Abstract
Ankyrin-B is a multifunctional adapter protein responsible for localization and stabilization of select ion channels, transporters, and signaling molecules in excitable cells including cardiomyocytes. Ankyrin-B dysfunction has been linked with highly penetrant sinoatrial node (SAN) dysfunction and increased susceptibility to atrial fibrillation. While previous studies have identified a role for abnormal ion homeostasis in ventricular arrhythmias, the molecular mechanisms responsible for atrial arrhythmias and SAN dysfunction in human patients with ankyrin-B syndrome are unclear. Here, we develop a computational model of ankyrin-B dysfunction in atrial and SAN cells and tissue to determine the mechanism for increased susceptibility to atrial fibrillation and SAN dysfunction in human patients with ankyrin-B syndrome. Our simulations predict that defective membrane targeting of the voltage-gated L-type Ca2+ channel Cav1.3 leads to action potential shortening that reduces the critical atrial tissue mass needed to sustain reentrant activation. In parallel, increased fibrosis results in conduction slowing that further increases the susceptibility to sustained reentry in the setting of ankyrin-B dysfunction. In SAN cells, loss of Cav1.3 slows spontaneous pacemaking activity, whereas defects in Na+/Ca2+ exchanger and Na+/K+ ATPase increase variability in SAN cell firing. Finally, simulations of the intact SAN reveal a shift in primary pacemaker site, SAN exit block, and even SAN failure in ankyrin-B-deficient tissue. These studies identify the mechanism for increased susceptibility to atrial fibrillation and SAN dysfunction in human disease. Importantly, ankyrin-B dysfunction involves changes at both the cell and tissue levels that favor the common manifestation of atrial arrhythmias and SAN dysfunction.
Keywords: arrhythmia (mechanisms), atrial fibrillation; sinus node disease; ankyrin-B
ankyrins are a family of multifunctional polypeptides (ankyrin-R, ankyrin-G, ankyrin-B expressed in heart) required for proper membrane expression of ion channels, transporters, and signaling molecules in both excitable and nonexcitable cells (41). Importantly, ankyrin dysfunction has been closely linked to human disease. In heart, defects in both ankyrin-G- and ankyrin-B-dependent pathways have been associated with increased susceptibility to cardiac arrhythmia. In particular, loss of ankyrin-B function has been linked to both congenital and acquired arrhythmias (9, 19, 22, 27, 31, 33, 34). Several mutations in ANK2 (encodes ankyrin-B) have been identified as the cause of a complex cardiac phenotype in humans characterized by prolongation of the QT interval on the electrocardiogram coupled with increased susceptibility to ventricular arrhythmias and sudden death (27, 33–35, 40). Initially described as long-QT type 4 based on the electrocardiographic and electrophysiology findings, the disease has more recently been reclassified as “ankyrin-B syndrome” because of the presence of additional supraventricular phenotypes not found in classic long-QT syndrome. Specifically, in addition to ventricular tachyarrhythmias, human patients with ankyrin-B syndrome show highly penetrant sinus node dysfunction coupled with increased susceptibility to spontaneous and inducible atrial fibrillation (9, 27, 34). Thus an important unanswered question is, How does loss of ankyrin-B give rise to this broad spectrum of distinct arrhythmias?
Recent findings from our group and others have provided important clues into the molecular and cellular mechanism for arrhythmia in the setting of ankyrin-B dysfunction. In ventricular myocytes, increased susceptibility to proarrhythmogenic calcium-dependent afterdepolarizations has been observed, likely because of, in part, the loss of membrane localization of Na+/K+ ATPase (NKA) and Na+/Ca2+ exchanger (NCX) and associated imbalance in intracellular ion homeostasis (5, 8, 34, 44). In atrial and sinoatrial node (SAN) cells, L-type Ca2+ current is uniquely affected because of loss of membrane targeting of Cav1.3 (not expressed in ventricle) (9, 27). In parallel, ankyrin-B-deficient (ankyrin-B+/−) mice show a decrease in atrial action potential (AP) duration (APD) together with slow and variable SAN cell spontaneous firing rate (9). While these studies provide important insight into the underlying cellular defects, the link between specific cell/tissue changes and arrhythmogenesis remains unclear.
Mathematical modeling of excitable cells has been applied extensively to analyze the molecular and cellular basis of human disease (6, 17, 39). In previous work, we used a computational approach to analyze the substrate for stress-induced ventricular arrhythmias in ankyrin-B syndrome (44). We found that loss of NCX and NKA membrane targeting resulted in calcium overload under stress conditions, promoting inappropriate release of calcium from the sarcoplasmic reticulum and even afterdepolarizations. Here, we take a similar approach to define the substrate for arrhythmias across the full spectrum of arrhythmias observed clinically.
METHODS
Simulation of normal (wild-type) and ankyrin-B+/− atrial APs.
Ion channel kinetics for the atrial cardiomyocyte were simulated using two different well-validated models of the human atrial cardiomyocyte (7, 16). Modifications to the equations were made to account for experimentally measured changes in NCX, NKA, and L-type Ca2+ channel membrane in ankyrin-B+/− cardiomyocytes (9, 31, 32, 34, 35). Specifically, NCX and NKA surface expressions were reduced by 40 and 25%, respectively, from their wild-type (WT) values, consistent with experimental measurements in ankyrin-B+/− cardiomyocytes (27, 34). Furthermore, the surface expression of the L-type Ca2+ channel was reduced to produce a 40% decrease in the L-type Ca2+ current peak current at a test potential of −10 mV as measured experimentally in ankyrin-B+/− atrial myocytes (9). Single cell models were paced from rest to steady state (1,000 s of pacing to produce beat-to-beat change in APD < 0.1%) over a range of pacing cycle lengths (CLs from 2,000 to 300 ms; stimulus amplitude = −20 μA/μF; stimulus duration = 2 ms) using a conservative stimulus (18). The steady-state values for all state variables were used as initial conditions for subsequent simulations. In the case of multicellular simulations, steady-state values from pacing the single cell at CL of 300 ms were used as initial conditions (Table A1).
Table A1.
Initial conditions for state variables in mathematical model of human atrial AP*
| State Variable | Definition | WT | Ankyrin-B+/− |
|---|---|---|---|
| m | Fast Na+ current (INa) activation gate | 0.004147463955 | 0.00318851486 |
| h | INa inactivation gate | 0.9421032891 | 0.9598653389 |
| j | INa slow inactivation gate | 0.9319148046 | 0.9688717543 |
| d | L-type Ca2+ current (ICa) activation gate | 1.793553646e-04 | 0.0001466632492 |
| f | ICa voltage-dependent inactivation gate | 0.8466729054 | 0.8891946221 |
| fca | ICa calcium-dependent inactivation gate | 0.5727907784 | 0.5594239293 |
| Xr | Rapidly activating K+ current activation gate | 0.02303434883 | 0.01534625493 |
| Xs | Slowly activating K+ current activation gate | 0.03616188931 | 0.03017466983 |
| Oa | Transient outward K+ current (Ito) activation gate | 0.03436021906 | 0.03141789388 |
| Oi | Ito inactivation gate | 0.9987671846 | 0.9991357877 |
| ua | Ultrarapid K+ current (IKur) activation gate | 0.006237057018 | 0.00527170832 |
| ui | IKur inactivation gate | 0.9891443725 | 0.9897469285 |
| U | Ryanodine receptor (RyR) Ca2+-dependent activation gate | 4.537711703e-16 | 2.174552101e-16 |
| v | RyR Ca2+-dependent inactivation gate | 1.0 | 1.0 |
| W | RyR voltage-dependent inactivation gate | 0.9990895327 | 0.9991716587 |
| [Ca2+]i, mM | Ca2+ concentration in myoplasm | 0.0002599348619 | 0.0002745599852 |
| [Ca2+]Rel, mM | [Ca2+] in sarcoplasmic reticulum release compartment | 0.4298392427 | 0.521326844 |
| [Ca2+]Up, mM | [Ca2+] in sarcoplasmic reticulum uptake compartment | 1.960512954 | 2.041848657 |
| [Ca2+]cmdn, mM | Ca2+-bound calmodulin | 0.004923177073 | 0.005171523579 |
| [Ca2+]trpn, mM | Ca2+-bound troponin | 0.0239435873 | 0.02481321084 |
| [Ca2+]csqn, mM | Ca2+-bound calsequestrin | 3.495084787 | 3.945479851 |
| [Na+]i, mM | Na+ concentration in myoplasm | 15.19468146 | 16.43681531 |
| [K+]i, mM | K+ concentration in myoplasm | 134.9547735 | 133.6894531 |
| Vm, mV | Transmembrane potential | −79.01495665 | −80.62135663 |
Single cell was paced to steady state at cycle length (CL) = 300 ms. AP, action potential; WT, wild-type.
