Abstract
Endothelial nitric oxide synthase (eNOS) was assumed to be the only source of nitric oxide (NO) involved in the regulation of human coronary blood flow (CBF). However, our recent first-in-human study using the neuronal NOS (nNOS)-selective inhibitor S-methyl-L-thiocitrulline (SMTC) showed that nNOS-derived NO also plays a role. In this study, we investigated the relative contribution of nNOS and eNOS to the CBF response to a pacing-induced increase in cardiac workload. Incremental right atrial pacing was undertaken in patients with angiographically normal coronary arteries during intracoronary infusion of saline vehicle and then either SMTC or NG-monomethyl-l-arginine (l-NMMA; which inhibits both eNOS and nNOS). Intracoronary SMTC (0.625 μmol/min) and l-NMMA (25 μmol/min) reduced basal CBF to a similar extent (−19.2 ± 3.2% and 25.0 ± 2.7%, respectively; n = 10 per group). Pacing-induced increases in CBF were significantly blunted by l-NMMA (maximum CBF: 83.5 ± 14.2 ml/min during saline vs. 61.6 ± 9.5 ml/min during l-NMMA; P < 0.01). By contrast, intracoronary SMTC had no effect on the maximum CBF during pacing (98.5 ± 12.9 ml/min during saline vs. 102.1 ± 16.6 ml/min during SMTC; P = not significant). l-NMMA also blunted the pacing-induced increase in coronary artery diameter (P < 0.001 vs. saline), whereas SMTC had no effect. Our results confirm a role of nNOS in the regulation of basal CBF in humans but show that coronary vasodilation in response to a pacing-induced increase in cardiac workload is exclusively mediated by eNOS-derived NO.
Keywords: coronary blood flow, endothelial nitric oxide synthase, neuronal nitric oxide synthase, pacing, vascular
changes in coronary blood flow (CBF) are fundamentally important for matching myocardial perfusion to increases in cardiac workload in response to physiological stresses, such as exercise. Dynamic alterations in coronary microvascular tone and resistance are the primary driver for such changes in CBF and are dependent upon the local concentration of vasoactive mediators such as adenosine, prostaglandins, nitric oxide (NO), and endothelium-derived hyperpolarizing factors (EDHFs) as well as neural inputs (7, 11, 18). NO is well known to play an important role in the regulation of CBF both under basal conditions and in response to shear stress or agonist stimulation (19).
In the physiological setting, NO may be synthesized by endothelial or neuronal NO synthase (eNOS and nNOS, respectively) (19). The local regulation of vascular tone by NO has been considered to be largely dependent on the activity of endothelial eNOS. However, recent first-in-human data indicate that NO derived from nNOS also has an important physiological role in the regulation of peripheral and CBF (25, 26). Using a local infusion of the nNOS-selective inhibitor, S-methyl-l-thiocitrulline (SMTC), we found that basal flow was significantly reduced both in the forearm and coronary circulations in the absence of changes in the eNOS-mediated vasodilatation to shear stress, ACh, or substance P (25, 26). Local infusion of SMTC also inhibited the increase in forearm blood flow induced by mental stress (26), suggesting that nNOS-derived NO may also be involved in mediating changes in flow in response to some physiological stressors. These findings concur with evidence from animal studies in which nNOS-derived NO has been found to regulate blood flow in several vascular beds (5, 14, 27, 29).
A local release of NO (along with that of other paracrine mediators) has been shown to contribute significantly to the increase in myocardial blood flow that occurs in response to exercise or other settings of increased metabolic demand such as pacing (2, 3, 9, 15, 22, 28). However, the relative contribution of constitutive NOS isoforms to the CBF response to an increase in cardiac workload is unknown. The aim of the present study was to define the role of eNOS and nNOS in mediating the increase in CBF in response to incremental atrial pacing in subjects undergoing cardiac catheterization.
