Abstract
Hydrogen sulfide (H2S) therapy protects nondiabetic animals in various models of myocardial injury, including acute myocardial infarction and heart failure. Here, we sought to examine whether H2S therapy provides cardioprotection in the setting of type 2 diabetes. H2S therapy in the form of sodium sulfide (Na2S) beginning 24 h or 7 days before myocardial ischemia significantly decreased myocardial injury in db/db diabetic mice (12 wk of age). In an effort to evaluate the signaling mechanism responsible for the observed cardioprotection, we focused on the role of nuclear factor E2-related factor (Nrf2) signaling. Our results indicate that diabetes does not alter the ability of H2S to increase the nuclear localization of Nrf2, but does impair aspects of Nrf2 signaling. Specifically, the expression of NADPH quinine oxidoreductase 1 was increased after the acute treatment, whereas the expression of heme-oxygenase-1 (HO-1) was only increased after 7 days of treatment. This discrepancy was found to be the result of an increased nuclear expression of Bach1, a known repressor of HO-1 transcription, which blocked the binding of Nrf2 to the HO-1 promoter. Further analysis revealed that 7 days of Na2S treatment overcame this impairment by removing Bach1 from the nucleus in an Erk1/2-dependent manner. Our findings demonstrate for the first time that exogenous administration of Na2S attenuates myocardial ischemia-reperfusion injury in db/db mice, suggesting the potential therapeutic effects of H2S in treating a heart attack in the setting of type 2 diabetes.
Keywords: hydrogen sulfide, type 2 diabetes, myocardial infarction, nuclear factor E2-related factor
the endogenously produced gasotransmitter hydrogen sulfide (H2S) exerts cytoprotective effects in various models of cardiac injury. Specifically, treatment with H2S before, during, or after myocardial ischemia decreases injury (3, 6, 25). Furthermore, chronic H2S therapy improves survival and protects against ischemic-induced heart failure (2). These cytoprotective effects have been attributed to the ability of H2S to upregulate antioxidant defenses and to reduce apoptosis, inflammation, and mitochondrial injury (22). Increasing evidence suggests that hyperglycemia-induced reactive oxygen species production contributes to oxidative stress, resulting in myocardial damage (1). Thus the reported antioxidant effects of H2S may be of critical importance in the diabetic myocardium. H2S upregulates endogenous antioxidant defenses by activating nuclear factor E2-related factor (Nrf2), a member of the NF-E2 family of nuclear basic leucine zipper transcription factors (3). Nrf2 regulates the gene expression of a number of enzymes that serve to detoxify pro-oxidative stressors (7), including heme-oxygenase-1 (HO-1) and NADPH quinine oxidoreductase 1 (NQO1), by binding to the antioxidant response element (ARE) found in the promoter region of the gene (30, 38).
H2S is produced enzymatically in mammalian species via the action of three enzymes in the cysteine biosynthesis pathway: cystathionine-γ-lyase (CSE), cystathionine-β-synthase (CBS), and 3-mercaptopyruvate sulfurtransferase (3-MST). Several studies suggest that streptozotocin (STZ)-induced diabetes (model of type 1 diabetes) increases the expression of CSE and CBS in the pancreas, kidney, and liver (36, 37). In contrast, additional studies found that STZ does not alter the expression of either enzyme in the aorta, liver, kidney, or heart (4, 27). Despite this contrasting evidence about the effects of STZ-induced diabetes on the expression of CSE and CBS, there is clear evidence that circulating levels of H2S are decreased in animal models of diabetes (12, 27, 37) and may even contribute to the pathophysiology of diabetes (28). More importantly, lower circulating H2S levels have been detected in plasma samples taken from patients with type 2 diabetes mellitus (T2DM) (12, 32).
Recently, therapeutic strategies aimed at increasing the levels of H2S have been shown to provide protection against cardiomyopathy and vascular dysfunction in models of STZ-induced diabetes (5, 27). However, it has yet to be determined whether it has similar effects in the setting of T2DM. Therefore, the purpose of this study was to investigate the potential cardioprotective effects of H2S therapy in the setting of T2DM using a well-established in vivo mouse model of myocardial ischemia-reperfusion (MI/R) injury.
MATERIALS AND METHODS
Animals.
Male nondiabetic (C57BLKS/J) and diabetic (BKS.Cg-Dock7M+/+Leprdb/J mice; Jackson Laboratory, Bar Harbor, ME) mice were used at 12 wk of age. All experimental mouse procedures were approved by the Institute for Animal Care and Use Committee at Emory University School of Medicine and conformed to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH; Publication No. 86-23, revised 1996) and with federal and state regulations.
Materials.
Sodium sulfide (Na2S; catalog No. 407410; Sigma Aldrich) was dissolved in saline and administered by using a 32-gauge needle at a dose of 0.1 mg/kg in a final volume of 50 μl via a tail vein injection. Mice received Na2S either 24 h before experimentation or as a daily injection for 7 days. Saline was administered in the same manner for the respective vehicle groups. In all cases, Na2S was prepared just before use. Groups of mice also received 1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio) butadiene (U0126; 0.1 mg/kg).
Blood glucose determination.
Blood obtained via a tail snip was screened using a Xtra glucose-monitoring system (Precision).
MI/R protocol and myocardial injury assessment.
Surgical ligation of the left coronary artery, myocardial infarct size determination, and Troponin I measurements were performed similar to methods described previously (3).
Oxidative stress.
The degree of lipid peroxidation was determined by evaluating the levels of malondialdehyde in heart tissue using a commercially available thiobarbituric acid reactive substances assay kit according to the manufacturer's instructions (catalog No. ALX-850-287-KI01; Enzo Life Sciences). Concentrations of 8-isoprostane in the heart were determined by 8-isoprostane EIA kit according to manufacturer's instruction (catalog No. 516351; Cayman Chemicals).
Subcellular fractionation and Western blot analysis.
Samples of the heart were homogenized and fractionated into whole cell and nuclear fractions as described previously (3). Equal amounts of protein were loaded into lanes of polyacrylamide-SDS gels, and Western blot analysis was performed as previously described (3).
Chromatin immunoprecipitation.
Chromatin immunoprecipitation studies were performed with Imprint chromatin immunoprecipitation (ChIP) kit (Sigma-CHP1) according to the manufacturer's instrunctions. Briefly, heart samples (40 mg) were cross-linked with 1% formaldehyde, homogenized, and sonicated to shear DNA. A portion of the precleared chromatin was stored and labeled as input DNA. Equal amounts of chromatin were immunoprecipitated with the following antibodies: Nrf2 (Santa Cruz), Bach1 (Santa Cruz), and control IgG. The chromatin was washed and the crosslinks were hydrolyzed. The DNA was then purified through DNA binding column and subjected to quantitative PCR analysis using SYBER Green-based detection according to the manufacturer's instructions (Applied Biosystems). PCR primers were designed to amplify the fragment of the HO-1 and NQO1 promoter region containing the Nrf2 or Bach1 binding motif (ARE). The primers are as follows: HO-1, forward, 5′-AGG TAC ACA TCC AAG CCG AGA A-3′; HO-1, reverse, 5′-CTC TGG ACA CCT GAC CCT TCT G-3′; NQO1, forward, 5′-GCA GTT TCT AAG AGC AGA ACG-3′; NQO1, reverse, 5′-GTA GAT TAG TCC TCA CTC AGC CG-3′. Results were calculated according the manufacturer's recommendations and presented as fold enrichment to the input DNA.
