Abstract
The present study investigated transient receptor potential vanilloid type 4 (TRPV4) ion channels in pancreatic stellate cells (PSCs) isolated from rats with high-fat and alcohol diet (HFA)-induced chronic pancreatitis. TRPV4 is a calcium-permeable nonselective ion channel responsive to osmotic changes, alcohol metabolites arachidonic acid, anandamide, their derivatives, and injury-related lipid mediators. Male Lewis rats were fed HFA for 6–8 wk before isolation and primary culture of PSCs. Control PSCs were harvested from rats fed standard chow. Immunoreactivity for cytoskeletal protein activation product α-smooth muscle actin (α-SMA) and platelet-derived growth factor receptor-β subunit (PDGFR-β) characterized the cells as PSCs. TRPV4 expression increased in PSCs of HFA-fed rats and control cultures after alcohol treatment (50 mM). Cell responses to activation of inducible TRPV4 were assessed with live cell calcium imaging. Threefold increased and sustained intracellular calcium mobilization responses occurred in 70% of pancreatic stellate cells from HFA-fed rats in response to TRPV4 activators arachidonic acid, lipid second messenger, phorbol ester 4 α-phorbol 12,13-didecanoate (4αPDD), and 50% hypoosmotic media compared with relatively unresponsive PSCs from control rats. Activation responses were attenuated by nonselective TRPV channel blocker ruthenium red. Tumor necrosis factor-α (TNF-α, 1 ng/ml, 16 h) increased responses to 4αPDD in control PSCs. These findings implicate TRPV4-mediated calcium responses inducible after HFA exposure and inflammation in reactive responses of activated PSCs that impair pancreatic function, such as responsiveness to cytokines and the deposition of collagen fibrosis that precipitates ductal blockage and pain.
Keywords: calcium imaging, dendritic cells, fibrosis, pain, pancreatitis, pancreatic cancer, transient receptor potential vanilloid ion channel, innate immunity, stress response, TNF-α, hypoosmolarity
pancreatic disease is precipitated by genetic and environmental factors such as alcohol, smoking, and high-fat diet (40–42). The pancreatic inflammation and fibrosis are promoted by activated pancreatic stellate cells (PSCs) in response to alcohol, alcohol oxidative metabolites such as acetyldehyde, and other injury-related lipid mediators in the local microenvironment. Activated PSCs are innate immunity cells that enlarge, change shape, and initiate inflammatory processes. One of the key features of chronic pancreatitis is stellate cell deposition of collagen fibrosis in the gland that eventually leads to constrictive interference of secretory function (3). These spindle-shaped, α-smooth muscle actin (α-SMA)-positive myofibroblast-like cells were identified around the acini, pancreatic ducts, and vessels of the human pancreas by Satome and colleagues (28). A method of isolating and culturing these cells from rat pancreas was established by Apte et al. (4) and Bachem et al. (10) who gave them the name pancreatic stellate cells. Activated PSCs are similar in function and morphology to hepatic stellate cells and are principal effectors of progressive inflammation and fibrosis (6, 20, 32). These dendritic cells residing in both organs are derived from myeloid precursor cells in parallel with macrophages.
Evidence from both clinical and experimental studies indicate a role for PSCs in ethanol and high fat-induced pancreatic inflammation (3, 7–9, 19, 44). Activated PSCs have been identified in vivo in tissue from patients with chronic alcoholic pancreatitis and from animals with experimental chronic pancreatitis (21, 36). They are located within tissue areas with the collagen fibrosis they produce (2). Comparisons indicate that injury-related microenvironment conditions can be mimicked in vitro by exposure of PSCs to ethanol, pro-inflammatory cytokines, fatty acids, and acetaldehyde. In vitro studies have established that PSCs are activated directly by ethanol and its oxidative metabolites, acetaldehyde, epoxyalcohols (HEETs), and epoxides (EETs). Also of particular interest is the observation that rat PSCs show alcohol dehydrogenase activity, indicating that, apart from hepatic and pancreatic acinar cells, ethanol can also be metabolized by the periacinar stellate cells in the pancreas. Persistently activated PSCs migrate to sites of tissue damage; undergo regulated contraction, proliferate, phagocytosize; and generate products that modulate the extracellular matrix either by facilitating repair or promoting fibrosis (25, 26, 30). For example, collagen is colocalized in human PSCs with lipid peroxidation-derived aldehydes in chronic pancreatitis (15). PSC activation is characterized by increased α-SMA and/or desmin expression and increased production of extracellular matrix proteins such as collagens I and III, fibronectin, and laminin, which contribute to the pancreatic fibrosis that precipitates ductal blockage, pain, and impaired function (5, 37, 44).
Lipid peroxidation-derived aldehydes are activators of the ion channel transient receptor potential (TRP) family member TRPV4 (transient receptor potential vanilloid type 4) (33). TRPV4 is the mammalian homologue of the Osm-9 Caenorhabditis elegans gene critical in osmotic and mechanical avoidance. The mammalian TRPV4 channel along with other TRP channels is emerging as a front line candidate for molecular detection and integration of chemical, thermal, osmotic, and mechanical stimuli particularly in sensory neurons (1, 12, 16, 18, 27). In the present study, the role of TRPV4 channels in the development of chronic pancreatitis was investigated in PSCs isolated from rats fed a liquid high-fat and alcohol (HFA) diet for 6–8 wk. Activation of TRPV4 in response to specific agonists (arachidonic acid, 4αPDD) and 50% hypoosmotic media was assessed by live cell calcium microfluorimetry, and reduction by a nonselective TRPV channel blocker was tested. TRPV4 protein expression increase was determined with Western blot and immunochemical analyses.
METHODS
Animals and Diet
All procedures were consistent with the guidelines of the policies for Ethical Treatment of Research Animals published by the International Association for the Study of Pain and approved by the Animal Care and Use Committee at our institution. Male Lewis rats weighing between 200 and 250 g (Harlan Sprague-Dawley) were used for this study. Animals were kept in a temperature-constant (23° ± 2°C) room on a 12:12 h dark-light reversed cycle. The liquid HFA diet (LD 101A Micro-stabilized alcohol rodent liquid diet mix, with LD 104 maltose, Test-Diet, Richmond, IN) was prepared fresh each day and consisted of 20% fat from corn oil, safflower oil, and lard (39). The 30.3% protein, 5% fiber, vitamins, and minerals were added as a dry powder to water, apple juice (10%), and alcohol (wt/vol, 95% ethyl alcohol). The dose of alcohol was progressively increased from 4% to 6% as follows: 4% alcohol for the first week, 5% for second week, and 6% for the third through the eighth week. Each rat consumed between 50 and 70 g of liquid diet with alcohol per day. Body weight was monitored weekly. Animals were observed closely daily, and no evidence of alcohol intoxication (no ataxia or lethargy) was noted.
Pancreatic Stellate Cell Isolation and Culture
PSCs were isolated with a density gradient centrifugation (Nycodenz gradient) method adopted from Apte et al. (4). For each experiment, two 200- to 300-g rats were euthanized, and pancreatic tissue was taken, minced with surgical scissors, and digested with Gey's balanced salt solution (GBSS) containing 0.02% pronase, 0.05% collagenase P, and 0.1% DNAse at 37°C for 40 min. Digested tissue was pipetted vigorously and then filtered through a nylon mesh with 150-μm openings. After centrifugation, the supernatant was discarded, and the cells were resuspended in 9.5 ml GBSS containing 0.3% BSA. The cell suspension was mixed with 8 ml of 28.7% (wt/vol) of Nycodenz in GBSS without salt (NaCl). The gradient was prepared by layering the Nycodenz cell suspension beneath 6 ml GBSS with BSA in a 50-ml centrifuge tube. The gradient mixture was centrifuged for 20 min at 1,400 g. The PSCs separated into a band at the interface of the Nycodenz cushion and the GBSS with BSA. The band was harvested, and the cells were washed and resuspended in Iscove's modified Dulbecco's medium containing 10% fetal calf serum, 4 mM glutamine, and antibiotics (penicillin 100 U/ml; streptomycin 100 μg/ml). Cells were seeded in a density of 50,000 cells per well in plastic six-well culture plates in Dulbecco's medium with fetal calf serum, glutamine, and antibiotics as detailed above. Cells were maintained at 37°C in a humidified atmosphere of 5% CO2 air.
