Abstract
Overexpression of mitochondrial uncoupling proteins (UCPs) attenuates ischemia-reperfusion (I/R) injury in cultured cardiomyocytes. However, it is not known whether UCPs play an essential role in cardioprotection in the intact heart. This study evaluated the cardioprotective efficacy of UCPs against I/R injury and characterized the mechanism of UCP-mediated protection in addition to the role of UCPs in ischemic preconditioning (IPC). Cardiac UCP3 knockout (UCP3−/−) and wild-type (WT) mice hearts were subjected to ex vivo and in vivo models of I/R injury and IPC. Isolated UCP3−/− mouse hearts were retrogradely perfused and found to have poorer recovery of left ventricular function compared with WT hearts under I/R conditions. In vivo occlusion of the left coronary artery resulted in twofold larger infarcts in UCP3−/− mice compared with WT mice. Moreover, the incidence of in vivo I/R arrhythmias was higher in UCP3−/− mice. Myocardial energetics were significantly impaired with I/R, as reflected by a decreased ATP content and an increase in the AMP-to-ATP ratio. UCP3−/− hearts generated more reactive oxygen species (ROS) than WT hearts during I/R. Pretreatment of UCP3−/− hearts with the pharmacological uncoupling agent carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone improved postischemic functional recovery. Also the protective efficacy of IPC was abolished in UCP3−/− mice. We conclude that UCP3 plays a critical role in cardioprotection against I/R injury and the IPC phenomenon. There is increased myocardial vulnerability to I/R injury in hearts lacking UCP3. The mechanisms of UCP3-mediated cardioprotection include regulation of myocardial energetics and ROS generation by UCP3 during I/R.
Keywords: energetics, mitochondria
ischemic heart disease is one of the leading causes of morbidity and mortality in the United States, resulting in heart failure, arrhythmias, and sudden death (6). Ischemia-reperfusion (I/R) injury severely compromises energy metabolism and causes cellular damage through increased reactive oxygen species (ROS) generation, calcium overload, acidosis, and disrupted cellular ultrastructure (6, 33, 35, 39). Mitochondria play a critical role in ROS generation, ionic homeostasis, calcium storage, regulation of cellular metabolism, modulation of membrane potential, and cell survival. Cardiomyocytes use ATP to support contraction and ionic gradients as well as protein synthesis and degradation. Mitochondria occupy ∼30% of cardiomyocyte volume and produce over 90% of ATP. Therefore, preservation of the functional and structural integrity of mitochondria is essential for successful cardioprotection and myocardial tolerance to I/R stress.
One mechanism that may enhance myocardial tolerance to I/R stress and improve cardioprotection is the modulation of the mitochondrial electrochemical gradient by mitochondrial uncoupling proteins (UCPs) (5, 27, 32, 36, 37). Specifically, it has been suggested that mild to moderate mitochondrial uncoupling by the small reversible H+ leak mediated by UCPs is protective against cellular I/R injury by reducing ROS generation (5, 37). Pharmacological uncoupling has been shown to improve postischemic function in the isolated perfused heart (8, 32). Furthermore, overexpression of UCPs in cardiomyocytes was found to prevent cell death by preserving mitochondrial function and structure during oxidative stress (5, 37). However, it is not known whether UCPs play a role in maintaining cardiac function and preventing myocardial damage in intact heart in the setting of in vivo and ex vivo I/R stress.
As members of the superfamily of anion carrier proteins, UCPs are located in the mitochondrial inner membrane and regulate proton leak (23). UCP1 was the first UCP to be characterized in brown adipocytes and is responsible for nonshivering thermogenesis. UCP2 and UCP3 were later identified in various tissues, including heart, but appear to play no significant role in thermogenesis, despite sharing sequence homology with UCP1 (4, 23, 12). Previous studies showed that UCP3 is downregulated in the nonischemic failing heart and upregulated in the viable myocardium of chronically infarcted hearts (9, 31, 34). Also, increased cardiac UCP3 expression was found with diabetes, fasting, increased fatty acid provision, and thyroid hormone treatment (10, 23). This suggests a possible role of UCP3 in the response to oxidative or metabolic stress in the heart.
Accordingly, stable transfection of UCP1 was found to confer resistance to hypoxia/reoxygenation in cardiomyocytes, and expression of UCP1 in the mouse heart was protective against I/R injury with regulation of oxidative stress (5, 17). Moreover, UCP2 and UCP3 were shown to augment cardiomyocyte tolerance to anoxia/reperfusion injury and oxidative stress (27, 37). The ischemic preconditioning (IPC) cell survival program was thought to be involved in this process.
Although IPC is shown to be one of the most powerful and reproducible cardioprotective interventions that limit infarct size with reduced cardiomyocyte necrosis and apoptosis, the exact mechanism of IPC remains unclear (6, 7). Mild H+ leak during brief and longer episodes of I/R by UCPs may regulate ionic homeostasis, ROS generation, and ATP production that may result in the IPC phenomenon.
Based on these findings, we hypothesized that UCPs, particularly UCP3, play a critical role in both cardioprotection against I/R injury and induction of IPC by regulating mitochondrial ionic homeostasis, ATP, and ROS generation. UCPs may prevent myocardial necrosis and lethal arrhythmias in oxidative stress by preserving mitochondrial functional and structural integrity. To test these hypotheses, we assessed the effects of loss of cardiac UCP3 function using ex vivo and in vivo models of I/R injury and IPC in the present studies.
METHODS
The research protocol and the experimental procedures were reviewed and approved by the Yale University Animal Care and Use Committee. All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication no. 5377-3, 1996).
Animals.
Ten- to 12-wk-old male mice were used in this study. Mice (C57BL/6) overexpressing UCP2 and UCP3 (hUCP2/3) and UCP2−/− have been previously characterized (19). Swiss black UCP3−/− transgenic mice were generously provided by Dr. Toren Finkle from the National Institutes of Health (29). Wild-type (WT) mice were of the same strain as the corresponding transgenic mice. During experimental procedures, mice were anesthetized by using 50–100 mg/kg ip pentobarbital sodium and/or 0.5–2.5% isoflurane for deep anesthesia or termination. The adequacy of anesthesia was continuously monitored by the response to painful stimuli such as toe pinch, heart rate, respiratory rate, and electrocardiogram. To maintain optimal anesthesia during the procedure, isoflurane dose was adjusted, and additional doses of pentobarbital were given as needed. All experiments were terminal, and therefore no postoperative analgesics were required.
Ex vivo assessment of I/R injury.
