Abstract
There are currently two medical treatments approved in Canada that offer survival benefits for patients with metastatic castration-resistant prostate cancer that progresses on or after docetaxel-based chemotherapy, and evidence is accumulating on the efficacy of further interventions in this setting. The current and emerging strategies are based on a variety of mechanisms (cytotoxicity, hormonal inhibition, radiopharmacy and immunotherapy) and there is nothing to suggest that patients will be unable to benefit from several or even all of these agents when used sequentially. Given the possibility of multiple lines of treatment for patients whose disease progresses on or after docetaxel, the challenge for clinicians will be to determine the optimum treatment pathway for each individual. That challenge is already being faced, albeit on a limited scale, now that both cabazitaxel (chemotherapy) and abiraterone (hormonal agent) are available for use post-docetaxel.
Introduction
As recently as the beginning of the 21st century, metastatic castration-resistant prostate cancer (mCRPC) had a bleak prognosis. The available treatments, such as radiotherapy, bone-seeking isotopes, bisphosphonates, chemotherapy, cortico-steroids and analgesics, offered palliation of symptoms, but no improvement in survival.1In the past 8 years, the outlook has changed dramatically. First, the landmark TAX327 trial of docetaxel demonstrated that, contrary to previous experience,2 mCRPC was responsive to chemotherapy in terms of patient survival.1 Then, in 2010, after 6 years with no treatment offering a survival benefit in the post-docetaxel setting, phase III data on cabazitaxel showed that patients could derive further survival benefit from second-line chemotherapy.3 In 2011, the hormonal agent abiraterone was also reported to improve survival in patients previously treated with docetaxel.4 Both cabazitaxel and abiraterone are now approved for use in Canada for the treatment of mCRPC that has progressed during or after docetaxel-based chemotherapy. In addition, evidence is accumulating from trials of other agents, not yet approved in Canada, that offer a survival benefit in mCRPC.
With these ongoing therapeutic developments, the multi-disciplinary team caring for men with mCRPC has a growing choice of agents to use in the post-docetaxel setting. The recent and emerging treatments vary widely in their mode of action (cytotoxicity, hormonal inhibition, radiopharmacy, immunotherapy), and there is no suggestion, to date, that patients will be able to benefit from only one of the post-docetaxel options. Indeed, the possibility has been mooted of mCRPC entering an age of chronic-disease-style management, with an array of treatments, each improving the survival of the individual.5 Even with the choice narrowed to the two agents currently approved for use post-docetaxel, it is anticipated that patients will be able to derive a survival advantage from both cabazitaxel and abiraterone.6 The key question for clinicians and their patients is: how can these treatments be sequenced to maximize each individual’s survival?
This article presents an overview of the evidence base for the approved and emerging treatments for mCRPC post-docetaxel, and considers how to ensure that eligible patients are able to benefit from the two treatments currently available (cabazitaxel and abiraterone).