RT-PCR
RT-PCR was performed in primary cultured PSCs to confirm TRPV4 gene expression. PSCs were grown to confluence in a six-well cluster with culture medium. Total RNA was extracted from PSCs through TRIzol (Invitrogen) suspension, chloroform separation, and 2-proponol precipitation. Total RNA was treated with TURBO DNA-free (Invitrogen) to eliminate DNA contamination and RNA concentration was measured. Reverse transcription was performed as the following: 5 × first-strand buffer (2 μl), 10 mM dNTP (0.5 μl), 50 μM random hexamer (1 μl), diethyl pyrocarbonate H2O, 0.mM dithiothreitol (DTT; 1 μl), 10 U/μl RNase inhibitor (1 μl), and 1 μl SuperScript II reverse transcriptase (Invitrogen). Samples were incubated at 42°C for 50 min and inactivated at 70°C for 15 min. PCR was performed for 35 cycles using the following temperature protocol: 94°C (30 s), 56°C (60 s), and 72°C (30 s), with an initial step at 94°C (2 min) to activate the platinum Taq polymerase (Invitrogen) followed by 5 min extension at 72°C by PTC-100 programmable thermal controller (MJ Research, Waltham, MA). TRPV4 gene-specific primer is as follows: ATCAACTCGCCCTTCAGAGA (forward) and GGTGTTCTCTCGGGTGTTGT (reverse) (23). The predicted size of amplicon is 339 bp for TRPV4. Reaction products were separated on 1% agarose gel in Tris-acetate EDTA buffer, stained with 0.5% ethidium bromide, and analyzed with Fotodyne software (Fotodyne, Hartland, WI).
Ratiometric Live Cell Imaging of Calcium Mobilization in Pancreatic Stellate Cells
PSCs were plated at the density of 4 × 104/cm2 on a microscope coverslip (Ø1.2 cm) and kept in normal cell culture media for 48 h to form a single layer. Cells were loaded with a dual excitation Ca2+ indicator dye acetoxy-methyl-ester fura-2 (5 μM, fura-2AM, Molecular Probes), which diffused across the cell membrane in Krebs solution for 1 h at room temperature in the dark, before imaging was performed. Cells were then washed three times with Krebs solution. After a wash to remove extracellular fura-2, the indicator was allowed to deesterify for 30 min. During this process, the charged Ca2+ indicator is trapped inside the cell body, and any changes in the 340-to-380 emission ratio is an indication of intracellular Ca2+ mobilization. The coverslip with PSCs was mounted in a recording chamber containing 1 ml Krebs solution attached to the stage of an inverted microscope at room temperature. Fluorescence studies were performed on populations of cells with 10–20 cells in each microscope field of each plate using a Nikon TE-2000 (Nikon Instruments, Melville, NY) inverted epifluorescence microscope equipped with a ×20 0.9 quartz objective and a cool-snap HQ digital camera connected to a computer using Nikon Element program software. Fura-2 AM-loaded PSCs were excited alternately at wavelengths of 340 nm and 380 nm with a Sutter Lambda LS high-speed shutter system. The ratio of emitted light (340/380) detected at 510 nm was used as an indirect ratiometric measurement of intracellular Ca2+ activity. The composition of Krebs solution was as follows (in mM): 130 NaCl, 5.5 KCl, 2.5 CaCl2, 1 MgCl2, 20 HEPES, and 10 glucose, pH 7.2–7.4.
All experiments were done at room temperature (22–23°C). Microscope fields with 10–20 cells each were globally selected to measure the changes of intracellular calcium activity. Cells were stimulated with arachidonic acid (AA), a lipid messenger and 4αPDD, a potent TRPV4 agonist, or hypotonic bath solution. AA and 4αPDD were diluted with Krebs solution and applied by bath perfusion immediately after baseline recording. Ruthenium red (RR), a TRPV channel blocker, was applied in the bath 10 min before the agonist addition. Each experimental condition was repeated three times in different cell preparations.
Immunofluorescent Study in Primary Cultured PSCs
The cells, attached to the coverslip, were fixed with 4% paraformaldehyde for 20 min, washed in 0.1 M PBS (pH 7.3), permeabilized with 0.1% Triton X100, and incubated with primary antibody overnight at room temperature. Primary antibodies utilized included rabbit anti-platelet-derived growth factor receptor-β (PDGFR-β) (1:500, Santa Cruz), rabbit anti-TRPV4 (1:500, Abcam, Cambridge, MA), and mouse anti-α smooth muscle actin (1:200, Sigma). After washing was completed, cells were incubated with a FITC- or Texas red-labeled secondary antibody (1:1,000 dilutions of goat anti-mouse IgG or goat anti-rabbit IgG, Invitrogen). Fluorescent signals were detected with a Nikon Eclipse E2000 microscope. Samples were excited at 488 nm or 594 nm, and emitted fluorescence was recorded at 525 nm. The excitation levels were held constant between all samples. Quantification of PDGFR-β or TRPV4 expression was performed by Metamorph Imaging system offline on digital images collected under constant conditions between samples. The mean intensity of fluorescence was measured after subtraction of background fluorescence. Background level was set in regions from control cell samples and utilized throughout the session in which all fields were photographed during the same session using the same microscope settings. Cells were counted from four randomly selected fields on each slide from each treatment in three different preparations. A minimum of 20 cells appeared in the fields under study using the ×20 objective lens. Data are expressed as relative fluorescence intensity values.
In Situ TRPV4 Immunohistochemical Study in Pancreatic Tissue
Paraffin-embedded pancreas tissue sections (5 μm) from control and HFA-fed rats were batch processed for immunostaining of TRPV4 and colabeling with α-SMA.The sections were deparaffinized and rehydrated in Citrisolv and concentration series of EtOH. The sections were washed with 0.1 M phosphate-buffered saline (PBS, pH 7.3) and blocked with 3% normal goat serum (30 min, RT). Sections were incubated overnight at room temperature with rabbit anti-TRPV4 antibody (1:1,000, Abcam), mouse anti-α-SMA (1:1,000, Sigma), or mouse anti-desmin antibody (1:500, Sigma). Subsequently, sections were incubated with secondary antibodies, goat anti-rabbit IgG Alexa Fluor 594, and goat anti-mouse IgG Alexa Fluor 488 (1:1,000, 1 h, Invitrogen). Sections were coverslipped with glycerol-based mounting media (Vector Laboratories, Burlingame, CA). Sections incubated without primary antibody were included in each staining experiment as negative controls (data not shown). Histological stain of fibrotic collagen was achieved in defatted paraffin sections (5 μm) with Sirius red (0.1%, 60 min, EMS Hatfield, PA) and hematoxylin and eosin (21).
Staining was visualized and intensity analyzed using a Nikon E1000 microscope equipped with the MetaVue and ACT-1 programs. Five randomly selected sections from each animal were photographed for study using the ×20 objective lens. Background level was set in a region without staining in a pancreas section from the control group, the excitation levels were held constant between all samples, and the same microscope settings were maintained throughout the data collection session in which all fields and all groups were photographed. Data are expressed as relative fluorescence intensity values.
