Abstract
Cannabinoids have been shown to exert analgesic and anti-inflammatory effects, and the effects of cannabinoids are mediated primarily by cannabinoid receptors 1 and 2 (CB1and CB2). Both CB1 and CB2 are present in bladders of various species, including human, monkey, and rodents, and it appears that CB2 is highly expressed in urothelial cells. We investigated whether treatment with the CB2 agonist GP1a alters severity of experimental cystitis induced by acrolein and referred mechanical hyperalgesia associated with cystitis. We also investigated whether the mitogen-activated protein kinases (MAPK), ERK1/2, p38, and JNK are involved in the functions of CB2. We found that treatment with the selective CB2 agonist GP1a (1–10 mg/kg, ip) inhibited the severity of bladder inflammation 3 h after intravesical instillation of acrolein in a dose-dependent manner, and inhibition reached significance at a dose of 10 mg/kg (P < 0.05). Treatment with GP1a (10 mg/kg) inhibited referred mechanical hyperalgesia associated with cystitis (P < 0.05). The inhibitory effects of the CB2 agonist were prevented by the selective CB2 antagonist AM630 (10 mg/kg, sc). We further demonstrated the inhibitory effects of CB2 appear to be at least partly mediated by reducing bladder inflammation-induced activation of ERK1/2 MAPK pathway. The results of the current study indicate that CB2 is a potential therapeutic target for treatment of bladder inflammation and pain in patients.
Keywords: cannabinoid receptor 2, GP1a, cystitis, hyperalgesia, mice
painful bladder disorders, such as interstitial cystitis/painful bladder syndrome (IC/PBS), are relatively common. IC/PBS is a chronic painful disorder characterized by increased frequency, urgency, and bladder pain, and it is estimated to affect 2.7% to 6.5% of American women (1, 5, 20). The etiology and pathogenesis of IC/PBS remain unknown. It is highly probable that the etiology of IC/PBS is a multifactorial condition, and no treatment or combination of treatments has been found to be consistently effective in alleviating symptoms in IC/PBS patients. (1, 5, 20). Cannabinoids have been shown to have analgesic and anti-inflammatory effects, and the effects of cannabinoids are mediated primarily by cannabinoid receptors 1 and 2 (CB1and CB2), both coupled to inhibitory G proteins (2, 12, 17, 19). CB1 is predominantly located in the central nervous system (CNS), whereas CB2 is primarily present in peripheral tissues (2, 12, 29). Therefore, selective CB2 agonists may exert actions without inducing undesirable CNS effects related to activation of CB1, including hypoactivity, hypothermia, and catalepsy (2, 12, 29, 46, 58).
Both CB1 and CB2 are present in bladders of various species, including humans, monkeys, and rodents (26, 32, 49, 69–70), and it appears that CB2 is highly expressed in urothelial cells (26, 32, 49, 69). Intravesical administration of a selective CB1 agonist inhibited sensitization of bladder afferent nerves induced by bladder inflammation (73). In vitro electrically evoked contraction of bladder strips was inhibited by treatment with CB1 or CB2 agonists (70). Treatment with a selective CB2 agonist increased micturition intervals and volumes in normal rats (27), and improved bladder function of rats after partial urethral obstruction (28). Fatty acid amide hydrolase (FAAH), an enzyme that specifically degrades anandamide, an endogenous cannabinoids (or endocannabinoid), is present in urothelium (50, 65). Inhibition of FAAH activity also increased micturition intervals and volumes in rats (65), and the effects of FAAH inhibition were prevented by a selective CB2 antagonist. These studies support a role of cannabinoid receptors, particularly CB2, in regulating bladder functions under physiological and pathophysiological conditions.
Cyclophosphamide (CYP) is an antineoplastic alkylating agent commonly used to treat cancer patients (15), and an undesirable clinical side effect of CYP is hemorrhagic cystitis. CYP is metabolized by the liver to acrolein that is accumulated in urine, and acrolein is primarily responsible for CYP-induced cystitis (13, 15). CYP/acrolein-induced cystitis in rodents is commonly used as an experimental model to study mechanisms underlying cystitis and associated visceral pain (7, 8, 25). Recently, a highly specific CB2 agonist, N-(piperidin-1-yl)-1-(2,4-dichlorophenyl)-1,4-dihydro-6-methylindeno[1,2-c]pyrazole-3-carboxamide (GP1a) has been described (22, 53). Systemic treatment with GP1a decreased serum IL-6 concentrations, reduced neutrophil recruitment in lung tissues, and increased mean survival time in a mouse model of sepsis induced by cecal ligation and puncture (68). In the present study, we investigated whether treatment with the CB2 agonist GP1a alters severity of experimental cystitis induced by acrolein and referred hyperalgesia associated with cystitis. We also investigated whether the mitogen-activated protein kinases (MAPK), ERK1/2, p38 and JNK are involved in the functions of CB2.
MATERIALS AND METHODS
Animals.
Female C57BL/6NH mice (10–12 wk old) were obtained from Harlan (Indianapolis, IN). Experiments were conducted in accordance with National Institutes of Health Guidelines, and all protocols were reviewed and approved by the Animal Care and Use Committee of the University of Wisconsin.
Histological evaluation of cystitis.
Mice were anesthestized with avertin (250 mg/kg, Sigma-Aldrich, St. Louis, MO), injected intraperitoneally (ip), and cystitis was induced by intravesical instillation of acrolein (1 mM, 150 μl total volume; Ultra Scientific, Kingstown, RI) via a urethral catheter (PE 10, i.d. 0.28 mm, o.d. 0.61 mm; Becton Dickinson, Sparks, MD). The dose and volume of acrolein were chosen on the basis of results of preliminary experiments to induce cystitis of moderate severity. Control mice received an equivalent volume of intravesical saline (0.9%) instead of acrolein.
Three hours after instillation of acrolein or saline, mice were deeply anesthetized with pentobarbital (50 mg/kg, ip) and perfused with saline through a canula inserted into the left ventricle. Bladders were removed and weighed, and bladder weight (mg) was normalized to body weight (g). Bladders were divided into two parts; the caudal part including the neck region was fixed in 4% paraformaldehyde for 4 h at 4°C and cryoprotected with 30% sucrose in phosphate-buffered saline (PBS) at 4°C. Tissue sections were made with a cryostat at a thickness of 10 μm. Every 4th section was stained with hematoxylin and eosin for morphological analysis, and 4–6 sections from each bladder were examined microscopically. The urothelium/suburothelium of the remainder of the bladders was mechanically separated from the detrusor using fine forceps as previously described (40, 75). Tissues were stored at −80°C until analyzed (see below).
Peripheral nociception testing.
The effects of cystitis on response to peripheral application of mechanical stimuli were also evaluated. The individual performing testing was unaware of the pretreatments of mice. Mechanical sensitivity of the hind paws was assessed using von Frey monofilaments and the up-down method (10). Mice were placed in individual Plexiglas chambers with a wire mesh floor, and allowed to acclimate for at least 30 min or until cage exploration stopped. Sensitivity of the hind paws was assessed with a series of six von Frey filaments of increasing stiffness. Stimulus-related retraction of the tested paw was considered a withdrawal response, and the 50% paw withdrawal threshold was determined by the nonparametric method of Dixon (10). Previous studies have reported enhanced mechanical sensitivity of both the hind paws and pelvic region of mice in experimental cystitis (48, 66, 74) and similar results are reported, regardless of the area tested (48, 66).