Measurement of hydrogen sulfide and sulfane sulfur.
Hydrogen sulfide and sulfane sulfur levels were measured in tissue and blood according to previously described methods (23). Fresh tissue was homogenized in 5 vol of PBS (pH 7.4). For measurement of hydrogen sulfide, 0.2 ml of the sample homogenate was placed in a small glass vial (5182-0553; Agilent Technologies, Santa Clara, CA) along with 0.4 ml of 1 M sodium citrate buffer, pH 6.0, and sealed. The mixture was incubated at 37°C for 10 min with shaking at 125 rpm on a rotary shaker (Fisher Scientific) to facilitate the release of H2S gas from the aqueous phase. After the mixture was shaked, 0.1 ml of head-space gas was applied to a gas chromatograph (7890A GC System; Agilent) equipped with a dual plasma controller and chemiluminescence sulfur detector (355; Agilent) and a data processor. The carrier gas was helium with a flow rate of 2.4 ml/min. For the measurement of H2S released from bound sulfane sulfur, 0.1 ml of the sample homogenates and 0.1 ml of 15 mM DTT in 0.1 mM Tris·HCl (pH 9.0) were placed in a small glass vial, sealed, and incubated at 37°C for 50 min. After incubation, 0.4 ml of 1 M sodium citrate buffer was injected through the rubber stopper and the mixture was incubated at 37°C for 10 min with shaking at 125 rpm on a rotary shaker to facilitate the release of H2S gas from the aqueous phase. After the mixture was shaked, 0.1 ml of head-space gas was applied to a gas chromatograph as detailed above. For the measurement of H2S and sulfane sulfur in blood, 0.1 ml and 0.05 ml of whole blood was used for each measurement, respectively. The concentrations of H2S in the samples were calculated using a standard curve of Na2S as a source of H2S. Chromatographs were captured and analyzed with Agilent ChemStation software (B.04.03). For tissue, the amount of H2S is reported as nanomole per milligram wet weight. For the blood, the amount of H2S is reported as micromoles.
Enzymatic production of hydrogen sulfide.
Enzyme reactions to determine the production of H2S from 3-MST were performed as described previously (24). Briefly, heart tissue was homogenized in buffer containing 100 mM potassium phosphate (pH 7.4), 1 mM DTT, and protease inhibitor cocktail complete. For enzyme reactions, 0.2 ml of the homogenate was incubated with 0.022 ml of substrate mix (10 mM L-cysteine and 0.5 mM α-ketoglutarate) in a sealed vial at 37°C for 50 min. After 0.444 ml of 1 M sodium citrate buffer (pH 6.0) was added, the mixtures were incubated at 37°C for 10 min with shaking on a rotary shaker to facilitate a release of H2S gas from the aqueous phase. After the mixtures were shaked, 0.1 ml of head-space gas was applied to a gas chromatograph as described above.
Enzyme reactions to determine the production of H2S from CBS and CSE were performed as described previously (37) with slight modification. Briefly, heart tissue was homogenized in buffer containing 100 mM potassium phosphate (pH 7.4). For enzyme reactions, 0.172 ml of homogenate was incubated with 0.028 ml of substrate mix (10 mM L-cysteine and 2 mM pyridoxal 5′-phosphate) in a sealed vial at 37°C for 30 min. After 0.400 ml of 1 M sodium citrate buffer (pH 6.0) was added, the mixtures were incubated at 37°C for 10 min with shaking on a rotary shaker to facilitate a release of H2S gas from the aqueous phase. After mixture was shaken, 0.1 ml of head-space gas was applied to a gas chromatograph as described above. For both reactions, the H2S concentration of each sample was calculated against a calibration curve of Na2S and results are expressed as nanomole H2S formed per milligram of soluble protein (determined by using the DC protein assay; Bio-Rad).
Isolation of mRNA and taqman quantitative PCR.
RNA was isolated using the RiboPure kit according to manufacturer's instructions (Ambion). Reverse transcription was performed in a standard fashion with QuantiTect Reverse Transcription Kit (QIAGEN) supplemented with DNase treatment. Taqman quantitatie PCR was carried out according to the manufacturer's instructions using probe sets for Cbs, Cth, and Mpst and 18S (Applied Biosystems). Analysis was carried out using the ΔΔ-CT method with 18S correction and reported as relative fold change versus nondiabetic controls.
Statistical analysis.
All data in this study are expressed as means ± SE. Differences in data between the groups were compared using Prism 4 (GraphPad Software) with Student's paired two-tailed t-test or one-way ANOVA where appropriate. For the one-way ANOVA, if a significant variance was found, the Tukey test was used as the post hoc analysis. A P value less than 0.05 was considered significant.
RESULTS
Sulfide levels are decreased in diabetic mice.
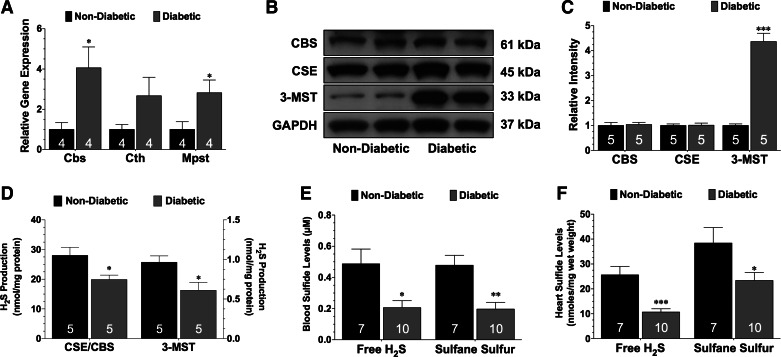
Diabetic mice exhibited the typical characteristics of a severe diabetic phenotype when compared with nondiabetic mice, including marked obesity and hyperglycemia (Table 1). Initial studies examined the effects of diabetes on the gene and protein expression of the three known H2S-producing enzymes, as well as the levels of circulating and myocardial sulfide. Quantitative PCR analysis revealed that the gene expression of all three enzymes were elevated in the diabetic heart compared with the nondiabetic heart (Fig. 1A). The protein expression of CBS and CSE were unaltered in the diabetic heart compared with the nondiabetic heart (Fig. 1B). However, the expression of 3-MST was significantly upregulated (P < 0.001 vs. nondiabetic). Further studies revealed that the biosynthesis of H2S from the pyridoxal-5′-phosphate-dependent enzymes, CBS and CSE, as well as from 3-MST was decreased in the diabetic heart (Fig. 1D; P < 0.05 vs. nondiabetic). Finally, free H2S and sulfane sulfur levels were significantly lower in the blood and heart of diabetic mice compared with nondiabetic mice (Fig. 1, E and F; P < 0.05).
Table 1.