Simulation of reentry in atrial tissue.
Spiral wave reentry was induced in the two-dimensional tissue model by cross-field stimulation of two perpendicular rectilinear waves. Fibrosis was simulated by replacing a percentage of normal cells (3% for WT, and 9% for ankyrin-B+/−) with poorly coupled inexcitable cells. Inexcitable cells were modeled as passive elements (36) with a leak conductance to produce a rest potential of −40 mV in agreement with experimental measurements from isolated adult atrial fibroblasts (21). Two different random fibroblast distributions were implemented [pseudo-random numbers generated using the rand() command in the stdlib.h C standard library]: 1) “diffuse,” where elements were distributed individually, and 2) “clustered,” where groups of up to five were designated at a time. The two-dimensional cable equation describing AP propagation was solved using an alternating direction implicit method, a mesh size of 200 μm and a fixed time step of 0.005 ms (20, 42).
Simulation of WT and ankyrin-B+/− SAN AP.
Ion channel kinetics underlying the SAN AP were simulated using a recently published model of the murine SAN cell (23). Importantly, this model includes separate formulations for both L-type Ca2+ channel subtypes found in the SAN (Cav1.3 and Cav1.2) and accurately reproduces spontaneous firing rate and AP morphology of the mouse SAN cell. Similar to the atrial cell model, NCX and NKA surface expressions were reduced by 40 and 25%, respectively. As ankyrin-B binds Cav1.3 but not Cav1.2, the surface expression of the Cav1.3 (but not Cav1.2) component of L-type Ca2+ channel was reduced by 40% as measured experimentally in ankyrin-B+/− SAN myocytes (27). Parametric analysis was performed by simultaneously varying Cav1.3 maximal conductance and NKA pumping rate or NCX scaling factor.
Intact SAN.
Detailed mathematical models were used to simulate SAN cell and atrial APs (Table A2) (7, 23). A model of the human atrial cell (7) was modified to produce an AP waveform more similar to the murine atrial AP (equations provided in appendix). The goal was not to create a model of the mouse atrial myocyte (insufficient data currently available) but rather to generate a waveform with physiological morphology (shown in appendix, Fig. 8) to couple with the murine SAN models. Importantly, measured and simulated APDs show agreement for both WT and ankyrin-B+/− murine atrial cells. Geometry of the intact SAN was represented as a two-dimensional 400 × 45 rectangular grid. Cell types in different regions were determined by histologically reconstructed sections through the rabbit right atrium (RA), as described (3, 13, 42), scaled to a spatial resolution of 9 μm to match dimensions of the mouse SAN (30). Regional differences in cell size, coupling, and ion channel expression within the SAN node were taken into account by making cell properties a function of space, following the previously described approach (3) (equations provided in appendix). Initial conditions for cells from different regions were derived from steady-state values for individual cell models following either 200 s of spontaneous activity (SAN cell) or 1,000 s of rapid pacing (atrial cell) (values provided in Tables A3 and A4). While these initial conditions do not represent true steady state for the coupled system, they greatly reduce transient initial fluctuations in CL and other properties. Ten seconds of spontaneous activity were simulated, and APs from different SAN regions were analyzed for activation pattern and rate.
Table A2.
Mathematical models for different regions of intact SAN
Table A3.
Initial conditions for state variables in mathematical model of mouse SAN AP*
| State Variable | Definition | WT | Ankyrin-B+/− |
|---|---|---|---|
| mTTXr | TTX-resistant fast Na+ current (INa,TTXr) activation gate | 0.4013731391 | 0.5175464965 |
| hTTXr | INa,TTXr inactivation gate | 0.2724609414 | 0.1709890012 |
| jTTXr | INa,TTXr slow inactivation gate | 0.02491755567 | 0.02006050186 |
| mTTXs | TTX-sensitive fast Na+ current (INa,TTXs) activation gate | 0.1078937365 | 0.161864092 |
| hTTXs | INa,TTXs inactivation gate | 0.4499459412 | 0.3048035189 |
| jTTXs | INa,TTXs slow inactivation gate | 0.0268422807 | 0.01625136245 |
| d12 | Cav1.2 L-type Ca2+ current (ICaL,1.2) activation gate | 4.556481192e-06 | 1.522040235e-05 |
| f12 | ICaL,1.2 voltage-dependent inactivation gate | 0.9968143283 | 0.9906700529 |
| fca | ICaL calcium-dependent inactivation gate | 0.7649209291 | 0.7686207865 |
| d13 | Cav1.3 L-type Ca2+ current (ICaL,1.3) activation gate | 0.0002036010996 | 0.0005560731373 |
| f13 | ICaL,1.3 voltage-dependent inactivation gate | 0.9809331115 | 0.9590375512 |
| dT | T-type Ca2+ current (ICaT) activation gate | 0.001625076871 | 0.004482631204 |
| fT | ICaT voltage-dependent inactivation gate | 0.426423268 | 0.2744185369 |
| Xr | Rapidly activating K+ current (IKr) activation gate | 0.4043762826 | 0.3367064343 |
| Xri | IKr inactivation gate | 0.925010631 | 0.8997897425 |
| Xs | Slowly activating K+ current activation gate | 0.01271025192 | 0.01089959964 |
| dst | Sustained inward Na+ (Ist) current activation gate | 0.6245901914 | 0.8464766153 |
| fst | Ist inactivation gate | 0.4537962624 | 0.4492530382 |
| Y | Hyperpolarization-activated current (If) activation gate | 0.02800253901 | 0.01852389239 |
| Oi | Transient outward K+ current (Ito) inactivation gate | 0.6106878506 | 0.4978937269 |
| Oa | Activation gate for Ito and Isus | 0.004626741836 | 0.006270214103 |
| Ro | Ryanodine receptor (RyR) open state | 7.577231176e-08 | 6.414963718e-08 |
| Ri | RyR inactivated state | 2.121940549e-08 | 1.757603047e-08 |
| Rri | RyR resting-inactivated state | 0.2154275978 | 0.2124565019 |
| Rr | RyR resting state | 0.7692198658 | 0.7754111992 |
| [Ca2+]i | Ca2+ concentration in myoplasm (mM) | 3.193034323e-05 | 3.235691602e-05 |
| [Ca2+]JSR | [Ca2+] in junctional sarcoplasmic reticulum (mM) | 0.1189292632 | 0.1631189264 |
| [Ca2+]NSR | [Ca2+] in network sarcoplasmic reticulum (mM) | 1.577923289 | 1.542832819 |
| [Ca2+]ss | [Ca2+] in subspace (mM) | 5.603557083e-05 | 7.408237329e-05 |
| fCMi | Fractional occupancy of calmodulin by Ca2+ | 0.0138611385 | 0.01401896089 |
| fCMs | Fractional occupancy of calmodulin by subspace Ca2+ | 0.02419980623 | 0.0313994341 |
| fTC | Fractional occupancy of troponin Ca2+ site by Ca2+ | 0.006346326788 | 0.006416675037 |
| fTMC | Fractional occupancy of troponin Mg+ site by Ca2+ | 0.1297414046 | 0.1266803519 |
| fTMM | Fractional occupancy of troponin Mg+ site by Mg+ | 0.7688005798 | 0.7715097855 |
| fCQ | Fractional occupancy of calsequestrin by JSR Ca2+ | 0.120498404 | 0.1601843848 |
| [Na+]i, mM | Na+ concentration in myoplasm | 8.11828204 | 7.785938742 |
| [K+]i, mM | K+ concentration in myoplasm | 139.8850767 | 140.2183952 |
| Vm, mV | Transmembrane potential | −64.52369393 | −58.40912806 |
Single cell underwent spontaneous activity for 200 s. Model equations for mouse SAN cell are found in original publication (23).
