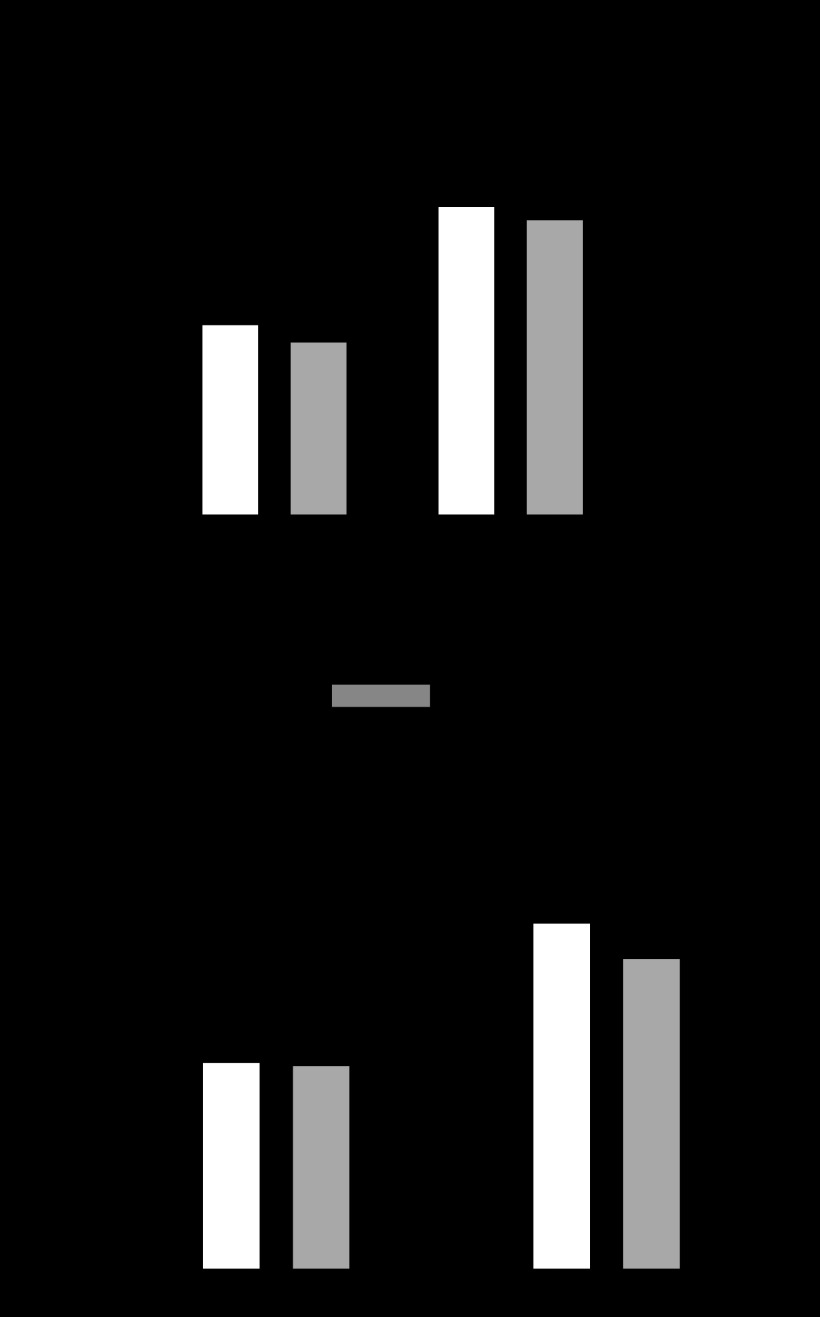Abstract
Nitric oxide (NO) release from endothelial NO synthase (eNOS) and/or neuronal NO synthase (nNOS) could be modulated by sympathetic nerve activity and contribute to increased blood flow after exercise. We examined the effects of brachial-arterial infusion of the nNOS selective inhibitor S-methyl-l-thiocitrulline (SMTC) and the nonselective NOS inhibitor NG-monomethyl-l-arginine (l-NMMA) on forearm arm blood flow at rest, during sympathetic activation by lower body negative pressure, and during lower body negative pressure immediately after handgrip exercise. Reduction in forearm blood flow by lower body negative pressure during infusion of SMTC was not significantly different from that during vehicle (−28.5 ± 4.02 vs. −34.1 ± 2.96%, respectively; P = 0.32; n = 8). However, l-NMMA augmented the reduction in forearm blood flow by lower body negative pressure (−44.2 ± 3.53 vs. −23.4 ± 5.71%; n = 8; P < 0.01). When lower body negative pressure was continued after handgrip exercise, there was no significant effect of either l-NMMA or SMTC on forearm blood flow immediately after low-intensity exercise (P = 0.91 and P = 0.44 for l-NMMA vs. saline and SMTC vs. saline, respectively; each n = 10) or high-intensity exercise (P = 0.46 and P = 0.68 for l-NMMA vs. saline and SMTC vs. saline, respectively; each n = 10). These results suggest that sympathetic activation increases NO release from eNOS, attenuating vasoconstriction. Dysfunction of eNOS could augment vasoconstrictor and blood pressure responses to sympathetic activation. However, neither eNOS nor nNOS plays an essential role in postexercise hyperaemia, even in the presence of increased sympathetic activation.