Isolation of protein from urothelium/suburothelium.
Tissues were homogenized with T-PER Tissue Protein Extraction Reagent (Thermo Scientific, Rockford, IL) containing protease and phosphatase inhibitors (Roche, Indianapolis, IN). Supernatants were collected by centrifugation at 10,000 g for 15 min at 4°C. Protein concentrations were determined using the BCA Protein Assay kit (Thermo Scientific). Protein samples were mixed 1:1 with Laemmli sample buffer (Bio-Rad, Hercules, CA), placed in boiling water for 5 min, and stored at −20°C until analyzed.
Semi-quantitative immunoblotting analysis.
Protein samples (20 μg/lane) were resolved on 10% SDS-polyacrylamide gels and transferred to nitrocellulose membranes (Bio-Rad). Membranes were blocked in 5% dry fat-free milk in 1 × TBST (20 mM Tris-HCl, 137 mM NaCl, 0.05% Tween-20; pH 7.5). After rinsing, membranes were incubated at 4°C overnight with the specific primary antibody. Membranes were then washed free of primary antibody and incubated for 1 h with appropriate secondary antibody conjugated to horseradish peroxidase at room temperature. Signals were revealed using a chemiluminescent detection reagent (Amersham, Arlington Heights, IL). Membranes were apposed to X-ray films, and films were developed. Membranes were then stripped and reprobed with a mouse anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody or rabbit anti-total ERK1/2, total p38, or total JNK as loading controls. Protein abundance was estimated by optical density measurement with ImageJ software (NIH, Bethesda, MD, USA). The values were normalized with the loading control (for CB2, GAPDH; for others, each corresponding nonphosphorylated protein). Abundance of p-ERK1 and 2 was analyzed individually, and the mean values were used for normalization. To compare the values between different treatment groups, the normalized values of each protein(s) were calculated as fold change based on values of control samples (set as 1). The primary antibodies used for immunoblotting were rabbit anti-murine CB2 (Cayman Chemical, Ann Arbor, MI, 1:1,000), monoclonal anti-GAPDH antibody (1:5,000) (Abcam, Cambridge, MA), rabbit anti-phospho-ERK1/2 (1:3,000), phospho-p38 (1:3,000), phospho-JNK (1:3,000), total-ERK1/2 (1:3,000), total p38 (1:2,000), and total JNK (1:2,000) and antibodies (Cell Signaling, Danvers, MA). The secondary antibodies were goat anti-rabbit IgG and goat anti-mouse IgG (both conjugated to horseradish peroxidase and used at 1:20,000 dilutions) (Santa Cruz, Biotechnology, Santa Cruz, CA). The specificity of CB2 antibody has been well described, and this antibody reveals the expression of CB2 in various tissues in wild-type, but not in CB2 knockout mice (3, 56, 63).
Treatment with CB2 agonist and antagonist, and ERK phosphorylation inhibitor.
The selective CB2 agonist (GP1a) and the selective CB2 antagonist (AM630) were obtained from Tocris (Bristol, UK). The Ki values for GP1a are 0.037 and 363 nM for the CB2 and CB1, respectively, representing a 4 log higher affinity of this compound for the CB2 (22, 53). The Ki values of AM630 are 31.2 nM and 5.3 μM for the CB2 and CB1, respectively (Tocris). U0126 (inhibitor of ERK1/2 phosphorylation) was purchased from Promega (Madison, WI). GP1a, AM630, and U0126 were dissolved with ethanol as stock solutions and diluted in saline to desired concentrations. GP1a (1–10 mg/kg, ip) or vehicle was given 10 min prior to instillation of acrolein or saline. AM 630 (10 mg/kg) or vehicle was given 10 min prior to injection of GP1a by subcutaneous injection (sc). In other experiments, U0126 (30 mg/kg, ip) or vehicle was given 15 min prior to acrolein or saline.
Statistical analysis.
Data are presented as arithmetic means ± SE; n = 6–8 in each treatment group. The data were analyzed using one-way ANOVA followed by Bonferroni post hoc comparisons (GraphPad Prism, San Diego, CA). P values < 0.05 were considered significant.
RESULTS
Effects of GP1a on severity of cystitis.
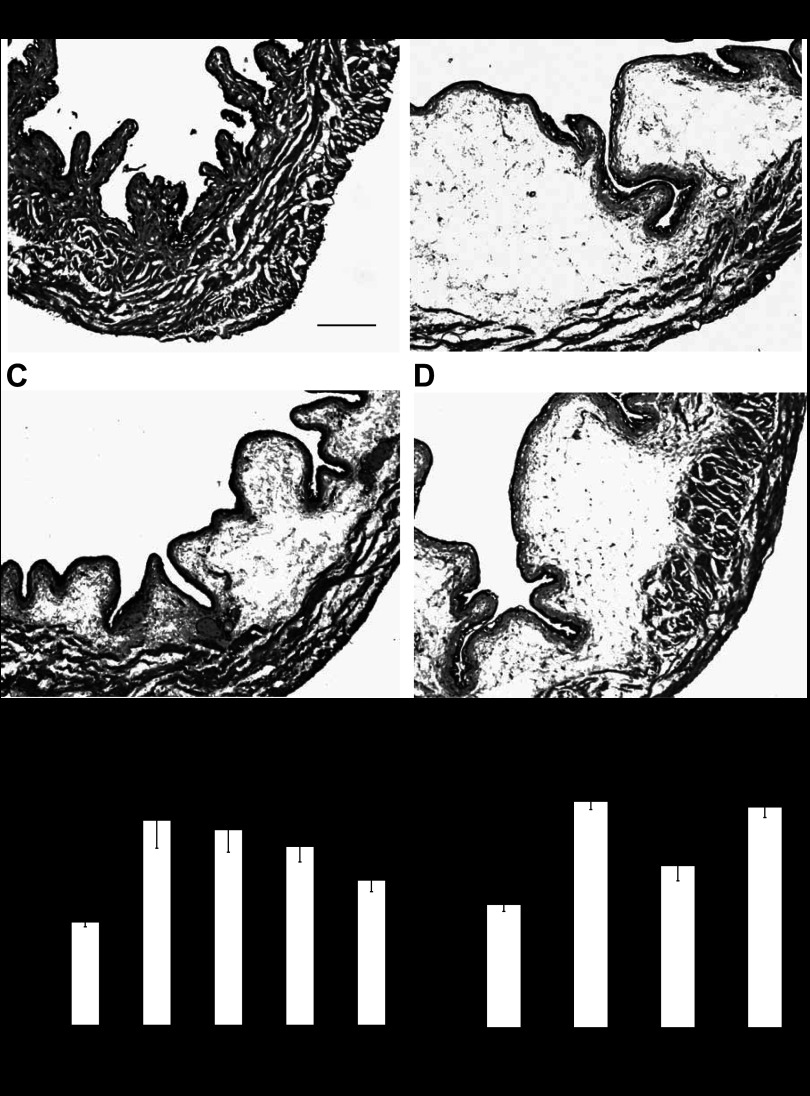
No signs of cystitis were observed in saline-treated animals (Fig. 1A). Three hours after intravesical instillation of 1 mM acrolein, histological examination of the bladders indicated the presence of cystitis characterized primarily by edema in the submucosal region (Fig. 1 B–D). Occasionally, areas of hemorrhage and mild infiltration of inflammatory cells were also observed. The weights of acrolein-treated bladders were significantly increased (2.34 ± 0.32 mg/g, n = 8) relative to saline-treated controls (1.18 ± 0.06 mg/g, n = 6) (P < 0.01), and this appeared to be primarily due to submucosal edema. Treatment with GP1a (1–10 mg/kg, ip) attenuated the increase of bladder weight in a dose-dependent manner (the correlation coefficient r2 between dose of GP1a and inhibition was 0.99), and inhibition reached significance at a dose of 10 mg/kg (P < 0.05 vs acrolein-treated). Inhibition of acrolein-induced increased bladder weight produced by GP1a (10 mg/kg) (1.58 ± 0.15 mg/g, n = 8) was reversed by pretreatment with the selective CB2 antagonist AM630 (2.15 ± 0.1 mg/g, n = 8) (10 mg/kg, P < 0.05 vs acrolein+GP1a-treated). Treatment with AM630 (10 mg/kg) alone did not affect acrolein-induced increase of bladder weight (2.21 ± 0.1 mg/g, P > 0.05 vs acrolein-treated, n = 6).
