Abstract
The present study sought to determine whether the protein catabolic response in skeletal muscle produced by chronic alcohol feeding was exaggerated in aged rats. Adult (3 mo) and aged (18 mo) female F344 rats were fed a nutritionally complete liquid diet containing alcohol (36% of total calories) or an isocaloric isonitrogenous control diet for 20 wk. Muscle (gastrocnemius) protein synthesis, as well as mTOR and proteasome activity did not differ between control-fed adult and aged rats, despite the increased TNF-α and IL-6 mRNA and decreased IGF-I mRNA in muscle of aged rats. Compared with alcohol-fed adult rats, aged rats demonstrated an exaggerated alcohol-induced reduction in lean body mass and protein synthesis (both sarcoplasmic and myofibrillar) in gastrocnemius. Alcohol-fed aged rats had enhanced dephosphorylation of 4E-BP1, as well as enhanced binding of raptor with both mTOR and Deptor, and a decreased binding of raptor with 4E-BP1. Alcohol feeding of both adult and aged rats reduced RagA binding to raptor. The LKB1-AMPK-REDD1 pathway was upregulated in gastrocnemius from alcohol-fed aged rats. These exaggerated alcohol-induced effects in aged rats were associated with a greater decrease in muscle but not circulating IGF-I, but no further increase in inflammatory mediators. In contrast, alcohol did not exaggerate the age-induced increase in atrogin-1 and MuRF1 mRNA or the increased proteasome activity. Our results demonstrate that, compared with adult rats, the gastrocnemius from aged rats is more sensitive to the catabolic effects of alcohol on protein synthesis, but not protein degradation, and this exaggerated response may be AMPK-dependent.
Keywords: mTOR, protein degradation, sarcopenia, atrophy, AMPK
chronic alcohol abuse produces a diverse array of hormonal and metabolic derangements, which alter body composition (51). One hallmark of excessive sustained alcohol consumption, evident in both humans and rodent models, is the reduction in lean body mass (LBM) and muscle mass (20, 56). The etiology of this wasting is due predominantly to a decrease in protein synthesis (48, 52, 53, 56, 67), mediated via an impairment in the kinase activity of mTOR (mammalian target of rapamycin) (50, 55), while alcohol-induced changes in muscle protein degradation are more controversial (73, 84). It is now recognized that mTOR resides in two distinct complexes (mTORC1 and mTORC2) and that alterations in protein-protein interactions within primarily mTORC1 directly regulate the rate of protein translation (23). For example, chronic alcohol consumption in rats and exposure of cultured myocytes to alcohol increase the binding of raptor (regulatory associated protein of mTOR) to mTOR (39, 55), which is consistent with the formation of a “closed confirmation” rendering mTOR less active (45). Other catabolic insults alter different protein-protein interactions within mTORC1 (14, 44, 65), but this level of granularity has not yet been investigated in response to chronic alcohol consumption.
The fraction of the U.S. population >65 years of age is rapidly growing. While chronic alcohol abuse has long been considered to promote premature aging, most data supporting this hypothesis have focused on the peripheral and central nervous system (3, 34, 77), with little research on whether aging alters the hormonal and metabolic response to alcohol (21). However, indirect evidence supports the possibility that alterations in protein homeostasis produced by excessive alcohol and as part of the aging process (i.e., sarcopenia) may interact to produce either an additive or synergistic effect on skeletal muscle mass. For example, aged rats are highly sensitive to the catabolic effect of the synthetic glucocorticoid dexamethasone, demonstrating a more pronounced muscle wasting than adult animals (12, 13) and the regenerative capacity of muscle is markedly diminished by alcohol feeding in aged rats (60). Therefore, to address these gaps in understanding, we designed experiments to test the hypothesis that the catabolic effects of alcohol on skeletal muscle protein balance are exaggerated in aged rats. While the primary endpoints pertaining to protein homeostasis were related to protein synthesis, we also assess the impact of alcohol and aging on proteasome activity, as well as circulating and tissue factors that are recognized to modulate muscle protein balance.
MATERIALS AND METHODS
Animal protocol.
Specific pathogen-free adult (3 mo) and aged (18 mo) female Fischer 344 (F344) rats were purchased from the National Institute on Aging colony at Taconic (Hudson, NY). Rats were housed under a 12:12-h light-dark cycle and initially received standard rat chow (LabDiet 5001; PMI Nutrition International, St. Louis, MO) and water ad libitum for at least 1 wk before experiments were performed. Thereafter, rats were randomized to a alcohol- or control-fed group, as we have previously described (79). Each group was maintained for 20 wk on the Lieber-DeCarli liquid diet (Bio-Serv, Frenchtown, NJ) (58). Female rats consuming the ethanol-containing diet initially received 12% of total calories from ethanol, and this percentage was gradually increased each week by 12%, so all animals consumed a maximum of 36% of caloric intake from alcohol. Time-matched pair-fed control animals received a liquid diet in which isocaloric maltose-dextran was substituted for ethanol. While total caloric intake was experimentally controlled, no attempt was made to match the pattern of food intake between control and alcohol-fed rats. Consumption of the liquid diet was assessed daily, and animals were weighed weekly. This duration of alcohol feeding has been previously reported to lead to muscle wasting and alterations in muscle protein balance in both male and female rats (52, 53, 55, 56). All experiments described herein were approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University and adhered to the National Institutes of Health (NIH) guidelines for the use of experimental animals.
Whole body composition and muscle protein synthesis.
The liquid diet was removed from cages at ∼0500, at which time there was liquid remaining in all feeding tubes. Body composition was then determined noninvasively in conscious rats between 0600 and 0700 using a 1H-NMR analyzer (Bruker LF90 proton-NMR Minispec; Bruker Optics, Woodlands, TX), as previously described (49). In vivo protein synthesis in gastrocnemius and soleus was determined between 0800 and 1000 (i.e., ∼3–5 h after removal of food) using the flooding-dose technique (85). The order of adult and aged control- and alcohol-fed rats was randomized to minimize potential changes that might result from differences in duration of fasting from the beginning to end of the protocol. Rats were anesthetized using intraperitoneal pentobarbital sodium (100 mg/kg), and a catheter was inserted in the carotid artery. Arterial blood was collected for measuring the plasma concentration of ethanol and various hormones, and then a bolus injection of l-[2,3,4,5,6-3H]phenylalanine [Phe; 150 mM, 30 μCi/ml; 1 ml/100 g body wt (BW)] was injected via the jugular vein. Serial arterial blood samples were drawn at 2, 6, and 10 min after Phe injection for measurement of Phe concentration and radioactivity. Immediately after the final blood sample, skeletal muscles were excised, a portion was frozen between liquid-nitrogen cooled aluminum blocks, and the remaining fresh muscle was directly homogenized. Blood was centrifuged, and plasma was collected. All tissue and plasma samples were stored at −80°C until analyzed. The frozen muscle was powdered under liquid nitrogen and a portion was used to estimate the global rate of incorporation of [3H]Phe into protein, whereas another portion of the frozen, powdered muscle was used to separate the myofibrillar and sarcoplasmic proteins, as described by our laboratory (85).
Western blot analysis.
Fresh tissue was homogenized in ice-cold homogenization buffer consisting of (in mmol/l) 20 HEPES (pH 7.4), 2 EGTA, 50 sodium fluoride, 100 potassium chloride, 0.2 EDTA, 50 β-glycerol phosphate, 1 DTT, 0.1 phenylmethane-sulfonylfluoride, 1 benzamidine, and 0.5 sodium vanadate using a Polytron homogenizer and clarified by centrifugation (49, 50, 52, 53, 55). Equal amounts of protein per sample were subjected to standard SDS-PAGE for total and phosphorylated ribosomal protein S6 kinase-1 (S6K1; Thr389; Cell Signaling, Beverly, MA) and eukaryotic initiation factor 4E binding protein-1 (4E-BP1; Thr-37/46; Bethyl Laboratories, Montgomery, TX). In addition, total and phosphorylated (Thr-246) PRAS40, total and phosphorylated (Ser-792) raptor, total and phosphorylated (Thr-172) 5′-AMP-activated kinase (AMPKα), as well as total GβL (also called mLST8), Deptor (DEP-domain containing partner of mTOR), RagA, and RagC, and REDD1 (regulated in development and DNA damage responses) were also determined by Western blot analysis. Blots were developed with enhanced chemiluminescence Western blotting reagents and then exposed to X-ray film in a cassette equipped with a DuPont Lightning Plus intensifying screen. After development, the film was scanned (Microtek ScanMaker IV) and analyzed using NIH Image 1.6 software.
Muscle was also homogenized in 3[(3-cholamidopropyl)dimethylammonio]-propanesulfonic acid (CHAPS) buffer consisting of (in mmol/l) 40 HEPES at pH 7.5, 120 NaCl, 1 EDTA, 10 pyrophosphate, 10 β-glycerol phosphate, 50 sodium fluoride, 1.5 sodium vanadate, 0.3% CHAPS, and 1 protease inhibitor cocktail tablet (44). The homogenate was mixed on a platform rocker and clarified by centrifugation. An aliquot of the resulting supernatant was combined with anti-raptor antibody, and immune complexes were isolated with a goat anti-rabbit BioMag IgG beads (PerSeptive Diagnostics, Cambridge, MA). Beads were collected, washed with CHAPS buffer, precipitated by centrifugation, and subjected to SDS-PAGE and analysis as above.
RNA extraction and real-time quantitative PCR.