Table A4.
Initial conditions for state variables in mathematical model of mouse atrial AP*
| State Variable | Definition | WT | Ankyrin-B+/− |
|---|---|---|---|
| M | Fast INa activation gate | 0.00135132373 | 0.001504400009 |
| H | INa inactivation gate | 0.9873598055 | 0.9854360416 |
| J | INa slow inactivation gate | 0.9918930493 | 0.9908065458 |
| D | ICa activation gate | 0.0009336318521 | 0.001011615048 |
| F | ICa voltage-dependent inactivation gate | 0.971452238 | 0.9965778666 |
| fca | ICa calcium-dependent inactivation gate | 0.6432093256 | 0.8230278873 |
| Xr | Rapidly activating K+ current activation gate | 0.004254403903 | 6.234082795e-05 |
| Xs | Slowly activating K+ current activation gate | 0.01764536694 | 0.01599482291 |
| Oa | Transient outward K+ current (Ito) activation gate | 0.001906509835 | 0.002030472113 |
| Oi | Ito inactivation gate | 0.9999848553 | 0.9999849009 |
| ua | Ultrarapid K+ current (IKur) activation gate | 0.01217305509 | 0.0003118014711 |
| ui | IKur inactivation gate | 0.9667493143 | 0.9927894458 |
| aKss | Noninactivating steady-state K+ current (IKss) activation gate | 0.8135516746 | 0.7868359662 |
| U | Ryanodine receptor (RyR) Ca2+-dependent activation gate | 8.021780287e-32 | 0 |
| V | RyR Ca2+-dependent inactivation gate | 1.0 | 1.0 |
| W | RyR voltage-dependent inactivation gate | 0.9993892777 | 0.9993657578 |
| [Ca2+]i, mM | Ca2+ concentration in myoplasm | 0.0001938318203 | 7.524196035e-05 |
| [Ca2+]Rel, mM | [Ca2+] in sarcoplasmic reticulum release compartment | 1.006559386 | 1.481621283 |
| [Ca2+]Up, mM | [Ca2+] in sarcoplasmic reticulum uptake compartment | 3.945619176 | 1.916274235 |
| [Ca2+]cmdn, mM | Ca2+-bound calmodulin | 0.00376544676 | 0.001532272634 |
| [Ca2+]trpn, mM | Ca2+-bound troponin | 0.01955555503 | 0.009156042428 |
| [Ca2+]csqn, mM | Ca2+-bound calsequestrin | 5.571692766 | 6.493721346 |
| [Na+]i, mM | Na+ concentration in myoplasm | 20.28987641 | 13.367893 |
| [K+]i, mM | K+ concentration in myoplasm | 129.5105865 | 136.7678717 |
| Vm, mV | Transmembrane potential | −85.80492414 | −85.16157828 |
Single cell was paced to steady state at CL = 300 ms.
All computer code was written in C++, compiled using Intel Composer XE 2011 for Linux and executed on a Dell PowerEdge R515 server (Dual 6 core, 32 GB RAM running CentOS-6.2). Simulation activation data were visualized in MATLAB (R2012a).
Histology of atrium from WT and ankyrin-B+/− mice.
Hearts from age- and sex-matched (2-mo-old males) WT and ankyrin-B+/− mice were fixed in 4% paraformaldehyde. Tissues were sectioned and stained with Masson's trichrome. Light microscopy images were analyzed using ImageJ. Hearts were obtained after animals were euthanized by acute CO2 asphyxiation followed by cervical dislocation in accordance with the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health, and the protocols were approved by the Institutional Animal Care and Use Committee of Ohio State University.
RESULTS
Increased fibrosis in ankyrin-B+/− atria.
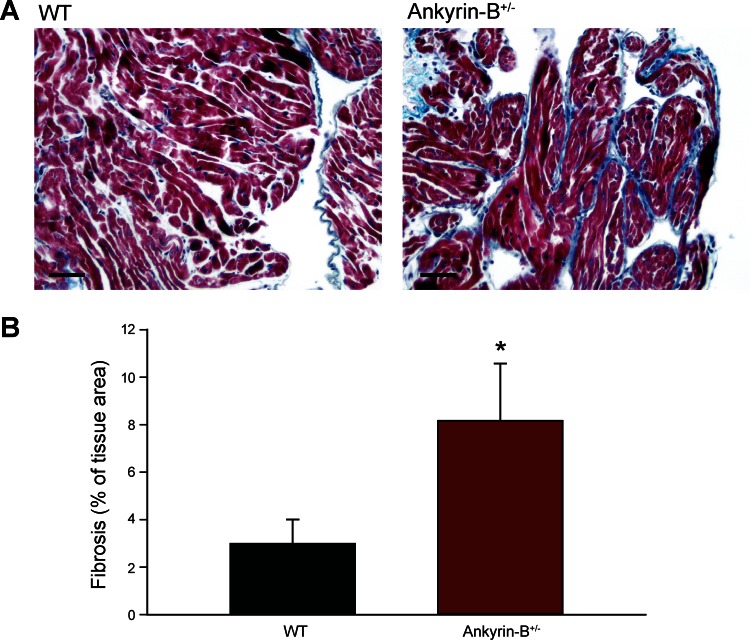
Ankyrin-B dysfunction alters membrane expression of select ion channels, pumps, and exchangers that may regulate the substrate for atrial fibrillation and/or sinus node dysfunction (9). In previous studies, we defined the atrial and SAN cellular phenotype associated with ankyrin-B dysfunction (9, 27). However, changes in atrial structure and/or dimension have been shown to increase susceptibility to arrhythmia (2, 25). Therefore, we first evaluated whether loss of ankyrin-B function altered gross atrial structure through histological staining of normal and ankyrin-B+/− mouse atrial sections. Interestingly, we found almost a threefold increase in the extent of fibrosis in atria from ankyrin-B+/− animals compared with age- and sex-matched WT mouse atria (Fig. 1). These data indicate that in addition to cellular level changes in ion channel membrane targeting, ankyrin-B dysfunction results in structural remodeling that may influence the substrate for arrhythmias.
Fig. 1.
Increased fibrosis in ankyrin-B+/− atria. A: Masson trichrome staining of atrial sections from wild-type (WT) and ankyrin-B+/− mice. Blue staining indicates collagen. B: summary data on fibrosis as percentage of total tissue area in WT and ankyrin-B+/− atrial sections. *P < 0.05, n = 5. Scale bar = 50 μm.
Altered cellular dynamics in ankyrin-B+/− atrial cell.