Keywords: endothelial nitric oxide synthase, exercise, forearm blood flow, neuronal nitric oxide synthase, sympathetic vasoconstriction
nitric oxide (no), synthesized from l-arginine and molecular oxygen by NO synthases (NOS) (21–23), plays an important role in regulating arteriolar tone, peripheral resistance, and blood flow, acting as a tonic dilator opposing sympathetically mediated vasoconstriction (28). Although it has been considered that NO derived from endothelial NOS (eNOS) is the major regulator of local blood flow, it is now clear that NO synthesised from the neuronal NOS isoform (nNOS) plays an equally important role. In humans, brachial artery infusion of S-methyl-l-citrulline (SMTC), a selective inhibitor of nNOS, reduces basal forearm blood flow (FBF) but does not block the eNOS-mediated vasodilator response to ACh or increased shear stress (24, 25). This suggests that nNOS-derived NO contributes to the tonic regulation of basal arteriolar tone and hence blood flow. nNOS is expressed mainly in nonadrenergic, noncholinergic nitrergic nerves, and nNOS inhibition suppresses the vasodilatory response to mental stress (thought to be a nitrergically mediated response) (25), suggesting that nitrergic nerves might be the source of nNOS influencing basal arteriolar tone. However, other tissues expressing nNOS such as skeletal muscle (20) and vascular smooth muscle (16) could be involved.
Animal studies and indirect observations in humans suggest that sympathetic nerve activity may stimulate NO release from eNOS and/or nNOS (18, 27), and NO release by this or other mechanisms could contribute to functional sympatholysis, the local attenuation of sympathetic vasoconstriction in exercising muscle and/or to direct vasodilation after exercise. A role for NOS and particularly nNOS in functional sympatholysis is supported by some (1, 19, 26) but not all (6) animal studies. However, human studies using nonspecific inhibitors of NOS suggest the role of NOS in regulation of blood flow immediately after local exercise is small or absent (7, 8, 10, 11) but have not been performed in the presence of the increased sympathetic activation expected during systemic exercise.
We tested the hypothesis that NO release from nNOS and/or eNOS regulates local FBF during reflex sympathetic activation at rest and after exercise. We used nonselective and selective NOS inhibitors to determine the role of eNOS-derived and nNOS-derived NO in 1) opposing sympathetically mediated increases in arteriolar tone in the forearm during low body negative pressure (LBNP) applied at rest and 2) contributing to the increase in blood flow after handgrip exercise during LBNP. We also examined effects of NOS inhibition on blood flow responses after exercise without LBNP and the effects of propranolol on the blood flow response to LBNP to determine whether β-adrenergic responses are implicated in this response.
METHODS
The studies were approved by the local Research Ethics Committee (St. Thomas' Hospital), and all participants provided written informed consent. The studies conformed to the standards set by the latest revision of the Declaration of Helsinki.
Healthy male volunteers, aged 25.9 ± 6.9 years, were recruited by local advertisement. Subjects on regular medication with hypertension, hyperlipidaemia, or any significant abnormality on hematological or biochemical screening were excluded. Participants were asked to abstain from caffeine for at least 12 h before the studies.