After attaining optimal anesthesia with injection of 100 mg/kg ip pentobarbital sodium, hearts were rapidly excised following anterior thoracotomy and arrested in ice-cold Krebs-Henseleit (KH) buffer containing 7 mmol/l glucose, 0.4 mmol/l oleate, 1% BSA, and 10 μU/ml insulin. A left-ventricular balloon was inflated to achieve a diastolic pressure of 5 mmHg during baseline perfusion, and its volume was kept constant during the experiment. As previously described, isolated mouse hearts were retrogradely perfused with KH buffer and subjected to I/R injury (30 min of either low-flow or no-flow ischemia followed by reperfusion for 30 min) (28, 35). Developed pressure and heart rate were continuously monitored. Hearts were freeze-clamped in liquid nitrogen at the end of the experiment. IPC was induced by three cycles of 4 min of no-flow ischemia followed with 4 min of reperfusion before 30 min of ischemia and 30 min of reperfusion. Pharmacological uncoupling was induced in UCP3−/− mouse hearts by perfusing hearts for 5 min with 100 nM carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone (FCCP).
In vivo assessment of I/R injury.
Open-chest left coronary artery (LCA) ligation technique was used to induce I/R injury following induction with 50 mg/kg ip pentobarbital sodium and maintaining deep anesthesia as described above (28). All transgenic and WT mice were intubated and ventilated with a small rodent ventilator (Harvard Apparatus, Holliston, MA). The core temperature was maintained at 37°C with a heating pad. A left side thoracotomy was performed. The LCA was ligated with an 8–0 nylon suture, and the heart was subjected to 20 min of ischemia. Next, 2 h of reperfusion were maintained after removing the suture. Continuous monitoring of electrocardiograms confirmed ischemic ST-segment elevation during coronary occlusion (AD Instruments). Following I/R, blood samples were collected, and the heart was then excised. The serum troponin I was determined by enzyme-linked immunosorbent assay (ELISA). Infarct area (IA) and area at risk (AAR) were assessed with triphenyltetrazolium chloride and Evans blue staining, as described previously (28). Hearts were fixed and sectioned into 1-mm slices. Tissues were photographed using a Leica microscope. The National Institutes of Health Image J software was used for quantification. Also, the hearts were freeze-clamped in liquid nitrogen at the end of I/R without staining after some of the experiments. The frozen tissue samples were used for various biochemical analyses, including nucleotide profile and ROS measurements.
Heart rhythm analysis.
Continuous electrocardiographic monitoring (Powerlab; ADInstruments) was performed during in vivo myocardial I/R with LCA ligation. Heart rate and rhythm were analyzed throughout the experiment. The incidence and type of arrhythmias, including atrioventricular block, premature ventricular contraction, and ventricular arrhythmias, were evaluated during I/R based on limb lead recordings.
Imaging.
In vivo left ventricular (LV) systolic function and structure were evaluated by echocardiography (45-MHz probe; VisualSonics Vevo 770, Toronto, Canada) while animals were under light anesthesia (0.5–1% isoflurane). Two-dimensionally guided M-mode images of the LV were acquired in the long and short axes to assess LV cavity dimensions, anterior and posterior wall thicknesses, and fractional shortening. Some hearts were fixed in paraformaldehyde and then embedded in paraffin. Samples were cut into thin sections and stained with hematoxylin-eosin or Masson's trichrome stain. Myocardial morphology and ultrastructure were examined from photomicrographs (Microphot-FXA; Nikon).
Myocardial nucleotide profile and ROS measurement.
Hearts were rapidly excised and freeze-clamped in liquid nitrogen following I/R stress. The adenine nucleotide content of perchloric acid extracts of myocardial tissue was measured by high-performance liquid chromatography (Waters 510, Milford, MA) (5, 33). Lucigenin-derived chemiluminescence (LDCL) was used for assessment of ROS in myocardial tissue extracts. LDCL quantifies superoxide anion (O2−), which generated from mitochondrial inner membrane in the mitochondrial respiratory chain (14, 24). LDCL of heart homogenate (100 μg protein) was measured using a luminometer after 15 min of incubation in the presence of 75 μM lucigenin (24). Also, generation of another mitochondrial ROS, hydrogen peroxide (H2O2), was measured by using 2,7-dichlorofluorescein (DCF) diacetate fluorescence. Myocardial tissue (100 μg protein) was incubated with 100 μM DCF, and the fluorescence was measured by a multiplate reader at 480 nm excitation and 530 nm emission as defined previously (5, 33).
Chemicals.
All chemicals were obtained from Sigma and Fisher Scientific. The ELISA kit for specific mouse troponin I measurement was purchased from Life Diagnostics (West Chester, PA). FCCP was dissolved in dimethyl sulfoxide (DMSO). The concentration of DMSO within the KH buffer was kept under 0.05%. The control experiments were performed with corresponding DMSO concentration.
Statistical analysis.
Data are presented as means ± SE, and n represents the number of animals used for experiments. Comparisons between groups were performed by the Student's t-test or ANOVA with the Student-Newman-Keuls post hoc correction. Categorical data were analyzed by the chi-squared test. A value of P < 0.05 was considered statistically significant.
RESULTS
Loss of UCP3 results in decreased left ventricular contractile function ex vivo following I/R.
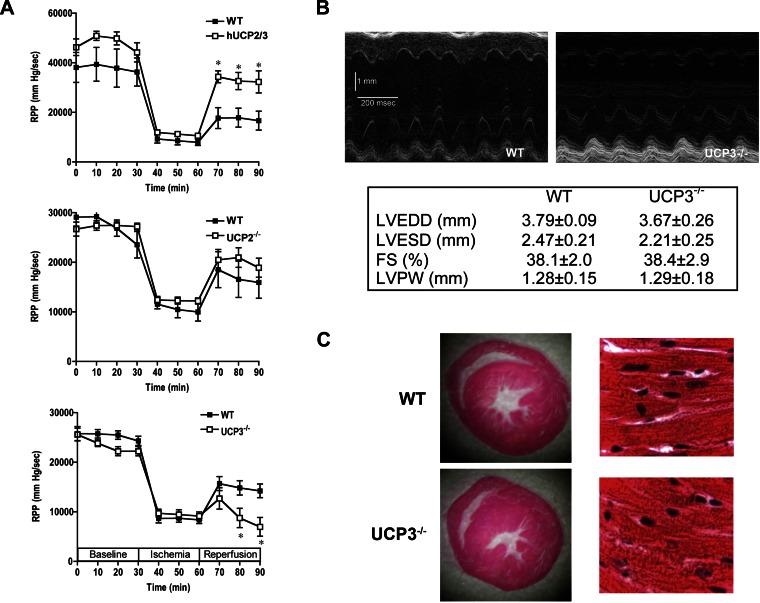
The transgenic mice with overexpression of UCP2/3 or deletion of UCP2 (UCP2−/−) or UCP3 (UCP3−/−) hearts were subjected to ex vivo I/R injury to evaluate the LV function. Isolated perfused hUCP2/3 hearts (n = 6) had a twofold greater recovery of function compared with WT hearts (n = 6) following I/R (Fig. 1A). Because of the potential confounding effects of UCP overexpression on mitochondrial function (11, 36), hearts from mice with germline deletion of UCP2 (UCP2−/−) or UCP3 (UCP3−/−) were subjected to perfusion using the I/R protocol. There were no significant differences in baseline LV function in either UCP2−/− or UCP3−/− hearts compared with their respective WT counterparts (n = 6 in each group, Fig. 1A). Furthermore, LV contractile function decreased to a similar extent during ischemia in WT hearts and UCP2−/− or UCP3−/− hearts. During reperfusion, WT and UCP2−/− hearts had similar degrees of recovery of function, suggesting that UCP2 is not essential for cardioprotection against I/R injury in the mouse heart (Fig. 1A). In contrast, postischemic LV contractile function was 50% lower in UCP3−/− hearts compared with WT hearts (WT: 14,230 ± 1,377 mmHg/min, UCP3−/−: 6,970 ± 1,885 mmHg/min; Fig. 1A). This demonstrated that UCP3 has an important role in myocardium during I/R stress, and therefore UCP3−/− mice were used for further experiments.