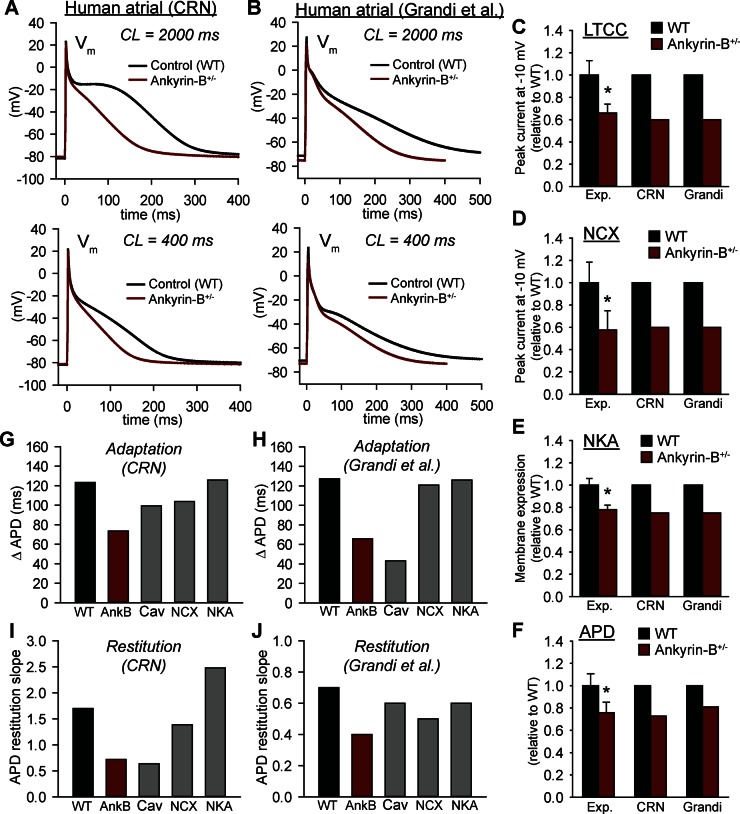
Altered cellular dynamics have been linked to increased susceptibility to formation of wave break and cardiac fibrillation (43). Therefore, we next determined whether ankyrin-B dysfunction alters the dynamic response of the atrial cell AP to pacing. Mathematical models of ankyrin-B+/− and WT cells were paced to steady state over a CL range from 2,000 to 300 ms (Fig. 2). We also determined the APD frequency response to isolated defects in NCX, NKA, and Cav1.3. To verify model independence of findings, simulations were performed using two different models of the human atrial AP: one from Courtemanche, Ramirez, and Nattel (7) that was among the first developed (referred to as “CRN”) and a recent model from Grandi and colleagues (16) (referred to as “Grandi”) that includes a more advanced formulation for Ca2+ handling (Fig. 2, A and B). Importantly, the CRN and Grandi models predicted a similar decrease in APD with ankyrin-B deficiency that agrees with experiment (9) (Fig. 2, A, B, and F). While data on maximum action potential upstroke velocity (dV/dtmax) in ankyrin-B-deficient cells are not available, studies in isolated human atrial myocytes show a value of 203 ± 11 ms with no difference between sinus rhythm and atrial fibrillation patients (45). For comparison, at the same CL (800 ms) we observe a dV/dtmax of 205 ms in sinus rhythm (for CRN) and 198 ms in ankyrin-B+/− (not shown). Interestingly, the ankyrin-B+/− cells displayed a reduced capacity for APD adaptation with rate: APD decreases by 74 ms in CRN ankyrin-B+/−, as CL decreases from 2,000 to 400 ms, compared with 123 ms in WT, with a similar difference observed in the Grandi model (Fig. 2, G and H). Loss of Cav1.3 is the primary determinant of altered APD adaption in both the CRN and Grandi models as Cav1.3 deficiency shows decreased APD at all CLs, similar to the ankyrin-B+/− cell (Fig. 2, G and H). Interestingly, loss of NCX had a greater impact on APD adaptation in the CRN model than in the Grandi model. Nonetheless, independent of model, the role of NCX was secondary to Cav1.3 with little to no role for NKA.
Fig. 2.
Rate dependence of action potential (AP) duration (APD) in mathematical model of ankyrin-B+/− (AnkB) atrial myocyte. A and B: 2 different models of the human atrial AP (7, 16) were used to simulate atrial APs from control (WT, black) and ankyrin-B+/− (red) myocytes during slow [cycle length (CL) = 2,000 ms] and rapid (CL = 400 ms) pacing. C–F: simulated and measured (9) L-type Ca2+ current (LTCC), Na+/Ca2+ exchanger (NCX), Na+/K+ ATPase (NKA), and APD are shown for model validation. G and H: APD adaptation for WT, ankyrin-B+/−, Cav1.3-deficient (Cav), NCX-deficient (NCX) and NKA-deficient (NKA) cells. Adaptation curves were created by pacing cell to steady state over a range of CLs. Summary data are shown as change in APD as CL decreases from 2,000 to 400 ms. I and J: slope of APD restitution curves for WT, ankyrin-B+/−, Cav1.3-deficient, NCX-deficient, and NKA-deficient cells. Restitution curves were created by applying a premature stimulus following steady-state pacing at CL of 1,000 ms, and summary data are shown as maximal slope of the curve. CRN, Courtemanche, Ramirez, and Nattel (7) model; Grandi, Grandi et al. (16) model; Exp, experiment; Vm, transmembrane potential. *P < 0.05 vs. WT.
We also determined APD in response to a premature stimulus applied at a variable diastolic interval following steady-state pacing (APD restitution). The maximal slope of the restitution curve is shallower in the ankyrin-B+/− cell compared with WT, indicating a decreased susceptibility to proarrhythmogenic AP alternans (Fig. 2, I and J). Whereas the shallow restitution curve in the ankyrin-B+/− CRN model depended almost exclusively on loss of Cav1.3, the Grandi model showed a more equal contribution from Cav1.3, NCX, and NKA. Thus these simulations demonstrate that loss of Cav1.3 (and to a lesser extent NCX) contributes to abnormal APD adaptation and restitution in ankyrin-B+/− atrial myocytes. Whereas we expect that APD shortening observed in ankyrin-B+/− is proarrhythmic, the changes to restitution are likely antiarrhythmic as steep restitution has been linked to increased susceptibility to complex AP dynamics and fibrillation (43).
Two-dimensional model of atrial tissue.
Our experimental and computational results show complex electrical and structural remodeling in ankyrin-B+/− hearts. Based on these findings, we hypothesized that increased susceptibility to atrial fibrillation in the setting of ankyrin-B dysfunction is due to the presence of multiple defects at the cell and tissue level. Specifically, we hypothesized that decreased APD and slowed conduction (due to fibrosis) dramatically decrease ankyrin-B+/− AP wavelength, a measure of the spatial extent of the propagating AP (estimated as the product of conduction velocity and APD). To evaluate the role of cell and tissue level changes in atrial arrhythmias, we used a two-dimensional model of atrial tissue in which human WT or ankyrin+/− cell models (based on CRN model) are coupled together via gap junctions in a two-dimensional grid (Fig. 3). Fibrosis was introduced by randomly dispersing inexcitable cells. Conduction velocities in longitudinal and transverse directions were determined using plane wave stimuli. Reentry was then initiated using a cross-field stimulation protocol. The size of the two-dimensional grid was systematically reduced (while retaining aspect ratio of 2.5) until it could no longer support even one cycle of reentry. Duration of reentrant activation, average CL, and activation maps were determined at each grid size and compared for the WT and ankyrin-B+/− model with changes at both the cell and tissue level (3 and 9% fibrosis in WT and ankyrin-B+/−, respectively) (Fig. 3). Reentry dynamics displayed a complex pattern in the ankyrin-B+/− model relative to WT. Specifically, duration of reentry tended to be shorter than that of WT for tissue sizes > 10 cm2 but longer for grid sizes < 10 cm2. Importantly, whereas the WT model could not support more than one cycle of reentry in grids smaller than 9.6 cm2, the ankyrin-B+/− model supported at least one cycle of reentry in a grid as small as 6 cm2. Activation maps during one cycle of reentry reveal a shorter functional line of conduction block and earlier activation times in the ankyrin-B+/− model compared with WT (Fig. 3, E and F). Thus, unexpectedly, ankyrin-B dysfunction increases the likelihood of conduction block at larger tissue sizes but decreases the critical tissue mass that can support reentry.
Fig. 3.
Increased susceptibility to sustained reentry in ankyrin-B+/− atrial tissue. Simulated duration of reentry as a function of tissue area in heterogeneous grids comprised of WT and ankyrin-B+/− cells [based on CRN model (7)] interspersed with poorly coupled electrically inexcitable cells (fibroblasts) at levels corresponding to experimental measurements (3 and 9% for WT and ankyrin-B+/−, respectively) (A); homogeneous (0% fibrosis) 2-dimensional grids comprised of WT (black), ankyrin-B+/− (red), Cav1.3-deficient (gray), or NCX/NKA-deficient (green) cells (B); heterogeneous grids comprised of ankyrin-B+/− cells interspersed with varying degrees of fibroblasts: 0 (black), 3 (gray), and 9% (red) (C); heterogeneous grids comprised of ankyrin-B+/− cells with 9% fibrosis distributed in a diffuse (red) or clustered (gray) pattern (D). E–H: simulated activation maps during 1 cycle of reentry in heterogeneous WT and ankyrin-B+/− grids (E and F) and homogeneous grids with no fibrosis (G and H). Isochrone lines are labeled with corresponding activation time (in ms). I and J: simulated APs at a point remote from the reentry core during 1 s of reentrant activation in heterogeneous (I) and homogeneous (J) WT and ankyrin-B+/− grids. K: average CL during 2 s of sustained reentry in WT and ankyrin-B+/− grids with varying levels of fibrosis (0, 3, and 9%). Longitudinal (L) and transverse conduction velocity (CV; M) of a plane wave propagating in WT and ankyrin-B+/− grids with varying levels of fibrosis.