FBF studies were undertaken in a quiet temperature-controlled vascular laboratory (23°C to 25°C) after at least 30 min of rest. A 27-gauge needle was inserted into the brachial artery under local anesthesia by using less than 0.2 ml 1% lignocaine, and saline vehicle or drugs infused at 1 ml/min by constant rate infusion pump. FBF was measured by venous occlusion plethysmography by using electrically calibrated strain gauges (14). Drugs were infused for at least 5 min, and blood flow was measured over the final 1 min of infusion, with the mean of 5 measurements used for analysis. The protocols below were used to assess effects of the nonselective NOS inhibitor NG-monomethyl-l-arginine (l-NMMA; 2 μmol/min) and the nNOS specific NOS inhibitor SMTC (0.2 μmol/min) on reflex sympathetic activation induced by LBNP and handgrip exercise (Fig. 1). These doses of l-NMMA and SMTC were chosen because they produce the same reduction in basal FBF [with effects that are near maximal within 5 min (25, 28) and that for SMTC is specific for nNOS, inhibiting nitrergic responses but being without effect on eNOS-stimulated responses]. Furthermore, these doses of l-NMMA and SMTC inhibit eNOS and nNOS and nNOS, respectively, under high-flow conditions such as during activation of eNOS by shear stress (24) or substance P (4), and nNOS by mental stress (25). SMTC at a purity >99% was obtained from Calbiochem UK and prepared to standards suitable for human use in a nationally accredited pharmaceutical manufacturing facility as previously described (25). Pharmaceutical grade l-NMMA was purchased from Bachem (Switzerland).
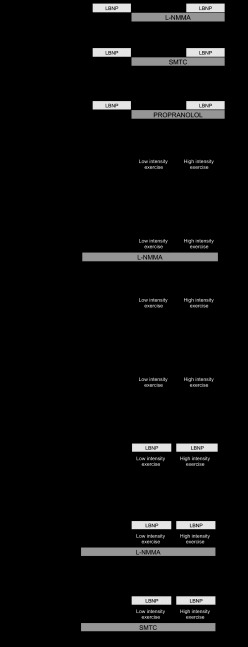
Fig. 1.
Schematic of study protocols. Forearm blood flow (FBF) was measured by using venous occlusion plethysmography. A: effect of low body negative pressure (LBNP) on FBF was measured during infusion of NG-monomethyl-l-arginine (l-NMMA; 2 μmol/min) and vehicle, and S-methyl-l-thiocitrulline (SMTC; 0.2 μmol/min) and vehicle. B: effect of LBNP on FBF was measured during infusion of propranolol and vehicle. C: effect of low- and high-intensity hand-grip exercise on FBF was measured in the presence of l-NMMA and vehicle and also with vehicle throughout. D: effect of simultaneous LBNP with low- and high-intensity exercise on FBF was measured in the presence of l-NMMA, SMTC, and saline control in a cross-over study.
Effects of SMTC and l-NMMA on FBF during sympathetic activation.
Saline vehicle was infused for 15 min to establish baseline FBF, followed by infusion of SMTC (0.2 μmol/min for 15 min; n = 8) and, in a separate group of subjects, l-NMMA (2 μmol/min for 15 min; n = 8). During the final 3 min of each infusion period, reflex sympathetic activation was induced by applying −20 mmHg LBNP using a lower body chamber sealed above the level of the iliac crest. This level of LBNP simulates mild orthostatic stress, unloading the cardiopulmonary mechanoreceptors, leading to a reflex increase in sympathetic output and reproducible reduction in FBF (15, 31).
Effects of β-adrenergic blockade on FBF during sympathetic activation.
After baseline FBF was established, −20 mmHg LBNP was applied for 5 min. After return of FBF to baseline, propranolol (50 μg/min; n = 12) was infused for 5 min under basal conditions and during a further application of LBNP (−20 mmHg for 5 min).
Effects of l-NMMA on FBF immediately after handgrip exercise.
After baseline FBF during infusion of saline was established, subjects performed handgrip exercise using a modified Grip dynamometer (US Gauge) for 3 min at 30 pulls/min at low intensity (30% maximal voluntary contraction) and for 3 min during high-intensity (80% maximal voluntary contraction) handgrip exercise. After ∼25 min recovery, l-NMMA (2 μmol/min; n = 11) was infused for 7 min at rest, during further 3-min periods of low- and high-intensity handgrip exercise, and during FBF measurements immediately after exercise. These were made for 1 min immediately after the cessation of handgrip exercise with the mean of at least 5 measurements used for analysis. To control for a carry-over effect of the first period of exercise, this protocol was repeated in a separate group of subjects (n = 11) with the infusion of saline control in place of l-NMMA during the second two periods of exercise.
Effects of SMTC and l-NMMA on FBF immediately after exercise during LBNP.