Body weights and blood glucose levels
| Group | n | Body Weight, g | Blood Glucose, mg/dL |
|---|---|---|---|
| Nondiabetic | 5 | 25.7 ± 0.6 | 199 ± 19 |
| Diabetic | 5 | 44.4 ± 0.4*** | 456 ± 25*** |
| Diabetic + vehicle | 8 | 46.7 ± 0.9*** | 506 ± 27*** |
| Diabetic + Na2S the day before experimentation | 8 | 46.9 ± 1.8*** | 508 ± 49*** |
| Diabetic + Na2S for 7 days before experimentation | 10 | 46.1 ± 1.1*** | 490 ± 29*** |
| Diabetic + vehicle | 8 | 43.5 ± 1.1*** | 450 ± 28*** |
| Diabetic + U0126 for 7 days | 8 | 47.5 ± 2.2*** | 523 ± 21*** |
| Diabetic + U0126 + Na2S for 7 days | 8 | 47.2 ± 1.1*** | 448 ± 32*** |
Values are means ± SE.
P < 0.001 vs. nondiabetic. Na2S, hydrogen sulfide therapy in the form of sodium sulfide.
Fig. 1.
Diabetes reduces sulfide levels. A: mRNA expression of the genes that encode for cystathionine-β-synthase (Cbs), cystathionine-γ-lyase (Cth), and 3-mercaptopyruvate sulfutransferase (Mpst) in the hearts of nondiabetic and diabetic mice. B and C: representative immunoblots and densitometric analysis of cystathionine-γ-lyase (CSE), CBS, and 3-mercaptopyruvate sulfurtransferase (3-MST) proteins in the hearts of nondiabetic and diabetic mice. D: biosynthesis of hydrogen sulfide (H2S) from the pyridoxal-5′-phosphate (PLP)-dependent enzymes (CBS and CSE) and from 3-MST. E: circulating and myocardial free (F) H2S and sulfane sulfur levels in nondiabetic and diabetic mice. Values are means ± SE. Numbers inside of the bars indicate the number of animals that were investigated in each group. Data were compared with a Student's t-test. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. nondiabetic.
Pretreatment with Na2S therapy reduces myocardial injury after ischemia-reperfusion.
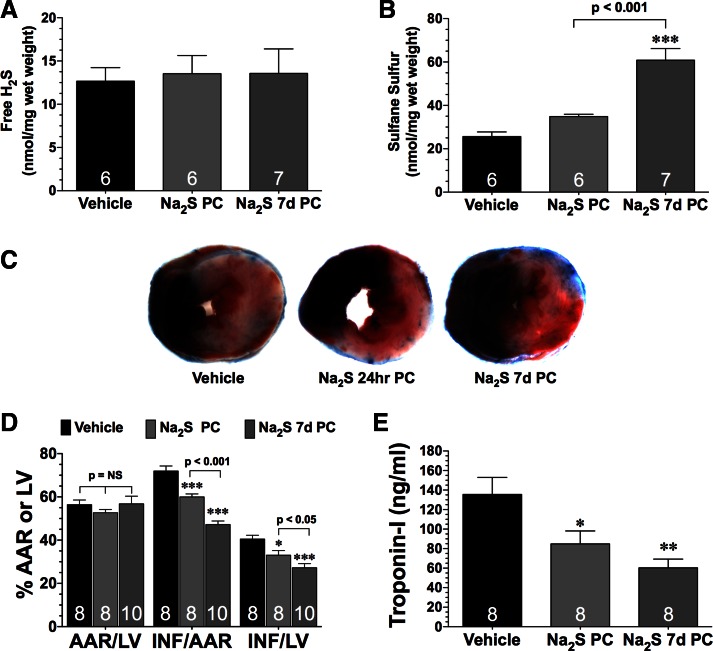
Given that diabetes is a condition in which preconditioning strategies may have clinical relevance, studies were conducted to determine whether Na2S therapy could increase sulfide levels and provide protection against acute MI/R injury. For these studies, diabetic mice received either a single tail vein injection of Na2S the day before experimentation (Na2S PC; 0.1 mg/kg) or Na2S for 7 days before experimentation (Na2S 7d PC) or vehicle. Free H2S levels were not different between the groups (Fig. 2A). However, sulfane sulfide levels were slightly higher in the hearts of the Na2S PC mice (P = not significant) and significantly increased in the hearts of Na2S 7d PC mice (Fig. 2B; P < 0.001 vs. vehicle and Na2S PC). Additional groups of mice were subjected to 30 min of ischemia and 2 h of reperfusion. Representative midventricular photomicrographs of hearts from the different groups of mice are shown in Fig. 2C. Pretreatment with Na2S for 24 h significantly decreased infarct size relative the area at risk (INF/AAR) by 17% and INF relative to the left ventricle (INF/LV) by 19% compared with vehicle-treated mice (Fig. 2D; P < 0.001). Pretreatment with Na2S for 7 days significantly decreased INF/AAR by 35% and decreased INF/LV by 33% when compared with vehicle-treated mice (Fig. 2D; P < 0.001). Comparing the infarct size lowering effects between the two therapeutic approaches reveals that the 7-day pretreatment strategy was 51% more effective in reducing INF/AAR than the acute strategy (P < 0.05). Likewise, both strategies significantly decreased circulating Troponin I levels when compared with the vehicle-treated mice (Fig. 2E; P < 0.05). These changes were independent of any effects on body weight or blood glucose levels (Table 1).
Fig. 2.
H2S therapy in the form of sodium sulfide (Na2S) pretreatment reduces the extent of myocardial injury in diabetic mice after ischemia-reperfusion. Myocardial free H2S (A) and sulfane sulfur (B) levels were from diabetic mice treated with vehicle or a single tail vein injection of Na2S the day before experimentation (Na2S PC; 0.1 mg/kg) or Na2S for 7 days before experimentation (Na2S 7d PC). C: representative midventricular photomicrographs of hearts, myocardial infarct size relative to the area-at-risk (INF/AAR) and INF relative to the left ventricle (INF/LV) (D), and circulating Troponin I levels (E) from the experimental groups after 30 min of ischemia and 2 h of reperfusion. Values are means ± SE. Data were compared through the use of a 1-way ANOVA with a Tukey test as the post hoc analysis. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. vehicle.
Na2S therapy reduced MI/R-induced oxidative stress and apoptosis.
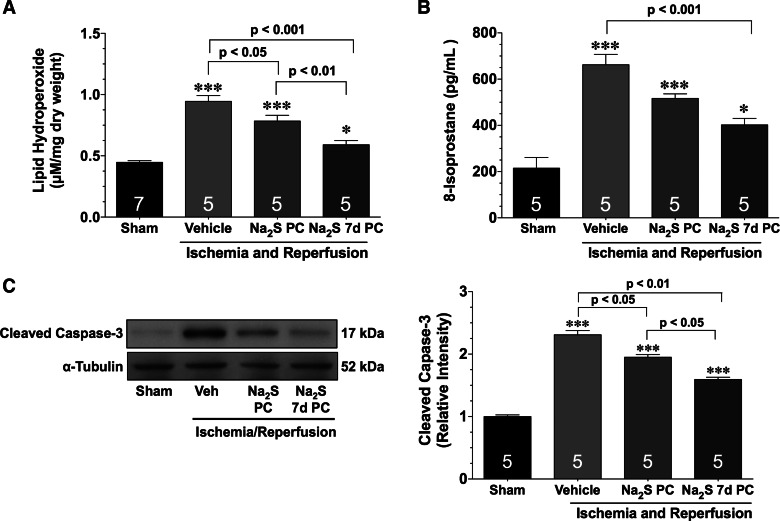
In response to MI/R injury, lipid peroxidation and 8-isoprostane levels increased in all of the groups (Fig. 3, A and B). Lipid peroxidation levels were significantly lower in the Na2S PC mice compared with the vehicle-treated mice (P < 0.05). However, both markers of oxidative stress were significantly lower in the Na2S 7d PC mice (P < 0.001 vs. vehicle). Furthermore, lipid peroxidation levels were significantly lower in the Na2S 7d PC mice compared with the Na2S PC mice (P < 0.01), whereas 8-isoprostane levels trended lower. MI/R also increased the expression of cleaved caspase-3 in the hearts of all the groups (Fig. 3, C and D; P < 0.001 vs. sham). The hearts of both groups of mice treated with Na2S exhibited a significant reduction in cleaved caspase-3 expression compared with vehicle-treated mice (P < 0.05 for Na2S PC and P < 0.01 for Na2S 7d PC). Additionally, the Na2S 7d PC mice displayed a lower expression compared with the Na2S PC mice (P < 0.05).