Total RNA was extracted using Tri-reagent (Molecular Research Center, Cincinnati, OH) and RNeasy mini kit (Qiagen, Valencia, CA) following the manufacturers' protocols. Gastrocnemius was homogenized in tri-reagent followed by chloroform extraction, according to the manufacturer's instructions. An equal volume of 70% ethanol was added to the aqueous phase, and the mixture was loaded on a Qiagen mini spin column. The Qiagen mini kit protocol was followed from this step onward, including the on-column DNase I treatment to remove residual DNA contamination. RNA was eluted from the column with RNase-free water, and an aliquot was used for quantitation (NanoDrop 2000, Thermo Fisher Scientific, Waltham, MA). Quality of the RNA was analyzed on a 1% agarose gel. Total RNA (1 μg) was reverse transcribed using superscript III RT (Invitrogen, Carlsbad, CA) following the manufacturer's instructions. Real-time quantitative PCR was performed using 25 ng of cDNA in a StepOnePlus system using TaqMan gene expression assays (Applied Biosystems, Foster City, CA) for atrogin-1, F-box protein 32; Rn00591730_m1; muscle RING-finger 1, MuRF1; Rn00590197_m1; interleukin, IL-6 (Rn01410330_m1); IL-1, Rn00566700_m1; tumor necrosis factor (TNF)-α, Rn01525859_g1; nitric oxide synthase (NOS)-2, Rn00561646_m1; insulin-like growth factor-I, IGF-I; Rn00710306_n1 - all IGF-I transcripts; IGF binding protein (IGFBP)-4, Rn01464112_m1; and IGFBP-5, Rn00563116_m1; L32, Rn00820748_g1; and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), Rn01775763 g1. The comparative quantitation method 2−ΔΔCt was used in presenting gene expression of target genes in reference to the endogenous control.
Proteasome activity.
Fresh gastrocnemius was homogenized in cell lysis buffer containing (in mM) 25 HEPES, 5 MgCl2, 5 EDTA, 5 DTT, pH 7.5, followed by centrifugation at 14,000 rpm (55). The protein concentration in the supernatant was determined using the BCA protein assay kit (Pierce, Rockford, IL). The proteasome enzymatic activity was measured by using a proteasome 20S assay kit (Enzo Life Sciences, Farmingdale, New York) following the manufacturer's instructions. Briefly, the protein extract from gastrocnemius was used to assess proteasome 20S activity by measuring the hydrolysis of a fluorogenic peptidyl substrate Suc-Leu-Leu-Val-Tyr-AMC (AMC, 7-amino-4-methylcoumarin). This substrate was cleaved by the proteasome activity, and the subsequently released free AMC was then detected by a fluorometer (excitation wavelength 380 nm; emission wavelength 460 nm). The fluorescence signal was monitored before and 1 h after incubation at 37°C. The change in fluorescence signal was normalized to protein content. Each sample/substrate combination was measured both in the presence and in the absence of the specific 20S proteasome inhibitor MG132 (Boston Biochem, Cambridge, MA) to account for any nonproteasomal degradation of the substrate, as previously described (57).
Plasma concentrations.
Plasma insulin (Alpco; Salem, NH) and IGF-I (R&D Systems, Minneapolis, MN) concentrations were measured using commercial ELISAs. The plasma glucose and alcohol concentrations were determined by a rapid analyzer (Analox Instruments, Lunenburg, MA). Levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined by standard enzymatic procedures (Sigma-Aldrich; St. Louis, MO). Finally, the plasma branched-chain amino acid concentrations were determined using reverse-phase HPLC after precolumn derivatization of amino acids with phenylisothiocyanate. The plasma concentrations of glucose, insulin, IGF-I, branched-chain amino acids, and alcohol were determined on blood collected immediately prior to injection of radiolabeled phenylalanine.
Statistical analysis.
Data for each condition are summarized as means ± SE, where the number of rats per group is indicated in the figure or table legend. Statistical evaluation of the data was performed using ANOVA followed post hoc by Student-Neuman-Keuls test. Differences were considered significant when P < 0.05.
RESULTS
Body composition.
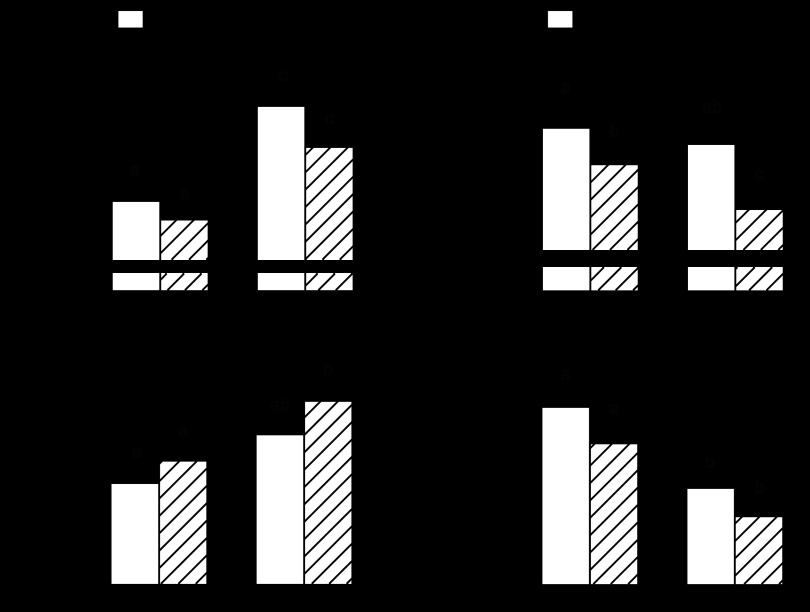
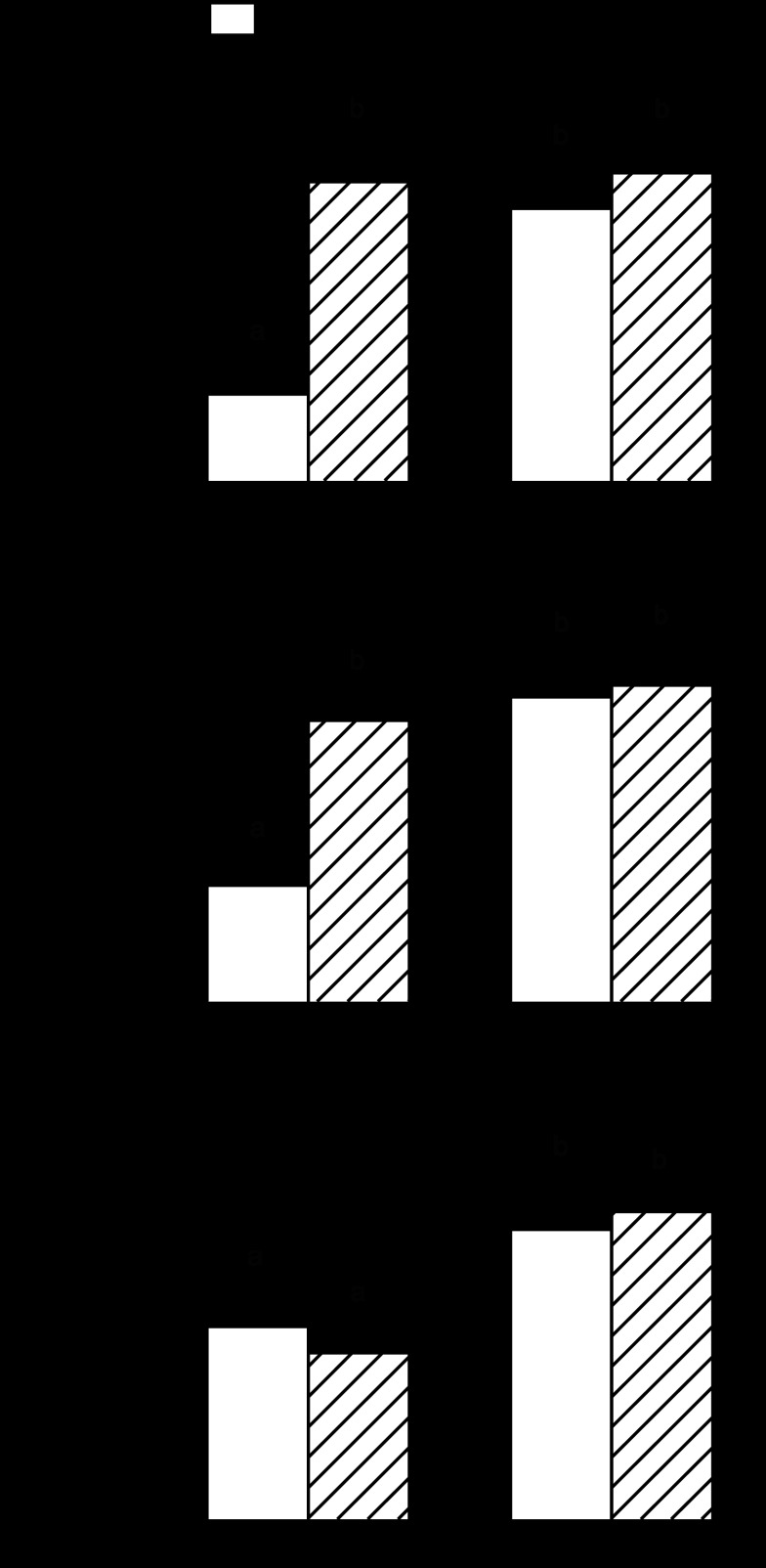
The body weight of adult rats fed the alcohol-containing diet tended to be reduced (11%), compared with pair-fed control rats, but this change did not achieve statistical significance (Fig. 1A). The body weight of both groups of aged rats was increased, compared with control-fed adult rats, but the body weight of pair-fed alcohol-consuming aged rats was reduced 17%. Alcohol consumption in adult rats significantly decreased the percent LBM and tended to increase the percent body fat, compared with control-fed rats of the same age (Fig. 1, B and C, respectively). The percentage of LBM was reduced, and the percentage of fat mass was increased in alcohol-fed aged rats, compared with both adult groups. The qualitative changes in the mass of the gastrocnemius, a representative fast-twitch muscle, were comparable to those observed for LBM (data not shown). However, because of the difference in body weight between adult and aged rats, we also normalized the gastrocnemius weight to total body weight. In doing so, the relative gastrocnemius weight was lower in aged rats than adult animals and, although not statistically significant, there was a trend for alcohol-fed rats in both groups to have a lower relative gastrocnemius weight than the age-matched pair-fed control rats (Fig. 1D).
Fig. 1.
Effect of alcohol consumption on total body weight (A), lean body mass (B), fat mass (C), and gastrocnemius mass (D) of adult and aged female rats. Whole-body fat mass and lean body mass were determined in vivo using 1H-NMR and normalized to the body weight (BW) of each rat. Values are expressed as means ± SE; n = 8 or 9 rats per group. a,b,cValues with different letters are statistically different (P < 0.05), while those values with the same letter are not statistically different.