METHODS
The study conformed to the standards set by the latest revision of the Declaration of Helsinki and was approved by the local Research Ethics Committee. All participants provided written informed consent. Twenty patients (11 male, 9 female; mean age 57 ± 3.2 years) undergoing diagnostic cardiac catheterization for atypical chest pain, who had angiographically smooth and unobstructed coronary arteries, were included in the study. Subjects with valvular heart disease, left ventricular hypertrophy, reduced ejection fraction, or significant renal, hepatic, or inflammatory disease were excluded. Studies were performed in the morning after an overnight fast. Any vasoactive drugs were discontinued, and subjects refrained from alcohol, caffeinated drinks, and smoking for ≥24 h before the study.
Standard diagnostic coronary angiography was performed via the right femoral arterial in a quiet, temperature-controlled cardiac catheterization laboratory with digital cineangiography. After completion of the diagnostic procedure and confirmation of angiographically normal arteries, a temporary pacing lead was positioned in the right atrium via the femoral venous approach. A 0.014-inch intracoronary Doppler wire (FloWire; Volcano Therapeutics, Rancho Cordova, CA) was advanced through a 6F guiding catheter into a straight, nonoverlapping and side-branch free segment of the proximal left coronary artery (either the left anterior descending or circumflex artery, as detailed in Table 1). The Doppler wire was interfaced with a real-time spectral analysis system (ComboMap Pressure and Flow system; Volcano Therapeutics) to derive continuous Doppler traces and corresponding average peak velocity (APV) values. Contrast angiographic images were obtained with the study artery positioned at the isocenter without altering the angle of projection during the study. Changes in epicardial coronary artery diameter were measured by using an automated quantitative coronary angiography (QCA) edge detection system (Philips), in a 2.5–5 mm length segment of vessel ∼2.5 mm distal to the tip of the Doppler wire. The ECG was continuously monitored. Intracoronary infusions of SMTC (0.0625 μmol/min), NG-monomethyl-l-arginine (l-NMMA; 25 μmol/min), or saline vehicle were administered via the guiding catheter at a rate of 2 ml/min (25). These doses of SMTC and l-NMMA were previously shown to inhibit either nNOS alone or both nNOS and eNOS, respectively (25). SMTC was obtained from Calbiochem and prepared to standards suitable for human use in a nationally accredited pharmaceutical manufacturing facility, as previously described (26). l-NMMA was purchased from Bachem (Switzerland).
Table 1.
Baseline characteristics of patients
| S-methyl-l-thiocitrulline | NG-monomethyl-l-arginine | P | |
|---|---|---|---|
| Number | 10 | 10 | |
| Age, years | 58.7 ± 2.9 | 57.1 ± 3.7 | 0.73 |
| Male/female, n | 6/4 | 5/5 | |
| Cardiovascular risk factors, n | |||
| Smoker | 4 | 4 | |
| Hypertension | 5 | 3 | |
| Diabetes mellitus | 4 | 2 | |
| Hypercholesterolemia | 3 | 7 | |
| Family history | 1 | 1 | |
| Blood pressure, mmHg | |||
| Systolic | 128 ± 7.9 | 139 ± 7.8 | 0.30 |
| Diastolic | 77 ± 4.1 | 74 ± 2.3 | 0.79 |
| Study artery, n | |||
| Left anterior descending | 5 | 6 | |
| Left circumflex | 5 | 4 |
Values are means ± SE.