Fig. 1.
Effects of ischemia and reperfusion (I/R) on left ventricular function. The overexpression of uncoupling protein (UCP) 2 and UCP3 (hUCP2/3) in hearts results in better postischemic recovery of function compared with wild-type (WT) hearts (A). Left ventricular contractile functional recovery was similar in WT and UCP2−/− hearts following I/R (A). However, the recovery of function was significantly lower in UCP3−/− hearts compared with WT hearts (A). Representative M-mode echocardiograms from WT and UCP3−/− mice and echocardiographic analysis of left ventricular size and function, which reveals comparable cardiac function and structure in UCP3−/− and WT hearts in vivo (B). Myocardial architecture on hematoxylin- and eosin-stained sections was grossly similar in WT and UCP3−/− hearts (C). RPP, rate pressure product; LVEDD, LV end diastolic dimension; LVESD, LV end systolic dimension; FS, fractional shortening; LVPW, LV posterior wall. *P < 0.05 compared with WT.
Structural evaluation of UCP3−/− hearts.
Cardiac function and morphology were evaluated in UCP3−/− and WT animals to exclude possible baseline differences contributing to the poorer postischemic functional recovery. Chamber cavity size, wall thickness, and LV systolic function were similar in both groups (Fig. 1B) based on echocardiographic analysis. Cross sections of myocardial tissue at the midventricular level demonstrated similar structural morphology and confirmed the echocardiographic findings (Fig. 1C). Examination of hematoxylin- and eosin-stained specimens revealed preserved myocardial architecture (Fig. 1C). Thus, there was no significant baseline morphological difference between UCP3−/− and WT hearts.
Increased vulnerability to I/R injury in UCP3−/− hearts in vivo.
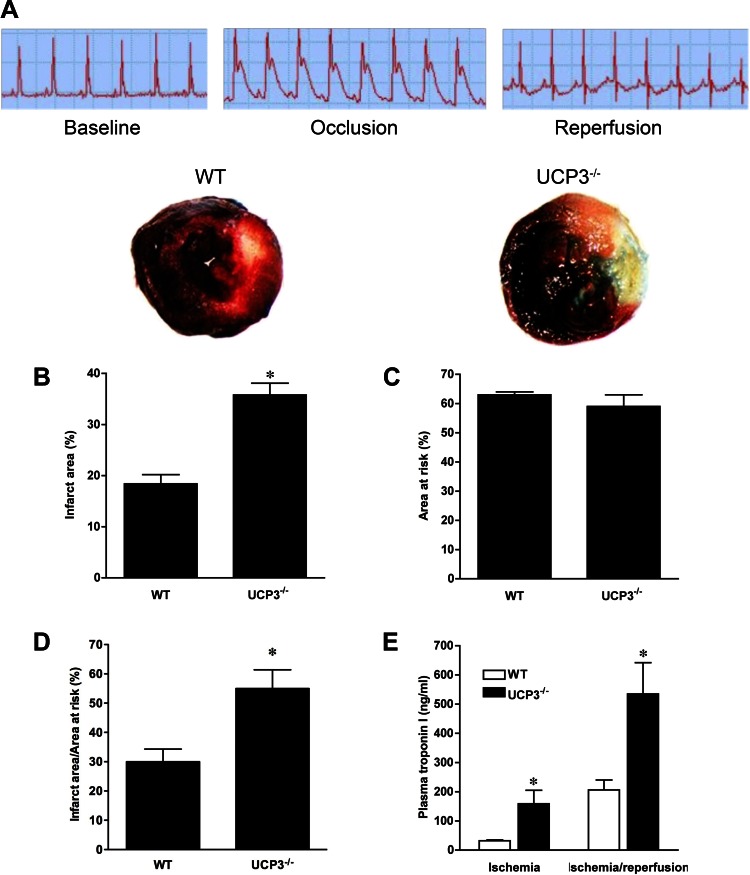
In vivo I/R injury was produced by LCA ligation in WT and UCP3−/− animals to confirm the critical role of UCP3 in the response of I/R stress in the heart. Marked ST-segment elevation, an indicator of effective LCA ligation and injury in the LCA territory, was observed during 20 min of ischemia in all mice, which improved during reperfusion (Fig. 2A). The IA was two times as large in UCP3−/− hearts than in WT hearts following I/R (35.8 ± 2.3% of the LV vs. 18.4 ± 1.8% of the LV, n = 7–8, P < 0.05; Fig. 2B), whereas the AAR was comparable in the two groups (WT: 63 ± 1% of the LV, UCP3−/−: 59 ± 4% of the LV, P > 0.05; Fig. 2C). Accordingly, there was a significant difference in the IA to AAR ratio (WT: 30.6 ± 1.5%, UCP3−/−: 54.7 ± 2.4%, P < 0.05; Fig. 2D). In parallel with the infarct size, UCP3−/− hearts released significantly greater amounts of troponin I at the end of ischemia (159 ± 46 ng/ml, n = 5) and at the end of 2 h of reperfusion (535 ± 107 ng/ml, n = 8) compared with WT hearts (32 ± 3 ng/ml during ischemia and 206 ± 34 ng/ml during I/R, n = 4–9, P < 0.05; Fig. 2E). In the absence of injury, both groups had similar serum troponin I concentrations (0.59 ± 0.21 vs. 0.37 ± 0.09 ng/ml, n = 6–7, P > 0.05).
Fig. 2.
In vivo myocardial infarction following left coronary artery (LCA) ligation in WT and UCP3−/− animals. ST-segment elevation, indicative of myocardial infarction, was observed during 20 min of ischemia in WT and UCP3−/− mice, which improved during reperfusion (A). Representative cross sections of WT and UCP3−/− hearts stained with triphenyltetrazolium chloride and Evan's blue dye depict infarct area (IA) and area at risk (AAR), respectively. UCP3−/− hearts had greater IA than WT hearts following I/R (B), whereas the AAR was comparable in the two groups (C). Thus, the IA to AAR was higher in UCP3−/− hearts (D). Also, UCP3−/− hearts released more troponin I following ischemia and I/R injury (E) as a result of further myocardial necrosis. *P < 0.05 compared with WT.