FBF remained persistently elevated after exercise despite a prolonged period of recovery. This final protocol was, therefore, performed on separate days. After baseline FBF was established, subjects had an infusion of either saline (n = 10), SMTC (0.2 μmol/min; n = 10), or l-NMMA (2 μmol/min; n = 10) for 7 min at rest, during 3-min periods of low- and high-intensity handgrip exercise, and during FBF measurements 1 min after exercise. This was done in the presence of −20 mmHg LBNP, which commenced immediately before exercise and continued after exercise during the measurement of FBF.
Statistical analysis.
Data were summarized as means ± SE. Vasoconstrictor responses to SMTC, l-NMMA, and LBNP were expressed as the absolute decrease in FBF and percent reduction in FBF from baseline. Vasodilator responses to exercise were expressed as the absolute FBF. This method of expressing FBF responses has previously been shown to be the most reproducible (29). Effects of the NOS inhibitors on the blood flow responses were analyzed by one-way ANOVA or by ANOVA for repeated measures as appropriate. All tests were two-tailed, and differences were considered significant when P < 0.05.
RESULTS
Propranolol does not affect the FBF response to LBNP.
Propanolol was used to examine the possible contribution of β-adrenergic activation to opposing the vasoconstrictor response to sympathetic stimulation by LBNP. Propranolol had no significant effect on basal FBF (data not shown) or on the response to LBNP (−34 ± 3.5 vs. −27 ± 4.2% reduction during saline and propranolol, respectively; P = 0.21).
l-NMMA but not SMTC augments the FBF response to LBNP.
l-NMMA and SMTC were used to examine the possible contributions of NOS (eNOS and nNOS) and nNOS, respectively, in opposing the vasoconstrictor response to sympathetic stimulation by LBNP. At rest and in the absence of LBNP, both l-NMMA (2 μmol/min) and SMTC (0.2 μmol/min) significantly reduced FBF by 25.8 ± 3.0% (from 2.76 ± 0.20 to 2.02 ± 0.12 ml·min−1·100 ml−1; P < 0.001; Fig. 2A) and 20.6 ± 3.3% (from 3.42 ± 0.31 to 2.71 ± 0.27 ml min/100 ml; P < 0.01, P = 0.38 compared with l-NMMA; Fig. 2B), respectively. LBNP (−20 mmHg) reduced FBF by 23.4 ± 5.7% (from 2.75 ± 0.20 to 2.12 ± 0.22 ml·min−1·100 ml−1; P < 0.01) during saline vehicle infusion. In the presence of l-NMMA, LBNP reduced flow by 44.2 ± 3.5% (from 2.02 ± 0.12 to 1.14 ± 0.11 ml·min−1·100 ml−1), significantly more than during saline vehicle (P < 0.01 vs. saline; Fig. 2A). By contrast, the reduction in FBF by LBNP during infusion of SMTC (28.5 ± 4.0% reduction from 2.71 ± 0.27 to 1.92 ± 0.18 ml·min−1·100 ml−1) did not differ significantly from that observed during saline infusion (a 34.1 ± 3.0% reduction, from 3.42 ± 0.31 to 2.24 ± 0.20 ml·min−1·100 ml−1; P = 0.32; Fig. 2B). Thus the relative reduction in FBF in response to LBNP was significantly greater in the presence of l-NMMA than in the presence of SMTC (44.2 ± 3.5 vs. 28.5 ± 4.0%; P < 0.01; Fig. 2C).
Fig. 2.
A: FBF at rest and during LBNP during infusion of saline and l-NMMA. *P < 0.01 vs. saline with no LBNP; **P < 0.001 vs. saline with no LBNP; †P < 0.001 vs. l-NMMA with no LBNP. B: FBF at rest and during LBNP during infusion of saline and SMTC. *P < 0.001 vs. saline with no LBNP; **P < 0.01 vs. saline with no LBNP; †P < 0.01 vs. SMTC with no LBNP. C: percent change in FBF during l-NMMA and SMTC with LBNP. *P < 0.01 vs. SMTC (+, LBNP; −, no LBNP).
l-NMMA does not affect the FBF response immediately after exercise.
