Abstract
Cardiac metabolism remains altered for an extended period of time after myocardial infarction. Studies have shown fibroblasts from normal hearts express KATP channels in culture. It is unknown whether fibroblasts from infarcted hearts express KATP channels and whether these channels contribute to scar and border zone electrophysiology. KATP channel subunit expression levels were determined in fibroblasts isolated from normal hearts (Fb), and scar (sMI-Fb) and remote (rMI-Fb) regions of left anterior descending coronary artery (LAD) ligated rat hearts. Whole cell KATP current density was determined with patch clamp. Action potential duration (APD) was measured with optical mapping in myocyte-only cultures and heterocellular cultures with fibroblasts with and without 100 μmol/l pinacidil. Whole heart optical mapping was used to assess KATP channel activity following LAD ligation. Pinacidil activated a potassium current (35.4 ± 7.5 pA/pF at 50 mV) in sMI-Fb that was inhibited with 10 μmol/l glibenclamide. Kir6.2 and SUR2 transcript levels were elevated in sMI-Fb. Treatment with Kir6.2 short interfering RNA decreased KATP currents (87%) in sMI-Fb. Treatment with pinacidil decreased APD (26%) in co-cultures with sMI-Fb. APD values were prolonged in LAD ligated hearts after perfusion with glibenclamide. KATP channels are present in fibroblasts from the scar and border zones of infarcted hearts. Activation of fibroblast KATP channels could modulate the electrophysiological substrate beyond the acute ischemic event. Targeting fibroblast KATP channels could represent a novel therapeutic approach to modify border zone electrophysiology after cardiac injury.
Keywords: arrhythmia, ATP-sensitive potassium channels, electrophysiology, fibroblasts, myocardial infarction
myocardial infarction is often associated with the development of malignant ventricular arrhythmias and remains a major cause of mortality in the United States. Unfortunately, the underlying mechanisms responsible for initiation and maintenance of cardiac arrhythmias remain poorly understood. Fibrosis is associated with many forms of cardiovascular disease including ischemic cardiomyopathies and is recognized as a major contributing cause of arrhythmias. Fibrosis is classically thought to contribute to cardiac electrophysiology indirectly by creating physical barriers to electrical conduction. However, numerous in vitro and in vivo studies have suggested direct electrical coupling between myocytes and fibroblasts, which may contribute to the electrophysiology of the normal and diseased heart (3, 4, 13, 25, 29, 31, 40). In addition, several studies have demonstrated the electrophysiological properties of fibroblasts are altered in response to cardiac injury (8, 22, 47, 48).
Significant interest in the electrophysiological properties of fibroblasts and myofibroblasts has recently emerged. It has been shown that potassium currents in ventricular fibroblasts are able to modulate membrane potentials and are involved in electrical signaling (7, 44, 50). Moreover, fibroblasts also express calcium-dependent potassium channels (52) and other nonselective channels (8, 9, 19–21, 43). Recent studies have indicated fibroblasts isolated from normal hearts and maintained in culture express KATP channel subunits (1, 7, 26). Patch clamp studies also demonstrate that KATP currents appear progressively over time in culture and are associated with the differentiation of fibroblasts into an in vitro myofibroblast phenotype as determined by the expression of α-smooth muscle actin. These data suggest KATP channels may represent a significant potassium conductance in cardiac fibroblasts under pathological conditions. However, it is currently unknown if fibroblasts from injured hearts express KATP channels and whether the activation of these channels modulates myocyte electrophysiological properties.
KATP channels are characterized by their sensitivity to ATP/ADP levels and are activated by the potassium channel openers pinacidil, diazoxide, and nicorandil and inhibited by intracellular ATP and the sulfonylurea drugs glibenclamide and tolbutamide (12, 28, 37, 38). Different combinations of pore-forming Kir6.x (Kir6.1 or Kir6.2) and sulfonylurea receptor (SUR1, SUR2A, or SUR2B) subunits constitute KATP channels with distinct electrophysiological and pharmacological properties (2, 36). Activation of fibroblasts KATP channels may play an important role in infarcted hearts, particularly in the border zone and scar regions where fibroblasts are more numerous. Studies have shown there is a reduction of ATP levels and enzymes involved in ATP production immediately after ischemic events and that these changes persist in the infarcted myocardium for several weeks after injury (11, 16). These data suggest conditions that would lead to activation of KATP channels are present beyond the acute ischemic event. Moreover fibroblast KATP currents could also contribute to border zone electrophysiology in response to reinfarction events.
Here, we report for the first time myocardial infarction induces expression of functional KATP channels in scar and border zone fibroblasts. Activation of these channels modulates myocyte repolarization properties, alters the electrophysiological substrate, and may affect arrhythmia formation after myocardial infarction.
METHODS
All procedures complied with the standards for the care and use of animal subjects as stated in the Guide for the Care and Use of Laboratory Animals (NIH publication no. 85-23, revised 1996). Protocols were approved by the Institutional Animal Care and Use Committee of the New York University School of Medicine (protocol number 100407-02).
Myocardial infarction model.
Myocardial infarction was induced in male Wistar-Hannover rats weighing 170 to 250 g by ligating the left anterior descending coronary artery (LAD) as previously described (39). Anesthesia was induced with inhaled isoflurane (5%). The rat was intubated and ventilated with 100% oxygen. Anesthesia was maintained with 3.5% isoflurane. Meloxicam (1 mg/kg) and lidocaine (6 mg/kg) were administered by intramuscular injection. The surgical area was shaved and cleaned. An incision was made on the chest to the left of the sternum. The third and fourth ribs were transected, and the intercostal muscles were dissected. The thoracic cavity was entered, and the heart was exteriorized through the incision. A curved needle was used to pass 6-0 suture under the LAD. The suture was tied, and the heart was placed back into the thoracic cavity. The thoracic wall and the skin incision were closed. The animal was resuscitated using positive pressure ventilation and placed in an oxygen-rich environment. Fibroblasts were isolated from hearts 1 wk after the LAD ligation procedure. Only hearts with visible transmural infarcts were used in the study.
Fibroblast isolation.