Fig. 1.
Representative images of bladders from mice treated with saline (A), acrolein (B), acrolein+GP1a (C), and acrolein+AM630+GP1a (D) 3 h prior to death. Acrolein induced cystitis characterized by edema in the submucosal region, areas of hemorrhage, and mild infiltration of inflammatory cells. E: treatment with GP1a reduced severity of cystitis in a dose-dependent manner. F: effect of GP1a (10 mg/kg) was reversed by AM630 (10 mg/kg). Scale bar indicates 100 μm. n = 6 (saline-treated controls). n = 8 (other treatment groups). *P < 0.01 vs. saline-treated group. #P < 0.05 vs. acrolein-treated group. @P < 0.05 vs. acrolein+GP1a-treated.
Treatment with GP1a (10 mg/kg) or AM630 (10 mg/kg) in mice instilled with saline did not affect bladder weight (1.16 ± 0.05 and 1.09 ± 0.09 mg/g, respectively, n = 6, P > 0.05 vs saline-treated controls), and no histological evidence of cystitis was observed (not shown).
Presence of CB2 in urothelium/suburothelium.
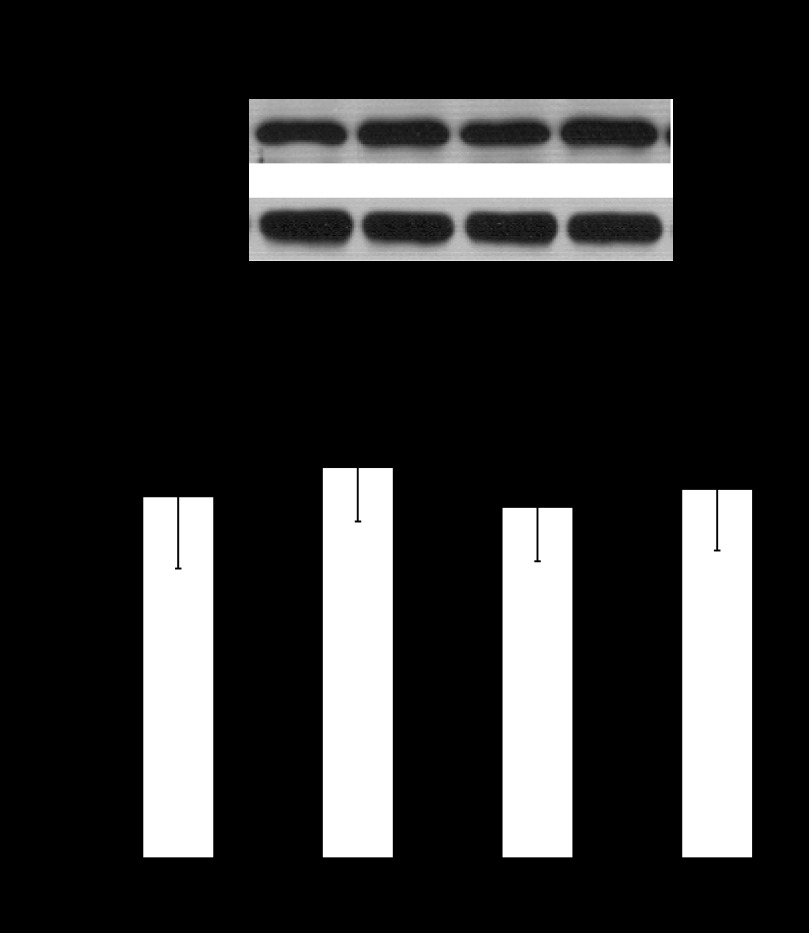
We investigated whether CB2 is present in mouse urothelium/suburothelium using immunoblotting. As shown in Fig. 2, CB2 protein occurred as a single band at about 45 kDa on denaturing acrylamide gel, as reported previously (49). Exposure to acrolein for 3 h did not alter CB2 abundance in urothelium/suburothelium. Also, treatment with GP1a or AM630+GP1a did not affect abundance of CB2 (Fig. 2).
Fig. 2.
Semiquantitative immunoblotting analysis of CB2 in urothelium/suburothelium. Representive immunoblots are shown. The values determined by densitometry were normalized to those of GAPDH (loading control) in each sample. Exposure to acrolein for 3 h did not alter CB2 abundance in urothelium. Also, treatment with acrolein+GP1a or acrolein+AM630+GP1a did not affect abundance of CB2. n = 6 (saline-treated controls). n = 8 (other treatment groups).
Effects of GP1a on enhanced mechanical sensitivity associated with cystitis.
The basal mechanical sensitivity threshold was about 2.8 g in all groups (Fig. 3). Intravesical instillation of saline did not affect peripheral mechanical sensitivity (Fig. 3). The mechanical sensitivity threshold was reduced 3 h after instillation of acrolein (0.22 ± 0.05 g, n = 8) (P < 0.01 vs saline-treated controls). Treatment with GP1a (10 mg/kg) attenuated increased mechanical sensitivity (1.74 ± 0.35 g, n = 8) (P < 0.05 vs acrolein-treated), and the effect of GP1a was reversed by the selective CB2 antagonist AM630 (10 mg/kg) (0.21 ± 0.04 g, n = 8) (P < 0.05 vs acrolein+GP1a-treated) (Fig. 3). Treatment with AM630 (10 mg/kg) alone did not affect acrolein-induced increase of mechanical sensitivity (0.2 ± 0.04 g, n = 6, P > 0.05 vs acrolein treated).