Skeletal muscle protein synthesis and mTOR activity.
The rate of total protein synthesis in gastrocnemius did not differ between control-fed adult and aged rats (Fig. 2). There was also no difference in the synthetic rate for sarcoplasmic or myofibrillar proteins between these groups. In contrast, alcohol feeding reduced total, sarcoplasmic, and myofibrillar protein synthesis by 15–20% in adult rats. Rates of synthesis for total, sarcoplasmic, and myofibrillar proteins were decreased in aged rats fed alcohol (45–55%), and the reductions in each were greater than that seen in the alcohol-fed adult rats (Fig. 2).
Fig. 2.
Effect of alcohol consumption on in vivo-determined protein synthesis in gastrocnemius of adult and aged female rats: total protein synthesis (A), sarcoplasmic protein synthesis (B), and myofibrillar protein synthesis (C). Values are expressed as means ± SE; n = 8 or 9 rats per group. a,b,cValues with different letters are statistically different (P < 0.05), while values with the same letter are not statistically different.
In contrast, no significant age or alcohol-induced change in mass or protein synthesis was detected in the soleus, a predominantly slow-twitch muscle (data not shown), and therefore, no subsequent analysis was performed on this muscle type.
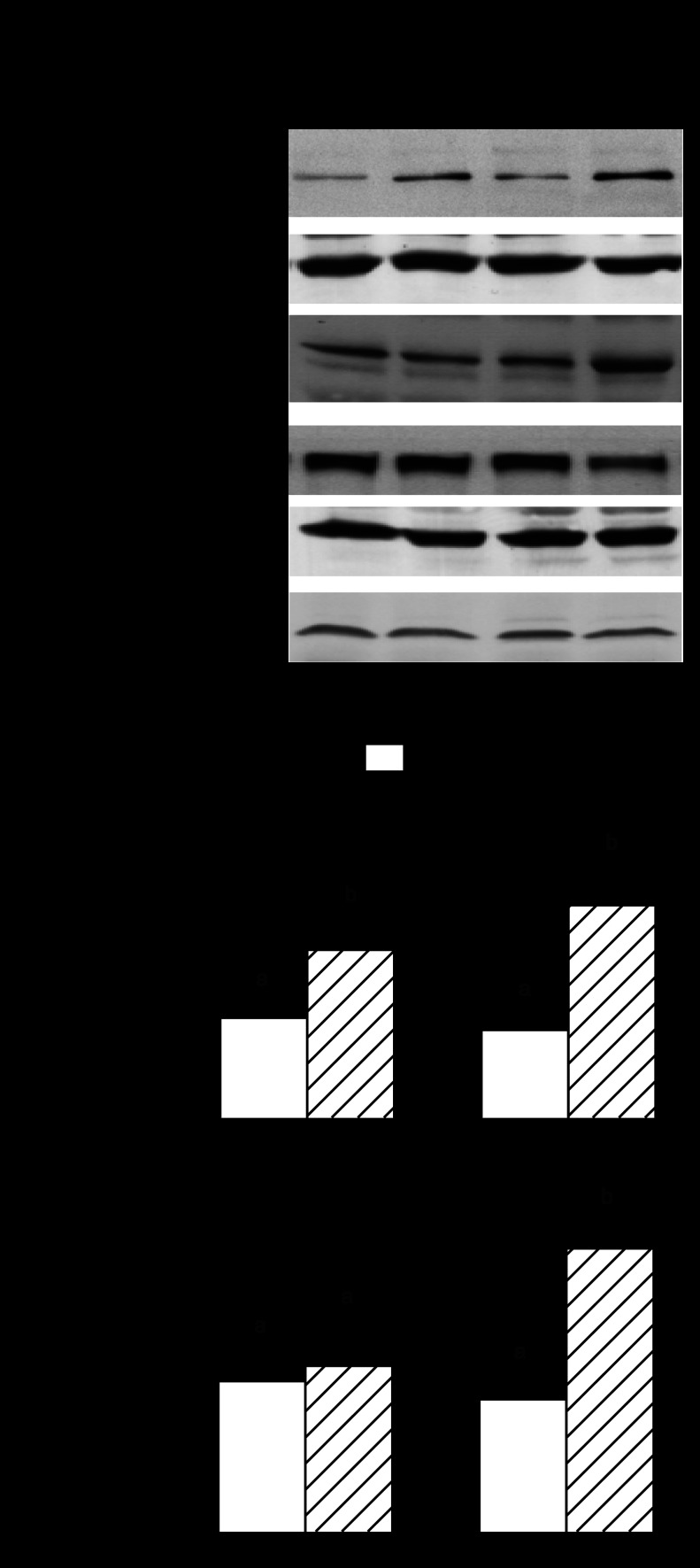
mTOR regulates protein translation, at least in part, by phosphorylation of downstream targets S6K1 and 4E-BP1 (23). In adult rats, an alcohol-induced decrease in mTOR, 4E-BP1, and S6K1 phosphorylation was detected, independent of a change in the total amount of these individual proteins (Fig. 3). A similar alcohol-induced decrease in mTOR and S6K1 phosphorylation occurred in gastrocnemius of aged rats, while the reduction in 4E-BP1 phosphorylation was greater in aged rats, compared with alcohol-fed adult rats. We failed to detect a significant change in either the phosphorylation state or total amount of these three proteins in gastrocnemius from adult and aged rats fed the control diet.
Fig. 3.
Effect of alcohol consumption on total and phosphorylated mTOR (A), 4E-BP1 (B), and S6K1 in gastrocnemius of adult and aged female rats (C). Values are expressed as means ± SE; n = 8–9 rats per group. Bar graphs, quantitation of all Western blot data (D) for Ser2481-phosphorylated mTOR (autophosphorylation site), Thr37/46-phosphorylated 4E-BP1 and Thr389-phosphorylated S6K1 normalized to the total amount of the respective protein, and the control-fed value set at 100 arbitrary units (AU). C, control-fed; A, alcohol-fed. a,b,cValues with different letters are statistically different (P < 0.05), while those values with the same letter are not statistically different.
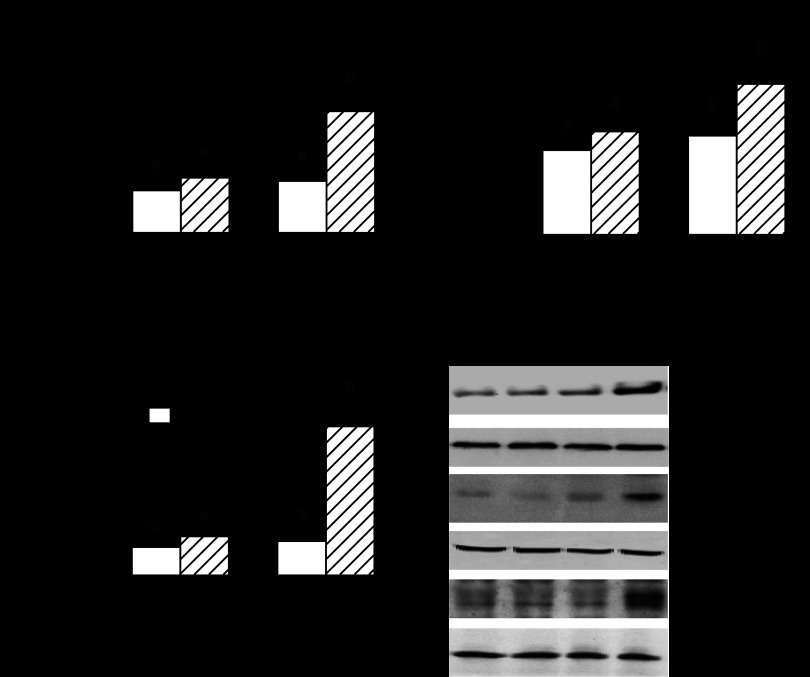
The mTORC1 complex is composed of at least five proteins: mTOR, raptor, GβL, PRAS40, and Deptor (23). Western blot analysis of whole muscle homogenates did not demonstrate a significant age- and/or alcohol-induced change for the total amount of raptor, mTOR, PRAS40, or GβL (Fig. 4A). Although the total amount of raptor did not differ between groups, Ser-792 phosphorylation of raptor was increased in alcohol-fed adult and aged rats (Fig. 4, A and B). Aged, but not adult, rats had a twofold increase in Deptor when fed the alcohol-containing diet (Fig. 4, A and C).
Fig. 4.
Effect of alcohol consumption on protein components of mTORC1 in gastrocnemius of adult and aged female rats. Values are expressed as means ± SE; n = 8 or 9 rats per group. Western blot data (A) were normalized to the amount of total raptor (B), or deptor (DEP-domain containing partner of mTOR) (tubulin) (C), with the control-fed value for adult rats set at 100 AU. a,bValues with different letters are statistically different (P < 0.05), while those values with the same letter are not statistically different.
The activity of mTORC1 is controlled, in part, by various protein-protein interactions. In this regard, raptor functions as a scaffold protein recruiting substrates to mTORC1 via short TOS (mTOR signaling) motifs in its substrates, thereby regulating kinase activity (64). Therefore, raptor was immunoprecipitated from gastrocnemius and then immunoblotted for PRAS40, 4E-BP1, Deptor, and raptor. Alcohol feeding increased the binding of both mTOR and Deptor to raptor in adult rats, and this increased binding was exaggerated in aged animals fed alcohol (Fig. 5). Conversely, alcohol reduced the amount of the raptor-4EBP1 complex in gastrocnemius from aged rats to a greater extent than in adult animals. The interaction of mTOR and 4E-BP1 with raptor did not differ in control-fed adult and aged rats, but there was an increased assembly of the Deptor-raptor complex in aged rats.
Fig. 5.