Survival post-docetaxel: The evidence base
Current options
Cabazitaxel
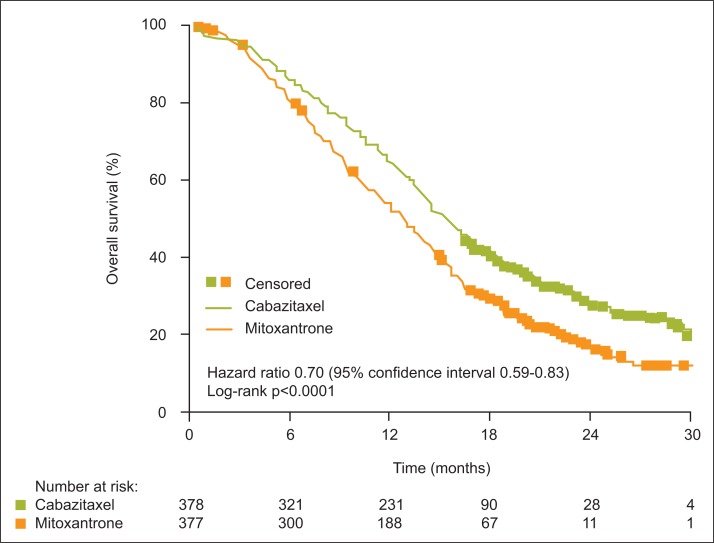
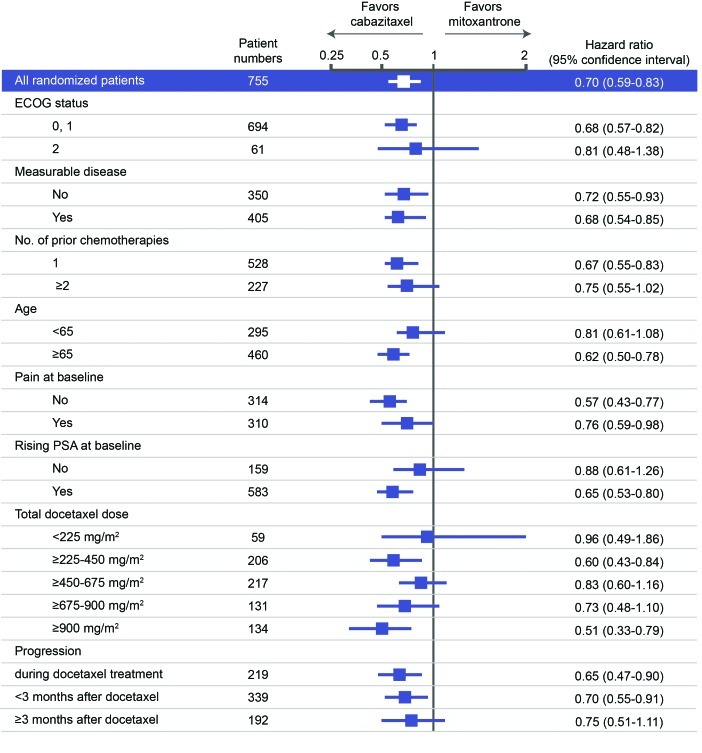
The rationale for use of cabazitaxel in mCRPC post-docetaxel (the TROPIC trial of cabazitaxel versus mitoxantrone in this setting, and the ongoing cabazitaxel early-access program [EAP]) has been discussed in detail elsewhere by Saad and Asselah in this supplement, page S5.7 In brief, TROPIC showed that cabazitaxel improved median overall survival (cabazitaxel, 15.1 months; mitoxantrone, 12.7 months; Fig. 1), and that the benefit applied to all subgroups analyzed (Fig. 2).3 Interim results from the EAP indicate improvement in pain control with continuing treatment, and stable scores for anxiety/depression, mobility and self-care.8,9
Fig. 1.
Overall survival in the TROPIC trial of cabazitaxel versus mitoxantrone.3 Used with permission from The Lancet.
Fig. 2.
Subgroup analysis of survival data from the TROPIC trial.3 Used with permission from The Lancet. ECOG: Eastern Cooperative Oncology Group; PSA: prostate-specific antigen.