To determine the relative contribution of cell and tissue level remodeling to the altered reentry dynamics observed in ankyrin-B+/− tissue, we next compared reentrant activation in homogeneous WT and ankyrin-B+/− grids (lacking fibrosis) (Fig. 3B). Eliminating fibrosis eliminated the complex relationship between duration of reentry and grid size, suggesting that fibrosis promotes complex reentry dynamics seen in ankyrin-B+/− tissue. Importantly, loss of ion channel membrane targeting alone is sufficient to produce a dramatic decrease in activation CL (compare values for 0% fibrosis in Fig. 3K) and critical mass. Loss of Cav1.3 is the primary determinant of reduced critical mass in ankyrin-B+/− tissue (Fig. 3B), consistent with our findings that Cav1.3-deficiency is largely responsible for decreased APD in the ankyrin-B+/− atrial cell (Fig. 2).
To further clarify the role of fibrosis, we compared activation and reentry dynamics in ankyrin-B+/− tissue with 0 (homogeneous), 3 (similar to WT), and 9% fibrosis (Fig. 3C). As the degree of fibrosis increases, the relationship between reentry duration and tissue size also became increasingly more complex with increased likelihood of block at larger tissue sizes but decreased likelihood of block at smaller sizes. The net result is a leftward (smaller) shift in the critical mass to support reentry with increasing fibrosis. Increasing fibrosis decreased conduction velocity to a similar degree in WT and ankyrin-B+/− tissue (Fig. 3L). We also examined the difference between activation and reentry dynamics in the ankyrin-B+/− tissue with a diffuse or “stringy” pattern of fibrosis where clusters of up to five cells were allowed (Fig. 3D). We observed a similar effect of fibrosis on conduction velocity and reentry dynamics independent of fibrosis pattern. Thus structural remodeling in ankyrin-B+/− contributes to the decrease in critical tissue mass to support reentry by decreasing conduction velocity. Fibrosis also introduces complex reentry dynamics by increasing the likelihood that the reentrant wave front will terminate by encountering an inexcitable barrier (fibrotic cluster or tissue edge). In summary, our simulations suggest that loss of ankyrin-B function decreases the critical tissue mass for sustained reentry through cellular changes in ion channel membrane expression (decreased APD) with a secondary contribution from altered tissue structure (decreased conduction velocity). Altered tissue structure also introduces complex reentry dynamics by increasing the likelihood for wave break.
Mathematical model of SAN pacemaking.
Ankyrin-B dysfunction is associated with severe and highly penetrant SAN dysfunction, in addition to atrial and ventricular arrhythmias (27). Similarly, ankyrin-B+/− mice show bradycardia coupled with increased heart rate variability (27). At the cellular level, loss of Cav1.3 (normally highly expressed in SAN), NCX, and NKA is observed in SAN cells from ankyrin-B+/− mice. To determine the mechanism for SAN dysfunction in ankyrin-B syndrome, we first incorporated measured loss of Cav1.3, NCX, and NKA into a recently published model of the murine SAN cell (23). Importantly, this model includes separate formulations for Cav1.2 and Cav1.3 channel populations and accurately simulates normal murine AP and calcium cycling. In contrast to the WT SAN cell, which showed regular and rapid spontaneous activation, the ankyrin-B+/− SAN cell displayed highly irregular activity (Fig. 4). Fourier analysis was performed to quantify changes in activation rate and pattern. Whereas the WT SAN cell shows a single peak at 4.7 Hz in agreement with experimental measurements from isolated SAN cells [4.8 ± 0.5 Hz (27)], the ankyrin-B+/− SAN cell shows multiple peaks at lower frequencies as well as a broadening of peaks reflecting an overall slowing of spontaneous activation coupled with increased variability (Fig. 4), consistent with findings in the ankyrin-B+/− mouse and human patients (27). To determine the mechanism for SAN dysfunction in ankyrin-B+/−, we evaluated spontaneous activity in four scenarios: 1) loss of Cav1.3 alone, 2) loss of NCX alone, 3) loss of NKA alone, and 4) coupled loss of NCX and NKA. Cav1.3 loss was sufficient to produce a slowing of spontaneous firing rate, without affecting variability (single peak at 4.2 Hz). In contrast, loss of NCX or NKA together produced a slight increase in firing rate (Fig. 4B). To investigate in detail the sensitivity of SAN pacemaking to changes in expression of Cav1.3, NCX, and NKA, we analyzed changes in SAN CL as we simultaneously varied Cav1.3 and NKA expression or Cav1.3 and NCX expression (Fig. 5). While, in general, CL was most sensitive to changes in Cav1.3, a 30–40% reduction in NKA (depending on Cav1.3 expression) induced a transition from regular periodic firing to a more irregular pattern (“chaotic”) characteristic of ankyrin-B+/− (Fig. 5A). A similar transition was observed as NCX was varied but only after a relatively large degree of loss (50–60% reduction, Fig. 5B). Thus, whereas loss of Cav1.3 is responsible for slowing of firing rate, loss of NKA (and to a lesser degree, NCX) provide a mechanism for the increase in variability observed in ankyrin-B+/− SAN cells.
Fig. 4.
Slow and irregular spontaneous activity in ankyrin-B+/− sinoatrial node cell. Simulated spontaneous APs (A) and corresponding power spectra (B) from WT, ankyrin-B+/−, Cav1.3-deficient, and NCX/NKA-deficient sinoatrial node cells. Power spectra were computed using fast Fourier transform in MATLAB on Vm(t) (100-s duration). Arrows in B indicate location of dominant frequency in WT.
Fig. 5.
Regulation of sinoatrial node cell firing rate pattern by Cav1.3, NKA, and NCX. Parametric analysis was performed by simultaneously varying maximal conductance of Cav1.3 L-type Ca2+ current (ICaL,1.3) and maximal NKA pump rate (A) or maximal conductance of ICaL,1.3 and NCX scaling factor (B). Each parameter was varied from 0.3 to 1 times its control value in increments of 0.02 to produce 1,225 simulations for each panel. CL following 200 s of spontaneous activity was plotted for each simulation (color bar on right indicates value). Three discrete phases of activity [regular (a), chaotic (b), and failure (c)] were observed (representative AP traces shown at right).
Mathematical model of intact SAN.
Regular SAN activity depends on the intricate structure of the distributed pacemaker complex in the context of the intact RA. Our experimental data indicate that ankyrin-B dysfunction results in both electrical and structural remodeling of the RA (see Fig. 1). To determine the interplay between cellular and tissue level changes on SAN dysfunction in ankyrin-B+/− hearts, we used a two-dimensional physiological model of the intact SAN (Fig. 6) (3, 13, 42). The WT model displayed regular spontaneous activation that initiated from a point in the central node and spread through the peripheral node and into the surrounding atrial tissue with a conduction velocity that agrees with experimental measurements (3) (Fig. 6, B and D). Interestingly, the ankyrin-B+/− node showed a shift in the primary activation site toward the periphery as system approached steady state (activation failure in central SAN region indicated by white area in Fig. 6C). Furthermore, exit block occurred after only one beat, and eventually spontaneous activity terminated altogether at steady state (Fig. 6C). While these simulations do not rule out the possibility that in three dimensions, pacemaking would be sustained at an alternative site, they do predict a dramatic shift in location of the primary pacemaking site consistent with experimental measurements in intact ankyrin-B+/− SAN (15). To determine the mechanism for SAN dysfunction in the ankyrin-B+/− model, we evaluated sensitivity of RA activation to each component of ankyrin-B-deficiency in the model (Fig. 6, G–I). Consistent with findings in the single SAN cell, loss of Cav1.3 had the greatest impact on RA activation as determined by the number of successful beats (Fig. 3G) and CL of RA activation (Fig. 3H). Interestingly, whereas loss of NCX or NKA had little impact on RA activation, increasing the degree of fibrosis dramatically increased RA CL and latency likely because of increased loading of surviving SAN cells by surrounding atrial cells. Finally, we determined the effect of uniform uncoupling on SAN function in the ankyrin-B+/− model (Fig. 6, E and F). Interestingly, in contrast to fibrosis that promotes SAN dysfunction, a moderate level of uniform uncoupling had protective effects on SAN function.