Effect of alcohol consumption on binding of various proteins to raptor in gastrocnemius of adult and aged female rats. Values are expressed as means ± SE; n = 5–6 rats per group. Raptor was immunoprecipitated (IP) and then immunoblotted for mTOR (A), 4E-BP1 (B), Deptor (C), PRAS40 (D), and raptor (E). Quantitation of Western blot data were normalized to amount of raptor in IP, with the control-fed value for adult rats set at 100 AU. The black vertical line in the 4EBP-1 blot indicates a spliced figure, which was necessitated because the order of the adult and aged rats was inverted; however, all samples on this blot were run simultaneously on the same gel, just their order was reversed. a,b,cValues with different letters are statistically different (P < 0.05), while those values with the same letter are not statistically different.
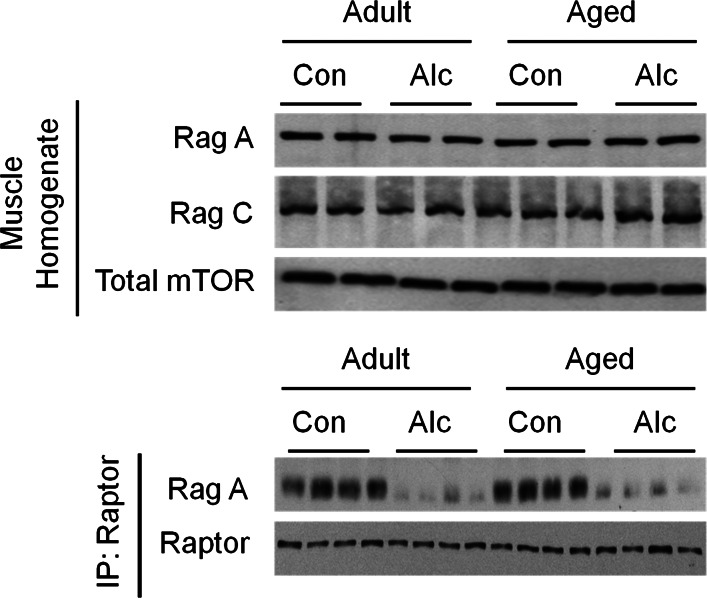
Finally, the Rag GTPase proteins (i.e., RagA-D) are amino acid-specific regulators of mTORC1, which interact directly with raptor (76). Western blot analysis of whole tissue homogenate indicated the total amount of RagA and RagC did not differ with alcohol or aging (Fig. 6, top). In contrast, the amount of RagA-raptor complex was reduced >90% by alcohol feeding, regardless of the age of the rat (Fig. 6, bottom).
Fig. 6.
Effect of alcohol consumption on total RagA and RagC, as well as the binding of RagA to raptor in gastrocnemius of adult and aged female rats. Top: Western blot for the total amount of RagA and RagC in whole muscle homogenate, where mTOR was used as a loading control. There were no age and/or alcohol effect on total RagA or RagC (n = 8 or 9 rats per group). Bottom: raptor was immunoprecipitated and RagA and raptor immunoblotted. Alcohol consumption, regardless of age, reduced binding of RagA to raptor (n = 4 per group).
Upstream protein factors regulating protein synthesis.
The general energy state of muscle is sensed by AMPK, which is activated (phosphorylated) by various catabolic agents, thereby inhibiting mTOR kinase activity (33). AMPK is an LKB1 (liver kinase B1) substrate, and increased LKB1 Ser-428 phosphorylation is important in activating AMPK. Moreover, REDD1 is a novel stress-response gene activated in an AMPK-dependent manner (16). The total and phosphorylated levels of AMPK and LKB1 and total REDD1 did not differ in gastrocnemius from alcohol-fed adult rats or in control aged rats, compared with adult control values (Fig. 7). However, there was a 50% increase in LKB1 phosphorylation, a 3-fold increase in AMPK phosphorylation, and a nearly 5-fold increase in REDD1 protein in gastrocnemius of aged rats consuming alcohol, demonstrating upregulation of the LKB-AMPK-REDD1 pathway.
Fig. 7.
Effect of alcohol consumption on total and phosphorylated AMPK (A) and LKB1 (B), as well as total REDD1 (regulated in development and DNA damage responses) protein (C) in gastrocnemius of adult and aged female rats. Values are expressed as means ± SE; n = 8 or 9 rats per group. Bar graphs, quantitation of all Western blot data (D) for Thr172- phosphorylated AMPK and Ser428-phosphorylated LKB1 or REDD1 normalized to the total amount of the respective protein or loading control (i.e., eIF4E), and the control-fed value from adult rats set at 100 AU. a,bValues with different letters are statistically different (P < 0.05), while those values with the same letter are not statistically different.
Protein degradation.
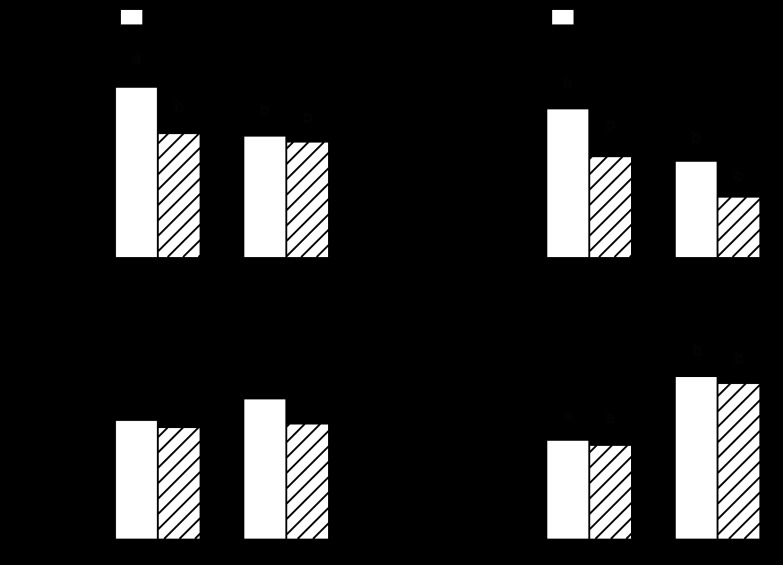
Because in vivo determinations of muscle protein breakdown are not routinely performed, we assessed accepted surrogate markers of proteolysis (5, 78). Alcohol consumption in adult animals increased the mRNA content for both atrogin-1 and MuRF1 in gastrocnemius (Fig. 8, A and B). A comparable increase in both atrogenes was observed in control-fed aged rats, compared with adult control animals. However, atrogin-1 and MuRF1 mRNA content was similarly elevated in both control and alcohol-fed aged rats. Finally, in vitro proteasome activity in gastrocnemius was not altered by alcohol feeding in adult rats but was increased 25% in control-fed aged rats (Fig. 8C). The magnitude of the age-induced increase in proteasome activity did not differ between control- and alcohol-fed aged rats.
Fig. 8.
Effect of alcohol consumption on surrogate markers of protein breakdown in gastrocnemius of adult and aged female rats: atrogin-1 mRNA (A), MuRF1 mRNA (B), and proteasome activity (C). Values are expressed as means ± SE; n = 8–9 rats per group. a,bValues with different letters are statistically different (P < 0.05), while those values with the same letter are not statistically different.
IGF system.
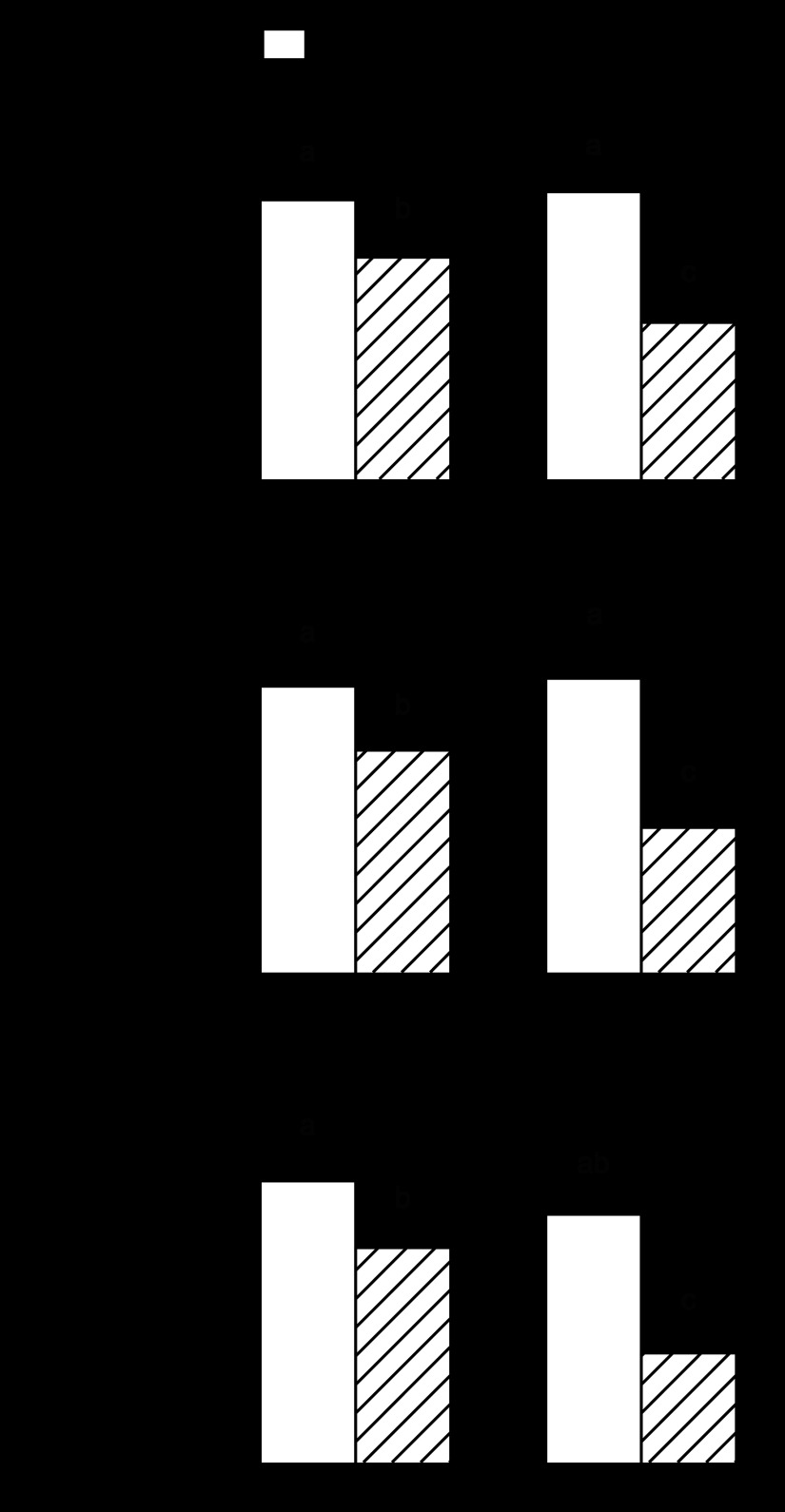
Alterations in circulating and local concentrations of IGF-I and its various binding proteins can impact muscle protein metabolism (22). Alcohol feeding in adult rats decreased both the plasma concentration and muscle mRNA content for IGF-I by ∼30% (Fig. 9, A and B). A similar reduction in blood and muscle IGF-I was observed in control-fed aged rats. However, whereas alcohol feeding did not further reduce the plasma IGF-I concentration in aged rats, this group exhibited the lowest IGF-I mRNA content in gastrocnemius. IGFBP-4 and IGFBP-5 can regulate IGF-I bioavailability, and changes in these binding proteins have been noted in some types of muscle disease (54). We detected no alcohol- or age-related change in IGFBP-4 mRNA in gastrocnemius (Fig. 9C). In contrast, IGFBP-5 mRNA was increased 50% in both groups of aged rats compared with adult animals, but muscle IGFBP-5 was not altered by alcohol feeding (Fig. 9D).
