wheel running, as, at least in humans, plasma IL-6 peaks at ∼4 h and remains elevated for up to ∼24 h following acute exercise (17, 38). Additionally, it is now clear that IL-6 has potent anti-inflammatory (38), insulin-sensitizing (1, 10, 20), and lipolytic (1) actions. It also has been suggested that IL-6 confers favorable regulatory effects on hepatic fatty acid oxidation (39). Thus, taking into account these recent advances with our understanding of IL-6 biology, our current data suggest that the loss of repeated activity-induced increases in circulating IL-6 may have contributed to the decreased hepatic fatty acid oxidation seen in the OLETF-WL4wk rats and to the development of NAFLD.
As previously reviewed, mitochondrial abnormalities and impaired β-oxidative capacity have been implicated in the pathogenesis of NAFLD (53). We have previously shown that mitochondrial dysfunction precedes hepatic steatosis development in OLETF rats under sedentary conditions (44). However, when given access to running wheels, increasing daily physical activity increased hepatic mitochondrial function and content and prevented NAFLD development, even in the presence of hyperphagia in OLETF rats (42, 43). Similar to our previous report of a loss of hepatic mitochondrial function with 7 days of physical inactivity (42), the present study showed activity-induced improvements in mitochondrial function, including total mitochondrial palmitate oxidation and mitochondrial enzyme activities (citrate synthase and β-HAD) were lost after 4 wk of inactivity. This loss of hepatic mitochondrial function likely contributed to the increases in hepatic TAGs witnessed in the WL4wk rats.
Somewhat contrary to our original hypothesis based on the rapid changes induced with 7 days of inactivity (42), there were residual benefits of chronic voluntary wheel running on fatty liver disease that remained after 4 wk of inactivity, even under conditions of overnutrition. Recent work in NAFLD patients highlights that greater than 25% of hepatic TAG accumulation can be accounted for by de novo lipogenesis (14), pointing to the importance of this metabolic pathway in NAFLD. In addition, recent studies have indicated that the fatty acid transporter CD36/FAT is upregulated in NAFLD patients (31). We previously reported that some proteins associated with de novo lipogenesis (FAS, SCD-1) remained improved in animals that were physically inactive for 7 days compared with chronically sedentary animals (42). Here, we report that physical activity-induced suppression of hepatic CD36, SREBP-1c, ACC, FAS, and SCD-1 remained largely reduced after being in a physically inactive state for 4 wk. SREBP-1c is considered to be a primary transcription factor controlling lipogenesis, and because ACC and FAS are the first two committed steps in de novo fatty acid synthesis, their continued suppression likely contributes to the residual benefits of physical activity in its suppression of hepatic TAG accumulation. Another candidate molecule perhaps contributing to the residue benefits of chronic activity is SCD-1. Hepatic SCD-1 is known to contribute to the abnormal partitioning of fatty acids by increasing ACC activity and decreasing fatty acid oxidation, shunting substrates to fatty acid synthesis (13, 22). Interestingly, activity-induced reductions in SCD-1 protein content were completely maintained following 4 wk of physical inactivity and hyperphagia. When taken together, we speculate that these reductions in CD36, SREBP-1c, FAS, ACC, and SCD-1 that remained even after 4 wk of physical inactivity in an environment of overnutrition may be highly important in the continued suppression of hepatic TAG accumulation in previously active animals compared with sedentary animals. Moreover, it appears likely that these proteins remained suppressed as a result of peripheral factors remaining lower (FFAs, TAGs, glucose) in the WL4wk animals compared with OLETF-SED animals. This highlights both hepatic and peripheral adaptations that are conferring protection in these animals.
The benefits of daily physical activity and energy expenditure in the prevention of obesity, systemic inflammation, and hepatic steatosis are striking in this animal model. Transitioning from 16 wk of physical activity to 4 wk of physical inactivity resulted in modest body weight gain and in several peripheral and hepatic changes associated with NAFLD development and progression, with many of the activity-induced changes in systemic inflammation and cytokine levels being lost. There was a significant increase in hepatic TAG accumulation seen after 4 wk of inactivity, which occurred in conjunction with a loss of activity-induced increases in hepatic mitochondrial function and fatty acid oxidation. Importantly, however, the increased hepatic TAGs still remained largely lower compared with chronically sedentary animals, a finding linked to a sustained reduction in proteins related to fatty acid uptake and hepatic de novo lipogenesis.