Fig. 3.
Na2S pretreatment reduces oxidative stress and apoptosis after myocardial ischemia and reperfusion. Lipid hydroperoxide (A), 8-isoprostane levels (B), and representative immunoblots and densitometric analysis (C) of cleaved caspase-3 expression from hearts of sham, vehicle (Veh), Na2S PC, and Na2S 7d PC mice after 30 min of ischemia and 1 h of reperfusion were shown. Values are means ± SE. Data were compared through the use of a 1-way ANOVA with a Tukey test as the post hoc analysis. *P < 0.05 and ***P < 0.001 vs. sham.
Na2S treatment strategies affect the expression of NQO1 and HO-1 differently.
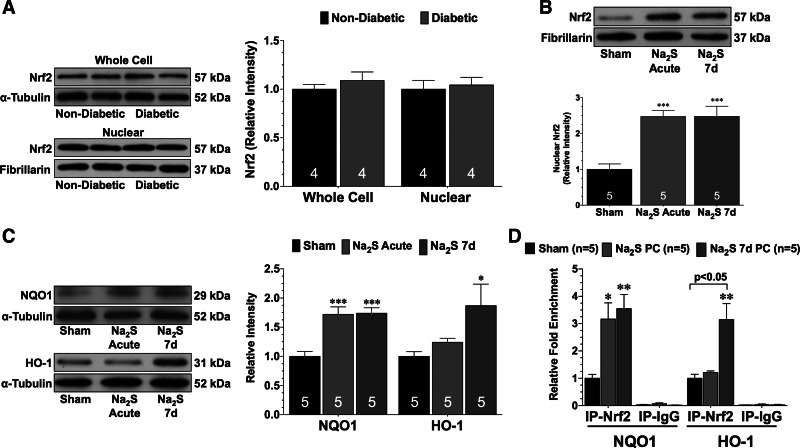
Experiments were then conducted to elucidate potential mechanisms responsible for the cardioprotective effects of Na2S pretreatment. As an initial measure, it was determined whether diabetes altered the expression of Nrf2. For these experiments, nondiabetic and db/db diabetic mice were euthanized at 12 wk of age. Western blot analysis revealed no difference in the whole cell or nuclear expression of Nrf2 between nondiabetic and diabetic mice (Fig. 4A). Next, experiments were conducted to determine whether Na2S pretreatment induced Nrf2 signaling in the diabetic heart. For these experiments, both the acute and 7-day strategies were investigated. For the acute strategy (Na2S acute), Na2S was administered to diabetic mice and heart tissue was collected 1 and 24 h later. One hour after the administration of Na2S, the nuclear expression of Nrf2 was increased (Fig. 4B; P < 0.001 vs. sham). Analysis at 24 h revealed that NQO1 levels were significantly increased (Fig. 4C; P < 0.001 vs. sham). In contrast, HO-1 levels were not increased at this time point. Analysis revealed that the nuclear expression of Nrf2 remained elevated after 7 days treatment (Na2S 7d; Fig. 4B; P < 0.001 vs. sham). Furthermore, NQO1 levels also remained elevated in the hearts of mice treated with Na2S for 7 days (Fig. 4C; P < 0.001 vs. sham). Importantly, 7 days of Na2S treatment increased HO-1 levels in the hearts of diabetic mice (P < 0.05 vs. sham).
Fig. 4.
Na2S increases the nuclear expression of nuclear factor E2-related factor (Nrf2). A: representative immunoblots and densitometric analysis of whole cell and nuclear Nrf2 from the hearts of nondiabetic and diabetic mice. B: representative immunoblots and densitometric analysis of nuclear Nrf2 in the hearts of diabetic mice treated with either a single injection of Na2S (Na2S acute) or with daily injections for 7 days (Na2S 7d). C: representative immunoblots and densitometric analysis of NADPH quinine oxidoreductase 1 (NQO1) and heme-oxygenase-1 (HO-1) in the hearts of sham, Na2S acute, and Na2S 7d mice. D: chromatin immunoprecipitation (ChIP) analysis of Nrf2 binding to the NQO1 or HO-1 promoter in the hearts of sham, Na2S acute, and Na2S 7d mice. A parallel ChIP assay was performed with IgG as a ChIP assay control. Data in A were compared with a Student's t-test. All other data were compared through the use of a 1-way ANOVA with a Tukey test as the post hoc analysis. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. sham. IP, immunoprecipitation.
Next, ChIP assays were performed to examine the binding of Nrf2 to the ARE in the NQO1 and HO-1 promoters. As shown in Fig. 4D, Na2S therapy enhanced the binding of Nrf2 to the NQO1 promoter after both a single administration and 7 days of treatment (P < 0.05 vs. sham). In contrast, a single administration of Na2S did not increase the binding of Nrf2 to the HO-1 promoter. However, after 7 days of Na2S treatment, an increase in binding was observed (P < 0.05 vs. sham).
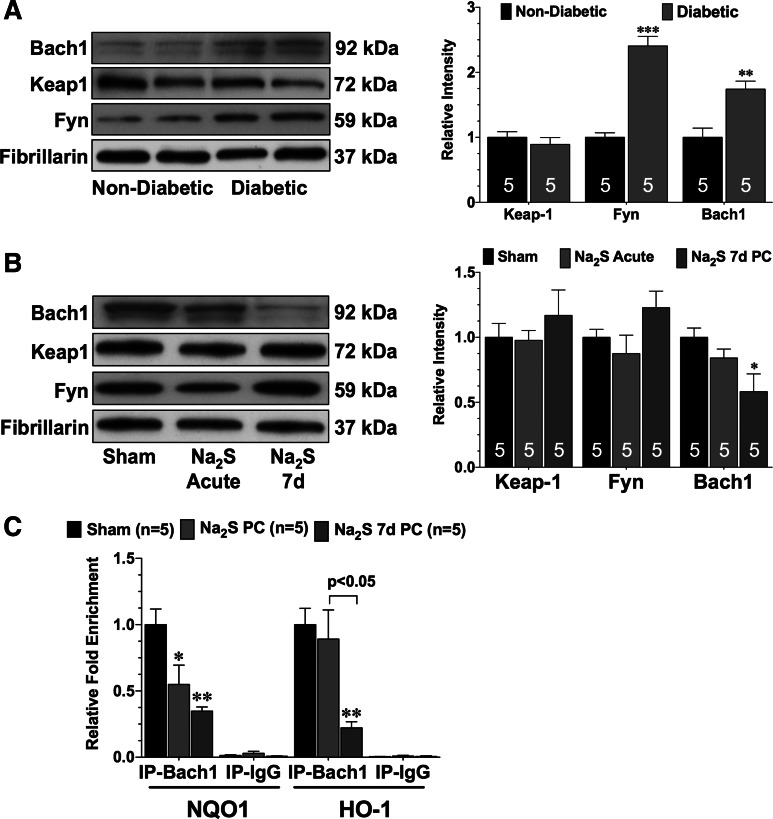
Pretreatment with Na2S does not alter the nuclear expression of kelch-like ECH-associated protein 1 or fyn in the diabetic heart but does reduce the expression of Bach1.