Abiraterone
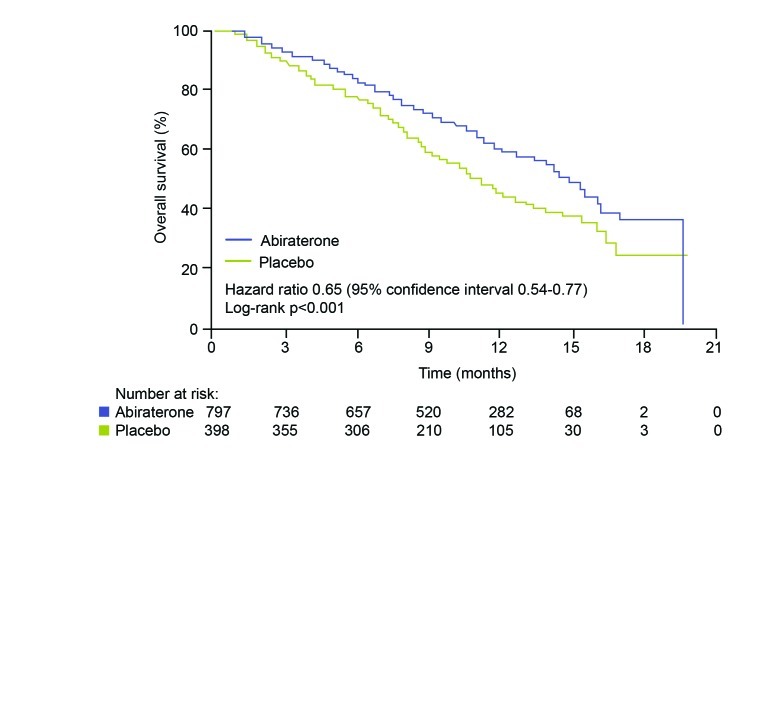
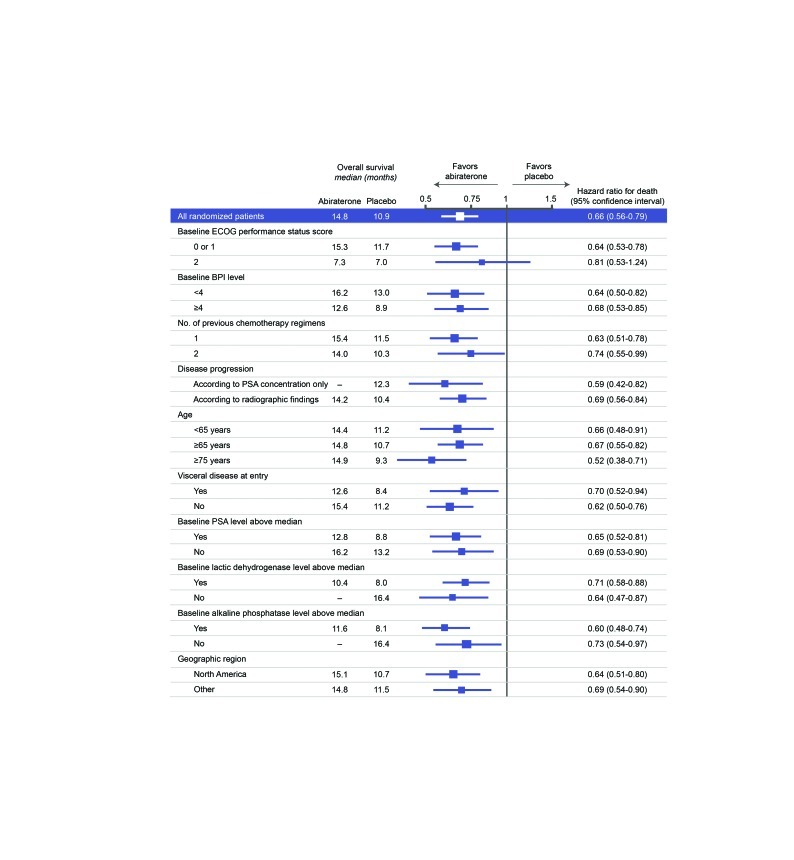
The decision to investigate abiraterone in mCRPC came from the observation that enzymes involved in androgen synthesis are unregulated in the condition, leading to increased androgen levels in the tumor.10 Abiraterone acetate blocks cytochrome p450 c17 (CYP17), an enzyme required for testosterone synthesis; early trials of the agent showed promising anti-tumor activity in patients with mCRPC both before and after chemotherapy.4 The phase III COU-AA-301 trial compared abiraterone 1000 mg/day plus prednisone 10 mg/day (n=797) with placebo plus prednisone 10 mg/day (n=398) in men with mCRPC who had previously received chemotherapy.4 COU-AA-301 showed that abiraterone improved median overall survival (Fig. 3); at the latest analysis, men in the abiraterone group survived 15.8 months, compared with 11.2 months in the placebo group.11 Furthermore, the initial trial report indicated that the survival benefit applied to all subgroups analyzed (Fig. 4).4
Fig. 3.