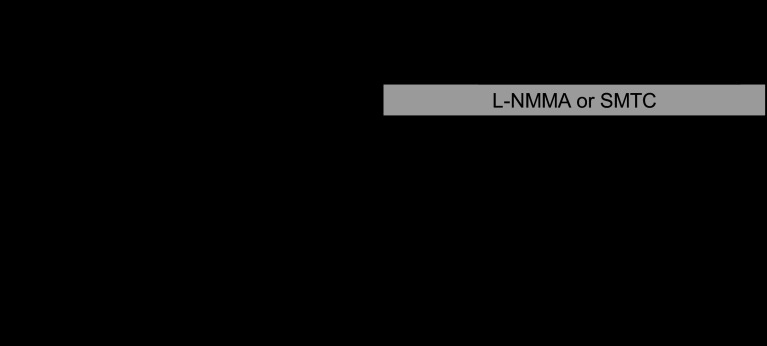
The study protocol is summarized in Figure 1. All subjects underwent two periods of incremental right atrial pacing separated by a 10-min interval. The pacing rate was increased by 20 beats/min every 2 min up to a maximum heart rate of 150 beats/min or the occurrence of rate-related temporary atrio-ventricular block (6, 23). The first pacing protocol was performed during intracoronary saline vehicle infusion and the second during either intracoronary SMTC or l-NMMA infusion (1 inhibitor per patient). The NOS inhibitors were infused for at least 7 min before commencing pacing. The APV, epicardial coronary artery diameter, heart rate, and aortic pressure were measured at baseline and after each increase in heart rate. To avoid hyperemic effects of angiographic contrast influencing CBF calculations, APV recordings at each time-point were always taken before coronary angiography and the protocol progressed to the next stage only after the APV value had returned to its precontrast value.
Fig. 1.
Schematic diagram of the protocol. Incremental pacing was performed up to 150 beats/min, with measurements of average peak velocity (APV), blood pressure, ECG, and coronary angiography. SMTC, S-methyl-L-thiocitrulline; l-NMMA, NG-monomethyl-l-arginine.
Data analysis and statistics.
All data were recorded digitally and analyzed offline in a blinded fashion at the end of the study. CBF was calculated, as previously described (8), as the product of APV and the QCA-derived coronary artery diameter (1/2 × APV × coronary cross-sectional area). The cardiac workload was calculated as mean aortic pressure × heart rate. Statistical comparisons were made by using a repeated-measures ANOVA or a one-way ANOVA, as appropriate. All tests were two-tailed, and differences were considered significant when P < 0.05. Data are shown as means ± SE.
RESULTS
The baseline characteristics of the study subjects did not differ between those randomized to receive SMTC or l-NMMA (Table 1). Neither intracoronary SMTC nor l-NMMA caused any change in heart rate or systemic blood pressure, as reported previously (25, 26). The blood pressure was 98.2 ± 4.5 mmHg before and 103.6 ± 5.9 mmHg after l-NMMA and was 94.4 ± 5.5 mmHg before and 106.6 ± 8.1 mmHg after SMTC. None of the subjects developed adverse reactions, symptoms of ischemia, or changes in the surface ECG during either infusion.
Effects of l-NMMA and SMTC on basal CBF and coronary diameter.
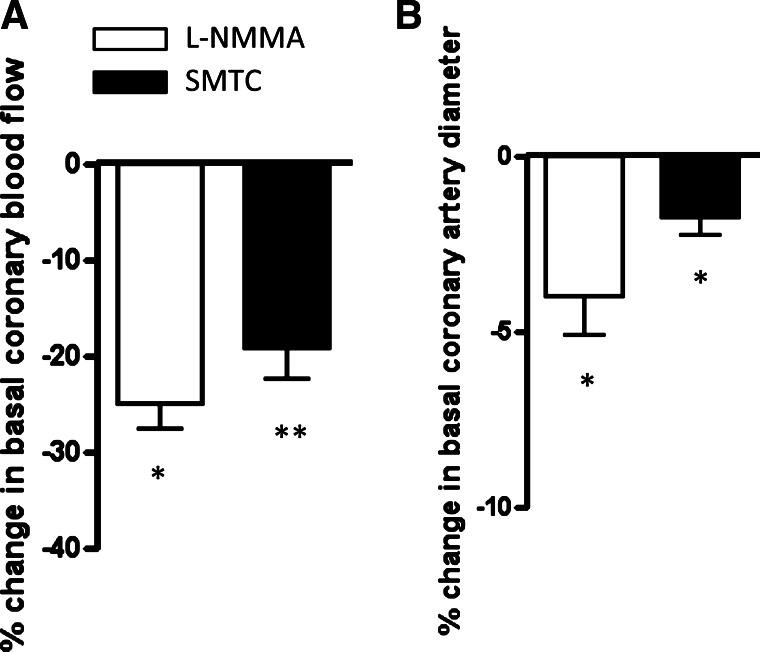
Intracoronary infusion of either l-NMMA (25 μmol/min) or STMC (0.625 μmol/min) reduced basal CBF to a similar extent (Fig. 2A). Likewise, l-NMMA or SMTC infusion both caused a similar reduction in basal coronary artery diameter (Fig. 2B).