In an effort to determine the possible mechanisms underlying the diminished binding of Nrf2 to the ARE in the HO-1 promoter, the expression of three proteins known to influence the signaling or activation of Nrf2 were evaluated: Kelch-like ECH-associated protein 1 (Keap1), Fyn, and Bach1. The expression of each was first evaluated in the nuclear fraction of heart tissue taken from nondiabetic and diabetic mice and then evaluated in the nuclear fraction of heart tissue taken from vehicle, Na2S PC, and Na2S 7d PC-treated mice. The nuclear expression of Keap1 was similar in the hearts of nondiabetic and diabetic sham mice (Fig. 5A), and no significant changes were observed in either group of mice treated with Na2S (Fig. 5B). The expression of Fyn kinase, a key regulator of Nrf2 nuclear export (11), was then evaluated. The nuclear expression of Fyn was significantly elevated in the hearts of diabetic mice when compared with nondiabetic mice (Fig. 5A; P < 0.001). However, neither pretreatment strategy of Na2S altered the nuclear expression of Fyn (Fig. 5B). Experiments then focused on Bach1, a stress responsive transcription factor that has been reported to repress the transcription of HO-1 by competing with Nrf2 in binding to the promoter of HO-1 (15). As with Fyn, the nuclear expression of Bach1 was significantly elevated in the hearts of diabetic mice compared with nondiabetic mice (Fig. 5A; P < 0.05). Importantly, the expression of Bach1 trended lower in the Na2S PC mice (Fig. 5B; P = not significant vs. sham) and was markedly decreased in the hearts of Na2S 7d PC (P < 0.05 vs. sham). Further experiments were then conducted to determine whether the changes in the nuclear levels of Bach1 were accompanied by a decrease in the binding of Bach1 to the NQO1 and HO-1 promoters (Fig. 5C). ChIP analysis revealed that significantly less Bach1 was bound to the NQO1 promoter in the hearts of Na2S PC and Na2S 7d PC (P < 0.05 vs. sham). In contrast, a single administration of Na2S did not result in less Bach1 bound to the HO-1 promoter in the diabetic heart. However, after 7 days of Na2S treatment, significant decrease was observed (P < 0.05 vs. sham).
Fig. 5.
Na2S treatment for 7 days increases the phosphorylation of Erk and decreases the nuclear expression of Bach1. A: representative immunoblots and densitometric analysis of Bach1, kelch-like ECH-associated protein 1 (Keap1), and Fyn kinase in the nuclear fractions of hearts taken from nondiabetic and diabetic mice. B: representative immunoblots and densitometric analysis of Bach1, Keap1, and Fyn kinase in the nuclear fractions of hearts taken from sham, Na2S acute, and Na2S 7d mice. C: ChIP analysis of Bach1 binding to the NQO1 or HO-1 promoter in the hearts of sham, Na2S acute, and Na2S 7d mice. Values are means ± SE. Data in A were compared with a Student's t-test. All other data were compared through the use of a 1-way ANOVA with a Tukey test as the post hoc analysis. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. sham or nondiabetic. IP, immunoprecipitation.
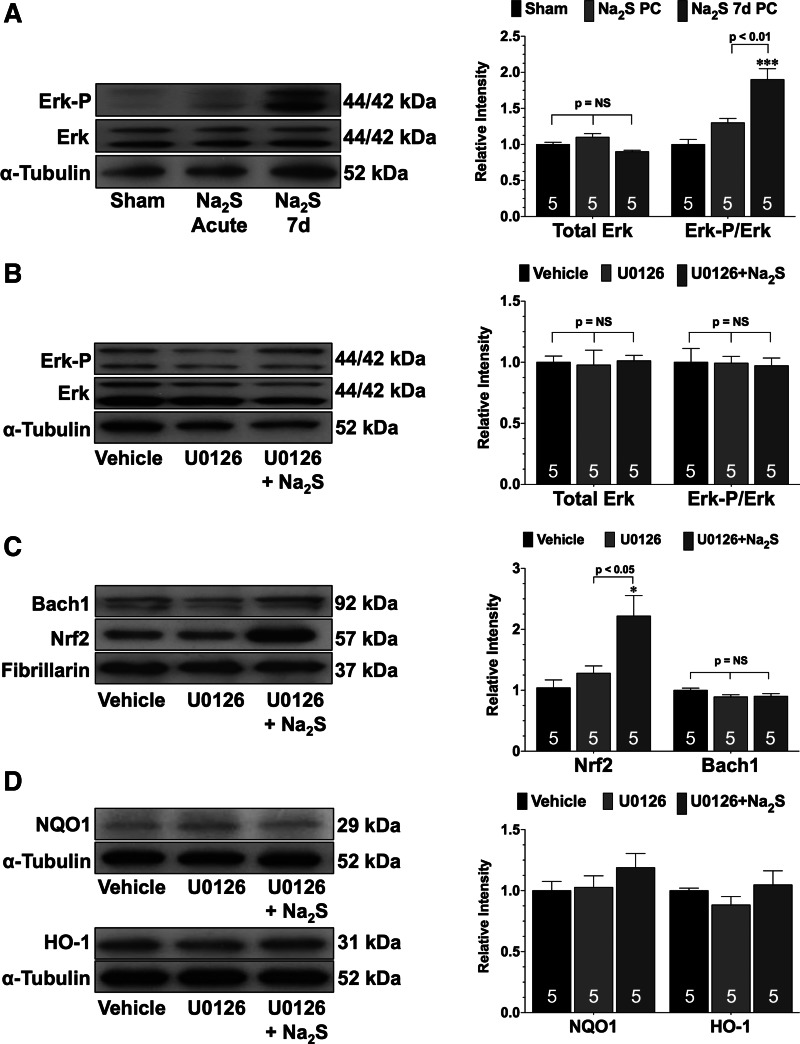
Pretreatment with Na2S for 7 days removed Bach1 from the nucleus by activating Erk.