Fibroblasts from the left ventricles of LAD ligated and age-matched normal (Fb) rat hearts were isolated by enzymatic digestion as previously described (14). Fibroblasts from LAD ligated hearts were separately isolated from the scar and border zone (sMI-Fb) and from noninfarcted remote (rMI-Fb) regions. The cells isolated from infarcted hearts were likely a mixture of fibroblasts and myofibroblasts. The majority of cells in the rMI-Fb group were likely fibroblasts, whereas the majority of cells in the sMI-Fb group were myofibroblasts. Rats were first euthanized by CO2 inhalation. Hearts were then extracted via thoracotomy. The left ventricular tissue was dissected, washed, minced, and subjected to repeated digestions at 37°C in a solution containing a mixture of 100 units/ml of collagenase I and 0.1% trypsin. After each digestion, the tissue was mechanically dissociated using a wide mouth pipet, the supernatant containing dissociated cells was collected, and cells were resuspended in L-15 medium. Cells from all digestions were pooled and resuspended in Medium-199 supplemented with 10% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, 1% nonessential aminoacids, and 20 μg/ml vitamin B12. The cells were plated and incubated for 2 h to allow for the preferential attachment of fibroblasts. The supernatant was then replaced with fresh culture medium. All experiments were performed 4 days after isolation.
Neonatal myocyte isolation.
Ventricular myocytes were isolated using an enzymatic digestion protocol as previously described (41). Myocytes were isolated from 0 to 2-day-old rat hearts (Wistar-Hannover) anesthetized with isoflurane followed by decapitation. The hearts were removed and pooled in chilled calcium and magnesium free HBSS. Hearts were washed twice in chilled HBSS, and the ventricular tissue was minced and digested at 37°C several times in a solution containing 0.125% trypsin and 60 μg/ml pancreatin at 37°C for 10 min. After each digestion, the tissue was mechanically dissociated using a wide mouth pipet, the supernatant containing dissociated cells was collected, and cells were resuspended in L-15 medium. Cells from all digestions were pooled and passed through a 70 μm cell strainer. Isolated cells were resuspended for plating in M-199 supplemented with 10% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, 1% nonessential aminoacids, and 20 μg/ml vitamin B12. Dissociated cells were preplated to separate myocytes and fibroblasts. Myocytes were resuspended in 10% FBS medium with 0.1 mmol/l bromodeoxyruridine and plated at a density 3.1 × 103 cells/mm2 on collagen-treated Petri dishes. Myocytes were maintained in with M-199 supplemented with 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mg/ml L-thyroxine, 0.1 mg/ml insulin, 0.5 mg/ml transferrin, 2.5 mg/ml ascorbic acid, 1 nmol/l lithium chloride, and 1 nmol/l sodium selenite (Sigma) starting 2 days after isolation.
Whole cell voltage clamp.
Isolated fibroblasts were cultured on glass cover slips and mounted on a recording chamber (RC-21BRW; Warner Instruments). Ionic currents were recorded in the whole cell patch clamp configuration with a patch clamp amplifier (Axopatch 200B; Axon Instruments) connected to an analog to digital converter (Digidata acquisition system; Clampex version 9.1 software; Axon Instruments). Additional patch clamp experiments were performed using sMI-Fb transfected with Kir6.2 short interfering RNA (siRNA). Patch electrodes were filled with an internal solution containing (in mmol/l) 12 NaCl, 20 KCl, 110 K-aspartate, 1 CaCl2, 1 MgCl2, 2 K2ATP, 10 EGTA, and 10 HEPES (pH adjusted to 7.2 with KOH). The extracellular solution was a modified Tyrode's solution containing (in mmol/l) 140 NaCl, 5.4 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose (pH adjusted to 7.4 with NaOH). Recordings were obtained in modified Tyrode's solution (control), modified Tyrode's solution containing 100 μM pinacidil, and modified Tyrode's solution containing 100 μM pinacidil and 10 μM glibenclamide. All experiments were performed at room temperature (23–25°C). Cell capacitance was measured by integrating the area under the capacitive transient elicited by 5-mV depolarizing steps from a holding potential of 0 mV. Series resistance and whole cell capacitance were electronically compensated. Whole cell current-voltage relationships were determined from a holding potential of −50 mV with a protocol consisting of 1.5-s voltage steps applied from −120 to 50 mV in 10-mV increments. The amplitudes of whole cell membrane currents at the end of the voltage steps were normalized to cell capacitance. Recordings were analyzed with pCLAMP (Axon Instruments) and Origin 7.0 (OriginLab) software packages. Additional patch clamp experiments were performed using sMI-Fb transfected with Kir6.2 siRNA.
Transcript analysis.
Quantitative RT-PCR was performed to determine KATP channel subunit transcript expression levels. Total RNA was extracted using the TRIzol reagent as specified by the manufacturer (Life Technologies). cDNA was synthesized from Fb, rMI-Fb, and sMI-Fb using the Superscript III First Strand Synthesis SuperMix (Life Technologies). Quantitative RT-PCR was performed using the Power Sybr Green PCR master mix (Applied Biosystems) with specific primers for Kir6.1 (GAAAGGCATCACGGAGAAGA and CTCCAAACCCAATGGTCACT), Kir6.2 (CCTCCTATCTGGCTGACGAG and GTGGGCACTTTAACGGTGTT), SUR1 (TCCAGAAGGTGGTGATGACA and AGGTCTGCACTCAGGATGGT), SUR2 (GCCTTTGTTCGAAAGAGCAG and GCTGTCATGACTACTTTCTGCAA), and actin (AGATTACTGCCCTGGCTCCT and TAGAGCCACCAATCCACACA). The transcript expression for each KATP channel subunit was normalized to actin expression.
siRNA transfection.
Transfection of sMI-Fb with Kir6.2 (sc-270034; Santa Cruz) and control (AM1631; Life Technologies) siRNA was performed using the siPORT NeoFx transfection agent (Life Technologies) following the manufacturer's protocol. The control siRNA is composed of scrambled sequence that will not lead to the specific degradation of any known cellular mRNA. Briefly, sMI-Fb were trypsinized and resuspended in normal growth medium at a density of 1 × 105 cells/ml. siPORT NeoFx transfection agent (9 μl) was diluted in 100 μl of OPTI-MEM (Life Technologies), and the mixture was incubated at room temperature for 10 min. The siRNA was also diluted at a final concentration of 30 nmol/l in 100 μl of OptiMEM and then mixed with the diluted NeoFX transfection agent. The final mixture was incubated at room temperature for 10 min and combined with 2.3 ml of the sMI-Fb cell suspension. The cells were plated and incubated at 37°C for 24 h followed by a media change. Quantification of mRNA levels was performed 48 h after transfection to determine knockdown efficiency.
Preparation of myocyte and fibroblast cocultures.