Fig. 2.
Effect of SMTC and l-NMMA on basal coronary flow and epicardial artery diameter. A: percent change ± SE in basal coronary blood flow after SMTC (n = 10) or l-NMMA (n = 10). B: percent change ± SE in epicardial artery diameter. There was no significant difference between responses to SMTC and l-NMMA. *P < 0.01 and **P < 0.001 for l-NMMA or SMTC vs. baseline.
Effects of l-NMMA and SMTC on response to incremental pacing.
As expected, incremental pacing during saline vehicle led to an increase in CBF in all subjects (Figs. 3 and 4). During l-NMMA infusion, the maximal pacing-induced increase in CBF was significantly blunted (an increase from 56.8 ± 9.27 to 83.5 ± 14.2 ml/min during saline compared with an increase from 45.5 ± 6.76 to 61.6 ± 9.49 ml/min during l-NMMA; P < 0.05 by 2-way ANOVA). By contrast, during STMC infusion, there was no reduction in the pacing-induced increase in CBF compared with saline vehicle (an increase from 67.2 ± 8.98 to 98.5 ± 12.87 ml/min during saline infusion vs. an increase from 54.7 ± 7.03 to 102.1 ± 16.57 ml/min during SMTC; P = not significant by 2-way ANOVA; Fig. 4C). The results were similar when CBF was related to cardiac workload instead of heart rate (Fig. 4, B and D). We also looked at the effects of l-NMMA or SMTC on coronary vascular resistance during pacing. Coronary resistance fell significantly with pacing and at peak pacing was significantly higher with l-NMMA compared with saline (1.77 ± 0.25 vs. 1.44 ± 0.32 mmHg/ml/min; P < 0.01). However, in the presence of SMTC, the coronary resistance at peak pacing was similar to that during saline (1.02 ± 0.05 vs. 1.14 ± 0.17 mmHg·ml−1·min−1; P = not significant).
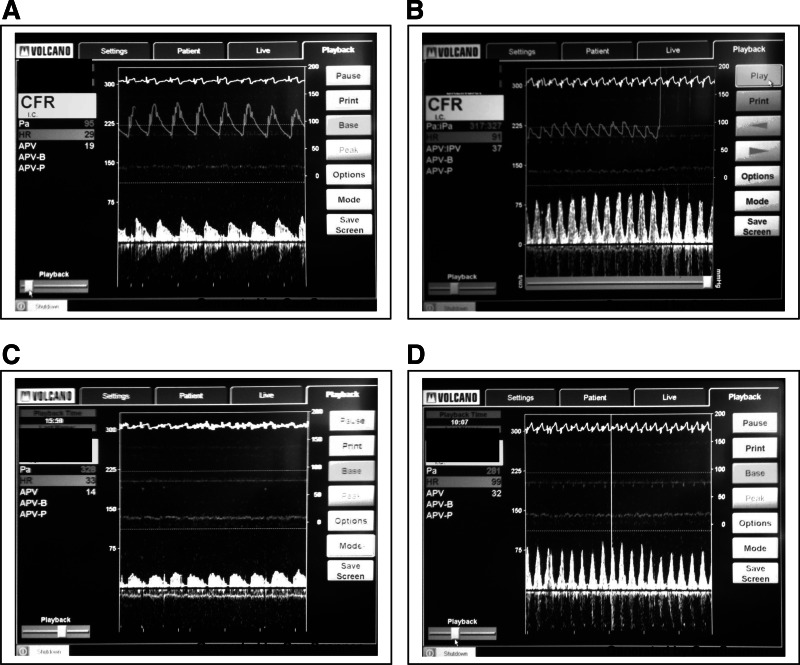
Fig. 3.