Fig. 6.
Effect of ankyrin-B dysfunction on pacemaking. A: mathematical model of intact sinoatrial node (SAN) based on realistic geometry with cell types determined by immunohistochemistry (3, 13, 42). WT (B) and ankyrin-B+/− (C) activation maps corresponding to one complete cycle (top) and spontaneous APs (bottom) recorded from SAN (black lines) and right atrial free wall (red lines). For ankyrin-B+/−, spontaneous SAN APs are shown for 2 sites (black and gray lines, locations indicated by asterisks) on either side of central block region (white area denoted by arrow). D: simulated and measured (3) conduction velocity from SAN into right atrium (RA). Ankyrin-B dysfunction results in shift of primary pacemaker site toward periphery (central SAN region fails to activate), sinus exit block, and ultimately sinoatrial node failure. E and F: comparison of activation of RA free wall and septum in WT and ankyrin-B+/− for different degrees of fibrosis and different values of cell-to-cell coupling. RA activation properties characterized by number of free wall activations (G), CL of RA activation (H), and latency to first activation (I) in WT, ankyrin-B+/−, Cav1.3-deficient, NKA-deficient, NCX-deficient, and WT with 9% fibrosis. Rg, gap junction resistance.
DISCUSSION
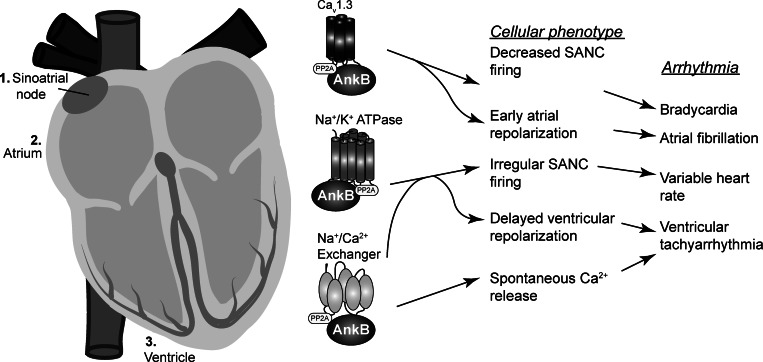
Our studies identify ionic mechanisms underlying sustained atrial fibrillation and SAN dysfunction in cardiac disease. Specifically, we find that loss of Cav1.3 leads to AP shortening which reduces the AP spatial wavelength and the critical tissue size needed to sustain reentry. In parallel, increased fibrosis also reduces spatial wavelength but by slowing conduction. In the SAN, loss of Cav1.3 contributes to a general slowing of activation, whereas defects in NKA and NCX to a lesser degree underlie increased variability (Fig. 7). Simulations of pacemaking in the intact SAN reveal a shift in primary pacemaking site, episodes of SAN exit block, and even failure in ankyrin-B+/−. In previous computational studies, we have shown that ventricular arrhythmias associated with ankyrin-B deficiency are likely the cause of Ca2+ overload secondary to defects in NCX and NKA membrane targeting (44). In contrast, results from the current study predict that loss of Cav1.3 in ankyrin-B+/− atrial and SAN cells compensates for loss of NCX and NKA, resulting in very little net change in intracellular Ca2+ cycling. Instead, defects in repolarization and/or spontaneous depolarization lead to distinct arrhythmia phenotypes in these specific cardiac regions (Fig. 7).
Fig. 7.
Broad spectrum of arrhythmia phenotypes and proposed underlying cellular/molecular mechanisms in ankyrin-B syndrome. Loss of ankyrin-B affects a myriad of ion channels and transporters. Loss of Cav1.3 promotes early atrial repolarization and slowing of SAN cell spontaneous firing which contributes to a substrate for sinus node dysfunction and atrial fibrillation. Loss of NKA promotes irregular, chaotic SAN firing and delayed ventricular repolarization leading to a substrate for sinus node dysfunction and ventricular tachyarrhythmias. Finally, loss of NCX promotes overload of Ca2+ in the sarcoplasmic reticulum, which is an important determinant of the proarrhythmogenic substrate in the ventricle. PP2A, protein phosphatase 2A.
Loss-of-function ankyrin-B defects have been observed in both congenital (ankyrin-B syndrome) and acquired disease (19, 22, 33–35). In the setting of ankyrin-B syndrome, it is clear that ankyrin-B dysfunction is responsible for changes in membrane expression of ion channels leading ultimately to increased susceptibility to arrhythmias. Although the situation in acquired disease is undoubtedly more complex, it is interesting to consider the possibility that ankyrin-B dysfunction underlies electrical and structural remodeling known to precipitate arrhythmias in heart disease including heart failure. An important question going forward is, What leads to ankyrin-B defects in acquired disease? Recent studies suggest that activation of the calcium-dependent protease calpain is an important precursor to loss of ankyrin-B under stress conditions (22). Future studies will determine whether this pathway is involved across disease pathologies and whether therapeutic interventions aimed at calpain may prevent ankyrin-B dysfunction and associated arrhythmias.
Our results indicate that loss of ankyrin-B regulates the arrhythmia substrate at the cellular level by altering membrane expression and at the tissue level by inducing fibrosis. We also predict that fibrosis has a very different impact on SAN function than uniform uncoupling. Specifically, whereas fibrosis compromises SAN activity, moderate uniform uncoupling actually preserves function. These findings are consistent with recent computational studies showing a similar relationship between electrical remodeling, structural remodeling, and AP wavelength (25). Our findings also agree with previous work showing that fibrosis slows conduction and produces fragmented wave fronts conducive to multiple reentry circuits (4, 38, 46). An important limitation to consider when interpreting these results is that the exact degree of coupling between fibroblasts and myocytes in vivo is unknown. Here, we assume poor coupling based on studies showing sparse gap junction formation between fibroblasts and myocytes in vivo (10, 24). Whether this coupling changes in disease or in different regions of the heart remains to be determined. Furthermore, while the molecular pathway for ankyrin-B-dependent regulation of ion channel membrane expression has been well characterized, the pathway linking ankyrin-B dysfunction to changes in fibrosis is unknown. However, fibrosis is a common finding in atrial fibrillation and has been shown to be regulated at least in part by reactive oxygen species-dependent activation of matrix metalloproteinases (14). Interestingly, calpain activation has also been identified as a potential inducer of fibrosis through TGF-β activation (29). It will be important for future studies to determine whether ankyrin-B dysfunction induces changes in tissue structure through similar or distinct pathways. It is also important to note that fibrosis measurements in this study come from age-matched WT and ankyrin-B+/− mice. We anticipate that fibrosis in ankyrin-B-deficient atria will vary with age and species.
Our findings add to mounting evidence that a common mechanism may give rise to atrial arrhythmias and SAN dysfunction (12, 28). Fibrosis helps create the substrate for sustained reentry in the atria by slowing conduction while contributing to SAN dysfunction (slowed activation, intermittent exit block) by altering the source-sink relationship between SAN and surrounding atrial tissue. At the same time, loss of Cav1.3 reduces duration of atrial APs (favorable for sustained reentry) while slowing spontaneous SAN cell activation. Thus ankyrin-B dysfunction involves changes at both the cell and tissue level that favor the common manifestation of atrial arrhythmias and sinus node dysfunction. Interestingly, severe bradycardia has been reported in the Cav1.3 null (α1D−/−) mouse (37). While spontaneous atrial tachyarrhythmias have not been observed, it will be important to determine if these animals are susceptible to induced arrhythmias. However, it is interesting to consider the possibility that atrial arrhythmias are more common in ankyrin-B+/− due to the presence of multiple proarrhythmic defects at the cell and tissue level (e.g., loss of multiple ion channels combined with increased fibrosis).