Fig. 9.
Effect of alcohol consumption on IGF-I in plasma (A) and muscle IGF-I (B), muscle IGF binding protein (IGFBP)-4 (C), and IGFBP-5 (D) mRNA content in gastrocnemius of adult and aged female rats. mRNA values were normalized to L32. Values are expressed as means ± SE; n = 8 or 9 rats per group. a,b,cValues with different letters are statistically different (P < 0.05), while values with the same letter are not statistically different.
Cytokines and inflammatory mediators.
Cytokines and inflammatory mediators have been implicated in the etiology of muscle wasting (24). Alcohol increased TNF-α mRNA content three-fold in the gastrocnemius from adult animals (Fig. 10A). Muscle TNF-α mRNA was increased twofold in control-fed aged rats, but alcohol-feeding did not lead to a further increase. IL-6 mRNA content was also increased in alcohol-fed adult animals (Fig. 10B). The gastrocnemius from both control- and alcohol-fed aged rats showed a fourfold increase in IL-6 mRNA. In contrast, we detected no alcohol- or age-induced change in either IL-1β or NOS2 mRNA in gastrocnemius (Fig. 10, C and D).
Fig. 10.
Effect of alcohol consumption on inflammatory mediators in gastrocnemius of adult and aged female rats: TNFα mRNA (A), IL-6 mRNA (B), IL-1β (C), and NOS2 mRNA (D). mRNA values were normalized to L32. Values are expressed as means ± SE; n = 8 or 9 rats per group. a,b,cValues with different letters are statistically different (P < 0.05), while those values with the same letter are not statistically different.
Metabolic substrates and hormones.
The total amount of alcohol consumed by aged rats tended to be greater than adult rats (3.7 ± 0.3 vs. 3.0 ± 0.2 g ethanol·rat−1·day−1, respectively; P = 0.07); however, when normalized per kilogram of body weight, alcohol consumption was lower in aged compared with adult rats (10.6 ± 0.9 vs. 13.1 ± 0.7 g ethanol·kg−1·day−1, respectively; P < 0.05). The blood alcohol concentration (BAC) determined near the beginning of the light cycle did not differ between adult and aged rats, averaging ∼16 mM (∼70 mg/dl; 0.07%; Table 1). There were no alcohol- or age-induced changes in the concentration of insulin, a known anabolic hormone. The constant insulin levels coupled with the lack of alcohol- or age-induced change in plasma glucose, suggests the absence of overt insulin resistance. Finally, the plasma concentration of each branched-chain amino acid (leucine, isoleucine, and valine) did not differ in alcohol-fed or aged rats, compared with control values. Finally, ALT and AST were determined to estimate hepatic damage. As presented in Table 1, ALT and AST did not differ between control-fed adult and aged rats, but both liver enzymes were increased to a comparable extent by alcohol feeding.
Table 1.
Effect of alcohol consumption on plasma concentrations of various substances in adult and aged rats
| Adult |
Aged |
|||
|---|---|---|---|---|
| Control | Alcohol | Control | Alcohol | |
| Alcohol, mmol/l | ND | 15.4 ± 2.3 | ND | 16.1 ± 2.8 |
| Glucose, mmol/l | 5.4 ± 0.2 | 5.3 ± 0.1 | 5.5 ± 0.3 | 5.2 ± 0.2 |
| Insulin, pmol/l | 162 ± 12 | 153 ± 15 | 151 ± 19 | 164 ± 27 |
| Leucine, μmol/l | 156 ± 9 | 151 ± 7 | 139 ± 18 | 147 ± 12 |
| Isoleucine, μmol/l | 117 ± 21 | 98 ± 13 | 114 ± 16 | 117 ± 8 |
| Valine, μmol/l | 133 ± 11 | 124 ± 8 | 115 ± 8 | 137 ± 10 |
| ALT, U/l | 46 ± 5a | 84 ± 8b | 55 ± 6a | 89 ± 12b |
| AST, U/l | 94 ± 8a | 145 ± 12b | 103 ± 21a | 164 ± 17b |
Values are expressed as means ± SE; n = 8 or 9 rats per group. There were statistically significant differences among the four groups for any listed parameter.
ALT, alanine aminotransferase; AST, aspartate aminotransferase. ND, not detectable.
Values in the same row with a different superscript letter are statistically different (P<0.05); values with the same letter are not different.
DISCUSSION
The current data support our hypothesis that the catabolic effect of alcohol on gastrocnemius is exaggerated in aged rats, and this response appears largely mediated by mTOR-dependent changes in protein synthesis. To place these new data in the context of existing literature, we will first discuss pertinent and novel changes observed in response to alcohol and aging alone before concluding with a discussion of the age-alcohol interaction on protein balance.
Alcohol-induced changes in adult female rats.
The current data are consistent with previous reports indicating that alcohol decreases both sarcoplasmic and myofibrillar protein synthesis without an apparent increase in protein degradation (71, 83). The alcohol-induced decrease in gastrocnemius protein synthesis is caused, at least in part, by a decreased mTOR kinase activity, as evidenced by the decreased autophosphorylation of mTOR, as well as decreased phosphorylation of the downstream substrates 4E-BP1 and S6K1 (50, 52, 55, 56). Although the activity of mTORC1 is regulated by various protein-protein interactions, changes have only been reported under in vitro conditions with relatively short-term incubation of myocytes with alcohol (37, 39, 40). Although there was no difference in the total amount of raptor, Deptor, PRAS40, GβL, or RagA/C in homogenates of gastrocnemius from control- and alcohol-fed adult rats, alcohol-induced changes were detected for several potentially important protein-protein interactions within mTORC1. For example, alcohol-fed rats have an increased binding of Deptor to raptor with a concomitant reduction in the binding of 4E-BP1 with raptor. This alcohol-induced increase in Deptor-raptor complex formation is particularly noteworthy, as Deptor is a negative regulator of mTORC1 activity (69). Previous studies have reported that overexpression of Deptor increases cell proliferation, while Deptor knockdown can reverse the muscle atrophy produced by disuse (43). Moreover, increased Deptor-raptor binding occurs in myocytes cultured with alcohol (39). The alcohol-induced reduction in raptor-4EBP1 binding is consistent with the scaffold function of raptor and the necessary recruitment of 4E-BP1 prior to its phosphorylation and release (36). Moreover, the increased raptor S792-phosphorylation in alcohol-fed rats is consistent with inhibition of mTOR kinase in other catabolic conditions (29). Whereas the increased raptor phosphorylation is typically attributed to activation of the cellular energy sensor AMPK (33), this pathway does not appear activated because the phosphorylation of AMPK and its upstream kinase LKB1 and a downstream substrate REDD1 did not differ between adult control- and alcohol-fed rats. We also detected a reduced binding of RagA with raptor in response to alcohol, a situation that would be expected to impair protein synthesis (76) and that has only been previously reported in myocytes incubated short-term with alcohol (38).
Relatively less is known regarding alcohol-induced changes in muscle proteolysis. We have reported that while acute alcohol intoxication increases the mRNA content for the ubiquitin-E3 ligases MuRF1 and atrogin-1, there was no evidence of increased proteolysis based on either 3-methylhistidine release from isolated perfused muscles or tyrosine release from incubated epitrochlearis muscles (84). Increased MuRF1 and atrogin-1 have also been observed in muscle from alcohol-fed rats (53, 66). Although our current results confirm these earlier observations, proteasome activity did not differ between control and alcohol-fed rats. These data would suggest that if chronic alcohol intake increases proteolysis in gastrocnemius, the mechanism must be mediated by other pathways for protein breakdown or that the degradation of a relatively small number of specific proteins is enhanced (90).
Excess production of proinflammatory cytokines can decrease muscle protein synthesis (24), and an increase in muscle TNF-α and IL-6 mRNA is observed in some (8, 66) but not other studies (62) in response to chronic alcohol feeding. Our current data confirm alcohol-fed rats have a selective increase in TNF-α and IL-6 mRNA in gastrocnemius, but no detectable change in IL-1β or NOS2. The lack of alcohol-induced change in NOS2 was unexpected as this inflammatory mediator has potent inhibitory effects on muscle protein synthesis (25). Finally, alcohol consumption decreases both circulating and muscle IGF-I (52, 55, 75), which may represent a potential mechanism for the decreased muscle protein synthesis (18, 22). Our results confirm that alcohol-fed rats have both a reduction in plasma IGF-I and muscle IGF-I mRNA, but no change in either IGFBP-4 or IGFBP-5, which can modulate IGF-I bioavailability (22). We speculate that, collectively, the features observed in adult alcohol-fed mice (i.e., atrophy, decreased protein synthesis, decreased circulating and tissue IGF-I, and increased atrogenes and specific inflammatory cytokines) are consistent with a premature aging-like phenotype (described below). Other characteristics of this phenotype, such as fibrosis, lipid accumulation, and impaired regeneration, will need to be assessed in future studies.