Western Blot Analysis of TRPV4 Expression in Pancreatic Stellate Cells
PSCs isolated from HFA-fed and control Lewis rats were cultured for 48 h, washed with PBS (4°C), and lysed into RIPA buffer containing protease inhibitor and phosphatase inhibitor cocktail (Sigma). Equal amounts of protein (15 μg) were size fractionated by 10% (wt/vol) sodium dodecyl sulfate-polyacryl-amide gel electrophoresis and transferred onto a PVDF membrane (Bio-Rad, Hercules, CA). After being blocked with 5% (wt/vol) nonfat milk in washing buffer (0.1% Tween 20 in 0.1 M PBS, pH 7.4) at 4°C for overnight, the membrane was incubated with rabbit-anti-TRPV4 primary antibody (1:500 Abcam) and mouse-anti-β-actin antibody from Santa Cruz (1:10,000, Santa Cruz, CA), diluted in 1% (wt/vol) nonfat milk in washing buffer at room temperature for 2 h. The blots were then washed three times for 10 min each in washing buffer and incubated with horseradish peroxidase conjugated IgG, (1:10,000, GE Healthcare, Piscataway, NJ) diluted in 1% (wt/vol) nonfat milk in washing buffer at room temperature for 1 h. The membrane was washed with washing buffer three times for 10 min each and incubated with Chemiluminescence reagents (ECL plus kit, GE Healthcare, Piscataway, NJ) for 5 min. The blots were exposed on CL XPosure film (Thermo Scientific, Rockford, IL), and the intensity of specific immunoreactive bands was quantified using densitometric scanning analysis and density detection software (Image J, NIH). The expression of β-actin was the internal control. The relative density of the immunoblot bands from the HFA-fed and normal control animals were calculated by normalizing the density of TRPV4 blots over the density of β-actin blots for the same conditions, respectively.
Statistical Analysis
Paired treatment comparisons were analyzed with Student's t-tests. Group data with different test reagent concentrations were analyzed with one-way analysis of variance (ANOVA) followed by Tukey post hoc test. Multiple comparisons were analyzed with two-way ANOVA, followed by Bonferroni post hoc test. P values ≤0.05 were considered statistically significant.
RESULTS
Characterization of PSCs and Their Activation by Alcohol
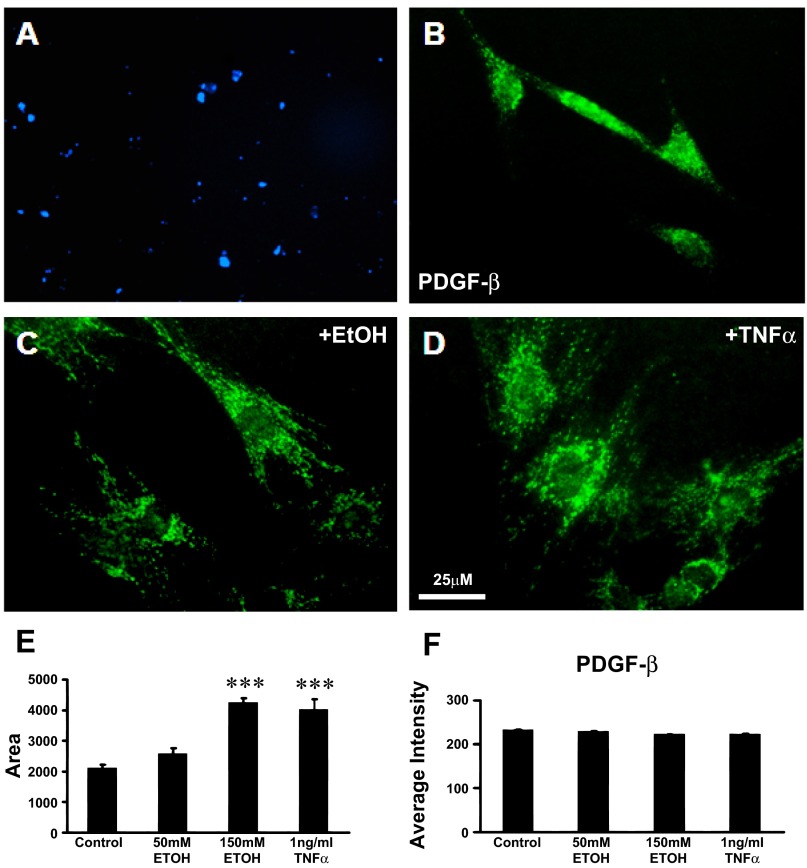
PSCs activation is characterized by the loss of autofluorescent vitamin A droplets and expression of α-SMA and PDGFR-β (Fig. 1) (24, 28).
Fig. 1.
Pancreatic stellate cell (PSC) activation is characterized by the loss of autofluorescent vitamin A droplets and expression of α-smooth muscle actin (α-SMA) and platelet-derived growth factor-β (PDGFR-β). A: vitamin A fat droplets were evident as blue autofluorescence under UV light in the cytoplasm of PSCs after 24 h in culture. This is a characteristic of quiescent stellate cells. B: cytoplasm of PSCs in culture stained positively for the β subunit of the PDGF receptor (PDGF-β). C: after EtOH (150 mM) stimulation for 24 h, PSCs underwent activation with morphological and functional changes typical of myofibroblast-like cells. The cell body of PSCs expanded 2.5-fold, and PDGF-β receptor was positively stained. D: after tumor necrosis factor-α (TNF-α, 1 ng/ml) exposure for 24 h, the cell bodies of PSCs were enlarged. E and F: histograms show measurements of the soma area of PSCs (E) and the relative staining density (F) of PDGF-β receptor when treated with 50 mM (n = 60) or 150 mM EtOH (n = 50) or 1 ng/ml TNF-α (n = 60) for 24 h. The cell body size of PSCs significantly increased with exposure to the high dose of EtOH and to TNF-α. The intensity of the PDGF-β receptor-like immunoreactivity remained constant as the cell body area expanded, suggesting an overall increase in PDGF-β receptor in the PSCs soma as reported by others previously [EtOH vs. control (n = 70); TNF-α vs. control, ***P < 0.001, Student's t-test].
Vitamin A in quiescent PSCs in the culture model.
Examination of the PSCs with a UV microscope filter revealed the presence of punctuate blue autofluorescent vitamin A droplets after 24 h in culture indicating a quiescent state before the in vitro activation studies using alcohol and tumor necrosis factor-α (TNF-α) (Fig. 1A). Vitamin A autofluorescence droplets disappeared after PSC activation with ethanol and TNF-α treatment (data not shown).
PDGF-β receptor expressed in PSCs.
The cytoplasm of the PSCs in culture stained positively for the β subunit of platelet-derived growth factor receptor-β (PDGF-β), a specific marker and transforming factor in pancreatic periacinar cells (Fig. 1B). After PSCs were treated with 150 mM (0.87%) EtOH for 24 h, the cell bodies of the PSCs were enlarged and the cell body areas of PDGF-β receptor expression expanded (Fig. 1C). After PSCs were treated with the cytokine TNF-α (1 ng/ml, 16 h), the cell bodies of PSCs were also enlarged (Fig. 1D). Histograms show the cell body area measurements (Fig. 1E) and relative staining density of PDGF-β receptor (Fig. 1F) for control PSCs (n = 70) and PSCs treated 24 h with 50 mM EtOH (0.29%; n = 60), 150 mM EtOH (0.87%; n = 50), or 1 ng/ml TNF-α (n = 60). The cell body area of the PSC was significantly enlarged in a dose-dependent manner suggesting cellular stress with the higher dose. The relative intensity of PDGF-β receptor-like immunoreactivity remained constant as the cell body area expanded, suggesting that overall the PDGFR-β content was significantly increased within the PSCs.
TRPV4 expression in primary cultured PSCs.
Immunohistochemical study data revealed that PSCs from control rats had a minimum level of TRPV4-like immunoreactivity. In the PSCs treated with 50 mM (0.29%, vol/vol) EtOH for 24 h, the average fluorescent intensity for TRPV4 expression was significantly increased in the cell cytoplasm (n = 108) compared with TRPV4 expression in control PSCs (n = 145) (Fig. 2 A–C, P < 0.001).
Fig. 2.