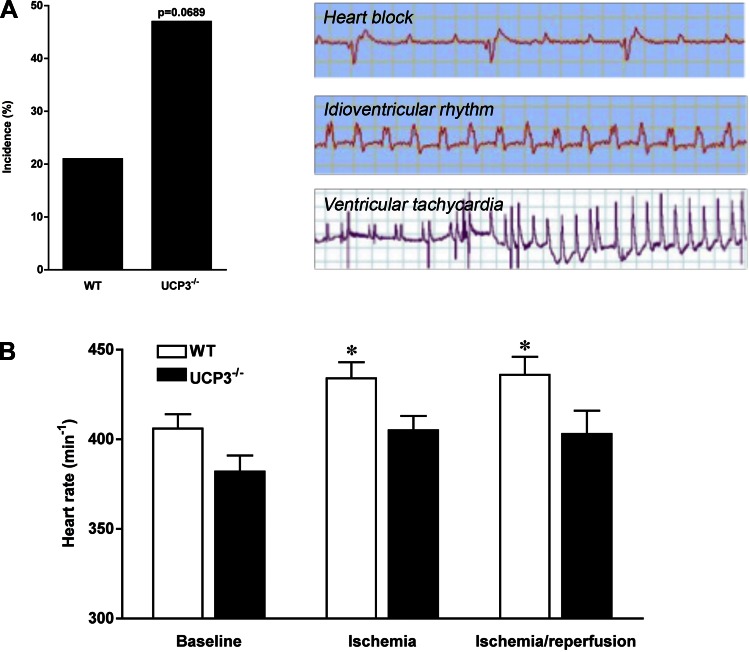
I/R arrhythmias in UCP3−/− hearts in vivo.
The incidence of I/R arrhythmias was higher in UCP3−/− (47%, 8 out of 17 mice) compared with WT (21%, 3 out of 14 mice; P = 0.0689; Fig. 3A) mice following LCA ligation. The most common arrhythmia was high-grade atrioventricular block (2/14 in WT, 7/17 in UCP3−/−, P = 0.05; Fig. 3A). Premature ventricular contractions (3/17 in UCP3−/−, 3/14 in WT), nonsustained ventricular tachycardia (2/17 in UCP3−/−, 0/14 in WT), and idioventricular rhythm (1/17 in UCP3−/−, 0/14 in WT) also occurred (Fig. 3A). The heart rate response to I/R stress was blunted in UCP3−/− mice (382 ± 9 to 403 ± 13 beats/min, not significant), but the heart rate increased in WT mice in response to I/R (406 ± 8 to 436 ± 10 beats/min, P < 0.05; Fig. 3B). Thus, UCP3−/− hearts are more susceptible to I/R arrhythmias in association with impaired impulse conduction and formation.
Fig. 3.
There was a strong trend toward a greater incidence of I/R arrhythmias in UCP3−/− mice compared with WT mice (P = 0.0689; A). Various types of arrhythmia, including atrioventricular block, premature ventricular contractions, couplets, triplets, nonsustained ventricular tachycardia, and idioventricular rhythm, were observed during I/R injury (A). The heart rate response to I/R stress was blunted in UCP3−/− mice compared with WT mice (B). Thus, both impulse conduction and formation are impaired in UCP3−/− hearts. *P < 0.05 compared with baseline.
Mechanisms of UCP3-mediated myopreservation in I/R include maintenance of myocardial energetics and modulation of ROS generation.
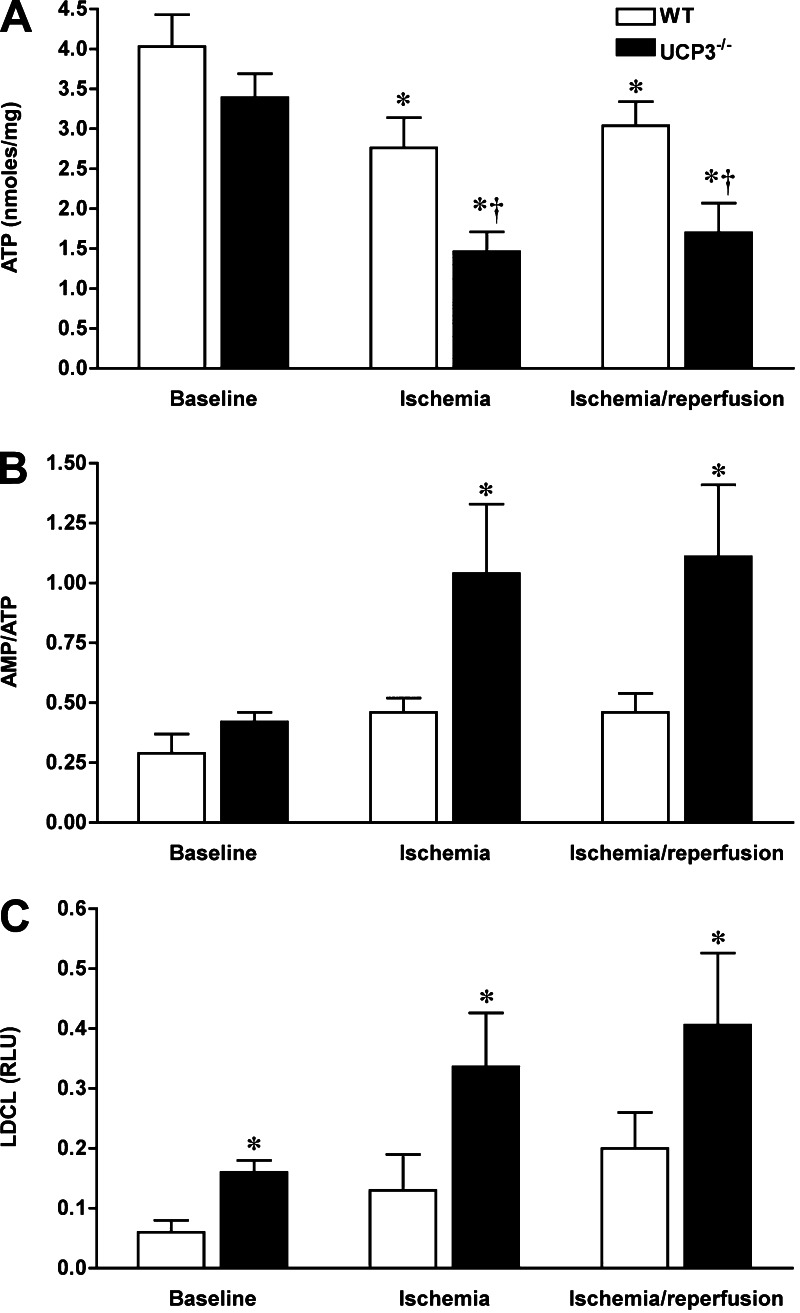
Because mitochondrial ATP production is essential for cell integrity and survival and sensitive to stress conditions, myocardial adenine nucleotide content was measured following in vivo I/R injury with LCA ligation. Immediately after 20 min of ischemia, the myocardial content of ATP was significantly reduced in both WT (from 4.03 ± 0.4 to 2.76 ± 0.28 nmol/mg protein, n = 6–7, P < 0.05) and UCP3−/− (from 3.39 ± 0.3 to 1.46 ± 0.25 nmol/mg protein, n = 6, P < 0.05; Fig. 4A) hearts. However, the reduction in ATP content in UCP3−/− hearts was more severe than in WT hearts (P < 0.05), whereas the amount of ATP in both groups was comparable under basal conditions. Also, UCP3−/− hearts had a lower content of ATP after 2 h of reperfusion compared with WT hearts (3.04 ± 0.3 vs. 1.7 ± 0.37 nmol/mg protein, n = 7–9, P < 0.05). Moreover, the AMP-to-ATP ratio, a marker of metabolic stress, was higher in UCP3−/− hearts than in WT hearts (1.04 ± 0.29 in ischemia, 1.11 ± 0.3 in reperfusion, P < 0.05; Fig. 4B). Thus I/R injury caused more severe disruption in myocardial energetics in UCP3−/− hearts than in WT hearts.