Limitations.
While our mathematical model of the ankyrin-B+/− cardiomyocyte accounts for many known ankyrin-B targets, it has important limitations based on the available experimental data. Although the model accounts for the loss of NCX, NKA, and Cav1.3, it does not consider the loss of the inositol 1,4,5-trisphosphate receptor in atrial cardiomyocytes. Furthermore, it is likely that dysregulation of kinase/phosphatase balance secondary to loss of protein phosphatase 2a targeting will play an important role in regulating the arrhythmia substrate and/or trigger (11). Our model will provide the framework into which new data on the function of these proteins in atrial cardiomyocytes may be incorporated. It is also important to note that for the sake of simplicity, these studies do not account for the detailed physiology of the three-dimensional atrium. Nevertheless, there is good agreement between the fundamental behavior in our study and that observed using higher dimensional models (25). Finally, while simulation results are considerably robust across models, important distinctions exist, particularly with respect to AP restitution (Fig. 2), that may have implications for behavior at the tissue level.
Appendix
Initial conditions for state variables in mathematical model of human atrial AP can be found in Table A1.
Mathematical model of the intact SAN.
Mathematical models used to represent electrical activity of different cell types in the intact SAN are provided in Table A2. Gap junction resistance and ion channel conductances were scaled from the SAN center to the periphery according to Eqs. 1–4 to account for heterogeneity of the SAN cell population (3, 23, 26). The model of the atrial cell was modified to produce an AP more similar to the mouse atrial AP (Eqs. 5–9). Initial conditions for cells in the intact node are provided in Tables A3 and A4.
Equations for heterogeneity in SAN region.
Gap junction resistance (in Ωcm2) is as follows:
| (1) |
where x is distance (in cm).
Conductance of fast sodium current (in mS/cm2) is as follows:
| (2) |
Conductance of Cav1.2 L-type Ca2+ current (in ms/cm2) is as follows:
| (3) |
Conductance of rapid delayed rectifier K+ current (in mS/cm2) is as follows:
| (4) |
Mathematical model of murine atrial AP waveform.
Equations and parameters that differ from original publication are provided below. All other equations and parameters may be found in original publication (7). Importantly, there is good agreement between APD in measured and simulated WT and ankyrin-B+/− mouse atrial cells (Fig. A1).
Fig. A1.
Modified model of murine atrial action potential waveform. A: simulated WT and ankyrin-B+/− action potentials following steady-state pacing at CL = 1,000 ms. B: simulated and measured (9) APD. APD90, APD at 90% repolarization.
L-type Ca2+ current is as follows:
| (5) |
Regarding ultrarapid delayed rectifier K+ current, equations derived from model of mouse ventricular AP (1) are as follows:
 |
Regarding transient outward K+ current, equations derived from model of mouse ventricular AP (1) are as follows:
 |
Regarding noninactivating steady-state K+ current, equations derived from model of mouse ventricular AP (1) are as follows:
 |
Ryanodine receptor Ca2+ release channel is as follows:
| (9) |
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grants HL-096805 and HL-114893 (to T. J. Hund), HL-084583 and HL-083422 (to P. J. Mohler), and HL-079031, HL-096652, HL-113001, and HL-070250 (to M. E. Anderson); National Science Foundation Grant DMS-1022466 (to C. C. Mitchell); the Gilead Sciences Research Scholars Program in Cardiovascular Disease (to T. J. Hund); Saving Tiny Hearts Society (to P. J. Mohler); American Heart Association (to P. J. Mohler); and the Fondation Leducq Transatlantic Alliance for CaMKII Signaling (08CVD01) (to M. E. Anderson and P. J. Mohler).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.M.W., P.G., S.H., K.Z., C.C.M., M.E.A., P.J.M., and T.J.H. conception and design of research; R.M.W., P.G., S.H., K.Z., P.J.M., and T.J.H. performed experiments; R.M.W., P.G., K.Z., C.C.M., M.E.A., P.J.M., and T.J.H. analyzed data; R.M.W., P.G., S.H., K.Z., C.C.M., M.E.A., P.J.M., and T.J.H. interpreted results of experiments; R.M.W., P.G., S.H., P.J.M., and T.J.H. prepared figures; R.M.W., P.G., P.J.M., and T.J.H. drafted manuscript; R.M.W., P.G., S.H., K.Z., C.C.M., M.E.A., P.J.M., and T.J.H. edited and revised manuscript; R.M.W., P.G., S.H., K.Z., C.C.M., M.E.A., P.J.M., and T.J.H. approved final version of manuscript.
REFERENCES
- 1. Bondarenko VE, Szigeti GP, Bett GC, Kim SJ, Rasmusson RL. Computer model of action potential of mouse ventricular myocytes. Am J Physiol Heart Circ Physiol 287: H1378–H1403, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Burstein B, Comtois P, Michael G, Nishida K, Villeneuve L, Yeh YH, Nattel S. Changes in connexin expression and the atrial fibrillation substrate in congestive heart failure. Circ Res 105: 1213–1222, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Butters TD, Aslanidi OV, Inada S, Boyett MR, Hancox JC, Lei M, Zhang H. Mechanistic links between Na+ channel (SCN5A) mutations and impaired cardiac pacemaking in sick sinus syndrome. Circ Res 107: 126–137, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res 65: 40–51, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Camors E, Mohler PJ, Bers DM, Despa S. Ankyrin-B reduction enhances Ca spark-mediated SR Ca release promoting cardiac myocyte arrhythmic activity. J Mol Cell Cardiol 52: 1240–1248, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Christensen MD, Dun W, Boyden PA, Anderson ME, Mohler PJ, Hund TJ. Oxidized calmodulin kinase II regulates conduction following myocardial infarction: a computational analysis. PLoS Comput Biol 5: e1000583, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Courtemanche M, Ramirez RJ, Nattel S. Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model. Am J Physiol Heart Circ Physiol 275: H301–H321, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Cunha SR, Bhasin N, Mohler PJ. Targeting and stability of Na/Ca exchanger 1 in cardiomyocytes requires direct interaction with the membrane adaptor ankyrin-B. J Biol Chem 282: 4875–4883, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Cunha SR, Hund TJ, Hashemi S, Voigt N, Li N, Wright P, Koval O, Li J, Gudmundsson H, Gumina RJ, Karck M, Schott J, Probst V, Le Marec H, Anderson ME, Dobrev D, Wehrens XH, Mohler PJ. Defects in ankyrin-based membrane protein targeting pathways underlie atrial fibrillation. Circulation 124: 1212–1222, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Maziere AM, van Ginneken AC, Wilders R, Jongsma HJ, Bouman LN. Spatial and functional relationship between myocytes and fibroblasts in the rabbit sinoatrial node. J Mol Cell Cardiol 24: 567–578, 1992 [DOI] [PubMed] [Google Scholar]