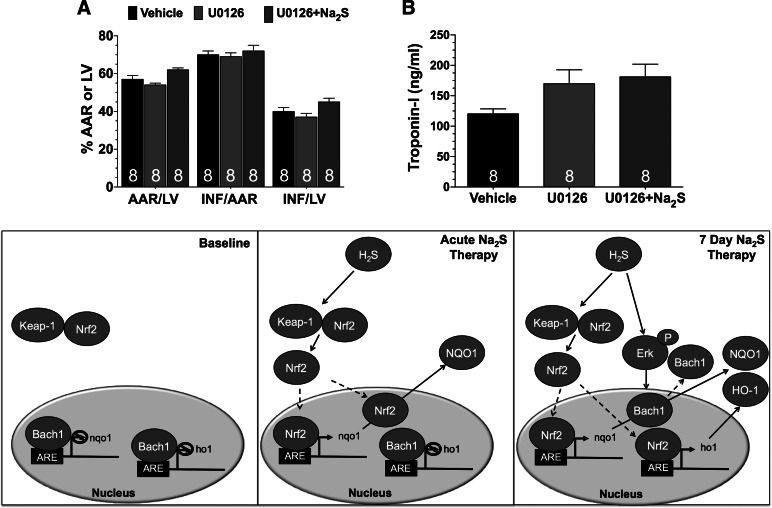
Signaling through Erk has been reported to regulate the nuclear localization of Bach1 (9). Analysis revealed that the expression of phosphorylated Erk was not increased in the hearts of Na2S PC-treated mice (Fig. 6A). However, Erk phosphorylation was significantly increased in the hearts of Na2S 7d PC mice (P < 0.001 vs. sham and P < 0.01 vs. Na2S PC). Experiments then focused on the importance of Erk in mediating the Na2S-induced removal of Bach1 from the nucleus. Since the nuclear expression of Bach1 was only significantly decrease in the hearts of the Na2S 7d PC-treated mice, these additional studies only focused on 7 days of pretreatment. For these experiments, mice received vehicle, the Erk1/2 signaling inhibitor U0126 (U0126), or U0126 and Na2S (U0126 + Na2S) for 7 days. In the presence of U0126, Na2S pretreatment failed to increase the phosphorylation of Erk (Fig. 6B). Na2S pretreatment did, however, increase the nuclear expression of Nrf2 in the presence of U0126 (Fig. 6C; P < 0.05 vs. sham and U0126) but failed to reduce the nuclear expression of Bach1 (Fig. 6C) and failed to increase the expressions of NQO1 or HO-1 (Fig. 6D). Additionally, Na2S pretreatment failed to provide protection against MI/R injury when administered in the presence of U0126 (Fig. 7).
Fig. 6.
Na2S treatment for 7 days fails to decrease the nuclear expression of Bach1 when Erk is inhibited. A: representative immunoblots and densitometric analysis of total Erk and phosphorylated Erk in the hearts of sham, Na2S acute, and Na2S 7d mice. Representative immunoblots and densitometric analysis of total Erk and phosphorylated Erk (B), nuclear Bach1 and Nrf2 (C), and NQO1 and HO-1 (D) in the hearts of diabetic mice treated with vehicle, U0126, or U0126 and Na2S (U0126 + Na2S) for 7 days are shown. Values are means ± SE. Data were compared through the use of a 1-way ANOVA with a Tukey test as the post hoc analysis. *P < 0.05, ***P < 0.001 vs. sham or vehicle. NS, not significant.
Fig. 7.
Pretreatment with Na2S for 7 days does not provide protection against myocardial ischemia and reperfusion injury when Erk is inhibited. Myocardial INF/AAR and INF/LV (A) and circulating Troponin I levels (B) from following 30 min of ischemia and 2 h of reperfusion are shown. Mice were treated with vehicle, U0126, or U0126 and Na2S (U0126 + Na2S) for 7 days before myocardial ischemia. Bottom: schematic depicting the proposed signaling mechanism by which H2S activates Nrf2 signaling. Values are means ± SE. Data were compared through the use of a 1-way ANOVA with a Tukey test as the post hoc analysis. ARE, antioxidant response element.
DISCUSSION
Our main findings in this study are 1) Na2S therapy attenuates MI/R injury in the setting of T2DM; 2) the activity of the three H2S-producing enzymes is decreased in the hearts of db/db mice; 3) Nrf2 signaling induced by Na2S is partially impaired in the setting of T2DM by Bach1; and 4) Na2S therapy for 7 days overcomes this impairment by removing Bach1 from the nucleus in a Erk-dependent manner.
A main finding of our study relates to the effects of T2DM on the activity and expression of the H2S-producing enzymes. Similar to previous reports using type 1 diabetic models, we found that T2DM increased the gene expression of Cbs and Cth. More importantly, for the first time we report that the gene expression of Mpst was upregulated. Although the increase in the gene expression of Cbs and Cth did not result in an increase in protein levels of these two enzymes, we did find that the protein expression of 3-MST was significantly upregulated. This suggests that 3-MST could be regulated differently than the other two enzymes in the setting of T2DM. However, the mechanism(s) behind this regulation remain to be elucidated. We also report here for the first time that T2DM lowers cardiac sulfide levels. Suzuki et al. (27) suggest that the elevated levels of glucose seen in diabetes may cause tissues to increase their consumption of H2S, resulting in lower levels. Although this postulated mechanism could contribute to the lower circulating and cardiac levels seen in the current study, we provide novel evidence that the enzymatic activity of the pyridoxal-5′-phosphate-dependent enzymes (CBS and CSE) and 3-MST were decreased in the diabetic heart. Therefore, we suggest that the lower levels of H2S seen in the setting of diabetes are caused by the decreased production of endogenous H2S and/or the increased tissue consumption of the available H2S.
Currently, the mechanism(s) by which T2DM alters the expression and activity of the enzymes is unknown. There is evidence that the transcription factor specificity protein 1 (Sp1) regulates the hyperglycemia-induced upregulation of CSE gene expression in pancreatic β-cells (29). However, it is not known if Sp1 regulates the cardiac gene expression of CSE in the setting of diabetes/hyperglycemia or if Sp1 regulates the expression of CBS or 3-MST under any conditions. In regards to enzymatic activity, the activity of 3-MST is inhibited by oxidative stress (21). Additionally, thioredoxin interacts with and regulates the H2S-producing activity of 3-MST (20, 21). Recent work also demonstrates that hyperglycemia associated with STZ-induced diabetes increases the susceptibility of the heart to I/R injury by enhancing the nitrative inactivation of thioredoxin (35). Therefore, the oxidative stress associated with T2DM may potentially alter the activity of the enzymes. However, further work is needed to test this hypothesis and to determine whether thioredoxin can regulate CBS and CSE in a manner similar to 3-MST.
Another main finding of the current study relates to the cardioprotective effects of H2S in the setting of T2DM. H2S therapy provides protection against several models of myocardial injury in the setting of type 1 diabetes by alleviating apoptosis and oxidative stress (5, 8, 27). In agreement, the current study also found that H2S attenuates apoptosis and oxidative stress in the setting of diabetes. More importantly, it provides evidence for the first time that H2S therapy reduces myocardial injury in the T2DM heart. This is an important observation given that T2DM encompasses roughly 90% of diabetic patients (13). Our study also highlights the complexity of therapeutic intervention for the diabetic heart after ischemia, since the robust cardioprotective effects of H2S that have previously been reported in the nondiabetic state (3) were diminished in the diabetic heart. Finally, our study revealed that the 7 days of treatment significantly increased the levels of sulfane sulfur in the heart, suggesting that correcting the diabetes-induced deficit of cardiac H2S levels may be responsible for the enhanced protection. Sulfane sulfur or bound sulfur has been suggested to be an important storage pool that regulates the amount of bioavailable free H2S (17, 33). Moreover, there is evidence to suggest that free H2S is released from bound sources under acidic conditions (19). Therefore, during myocardial ischemia it is likely that bound sulfane sulfur becomes a source of free H2S, which can then serve as a signaling molecule to protect the heart against ischemic injury.