Age-induced changes.
Whether an absolute age-related decrease in muscle mass is detected in rats appears dependent on a complex interaction of multiple factors, including animal husbandry, rat strain, muscle type, sex, type of food provided, presence of comorbid conditions, and a broad definition of what age constitutes “old” in rats (35, 41, 91). However, essentially all studies report the presence of sarcopenia when muscle mass is normalized to body weight, and such was the case in the current study. The confounders mentioned above may have also influenced other metabolic and hormonal endpoints related to muscle protein balance, thereby contributing to the discrepancy on age-induced changes in the literature. For example, although some studies have shown aging decreases (4, 70, 88) or even increases in mixed or myofibrillar muscle protein synthesis (47, 80), other studies find the synthetic rates for mitochondrial, sarcoplasmic, and myosin heavy chain fractions in muscle are well maintained during aging (11, 27, 81, 82). Our data support these latter reports and reveal no difference in the synthetic rate for total, myofibrillar or sarcoplasmic proteins in gastrocnemius. The lack of an age-induced change in muscle protein synthesis is consistent with the similar content of total and phosphorylated proteins controlling mRNA translation (e.g., mTOR, 4E-BP1, S6K1, raptor, Deptor, RagA/C). Moreover, there was no change in the phosphorylation state of either AMPK or LKB1 or total REDD1, similar to previous studies (74), suggesting that aging did not alter constitutive AMPK activity in gastrocnemius.
Age-induced changes in atrogene expression and their physiological importance, if any, in regulating muscle proteolysis remains unclear. For example, several studies have reported no age-induced change in MuRF1 and atrogin-1 (7, 26, 89), while others showed an increased (1, 9, 10) or decreased (15, 18) expression of these atrogenes. We observed a two- to three-fold increase in both atrogin-1 and MuRF1 mRNA in aged rats, which was associated with a concomitant stimulation of in vitro-determined proteasome activity. These data are consistent with the elevated rate of proteolysis observed in aged humans (87) and elevated rate of 3-methylhistidine excretion in rats (63). Although these data suggest sarcopenia may be due solely to an increase in proteolysis, as opposed to a decrease in protein synthesis, the assessment of protein metabolism was conducted in the postabsorptive state (∼3–5 h after removal of food), and aging has been shown to attenuate the anabolic response of muscle protein synthesis to nutrient stimulation (2, 6). Hence, the relative importance of changes in protein synthesis and degradation as a cause for sarcopenia may vary depending on nutritional fluctuations.
Circulating IGF-I was reduced in control-fed aged rats, consistent with previous observations (17, 86). Locally produced IGF-I is also implicated in maintenance of muscle mass. However, data on IGF-I mRNA in skeletal muscle per se are often contradictory, with our study and others (9, 59, 86) showing a decrease, but others reporting either no change or an increased muscle IGF-I with aging (11, 18, 30, 32). Our current data do little to reconcile these highly diverse findings. Finally, aging can increase the circulating concentration of a number of potentially catabolic inflammatory mediators (68, 82), as well as the TNF-α mRNA content in muscle (9). In this regard, both TNF-α and IL-6 mRNA were increased in gastrocnemius from aged rats.
Alcohol-aging interaction.
The primary focus of the current study was to elucidate the combined effect of chronic alcohol consumption and aging on muscle protein balance. Our results highlight that aged rats have an increased sensitivity to the catabolic effects of dietary alcohol. A comparable increased sensitivity to excess glucocorticoids has been reported in aged rats (13). Sustained alcohol consumption in aged female rats produced an exaggerated decline in both LBM and the absolute mass of the gastrocnemius. Likewise, the alcohol-induced decrease in gastrocnemius protein synthesis (global, sarcoplasmic, and myofibrillar) was of greater magnitude in aged rats. No such age-alcohol interaction was detected for muscle weight or protein synthesis in the soleus muscle, which is consistent with the preferential effect of alcohol feeding on fast-twitch fibers (e.g., gastrocnemius) vs. slow-twitch fibers (soleus) (55). The mechanism for this relatively selective atrophy in response to alcohol is not known.
This exaggerated drop in muscle protein synthesis was associated with a concomitant reduction in the extent of phosphorylated 4E-BP1, but not mTOR or S6K1 phosphorylation. Of the proteins that constitute the mTORC1 complex, only the negative-regulatory protein Deptor was increased by the combination of alcohol and aging. As a result of these changes, we detected an increased formation of the Deptor-raptor complex, with a reduction in the 4E-BP1-raptor complex. We speculate these changes in mTORC1 are causally related to the accentuated decrease in muscle protein synthesis in the alcohol-fed aged rats (38, 43, 44). These changes in protein-protein interaction within mTORC1 may be LKB1-AMPK-REDD1-dependent, as this signaling pathway was only activated in muscle from alcohol-fed aged rats. However, AMPK inhibition of mTOR activity and protein synthesis is typically mediated by increased phosphorylation of raptor (30, 72), which showed no additive effect. It is noteworthy that it was the combination of alcohol and aging that stimulated the AMPK pathway, an activation that was not seen with either factor alone.
The physiological mechanism for this enhanced catabolic response remains to be elucidated. Of the parameters assessed, we detected no interaction of alcohol and aging on the plasma concentrations of IGF-I, insulin, or branched-chain amino acids, as well as no additional increase in TNF-α or IL-6 within gastrocnemius. In contrast, we detected a further reduction in IGF-I mRNA content in muscle of alcohol-fed aged rats, suggesting a possible mechanism.
In contrast to protein synthesis, aged rats fed alcohol had no further increase in atrogene expression or proteasome activity in gastrocnemius, compared with alcohol-fed adult rats. While our data suggest the increased sensitivity of older rats toward the catabolic effect of alcohol is predominantly mediated by a change in protein synthesis, we cannot exclude the possibility that the combination of aging and alcohol enhances other pathways of protein degradation, which were not part of this investigation. In this regard, acute alcohol intoxication has been reported to increase autophagy in cardiac muscle via an AMPK-dependent mechanism (28).
On the basis of our data, we cannot reach a definitive conclusion as to whether the increased sensitivity observed in alcohol-fed aged rats is mediated by a difference in the BAC. While alcohol consumption per kilogram body weight was 20% lower in aged rats and there was no difference in the BAC between groups determined at the beginning of the light cycle, we cannot exclude the possibility that aged rats had higher BAC during the dark cycle when the majority of alcohol is consumed. Such an age-related increase in BAC might be predicted if alcohol clearance is decreased by aging. However, results from pharmacokinetic studies on ethanol metabolism in rats are conflicting (31, 46), thereby rendering any conclusion equivocal. We can also not exclude the possibility that an alcohol- and/or aging-induced decrease in spontaneous physical activity did not contribute, in part, to the observed muscle phenotype. Finally, although a similar degree of hepatic injury in alcohol-fed adult and aged rats is suggested by the similar increment in AST and ALT levels, we have previously reported exaggerated hepatic lipidosis and inflammation in alcohol-fed aged rats (79). Therefore, detailed studies that assess hepatic function directly are needed to determine whether metabolic disturbances secondary to hepatic dysfunction (e.g., hyperammonemia) are causally related to the muscle wasting detected in alcohol-fed aged rats.
Our results demonstrate that chronic alcohol consumption has a greater catabolic effect on muscle wasting in aged vs. adult female rats. The mechanism for this exaggerated response in gastrocnemius is likely mediated by changes in protein-protein interactions within mTORC1 via an AMPK-dependent mechanism.
The potential clinical relevance of in vivo determined data is dependent in large part on the fidelity of the preclinical animal model used. In this regard, while there is no single best model, our study used the F344 rat strain because it is one of the best characterized rodent models of gerontological research (91). Additionally, the Lieber-DeCarli alcohol-containing diet has been extensively used for almost 50 years (58). Rats provided this liquid diet consume a relatively large quantity of alcohol, compared with humans classified as heavy drinkers (2 drinks/day). For example, the typical caloric intake for men is ∼2,000 kcal/day. Therefore, in humans where alcohol constitutes 36% of total calories of dietary intake (i.e., 720 kcal ethanol/day), ∼100 g of ethanol (7 kcal/g ethanol) would be ingested daily. As a “standard drink” averages 14 g ethanol, humans would have to consume at least 7 standard drinks per day to match that ingested by the alcohol-fed rats. However, this increased intake in rats is also coupled with a several-fold greater rate of alcohol clearance, compared with humans (19, 42). As a result, chronic alcohol ingestion in both rats and humans yields comparable circulating alcohol concentrations of 100–200 mg/dl. Collectively, our results suggest that sustained excessive alcohol consumption by the elderly should be discouraged to minimize the sarcopenia typically seen in this patient population, as muscle mass and strength are predictive of disability and all-cause mortality (61).
GRANTS
This project was supported by National Institutes of Health RC2 AA019403 and R01 HL 091097 (to D. H. Korzick) and R37 AA011290 (to C. H. Lang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: D.H.K., D.R.S., and C.H.L. conception and design of research; D.H.K., D.R.S., A.M.P., and C.H.L. performed experiments; D.H.K., A.M.P., and C.H.L. interpreted results of experiments; D.H.K., D.R.S., and C.H.L. drafted manuscript; D.H.K., D.R.S., A.M.P., and C.H.L. edited and revised manuscript; D.H.K., D.R.S., A.M.P., and C.H.L. approved final version of manuscript; A.M.P. and C.H.L. analyzed data; C.H.L. prepared figures.
ACKNOWLEDGMENTS
We are grateful to Gina Deiter for excellent technical assistance.
Present address for D. R. Sharda: Department of Biology, Olivet Nazarene University, Bourbonnais, IL 60914.