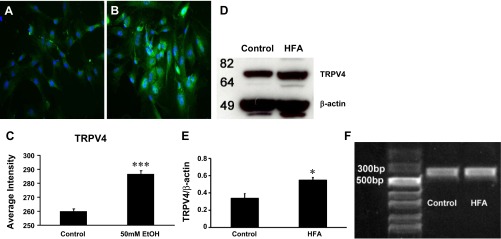
A: PSCs in the control group had minimal transient receptor potential vanilliod type 4 (TRPV4) immunoreactivity (green) (n = 145). 4,6-Diamidino-2-phenylindole (DAPI, blue) nuclear stain is shown. B: TRPV4 expression increased in the cytoplasm (n = 108) of PSCs after exposure to EtOH (50 mM) for 24 h. C: histogram shows the average fluorescent intensity of TRPV4 in alcohol-treated PSCs was significantly increased (***P < 0.001, Student's t-test). D: Western immunoblot analysis was used to compare TRPV4 protein in PSCs pooled from rats chronically fed an alcohol and high-fat diet (HFA, 6%, 5–6 wk; n = 4) with TRPV4 in PSC from control rats fed regular rodent chow (n = 4). TRPV4 expression was increased in the HFA-fed group. E: bar graph summarizes the relative density of the TRPV4 expression in immunoblot bands for both HFA-fed and control groups fed normal chow normalized with β-actin. There was approximately a 20% increase in expression for the PSCs isolated from HFA-fed rats over cells from control rats fed a normal diet. (*P < 0.05). F: RT-PCR was performed with primary cultured PSCs from control and HFA-fed rats. The predicted PCR amplicon sizes for TRPV4 is 339 bp. The PCR marker contains a 50–2000 bp ladder (Invitrogen, Grand Island, NY).
Western blot analysis was also utilized to compare TRPV4 in PSCs taken from HFA pancreatitis rats with PSCs from control rats fed normal chow (Fig. 2D). The bar graph summarizes the relative density of the immunoblot bands for TRPV4 expression normalized against β-actin in both control and HFA-fed groups. The TRPV4 expression in PSCs from HFA-fed rats was increased 20% over levels in PSCs from control rats (0.55 ± 0.03 vs. 0.34 ± 0.05; n = 4 each group; Fig. 2E, P < 0.05).
We evaluated TRPV4 channel gene expression by RT-PCR in primary cultured PSCs isolated from normal control and HFA-fed rats. PSCs from both groups demonstrated a clear PCR product of TRPV4 (339 bp) (n = 4 each group; Fig. 2F).
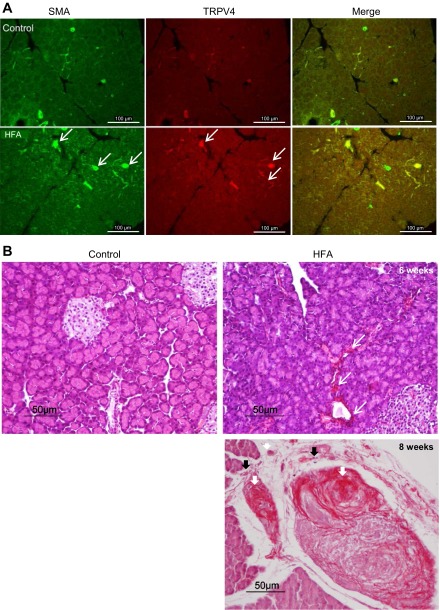
TRPV4 and α-SMA protein expression in pancreas.
We verified activation of PSCs in animals fed HFA by examining immunohistochemical staining of α-SMA (Fig. 3A). α-SMA is normally present in vessel and duct walls, as evidenced by immunostaining in normal pancreatic tissue. Despite variations from sample to sample, there was a marked increase in pancreatic α-SMA in the HFA-fed group with chronic pancreatitis compared with groups fed normal chow. A low level of TRPV4 immunoreactivity appears on the vessel and duct walls in normal rat pancreas. TRPV4 immunoreactivity was increased on the vessel and duct walls, in some unidentified fibers of the interlobular area of the pancreas, as well as overall in pancreas from rats in the HFA-fed group (Fig. 3A).
Fig. 3.
A: expression of α-SMA (white arrows), another indication of PSC activation and fibrinogenesis, was readily evident in pancreas of rats fed with HFA. A low level of TRPV4 immunoreactivity can be seen in blood vessels and ducts in normal pancreatic tissue section. TRPV4 immunoreactivity was increased on the vessel and duct walls as well as in some unidentified fibers of interlobular area of the pancreas of HFA-fed group (white arrows). B: hematoxylin and eosin and Sirius red staining of pancreatic tissue sections. The normal control pancreas sections showed clusters of healthy acinar and islet of Langerhans. In pancreatic tissue taken from rats fed HFA, the presence of glandular atrophy, intralobular, interlobular, and periductal fibrosis (in red, white arrows) and inflammatory cell infiltration (black arrows) can be seen after 6 wk (top right) and is greatly increased by 8 wk (bottom right) on the HFA diet.
HFA induced fibrosis in rat pancreas.
Pancreatic sections from rats fed HFA diet for 6 and 8 wk revealed the presence of glandular atrophy, intralobular, interlobular, and periductal fibrosis, and inflammatory cells infiltrations evidenced by staining with Sirius red and hematoxylin and eosin (Fig. 3B). The presence of pancreatic fibrosis is provided as another indication of the chronic nature of the HFA pancreatitis model and the induced activation of PSCs.
Effects of Alcohol on Functional TRPV4 Ion Channels in PSCs
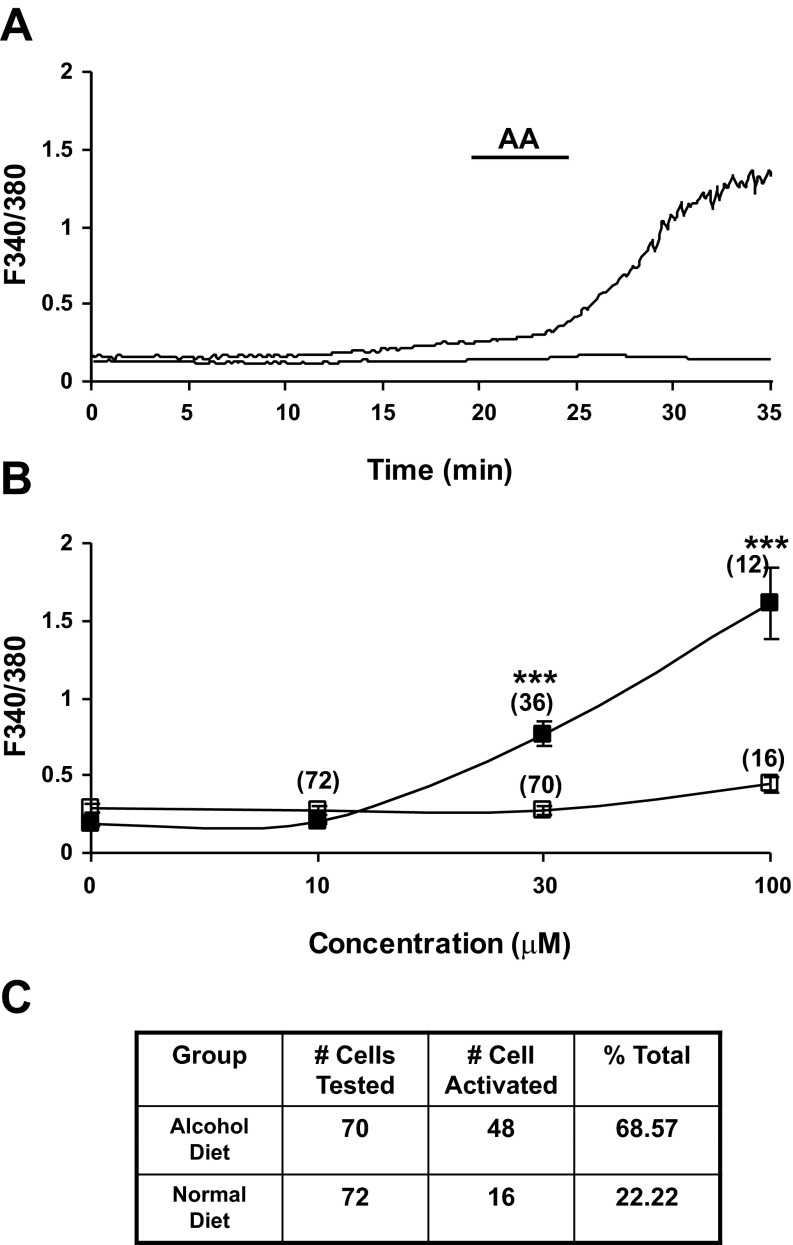
Alcohol increases PSC cytosolic calcium mobilization responses to arachidonic acid.