Fig. 3.
Intravesical instillation of saline did not affect peripheral mechanical sensitivity. The mechanical sensitivity threshold was reduced 3 h after instillation of acrolein. Treatment with GP1a attenuated increased mechanical sensitivity, and the effect of GP1a was reversed by the selective CB2 antagonist AM630. Baseline (open bar) and 3 h after treatment with saline or acrolein (gray bar). n = 6 (saline-treated controls). n = 8 (other treatment groups). *P < 0.01 vs. saline-treated. #P < 0.05 vs. acrolein-treated. @P < 0.05 vs. acrolein+GP1a-treated.
Treatment with GP1a (10 mg/kg) or AM630 (10 mg/kg) in mice instilled with saline did not affect the mechanical sensitivity (2.61 ± 0.33 and 2.7 ± 0.23 g, respectively, n = 6, P > 0.05 vs saline-treated controls).
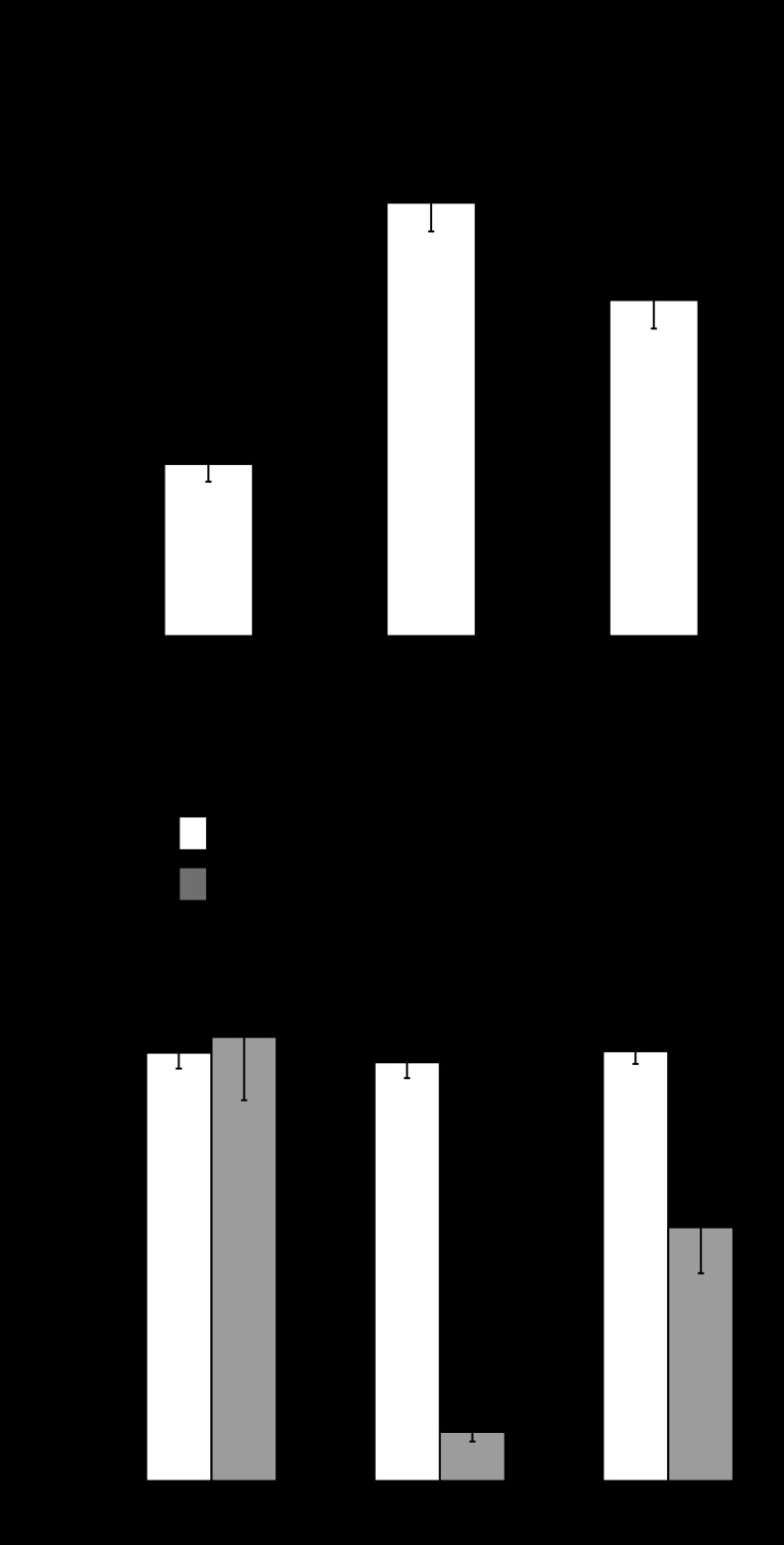
Effects of GP1a on MAPK activation.
The basal content of p-ERK1/2 in the urothelium/suburothelium was low as shown in Fig. 4A. Phosphorylation of ERK1/2 was increased 3 h after intravesical instillation of acrolein (6.31 ± 0.32, n = 8) (Fig. 4B, P < 0.01 vs saline-treated), and this effect of acrolein was attenuated by treatment with GP1a (10 mg/kg) administered 10 min before acrolein (4.13 ± 0.63, n = 8) (Fig. 4B, P < 0.05 vs acrolein-treated). The effect of GP1a on p-ERK1/2 abundance was reversed by treatment with the selective antagonist AM630 (10 mg/kg) (5.73 ± 0.32, n = 8) (Fig. 4B, P < 0.05 vs acrolein+GP1a-treated).
Fig. 4.
Semiquantitative immunoblotting analysis of phosphorylation of ERK1/2 (open bar), p38 (gray bar), and JNK (solid bar) in urothelium/suburothelium. Representive immunoblots are shown. Phosphorylation of ERK1/2 was increased 3 h after intravesical instillation of acrolein, and this effect of acrolein was attenuated by treatment with GP1a. The effect of GP1a was reversed by treatment with the selective antagonist AM630. n = 6 (saline-treated controls). n = 8 (other treatment groups). *P < 0.01 vs. saline-treated. #P < 0.05 vs. acrolein-treated. @P < 0.05 vs. acrolein+GP1a-treated.
Similarly, phosphorylation of JNK was increased 3 h after instillation of acrolein (Fig. 4, P < 0.01 vs saline-treated). Treatment with GP1a or AM630+GP1a did not prevent increased phosphorylation of JNK (P > 0.05 vs acrolein-treated) (Fig. 4).
Instillation of acrolein failed to induce phosphorylation of p38. Treatment with GP1a or AM630+GP1a did not affect phosphorylation of p38 (Fig. 4).
Effects of ERK1/2 phosphorylation inhibitor U0126.