Perspectives and Significance
The consequences of being physically inactive in an environment of nutrient excess are likely promoting the development and progression of NAFLD. With a relatively short-term transition (4 wk) to a physically inactive state, NAFLD developed and markers of hepatic mitochondrial function were lost in the hyperphagic OLETF rat. However, some hepatic protection was maintained in previously active animals, which was related to a sustained reduction in markers of hepatic fatty acid uptake and lipogenesis. Future studies are needed to better understand the mechanisms by which prior physical activity protects the liver, how long the beneficial effects of prior exercise on hepatic health persist, and whether these mechanisms are different when fatty liver disease is prevented by other lifestyle therapies.
GRANTS
This work was partially supported by National Institutes of Health (NIH) Grants HL-36088 (to M. H. Laughlin), R01 DK-088940 (to J. P. Thyfault), T32 AR-048523 (to N. T. Jenkins), and F32 DK-83182 (to R. S. Rector), and by VA Grant VHA-CDA2 IK2BX001299-01 (to R. S. Rector).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.A.L., G.M.E.M., M.L.R., N.T.J., and R.S.R. performed experiments; M.A.L., G.M.E.M., M.L.R., N.T.J., F.W.B., M.H.L., J.A.I., J.P.T., and R.S.R. analyzed data; M.A.L., G.M.E.M., M.L.R., N.T.J., F.W.B., M.H.L., J.A.I., J.P.T., and R.S.R. interpreted results of experiments; M.A.L. and R.S.R. prepared figures; M.A.L. and R.S.R. drafted manuscript; M.A.L., G.M.E.M., M.L.R., N.T.J., F.W.B., M.H.L., J.A.I., J.P.T., and R.S.R. edited and revised manuscript; M.A.L., G.M.E.M., M.L.R., N.T.J., F.W.B., M.H.L., J.A.I., J.P.T., and R.S.R. approved final version of manuscript; F.W.B., M.H.L., J.A.I., J.P.T., and R.S.R. conception and design of research.
ACKNOWLEDGMENTS
The OLETF and LETO rats were a generous gift of the Tokushima Research Institute, Otsuka Pharmaceutical (Tokushima, Japan). The authors would like to thank Suzie Ridenhour, Craig Meers, Mahir Khan, and Ben Pape for excellent technical assistance to this work and Whitney Collins for help with animal husbandry. This work was supported with resources and the use of facilities at the Harry S. Truman Memorial Veterans Hospital in Columbia, MO.
REFERENCES
- 1. Al-Khalili L, Bouzakri K, Glund S, Lonnqvist F, Koistinen HA, Krook A. Signaling specificity of interleukin-6 action on glucose and lipid metabolism in skeletal muscle. Mol Endocrinol 20: 3364–3375, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Bass A, Brdiczka D, Eyer P, Hofer S, Pette D. Metabolic differentiation of distinct muscle types at the level of enzymatic organization. Eur J Biochem 10: 198–206, 1969 [DOI] [PubMed] [Google Scholar]
- 3. Bellentani S, Saccoccio G, Masutti F, Croce LS, Brandi G, Sasso F, Cristanini G, Tiribelli C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med 132: 112–117, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Bi S, Scott KA, Hyun J, Ladenheim EE, Moran TH. Running wheel activity prevents hyperphagia and obesity in Otsuka Long-Evans Tokushima Fatty rats: role of hypothalamic signaling. Endocrinology 146: 1676–1685, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Booth FW, Laye MJ, Lees SJ, Rector RS, Thyfault JP. Reduced physical activity and risk of chronic disease: the biology behind the consequences. Eur J Appl Physiol 102: 381–390, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Comprehens Physiol 1143–1211, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest 114: 147–152, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 40: 1387–1395, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology 42: 987–1000, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G, Prelovsek O, Hohnen-Behrens C, Watt MJ, James DE, Kemp BE, Pedersen BK, Febbraio MA. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 55: 2688–2697, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Chakravarthy MV, Booth FW. Eating, exercise, and “thrifty” genotypes: connecting the dots toward an evolutionary understanding of modern chronic diseases. J Appl Physiol 96: 3–10, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology 114: 842–845, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Dobrzyn P, Dobrzyn A, Miyazaki M, Cohen P, Asilmaz E, Hardie DG, Friedman JM, Ntambi JM. Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver. Proc Natl Acad Sci USA 101: 6409–6414, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 115: 1343–1351, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eder K, Baffy N, Falus A, Fulop AK. The major inflammatory mediator interleukin-6 and obesity. Inflamm Res 58: 727–736, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 43: S99–S112, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Fischer CP, Berntsen A, Perstrup LB, Eskildsen P, Pedersen BK. Plasma levels of interleukin-6 and C-reactive protein are associated with physical inactivity independent of obesity. Scan J Med Sci Sports 17: 580–587, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 307: 491–497, 2012 [DOI] [PubMed] [Google Scholar]
- 19. Gauthier MS, Couturier K, Latour JG, Lavoie JM. Concurrent exercise prevents high-fat-diet-induced macrovesicular hepatic steatosis. J Appl Physiol 94: 2127–2134, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Holmes AG, Mesa JL, Neill BA, Chung J, Carey AL, Steinberg GR, Kemp BE, Southgate RJ, Lancaster GI, Bruce CR, Watt MJ, Febbraio MA. Prolonged interleukin-6 administration enhances glucose tolerance and increases skeletal muscle PPARα and UCP2 expression in rats. J Endocrinol 198: 367–374, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Hsieh SD, Yoshinaga H, Muto T, Sakurai Y. Regular physical activity and coronary risk factors in Japanese men. Circulation 97: 661–665, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Hulver MW, Berggren JR, Carper MJ, Miyazaki M, Ntambi JM, Hoffman EP, Thyfault JP, Stevens R, Dohm GL, Houmard JA, Muoio DM. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab 2: 251–261, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krogh-Madsen R, Thyfault JP, Broholm C, Mortensen OH, Olsen RH, Mounier R, Plomgaard P, van Hall G, Booth FW, Pedersen BK. A 2-wk reduction of ambulatory activity attenuates peripheral insulin sensitivity. J Appl Physiol 108: 1034–1040, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Kump DS, Booth FW. Alterations in insulin receptor signalling in the rat epitrochlearis muscle upon cessation of voluntary exercise. J Physiol 562: 829–838, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kump DS, Booth FW. Sustained rise in triacylglycerol synthesis and increased epididymal fat mass when rats cease voluntary wheel running. J Physiol 565: 911–925, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laye MJ, Rector RS, Warner SO, Naples SP, Perretta AL, Uptergrove GM, Laughlin MH, Thyfault JP, Booth FW, Ibdah JA. Changes in visceral adipose tissue mitochondrial content with type 2 diabetes and daily voluntary wheel running in OLETF rats. J Physiol 587: 3729–3739, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Laye MJ, Thyfault JP, Stump CS, Booth FW. Inactivity induces increases in abdominal fat. J Appl Physiol 102: 1341–1347, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Li Z, Yang S, Lin H, Huang J, Watkins PA, Moser AB, Desimone C, Song XY, Diehl AM. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology 37: 343–350, 2003 [DOI] [PubMed] [Google Scholar]
- 29. McGinnis JM, Foege WH. Actual causes of death in the United States. JAMA 270: 2207–2212, 1993 [PubMed] [Google Scholar]
- 30. Mikus CR, Oberlin DJ, Libla JL, Taylor AM, Booth FW, Thyfault JP. Lowering physical activity impairs glycemic control in healthy volunteers. Med Sci Sports Exerc 44: 225–231, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miquilena-Colina ME, Lima-Cabello E, Sanchez-Campos S, Garcia-Mediavilla MV, Fernandez-Bermejo M, Lozano-Rodriguez T, Vargas-Castrillon J, Buque X, Ochoa B, Aspichueta P, Gonzalez-Gallego J, Garcia-Monzon C. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia, and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut 60: 1394–1402, 2011 [DOI] [PubMed] [Google Scholar]