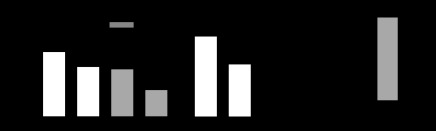
l-NMMA was used to examine the possible contribution of NOS (both eNOS and nNOS) to the increase in FBF immediately after exercise. During saline infusion, FBF increased from a baseline of 2.70 ± 0.29 to 11.06 ± 1.16 and 17.86 ± 1.06 ml·min−1·100 ml−1immediately after low- and high-intensity handgrip exercise, respectively. An increase in FBF persisted after exercise despite a 25-min period of recovery (2.78 ± 0.17 ml·min−1·100 ml−1before exercise vs. 5.65 ± 0.36 ml·min−1·100 ml−1after recovery). Nevertheless, the FBF elicited by the second period of low- and high-intensity handgrip in the presence of l-NMMA was not significantly different to that observed during saline infusion (Fig. 3A; P = 0.22 and P = 0.32, respectively). In the control study where saline vehicle was infused in place of l-NMMA, a similar persistent increase in FBF was seen after 25-min recovery from the first period of exercise (2.70 ± 0.29 ml·min−1·100 ml−1before exercise vs. 4.97 ± 0.51 ml·min−1·100 ml−1after recovery). However, the FBF elicited immediately after the first exercise periods was not significantly different to that during the second exercise periods of low- and high-intensity exercise (P = 0.73 and P = 0.63, respectively).
Fig. 3.
A: comparison of FBF after undertaking low- and high-intensity exercise, during saline or l-NMMA infusion. There was no significant difference in FBF during l-NMMA infusion when compared with saline during either low-intensity exercise (P = 0.22, n = 11) or high-intensity exercise (P = 0.32, n = 11). B: a comparison of the FBF immediately after low- and high-intensity exercise and LBNP while infusing saline, l-NMMA, or SMTC. There is no significant difference in FBF during l-NMMA or SMTC infusion when compared with saline, for either low-intensity exercise (P = 0.91 and P = 0.44, respectively; n = 10) or high-intensity exercise (P = 0.46 and P = 0.68, respectively; n = 10).
Neither l-NMMA nor SMTC affects the FBF response immediately after exercise during LBNP.
l-NMMA and SMTC were used to examine the possible contributions of NOS (eNOS and nNOS) and nNOS, respectively, to the increase in FBF immediately after exercise in the presence of additional sympathetic stimulation with LBNP. On separate study days, l-NMMA (2 μmol/min) and SMTC (0.2 μmol/min) reduced resting blood flow to a similar degree, by 23.2 ± 2.2% (from 5.40 ± 0.72 to 4.21 ± 0.61 ml·min−1·100 ml−1; P < 0.001) and 19.6 ± 2.5% (from 5.46 ± 0.73 to 4.42 ± 0.60 ml·min−1·100 ml−1; P < 0.001), respectively (P = 0.29 for l-NMMA vs. SMTC). The FBF in response to low- and high-intensity exercise during simultaneous LBNP (−20 mmHg) in the presence of l-NMMA was not significantly different to that during saline (P = 0.91 and P = 0.44 for low and high intensity, respectively), SMTC compared with saline (P = 0.46 and P = 0.68 for low and high intensity, respectively), and SMTC compared with l-NMMA (P = 0.31 and P = 0.76 for low and high intensity, respectively; Fig. 3B). Results did not differ when data were analyzed using a one-way ANOVA on all subjects or a repeated-measurements ANOVA restricted to subjects that attended all study days and thus had data during infusion of saline, l-NNMA, or SMTC.
DISCUSSION
Until recently it had been assumed that the NO that regulates local blood flow under physiological conditions in humans derives exclusively from eNOS. However, first-in-human studies with the nNOS-selective inhibitor SMTC showed that the basal regulation of vascular tone in both the forearm and coronary circulations is mediated by nNOS, whereas eNOS mediates relaxant responses to pharmacological and shear stress stimuli (24, 25). Cutaneous vasodilatation induced by heat stress is also mediated by nNOS in humans (17). The autonomic nervous system could activate local release of NO from nNOS through a neuronal link and/or eNOS through local release of neurotransmitters such as norepinephrine. To our knowledge, this is the first study to examine the role of nNOS- and eNOS-mediated vasomotor responses to physiological stimuli that modulate activity of the sympathetic and possibly other neural pathways within the autonomic nervous system. Nonisoform selective NOS inhibition with l-NMMA augmented the forearm vasoconstrictor response to LBNP, whereas selective nNOS inhibition (despite causing a similar reduction in basal FBF to that observed with l-NMMA) had no significant effect on the vasoconstrictor response to LBNP. This suggests that reflex sympathetic vasoconstriction at rest is partially opposed by eNOS- but not by nNOS-derived NO. These results are consistent with observations in the rat hind limb where nonselective NOS inhibition with NG-nitro-l-arginine methyl ester (l-NAME) increases muscle vasoconstriction in response to lumbar sympathetic nerve stimulation (27).