In vehicle-treated mice, acrolein induced increase of bladder weight (2.72 ± 0.18 mg/g, n = 6, P < 0.01 vs saline-treated controls) and enhanced mechanical sensitivity (0.31 ± 0.06 g, n = 6, P < 0.01 vs saline-treated controls). Treatment with ERK1/2 phosphorylation inhibitor U0126 (30 mg/kg, ip), applied 15 min before instillation of acrolein, attenuated the increase of bladder weight (2.11 ± 0.18 mg/g, n = 6) (Fig. 5A, P < 0.05 vs acrolein-treated) and enhanced mechanical sensitivity-induced by acrolein (1.61 ± 0.29 g) (Fig. 5B, P < 0.05 vs acrolein-treated).
Fig. 5.
Treatment with U0126 (30 mg/kg), an inhibitor of ERK1/2 phosphorylation, attenuated the increase of bladder weight (A) and the reduction in mechanical sensitivity threshold-induced by acrolein (B). n = 6. *P < 0.05, **P < 0.01 vs. saline-treated, respectively. #P < 0.05 vs. acrolein-treated.
In mice instilled with saline, treatment with U0126 (30 mg/kg) did not affect the bladder weight (1.15 ± 0.09 mg/g, n = 6, P > 0.05 vs saline-treated controls) or mechanical sensitivity (2.61 ± 0.22 g, n = 6, P > 0.05 vs saline-treated controls).
DISCUSSION
In the present study, we found 1) CB2 is present in urothelium and acute inflammation did not appear to alter expression of CB2 in urothelium; 2) treatment with a selective CB2 agonist, GP1a, reduced severity of acrolein-induced acute cystitis; 3) the CB2 agonist also inhibited enhanced peripheral sensitivity to mechanical stimuli associated with cystitis; 4) the effects of GP1a were reversed by the CB2-selective antagonist AM630; and 5) the effects following CB2 activation may be partly mediated by MAPK ERK1/2 pathways.
An emerging body of research reveals that activation of CB2 inhibits tissue inflammation (3, 12, 21, 57, 60). GW405833, a selective CB2 agonist, attenuated carrageenan-induced edema in hind paws of rats, and this effect was reversed by a selective CB2 antagonist (12). Quartilho et al. (60) reported similar observations using a different CB2 agonist (AM1241) to prevent carrageenan-induced edema, and the inhibitory effect of AM1241 was completely blocked by a selective CB2 antagonist, whereas a selective CB1 receptor antagonist had no effect. These authors further demonstrated that activation of CB2 not only prevented, but also reversed, inflammation-induced edema (60). In other studies, treatment with a selective CB2 agonist reduced arachidonic acid-induced edema in the ear (31), and edema of hind paw elicited by intraplantar injection of lipopolysaccharide (38). These studies clearly support the anti-inflammatory effects of CB2, although the precise mechanisms underlying CB2 activation-induced inhibition of inflammation remain to be clarified.
There is very limited information available regarding the inhibitory activity of CB2 relative to inflammation of visceral structures. Storr et al. (64) reported that treatment with a selective CB2 agonist inhibited (while a selective CB2 antagonist exacerbated) severity of experimental colitis. Treatment with a selective CB2 agonist attenuated the inflammatory responses in cardio and hepatic ischemia/reperfusion injuries in mice (33, 51–52). In the present study, treatment with the selective CB2 agonist GP1a reduced severity of bladder inflammation, and the inhibitory effect of GP1a was reversed by the selective CB2 antagonist AM630. Previous studies have shown that CB2 is present in bladders, particularly in urothelium, in various species (26, 32, 49, 69–70). We provide further evidence that CB2 is expressed in the urothelium of mouse bladders. While urothelium has historically been viewed as a simple barrier separating the bladder wall from urine, increasing evidence also suggests that the urothelium plays a critical role in physiological and pathophysiological processes in the bladder (6, 71–72, 75–76). Specifically, urothelial cells have the capacity to secrete a variety of signaling molecules such as PGE2, nerve growth factor, nitric oxide, and cytokines in response to various stimuli (6, 71–72, 75–76). Conceivably, chemical mediators derived from urothelial cells could significantly influence bladder function and processes involved in bladder inflammation. CB2 is coupled to inhibitory G proteins, and activation of CB2 inhibits adenylyl cyclase (17) and release of inflammatory cytokines in inflammatory cells (39, 52) and tumor cells (43). It is possible that activation CB2 may inhibit production and release of inflammatory mediators from urothelial cells, reducing the local inflammatory response of the bladder after acrolein treatment. However, inflammatory responses are complicated, and many biological events are involved. CB2 activation may also affect other cellular components, such as infiltration by immune cells, contributing to the anti-inflammatory effects of CB2, and this will be the focus of future studies.
It has been shown that activation of CB2 exerts anti-nociceptive effects (35–36, 45, 54). Treatment with CB2 agonists reduced the second phase of nocifensive behaviors elicited by intraplantar injection of formalin in mice (4) and allodynia elicited by L5-L6 spinal nerve ligation in rats (4). These effects of CB2 agonists were prevented by selective CB2 antagonists (4). In other reports, treatment with CB2 agonists suppressed carrageenan-evoked thermal and mechanical hyperalgesia in rodents (12, 30, 55) and cancer-induced pain (43–44). Little is known about the effects of CB2 activation on visceral pain. One hallmark of visceral pain is perception of pain arising from somatic sites distant from the area of visceral injury (referred hyperalgesia) (7–8, 42), and this is a relatively common finding in patients with IC/PBS (67). Sensitization of primary afferents at the site of injury plays an important role in development of referred hyperalgesia (8, 78). CB2 is present in dorsal root ganglia (DRG) afferent neurons, and CB2 expression in DRG is upregulated by pathological pain states (34). Gratzke et al. (26) demonstrated that CB2 is present in sensory nerve fibers in the bladder. Activation of CB2 inhibits capsaicin-induced increase of intracellular calcium in DRG afferent neurons (61), and release of calcitonin gene-related peptide from afferent nerve fibers in bladders (32) and spinal cord slices (4). Nackley et al. (55) reported that enhanced C-fiber activity in response to electrical stimulation was inhibited by a CB2 agonist. We found that treatment with the CB2 agonist GP1a inhibited bladder inflammation-associated enhanced peripheral mechanical sensitivity. Conceivably, activation CB2 attenuates production and release of inflammatory mediators from urothelial cells that are involved in sensitization of afferent nerves. Further, activation of CB2 may exert a direct inhibitory action on afferent nerves. The results from the present study, together with previous findings, suggest that suppression of referred mechanical hyperalgesia associated with bladder inflammation by the CB2 agonist may result from the combined effects of reduced inflammation and inhibition of afferent nerve activity following CB2 activation.