Overall survival in the COU-AA-301 trial of abiraterone versus placebo.4Used with permission from The New England Journal of Medicine.
Fig. 4.
Subgroup analysis of survival data from the COU-AA-301 trial.4 Used with permission from The New England Journal of Medicine. ECOG: Eastern Cooperative Oncology Group; BPI: Brief Pain Inventory; PSA: prostate-specific antigen.
TROPIC and COU-AA-301: key differences in trial design
It is inappropriate to draw direct comparisons between TROPIC and COU-AA-301, because the trials differed in various parameters (Table 1).
Table 1.
| Trial parameter | TROPIC | COU-AA-301 | Comment |
|---|---|---|---|
| Comparator | Mitoxantrone | Placebo | Mitoxantrone is an active agent, used in mCRPC for symptom palliation2 |
| Geographical range | North America, South America, Australia, Europe, Asia, Africa | North America, Australia, Europe | Inclusion of less developed countries brings potential for wide variation in patient care |
| Definition of disease progression | Evidence of PSA progression OR radiological progression OR symptomatic progression | Composite of PSA, radiological AND symptomatic progression | Potential that some TROPIC patients were responding to treatment when taken off study |
mCRPC: metastatic castration-resistant prostate cancer; PSA: prostate-specific antigen.
Emerging options
Phase III data are available on the following 3 agents, each showing a survival advantage in patients with mCRPC (Table 2). None of these treatments are approved for use in Canada.
Table 2.
Emerging therapies with phase III evidence of efficacy in mCRPC (not approved in Canada)
| Treatment | Type of agent | Patient status | Survival advantage versus placebo (months) | Comments |
|---|---|---|---|---|
| MDV310011 | Hormonal inhibitor | mCRPC, patients received up to two prior courses of chemotherapy | 4.8 | Promising agent for use after chemotherapy |
| Radium-22312 | Radiopharmaceutical | mCRPC, with at least two bone metastases, 58% received prior docetaxel | 2.8 | Appropriate only for patients with bone metastases, no outcomes data on patients who go on to receive chemotherapy |
| Sipuleucel-T13 | Therapeutic vaccine | mCRPC, 20% received prior chemotherapy, 16% received prior docetaxel | 4.1 | High-cost treatment, with data derived mainly from the chemotherapy-naïve setting14 |
mCRPC, metastatic castration-resistant prostate cancer.
MDV3100
MDV3100 is an androgen-receptor-signaling inhibitor that has shown activity in men with mCRPC with and without prior chemotherapy.12 In the phase III AFFIRM trial, all 1199 patients received at least two courses of chemotherapy for mCRPC, and were randomized 2:1 to receive MDV3100 160 mg/day or placebo. Interim data, announced at the 2012 Genitourinary Cancer meeting of the American Society of Clinical Oncology (ASCO-GU), showed that the novel agent produced a median overall survival benefit over placebo (MDV3100, 18.4 months; placebo, 13.6 months).