REFERENCES
- 1. Altun M, Besche HC, Overkleeft HS, Piccirillo R, Edelmann MJ, Kessler BM, Goldberg AL, Ulfhake B. Muscle wasting in aged, sarcopenic rats is associated with enhanced activity of the ubiquitin proteasome pathway. J Biol Chem 285: 39597–39608, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Attaix D, Mosoni L, Dardevet D, Combaret L, Mirand PP, Grizard J. Altered responses in skeletal muscle protein turnover during aging in anabolic and catabolic periods. Int J Biochem Cell Biol 37: 1962–1973, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Baird TJ, Vanecek SA, Briscoe RJ, Vallett M, Carl KL, Gauvin DV. Moderate, long-term, alcohol consumption potentiates normal, age-related spatial memory deficits in rats. Alcohol Clin Exp Res 22: 628–636, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am J Physiol Endocrinol Metab 273: E790–E800, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Capel F, Prod'homme M, Bechet D, Taillandier D, Balage M, Attaix D, Combaret L. Lysosomal and proteasome-dependent proteolysis are differentially regulated by insulin and/or amino acids following feeding in young, mature and old rats. J Nutr Biochem 20: 570–576, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Casas F, Pessemesse L, Grandemange S, Seyer P, Baris O, Gueguen N, Ramonatxo C, Perrin F, Fouret G, Lepourry L, Cabello G, Wrutniak-Cabello C. Overexpression of the mitochondrial T3 receptor induces skeletal muscle atrophy during aging. PLos One 4: e5631, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clary CR, Guidot DM, Bratina MA, Otis JS. Chronic alcohol ingestion exacerbates skeletal muscle myopathy in HIV-1 transgenic rats. AIDS Res Ther 8: 30, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clavel S, Coldefy AS, Kurkdjian E, Salles J, Margaritis I, Derijard B. Atrophy-related ubiquitin ligases, atrogin-1 and MuRF1 are up-regulated in aged rat tibialis anterior muscle. Mech Ageing Dev 127: 794–801, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Dalbo VJ, Roberts MD, Hassell SE, Brown RD, Kerksick CM. Effects of age on serum hormone concentrations and intramuscular proteolytic signaling before and after a single bout of resistance training. J Strength Cond Res 25: 1–9, 2011 [DOI] [PubMed] [Google Scholar]
- 11. Dardevet D, Sornet C, Attaix D, Baracos VE, Grizard J. Insulin-like growth factor-1 and insulin resistance in skeletal muscles of adult and old rats. Endocrinology 134: 1475–1484, 1994 [DOI] [PubMed] [Google Scholar]
- 12. Dardevet D, Sornet C, Savary I, Debras E, Patureau-Mirand P, Grizard J. Glucocorticoid effects on insulin- and IGF-I-regulated muscle protein metabolism during aging. J Endocrinol 156: 83–89, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Dardevet D, Sornet C, Taillandier D, Savary I, Attaix D, Grizard J. Sensitivity and protein turnover response to glucocorticoids are different in skeletal muscle from adult and old rats. Lack of regulation of the ubiquitin-proteasome proteolytic pathway in aging. J Clin Invest 96: 2113–2119, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dennis MD, Baum JI, Kimball SR, Jefferson LS. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem 286: 8287–8296, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deruisseau KC, Kavazis AN, Powers SK. Selective downregulation of ubiquitin conjugation cascade mRNA occurs in the senescent rat soleus muscle. Exp Gerontol 40: 526–531, 2005 [DOI] [PubMed] [Google Scholar]
- 16. DeYoung MP, Horak P, Sofer A, Sgroi D, Ellisen LW. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14–3-3 shuttling. Genes Dev 22: 239–251, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Donahue LR, Hunter SJ, Sherblom AP, Rosen C. Age-related changes in serum insulin-like growth factor-binding proteins in women. J Clin Endocrinol Metab 71: 575–579, 1990 [DOI] [PubMed] [Google Scholar]
- 18. Edstrom E, Ulfhake B. Sarcopenia is not due to lack of regenerative drive in senescent skeletal muscle. Aging Cell 4: 65–77, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Erickson CK. Ethanol clearance in nine inbred rat strains. Alcohol Clin Exp Res 8: 491–494, 1984 [DOI] [PubMed] [Google Scholar]
- 20. Fernandez-Sola J, Sacanella E, Estruch R, Nicolas JM, Grau JM, Urbano-Marquez A. Significance of type II fiber atrophy in chronic alcoholic myopathy. J Neurol Sci 130: 69–76, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Ferreira MP, Willoughby D. Alcohol consumption: the good, the bad, and the indifferent. Appl Physiol Nutr Metab 33: 12–20, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Frost RA, Lang CH. Alteration of somatotropic function by proinflammatory cytokines. J Anim Sci 82 Suppl: E100–E109, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Frost RA, Lang CH. mTor signaling in skeletal muscle during sepsis and inflammation: where does it all go wrong? Physiology (Bethesda) 26: 83–96, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frost RA, Lang CH. Regulation of muscle growth by pathogen-associated molecules. J Anim Sci 86: E84–E93, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Frost RA, Nystrom GJ, Lang CH. Endotoxin and interferon-gamma inhibit translation in skeletal muscle cells by stimulating nitric oxide synthase activity. Shock 32: 416–426, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Greig CA, Gray C, Rankin D, Young A, Mann V, Noble B, Atherton PJ. Blunting of adaptive responses to resistance exercise training in women over 75 y. Exp Gerontol 46: 884–890, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Guillet C, Zangarelli A, Mishellany A, Rousset P, Sornet C, Dardevet D, Boirie Y. Mitochondrial and sarcoplasmic proteins, but not myosin heavy chain, are sensitive to leucine supplementation in old rat skeletal muscle. Exp Gerontol 39: 745–751, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Guo R, Ren J. Deficiency in AMPK attenuates ethanol-induced cardiac contractile dysfunction through inhibition of autophagosome formation. Cardiovasc Res 94: 480–491, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30: 214–226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haddad F, Adams GR. Aging-sensitive cellular and molecular mechanisms associated with skeletal muscle hypertrophy. J Appl Physiol 100: 1188–1203, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Hahn HK, Burch RE. Impaired ethanol metabolism with advancing age. Alcohol Clin Exp Res 7: 299–301, 1983 [DOI] [PubMed] [Google Scholar]
- 32. Hamilton MT, Marsh DR, Criswell DS, Lou W, Booth FW. No effect of aging on skeletal muscle insulin-like growth factor mRNAs. Am J Physiol Regul Integr Comp Physiol 269: R1183–R1188, 1995 [DOI] [PubMed] [Google Scholar]
- 33. Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13: 251–262, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hervonen A, Jaatinen P, Sarviharju M, Kiianmaa K. Interaction of aging and lifelong ethanol ingestion on ethanol-related behaviors and longevity. Exp Gerontol 27: 335–345, 1992 [DOI] [PubMed] [Google Scholar]
- 35. Holloszy JO, Chen M, Cartee GD, Young JC. Skeletal muscle atrophy in old rats: differential changes in the three fiber types. Mech Ageing Dev 60: 199–213, 1991 [DOI] [PubMed] [Google Scholar]
- 36. Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 123: 569–580, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Hong-Brown LQ, Brown CR, Kazi AA, Huber DS, Pruznak AM, Lang CH. Alcohol and PRAS40 knockdown decrease mTOR activity and protein synthesis via AMPK signaling and changes in mTORC1 interaction. J Cell Biochem 109: 1172–1184, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hong-Brown LQ, Brown CR, Kazi AA, Navaratnarajah M, Lang CH. Rag GTPases and AMPK/TSC2/Rheb mediate the differential regulation of mTORC1 signaling in response to alcohol and leucine. Am J Physiol Cell Physiol 302: C1557–C1565, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hong-Brown LQ, Brown CR, Navaratnarajah M, Huber DS, Lang CH. Alcohol-induced modulation of rictor and mTORC2 activity in C2C12 myoblasts. Alcohol Clin Exp Res 35: 1445–1453, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hong-Brown LQ, Frost RA, Lang CH. Alcohol impairs protein synthesis and degradation in cultured skeletal muscle cells. Alcohol Clin Exp Res 25: 1373–1382, 2001 [PubMed] [Google Scholar]
- 41. Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol 89: 81–88, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Jones MK, Jones BM. Ethanol metabolism in women taking oral contraceptives. Alcohol Clin Exp Res 8: 24–28, 1984 [DOI] [PubMed] [Google Scholar]