The MAPK are a group of protein serine/threonine kinases that mediate signal transduction from the cell surface to the nucleus in response to a variety of extracellular stimuli (9). The MAPK consist of three major families of protein kinases, including ERK, p38, and JNK (9, 18), and activity of MAPK depends on their phosphorylation status (9, 18). There is substantial evidence that ERK1/2 is involved in inflammatory responses (62), as well as in the development of inflammatory and neuropathic pain (16, 37, 41, 77). Blockade of ERK1/2 phosphorylation reduces mechanical hyperalgesia of inflamed paws in rodents (24, 37) and development of referred hyperalgesia after intracolonic instillation of capsaicin or mustard oil in mice (23). Treatment of rats with CYP induced phosphorylation of ERK1/2 in bladders (14, 59), and ERK1/2 phosphorylation occurred primarily in urothelium (14). Furthermore, treatment with ERK1/2 phosphorylation inhibitor U0126 decreased bladder hyperreactivity and improved bladder capacity in CYP-treated rats, suggesting that ERK1/2 participates in inflammatory responses in the bladder (14). We confirmed that bladder inflammation induced phosphorylation of ERK1/2 in mice urothelium. Also, our findings that bladder inflammation induced phosphorylation of JNK, but not p38, in urothelium are consistent with previous observations (11, 13). We further demonstrated that GP1a selectively inhibited phosphorylation of ERK1/2 without affecting phosphorylation of JNK, and treatment of mice with U0126 inhibited bladder inflammation and the associated mechanical hyperalgesia of hind paws. Therefore, our results suggest that the ERK1/2 pathway may partly mediate the inhibitory effects of CB2 activation on bladder inflammation and the associated enhanced pain sensation.
Perspectives and Significance
Our findings demonstrate that CB2 is present in mouse urothelium/suburothelium and acute bladder inflammation does not appear to alter CB2 protein abundance. We further demonstrate that treatment with a selective CB2 agonist inhibits bladder inflammation and associated referred mechanical hyperalgesia. The inhibitory effects of CB2 appear to be at least partly mediated by reducing bladder inflammation-induced activation of ERK1/2 MAPK pathway. The results of the current study indicate that the CB2 is a potential therapeutic target for treatment of bladder inflammation and pain in patients. It should be noted that bladder inflammation and associated enhanced pain sensation involve complicated biological processes and other cellular components, and signaling pathways may also contribute to inhibitory effects of CB2 activation. These areas will, therefore, be the focus of future studies.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Z.-Y.W. and D.E.B. conception and design of research; Z.-Y.W. and P.W. performed experiments; Z.-Y.W. analyzed data; Z.-Y.W. interpreted results of experiments; Z.-Y.W. prepared figures; Z.-Y.W. drafted manuscript; Z.-Y.W. and D.E.B. edited and revised manuscript; Z.-Y.W., P.W., and D.E.B. approved final version of manuscript.
ACKNOWLEDGMENTS
This study was supported by NIH R-01 DK-066349 (to D. E. Bjoring).
REFERENCES
- 1. Alagiri M, Chottiner S, Ratner V, Slade D, Hanno PM. Interstitial cystitis: unexplained associations with other chronic disease and pain syndromes. Urology 49 (Suppl 5A): 52–57, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Anand P, Whiteside G, Fowler CJ, Hohmann AG. Targeting CB2 receptors and the endocannabinoid system for the treatment of pain. Brain Res Rev 60: 255–266, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barutta F, Piscitelli F, Pinach S, Bruno G, Gambino R, Rastaldi MP, Salvidio G, Di Marzo V, Cavallo Perin P, Gruden G. Protective role of cannabinoid receptor type 2 in a mouse model of diabetic nephropathy. Diabetes 60: 2386–2396, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beltramo M, Bernardini N, Bertorelli R, Campanella M, Nicolussi E, Fredduzzi S, Reggiani A. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur J Neurosci 23: 1530–1538, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, Nyberg L, Clemens JQ. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol 186: 540–544, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Birder LA. Urothelial signaling. Handb Exp Pharmacol 202: 207–231, 2011 [DOI] [PubMed] [Google Scholar]
- 7. Bon K, Lanteri-Minet M, Michiels JF, Menetrey D. Cyclophosphamide cystitis as a model of visceral pain in rats: a c-fos and Krox-24 study at telencephalic levels, with a note on pituitary adenylate cyclase activating polypeptide (PACAP). Exp Brain Res 122: 165–174, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Cervero F, Laird JM. Understanding the signaling and transmission of visceral nociceptive events. J Neurobiol 61: 45–54, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature 410: 37–40, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53: 55–63, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Chung CW, Zhang QL, Qiao LY. Endogenous nerve growth factor regulates collagen expression and bladder hypertrophy through Akt and MAPK pathways during cystitis. J Biol Chem 285: 4206–4212, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clayton N, Marshall FH, Bountra C, O'Shaughnessy CT. CB1 and CB2 cannabinoid receptors are implicated in inflammatory pain. Pain 96: 253–260, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Conklin DJ, Haberzettl P, Lesgards JF, Prough RA, Srivastava S, Bhatnagar A. Increased sensitivity of glutathione S-transferase P-null mice to cyclophosphamide-induced urinary bladder toxicity. J Pharmacol Exp Ther 331: 456–469, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Corrow KA, Vizzard MA. Phosphorylation of extracellular signal-regulated kinases in urinary bladder in rats with cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol 293: R125–R134, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Cox PJ. Cyclophosphamide cystitis—identification of acrolein as the causative agent. Biochem Pharmacol 28: 2045–2049, 1979 [DOI] [PubMed] [Google Scholar]