- 32. Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA 291: 1238–1245, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Moran TH, Bi S. Hyperphagia and obesity in OLETF rats lacking CCK-1 receptors. Philos Trans R Soc London Ser B, Biol Sci 361: 1211–1218, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moran TH, Bi S. Hyperphagia and obesity of OLETF rats lacking CCK1 receptors: developmental aspects. Dev Psychobiol 48: 360–367, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Morris RT, Laye MJ, Lees SJ, Rector RS, Thyfault JP, Booth FW. Exercise-induced attenuation of obesity, hyperinsulinemia, and skeletal muscle lipid peroxidation in the OLETF rat. J Appl Physiol 104: 708–715, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Olsen RH, Krogh-Madsen R, Thomsen C, Booth FW, Pedersen BK. Metabolic responses to reduced daily steps in healthy nonexercising men. JAMA 299: 1261–1263, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Pedersen BK. The diseasome of physical inactivity—and the role of myokines in muscle—fat cross talk. J Physiol 587: 5559–5568, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 88: 1379–1406, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 8: 457–465, 2012 [DOI] [PubMed] [Google Scholar]
- 40. Perseghin G, Lattuada G, De Cobelli F, Ragogna F, Ntali G, Esposito A, Belloni E, Canu T, Terruzzi I, Scifo P, Del Maschio A, Luzi L. Habitual physical activity is associated with intrahepatic fat content in humans. Diabetes Care 30: 683–688, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Rector RS, Thyfault JP. Does physical inactivity cause nonalcoholic fatty liver disease? J Appl Physiol 111: 1828–1835, 2011 [DOI] [PubMed] [Google Scholar]
- 42. Rector RS, Thyfault JP, Laye MJ, Morris RT, Borengasser SJ, Uptergrove GM, Chakravarthy MV, Booth FW, Ibdah JA. Cessation of daily exercise dramatically alters precursors of hepatic steatosis in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. J Physiol 586: 4241–4249, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol 294: G619–G626, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, Mikus CR, Laye MJ, Laughlin MH, Booth FW, Ibdah JA. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol 52: 727–736, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rector RS, Uptergrove GM, Borengasser SJ, Mikus CR, Morris EM, Naples SP, Laye MJ, Laughlin MH, Booth FW, Ibdah JA, Thyfault JP. Changes in skeletal muscle mitochondria in response to the development of type 2 diabetes or prevention by daily wheel running in hyperphagic OLETF rats. Am J Physiol Endocrinol Metab 298: E1179–E1187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rector RS, Uptergrove GM, Morris EM, Borengasser SJ, Laughlin MH, Booth FW, Thyfault JP, Ibdah JA. Daily exercise vs. caloric restriction for prevention of nonalcoholic fatty liver disease in the OLETF rat model. Am J Physiol Gastrointest Liver Physiol 300: G874–G883, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Roberts MD, Company JM, Brown JD, Toedebusch RG, Padilla J, Jenkins NT, Laughlin MH, Booth FW. Potential clinical translation of juvenile rodent inactivity models to study the onset of childhood obesity. Am J Physiol Regul Integr Comp Physiol 303: R247–R258, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Roytblat L, Rachinsky M, Fisher A, Greemberg L, Shapira Y, Douvdevani A, Gelman S. Raised interleukin-6 levels in obese patients. Obes Res 8: 673–675, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Song A, Nikolcheva T, Krensky AM. Transcriptional regulation of RANTES expression in T lymphocytes. Immunol Rev 177: 236–245, 2000 [DOI] [PubMed] [Google Scholar]
- 50. Srere PA. Citrate synthase. Methods Enzymol 13: 3–5, 1969 [Google Scholar]
- 51. Stephens BR, Granados K, Zderic TW, Hamilton MT, Braun B. Effects of 1 day of inactivity on insulin action in healthy men and women: interaction with energy intake. Metabolism 60: 941–949, 2011 [DOI] [PubMed] [Google Scholar]
- 52. Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 40: 181–188, 2008 [DOI] [PubMed] [Google Scholar]
- 53. Wei Y, Rector RS, Thyfault JP, Ibdah JA. Nonalcoholic fatty liver disease and mitochondrial dysfunction. World J Gastroenterol 14: 193–199, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yasari S, Paquette A, Charbonneau A, Gauthier MS, Savard R, Lavoie JM. Effects of ingesting a high-fat diet upon exercise-training cessation on fat accretion in the liver and adipose tissue of rats. Appl Physiol Nutr Metab 31: 367–375, 2006 [DOI] [PubMed] [Google Scholar]