The simplest explanation for the augmented vasoconstriction to sympathetic stimulation after NOS inhibition is that norepinephrine released from sympathetic nerves in response to LBNP stimulates eNOS through α- or β-adrenergic receptors, actions that have previously been described in animals and humans (18). The nonselective β-adrenergic receptor antagonist propranolol had no significant effect on the FBF response to LBNP, suggesting that eNOS-derived NO attenuates α-adrenergic mediated vasoconstriction. It is not possible to probe this with an α-antagonist since this would not distinguish between antagonism of direct α-receptor mediated vasoconstriction or of α-receptor mediated activation of eNOS.
To our knowledge, the present results are the first in the human to demonstrate that sympathetic activation stimulates NO release from eNOS, which attenuates vasoconstriction. This finding could have important potential clinical implications with eNOS dysfunction, secondary to cardiovascular factors, leading to enhanced vasoconstriction and hence an enhanced blood pressure response to sympathetic activation.
Increases in blood flow during exercise depend upon short-term adjustments and interplay between autonomic influences and local regulatory factors. Previous human and animal studies examining the role of NO during functional hyperemia have yielded conflicting results. Many animal models do suggest a potential role for NO, and more specifically nNOS-derived NO in regulating blood flow by blunting the vasoconstrictor response to α-adrenergic activation during dynamic exercise (functional sympatholysis) and/or contributing to exercise-induced vasodilation. These include a dog hind limb model of exercise (1) a rat model of exercise (5) and nNOS null mice (9, 12, 19, 26). In the mdx mouse, a mouse model of Duchenne Muscular Dystrophy where dystrophin deficiency results in reduced nNOS expression in skeletal muscle, the normal ability of muscle contraction to attenuate α-adrenergic vasoconstriction is defective (26). Kobayashi and colleagues have suggested that nNOS in skeletal muscle contributes to increased blood flow after mild exercise in mouse models (19).
In humans, a number of studies have shown a reduction in FBF during or immediately after exercise in response to nonselective NOS inhibition with l-NMMA or l-NAME (8, 10, 11, 30). However, the size of the effects has been small and, when compared with a vasoconstrictor control with similar effects on resting blood flow, l-NMMA has been found to have no significant effect on blood flow responses during exercise (7). Chavoshan and colleagues (2) examined the effect of a reflex increase in sympathetic efferent activity (induced by LBNP) on muscle perfusion (assessed from measurement of muscle oxygenation by near infra-red spectroscopy) during exercise. Systemic NOS inhibition with l-NAME completely reversed the blunted vasoconstrictor response to LBNP in the exercising forearm. However, such results could be influenced by the reflex response to the systemic effects of l-NAME (which include a rise in mean arterial blood pressure). Dinenno and Joyner (7) examined the effects of local NOS inhibition with l-NMMA or l-NAME on FBF responses during handgrip exercise and while stimulating release of endogenous norepinephrine (by intra-arterial infusion of tyramine). Neither NOS inhibitor restored the vasoconstrictor response to local tyramine-stimulated norepinephrine release during exercise, arguing against a role for NO in functional sympatholysis (7).
Our results using l-NMMA show no significant effect of l-NMMA to blunt blood flow immediately after exercise. Similarly, even in the face of increased sympathetic stimulation with LBNP, we found no significant effect of either l-NMMA or SMTC on FBF responses immediately after exercise. These results support the findings of Dinenno and Joyner (7) by using a different sympathetic stimulus and using both nonselective and selective inhibitors of NOS. It is likely that there are multiple mechanisms involved in exercise hyperaemia and functional sympatholysis including metabolic mediators such as adenosine, ATP, potassium, hypoxia, and hydrogen ions that may link blood flow to metabolic demands (3). Nonadrenergic, noncholinergic peptides such as calcitonin gene-related protein may also be involved (13). The present results and those of other investigators showing lack of effect of inhibition of NOS on exercise-induced hyperaemia are consistent with the proposal by Clifford and Hellsten that there is a redundancy of vasodilators contributing to exercise-induced hyperemia where one vasoactive compound may take over when the formation of another is inhibited (3).