Freshly isolated myocytes were seeded on collagen-treated Petri dishes to obtain confluent monolayers. Three days after seeding myocytes, Fb and sMI-Fb were trypsinized and plated on top of myocyte monolayers (2 × 105 cells per dish). Myocyte-only monolayers were used as a control. Heterocellular cultures were returned to the incubator and optically mapped 24 h after fibroblast plating.
Cell culture optical mapping.
Myocyte-only cultures and cocultures of myocytes and fibroblasts were optically mapped. Mapping studies were performed using an upright microscope (BX51WI; Olympus) equipped with a CMOS camera (MiCAM ULTIMA-L; SciMedia). Light from a mercury arc lamp was passed through a filter cube that reflects excitation light (filter: 480–550 nm; dichroic mirror: 570 nm) to the cells and passes the emitted light (>590 nm) to the camera. An electronic shutter was used to limit exposure time. Recordings were made at 250 frames/s with 14-bit resolution from a 100 × 100 pixel array, which provided a spatial resolution of 81.6 μm. Cells were loaded with the voltage-sensitive dye di-8-ANEPPS (135 μmol/l; Life Technologies). After dye loading, cells were maintained in a recording solution (1% FBS, 1% HEPES Hank's buffered salt solution at 37°C, pH 7.4) throughout the mapping procedure. Optical recordings were obtained in control recording solution with vehicle followed by recording solution containing 100 μmol/l pinacidil. Cells were stimulated at a basic cycle length of 400 ms using a bipolar electrode (250 μm diameter, 800 μm separation; FHC). Experiments were performed in the absence of motion reduction techniques. Conduction velocity (CV) and action potential duration (APD) at 70% repolarization were calculated as described previously (24, 48).
Whole heart isolation and optical mapping.
Normal and LAD-ligated hearts were Langendorff perfused as previously described (32, 46). Briefly, rats were intubated and anesthetized with 3–5% isoflurane. Heparin was administered (0.5 units/g ip) 15 min before heart isolation. Hearts were excised through a sternotomy and rinsed in ice-cold modified Tyrode's solution containing (in mmol/l) 1.8 CaCl2, 1.0 MgCl2, 1.2 KH2PO4, 130.0 NaCl, 4.7 KCl, 11.1 glucose, 24.0 NaHCO3, and 0.052 g/l albumin equilibrated with a 95% O2-5% CO2 gas mixture. Aortas were cannulated, and hearts were perfused with warm (37°C) modified Tyrode's solution at a constant pressure of 68–74 mmHg. Hearts were submerged in warm oxygenated Tyrode's solution during the experiments to limit transmural temperature gradients. Hearts were stained with the voltage-sensitive dye di-4-ANEPPS (Life Technologies) as previously described (24). Optical mapping experiments were performed using an upright Olympus microscope (MVX10) with a reflected light fluorescence attachment (BX-FLA) equipped with a CMOS camera (Mi-CAM Ultima-L; SciMedia). Excitation light from a 100 W mercury arc lamp (Olympus) entered a filter cube that reflected green excitation light (480–550 nm, dichroic mirror: 570 nm) to the heart and passed the emitted light (>610 nm) to the camera. Movies were acquired at 1,000 frames/s with 14-bit resolution from a 100 × 100-pixel array, which provided a spatial resolution of 31 μm. Blebbistatin (5 μmol/l; BioMol) was used to limit motion artifacts during optical recordings (10). Movies were acquired during perfusion with control Tyrode's solution and Tyrode's solution containing 10 μmol/l glibenclamide while pacing at a basic cycle length of 100 ms with 4 ms stimuli at twice diastolic threshold. Movies were filtered and signal averaged to improve the signal-to-noise ratio of the recordings. Pixels with low signal-to-noise ratio were excluded from analysis. Average APD at 70% repolarization (APD70) values were calculated as previously described (23, 48).
Statistical analysis.
Results are presented as means ± SE. Unpaired and paired Student's t-tests were used for statistical comparisons of siRNA and whole heart mapping data, respectively. One-factor ANOVA followed by post hoc Student's t-tests were used to compare the patch-clamp and quantitative RT-PCR data. Two-factor ANOVA followed by post hoc unpaired and paired Student's t-tests when appropriate were used to compare the cell culture optical mapping data. A P value of less than 0.05 was considered to be statistically significant.
RESULTS
Whole cell voltage clamp.
The presence of KATP currents was investigated with whole cell patch clamp by treating fibroblasts with the channel opener, pinacidil, and blocker, glibenclamide. Figure 1 shows representative current traces and the mean current voltage relationships obtained from Fb, rMI-Fb, and sMI-Fb. The mean cell capacitance was not significantly different between Fb (25.1 ± 3.9 pF), rMI-Fb (20.2 ± 3.5 pF), and sMI-Fb (23.3 ± 2.5 pF). Fibroblasts from Fb, rMI-Fb, and sMI-Fb showed linear current-voltage relationships in control conditions. Pinacidil treatment had no significant effect on the membrane current voltage relationships or reversal potentials for Fb and rMI-Fb. In sMI-Fb, treatment with pinacidil significantly activated a potassium current (35.4 ± 7.5 pA/pF at 50 mV) that was glibenclamide sensitive. The pinacidil-induced current was significantly increased in sMI-Fb compared with Fb and rMI-Fb. Consistent with the activation of a potassium current, pinacidil treatment of sMI-Fb resulted in a significant shift in the reversal potential from −29.4 ± 3.9 mV to −67.4 ± 2.8 mV (P < 0.0001). These data indicate that cardiac injury induces expression of a KATP current in sMI-Fb.
Fig. 1.
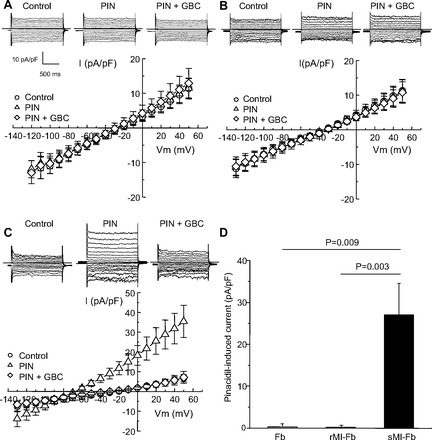
Effects of pinacidil (Pin) and glibenclamide (GBC) on whole-cell currents in fibroblasts isolated from normal hearts (Fb) and remote (rMI-Fb) and scar (sMI-Fb) regions. Representative current traces obtained during voltage steps from −120 mV to +50 mV from a holding potential of −50 mV and mean current-voltage relationships obtained under control conditions, in the presence of 100 μmol/l Pin and in the presence of Pin and 10 μmol/l GBC in Fb (n = 15; A), rMI-Fb (n = 14; B), and sMI-Fb (n = 10; C), are shown. Dashed lines indicate 0 current level. D: average Pin-induced current density at +50 mV for Fb, rMI-Fb, and sMI-Fb.