Fig. 4.
I/R injury causes more severe disruption in myocardial energetics and greater reactive oxygen species (ROS) generation in UCP3−/− hearts. The myocardial content of ATP in I/R injury was significantly reduced in both WT and UCP3−/− hearts, whereas the decline in ATP production was more severe in UCP3−/− hearts than in WT hearts (A). Consequently, metabolic stress, as reflected by the AMP-to-ATP ratio, was significantly higher in UCP3−/− hearts than in WT hearts (B). Following 20 min of ischemia, UCP3−/− hearts produced more ROS in the myocardium as shown by higher lucigenin-derived chemiluminescence (LDCL; C). RLU, relative light units. *P < 0.05 compared with WT. †P < 0.05 compared with control.
Metabolic response to I/R was also evaluated by determining AMP-activated protein kinase (AMPK) activity in myocardium. In the perfused heart, I/R resulted in activation of AMPK in WT hearts as evidenced by an increase in phosphorylation of Thr172 of the α-subunit of AMPK. Under nonischemic conditions, AMPK was activated in UCP3−/− hearts to the same level as WT hearts at the end of I/R, suggesting the presence of metabolic stress under basal conditions. However, I/R did not result in further activation of AMPK in the UCP3−/− hearts despite the greater increase in the AMP-to-ATP ratio. In contrast to the altered I/R-induced activation pattern of AMPK in UCP3−/− hearts, phosphorylation of Thr308 of protein kinase B was similar in WT and UCP3−/− hearts.
Because mitochondria generate the majority of ROS in the cell, an excessive ROS production causes significant damage to cellular proteins, lipids, and nucleic acids. The levels of ROS generation at baseline and during I/R stress were measured in UCP3−/− hearts compared with WT. In keeping with prior reports of the effects of UCPs (3), the baseline ROS generation was found to be significantly higher in UCP3−/− hearts compared with WT hearts based on LDCL measurement (n = 6, Fig. 4C). In parallel to the O2− level, UCP3−/− hearts produced higher H2O2 as demonstrated by DCF fluorescence (54 ± 3 vs. 34 ± 4% of maximum, P < 0.05). Following 20 min of in vivo ischemia with LCA ligation, UCP3−/− hearts demonstrated a more pronounced production of ROS, O2− [0.336 ± 0.09 vs. 0.13 ± 0.06 relative light units (RLU), P < 0.05; Fig. 4C], and H2O2 (54 ± 3 vs. 34 ± 4% of maximum DCF fluorescence, P < 0.05) in myocardial tissue. Following 20 min of ischemia, UCP3−/− hearts demonstrated more pronounced H2O2 generation with increased DCF level (38 ± 2 vs. 23 ± 3% increase from baseline, P < 0.05) and O2− production with higher lucigenin luminescence (0.336 ± 0.09 vs. 0.13 ± 0.06 RLU, P < 0.05) in myocardial tissue. After 2 h of reperfusion, LDCL was still greater in UCP3−/− hearts (0.406 ± 0.12 vs. 0.2 ± 0.06 RLU, P < 0.05), but the increase in DCF florescence was comparable in UCP3−/− hearts and WT (32 ± 3 vs. 28 ± 3%, P > 0.05). Although the initial response to I/R included H2O2 and O2− burst, H2O2 was washed out during longer reperfusion, and O2− remained prominent. Thus, increased vulnerability to I/R stress in the UCP3−/− heart is associated with increased ROS generation.
Pharmacological uncoupling preserves LV contractile function in I/R.
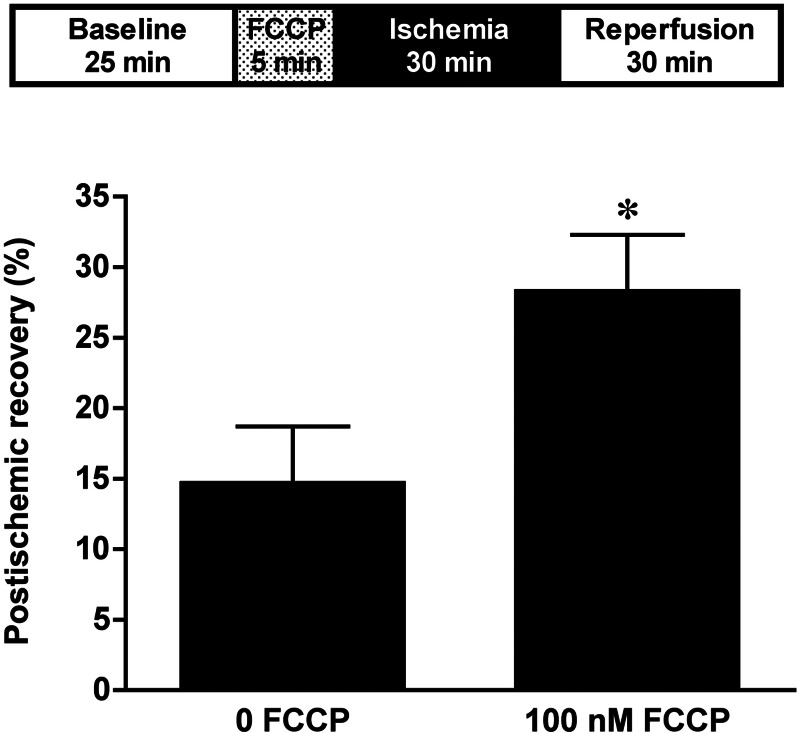
Previous studies have demonstrated that pharmacological uncoupling improves postischemic recovery of contractile function in WT hearts (30). The efficacy of the pharmacological mitochondrial uncoupler FCCP was used in isolated perfused UCP3−/− hearts to test our hypothesis that it is the uncoupling property of UCP3 that is important for its role in cardioprotection against I/R injury. Hearts were treated with 100 nM FCCP for 5 min, just before 30 min of no-flow global ischemia and 30 min of reperfusion (Fig. 5). FCCP treatment before I/R injury resulted in better LV functional recovery in UCP3−/− hearts compared with untreated hearts (28.3 ± 4 vs. 14.7 ± 4%, n = 5 in each group, P < 0.05). Thus pharmacological uncoupling with FCCP in the absence of UCP3 preserves LV contractile function in I/R injury by restoring uncoupling. This further supports the importance of mitochondrial uncoupling in tolerance to I/R injury.