Limitations.
It is important to note that our measurements were made immediately after exercise rather than during exercise and therefore the results do not necessarily mean that regulation of blood flow during exercise is independent of eNOS- or nNOS-derived NO. However, lack of effect of NOS inhibition after exercise is an important finding in its own right since studies in the nNOS null mouse implicate a role for nNOS in enhancing blood flow after exercise (19): our results suggest an important species difference and/or a phenotype of the nNOS null mouse that alters vascular reactivity independent of nNOS.
Our results pertain to healthy men, and cannot be extrapolated to women or subjects with cardiovascular risk factors. Due to the limited sample size we cannot exclude a small effect of nNOS to oppose sympathetically mediated tone or an effect of eNOS/nNOS on exercise blood flow. Our study involved acute NOS inhibition, and results could differ from those of studies involving chronic nNOS inhibition or absent nNOS such as in a knock-out murine model. Studies were undertaken in the human forearm and therefore do not allow separation of discrete fiber types, which may show a differential regulation of NOS activity. SMTC has relatively high specificity for nNOS and to be sure SMTC was acting specifically on nNOS, we used a concentration previously shown not to inhibit eNOS-mediated responses (24, 25) but which reduced basal FBF to a similar degree to concentrations of l-NMMA, that inhibit eNOS-mediated responses. However, should more specific inhibitors of nNOS become available for human use it would be advisable to use these to confirm our findings.
In conclusion, these results suggest that in healthy normotensive men, sympathetic activation may increase NO release from eNOS attenuating vasoconstrictor responses. Dysfunction of eNOS, as occurs in many conditions associated with increased cardiovascular risk, could, therefore, contribute to an increase in vascular resistance and blood pressure during sympathetic stimulation. However, neither eNOS nor nNOS plays an essential role in postexercise hyperemia, even in the presence of increased sympathetic activation.
GRANTS
This work was supported by British Heart Foundation Fellowship FS/09/062/27958 (to H. Shabeeh) and a National Institute for Health Research (NIHR) Biomedical Research Center award to Guy's and St. Thomas′ National Health Services Foundation Trust in partnership with King's College London and King's College Hospital National Health Services Foundation Trust and a Leducq Foundation Transatlantic Network of Excellence award (to B. Casadei and A. M. Shah).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.S., M.S., S.B., N.M., B.C., A.M.S., and P.C. completed conception and design of research; H.S., M.S., S.B., and P.C. performed experiments; H.S., M.S., S.B., N.M., B.C., A.M.S., and P.C. analyzed data; H.S., M.S., S.B., N.M., B.C., A.M.S., and P.C. interpreted results of experiments; H.S. and P.C. prepared figures; H.S., A.M.S., and P.C. drafted manuscript; H.S., M.S., S.B., N.M., B.C., A.M.S., and P.C. edited and revised manuscript; H.S., M.S., S.B., N.M., B.C., A.M.S., and P.C. approved final version of manuscript.
REFERENCES
- 1. Buckwalter JB, Taylor JC, Hamann JJ, Clifford PS. Role of nitric oxide in exercise sympatholysis. J Appl Physiol 97: 417–423, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Chavoshan B, Sander M, Sybert TE, Hansen J, Victor RG, Thomas GD. Nitric oxide-dependent modulation of sympathetic neural control of oxygenation in exercising human skeletal muscle. J Physiol 540: 377–386, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol 97: 393–403, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Cockcroft JR, Chowienczyk PJ, Brett SE, Ritter JM. Effect of NG-monomethyl-l-arginine on kinin-induced vasodilation in the human forearm. Br J Clin Pharmacol 38: 307–310, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Copp SW, Hirai DM, Ferguson SK, Musch TI, Poole DC. Role of neuronal nitric oxide synthase in modulating microvascular and contractile function in rat skeletal muscle. Microcirculation 18: 501–511, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Copp SW, Hirai DM, Schwagerl PJ, Musch TI, Poole DC. Effects of neuronal nitric oxide synthase inhibition on resting and exercising hindlimb muscle blood flow in the rat. J Physiol 588: 1321–1331, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dinenno FA, Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory? J Physiol 553: 281–292, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Endo T, Imaizumi T, Tagawa T, Shiramoto M, Ando S, Takeshita A. Role of nitric oxide in exercise-induced vasodilation of the forearm. Circulation 90: 2886–2890, 1994 [DOI] [PubMed] [Google Scholar]