- 16. Cruz CD, Cruz F. The ERK 1 and 2 pathway in the nervous system: from basic aspects to possible clinical applications in pain and visceral dysfunction. Curr Neuropharmacol 5: 244–252, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Demuth DG, Molleman A. Cannabinoid signalling. Life Sci 78: 549–563, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol 20: 55–72, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Elmes SJ, Winyard LA, Medhurst SJ, Clayton NM, Wilson AW, Kendall DA, Chapman V. Activation of CB1 and CB2 receptors attenuates the induction and maintenance of inflammatory pain in the rat. Pain 118: 327–335, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Erickson DR, Davies MF. Interstitial cystitis. Int Urogynecol J Pelvic Floor Dysfunct 9: 174–183, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Fernández-López D, Pazos MR, Tolón RM, Moro MA, Romero J, Lizasoain I, Martínez-Orgado J. The cannabinoid agonist WIN55212 reduces brain damage in an in vivo model of hypoxic-ischemic encephalopathy in newborn rats. Pediatr Res 62: 255–260, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Franklin JM, Vasiljevik T, Prisinzano TE, Carrasco GA. Cannabinoid 2 receptor- and beta arrestin 2-dependent upregulation of serotonin 2A receptors. Eur Neuropsychopharmacol In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Galan A, Cervero F, Laird JM. Extracellular signaling-regulated kinase-1 and -2 (ERK 1/2) mediate referred hyperalgesia in a murine model of visceral pain. Brain Res Mol Brain Res 116: 126–134, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Galan A, Lopez-Garcia JA, Cervero F, Laird JM. Activation of spinal extracellular signaling-regulated kinase-1 and -2 by intraplantar carrageenan in rodents. Neurosci Lett 322: 37–40, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Girard BM, Tompkins JD, Parsons RL, May V, Vizzard MA. Effects of CYP-induced cystitis on PACAP/VIP and receptor expression in micturition pathways and bladder function in mice with overexpression of NGF in urothelium. J Mol Neurosci 48: 730–743, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gratzke C, Streng T, Park A, Christ G, Stief CG, Hedlund P, Andersson KE. Distribution and function of cannabinoid receptors 1 and 2 in the rat, monkey and human bladder. J Urol 181: 1939–1948, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Gratzke C, Streng T, Stief CG, Downs TR, Alroy I, Rosenbaum JS, Andersson KE, Hedlund P. Effects of cannabinor, a novel selective cannabinoid 2 receptor agonist, on bladder function in normal rats. Eur Urol 57: 1093–1100, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Gratzke C, Streng T, Stief CG, Alroy I, Limberg BJ, Downs TR, Rosenbaum JS, Hedlund P, Andersson KE. Cannabinor, a selective cannabinoid-2 receptor agonist, improves bladder emptying in rats with partial urethral obstruction. J Urol 185: 731–736, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Guindon J, Hohmann AG. Cannabinoid CB2 receptors: a therapeutic target for the treatment of inflammatory and neuropathic pain. Br J Pharmacol 153: 319–334, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gutierrez T, Farthing JN, Zvonok AM, Makriyannis A, Hohmann AG. Activation of peripheral cannabinoid CB1 and CB2 receptors suppresses the maintenance of inflammatory nociception: a comparative analysis. Br J Pharmacol 150: 153–163, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hanus L, Breuer A, Tchilibon S, Shiloah S, Goldenberg D, Horowitz M, Pertwee RG, Ross RA, Mechoulam R, Fride E. HU-308: a specific agonist for CB(2), a peripheral cannabinoid receptor. Proc Natl Acad Sci USA 96: 14228–14233, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hayn MH, Ballesteros I, de Miguel F, Coyle CH, Tyagi S, Yoshimura N, Chancellor MB, Tyagi P. Functional and immunohistochemical characterization of CB1 and CB2 receptors in rat bladder. Urology 72: 1174–1178, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Horváth B, Magid L, Mukhopadhyay P, Bátkai S, Rajesh M, Park O, Tanchian G, Gao RY, Goodfellow CE, Glass M, Mechoulam R, Pacher P. A new cannabinoid CB2 receptor agonist HU-910 attenuates oxidative stress, inflammation and cell death associated with hepatic ischaemia/reperfusion injury. Br J Pharmacol 165: 2462–2478, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hsieh GC, Pai M, Chandran P, Hooker BA, Zhu CZ, Salyers AK, Wensink EJ, Zhan C, Carroll WA, Dart MJ, Yao BB, Honore P, Meyer MD. Central and peripheral sites of action for CB2 receptor mediated analgesic activity in chronic inflammatory and neuropathic pain models in rats. Br J Pharmacol 162: 428–440, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ibrahim MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP, Vanderah TW, Lai J, Porreca F, Makriyannis A, Malan TP., Jr Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci USA 100: 10529–10533, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ibrahim MM, Rude ML, Stagg NJ, Mata HP, Lai J, Vanderah TW, Porreca F, Buckley NE, Makriyannis A, Malan TP., Jr CB2 cannabinoid receptor mediation of antinociception. Pain 122: 36–42, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Ji RR, Gereau 4th RW, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev 60: 135–148, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kinsey SG, Mahadevan A, Zhao B, Sun H, Naidu PS, Razdan RK, Selley DE, Imad Damaj M, Lichtman AH. The CB2 cannabinoid receptor-selective agonist O-3223 reduces pain and inflammation without apparent cannabinoid behavioral effects. Neuropharmacology 60: 244–251, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Klegeris A, Bissonnette CJ, McGeer PL. Reduction of human monocytic cell neurotoxicity and cytokine secretion by ligands of the cannabinoid-type CB2 receptor. Br J Pharmacol 139: 775–786, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Klinger MB, Dattilio A, Vizzard MA. Expression of cyclooxygenase-2 in urinary bladder in rats with cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol 293: R677–R685, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Klinger MB, Sacks S, Cervero F. A role for extracellular signal-regulated kinases 1 and 2 in the maintenance of persistent mechanical hyperalgesia in ovariectomized mice. Neuroscience 172: 483–493, 2011 [DOI] [PubMed] [Google Scholar]
- 42. Laird JM, Souslova V, Wood JN, Cervero F. Deficits in visceral pain and referred hyperalgesia in Nav1.8 (SNS/PN3)-null mice. J Neurosci 22: 8352–8356, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lozano-Ondoua AN, Wright C, Vardanyan A, King T, Largent-Milnes TM, Nelson M, Jimenez-Andrade JM, Mantyh PW, Vanderah TW. A cannabinoid 2 receptor agonist attenuates bone cancer-induced pain and bone loss. Life Sci 86: 646–653, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lozano-Ondoua A, Hanlon K, Symons A, Largent-Milnes T, Havelin J, Ferland 3rd H, Chandramouli A, Owusu-Ankomah M, Nikolich-Zugich T, Bloom A, Jimenez-Andrade J, King T, Porreca F, Nelson M, Mantyh P, Vanderah T. Disease modification of breast cancer-induced bone remodeling by cannabinoid 2 receptor agonists. J Bone Miner Res 28: 92–107, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Malan TP, Jr, Ibrahim MM, Deng H, Liu Q, Mata HP, Vanderah T, Porreca F, Makriyannis A. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain 93: 239–245, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Malan TP, Jr, Ibrahim MM, Lai J, Vanderah TW, Makriyannis A, Porreca F. CB2 cannabinoid receptor agonists: pain relief without psychoactive effects? Curr Opin Pharmacol 3: 62–67, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Martin A, Garcia-Ovejero D, Molina-Holgado E. The endocannabinoid 2-arachidonoylglycerol reduces lesion expansion and white matter damage after spinal cord injury. Neurobiol Dis 38: 304–312, 2010 [DOI] [PubMed] [Google Scholar]