KATP channel subunit expression.
Figure 2 shows KATP channel subunit transcript expression levels in Fb, rMI-Fb, and sMI-Fb. Transcripts for Kir6.1, Kir6.2, and SUR2 were detected in Fb, rMI-Fb, and sMI-Fb. SUR1 was not detected in any of the three groups (n = 4). The expression of Kir6.2 and SUR2 transcripts were significantly increased in sMI-Fb compared with Fb and rMI-Fb.
Fig. 2.
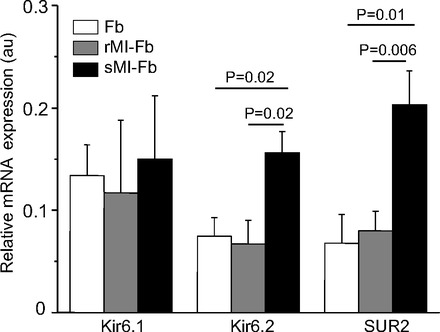
KATP channel subunit transcript expression levels. Kir6.1 (n = 3 for each group), Kir6.2 (n = 5 for each group), and SUR2 (n = 5 for each group) transcripts levels relative to actin in Fb, rMI-Fb, and sMI-Fb are shown. Au, arbitrary units.
Kir6.2 knockdown studies.
Figure 3 shows reducing Kir6.2 expression using siRNA alters the pinacidil-induced current in sMI-Fb. The amplitude of the pinacidil-induced current was significantly reduced in sMI-Fb cells treated with Kir6.2 siRNA compared with the cells treated with control siRNA. These data indicate that Kir6.2 is the main pore-forming subunit of sMI-Fb KATP channels. The residual pinacidil-induced current could be due to remaining Kir6.2 or possibly KATP channels composed of Kir6.1. Additionally, the reversal potential of Kir6.2 siRNA-treated cells was significantly increased (−5.4 ± 2.8 mV) compared with cells treated with control siRNA (−31.6 ± 8.5 mV, P = 0.007). These data indicate KATP channels contribute to the resting membrane conductance of fibroblasts isolated from the infarct scar.
Fig. 3.
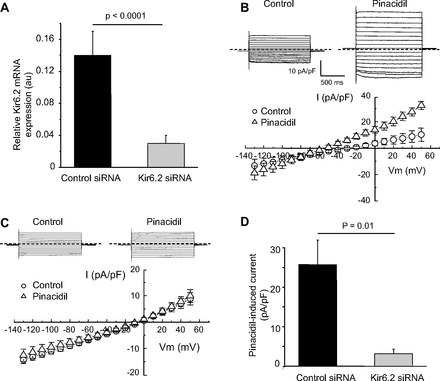
Effect of Kir6.2 short interfering RNA (siRNA) treatment on the pinacidil-induced current in sMI-Fb. A: average Kir6.2 transcripts levels in sMI-Fb treated with control siRNA (n = 3) and Kir6.2 siRNA (n = 3). Representative currents traces obtained from sMI-Fb during voltage steps from −120 mV to +50 mV from a holding potential of −50 mV and mean current voltage relationships obtained under control conditions and in the presence of 100 μmol/l pinacidil after treatment with control siRNA (n = 6; B) and Kir6.2 siRNA (n = 8; C) are shown. Dashed lines indicate 0 current level. D: average pinacidil-induced current density at +50 mV.
Culture optical mapping.
The effect of pinacidil on CV of myocyte-only and heterocellular cultures with Fb or sMI-Fb was evaluated using high resolution optical mapping. Figure 4 shows average CV values were significantly reduced (P < 0.0001) in heterocellular cultures of Fb and myocytes (0.15 ± 0.003 m/s) and sMI-Fb and myocytes (0.12 ± 0.003 m/s) compared with myocyte-only (0.18 ± 0.005 m/s) cultures. Average CV values were unaffected by the application of 100 μmol/l pinacidil. However, a trend toward increased conduction velocity was observed in heterocellular cultures with sMI-Fb treated with pinacidil compared with untreated cultures. This effect could be due to myocyte hyperpolarization associated with activation of fibroblast KATP channels (30). Figure 5 shows representative optical action potential traces and mean APD70 values obtained from myocyte-only and myocyte heterocellular cultures with Fb and sMI-Fb in control conditions and in the presence of pinacidil. APD70 values from myocyte cultures (94.5 ± 3.0 ms) were unchanged with addition of Fb (105.1 ± 5.2 ms) and significantly increased in the presence of sMI-Fb (125.4 ± 4.1 ms; P < 0.0001). APD70 values were prolonged in heterocellular cultures of myocytes and sMI-Fb compared with heterocellular cultures with Fb (P = 0.003). In myocyte cultures, APD70 was unaffected by the application of pinacidil (90.7 ± 3.2 ms). Average APD70 was significantly reduced in heterocellular cultures with Fb (90.7 ± 4.0 ms) and sMI-Fb (93.1 ± 2.4 ms) in response to pinacidil. A significantly greater pinacidil-induced reduction of APD70 was observed in heterocellular cultures with sMI-Fb (32.3 ± 3.0 ms) compared with Fb (14.0 ± 1.9 ms; P < 0.0001).
Fig. 4.
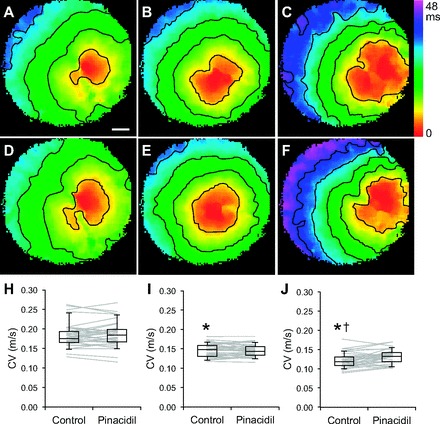
Conduction properties of heterocellular cultures in control conditions and in the presence of pinacidil. A–C: representative activation maps under control conditions from myocyte-only, myocyte and Fb, and myocyte and sMI-Fb cultures, respectively. Isochronal lines are drawn every 8 ms. Bar is 1 mm. D–F: representative activation maps after treatment with 100 μmol/l pinacidil from myocyte-only, myocyte and Fb, and myocyte and sMI-Fb cultures, respectively. Data points and box plots show average conduction velocity values obtained from individual myocyte only (n = 34; H), myocyte and Fb (n = 34; I), and myocyte and sMI-Fb (n = 34; J) cultures under control conditions and in the presence of pinacidil. *Significant difference compared with myocyte only cultures under control conditions; †significant difference compared with myocyte and Fb heterocellular cultures under control conditions.