For in vitro calcium mobilization studies, pancreatic stellate cells were harvested from Lewis rats fed high fat/ethanol (6%) for 5–6 wk, cultured for 2 days, and preloaded with fura-2 (5 μM, 1 h). Intracellular calcium mobilization responses evoked by bath application of AA were classified as two types in distinct populations of PSCs: 1) irregular but sustained calcium responses that lasted for hours (Fig. 4A); or 2) low-level oscillatory calcium mobilization responses composed of brief spike-like activity (data not shown). The PSCs from rats with HFA pancreatitis were highly activated. There was a statistically significant increase in evoked calcium mobilization responses to AA (10–100 μM) for PSCs isolated from HFA-fed rats compared with control PSCs, which were relatively quiescent (Fig. 4, A and C). In these experiments, 48 of 70 randomly selected cells were activated (68.57%, see Table). The majority of the activated cells showed irregular, sustained calcium responses persisting over 1 h (Fig. 4A). The F340-to-F380 ratio increased from a baseline of 0.2–0.3 to 1–1.5. Some of the activated cells showed multiple oscillatory spike calcium responses.
Fig. 4.
A: representative trace demonstrates the effect of arachidonic acid (AA, 100 μM) on the Ca2+ fluorescence ratio in PSCs. AA produced a sustained and robust increase in intracellular calcium mobilization responses (F340/F380 ratio) in PSCs harvested from HFA-fed rats (top trace). Cells were isolated from male Lewis rats fed HFA for 6–8 wk and placed in culture for 3 days. Calcium mobilization was seen in 48 of 70 PSCs in response to bath application of AA. In contrast, bath application of AA evoked no response or minimal response in PSCs (16 of 72) prepared from naïve control Lewis rats (bottom trace). B: change in peak fluorescence response ratio after AA was summarized as a concentration-response relationship. The strong activation response of PSCs taken from pancreas of HFA-fed rats increased in a dose-dependent manner (■, top line), while a minimal response was recorded from PSCs isolated from pancreas of control rats (□, bottom line). (***P < 0.001, two-way ANOVA, Bonferroni post hoc test). C: table shows the total number of PSCs tested and percentage of total cells activated by AA in both HFA fed and control rats.
In contrast, PSCs taken from rats fed normal chow had minimal or no calcium activity in response to bath application of AA (from 10–100 μM; Fig. 4, B and C). In these experiments, 72 cells from control rats were randomly selected and only 16 cells appeared to be slightly activated (22.2%, see table in Fig. 4C).
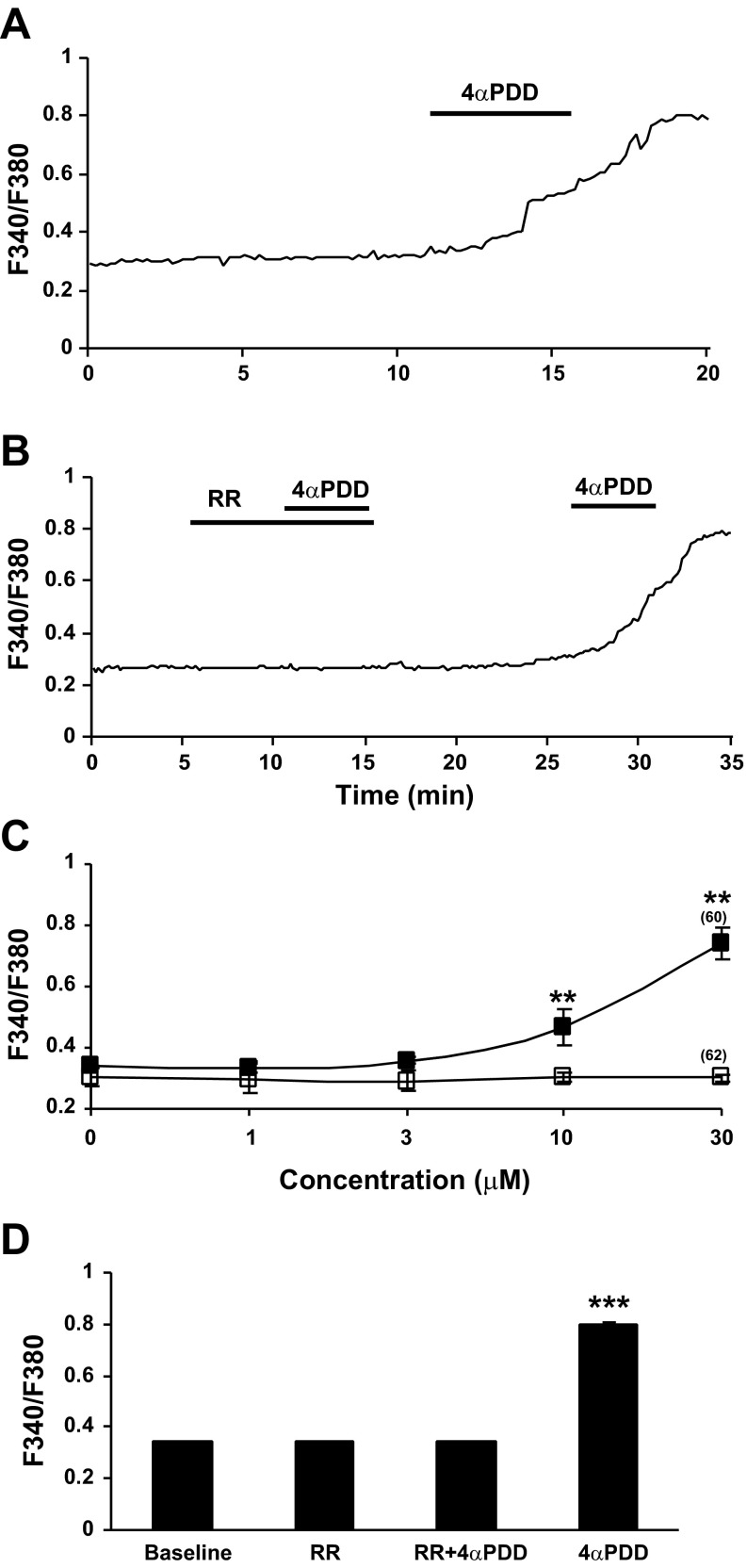
Calcium mobilization in PSCs induced by TRPV4 channel activation.
To determine whether intracellular calcium mobilization responses were TRPV4 mediated, 4αPDD was bath applied to PSCs isolated from rats fed HFA. Bath application of 4αPDD (30 μM) triggered an increase in intracellular calcium mobilization in stellate cells from HFA-fed rats (Fig. 5A). In these experiments, 99 cells were tested and 60 of the cells were activated (60.6%). A sustained increase in calcium mobilization is seen in most PSCs in response to 4αPDD in a dose-dependent manner (Fig. 5C). The F340-to-F380 ratio increased from a baseline average of 0.34 ± 0.006 to 0.74 ± 0.05. The activity was effectively blocked at baseline level by bath application of RR (3 μM 10 min) (Fig. 5, B and D, P < 0.001, ANOVA followed by Tukey post hoc test).
Fig. 5.