- 48. May V, Vizzard MA. Bladder dysfunction and altered somatic sensitivity in PACAP−/− mice. J Urol 183: 772–779, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Merriam FV, Wang ZY, Guerios SD, Bjorling DE. Cannabinoid receptor 2 is increased in acutely and chronically inflamed bladder of rats. Neurosci Lett 445: 130–134, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Merriam FV, Wang ZY, Hillard CJ, Stuhr KL, Bjorling DE. Inhibition of fatty acid amide hydrolase suppresses referred hyperalgesia induced by bladder inflammation. BJU Int 108: 1145–1149, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Montecucco F, Lenglet S, Braunersreuther V, Burger F, Pelli G, Bertolotto M, Mach F, Steffens S. CB2 cannabinoid receptor activation is cardioprotective in a mouse model of ischemia/reperfusion. J Mol Cell Cardiol 46: 612–620, 2009 [DOI] [PubMed] [Google Scholar]
- 52. Mukhopadhyay P, Rajesh M, Horváth B, Bátkai S, Park O, Tanchian G, Gao RY, Patel V, Wink DA, Liaudet L, Haskó G, Mechoulam R, Pacher P. Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free Radic Biol Med 50: 1368–1381, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Murineddu G, Lazzari P, Ruiu S, Sanna A, Loriga G, Manca I, Falzoi M, Dessì C, Curzu MM, Chelucci G, Pani L, Pinna GA. Tricyclic pyrazoles, 4: synthesis and biological evaluation of analogs of the robust and selective CB2 cannabinoid ligand 1-(2′,4′-dichlorophenyl)-6-methyl-N-piperidin-1-yl-1,4-dihydroindeno[1,2-c]pyrazole-3-carboxamide. J Med Chem 49: 7502–7512, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Nackley AG, Makriyannis A, Hohmann AG. Selective activation of cannabinoid CB2 receptors suppresses spinal fos protein expression and pain behavior in a rat model of inflammation. Neuroscience 119: 747–757, 2003 [DOI] [PubMed] [Google Scholar]
- 55. Nackley AG, Zvonok AM, Makriyannis A, Hohmann AG. Activation of cannabinoid CB2 receptors suppresses C-fiber responses and windup in spinal wide dynamic range neurons in the absence and presence of inflammation. J Neurophysiol 92: 3562–3574, 2004 [DOI] [PubMed] [Google Scholar]
- 56. Ofek O, Karsak M, Leclerc N, Fogel M, Frenkel B, Wright K, Tam J, Attar-Namdar M, Kram V, Shohami E, Mechoulam R, Zimmer A, Bab I. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc Natl Acad Sci USA 103: 696–701, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Palazuelos J, Aguado T, Pazos MR, Julien B, Carrasco C, Resel E, Sagredo O, Benito C, Romero J, Azcoitia I, Fernández-Ruiz J, Guzmán M, Galve-Roperh I. Microglial CB2 cannabinoid receptors are neuroprotective in Huntington's disease excitotoxicity. Brain 132: 3152–3164, 2009 [DOI] [PubMed] [Google Scholar]
- 58. Patel KD, Davison JS, Pittman QJ, Sharkey KA. Cannabinoid CB2 receptors in health and disease. Curr Med Chem 17: 1393–1410, 2010 [DOI] [PubMed] [Google Scholar]
- 59. Qiao LY, Gulick MA. Region-specific changes in the phosphorylation of ERK1/2 and ERK5 in rat micturition pathways following cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol 292: R1368–R1375, 2007 [DOI] [PubMed] [Google Scholar]
- 60. Quartilho A, Mata HP, Ibrahim MM, Vanderah TW, Porreca F, Makriyannis A, Malan TP., Jr Inhibition of inflammatory hyperalgesia by activation of peripheral CB2 cannabinoid receptors. Anesthesiology 99: 955–960, 2003 [DOI] [PubMed] [Google Scholar]
- 61. Sagar DR, Kelly S, Millns PJ, O'Shaughnessey CT, Kendall DA, Chapman V. Inhibitory effects of CB1 and CB2 receptor agonists on responses of DRG neurons and dorsal horn neurons in neuropathic rats. Eur J Neurosci 22: 371–379, 2005 [DOI] [PubMed] [Google Scholar]
- 62. Schuh K, Pahl A. Inhibition of the MAP kinase ERK protects from lipopolysaccharide-induced lung injury. Biochem Pharmacol 77: 1827–1834, 2009 [DOI] [PubMed] [Google Scholar]
- 63. Steffens S, Veillard NR, Arnaud C, Pelli G, Burger F, Staub C, Karsak M, Zimmer A, Frossard JL, Mach F. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature 434: 782–786, 2003 [DOI] [PubMed] [Google Scholar]
- 64. Storr MA, Keenan CM, Zhang H, Patel KD, Makriyannis A, Sharkey KA. Activation of the cannabinoid 2 receptor (CB2) protects against experimental colitis. Inflamm Bowel Dis 15: 1678–1685, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Strittmatter F, Gandaglia G, Benigni F, Bettiga A, Rigatti P, Montorsi F, Gratzke C, Stief C, Colciago G, Hedlund P. Expression of fatty acid amide hydrolase (FAAH) in human, mouse, and rat urinary bladder and effects of FAAH inhibition on bladder function in awake rats. Eur Urol 61: 98–106, 2012 [DOI] [PubMed] [Google Scholar]
- 66. Studeny S, Cheppudira BP, Meyers S, Balestreire EM, Apodaca G, Birder LA, Braas KM, Waschek JA, May V, Vizzard MA. Urinary bladder function and somatic sensitivity in vasoactive intestinal polypeptide (VIP)−/− mice. J Mol Neurosci 36: 175–187, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tripp DA, Nickel JC, Wong J, Pontari M, Moldwin R, Mayer R, Carr LK, Doggweiler R, Yang CC, Mishra N, Nordling J. Mapping of pain phenotypes in female patients with bladder pain syndrome/interstitial cystitis and controls. Eur Urol 62: 1188–1194, 2012 [DOI] [PubMed] [Google Scholar]
- 68. Tschöp J, Kasten KR, Nogueiras R, Goetzman HS, Cave CM, England LG, Dattilo J, Lentsch AB, Tschöp MH, Caldwell CC. The cannabinoid receptor 2 is critical for the host response to sepsis. J Immunol 183: 499–505, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tyagi V, Philips BJ, Su R, Smaldone MC, Erickson VL, Chancellor MB, Yoshimura N, Tyagi P. Differential expression of functional cannabinoid receptors in human bladder detrusor and urothelium. J Urol 181: 1932–1938, 2009 [DOI] [PubMed] [Google Scholar]
- 70. Tyagi P, Tyagi V, Yoshimura N, Chancellor M. Functional role of cannabinoid receptors in urinary bladder. Indian J Urol 26: 26–35, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vera PL, Wolfe TE, Braley AE, Meyer-Siegler KL. Thrombin induces macrophage migration inhibitory factor release and upregulation in urothelium: a possible contribution to bladder inflammation. PLoS One 5: e15904, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vizzard MA. Neurochemical plasticity and the role of neurotrophic factors in bladder reflex pathways after spinal cord injury. Prog Brain Res 152: 97–115, 2006 [DOI] [PubMed] [Google Scholar]
- 73. Walczak JS, Cervero F. Local activation of cannabinoid CB2 receptors in the urinary bladder reduces the inflammation-induced sensitization of bladder afferents. Mol Pain 7: 31, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang ZY, Wang P, Merriam FV, Bjorling DE. Lack of TRPV1 inhibits cystitis-induced increased mechanical sensitivity in mice. Pain 139: 158–167, 2008 [DOI] [PubMed] [Google Scholar]
- 75. Wang ZY, Wang P, Bjorling DE. Role of mast cells and protease-activated receptor-2 in cyclooxygenase-2 expression in urothelial cells. Am J Physiol Regul Integr Comp Physiol 297: R1127–R1135, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wang ZY, Bjorling DE. Tumor necrosis factor-α induces expression and release of interleukin-6 by human urothelial cells. Inflamm Res 60: 525–532, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. White JP, Cibelli M, Fidalgo AR, Nagy I. Extracellular signal-regulated kinases in pain of peripheral origin. Eur J Pharmacol 650: 8–17, 2011 [DOI] [PubMed] [Google Scholar]
- 78. Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci 19: 4644–4653, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]