Representative coronary Doppler spectral traces of APV. APV at baseline heart rate during saline vehicle (A), at peak heart rate during saline vehicle (B), at baseline heart rate during l-NMMA (C), and at peak heart rate during l-NMMA (D) is shown.
Fig. 4.
Effects of l-NMMA and SMTC on pacing-induced changes in coronary blood flow (CBF). A and B: response to l-NMMA with CBF plotted against heart rate and cardiac workload, respectively. C and D: response to SMTC with CBF plotted against heart rate and cardiac workload, respectively. Data at baseline and peak pacing are shown. *P < 0.05 for comparison of peak vs. basal CBF; †P < 0.05 for significant interaction between groups by 2-way ANOVA. Bpm, beats/min.
Incremental atrial pacing significantly increased coronary arterial diameter during saline infusion (Fig. 5). This response was significantly blunted during intracoronary infusion of l-NMMA (Fig. 5, A and B), whereas SMTC infusion had no significant effect (Fig. 5, C and D).
Fig. 5.
Effect of l-NMMA and SMTC on coronary artery diameter during incremental pacing. A and B: response to l-NMMA with coronary diameter plotted against heart rate and cardiac workload, respectively. C and D: response to SMTC with coronary diameter plotted against heart rate and cardiac workload, respectively. Data at baseline and peak pacing are shown. *P < 0.05 for comparison of coronary diameter at peak pacing vs. baseline; †P < 0.01 for significant interaction between groups by 2-way ANOVA; **P < 0.01. Bpm, beats/min.
DISCUSSION
Continuous short-term adjustments in CBF are fundamentally important to ensure a close match between myocardial tissue perfusion and cardiac workload (7). NO is known to be one of a number of paracrine mediators (including adenosine, prostaglandins, and EDHFs) that, in conjunction with autonomic inputs, determines optimal CBF. However, the relative contribution of different NOS isoforms to the regulation of epicardial and microvascular tone, and thus blood flow, in response to changes in metabolic demand remains unclear. Our results indicate that the change in CBF that occurs in response to increased cardiac workload, as induced through incremental cardiac pacing, is mediated by eNOS rather than nNOS even though nNOS has a significant effect on basal CBF.