The phorbol ester 4 α-phorbol 12,13-didecanoate (4αPDD) evoked intracellular calcium response was significantly elevated in PSCs isolated from rats with chronic exposure to a diet with HFA. A: sustained increase in calcium mobilization was observed in all pancreatic stellate cells in response to bath application of 4αPDD (30 μM). Cells were isolated from male Lewis rats fed HFA for 6–8 wk and cultured for 3 days. B: ruthenium red (RR, 3 μM, 10 min), a TRPV channel blocker, eliminated the F340/380 ratio elevation in response to 4αPDD (30 μM, 5 min). The response to 4αPDD was restored after a washout period. C: change in peak fluorescence ratio in response to 4αPDD was evident as a concentration-dependent relationship (n = 60, ■, top line), whereas PSCs isolated from control rats fed a normal diet had a minimum response (n = 62, □, bottom line). (**P < 0.01, two-way ANOVA, Bonferroni post hoc test). D: histogram summarizes the 4αPDD activation and RR blockade in PSCs. The significantly increased response to 4αPDD was absent in PSCs when RR was present in the bath (n = 19, baseline, RR and RR+4αPDD vs. 4αPDD, ***P < 0.001, one-way ANOVA, Tukey's multiple comparison test).
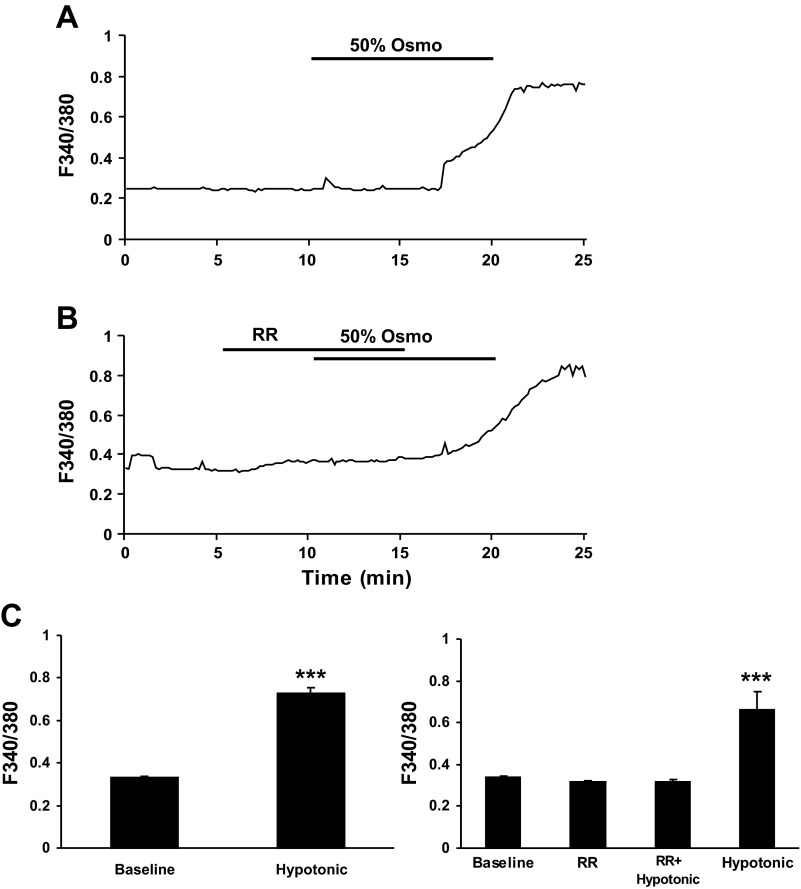
TRPV4 is a functional osmosensor in PSCs.
In light of the crucial role of TRPV4 in cell volume regulation in peripheral tissue osmosensation, we investigated the possibility that TRPV4 in PSCs are responsive to cell volume regulation. In primary cultured PSCs taken from HFA-fed rats, responses to hypotonic saline challenge (≈50% osmolarity or 150 mosmol) observed via live cell calcium imaging produced sustained calcium mobilization responses that were abrogated by RR (3 μM, 10 min) (Fig. 6, A and C, right, P < 0.001, ANOVA followed by Tukey post hoc test). In these experiments, 71 (53%) of the 134 cells tested were activated. The F340-to-F380 ratio increased from an average baseline of 0.33 ± 0.01 to 0.73 ± 0.02 (Fig. 6C, left, P < 0.001, paired t-test).
Fig. 6.
Hypotonic experimental solution challenge induced a sustained calcium mobilization (F340/F380 ratio) in primary cultures of PSCs taken from rats fed HFA diet. A: representative trace is shown illustrating the effect of hypotonic experimental solution (50% osmolarity, ≈150 mosmol) on the calcium response in PSCs. B: hypotonic experimental solution-induced calcium mobilization was abrogated by nonspecific TRPV channel blocker RR (3 μM, 10 min). C: histogram summarizes the statistically significant increase in F340/F380 ratio after hypotonic solution challenge (left; n = 71, hypotonic vs. baseline, ***P < 0.001, paired t-test). Pretreatment with RR effectively blocked the increased F340/F380 ratio (right; n = 25, baseline, RR, and RR+hypotonic vs. hypotonic, ***P < 0.001, one-way ANOVA, Turkey's multiple comparison test).
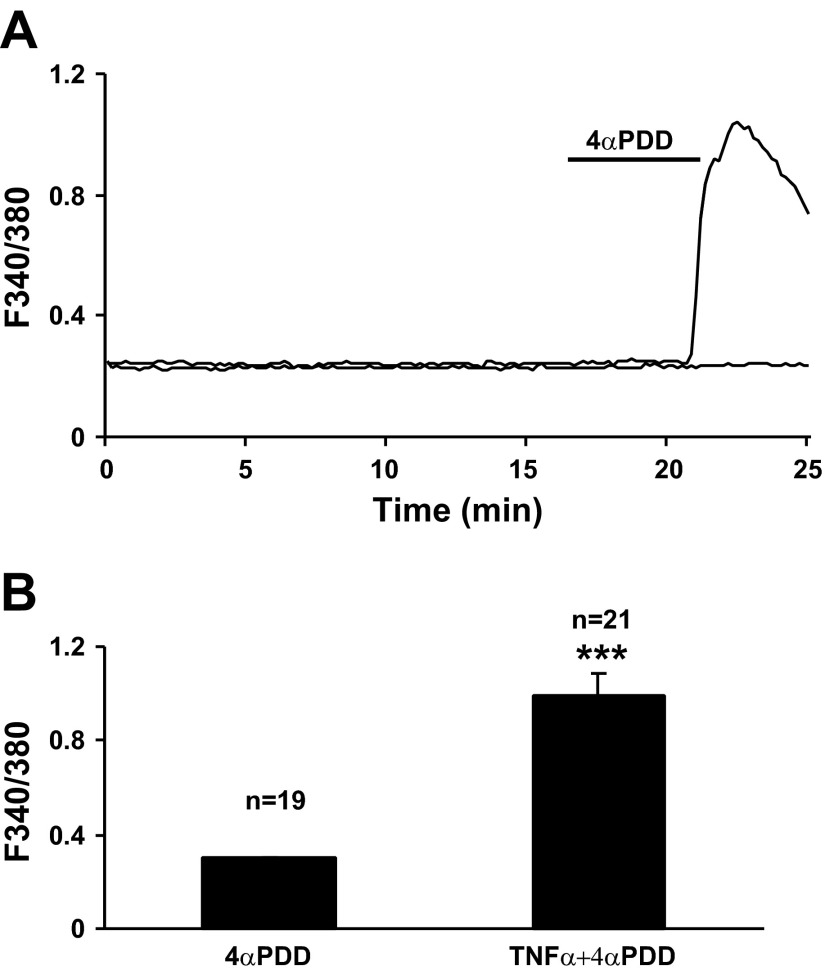
TNF-α enhances TRPV4-mediated calcium mobilization.