- 9. Fadel PJ, Zhao W, Thomas GD. Impaired vasomodulation is associated with reduced neuronal nitric oxide synthase in skeletal muscle of ovariectomized rats. J Physiol 549: 243–253, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gilligan DM, Panza JA, Kilcoyne CM, Waclawiw MA, Casino PR, Quyyumi AA. Contribution of endothelium-derived nitric oxide to exercise-induced vasodilation. Circulation 90: 2853–2858, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Gordon MB, Jain R, Beckman JA, Creager MA. The contribution of nitric oxide to exercise hyperemia in the human forearm. Vasc Med 7: 163–168, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Grange RW, Isotani E, Lau KS, Kamm KE, Huang PL, Stull JT. Nitric oxide contributes to vascular smooth muscle relaxation in contracting fast-twitch muscles. Physiol Genomics 5: 35–44, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Hasbak P, Lundby C, Olsen NV, Schifter S, Kanstrup IL. Calcitonin gene-related peptide and adrenomedullin release in humans: effects of exercise and hypoxia. Regul Pept 108: 89–95, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Hokanson DE, Sumner DS, Strandness DE., Jr An electrically calibrated plethysmograph for direct measurement of limb blood flow. IEEE Trans Biomed Eng 22: 25–29, 1975 [DOI] [PubMed] [Google Scholar]
- 15. Johnson JM, Rowell LB, Niederberger M, Eisman MM. Human splanchnic and forearm vasoconstrictor responses to reductions of right atrial and aortic pressures. Circ Res 34: 515–524, 1974 [DOI] [PubMed] [Google Scholar]
- 16. Kavdia M, Popel AS. Contribution of nNOS- and eNOS-derived NO to microvascular smooth muscle NO exposure. J Appl Physiol 97: 293–301, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Kellogg DL, Jr, Zhao JL, Wu Y. Neuronal nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. J Physiol 586: 847–857, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol 36: 1233–1238, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Kobayashi YM, Rader EP, Crawford RW, Iyengar NK, Thedens DR, Faulkner JA, Parikh SV, Weiss RM, Chamberlain JS, Moore SA, Campbell KP. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature 456: 511–515, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kobzik L, Reid MB, Bredt DS, Stamler JS. Nitric oxide in skeletal muscle. Nature 372: 546–548, 1994 [DOI] [PubMed] [Google Scholar]
- 21. Li H, Forstermann U. Nitric oxide in the pathogenesis of vascular disease. J Pathol 190: 244–254, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Michel T, Feron O. Nitric oxide synthases: which, where, how, and why? J Clin Invest 100: 2146–2152, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moncada S, Higgs A. The l-arginine-nitric oxide pathway. N Engl J Med 329: 2002–2012, 1993 [DOI] [PubMed] [Google Scholar]
- 24. Seddon M, Melikian N, Dworakowski R, Shabeeh H, Jiang B, Byrne J, Casadei B, Chowienczyk P, Shah AM. Effects of neuronal nitric oxide synthase on human coronary artery diameter and blood flow in vivo. Circulation 119: 2656–2662, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Seddon MD, Chowienczyk PJ, Brett SE, Casadei B, Shah AM. Neuronal nitric oxide synthase regulates basal microvascular tone in humans in vivo. Circulation 117: 1991–1996, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Thomas GD, Sander M, Lau KS, Huang PL, Stull JT, Victor RG. Impaired metabolic modulation of alpha-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc Natl Acad Sci USA 95: 15090–15095, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thomas GD, Victor RG. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol 506: 817–826, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet 2: 997–1000, 1989 [DOI] [PubMed] [Google Scholar]
- 29. Walker HA, Jackson G, Ritter JM, Chowienczyk PJ. Assessment of forearm vasodilator responses to acetylcholine and albuterol by strain gauge plethysmography: reproducibility and influence of strain gauge placement. Br J Clin Pharmacol 51: 225–229, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wray DW, Witman MA, Ives SJ, McDaniel J, Fjeldstad AS, Trinity JD, Conklin JD, Supiano MA, Richardson RS. Progressive handgrip exercise: evidence of nitric oxide-dependent vasodilation and blood flow regulation in humans. Am J Physiol Heart Circ Physiol 300: H1101–H1107, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zoller RP, Mark AL, Abboud FM, Schmid PG, Heistad DD. The role of low pressure baroreceptors in reflex vasoconstrictor responses in man. J Clin Invest 51: 2967–2972, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]