Previous work has suggested that NO plays an important role in the regulation of metabolically induced vasodilatation in both the peripheral and coronary circulation, but the evidence remains conflicting and incomplete. In animal models, selective inhibition of nNOS or nNOS gene deletion attenuates exercise-induced arteriolar vasodilatation and increase in blood flow in the skeletal muscle (10, 13, 16, 27). In nNOS knockout mice and in the mdx mouse, an animal model of Duchenne Muscular Dystrophy where dystrophin deficiency results in greatly reduced nNOS expression in skeletal muscle, the ability of muscle contraction to attenuate α-adrenergic vasoconstriction has been shown to be defective and lead to abnormal flow regulation (27). nNOS knockout mice show enhanced fatigue after mild exercise, which has been attributed to impaired muscle perfusion during exercise (16). However, similar results have not been replicated in humans. NO is also implicated in the regulation of coronary vascular tone in response to exercise-induced increases in cardiac workload (2, 15, 17). In humans, Quyyumi and colleagues (22) demonstrated that nonselective inhibition of NOS activity results in a significant reduction in both microvascular and epicardial vasodilatation during incremental cardiac pacing. In addition, atherosclerosis, which is known to reduce the local bioavailability of NO, blunts CBF and/or epicardial dilatation in response to other stimuli, such as bicycle exercise, cold pressor test, and pacing (12, 20, 21, 28, 30). In the current study, we specifically addressed the relative contribution of eNOS- and nNOS-derived NO to pacing-induced increases in CBF using an nNOS-specific inhibitor (SMTC) and a nonisoform selective inhibitor, l-NMMA. The responses to these two agents are consistent with a model in which eNOS-derived NO is the main contributor to the pacing-induced increase in CBF, whereas nNOS-derived NO has no effect in this setting even though it increases basal blood flow. eNOS may facilitate increases in CBF both through a reduction in microvascular resistance and an increase in epicardial coronary artery diameter, as observed in this study, and at least part of the mechanism may involve shear stress-induced increases in flow. The latter response is known to be mediated through endothelial eNOS (19). On the other hand, the effect of nNOS on basal CBF could involve NO release from perivascular nerves (24), although we have no direct evidence to support this idea. It should be noted that pacing-induced change in CBF is not necessarily a pure model of increased workload and may also be influenced by other factors such as cardiac mechanics and the duration of diastole. Nevertheless, it is an objective and easy-to-implement intervention in the invasive setting of a clinical cardiac catheterization laboratory.
An interesting observation is that NOS inhibition significantly attenuates but does not abolish changes in CBF. This is in keeping with the knowledge that pathways controlling metabolic regulation of blood flow have significant redundancy through interdependence on several factors, such as NO, adenosine, prostanoids, and EDHFs (7, 9, 11). Such redundancy may also account for the conflicting data on the influence of NO on vasodilation in exercising skeletal muscle where factors other than NO might have a greater role. It is of interest that not only did SMTC fail to reduce the pacing-induced increase in CBF but that the increase tended to be even greater in the presence of the inhibitor, suggesting a possible interaction between basal nNOS-dependent effects and other pathways involved in vascular regulation.
The increase in CBF during exercise is not only driven by the increase in heart rate but by other factors, such as autonomic and neurohumoral inputs. Whereas this study only examined the contributions of nNOS and eNOS to the tachycardia-dependent increase in CBF, our previous work showed that forearm vasodilatation induced by mental stress is significantly attenuated by selective nNOS inhibition, suggesting a possible role for local nNOS-derived NO in regulation of microvascular tone through modulation of autonomic inputs (26). Consistent with this possibility, nNOS has been found to be expressed in perivascular autonomic fibers as well as in the vessel wall (1, 4, 24). Our data, therefore, do not exclude that nNOS-derived NO (released by perivascular nerves) may also play a role in the increase in CBF that occurs during physiological exercise, and this needs to be examined in future studies. Experimental studies have suggested the presence of an inverse functional association between the expression of constitutive NOS isoforms in the vasculature whereby a reduction in eNOS expression can be partly compensated by an increase in nNOS activity (4). nNOS-derived NO might, therefore, play a greater role in the CBF response to increased metabolic demand in the setting of endothelial dysfunction.
In conclusion, this study provides the first direct evidence that increases in CBF in response to pacing-induced changes in cardiac workload in humans are mediated by eNOS rather than nNOS-derived NO and confirms that the latter plays an important role in setting basal coronary resistance.