PSCs isolated from naïve Lewis rat were pretreated with TNF-α (1 ng/ml) for 16 h before calcium imaging to determine the impact of inflammatory factors on TRPV4 activation. Bath application of 4αPDD (30 μM) significantly increased intracellular calcium mobilization (61.9%) compared with the baseline in 13 of 21 randomly selected PSCs (Fig. 7A). The intracellular calcium activity was irregular with sustained repetitive increases persisting over 1 h. The F340-to-F380 ratio increased from baseline (0.33 ± 0.02 to 0.99 ± 0.09, P < 0.001, paired t-test, Fig. 7B). Repeated oscillatory calcium mobilization responses were seen in some cells (data not shown). In untreated PSC cultures few cells responded to 4αPDD stimulation (n = 19 tested, Fig. 7, A and B). These data suggest that TNF-α potentiates downstream events resulting in increased TRPV4-mediated calcium mobilization in PSCs.
Fig. 7.
TNF-α promoted the TRPV4 activation response to 4αPDD in PSC cultures from control rats. A: pretreatment of control PSC cultures with TNF-α (1 ng/ml) for 16 h produced a robust and sustained intracellular calcium mobilization in response to bath application of 4αPDD (30 μM) (top trace). The activity was sustained over 1 h with a single application of 4αPDD. In this experiment, 13 cells were activated from a total of 21 cells selected. In contrast, responses to 4αPDD were minimal for untreated control PSCs (bottom trace). B: histogram summarizes the averaged F340/F380 ratio to bath application of 4αPDD after TNF-α pretreatment (n = 21). Statistically significant increases in responses were observed for cells with TNF-α pretreatment compared with the untreated control group (n = 19, ***P < 0.001, Student's t-test).
DISCUSSION
In this study live cell calcium imaging, immunostaining, Western blot, and RT-PCR techniques were combined to investigate effects of a chronic HFA pancreatitis on isolated PSCs and pancreatic tissue. Levels of PDGFR-β, a stellate cell transforming factor specific activator marker, and extracellular matrix protein marker α-SMA expression levels increased positively after long-term exposure to high-fat/alcohol, documenting that the cells under study were PSCs. This study demonstrated upregulated expression of TRPV4 protein in pancreatic tissues and isolated PSCs. There was inducible increase in TRPV4-mediated calcium mobilization in HFA activated PSCs compared with PSCs from control rats fed normal chow. Similar results were obtained in PSCs from control rats after in vitro treatments with alcohol or TNF-α. These results demonstrate that TRPV4 is a functional ion channel and a visceral cellular sensor in rat pancreatic stellate cells responsive to cellular level of alcohol/fatty acid metabolite accumulation, edema, and inflammation typical in pancreatitis. The present experiments have determined that TRPV4 channel activation is an inducible, underlying cellular mechanism promoting prolonged pancreatic stellate cell activation.
There is an ever-increasing body of evidence that TRPV4 is involved in visceral hypersensitivity and sensory integration. TRPV4 is found in many tissues, including visceral organs: kidneys, urinary bladder, colon, and pancreas in rodent and humans (11, 13, 14, 16, 17). TRPV4 is expressed in sensory neurons and functional evidence has been correlated with intense enrichment of TRPV4 expression in splanchnic, pancreatic, and pelvic colonic afferents traced to their cell bodies in the thoraco-lumbar and lumbo-sacral dorsal root ganglia (13, 17). The selective role of TRPV4 in high-threshold afferents would be expected to translate into a role in visceral pain perception in vivo (12). Decreased abdominal electromyographic responses to noxious colorectal distension have been shown in TRPV4 knockouts or in mice with downregulated TRPV4 expression [via intervertebral small interfering RNA (siRNA) delivery] (13, 29). Intracolonic administration of 4α-PDD in wild-type mice caused neuronal activation in the lumbar-sacral spinal cord and caused dose-dependent visceral hypersensitivity to colorectal distension (13). TRP channels are present in subsets of vagal afferent neurons that project to the stomach may confer temperature and mechanosensitivity on these cells (43) and contribute to acute pancreatitis pain as well (17). Interactive stress response events in conditions where alcohol metabolites and high fat upregulate TRPV4 channels in gastrointestinal structures and innervating sensory nerves may confer and promote hypersensitivity and overactivation.
AA is a polyunsaturated fatty acid, a cellular second messenger, and an alcohol metabolite metabolized further to both pro-inflammatory and anti-inflammatory molecules, such as prostaglandin E2. Metabolized AA is an endogenous chemical activator of TRPV4 via cytochrome P450 (CYP)-derived epoxyeicosatrienoic acids (EETs) (38). TRPV4 is also activated by hypotonic solution-induced cell swelling, which shares the same cytochrome P450 pathway with AA (22). The PSC activators described which trigger calcium mobilization in the present study could be effectively inhibited by TRPV channel blocker RR.
Chronic exposure to high fat (fatty acid) and alcohol causes PSCs to undergo molecular plasticity changes which maintain a hypersensitized state. Any additional stimuli including inflammatory insults such as an increase in blood level cytokines leads to tissue damage, deposition of collagen that can restrict ductal flow, and painful acute pancreatitis attacks. The present data suggest that both alcohol and TNF-α potentiate events resulting in increased TRPV4-mediated calcium mobilization in PSCs. The studies thus provide additional evidence that cytokines can directly modulate calcium-activated events in PSCs mediated through TRPV4 channel activation. Recent in vivo and in vitro studies from Apte and others (34, 35) show that both LPS and alcohol exert anti-apoptotic effects on PSCs and promote their activation, thereby increasing the process of pancreatic fibrogenesis. Importantly, PSCs are themselves capable of producing cytokines that might perpetuate their activation via an autocrine loop that includes upregulation of TRPV4 channel expression and pathological interactions with sensory nerves. Thus alcohol, high fat, and the cytokines produced by the PSCs themselves play a critical role in activation of TRPV4 channels and calcium-mediated downstream cellular activation events during pancreatic inflammation. It is now acknowledged that repeated episodes of acute pancreatitis can cause progressive damage to the pancreas: the necrosis-fibrosis sequence, a constant feature of chronic pancreatitis (31).
Perspectives and Significance
The present study more fully characterizes activation of TRPV4 on isolated PSCs taken from rats with HFA-induced pancreas. Conditions similar to those found in the inflamed pancreatitis, including cell swelling and increases in alcohol metabolites and TNF-α, promote increased TRPV4 expression and enhance TRPV4-mediated activation of PSCs as indicated by increases in intracellular calcium mobilization responses. The expression and responsivity increases imply that TRPV4 plays a role in pancreatic stellate cell activation during pancreatic inflammation.
GRANTS
This study was funded by National Institutes of Health Grant NINDS R01-039041 (to K. N. Westlund).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.P.Z., F.M., and S.M.A. performed experiments; L.P.Z., F.M., and S.M.A. analyzed data; L.P.Z., F.M., and K.N.W. interpreted results of experiments; L.P.Z., F.M., and K.N.W. prepared figures; L.P.Z. and F.M. drafted manuscript; K.N.W. conception and design of research; K.N.W. edited and revised manuscript; K.N.W. approved final version of manuscript.