Radium-223 chloride
Radium-223 chloride is a radiopharmaceutical that targets bone metastases with high-energy, short-range alpha particles.13 In the phase III ALSYMPCA trial, 922 patients with mCRPC and at least two bone metastases were randomized 2:1 to receive six injections of radium-223 (50 kBq/kg) every 4 weeks, or placebo, plus best supportive care in both treatment arms. About half of the patients had received prior docetaxel, and the remainder were either unsuitable for such treatment or had refused it. Overall survival, announced at ASCO-GU 2012, was significantly longer in the active treatment group (radium-223, 14.0 months, placebo, 11.2 months).13
Sipuleucel-T
Sipuleucel-T is a therapeutic cancer vaccine, derived ex vivo from the patient’s own peripheral blood mononuclear cells which are activated using a recombinant fusion protein. The activated cells are administered via three intravenous infusions conducted at 2-week intervals.14 It is a high-cost therapy, and is currently available in the USA.15 The phase III IMPACT study, in which 512 patients with mCRPC were randomized (2:1) to receive sipuleucel-T or placebo, has shown a survival advantage in the active treatment group (sipuleucel-T, 25.8 months; placebo, 21.7 months).14 Of note, however, only 20% of the sipuleucel recipients in this trial had received prior chemotherapy, and only 16% had received docetaxel.12–15
Managing the continuum of care
The importance of a rational, individualized approach to post-docetaxel therapy is already apparent in the clinical setting, even with just two approved agents offering a potential increase in survival. There is no evidence or expectation that patients will be able to derive benefit from only cabazitaxel or abiraterone; their mechanisms are entirely different. Indeed, many clinical teams now hope that, where clinically appropriate, their patients will be able to receive both of these treatments.
The choice of treatment for mCRPC that progresses post-docetaxel needs to be based on individual assessment, with the aim of offering as many survival-extending treatments as are deemed appropriate for the patient. To maximize exposure to such treatments, the disease management pathway should be considered as a whole, rather than opting for one or other treatment in isolation in the period following docetaxel. Furthermore, a decision to start either abiraterone or cabazitaxel when the patient shows evidence of disease progression after docetaxel, but not necessarily waiting for the development of symptoms, may maximize the opportunity of exploiting both therapeutic possibilities while the patient’s performance status is good. In addition, the decision whether to offer cabazitaxel or abiraterone as the initial treatment should be guided by consideration of the patient’s likelihood of receiving the alternative agent on further disease progression.
Given that the patient will have already received a course of docetaxel, possibly completed only a small number of weeks previously, there is an argument in favor of considering the hormonal option as the next intervention. In this way, the patient will have a period of treatment without the risk of cytotoxic side effects, and with the option of cabazitaxel at a later date. Where abiraterone is used initially in the post-docetaxel setting and the intention is to offer cabazitaxel subsequently, it will be imperative to closely monitor not only disease progression but also the patient’s performance status, to ensure the opportunity for cabazitaxel is not missed.
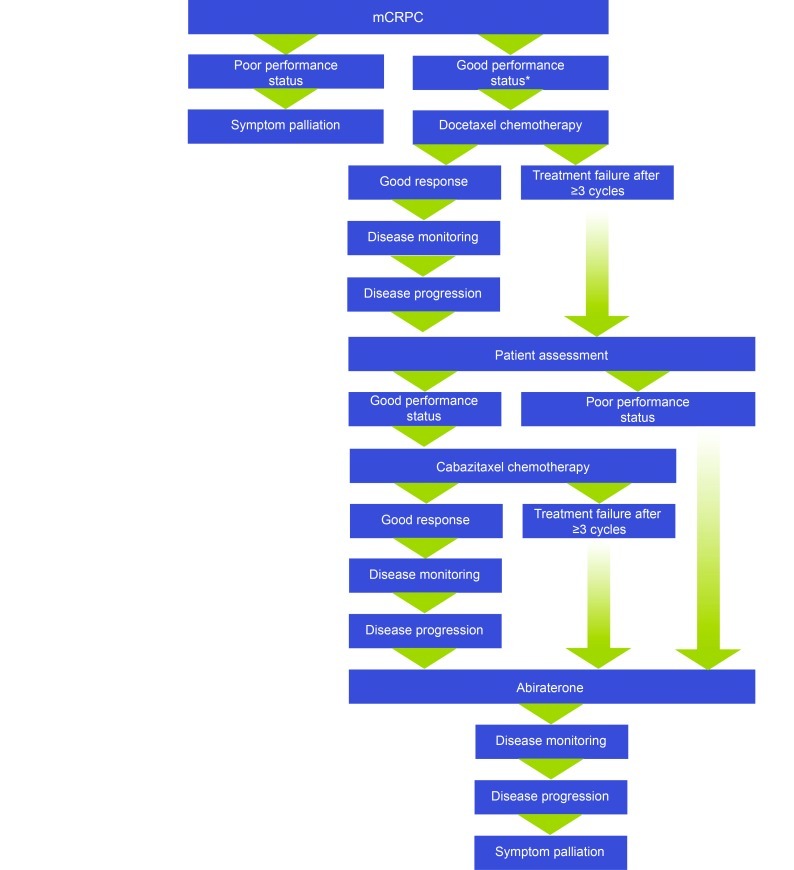