Fig. 5.
Treatment with the pharmacological uncoupler carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone (FCCP; 100 nM) before I/R improves left ventricle (LV) functional recovery in UCP3−/− hearts compared with untreated hearts. *P < 0.05 compared with 0 FCCP.
UCP3 plays an active role in IPC.
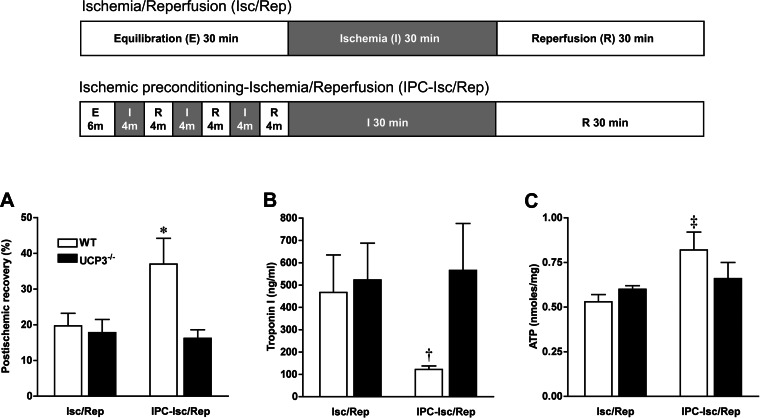
As defined above, the IPC protocol was applied ex vivo in isolated perfused heart to investigate the role of UCP3 in mechanisms of IPC. In WT hearts, myocardial contractile function was significantly improved by IPC when subjected to severe no-flow ischemia (no IPC: 19.7 ± 3.5%, IPC: 37.0 ± 7.2%, n = 6, P = 0.02; Fig. 6A), as it has been previously demonstrated by others (7). In contrast, there was no improvement in postischemic recovery of myocardial function with IPC in UCP3−/− hearts (no IPC: 17.8 ± 3.7%, IPC: 16.2 ± 2.4%, n = 6, P = 0.35). Thus, LV systolic function following IPC was significantly better in WT hearts compared with UCP3−/− hearts (P = 0.01; Fig. 6A). In parallel, there was significantly less troponin I released from WT hearts with IPC compared with UCP3−/− hearts (122 ± 16 vs. 566 ± 210 ng/ml, n = 6, P < 0.05; Fig. 6B). Myocardial ATP content was also greater with IPC in WT hearts compared with non-IPC (0.82 ± 0.1 vs. 0.53 ± 0.04 nmol/mg protein, n = 5–6, P = 0.01; Fig. 6C). In contrast, the amount of ATP in UCP3−/− hearts after I/R was not affected by IPC. Thus, the protective efficacy of IPC was abolished in UCP3−/− mice.
Fig. 6.
The role of UCP3 in ischemic preconditioning (IPC) was evaluated in the isolated working perfused heart subjected to severe ischemia with 3 cycles of 4 min of no-flow ischemia (Isc or I) followed with 4 min of reperfusion (Rep or R) before 30 min of ischemia and 30 min reperfusion (top). Postischemic contractile function was significantly improved by IPC in WT hearts, whereas there was no postischemic recovery of function with IPC in UCP3−/− hearts (A). In addition, there were less troponin I release (B) and greater ATP content in WT hearts with IPC compared with UCP3−/− hearts (C). Thus, IPC is abolished in UCP3−/− hearts. *P < 0.01 compared with I/R and UCP3−/−. †P < 0.05 compared with I/R and UCP3−/−. ‡P < 0.05 compared with I/R.
DISCUSSION
Mitochondrial ability to respond to oxidative and metabolic stress is critical for cardiomyocyte function and survival. This study demonstrates several novel findings regarding the vital role of mitochondrial UCP3 in effective mitochondrial responses to I/R stress and cardioprotection. Our results show that mitochondrial UCP3 plays an essential cardioprotective role in I/R injury by preserving myocardial contractility and limiting infarct size and myocardial necrosis in addition to decreasing the incidence of arrhythmias. Also, we demonstrate that the IPC phenomenon requires the presence of UCP3. The activation of UCP3 during I/R improves the cardiac energetic status by maintaining the high-energy phosphate content of the heart and modulating ROS generation. This establishes that the UCP3-regulated physiological H+ leak during oxidative stress is a cardioprotective phenomenon. We present the first direct evidence that UCP3 plays an essential role in the tolerance to I/R injury in the heart and plays a significant role in IPC.
Mitochondrial inner membrane functional and structural integrity is important for the prevention of detrimental I/R injury and postischemic recovery of contractile function. I/R leads to cardiac contractile and electrical dysfunction by altering mitochondrial bioenergetics, ionic homeostasis, and ROS generation, which are ultimately controlled by inner mitochondrial membrane proteins and electrochemical gradients. Thus, modulation of inner membrane function has a direct impact on cellular tolerance to oxidative or metabolic stress. As a mitochondrial integral inner membrane protein, UCP3 is responsible for this modulatory function.
Our findings demonstrate that activation of UCP3 is associated with myopreservation against I/R by maintaining myocardial energetic and reducing ROS generation. Uncoupling by endogenous UCP3 or a pharmacological agent is an essential component of cardioprotection during oxidative stress, since pharmacological uncoupling with FCCP demonstrated similar myopreservation in hearts lacking UCP3. This suggests that mild to moderate uncoupling in cardiomyocytes by UCP3 or FCCP prevents excessive ROS generation and therefore preserves ATP level. Accordingly, studies both in the brain and liver demonstrate that UCPs contribute to tissue protection against a variety of stressors that can increase ROS production (4, 12, 18). This indicates that UCPs are ultimate regulators of mitochondrial ROS production by modulating the inner mitochondrial membrane potential. UCPs have also been shown to protect against exogenous oxidant stress in cardiomyocytes (37).