- 11. DeGrande S, Nixon D, Koval O, Curran JW, Wright P, Wang Q, Kashef F, Chiang D, Li N, Wehrens XH, Anderson ME, Hund TJ, Mohler PJ. CaMKII inhibition rescues pro-arrhythmic phenotypes in model of human ankyrin-B syndrome. Heart Rhythm 9: 2034–2041, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dobrzynski H, Boyett MR, Anderson RH. New insights into pacemaker activity: promoting understanding of sick sinus syndrome. Circulation 115: 1921–1932, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Dobrzynski H, Li J, Tellez J, Greener ID, Nikolski VP, Wright SE, Parson SH, Jones SA, Lancaster MK, Yamamoto M, Honjo H, Takagishi Y, Kodama I, Efimov IR, Billeter R, Boyett MR. Computer three-dimensional reconstruction of the sinoatrial node. Circulation 111: 846–854, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Friedrichs K, Baldus S, Klinke A. Fibrosis in atrial fibrillation—role of reactive species and MPO. Front Physiol 3: 214, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Glukhov AV, Fedorov VV, Anderson ME, Mohler PJ, Efimov IR. Functional anatomy of the murine sinus node: high-resolution optical mapping of ankyrin-B heterozygous mice. Am J Physiol Heart Circ Physiol 299: H482–H491, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grandi E, Pandit SV, Voigt N, Workman AJ, Dobrev D, Jalife J, Bers DM. Human atrial action potential and Ca2+ model: sinus rhythm and chronic atrial fibrillation. Circ Res 109: 1055–1066, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hund TJ, Decker KF, Kanter E, Mohler PJ, Boyden PA, Schuessler RB, Yamada KA, Rudy Y. Role of activated CaMKII in abnormal calcium homeostasis and INa remodeling after myocardial infarction: Insights from mathematical modeling. J Mol Cell Cardiol 45: 420–428, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hund TJ, Kucera JP, Otani NF, Rudy Y. Ionic charge conservation and long-term steady state in the Luo-Rudy dynamic model of the cardiac cell. Biophys J 81: 3324–3331, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hund TJ, Wright PJ, Dun W, Snyder JS, Boyden PA, Mohler PJ. Regulation of the ankyrin-B-based targeting pathway following myocardial infarction. Cardiovasc Res 81: 742–749, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Joyner RW, Ramon F, Morre JW. Simulation of action potential propagation in an inhomogeneous sheet of coupled excitable cells. Circ Res 36: 654–661, 1975 [DOI] [PubMed] [Google Scholar]
- 21. Kamkin A, Kiseleva I, Isenberg G. Activation and inactivation of a non-selective cation conductance by local mechanical deformation of acutely isolated cardiac fibroblasts. Cardiovasc Res 57: 793–803, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Kashef F, Li J, Wright P, Snyder J, Suliman F, Kilic A, Higgins RS, Anderson ME, Binkley PF, Hund TJ, Mohler PJ. Ankyrin-B in heart failure: Identification of a new component of metazoan cardioprotection. J Biol Chem 287: 30268–30281, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kharche S, Yu J, Lei M, Zhang H. A mathematical model of action potentials of mouse sinoatrial node cells with molecular bases. Am J Physiol Heart Circ Physiol 301: H945–H963, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kohl P, Kamkin AG, Kiseleva IS, Noble D. Mechanosensitive fibroblasts in the sino-atrial node region of rat heart: interaction with cardiomyocytes and possible role. Exp Physiol 79: 943–956, 1994 [DOI] [PubMed] [Google Scholar]
- 25. Krogh-Madsen T, Abbott GW, Christini DJ. Effects of electrical and structural remodeling on atrial fibrillation maintenance: a simulation study. PLoS Comput Biol 8: e1002390, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kurata Y, Matsuda H, Hisatome I, Shibamoto T. Regional difference in dynamical property of sinoatrial node pacemaking: role of Na+ channel current. Biophys J 95: 951–977, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Le Scouarnec S, Bhasin N, Vieyres C, Hund TJ, Cunha SR, Koval O, Marionneau C, Chen B, Wu Y, Demolombe S, Song LS, Le Marec H, Probst V, Schott JJ, Anderson ME, Mohler PJ. Dysfunction in ankyrin-B-dependent ion channel and transporter targeting causes human sinus node disease. Proc Natl Acad Sci USA 105: 15617–15622, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee JM, Kalman JM. Sinus node dysfunction and atrial fibrillation: two sides of the same coin? Europace 15: 161–162, 2012 [DOI] [PubMed] [Google Scholar]
- 29. Letavernier E, Zafrani L, Perez J, Letavernier B, Haymann JP, Baud L. The role of calpains in myocardial remodelling and heart failure. Cardiovasc Res 96: 38–45, 2012 [DOI] [PubMed] [Google Scholar]
- 30. Liu J, Dobrzynski H, Yanni J, Boyett MR, Lei M. Organisation of the mouse sinoatrial node: structure and expression of HCN channels. Cardiovasc Res 73: 729–738, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Mohler PJ, Davis JQ, Bennett V. Ankyrin-B coordinates the Na/K ATPase, Na/Ca exchanger, and InsP3 receptor in a cardiac T-tubule/SR microdomain. PLoS Biol 3: e423, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mohler PJ, Healy JA, Xue H, Puca AA, Kline CF, Allingham RR, Kranias EG, Rockman HA, Bennett V. Ankyrin-B syndrome: enhanced cardiac function balanced by risk of cardiac death and premature senescence. PLoS One 2: e1051, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mohler PJ, Le Scouarnec S, Denjoy I, Lowe JS, Guicheney P, Caron L, Driskell IM, Schott JJ, Norris K, Leenhardt A, Kim RB, Escande D, Roden DM. Defining the cellular phenotype of “ankyrin-B syndrome” variants: human ANK2 variants associated with clinical phenotypes display a spectrum of activities in cardiomyocytes. Circulation 115: 432–441, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, Song LS, Haurogne K, Kyndt F, Ali ME, Rogers TB, Lederer WJ, Escande D, Le Marec H, Bennett V. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature 421: 634–639, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Mohler PJ, Splawski I, Napolitano C, Bottelli G, Sharpe L, Timothy K, Priori SG, Keating MT, Bennett V. A cardiac arrhythmia syndrome caused by loss of ankyrin-B function. Proc Natl Acad Sci USA 101: 9137–9142, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morita N, Sovari AA, Xie Y, Fishbein MC, Mandel WJ, Garfinkel A, Lin SF, Chen PS, Xie LH, Chen F, Qu Z, Weiss JN, Karagueuzian HS. Increased susceptibility of aged hearts to ventricular fibrillation during oxidative stress. Am J Physiol Heart Circ Physiol 297: H1594–H1605, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell 102: 89–97, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Rohr S. Myofibroblasts in diseased hearts: new players in cardiac arrhythmias? Heart Rhythm 6: 848–856, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Rudy Y, Silva JR. Computational biology in the study of cardiac ion channels and cell electrophysiology. Q Rev Biophys 39: 57–116, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schott JJ, Charpentier F, Peltier S, Foley P, Drouin E, Bouhour JB, Donnelly P, Vergnaud G, Bachner L, Moisan JP, Le Marec H, Paschal O. Mapping of a gene for long QT syndrome to chromosome 4q25–27. Am J Hum Genet 57: 1114–1122, 1995 [PMC free article] [PubMed] [Google Scholar]
- 41. Smith S, Curran J, Hund TJ, Mohler PJ. Defects in cytoskeletal signaling pathways, arrhythmia, and sudden cardiac death. Front Physiol 3: 122, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Swaminathan PD, Purohit A, Soni S, Voigt N, Singh MV, Glukhov AV, Gao Z, He JB, Luczak ED, Joiner ML, Kutschke W, Yang J, Donahue JK, Weiss RM, Grumbach IM, Ogawa M, Chen PS, Efimov IR, Dobrev D, Mohler PJ, Hund TJ, Anderson ME. Oxidized CaMKII causes sinus node dysfunction. J Clin Invest 121: 3277–3288, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weiss JN, Nivala M, Garfinkel A, Qu Z. Alternans and arrhythmias: from cell to heart. Circ Res 108: 98–112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wolf RM, Mitchell CC, Christensen MD, Mohler PJ, Hund TJ. Defining new insight into atypical arrhythmia: a computational model of ankyrin-B-syndrome. Am J Physiol Heart Circ Physiol 299: H1505–H1514, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Workman AJ, Kane KA, Rankin AC. The contribution of ionic currents to changes in refractoriness of human atrial myocytes associated with chronic atrial fibrillation. Cardiovasc Res 52: 226–235, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Zlochiver S, Munoz V, Vikstrom KL, Taffet SM, Berenfeld O, Jalife J. Electrotonic myofibroblast-to-myocyte coupling increases propensity to reentrant arrhythmias in two-dimensional cardiac monolayers. Biophys J 95: 4469–4480, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]