An alternative approach to treatment sequencing is to offer cabazitaxel as the first post-docetaxel treatment to patients who retain a good performance status.6,16 Advocates of the “cabazitaxel-first” strategy argue that it ensures delivery of cabazitaxel before a decline in performance status renders the patient ineligible for cytotoxic therapy; this strategy retains the option of subsequent abiraterone and thereby maximizes the likelihood of eligible patients receiving both of these licensed treatments (Fig. 5).
Fig. 5.
The “cabazitaxel-first” approach to mCRPC management post-docetaxel, as advocated in some centres.6,17,18 *The TAX327 trial of docetaxel versus mitoxantrone was conducted in patients with a Karnofsky performance status of ≥60% (able to self care with no more than occasional help).1 mCRPC: metastatic castration-resistant prostate cancer.
Regardless of which therapy is given first, it is important to offer the second post-docetaxel treatment while the patient is well enough to be able to tolerate and benefit from the agent. Of note, the issue of wellness is not simply a question of patient age. Guidelines from the International Society of Geriatric Oncology (SIOG) state that decisions on the management of advanced prostate cancer should be based on an assessment of underlying fitness and not on the chronologic age of the patient.17 An elderly patient with controlled comorbidities and good nutritional status, who does not rely on help in his activities of daily living, should be seen in the same light as a younger patient in terms of treatment eligibility.
In the near and longer-term future, the challenge facing multidisciplinary teams caring for men with mCRPC will be to formulate treatment pathways that make optimal use of all the agents that enter the treatment arena.19
Conclusion
The prospect of chronic-disease-style management for mCRPC is growing closer as evidence emerges on a variety of agents that offer not just symptom palliation, but also improved survival.1,3,12–14 Because the mechanisms of action of these agents are varied (cytoxicity, hormonal intervention, radiopharmacy and immunotherapy), there is hope that patients will be able to be benefit from several lines of treatment, each adding to overall survival.
Some of these new agents are still in the development stage. However, three are already approved for use in Canada: docetaxel-based chemotherapy is established in the first-line management of mCRPC, with cabazitaxel (chemotherapy) and abiraterone (hormonal treatment) now approved for use in the second line, when mCRPC progresses during or after docetaxel. With regard to the two approved post-docetaxel options, clinical experience thus far suggests that, in the absence of specific contraindications, patients may be able to benefit from both. However, questions remain over the rational sequence in which to deploy them. An argument in favor of the “abiraterone-first” approach is that the patient has already received docetaxel, and that hormonal therapy will offer a period free of cytotoxic side effects. In favor of the “cabazitaxel-first” approach is the argument that the patient’s performance status may decline during prior abiraterone treatment, such that the opportunity for subsequent cabazitaxel is lost. Either way, careful monitoring of disease progression and performance status will be essential throughout post-docetaxel treatment. In the longer term, of course, the sequencing quandary is likely to embrace a growing number of agents for this new-styled “chronic” cancer.