Fig. 5.
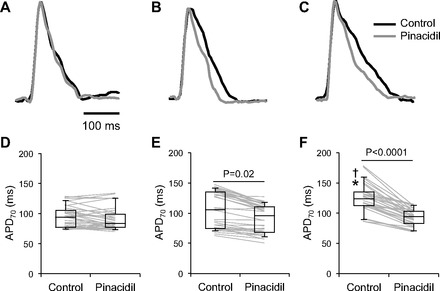
Action potential duration at 70% repolarization (APD70) of heterocellular cultures in control conditions and in the presence of pinacidil. Representative optical action potentials recorded from myocyte-only (A), myocyte and Fb (B), and myocyte and sMI-Fb (C) cultures under control conditions and in the presence of 100 μmol/l pinacidil are shown. Data points and box plots show average APD70 values obtained from individual myocyte only (n = 34; D), myocyte and Fb (n = 34; E), and myocyte and sMI-Fb (n = 34; F) cultures under control conditions and in the presence of pinacidil. *Significant difference compared with myocyte only cultures under control conditions; †significant difference compared with myocyte and Fb cultures under control conditions.
Whole heart optical mapping.
To determine whether KATP channels are active after cardiac infarction, APD70 was measured in whole hearts treated with glibenclamide. Figure 6 shows representative APD70 maps and average APD70 values for control and infarcted hearts under control conditions and after treatment with 10 μmol/l glibenclamide. These data demonstrate that average APD70 values were unchanged in normal hearts after perfusion with glibenclamide. In infarcted hearts, glibenclamide treatment significantly prolonged APD70 (44.6 ± 3.5 ms) compared with control conditions (38.4 ± 3.3 ms). These data indicate conditions are present for KATP currents to remain active several days after infarction. Myocyte KATP currents likely contribute to the observed changes in APD; however, these data are also consistent with the other findings of the study and suggest fibroblast KATP channels may actively contribute to the electrophysiology of infarcted hearts beyond the ischemic insult.
Fig. 6.
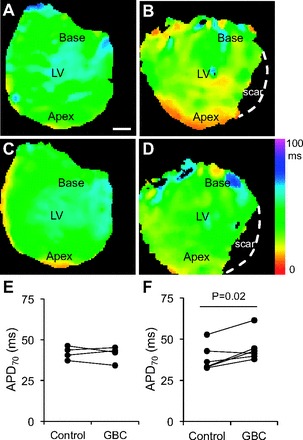
APD70 of normal and infarcted hearts treated with glibenclamide (GBC). A and B: representative APD70 maps obtained under control conditions from normal and left anterior descending coronary artery ligated hearts, respectively. Dashed white line indicates edge of heart in the scar region. LV, left ventricle. C and D: representative APD70 maps obtained during perfusion with 10 μmol/l GBC from normal and infarcted hearts, respectively. Graphs show average APD70 values obtained from individual normal (n = 4; E) and infarcted (n = 6; F) hearts under control conditions and in the presence of GBC.
DISCUSSION
This is the first study to demonstrate fibroblast from the scar and border zones of infarcted hearts express functional KATP channels. KATP currents were not present in fibroblasts from normal hearts or remote regions of infarcted hearts. Activation of KATP channels resulted in a shift in the reversal potential to more hyperpolarized values in fibroblasts from the scar and border zones of infarcted hearts. Numerous studies have demonstrated fibroblasts from normal hearts have a more depolarized resting membrane potential compared with myocytes (7, 29, 42, 48). When coupled to myocytes, fibroblasts have a depolarizing effect on myocyte resting membrane potential, which can lead to partial sodium channel inactivation. Recent studies have indicated positive shifts in the resting membrane potential of fibroblasts is the most critical factor promoting myocyte early after depolarizations (35). Activation of fibroblast KATP currents would decrease the depolarizing effect on myocytes and prevent early afterdepolarizations. On the other hand, activation of fibroblast KATP currents would also act to preserve myocyte excitability. This could lead to impulse regeneration in areas with small numbers of surviving myocytes resulting in slow heterogeneous conduction that may facilitate re-entry. Thus activation of fibroblasts KATP currents could modify the electrophysiological substrate in a manner that is anti- or pro-arrhythmic, depending on specific conditions.
Consistent with previous studies, transcript analysis demonstrated that ventricular fibroblasts express the KATP channel subunits Kir6.1, Kir6.2, and SUR2 (1, 7). Kir6.2 and SUR2 transcripts were upregulated in fibroblasts from the scar and border regions of infarcted hearts. Treatment with Kir6.2 siRNA significantly decreased KATP current density, suggesting that Kir6.2 is the pore-forming subunit of KATP channels in these cells. Additionally, a positive shift was observed in the reversal potential in fibroblasts from the scar and border zone regions treated with Kir6.2 siRNA, indicating KATP currents contribute to the background conductance of fibroblasts from infarct scars.
Myocardial ischemia results in a major reduction of APD, which has been attributed to opening of cardiac KATP channels (5, 15, 34, 49, 54). In addition to activation of KATP channels in myocytes, fibroblast KATP channels may play an important role in determining the electrophysiological properties of the infarcted area where fibroblasts are more numerous and where conditions allow for KATP channel activation. KATP channels are activated by a decrease in the ATP-to-ADP ratio (12, 37, 38). Several studies have shown a significant reduction of ATP content and enzymes critical for ATP production occurs in the peri-infarct region and that these changes persist for at least 6 wk after infarction (11, 16). These studies suggest fibroblast KATP channels could be active in the infarct border zone for many weeks following myocardial infarction.
Fibroblast KATP currents could also contribute to border zone electrophysiological abnormalities in response to reinfarction. Over the last two decades, improvements in interventional and medical therapies for the treatment of myocardial infarction have greatly improved survival outcomes. However, after the initial myocardial infarction event, reinfarction continues to be a major source of mortality (53). Recent studies have indicated reinfarction occurs in more than 10% of myocardial infarction patients, is associated with electrocardiographic abnormalities, and leads to death within 24 h in ∼10% of patients. Reinfarction episodes are associated with significant reductions in energetics and would lead to the activation of KATP currents.