In the present study, the direct role of the UCPs in cardioprotection was evaluated; however, the additional influence of free fatty acids on that function was not specifically determined. It is well established that free fatty acids can activate UCPs and mediate mitochondrial uncoupling independently (13, 16, 20, 38). During the stress of in vivo myocardial ischemia and infarction, circulating free fatty acid concentrations increase and could therefore potentially increase mitochondrial uncoupling, which could have a potential protective effect. Although the present ex vivo perfused heart studies were performed at relatively low concentrations of oleate, the in vivo studies would recapitulate the metabolic conditions present during myocardial ischemia and infarction in humans and demonstrate that, in the setting of increased free fatty acid concentrations, UCPs are necessary for myocardial protection. Although there may be increased uncoupling at higher free fatty acid concentrations, any beneficial effects of that coupling are likely offset by the deleterious effects of high free fatty acid concentrations on matching glycolysis and glucose oxidation, resulting in impaired postischemic functional recovery (25, 26).
While the present study demonstrates the importance of UCP3 in protecting against I/R injury in the mouse heart, UCP2 did not play a functionally significant role. Although prior studies have shown that UCP2 provides tissue- and cell-specific cytoprotection during ischemia, these studies have relied on overexpression of UCP2 to demonstrate the protective effects of this UCP (27, 36, 37). Some studies suggested that overexpression of UCPs may alter mitochondrial structure, resulting in changes in proton conductance that are independent of actual UCP function (11, 36). In addition, the absolute level of UCP2 transcript in the mouse heart is fivefold lower than UCP3 mRNA, supporting the notion that UCP3 plays a more significant role in cardioprotection in the mouse heart (2). Indeed, UCPs are tissue- and species-specific proteins with different functional importance in rat and mice heart tissue (2). As a result, our findings do not support the results of a study that showed that chronic hypobaric hypoxia for 7 days in rat heart was found to be associated with a reduction in left ventricular UCP3 gene expression (15). Although species and experimental settings are different in both studies, UCP3 is selectively more prominent in mouse heart, but UCP2 has more functional importance in rat heart.
Arrhythmias are one of the major determinants of survival during acute or chronic ischemic myocardial insult. Here we demonstrate that UCP3 expression is required to prevent I/R arrhythmias in addition to preventing myocyte necrosis and contractile dysfunction. This suggests the potential role of mitochondria-driven cardiomyocyte energetics and metabolism in the genesis of arrhythmias. Recently, it has been shown that the stability of the inner mitochondrial membrane potential in the postischemic heart is critical for restoration of electrical activity and suppression of reperfusion arrhythmias (1). Our finding supports this concept by demonstrating that UCP3-mediated mitochondrial inner membrane stability, which is also reflected by the ROS and ATP generation, is important for prevention of I/R arrhythmias. Also, impaired impulse formation with the blunted heart rate response to I/R stress in UCP3−/− heart is likely mediated with vagal stimulation and disrupted mitochondrial function (21). Probably, the higher incidence of I/R arrhythmias in UCP3−/− hearts is associated with alterations in the inner mitochondrial membrane potential, increased ROS generation, impaired mitochondrial energetics, and enhanced myocyte damage. Thus modulation of the electrochemical gradient in I/R by altering UCP expression or function may have important implications for the prevention of postischemic arrhythmias.
It is known that mitochondria play a major role in IPC (7, 8, 30, 32). However, the exact mitochondrial component and the mechanisms of the IPC are still controversial. In this study, we demonstrate that endogenous UCP3 is an important regulator of IPC, since preconditioning was completely abolished in UCP3−/− hearts. Maintenance of cellular high-energy phosphate stores and modulation of ROS generation by UCP3 during I/R injury are essential for the IPC phenomenon. Thus, UCP3 is an essential mitochondrial component of the mechanism of IPC.
In conclusion, UCP3 plays a critical role in cardioprotection during oxidative stress by preventing myocardial necrosis, contractile dysfunction, and arrhythmias during I/R injury while maintaining myocardial high-energy phosphates and suppressing detrimental ROS generation. UCP3 functions as a modulator of oxidative or metabolic stress in the heart. Stabilization of the mitochondrial inner membrane function by UCP3 may have important implications for myopreservation in the setting of I/R injury. Based on these findings, overexpression of UCP3 or pharmacological uncoupling may represent novel therapeutic strategies for cardioprotection against I/R injury.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01-HL-077310 (to R. R. Russell III) and R01-HL-080176 (to K. S. Russell).
DISCLOSURES
Authors have no conflict of interest, financial or otherwise, relevant to this study.
AUTHOR CONTRIBUTIONS
Author contributions: C.O. and R.R.R. conception and design of research; C.O. and M.P. performed experiments; C.O., M.P., and K.S.R. analyzed data; C.O. and R.R.R. interpreted results of experiments; C.O. and R.R.R. prepared figures; C.O. drafted manuscript; C.O., T.L.H., K.S.R., and R.R.R. edited and revised manuscript; C.O., M.P., T.L.H., K.S.R., and R.R.R. approved final version of manuscript.
ACKNOWLEDGMENTS
The present address of C. Ozcan: Div. of Cardiovascular Medicine, University at Buffalo School of Medicine, Buffalo, NY.
REFERENCES
- 1. Akar FG, Aon MA, Tomaselli GF, O'Rourke B. The mitochondrial origin of postischemic arrhythmias. J Clin Invest 115: 3527–3535, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alan L, Smolkova K, Kronusova E, Santorova J, Jezek P. Absolute levels of transcripts for mitochondrial uncoupling proteins UCP2, UCP3, UCP4, and UCP5 show different patterns in rat and mice tissues. J Bioenerg Biomembr 41: 71–78, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, Couplan E, Alves-Guerra MC, Goubern M, Surwit R, Bouillaud F, Richard D, Collins S, Ricquier D. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Gen 26: 435–439, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Bechmann I, Diano S, Warden CH, Bartfai T, Nitsch R, Horvath TL. Brain mitochondrial uncoupling protein 2 (UCP2): a protective stress signal in neuronal injury. Biochem Pharmacol 64: 363–367, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Bienengraeber M, Ozcan C, Terzic A. Stable transfection of UCP1 confers resistance to hypoxia/reoxygenation in a heart-derived cell line. J Mol Cell Cardiol 35: 861–865, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Bolli R, Becker L, Gross G, Mentzer R, Jr, Balshaw D, Lathrop DA. Myocardial protection at a crossroads: the need for translation into clinical therapy. Circ Res 95: 125–134, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Bolli R. Preconditioning: a paradigm shift in the biology of myocardial ischemia. Am J Physiol Heart Circ Physiol 292: H19–H27, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brennan JP, Southworth R, Medina RA, Davidson SM, Duchen MR, Shattock MJ. Mitochondrial uncoupling, with low concentration FCCP, induces ROS-dependent cardioprotection independent of