Abstract
Neurotransmitters can have diverse effects that occur over multiple time scales often making the consequences of neurotransmission difficult to predict. To explore the consequences of this diversity, we used the buccal ganglion of Aplysia to examine the effects of GABA release by a single interneuron, B40, on the intrinsic properties and motor output of the radula closure neuron B8. B40 induces a picrotoxin-sensitive fast IPSP lasting milliseconds in B8 and a slow EPSP lasting seconds. We found that the excitatory effects of this slow EPSP are also mediated by GABA. Together, these two GABAergic actions structure B8 firing in a pattern characteristic of ingestive programs. Furthermore, we found that repeated B40 stimulation induces a persistent increase in B8 excitability that was occluded in the presence of the GABA B receptor agonist baclofen, suggesting that GABA affects B8 excitability over multiple time scales. The phasing of B8 activity during the feeding motor programs determines the nature of the behavior elicited during that motor program. The persistent increase in B8 excitability induced by B40 biased the activity of B8 during feeding motor programs causing the motor programs to become more ingestive in nature. Thus, a single transmitter released from a single interneuron can have consequences for motor output that are expressed over multiple time scales. Importantly, despite the differences in their signs and temporal characteristics, the three actions of B40 are coherent in that they promote B8 firing patterns that are characteristic of ingestive motor outputs.
Keywords: multiple timescales, GABA, Aplysia, motor control
the impact of an individual synaptic connection can result from a combination of distinct signs, conductances, and time courses of its postsynaptic components. The different features of the postsynaptic response can be mediated by cotransmission of either fast transmitters (Barreiro-Iglesias et al. 2009; Beaulieu and Somogyi 1991; Burnstock 2009; Diaz-Rios et al. 2002; Katz and Harris-Warrick 1989) or neuropeptides (Harris et al. 2010; Nusbaum et al. 2001; Vilim et al. 1996a, 1996b, 2000; Weiss et al. 1992; Wood et al. 2000), or can even be mediated by a single transmitter activating different receptor types. For instance, exogenously applied serotonin induces a fast hyperpolarization and a slow depolarization in layer V pyramidal neurons of the prefrontal cortex by targeting different serotonin receptors (Araneda and Andrade 1991; Beique et al. 2004, 2007; Villalobos et al. 2005). Thus a single transmitter can have apparently conflicting effects. However, the behavioral consequences of these different features are difficult to study in vertebrate preparations. For instance, the distinct effects of exogenously applied transmitters do not necessarily indicate that in situ these effects result from activation of the same presynaptic source. Furthermore, the diversity of functional cell types within any neural network (Stevens 1998), the variability in the intrinsic properties of neurons within a given cell type (Marder and Goaillard 2006; Turrigiano 2008), and the number and types of synaptic connections experienced by any given neuron (Gulyas et al. 1999; Megias et al. 2001) contribute levels of complexity that can obscure the functional consequences of neurotransmitter release for behavior. Several features make invertebrate preparations well suited for examining the time scales with which neurotransmitters function including identified neurons and synapses, fewer neurons within a given circuit and observable motor output. For instance, the pharyngeal muscles of Drosophila have been used to demonstrate the functional consequences for motor output of acetylcholine activation of fast nicotinic and slow muscarinic-like excitatory receptors (Gorczyca et al. 1991), and the cholinergic L10 neuron in the abdominal ganglia of Aplysia was used to demonstrate that a single transmitter can have opposite effects on downstream neurons depending on firing frequency (Wachtel and Kandel 1967). We took advantage of the feeding central pattern generator (CPG) of Aplysia to examine the functional consequences of the integration of the different features of postsynaptic responses elicited by a single presynaptic neuron for motor output.
Activity of an identified interneuron (B40) elicits a multi-component response in the radula closure motor neuron B8. Each of these distinct components has functional consequences for the activity of B8 in feeding motor programs. In the first phase of buccal motor programs (protraction), the GABAergic interneuron B40 provides inhibitory drive that is implemented by fast IPSPs to B8. The IPSPs are apparently mediated by GABA as they are blocked by picrotoxin (Jing et al. 2003). B40 also induces a slow EPSP that during protraction is shunted by the fast IPSPs, but is expressed following the cessation of B40 firing, thus increasing B8 activity in the latter phase (retraction) of the motor program (Jing and Weiss 2002). These two actions of B40 determine the phasing of B8 activity in motor programs. The transmitter for this slow EPSP has not been identified. We report that the slow excitatory effects of this EPSP can be mimicked and occluded with either GABA or the GABA B receptor agonist baclofen, thus suggesting that the influence of B40 over the phasing of motor activity is mediated by the release of a single transmitter. Furthermore, we found a previously unpublished effect that B40 exerts on B8. Specifically, repeated B40 stimulation can increase the excitability of B8 over the course of minutes, an effect that is occluded by baclofen. Finally, we demonstrate that the B40-induced persistent increase in B8 excitability is likely to affect B8 activity in motor programs. Thus, a single neurotransmitter acts on multiple time scales to influence the nature of motor output in a coherent manner both within a single motor program and in subsequent motor programs.