Another major finding of our study relates to aspects of Nrf2 signaling that were impaired in the diabetic heart. This is based on the findings that the two Na2S treatment strategies affected the expression of NQO1 and HO-1 differently. The inability of a single injection of Na2S to upregulate the expression of HO-1 is in contrast with our previous findings in the nondiabetic heart (3) and may provide an explanation as to why the 7-day treatment strategy lowers oxidative stress and infarct size more than the acute. Therefore, we can speculate that NQO1 is sufficient to provide the moderate antioxidant observed after the acute treatment of Na2S; however, both NQO1 and HO-1 are able to provide the robust effects induced by the 7-day treatment. Importantly, our study revealed that the impairment in Nrf2 signaling as it relates to the regulation of HO-1 was initially caused by the inability of Nrf2 to bind to the ARE in the promoter of HO-1. This suggests two things: 1) although Nrf2 is present in the nucleus after a single injection of Na2S, something prevents it from binding to the HO-1 promoter; and 2) Na2S therapy for 7 days overcomes this impairment.
Under basal conditions, Keap1 represses the ability of Nrf2 to induce endogenous antioxidants by binding very tightly to Nrf2, anchoring it in the cytoplasm, and targeting it for ubiquitination and proteasome degradation (31). Upon stimulation, Nrf2 dissociates from Keap1 and translocates to the nucleus (16). H2S has recently been shown to sulfhydrate Keap1, which results in the release and translocation of Nrf2 to the nucleus (10, 34). Recent findings also indicate that Keap1 acts as a shuttle protein, suggesting that it has a functional role not only in the cytoplasm but also in the nucleus (18, 26). Therefore, it appears that under certain conditions Keap1 travels into the nucleus, dissociates Nrf2 from the ARE sequence, and escorts it out of the nucleus in an effort to turn the Nrf2 signaling pathway off (18). The results of the current study indicate that diabetes does not alter the ability of Na2S to disrupt the interaction between Keap1 and Nrf2, as evidenced by the findings that Na2S therapy increased the nuclear levels of Nrf2. Our analysis also found that nuclear Keap1 levels were not altered in the diabetic heart and that Na2S did not alter the expression of Keap1.
Although Keap1 is the major regulator of Nrf2 activation, there is evidence indicating multiple levels of Nrf2 regulation. For instance, Bach1 directly competes with Nrf2 in binding to the promoter of ARE-related genes leading to the negative regulation of Nrf2 signaling (14, 15). Our initial analysis found that the nuclear expression of Bach1 was increased in the diabetic heart, suggesting that it may contribute to the impairment of Nrf2 signaling in response to Na2S as it relates to HO-1. We found that an acute treatment of Na2S slightly reduced its nuclear expression, whereas 7 days of Na2S treatment significantly reduced the nuclear expression of Bach1. Given that this coincided with increased HO-1 expression, it can be suggested that Bach1 potentially causes the disruption of Nrf2/HO-1 signaling in the diabetic heart and that removal of Bach1 from the nucleus of the diabetic heart allows for Nrf2 to bind to the ARE in the HO-1 promoter. The latter was confirmed by ChIP analysis, which demonstrated that 7 days of Na2S treatment increased the binding of Nrf2 to the HO-1 promoter while at the same time reducing the binding of Bach1. Interestingly, the acute treatment of Na2S decreased the binding of Bach1 to the NQO1 promoter. This provides an explanation as to why NQO1 was increased after the acute treatment and suggests that the binding of Bach1 to the promoter of distinct Nrf2 target genes is differently regulated. It also suggests that in the heart binding of Bach1 to the NQO1 promoter is not as tightly regulated as binding to the HO-1 promoter. Additionally, inhibition of Erk1/2 signaling prevented Na2S therapy from removing Bach1 from the nucleus and subsequently prevented Na2S therapy from increasing the expression of NQO1 and HO-1. Inhibition of Erk1/2 signaling also prevented Na2S therapy from reducing myocardial injury after I/R, indicating that Erk1/2-mediated activation of Nrf2 signaling in part contributes to the cardioprotective effects of Na2S in the db/db diabetic heart. Currently, it is not known how H2S increases the phosphorylation of Erk. Previous studies suggest that it could be dependent on PKC (3). Alternatively, H2S could phosphorylate Erk in an PKC-independent manner. Therefore, further work is needed to determine the mechanisms by which H2S activates both Nrf2 and Erk signaling in the heart.
In summary, our findings demonstrate for the first time that T2DM decreases the levels of H2S in the heart and that exogenous administrations of Na2S attenuate MI/R injury in the setting of T2DM. Our results also provide important insights into the mechanism responsible for this cardioprotection. Specifically, Na2S treatment sets into motion events such as Erk phosphorylation, which ultimately lead to the removal of Bach1 from the nucleus and activation of Nrf2 signaling (Fig. 7C). Finally, given that H2S plays multiple protective roles in both the vasculature and the heart, it can be suggested that lower circulating and tissue H2S levels may contribute to the pathophysiology of diabetes (28) and strategies aimed at correcting this decline may serve as an effective therapeutic option in the treatment of heart disease in the setting of diabetes.
GRANTS
This work was supported by grants from the American Diabetes Association (7-09-BS-26) and the National Heart, Lung, and Blood Institute (5R01HL098481-03) (to J. W. Calvert). This work was also supported by funding from the Carlyle Fraser Heart Center (CFHC) of Emory University Hospital Midtown.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.F.P., C.K.N., and J.W.C. completed the conception and design of research; B.F.P., C.K.N., J.P.L., R.L.H., H.A., S.A., and J.W.C. performed experiments; B.F.P., C.K.N., J.P.L., R.L.H., H.A., S.A., and J.W.C. analyzed data; B.F.P., C.K.N., J.P.L., and J.W.C. interpreted results of experiments; B.F.P., C.K.N., J.P.L., and J.W.C. prepared figures; B.F.P. and J.W.C. drafted the manuscript; B.F.P., C.K.N., J.P.L., and J.W.C. edited and revised the manuscript; B.F.P., C.K.N., J.P.L., R.L.H., H.A., S.A., and J.W.C. approved the final version of manuscript.