KATP channel activation. Cardiovasc Res 72: 313–321, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Bugger H, Guzman C, Zechner C, Palmeri M, Russell KS, Russell RR. Uncoupling protein downregulation in doxorubicin-induced heart failure improves mitochondrial coupling but increases reactive oxygen species generation. Cancer Chemother Pharmacol 67: 1381–1388, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buroker NE, Young ME, Wei C, Serikawa K, Ge M, Ning XH, Portman MA. The dominant negative thyroid hormone receptor β-mutant Δ337T alters PPARα signaling in heart. Am J Physiol Endocrinol Metab 292: E453–E460, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Cadenas S, Buckingham J, Samec S, Seydoux J, Din N, Dulloo A, Brand MD. UCP2 and UCP3 rise in starved rat skeletal muscle but mitochondrial proton conductance is unchanged. FEBS Lett 462: 257–260, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Diano S, Matthews RT, Patrylo P, Yang L, Beal MF, Barnstable CJ, Horvath TL. Uncoupling protein 2 prevents neuronal death including that occurring during seizures: a mechanism for pre-conditioning. Endocrinology 144: 5014–5021, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Di Paola M, Lorusso M. Interaction of free fatty acids with mitochondria: Coupling, uncoupling and permeability transition. Biochim Biophys Acta 1757: 1330–1337, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Du G, Mouithys-Mickalad A, Sluse FE. Generation of superoxide anion by mitochondria and impairment of their functions during anoxia and reoxygenation in vitro. Free Radic Biol Med 25: 1066–1074, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Essop MF, Razeghi P, McLeod C, Young ME, Taegtmeyer H, Sack MN. Hypoxia-induced decrease of UCP3 gene expression in rat heart parallels metabolic gene switching but fails to affect mitochondrial respiratory coupling. Biochem Biophys Res Commun 314: 561–564, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Garlid KD, Orosz DE, Modriansky M, Vassanelli S, Jezek P. On the mechanism of fatty acid-induced proton transport by mitochondrial uncoupling protein. J Biol Chem 271: 2615–2620, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Hoerter J, Gonzalez-Barroso Md-M, Couplan E, Mateo P, Gelly C, Cassard-Doulcier AM, Diolez P, Bouillaud F. Mitochondrial uncoupling protein 1 expressed in the heart of transgenic mice protects against ischemic-reperfusion damage. Circulation 110: 528–533, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Horimoto M, Fulop P, Derdak Z, Wands JR, Baffy G. Uncoupling protein-2 deficiency promotes oxidant stress and delays liver regeneration in mice. Hepatology 39: 386–392, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Horvath TL, Diano S, Miyamoto S, Barry S, Gatti S, Alberati D, Livak F, Lombardi A, Moreno M, Goglia F, Mor G, Hamilton J, Kachinskas D, Horwitz B, Warden CH. Uncoupling proteins-2 and 3 influence obesity and inflammation in transgenic mice. Int J Obes Relat Metab Disord 27: 433–442, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Jezek P, Engstova H, Zackova M, Vercesi AE, Costa AD, Arruda P, Garlid KD. Fatty acid cycling mechanism and mitochondrial uncoupling proteins. Biochim Biophys Acta 1365: 319–327, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Katare RG, Ando M, Kakinuma Y, Arikawa M, Handa T, Yamasaki F, Sato T. Vagal nerve stimulation prevents reperfusion injury through inhibition of opening of mitochondrial permeability transition pore independent of the bradycardiac effect. J Thorac Cardiovasc Surg 137: 223–231, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Kato K, Yin H, Agata J, Yoshida H, Chao L, Chao J. Adrenomedullin gene delivery attenuates myocardial infarction and apoptosis after ischemia and reperfusion. Am J Physiol Heart Circ Physiol 285: H1506–H1514, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Krauss S, Zhang CY, Lowell BB. The mitochondrial uncoupling-protein homologues. Nat Rev Mol Cell Biol 6: 248–261, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Li Y, Zhu H, Kuppusamy P, Roubaud V, Zweier JL, Trush MA. Validation of lucigenin (bis-N-methylacridinium) as a chemilumigenic probe for detecting superoxide anion radical production by enzymatic and cellular systems. J Biol Chem 273: 2015–2023, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Lopaschuk G, Saddik M, Barr R, Huang L, Barker C, Muzyka R. Effects of high levels of fatty acids on functional recovery of ischemic hearts from diabetic rats. Am J Physiol Endocrinol Metab 263: E1046–E1053, 1992 [DOI] [PubMed] [Google Scholar]
- 26. Lopaschuk G, Wambolt R, Barr R. An imbalance between glycolysis and glucose oxidation is a possible explanation for the detrimental effects of high levels of fatty acids during aerobic reperfusion of ischemic hearts. J Pharmacol Exp Ther 264: 135–144, 1993 [PubMed] [Google Scholar]
- 27. McLeod CJ, Aziz A, Hoyt RF, Jr, McCoy JP, Jr, Sack MN. Uncoupling proteins 2 and 3 function in concert to augment tolerance to cardiac ischemia. J Biol Chem 280: 33470–33476, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Miller EJ, Li J, Leng L, McDonald C, Atsumi T, Bucala R, Young LH. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature 451: 578–582, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Mills EM, Banks ML, Sprague JE, Finkel T. Pharmacology: uncoupling the agony from ecstasy. Nature 426: 403–404, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Minners J, van den Bos EJ, Yellon DM, Schwalb H, Opie LH, Sack MN. Dinitrophenol, cyclosporin A, and trimetazidine modulate preconditioning in the isolated rat heart: support for a mitochondrial role in cardioprotection. Cardiovasc Res 47: 68–73, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Murray AJ, Cole MA, Lygate CA, Carr CA, Stuckey DJ, Little SE, Neubauer S, Clarke K. Increased mitochondrial uncoupling proteins, respiratory uncoupling and decreased efficiency in the chronically infarcted rat heart. J Mol Cell Cardiol 44: 694–700, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Nadtochiy SM, Tompkins AJ, Brookes PS. Different mechanisms of mitochondrial proton leak in ischaemia/reperfusion injury and preconditioning: implications for pathology and cardioprotection. Biochem J 395: 611–618, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ozcan C, Bienengraeber M, Dzeja PP, Terzic A. Potassium channel openers protect cardiac mitochondria by attenuating oxidant stress at reoxygenation. Am J Physiol Heart Circ Physiol 282: H531–H539, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Razeghi P, Young ME, Ying J, Depre C, Uray IP, Kolesar J, Shipley GL, Moravec CS, Davies PJ, Frazier OH, Taegtmeyer H. Downregulation of metabolic gene expression in failing human heart before and after mechanical unloading. Cardiology 97: 203–209, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Russell RR, III, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest 114: 495–503, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sack MN. Mitochondrial depolarization and the role of uncoupling proteins in ischemia tolerance. Cardiovasc Res 72: 210–219, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Teshima Y, Akao M, Jones SP, Marban E. Uncoupling protein-2 overexpression inhibits mitochondrial death pathway in cardiomyocytes. Circ Res 93: 192–200, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Winkler E, Klingenberg M. Effect of fatty acids on H+ transport activity of the reconstituted uncoupling protein. J Biol Chem 269: 2508–2515, 1994 [PubMed] [Google Scholar]
- 39. Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 357: 1121–1135, 2007 [DOI] [PubMed] [Google Scholar]