- 43. Kazi AA, Hong-Brown L, Lang SM, Lang CH. Deptor knockdown enhances mTOR activity and protein synthesis in myocytes and ameliorates disuse muscle atrophy. Mol Med 17: 925–936, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kazi AA, Pruznak AM, Frost RA, Lang CH. Sepsis-induced alterations in protein-protein interactions within mTOR complex 1 and the modulating effect of leucine on muscle protein synthesis. Shock 35: 117–125, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110: 163–175, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Kim YC, Kim SY, Sohn YR. Effect of age increase on metabolism and toxicity of ethanol in female rats. Life Sci 74: 509–519, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Kimball SR, O'Malley JP, Anthony JC, Crozier SJ, Jefferson LS. Assessment of biomarkers of protein anabolism in skeletal muscle during the life span of the rat: sarcopenia despite elevated protein synthesis. Am J Physiol Endocrinol Metab 287: E772–E780, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Koll M, Beeso JA, Kelly FJ, Simanowski UA, Seitz HK, Peters TJ, Preedy VR. Chronic alpha-tocopherol supplementation in rats does not ameliorate either chronic or acute alcohol-induced changes in muscle protein metabolism. Clin Sci (Lond) 104: 287–294, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Lang CH, Frost RA, Bronson SK, Lynch CJ, Vary TC. Skeletal muscle protein balance in mTOR heterozygous mice in response to inflammation and leucine. Am J Physiol Endocrinol Metab 298: E1283–E1294, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lang CH, Frost RA, Deshpande N, Kumar V, Vary TC, Jefferson LS, Kimball SR. Alcohol impairs leucine-mediated phosphorylation of 4E-BP1, S6K1, eIF4G, and mTOR in skeletal muscle. Am J Physiol Endocrinol Metab 285: E1205–E1215, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Lang CH, Frost RA, Summer AD, Vary TC. Molecular mechanisms responsible for alcohol-induced myopathy in skeletal muscle and heart. Int J Biochem Cell Biol 37: 2180–2195, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Lang CH, Frost RA, Svanberg E, Vary TC. IGF-I/IGFBP-3 ameliorates alterations in protein synthesis, eIF4E availability, and myostatin in alcohol-fed rats. Am J Physiol Endocrinol Metab 286: E916–E926, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Lang CH, Frost RA, Vary TC. Skeletal muscle protein synthesis and degradation exhibit sexual dimorphism after chronic alcohol consumption but not acute intoxication. Am J Physiol Endocrinol Metab 292: E1497–E1506, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Lang CH, Krawiec BJ, Huber D, McCoy JM, Frost RA. Sepsis and inflammatory insults downregulate IGFBP-5, but not IGFBP-4, in skeletal muscle via a TNF-dependent mechanism. Am J Physiol Regul Integr Comp Physiol 290: R963–R972, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Lang CH, Pruznak AM, Nystrom GJ, Vary TC. Alcohol-induced decrease in muscle protein synthesis associated with increased binding of mTOR and raptor: comparable effects in young and mature rats. Nutr Metab (Lond) 6: 4, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lang CH, Wu D, Frost RA, Jefferson LS, Kimball SR, Vary TC. Inhibition of muscle protein synthesis by alcohol is associated with modulation of eIF2B and eIF4E. Am J Physiol Endocrinol Metab 277: E268–E276, 1999 [DOI] [PubMed] [Google Scholar]
- 57. Lang SM, Kazi AA, Hong-Brown L, Lang CH. Delayed recovery of skeletal muscle mass following hindlimb immobilization in mTOR heterozygous mice. PLos One 7: e38910, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lieber CS, DeCarli LM. Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcohol 24: 197–211, 1989 [PubMed] [Google Scholar]
- 59. Marsh DR, Criswell DS, Hamilton MT, Booth FW. Association of insulin-like growth factor mRNA expressions with muscle regeneration in young, adult, and old rats. Am J Physiol Regul Integr Comp Physiol 273: R353–R358, 1997 [DOI] [PubMed] [Google Scholar]
- 60. Mendenhall CL, Rouster SD, Roselle GA, Grossman CJ, Ghosn S, Gartside P. Impact of chronic alcoholism on the aging rat: changes in nutrition, liver composition, and mortality. Alcohol Clin Exp Res 17: 847–853, 1993 [DOI] [PubMed] [Google Scholar]
- 61. Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci 57: B359–B365, 2002 [DOI] [PubMed] [Google Scholar]
- 62. Molina PE, Lang CH, McNurlan M, Bagby GJ, Nelson S. Chronic alcohol accentuates simian acquired immunodeficiency syndrome-associated wasting. Alcohol Clin Exp Res 32: 138–147, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mosoni L, Malmezat T, Valluy MC, Houlier ML, Attaix D, Mirand PP. Lower recovery of muscle protein lost during starvation in old rats despite a stimulation of protein synthesis. Am J Physiol Endocrinol Metab 277: E608–E616, 1999 [DOI] [PubMed] [Google Scholar]
- 64. Nojima H, Tokunaga C, Eguchi S, Oshiro N, Hidayat S, Yoshino K, Hara K, Tanaka N, Avruch J, Yonezawa K. The mammalian target of rapamycin (mTOR) partner, raptor, binds the mTOR substrates p70 S6 kinase and 4E-BP1 through their TOR signaling (TOS) motif. J Biol Chem 278: 15461–15464, 2003 [DOI] [PubMed] [Google Scholar]
- 65. Oshiro N, Takahashi R, Yoshino K, Tanimura K, Nakashima A, Eguchi S, Miyamoto T, Hara K, Takehana K, Avruch J, Kikkawa U, Yonezawa K. The proline-rich Akt substrate of 40 kDa (PRAS40) is a physiological substrate of mammalian target of rapamycin complex 1. J Biol Chem 282: 20329–20339, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Otis JS, Guidot DM. Procysteine stimulates expression of key anabolic factors and reduces plantaris atrophy in alcohol-fed rats. Alcohol Clin Exp Res 33: 1450–1459, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pacy PJ, Preedy VR, Peters TJ, Read M, Halliday D. The effect of chronic alcohol ingestion on whole body and muscle protein synthesis—a stable isotope study. Alcohol Alcohol 26: 505–513, 1991 [DOI] [PubMed] [Google Scholar]
- 68. Peake J, Della Gatta P, Cameron-Smith D. Aging and its effects on inflammation in skeletal muscle at rest and following exercise-induced muscle injury. Am J Physiol Regul Integr Comp Physiol 298: R1485–R1495, 2010 [DOI] [PubMed] [Google Scholar]
- 69. Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 137: 873–886, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pluskal MG, Moreyra M, Burini RC, Young VR. Protein synthesis studies in skeletal muscle of aging rats. I. Alterations in nitrogen composition and protein synthesis using a crude polyribosome and pH 5 enzyme system. J Gerontol 39: 385–391, 1984 [DOI] [PubMed] [Google Scholar]
- 71. Preedy VR, Macallan DC, Griffin GE, Cook EB, Palmer TN, Peters TJ. Total contractile protein contents and gene expression in skeletal muscle in response to chronic ethanol consumption in the rat. Alcohol 14: 545–549, 1997 [DOI] [PubMed] [Google Scholar]
- 72. Pruznak AM, Kazi AA, Frost RA, Vary TC, Lang CH. Activation of AMP-activated protein kinase by 5-aminoimidazole-4-carboxamide-1-beta-d-ribonucleoside prevents leucine-stimulated protein synthesis in rat skeletal muscle. J Nutr 138: 1887–1894, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Reilly ME, McKoy G, Mantle D, Peters TJ, Goldspink G, Preedy VR. Protein and mRNA levels of the myosin heavy chain isoforms Ibeta, IIa, IIx and IIb in type I and type II fibre-predominant rat skeletal muscles in response to chronic alcohol feeding. J Muscle Res Cell Motil 21: 763–773, 2000 [DOI] [PubMed] [Google Scholar]
- 74. Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, Befroy D, Pypaert M, Hardie DG, Young LH, Shulman GI. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab 5: 151–156, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rojdmark S, Brismar K. Decreased IGF-I bioavailability after ethanol abuse in alcoholics: partial restitution after short-term abstinence. J Endocrinol Invest 24: 476–482, 2001 [DOI] [PubMed] [Google Scholar]
- 76. Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320: 1496–1501, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sarviharju M, Jaatinen P, Hyytia P, Hervonen A, Kiianmaa K. Effects of lifelong ethanol consumption on drinking behavior and motor impairment of alcohol-preferring AA and alcohol-avoiding ANA rats. Alcohol 23: 157–166, 2001 [DOI] [PubMed] [Google Scholar]
- 78. Schakman O, Gilson H, Thissen JP. Mechanisms of glucocorticoid-induced myopathy. J Endocrinol 197: 1–10, 2008 [DOI] [PubMed] [Google Scholar]
- 79. Sharda DR, Miller-Lee JL, Kanski GM, Hunter JC, Lang CH, Kennett MJ, Korzick DH. Comparison of the agar block and Lieber-DeCarli diets to study chronic alcohol consumption in an aging model of Fischer 344 female rats. J Pharmacol Toxicol Methods 66: 257–263, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Smith GI, Reeds DN, Hall AM, Chambers KT, Finck BN, Mittendorfer B. Sexually dimorphic effect of aging on skeletal muscle protein synthesis. Biol Sex Differ 3: 11, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Timmerman KL, Dhanani S, Glynn EL, Fry CS, Drummond MJ, Jennings K, Rasmussen BB, Volpi E. A moderate acute increase in physical activity enhances nutritive flow and the muscle protein anabolic response to mixed nutrient intake in older adults. Am J Clin Nutr 95: 1403–1412, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Toth MJ, Matthews DE, Tracy RP, Previs MJ. Age-related differences in skeletal muscle protein synthesis: relation to markers of immune activation. Am J Physiol Endocrinol Metab 288: E883–E891, 2005 [DOI] [PubMed] [Google Scholar]
- 83. Vary TC, Deiter G. Long-term alcohol administration inhibits synthesis of both myofibrillar and sarcoplasmic proteins in heart. Metabolism 54: 212–219, 2005 [DOI] [PubMed] [Google Scholar]
- 84. Vary TC, Frost RA, Lang CH. Acute alcohol intoxication increases atrogin-1 and MuRF1 mRNA without increasing proteolysis in skeletal muscle. Am J Physiol Regul Integr Comp Physiol 294: R1777–R1789, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Vary TC, Lang CH. Assessing effects of alcohol consumption on protein synthesis in striated muscles. Methods Mol Biol 447: 343–355, 2008 [DOI] [PubMed] [Google Scholar]
- 86. Velasco B, Cacicedo L, Melian E, Fernandez-Vazquez G, Sanchez-Franco F. Sensitivity to exogenous GH and reversibility of the reduced IGF-I gene expression in aging rats. Eur J Endocrinol 145: 73–85, 2001 [DOI] [PubMed] [Google Scholar]
- 87. Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA 286: 1206–1212, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Welle S, Thornton C, Statt M, McHenry B. Postprandial myofibrillar and whole body protein synthesis in young and old human subjects. Am J Physiol Endocrinol Metab 267: E599–E604, 1994 [DOI] [PubMed] [Google Scholar]
- 89. Whitman SA, Wacker MJ, Richmond SR, Godard MP. Contributions of the ubiquitin-proteasome pathway and apoptosis to human skeletal muscle wasting with age. Pflügers Arch 450: 437–446, 2005 [DOI] [PubMed] [Google Scholar]
- 90. Wing SS, Lecker SH, Jagoe RT. Proteolysis in illness-associated skeletal muscle atrophy: from pathways to networks. Crit Rev Clin Lab Sci 48: 49–70, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yu BP, Masoro EJ, Murata I, Bertrand HA, Lynd FT. Life span study of SPF Fischer 344 male rats fed ad libitum or restricted diets: longevity, growth, lean body mass and disease. J Gerontol 37: 130–141, 1982 [DOI] [PubMed] [Google Scholar]