REFERENCES
- 1. Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87: 840–844, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Calvert JW, Gundewar S, Jha S, Elrod JW, Lefer DJ. Hydrogen sulfide therapy attenuates left ventricular dysfunction and reduces mortality in a murine model of heart failure. Circulation 118: S441–S331, 2008 [Google Scholar]
- 3. Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res 105: 365–374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Denizalti M, Bozkurt TE, Akpulat U, Sahin-Erdemli I, Abacioglu N. The vasorelaxant effect of hydrogen sulfide is enhanced in streptozotocin-induced diabetic rats. Naunyn Schmiedebergs Arch Pharmacol 383: 509–517, 2011 [DOI] [PubMed] [Google Scholar]
- 5. El-Seweidy MM, Sadik NA, Shaker OG. Role of sulfurous mineral water and sodium hydrosulfide as potent inhibitors of fibrosis in the heart of diabetic rats. Arch Biochem Biophys 506: 48–57, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA 104: 15560–15565, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fisher CD, Augustine LM, Maher JM, Nelson DM, Slitt AL, Klaassen CD, Lehman-McKeeman LD, Cherrington NJ. Induction of drug-metabolizing enzymes by garlic and allyl sulfide compounds via activation of constitutive androstane receptor and nuclear factor E2-related factor 2. Drug Metab Dispos 35: 995–1000, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Gao Y, Yao X, Zhang Y, Li W, Kang K, Sun L, Sun X. The protective role of hydrogen sulfide in myocardial ischemia-reperfusion-induced injury in diabetic rats. Int J Cardiol 152: 177–183, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Goven D, Boutten A, Lecon-Malas V, Boczkowski J, Bonay M. Prolonged cigarette smoke exposure decreases heme oxygenase-1 and alters Nrf2 and Bach1 expression in human macrophages: roles of the MAP kinases ERK(1/2) and JNK. FEBS Lett 583: 3508–3518, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Hourihan JM, Kenna JG, Hayes JD. The gasotransmitter hydrogen sulfide induces Nrf2-target genes by inactivating the keap1 ubiquitin ligase substrate adaptor through formation of a disulfide bond between Cys-226 and Cys-613. Antioxid Redox Signal. In press [DOI] [PubMed] [Google Scholar]
- 11. Jain AK, Jaiswal AK. GSK-3beta acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2. J Biol Chem 282: 16502–16510, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Jain SK, Bull R, Rains JL, Bass PF, Levine SN, Reddy S, McVie R, Bocchini JA. Low levels of hydrogen sulfide in the blood of diabetes patients and streptozotocin-treated rats causes vascular inflammation? Antioxid Redox Signal 12: 1333–1337, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jay D, Hitomi H, Griendling KK. Oxidative stress and diabetic cardiovascular complications. Free Radic Biol Med 40: 183–192, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Jyrkkanen HK, Kuosmanen S, Heinaniemi M, Laitinen H, Kansanen E, Mella-Aho E, Leinonen H, Yla-Herttuala S, Levonen AL. Novel insights into the regulation of antioxidant-response-element-mediated gene expression by electrophiles: induction of the transcriptional repressor BACH1 by Nrf2. Biochem J 440: 167–174, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Kaspar JW, Jaiswal AK. Antioxidant-induced phosphorylation of tyrosine 486 leads to rapid nuclear export of Bach1 that allows Nrf2 to bind to the antioxidant response element and activate defensive gene expression. J Biol Chem 285: 153–162, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47: 89–116, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Kimura H, Shibuya N, Kimura Y. Hydrogen sulfide is a signaling molecule and a cytoprotectant. Antioxid Redox Signal 17: 45–57, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lau A, Villeneuve NF, Sun Z, Wong PK, Zhang DD. Dual roles of Nrf2 in cancer. Pharmacol Res 58: 262–270, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levitt MD, Abdel-Rehim MS, Furne J. Free and acid-labile hydrogen sulfide concentrations in mouse tissues: anomalously high free hydrogen sulfide in aortic tissue. Antioxid Redox Signal 15: 373–378, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Mikami Y, Shibuya N, Kimura Y, Nagahara N, Ogasawara Y, Kimura H. Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem J 439: 479–485, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Nagahara N, Katayama A. Post-translational regulation of mercaptopyruvate sulfurtransferase via a low redox potential cysteine-sulfenate in the maintenance of redox homeostasis. J Biol Chem 280: 34569–34576, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Nicholson CK, Calvert JW. Hydrogen sulfide and ischemia-reperfusion injury. Pharmacol Res 62: 289–297, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Predmore BL, Kondo K, Bhushan S, Zlatopolsky MA, King AL, Aragon JP, Grinsfelder DB, Condit ME, Lefer DJ. The polysulfide diallyl trisulfide protects the ischemic myocardium by preservation of endogenous hydrogen sulfide and increasing nitric oxide bioavailability. Am J Physiol Heart Circ Physiol 302: H2410–H2418, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, Kimura H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal 11: 703–714, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Sodha NR, Clements RT, Feng J, Liu Y, Bianchi C, Horvath EM, Szabo C, Sellke FW. The effects of therapeutic sulfide on myocardial apoptosis in response to ischemia-reperfusion injury. Eur J Cardiothorac Surg 33: 906–913, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sun Z, Zhang S, Chan JY, Zhang DD. Keap1 controls postinduction repression of the Nrf2-mediated antioxidant response by escorting nuclear export of Nrf2. Mol Cell Biol 27: 6334–6349, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Suzuki K, Olah G, Modis K, Coletta C, Kulp G, Gero D, Szoleczky P, Chang T, Zhou Z, Wu L, Wang R, Papapetropoulos A, Szabo C. Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc Natl Acad Sci USA 108: 13829–13834, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Szabo C. Roles of hydrogen sulfide in the pathogenesis of diabetes mellitus and its complications. Antioxid Redox Signal 17: 68–80, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taniguchi S, Kimura T, Umeki T, Kimura Y, Kimura H, Ishii I, Itoh N, Naito Y, Yamamoto H, Niki I. Protein phosphorylation involved in the gene expression of the hydrogen sulphide producing enzyme cystathionine gamma-lyase in the pancreatic beta-cell. Mol Cell Endocrinol 350: 31–38, 2012 [DOI] [PubMed] [Google Scholar]
- 30. Tanito M, Agbaga MP, Anderson RE. Upregulation of thioredoxin system via Nrf2-antioxidant responsive element pathway in adaptive-retinal neuroprotection in vivo and in vitro. Free Radic Biol Med 42: 1838–1850, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Wakabayashi N, Dinkova-Kostova AT, Holtzclaw WD, Kang MI, Kobayashi A, Yamamoto M, Kensler TW, Talalay P. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci USA 101: 2040–2045, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Whiteman M, Gooding KM, Whatmore JL, Ball CI, Mawson D, Skinner K, Tooke JE, Shore AC. Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulphide. Diabetologia 53: 1722–1726, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Wintner EA, Deckwerth TL, Langston W, Bengtsson A, Leviten D, Hill P, Insko MA, Dumpit R, VandenEkart E, Toombs CF, Szabo C. A monobromobimane-based assay to measure the pharmacokinetic profile of reactive sulphide species in blood. Br J Pharmacol 160: 941–957, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang G, Zhao K, Ju Y, Mani S, Cao Q, Puukila S, Khaper N, Wu L, Wang R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of keap1 and activation of Nrf2. Antioxid Redox Signal. In press [DOI] [PubMed] [Google Scholar]
- 35. Yin T, Hou R, Liu S, Lau WB, Wang H, Tao L. Nitrative inactivation of thioredoxin-1 increases vulnerability of diabetic hearts to ischemia/reperfusion injury. J Mol Cell Cardiol 49: 354–361, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Yuan P, Xue H, Zhou L, Qu L, Li C, Wang Z, Ni J, Yu C, Yao T, Huang Y, Wang R, Lu L. Rescue of mesangial cells from high glucose-induced over-proliferation and extracellular matrix secretion by hydrogen sulfide. Nephrol Dial Transplant 26: 2119–2126, 2011 [DOI] [PubMed] [Google Scholar]
- 37. Yusuf M, Kwong Huat BT, Hsu A, Whiteman M, Bhatia M, Moore PK. Streptozotocin-induced diabetes in the rat is associated with enhanced tissue hydrogen sulfide biosynthesis. Biochem Biophys Res Commun 333: 1146–1152, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Zhu H, Itoh K, Yamamoto M, Zweier JL, Li Y. Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett 579: 3029–3036, 2005 [DOI] [PubMed] [Google Scholar]