GRANTS
This work was supported by supported by the British Heart Foundation (RE/08/003); a Foundation Leducq Transatlantic Network of Excellence Award; the Department of Health via a National Institute for Health Research Biomedical Research Center award to Guy's & St Thomas′ NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust; and an Academy of Medical Sciences award (N. Melikian).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.S., N.M., R.D., B.C., P.C., and A.M.S. contributed to the conception and design of research; H.S., N.M., R.D., and A.M.S. performed experiments; H.S., N.M., R.D., B.C., P.C., and A.M.S. analyzed data; H.S., N.M., R.D., B.C., P.C., and A.M.S. interpreted results of experiments; H.S. and A.M.S. prepared figures; H.S., N.M., R.D., B.C., P.C., and A.M.S. drafted the manuscript; H.S., N.M., R.D., B.C., P.C., and A.M.S. edited and revised the manuscript; H.S., N.M., R.D., B.C., P.C., and A.M.S. approved the final version of the manuscript.
REFERENCES
- 1. Bachetti T, Comini L, Curello S, Bastianon D, Palmieri M, Bresciani G, Callea F, Ferrari R. Co-expression and modulation of neuronal and endothelial nitric oxide synthase in human endothelial cells. J Mol Cell Cardiol 37: 939–945, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Berdeaux A, Ghaleh B, Dubois-Rande JL, Vigue B, Drieu La Rochelle C, Hittinger L, Giudicelli JF. Role of vascular endothelium in exercise-induced dilation of large epicardial coronary arteries in conscious dogs. Circulation 89: 2799–2808, 1994 [DOI] [PubMed] [Google Scholar]
- 3. Bernstein RD, Ochoa FY, Xu X, Forfia P, Shen W, Thompson CI, Hintze TH. Function and production of nitric oxide in the coronary circulation of the conscious dog during exercise. Circ Res 79: 840–848, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Boulanger CM, Heymes C, Benessiano J, Geske RS, Levy BI, Vanhoutte PM. Neuronal nitric oxide synthase is expressed in rat vascular smooth muscle cells: activation by angiotensin II in hypertension. Circ Res 83: 1271–1278, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Chi OZ, Liu X, Weiss HR. Effects of inhibition of neuronal nitric oxide synthase on NMDA-induced changes in cerebral blood flow and oxygen consumption. Exp Brain Res 148: 256–260, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Cotton JM, Kearney MT, MacCarthy PA, Grocott-Mason RM, McClean DR, Heymes C, Richardson PJ, Shah AM. Effects of nitric oxide synthase inhibition on basal function and the force-frequency relationship in the normal and failing human heart in vivo. Circulation 104: 2318–2323, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Deussen A, Ohanyan V, Jannasch A, Yin L, Chilian W. Mechanisms of metabolic coronary flow regulation. J Mol Cell Cardiol 52: 794–801, 2012 [DOI] [PubMed] [Google Scholar]
- 8. Doucette JW, Corl PD, Payne HM, Flynn AE, Goto M, Nassi M, Segal J. Validation of a Doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation 85: 1899–1911, 1992 [DOI] [PubMed] [Google Scholar]
- 9. Duffy SJ, Castle SF, Harper RW, Meredith IT. Contribution of vasodilator prostanoids and nitric oxide to resting flow, metabolic vasodilation, and flow-mediated dilation in human coronary circulation. Circulation 100: 1951–1957, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Fadel PJ, Zhao W, Thomas GD. Impaired vasomodulation is associated with reduced neuronal nitric oxide synthase in skeletal muscle of ovariectomized rats. J Physiol 549: 243–253, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garland CJ, Hiley CR, Dora KA. EDHF: spreading the influence of the endothelium. Br J Pharmacol 164: 839–852, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gordon JB, Ganz P, Nabel EG, Fish RD, Zebede J, Mudge GH, Alexander RW, Selwyn AP. Atherosclerosis influences the vasomotor response of epicardial coronary arteries to exercise. J Clin Invest 83: 1946–1952, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grange RW, Isotani