MATERIALS AND METHODS
Adult sea slugs (Aplysia californica) were obtained from Marinus (Long Beach, CA) and maintained at 14–15°C. Animals were anesthetized with isotonic MgCl2, and all experiments were performed at 14–15°C. Saline was perfused onto the preparation at 0.3 ml/min. Artificial salt water (ASW; in mM: 460 NaCl, 10 KCl, 55 MgCl2, 11 CaCl2, and 10 HEPES buffer, pH7.6) was applied for experiments in which biting motor programs (BMPs) were elicited. For experiments examining monosynaptic connections or in which spontaneous activity needed to be reduced, a high divalent saline (HiDi; in mM: 368 NaCl, 10 KCl, 101 MgCl2, 13.8 CaCl2, and 10 HEPES, pH 7.6) (Friedman and Weiss 2010) was superfused over the preparation. Pharmacological agents were superfused for 15–20 min into the recording dish and were all purchased from Sigma (St. Louis, MO). Picrotoxin was dissolved in ASW over night at 100 μM. GABA and baclofen were made fresh at 1 mM in either HiDi or ASW before each experiment and diluted as required. Both GABA and baclofen induce spontaneous activity in the buccal ganglion, requiring experiments examining the effects of GABA or baclofen on B8 excitability to be performed in HiDi. This decreased spontaneous activity in the buccal ganglion prevented interference with measures of B8 excitability. Only the experiment testing the effects of baclofen on B8 activity in CBI-2-triggered motor programs were performed in ASW, and the concentration of baclofen used did not elicit much spontaneous activity. Phaclofen, piperidine-4-sulfonic acid, and CG54626 (all vertebrate GABA B receptor antagonists) were all tested at 100 μM in a minimum of three animals each, but did not antagonize the effects of GABA, baclofen, or B40 on B8 excitability. Intracellular recordings were performed with borosilicate electrodes filled with 0.6 M K2SO4 and 60 mM KCl electrolyte solution. Electrodes were pulled with a Sutter Instrument (Novato, CA) Flaming/Brown micropipette puller and beveled with a stream of aluminum oxide in water to a final resistance of 7–9 MΩ for motor neurons and 10–12 MΩ for interneurons. Neurons were identified based on published physiological and locational criteria (Gardner and Kandel 1977; Hurwitz and Susswein 1996; Jing and Weiss 2002, 2005). Extracellular nerve recordings were performed by aspirating buccal nerve 2 (BN2), I2 nerve (I2N), and the radular nerve (RN) into polyethylene tubing. Electrodes were held in Molecular Devices (Sunnyvale, CA) HS-2A head stages and input to AxoClamp 2B (Molecular Devices) amplifiers. Intracellular and extracellular signals were then amplified by a CyberAmp 380 (Molecular Devices) and sent to a 1320A Digidata (Molecular Devices) for data acquisition. AxoScope v10 (Molecular Devices) was used to visualize and record experiments. Data were analyzed in Spike 2 (Cambridge Electronic Design) and organized in Excel (Microsoft) for later statistical analyses, which were performed in GraphPad Prism v5.01 (GraphPad Software). Error bars indicate SE, and an alpha of 0.05 was selected for significance tests. When ANOVA tests indicated significant effects, further individual comparisons were calculated with a Bonferroni correction. Normality was tested using the D'Agostino and Pearson omnibus normality test.
BMPs result from the coordination of two sets of muscles, those muscles that open and close the radula and those that protract and retract the radula. Because BMPs always begin with protraction followed by retraction, the functional nature of BMPs depends on the phasing of the opener/closer motor neuron activity over protraction and retraction phase. If the radula is open during protraction and then closed during retraction, the radula will extend out, clamp down on a food item, and draw it into the mouth (Kupfermann 1974; Morton and Chiel 1993a, b). This is referred to as an “ingestive program.” If the firing rate of the radula closer motor neuron B8 is low during protraction and high in the retraction phase, the BMP is ingestive as B8 will be contributing to the closure of the radula only during retraction. In this manner, B8 firing rate can be used as a monitor for the phasing of the opener/closer motor neurons with relation to protraction/retraction (Dacks et al. 2012; Friedman and Weiss 2010; Jing et al. 2004; Jing and Weiss 2001, 2002; Morton and Chiel 1993a, b; Proekt et al. 2004, 2007). Protraction phase is defined as the duration of activity on the I2N (Hurwitz et al. 1996; Jing and Weiss 2001, 2002; Morgan et al. 2000; Nargeot et al. 1999a, b) and retraction phase is defined as the duration of activity on BN2 after the cessation of protraction (Morton and Chiel 1993a, b; Nargeot et al. 1999a). Neurons were identified using published locational and physiological criteria (Hurwitz et al. 1997, 1999, 2003; Jing and Weiss 2001, 2002, 2005; Morton and Chiel 1993b; Rosen et al. 1991).