Several studies have demonstrated myocytes and fibroblasts can establish electrical communication through gap junction channels (13, 42, 48). Recent studies from our laboratory and others have suggested cardiac injury may be associated with an increase in fibroblast myocyte coupling (48, 56). These findings have raised the intriguing possibility that fibroblasts could contribute directly to cardiac electrophysiology. In this study, myocytes were cocultured with fibroblasts isolated from the scar and border regions of infarcted hearts and treated with the KATP opener, pinacidil, to investigate the possibility fibroblast KATP currents are able to modulate myocyte electrophysiology. Pinacidil had no significant effect on CV and APD values in myocyte-only cultures. This finding is consistent with other studies showing KATP subunit protein expression and channel density are low in neonatal hearts and progressively increases with maturation (6, 33). Consistent with previous studies, the addition of fibroblasts isolated from normal hearts or the scar and border zone regions of infarcted hearts to myocyte cultures resulted in significant decrease in CV (29, 48). In addition, CV values were significantly slower in heterocellular cultures with fibroblasts from infarcted hearts compared with cultures with fibroblasts from normal hearts (48). The decrease in CV is consistent with the depolarization of myocytes through the active coupling with fibroblasts due to their more positive resting membrane potential. The addition of fibroblast from normal hearts had no effect on APD of the heterocellular cultures, whereas fibroblast from the scar and border zone regions significantly prolonged APD. Fibroblast-induced effects on myocyte APD are dependent on cell size and density, coupling levels, and fibroblast resting membrane potential. The observed changes in APD are consistent with previous studies (18, 27, 35, 55).
Previous studies have shown automaticity in co-cultures of myocytes and fibroblasts from neonatal hearts can be suppressed with KATP channel openers (31). In this study, treatment of cocultures with myocytes and fibroblasts from normal or from the scar and border zone regions of infarcted hearts with the KATP channel opener, pinacidil, had no significant effect on CV values. On the other hand, treatment with pinacidil significantly decreased APD values in both types of cocultures. These findings are consistent with the activation of fibroblast KATP currents and modulation of myocyte repolarization through intercellular electrical coupling between myocytes and fibroblasts. APD changes induced by pinacidil in cultures with fibroblasts from normal hearts are inconsistent with the single cell patch recordings demonstrating the absence of functional KATP channels. These differences could be related to the plating and recording conditions. When fibroblasts are plated on top of myocytes, fibroblasts are subjected to mechanical forces that may trigger in vitro differentiation into a myofibroblast phenotype that could be associated with expression of functional KATP channels (1, 51). Importantly, the pinacidil-induced APD shortening effect was greater in the cocultures with fibroblasts from the scar and border zones of infarcted hearts. These data indicate KATP channels in scar and border zone fibroblasts can modulate the electrophysiology of surviving myocytes and may contribute to arrhythmogenesis in this region by decreasing wavelength and facilitating the formation of reentry.
Whole heart mapping studies of normal and infarcted hearts were performed to determine whether KATP channels are active after cardiac infarction. Perfusion with the KATP channel blocker, glibenclamide, prolonged APD in the infarcted hearts and had no effect on APD values in normal hearts. These data indicate KATP channels are active for at least 1 wk after myocardial infarction. Previous studies have shown myocyte KATP subunit transcript levels remain unchanged in the border zone 1 wk after infarction (17). Although activation of myocyte KATP currents likely contributes to the observed electrophysiological changes, these data are consistent with the other findings of the study and suggest fibroblast KATP channels actively contribute to the electrophysiology of infarcted hearts.
This study has limitations that are inherent to all studies that use an in vitro experimental approach. Isolated fibroblasts were maintained in culture for 4 days after isolation. It is possible the electrophysiological phenotype of fibroblasts could change during this period. Although culture conditions for all groups in this study were identical, further studies would be required to determine whether there is a differential response to the culture environment depending on the fibroblast source. Cultures of neonatal myocytes were used to evaluate the effects of fibroblast KATP currents on myocyte electrical properties. Adult myocytes cannot be used to generate the heterocellular cultures necessary to investigate interactions between myocytes and fibroblasts. The electrophysiological properties of neonatal and adult myocytes are different particularly with regards to KATP channel expression; therefore, further studies are necessary to evaluate the effect of fibroblast KATP currents on adult cardiac myocytes. It is possible that fibroblast paracrine factors can modify myocyte KATP channel expression and contribute to the electrophysiological changes observed in the heterocellular cultures. In addition, a recent study demonstrated fibroblast growth factor 2 could have cardioprotective effects through modification of Cx43 phosphorylation (45). Further experimentation would be required to evaluate these possibilities. Finally, the in vitro changes reported in this study do not provide direct evidence in terms of interaction between adult cardiomyocytes and fibroblasts in the intact heart. Further studies are needed to directly determine to what extent the changes in KATP expression contributed to altered conduction, repolarization, and arrhythmogenesis in injured hearts.
Conclusions
In this study, we demonstrate for the first time functional KATP channels composed of Kir6.2 and SUR2 subunits are present in fibroblasts from the scar and border zone regions of infarcted hearts. Activation of fibroblast KATP channels was shown to affect myocyte repolarization properties. Because cardiac metabolism is altered after myocardial infarction, fibroblast KATP channel activation could modulate the electrophysiological substrate for extended periods of time beyond the initial ischemic insult. These studies suggest targeting fibroblast KATP channel activity could represent a novel therapeutic approach to modify border zone electrophysiology after cardiac injury.
GRANTS
This work was supported by grants from the National Heart, Lung, and Blood Institute at the National Institutes of Health (HL-076751 to G. E. Morley, HL-085820 and HL-093563 to W. A. Coetzee, and T32-HL-098129 to C. Vasquez) and by a grant from the American Heart Association (12PRE11890017 to V. M. Mahoney).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.B., C.V., and G.E.M. completed conception and design of research; N.B., C.V., V.M.M., and M.J.S. performed experiments; N.B., C.V., V.M.M., M.J.S., and G.E.M. analyzed data; N.B., C.V., V.M.M., and G.E.M. interpreted results of experiments; N.B., C.V., V.M.M., and G.E.M. prepared figures; N.B., C.V., and G.E.M. drafted manuscript; N.B., C.V., W.A.C., and G.E.M. edited and revised manuscript; N.B., C.V., V.M.M., M.J.S., W.A.C., and G.E.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Ursula Andreo for technical expertise with the quantitative RT-PCR.