REFERENCES
- 1. Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JD. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron 39: 497–511, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Apte M, Pirola R, Wilson J. The fibrosis of chronic pancreatitis: new insights into the role of pancreatic stellate cells. Antioxid Redox Signal 15: 2711–2722, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Apte M, Pirola R, Wilson J. New insights into alcoholic pancreatitis and pancreatic cancer. J Gastroenterol Hepatol 24, Suppl 3: S51–S56, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut 43: 128–133, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Apte MV, Phillips PA, Fahmy RG, Darby SJ, Rodgers SC, McCaughan GW, Korsten MA, Pirola RC, Naidoo D, Wilson JS. Does alcohol directly stimulate pancreatic fibrogenesis? Studies with rat pancreatic stellate cells. Gastroenterology 118: 780–794, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Apte MV, Pirola RC, Wilson JS. Mechanisms of alcoholic pancreatitis. J Gastroenterol Hepatol 25: 1816–1826, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Apte MV, Pirola RC, Wilson JS. Molecular mechanisms of alcoholic pancreatitis. Dig Dis 23: 232–240, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Apte MV, Wilson JS. Mechanisms of pancreatic fibrosis. Dig Dis 22: 273–279, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Apte MV, Wilson JS. Stellate cell activation in alcoholic pancreatitis. Pancreas 27: 316–320, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grunert A, Adler G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 115: 421–432, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Birder LA. TRPs in bladder diseases. Biochim Biophys Acta 1772: 879–884, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blackshaw LA, Brierley SM, Hughes PA. TRP channels: new targets for visceral pain. Gut 59: 126–135, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Brierley SM, Page AJ, Hughes PA, Adam B, Liebregts T, Cooper NJ, Holtmann G, Liedtke W, Blackshaw LA. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology 134: 2059–2069, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Casas S, Novials A, Reimann F, Gomis R, Gribble FM. Calcium elevation in mouse pancreatic beta cells evoked by extracellular human islet amyloid polypeptide involves activation of the mechanosensitive ion channel TRPV4. Diabetologia 51: 2252–2262, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Casini A, Galli A, Pignalosa P, Frulloni L, Grappone C, Milani S, Pederzoli P, Cavallini G, Surrenti C. Collagen type I synthesized by pancreatic periacinar stellate cells (PSC) co-localizes with lipid peroxidation-derived aldehydes in chronic alcoholic pancreatitis. J Pathol 192: 81–89, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Cenac N, Altier C, Chapman K, Liedtke W, Zamponi G, Vergnolle N. Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology 135: 937–946, 946 e931–e932, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Ceppa E, Cattaruzza F, Lyo V, Amadesi S, Pelayo JC, Poole DP, Vaksman N, Liedtke W, Cohen DM, Grady EF, Bunnett NW, Kirkwood KS. Transient receptor potential ion channels V4 and A1 contribute to pancreatitis pain in mice. Am J Physiol Gastrointest Liver Physiol 299: G556–G571, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clapham DE. TRP channels as cellular sensors. Nature 426: 517–524, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Ellenrieder V, Schneiderhan W, Bachem M, Adler G. Fibrogenesis in the pancreas. Rocz Akad Med Bialymst 49: 40–46, 2004 [PubMed] [Google Scholar]
- 20. Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis mechanisms and treatment strategies. N Engl J Med 328: 1828–1835, 1993 [DOI] [PubMed] [Google Scholar]
- 21. Haber PS, Norris MD, Apte MV, Rodgers SC, Norton ID, Pirola RC, Roberts-Thomson IC, Wilson JS. Alcoholic pancreatitis and polymorphisms of the variable length polythymidine tract in the cystic fibrosis gene. Alcoholism Clin Exper Res 23: 509–512, 1999 [PubMed] [Google Scholar]
- 22. Hoffmann EK. Intracellular signalling involved in volume regulatory decrease. Cell Physiol Biochem 10: 273–288, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Marrelli SP, O'Neil RG, Brown RC, Bryan RM., Jr PLA2 and TRPV4 channels regulate endothelial calcium in cerebral arteries. Am J Physiol Heart Circ Physiol 292: H1390–H1397, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Menke A, Adler G. TGFbeta-induced fibrogenesis of the pancreas. Int J Gastrointest Cancer 31: 41–46, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest 117: 50–59, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Phillips PA, McCarroll JA, Park S, Wu MJ, Pirola R, Korsten M, Wilson JS, Apte MV. Rat pancreatic stellate cells secrete matrix metalloproteinases: implications for extracellular matrix turnover. Gut 52: 275–282, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Plant TD, Strotmann R. TRPV4. Handb Exp Pharmacol 189–205, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Saotome T, Inoue H, Fujimiya M, Fujiyama Y, Bamba T. Morphological and immunocytochemical identification of periacinar fibroblast-like cells derived from human pancreatic acini. Pancreas 14: 373–382, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Sipe WE, Brierley SM, Martin CM, Phillis BD, Cruz FB, Grady EF, Liedtke W, Cohen DM, Vanner S, Blackshaw LA, Bunnett NW. Transient receptor potential vanilloid 4 mediates protease activated receptor 2-induced sensitization of colonic afferent nerves and visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol 294: G1288–G1298, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Tahara J, Shimizu K, Shiratori K. Engulfment of necrotic acinar cells by pancreatic stellate cells inhibits pancreatic fibrogenesis. Pancreas 37: 69–74, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Tattersall SJ, Apte MV, Wilson JS. A fire inside: current concepts in chronic pancreatitis. Intern Med J 38: 592–598, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Uchida M, Ito T, Nakamura T, Igarashi H, Oono T, Fujimori N, Kawabe K, Suzuki K, Jensen RT, Takayanagi R. ERK pathway and sheddases play an essential role in ethanol-induced CX3CL1 release in pancreatic stellate cells. Lab Invest 93: 41–53, 2013 [DOI] [PubMed] [Google Scholar]
- 33. Vincent F, Acevedo A, Nguyen MT, Dourado M, DeFalco J, Gustafson A, Spiro P, Emerling DE, Kelly MG, Duncton MA. Identification and characterization of novel TRPV4 modulators. Biochem Biophys Res Commun 389: 490–494, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Vogelmann R, Ruf D, Wagner M, Adler G, Menke A. Effects of fibrogenic mediators on the development of pancreatic fibrosis in a TGF-β1 transgenic mouse model. Am J Physiol Gastrointest Liver Physiol 280: G164–G172, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Vonlaufen A, Phillips PA, Xu Z, Zhang X, Yang L, Pirola RC, Wilson JS, Apte MV. Withdrawal of alcohol promotes regression while continued alcohol intake promotes persistence of LPS-induced pancreatic injury in alcohol-fed rats. Gut 60: 238–246, 2011 [DOI] [PubMed] [Google Scholar]
- 36. Vonlaufen A, Wilson JS, Apte MV. Molecular mechanisms of pancreatitis: current opinion. J Gastroenterol Hepatol 23: 1339–1348, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Vonlaufen A, Wilson JS, Pirola RC, Apte MV. Role of alcohol metabolism in chronic pancreatitis. Alcohol Res Health 30: 48–54, 2007 [PMC free article] [PubMed] [Google Scholar]
- 38. Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424: 434–438, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Westlund KN, Vera-Portocarrero L. Rat models of pancreatitis. pain. Methods Mol Biol 851: 223–238, 2012 [DOI] [PubMed] [Google Scholar]
- 40. Whitcomb DC. Genetics and alcohol: a lethal combination in pancreatic disease? Alcohol Clin Exp Res 35: 838–842, 2011 [DOI] [PubMed] [Google Scholar]
- 41. Yadav D, Slivka A, Sherman S, Hawes RH, Anderson MA, Burton FR, Brand RE, Lewis MD, Gardner TB, Gelrud A, Disario J, Amann ST, Baillie J, Lawrence C, O'Connell M, Lowenfels AB, Banks PA, Whitcomb DC. Smoking is underrecognized as a risk factor for chronic pancreatitis. Pancreatology 10: 713–719, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zeng Y, Wang X, Zhang W, Wu K, Ma J. Hypertriglyceridemia aggravates ER stress and pathogenesis of acute pancreatitis. Hepatogastroenterology 59: 2012 [DOI] [PubMed] [Google Scholar]
- 43. Zhang L, Jones S, Brody K, Costa M, Brookes SJ. Thermosensitive transient receptor potential channels in vagal afferent neurons of the mouse. Am J Physiol Gastrointest Liver Physiol 286: G983–G991, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Zhang X, Cui Y, Fang L, Li F. Chronic high-fat diets induce oxide injuries and fibrogenesis of pancreatic cells in rats. Pancreas 37: e31–38, 2008 [DOI] [PubMed] [Google Scholar]