E, Lau KS, Kamm KE, Huang PL, Stull JT. Nitric oxide contributes to vascular smooth muscle relaxation in contracting fast-twitch muscles. Physiol Genomics 5: 35–44, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Ichihara A, Inscho EW, Imig JD, Navar LG. Neuronal nitric oxide synthase modulates rat renal microvascular function. Am J Physiol Renal Physiol 274: F516–F524, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Jones CJ, Kuo L, Davis MJ, DeFily DV, Chilian WM. Role of nitric oxide in the coronary microvascular responses to adenosine and increased metabolic demand. Circulation 91: 1807–1813, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Kobayashi YM, Rader EP, Crawford RW, Iyengar NK, Thedens DR, Faulkner JA, Parikh SV, Weiss RM, Chamberlain JS, Moore SA, Campbell KP. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature 456: 511–515, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kuo L, Chilian WM, Davis MJ. Interaction of pressure- and flow-induced responses in porcine coronary resistance vessels. Am J Physiol Heart Circ Physiol 261: H1706–H1715, 1991 [DOI] [PubMed] [Google Scholar]
- 18. Liu Y, Bubolz AH, Mendoza S, Zhang DX, Gutterman DD. H2O2 is the transferrable factor mediating flow-induced dilation in human coronary arterioles. Circ Res 108: 566–573, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med 329: 2002–2012, 1993 [DOI] [PubMed] [Google Scholar]
- 20. Nabel EG, Selwyn AP, Ganz P. Large coronary arteries in humans are responsive to changing blood flow: an endothelium-dependent mechanism that fails in patients with atherosclerosis. J Am Coll Cardiol 16: 349–356, 1990 [DOI] [PubMed] [Google Scholar]
- 21. Nabel EG, Selwyn AP, Ganz P. Paradoxical narrowing of atherosclerotic coronary arteries induced by increases in heart rate. Circulation 81: 850–859, 1990 [DOI] [PubMed] [Google Scholar]
- 22. Quyyumi AA, Dakak N, Andrews NP, Gilligan DM, Panza JA, Cannon RO., 3rd Contribution of nitric oxide to metabolic coronary vasodilation in the human heart. Circulation 92: 320–326, 1995 [DOI] [PubMed] [Google Scholar]
- 23. Rosano GM, Kaski JC, Arie S, Pereira WI, Horta P, Collins P, Pileggi F, Poole-Wilson PA. Failure to demonstrate myocardial ischaemia in patients with angina and normal coronary arteries Evaluation by continuous coronary sinus pH monitoring and lactate metabolism. Eur Heart J 17: 1175–1180, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Schuman EM, Madison DV. Nitric oxide and synaptic function. Annu Rev Neurosci 17: 153–183, 1994 [DOI] [PubMed] [Google Scholar]
- 25. Seddon M, Melikian N, Dworakowski R, Shabeeh H, Jiang B, Byrne J, Casadei B, Chowienczyk P, Shah AM. Effects of neuronal nitric oxide synthase on human coronary artery diameter and blood flow in vivo. Circulation 119: 2656–2662, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Seddon MD, Chowienczyk PJ, Brett SE, Casadei B, Shah AM. Neuronal nitric oxide synthase regulates basal microvascular tone in humans in vivo. Circulation 117: 1991–1996, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Thomas GD, Sander M, Lau KS, Huang PL, Stull JT, Victor RG. Impaired metabolic modulation of alpha-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc Natl Acad Sci USA 95: 15090–15095, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tousoulis D, Tentolouris C, Crake T, Toutouzas P, Davies G. Basal and flow-mediated nitric oxide production by atheromatous coronary arteries. J Am Coll Cardiol 29: 1256–1262, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Vallon V, Traynor T, Barajas L, Huang YG, Briggs JP, Schnermann J. Feedback control of glomerular vascular tone in neuronal nitric oxide synthase knockout mice. J Am Soc Nephrol 12: 1599–1606, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Yeung AC, Vekshtein VI, Krantz DS, Vita JA, Ryan TJ, Jr, Ganz P, Selwyn AP. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. N Engl J Med 325: 1551–1556, 1991 [DOI] [PubMed] [Google Scholar]