REFERENCES
- 1. Benamer N, Moha Ou, Maati H, Demolombe S, Cantereau A, Delwail A, Bois P, Bescond J, Faivre JF. Molecular and functional characterization of a new potassium conductance in mouse ventricular fibroblasts. J Mol Cell Cardiol 46: 508–517, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Burke MA, Mutharasan RK, Ardehali H. The sulfonylurea receptor, an atypical ATP-binding cassette protein, and its regulation of the KATP channel. Circ Res 102: 164–176, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Camelliti P, Devlin GP, Matthews KG, Kohl P, Green CR. Spatially and temporally distinct expression of fibroblast connexins after sheep ventricular infarction. Cardiovasc Res 62: 415–425, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Camelliti P, Green CR, LeGrice I, Kohl P. Fibroblast network in rabbit sinoatrial node: structural and functional identification of homogeneous and heterogeneous cell coupling. Circ Res 94: 828–835, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Carmeliet E. Cardiac transmembrane potentials and metabolism. Circ Res 42: 577–587, 1978 [DOI] [PubMed] [Google Scholar]
- 6. Chen F, Wetzel GT, Friedman WF, Klitzner TS. ATP-sensitive potassium channels in neonatal and adult rabbit ventricular myocytes. Pediatr Res 32: 230–235, 1992 [DOI] [PubMed] [Google Scholar]
- 7. Chilton L, Ohya S, Freed D, George E, Drobic V, Shibukawa Y, Maccannell KA, Imaizumi Y, Clark RB, Dixon IM, Giles WR. K+ currents regulate the resting membrane potential, proliferation, and contractile responses in ventricular fibroblasts and myofibroblasts. Am J Physiol Heart Circ Physiol 288: H2931–H2939, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Du J, Xie J, Zhang Z, Tsujikawa H, Fusco D, Silverman D, Liang B, Yue L. TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ Res 106: 992–1003, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. El Chemaly A, Guinamard R, Demion M, Fares N, Jebara V, Faivre JF, Bois P. A voltage-activated proton current in human cardiac fibroblasts. Biochem Biophys Res Commun 340: 512–516, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Fedorov VV, Lozinsky IT, Sosunov EA, Anyukhovsky EP, Rosen MR, Balke CW, Efimov IR. Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm 4: 619–626, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Feygin J, Hu Q, Swingen C, Zhang J. Relationships between regional myocardial wall stress and bioenergetics in hearts with left ventricular hypertrophy. Am J Physiol Heart Circ Physiol 294: H2313–H2321, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Findlay I. ATP4- and ATP.Mg inhibit the ATP-sensitive K+ channel of rat ventricular myocytes. Pflügers Arch 412: 37–41, 1988 [DOI] [PubMed] [Google Scholar]
- 13. Gaudesius G, Miragoli M, Thomas SP, Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ Res 93: 421–428, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Gustafsson AB, Brunton LL. Beta-adrenergic stimulation of rat cardiac fibroblasts enhances induction of nitric-oxide synthase by interleukin-1beta via message stabilization. Mol Pharmacol 58: 1470–1478, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Hicks MN, Cobbe SM. Effect of glibenclamide on extracellular potassium accumulation and the electrophysiological changes during myocardial ischaemia in the arterially perfused interventricular septum of rabbit. Cardiovasc Res 25: 407–413, 1991 [DOI] [PubMed] [Google Scholar]
- 16. Hu Q, Wang X, Lee J, Mansoor A, Liu J, Zeng L, Swingen C, Zhang G, Feygin J, Ochiai K, Bransford TL, From AH, Bache RJ, Zhang J. Profound bioenergetic abnormalities in peri-infarct myocardial regions. Am J Physiol Heart Circ Physiol 291: H648–H657, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Isidoro Tavares N, Philip-Couderc P, Papageorgiou I, Baertschi AJ, Lerch R, Montessuit C. Expression and function of ATP-dependent potassium channels in late post-infarction remodeling. J Mol Cell Cardiol 42: 1016–1025, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Jacquemet V, Henriquez CS. Loading effect of fibroblast-myocyte coupling on resting potential, impulse propagation, and repolarization: insights from a microstructure model. Am J Physiol Heart Circ Physiol 294: H2040–H2052, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kamkin A, Kiseleva I, Isenberg G. Activation and inactivation of a non-selective cation conductance by local mechanical deformation of acutely isolated cardiac fibroblasts. Cardiovasc Res 57: 793–803, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Kamkin A, Kiseleva I, Lozinsky I, Scholz H. Electrical interaction of mechanosensitive fibroblasts and myocytes in the heart. Basic Res Cardiol 100: 337–345, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Kamkin A, Kiseleva I, Wagner KD, Lozinsky I, Gunther J, Scholz H. Mechanically induced potentials in atrial fibroblasts from rat hearts are sensitive to hypoxia/reoxygenation. Pflügers Arch 446: 169–174, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Kiseleva I, Kamkin A, Pylaev A, Kondratjev D, Leiterer KP, Theres H, Wagner KD, Persson PB, Gunther J. Electrophysiological properties of mechanosensitive atrial fibroblasts from chronic infarcted rat heart. J Mol Cell Cardiol 30: 1083–1093, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Lader JM, Vasquez C, Bao L, Maass K, Qu J, Kefalogianni E, Fishman GI, Coetzee WA, Morley GE. Remodeling of atrial ATP-sensitive K+ channels in a model of salt-induced elevated blood pressure. Am J Physiol Heart Circ Physiol 301: H964–H974, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leaf DE, Feig JE, Vasquez C, Riva PL, Yu C, Lader JM, Kontogeorgis A, Baron EL, Peters NS, Fisher EA, Gutstein DE, Morley GE. Connexin40 imparts conduction heterogeneity to atrial tissue. Circ Res 103: 1001–1008, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lefroy DC, Fang JC, Stevenson LW, Hartley LH, Friedman PL, Stevenson WG. Recipient-to-donor atrioatrial conduction after orthotopic heart transplantation: surface electrocardiographic features and estimated prevalence. Am J Cardiol 82: 444–450, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Li GR, Sun HY, Chen JB, Zhou Y, Tse HF, Lau CP. Characterization of multiple ion channels in cultured human cardiac fibroblasts. PLoS One 4: e7307, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. MacCannell KA, Bazzazi H, Chilton L, Shibukawa Y, Clark RB, Giles WR. A mathematical model of electrotonic interactions between ventricular myocytes and fibroblasts. Biophys J 92: 