Footnotes
Competing interests: Dr. Catherine Sperlich has participated in advisory boards for Amgen, Janssen and Sanofi, and has received speaker honoraria from Amgen, Janssen, Novartis and Sanofi. Dr. Asselah has participated in advisory boards for Amgen and Sanofi, and has received speaker honoraria from Amgen and Sanofi.
This paper has been peer-reviewed.
References
- 1.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 2.Tannock IF, Osoba D, Stockier MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14:1756–64. doi: 10.1200/JCO.1996.14.6.1756. [DOI] [PubMed] [Google Scholar]
- 3.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 4.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornford P. Evolution of docetaxel-based therapy for metastatic castrate-resistant prostate cancer. Br J Med Surg Urol. 2010;3:225–30. [Google Scholar]
- 6.Malik ZI. Cabazitaxel: evidence and clinical experience. Br J Med Surg Urol. 2011;4(Suppl 1):S14–20. [Google Scholar]
- 7.Saad F, Asselah J. Chemotherapy for prostate cancer: clinical practice in Canada. Can Urol Assoc J. 2013;7:S5–S10. doi: 10.5489/cuaj.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bahl A, Masson S, Malik Z, et al. Cabazitaxel for metastatic castration-resistant prostate cancer (mCRPC): interim safety and quality-of-life (QOL) data from the U.K. early access program ( NCT01254279) J Clin Oncol. 2012;30(Suppl) abstract 44. [Google Scholar]
- 9.Sridhar SS, Winquist E, Hubay S, et al. Cabazitaxel early access program (EAP) - Canadian interim results: safety, QOL, and utility values in metastatic castration resistant prostate cancer (mCRPC) Ann Oncol. 2012;23(Suppl 9) abstract 960. [Google Scholar]
- 10.Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–25. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 11.Scher HI, Heller G, Molina A, et al. Evaluation of circulating tumor cell (CTC) enumeration as an efficacy response biomarker of overall survival (OS) in metastatic castration-resistant prostate cancer (mCRPC): planned final analysis (FA) of COU-AA-301, a randomized double-blind, placebo-controlled phase III study of abiraterone acetate (AA) plus low-dose prednisone (P) post docetaxel. J Clin Oncol. 2011;29(Suppl) abstract LBA4517. [Google Scholar]
- 12.Scher HI, Fizazi K, Saad F, et al. Effect of MDV3100, an androgen receptor signaling inhibitor (ARSI), on overall survival in patients with prostate cancer postdocetaxel: results from the phase III AFFIRM study. J Clin Oncol. 2012;30(Suppl) abstract LBA1. [Google Scholar]
- 13.Parker C, Heinrich D, O’Sullivan JM, et al. Overall survival benefit and safety profile of radium-223 chloride, a first-in-class alpha-pharmaceutical: results from a phase III randomized trial (ALSYMPCA) in patients with castration-resistant prostate cancer (CRPC) with bone metastases. J Clin Oncol. 2012;30(Suppl) abstract 8. [Google Scholar]
- 14.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 15.Abdulla A, Kapoor A. Emerging novel therapies in the treatment of castrate-resistant prostate cancer. Can Urol Assoc J. 2011;5:120–33. doi: 10.5489/cuaj.10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bahl A, Persad R. Metastatic castrate-resistant prostate cancer: new landscape, new challenges. Br J Med Surg Urol. 2011;4(Suppl 1):S9–13. [Google Scholar]
- 17.Droz JP, Balducci L, Bolla M, et al. Background for the proposal of SIOG guidelines for the management of prostate cancer in senior adults. Crit Rev Oncol Hematol. 2010;73:68–91. doi: 10.1016/j.critrevonc.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Malik ZI, Montazeri AH, Wong H, et al. Post docetaxel treatment for castration-resistant prostate cancer (CRPC): does sequencing matter? J Clin Oncol. 2012;30(Suppl) abstract e15135. [Google Scholar]
- 19.Francis J. Advances in treating metastatic castration-resistant prostate cancer. Cancer Nursing Practice. 11:32–7. [Google Scholar]