4121–4132, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miki T, Seino S. Roles of KATP channels as metabolic sensors in acute metabolic changes. J Mol Cell Cardiol 38: 917–925, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Miragoli M, Gaudesius G, Rohr S. Electrotonic modulation of cardiac impulse conduction by myofibroblasts. Circ Res 98: 801–810, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Miragoli M, Kadir SH, Sheppard MN, Salvarani N, Virta M, Wells S, Lab MJ, Nikolaev VO, Moshkov A, Hague WM, Rohr S, Williamson C, Gorelik J. A protective antiarrhythmic role of ursodeoxycholic acid in an in vitro rat model of the cholestatic fetal heart. Hepatology 54: 1282–1292, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miragoli M, Salvarani N, Rohr S. Myofibroblasts induce ectopic activity in cardiac tissue. Circ Res 101: 755–758, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Morley GE, Vaidya D, Samie FH, Lo C, Delmar M, Jalife J. Characterization of conduction in the ventricles of normal and heterozygous Cx43 knockout mice using optical mapping. J Cardiovasc Electrophysiol 10: 1361–1375, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Morrissey A, Parachuru L, Leung M, Lopez G, Nakamura TY, Tong X, Yoshida H, Srivastiva S, Chowdhury PD, Artman M, Coetzee WA. Expression of ATP-sensitive K+ channel subunits during perinatal maturation in the mouse heart. Pediatr Res 58: 185–192, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Nakaya H, Takeda Y, Tohse N, Kanno M. Effects of ATP-sensitive K+ channel blockers on the action potential shortening in hypoxic and ischaemic myocardium. Br J Pharmacol 103: 1019–1026, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nguyen TP, Xie Y, Garfinkel A, Qu Z, Weiss JN. Arrhythmogenic consequences of myofibroblast-myocyte coupling. Cardiovasc Res 93: 242–251, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature 440: 470–476, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Nichols CG, Lederer WJ. The mechanism of KATP channel inhibition by ATP. J Gen Physiol 97: 1095–1098, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Noma A. ATP-regulated K+ channels in cardiac muscle. Nature 305: 147–148, 1983 [DOI] [PubMed] [Google Scholar]
- 39. Pfeffer JM, Pfeffer MA, Braunwald E. Influence of chronic captopril therapy on the infarcted left ventricle of the rat. Circ Res 57: 84–95, 1985 [DOI] [PubMed] [Google Scholar]
- 40. Rohr S. Myofibroblasts in diseased hearts: new players in cardiac arrhythmias? Heart Rhythm 6: 848–856, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Rohr S, Fluckiger-Labrada R, Kucera JP. Photolithographically defined deposition of attachment factors as a versatile method for patterning the growth of different cell types in culture. Pflügers Arch 446: 125–132, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Rook MB, van Ginneken AC, de Jonge B, el Aoumari A, Gros D, Jongsma HJ. Differences in gap junction channels between cardiac myocytes, fibroblasts, and heterologous pairs. Am J Physiol Cell Physiol 263: C959–C977, 1992 [DOI] [PubMed] [Google Scholar]
- 43. Rose RA, Hatano N, Ohya S, Imaizumi Y, Giles WR. C-type natriuretic peptide activates a non-selective cation current in acutely isolated rat cardiac fibroblasts via natriuretic peptide C receptor-mediated signalling. J Physiol 580: 255–274, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shibukawa Y, Chilton EL, Maccannell KA, Clark RB, Giles WR. K+ currents activated by depolarization in cardiac fibroblasts. Biophys J 88: 3924–3935, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Srisakuldee W, Jeyaraman MM, Nickel BE, Tanguy S, Jiang ZS, Kardami E. Phosphorylation of connexin-43 at serine 262 promotes a cardiac injury-resistant state. Cardiovasc Res 83: 672–681, 2009 [DOI] [PubMed] [Google Scholar]
- 46. Vaidya D, Morley GE, Samie FH, Jalife J. Reentry and fibrillation in the mouse heart: a challenge to the critical mass hypothesis. Circ Res 85: 174–181, 1999 [DOI] [PubMed] [Google Scholar]
- 47. Vasquez C, Benamer N, Morley GE. The cardiac fibroblast: functional and electrophysiological considerations in healthy and diseased hearts. J Cardiovasc Pharmacol 57: 380–388, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vasquez C, Mohandas P, Louie KL, Benamer N, Bapat AC, Morley GE. Enhanced fibroblast-myocyte interactions in response to cardiac injury. Circ Res 107: 1011–1020, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Venkatesh N, Stuart JS, Lamp ST, Alexander LD, Weiss JN. Activation of ATP-sensitive K+ channels by cromakalim. Effects on cellular K+ loss and cardiac function in ischemic and reperfused mammalian ventricle. Circ Res 71: 1324–1333, 1992 [DOI] [PubMed] [Google Scholar]
- 50. Walsh KB, Zhang J. Neonatal rat cardiac fibroblasts express three types of voltage-gated K+ channels: regulation of a transient outward current by protein kinase C. Am J Physiol Heart Circ Physiol 294: H1010–H1017, 2008 [DOI] [PubMed] [Google Scholar]
- 51. Wang J, Chen H, Seth A, McCulloch CA. Mechanical force regulation of myofibroblast differentiation in cardiac fibroblasts. Am J Physiol Heart Circ Physiol 285: H1871–H1881, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Wang YJ, Sung RJ, Lin MW, Wu SN. Contribution of BKCa-channel activity in human cardiac fibroblasts to electrical coupling of cardiomyocytes-fibroblasts. J Membr Biol 213: 175–185, 2006 [DOI] [PubMed] [Google Scholar]
- 53. White HD, Reynolds HR, Carvalho AC, Pearte CA, Liu L, Martin CE, Knatterud GL, Dzavik V, Kruk M, Steg PG, Cantor WJ, Menon V, Lamas GA, Hochman JS. Reinfarction after percutaneous coronary intervention or medical management using the universal definition in patients with total occlusion after myocardial infarction: results from long-term follow-up of the Occluded Artery Trial (OAT) cohort. Am Heart J 163: 563–571, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wilde AA. Role of ATP-sensitive K+ channel current in ischemic arrhythmias. Cardiovasc Drugs Ther 7, Suppl 3: 521–526, 1993 [DOI] [PubMed] [Google Scholar]
- 55. Xie Y, Garfinkel A, Weiss JN, Qu Z. Cardiac alternans induced by fibroblast-myocyte coupling: mechanistic insights from computational models. Am J Physiol Heart Circ Physiol 297: H775–H784, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang Y, Kanter EM, Yamada KA. Remodeling of cardiac fibroblasts following myocardial infarction results in increased gap junction intercellular communication. Cardiovasc Pathol 19: E233–E240, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]