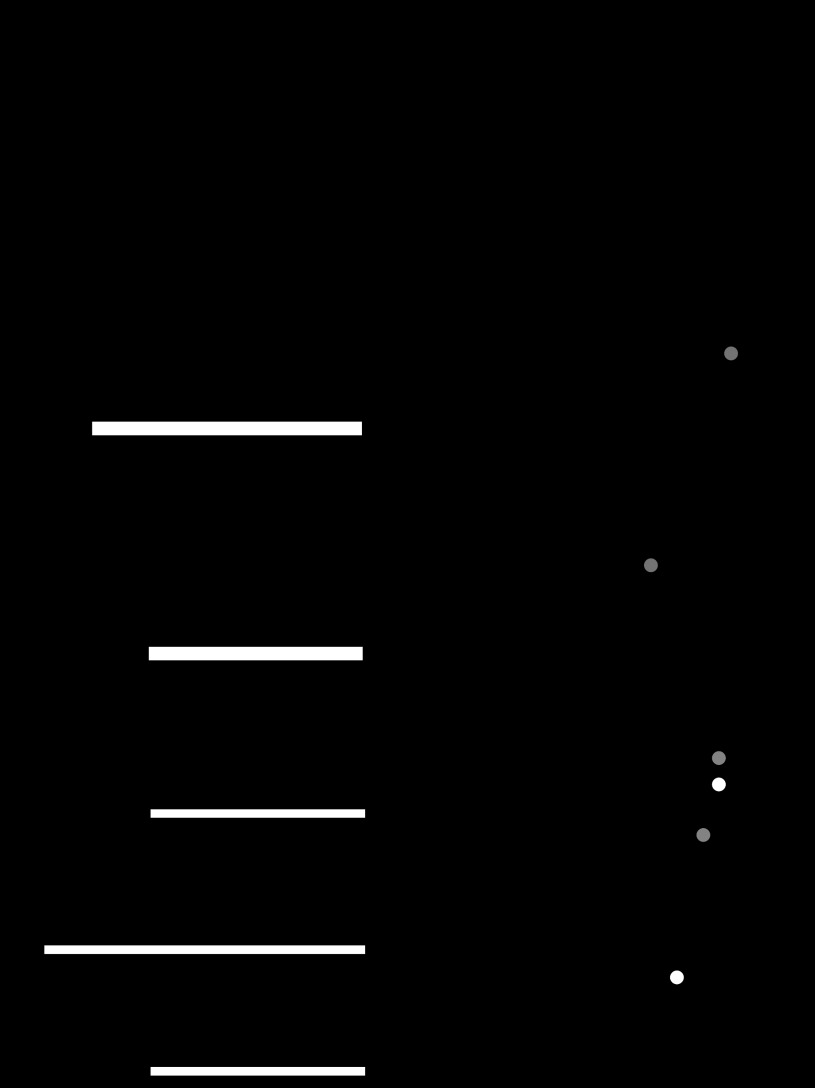
The synaptic interactions between CBI-2, B40, and B8 occur over multiple time scales. Figure 1 depicts these synaptic interactions on the orders of milliseconds, seconds, and minutes. CBI-2 elicits fast EPSPs in B40 causing B40 to spike which elicits fast IPSPs in B8. Thus, during protraction CBI-2 activates B40 which inhibits B8 on a time scale of milliseconds (Fig. 1A). Both CBI-2 (Dacks et al. 2012) and B40 (Jing and Weiss 2002) elicit slow EPSPs in B8 that last on the order of seconds (Fig. 1B). Thus at the end of protraction the shunting inhibition exerted by B40 on B8 ceases allowing the expression of the slow excitatory input (Jing and Weiss 2002). Finally, both CBI-2 (Dacks et al. 2012) and B40 (this study) can persistently upregulate B8 excitability on a time scale of minutes (Fig. 1C).
Fig. 1.
Schematic diagrams of the synaptic influences of CBI-2 and B40 on B8 over time scales of milliseconds, seconds, and minutes. A: on a time scale of milliseconds, CBI-2 elicits fast EPSPs in B40, and B40 elicits fast IPSPs in B8. CBI-2 and B40 are drawn with thicker lines to indicate that they are spiking while these faster synaptic effects occur. B: on a time scale of seconds, CBI-2 and B40 elicit slow EPSPs in B8 that are expressed after activity of CBI-2 or B40 ceases. C: on a time scale of minutes, both CBI-2 and B40 increase B8 excitability. Triangles represent excitatory input, whereas circles represent inhibitory input.
To measure the effects of GABA and baclofen on B8 excitability, B8 was injected with three levels of DC, eliciting 4, 7, or 15 spikes in 4 s, every 30 s to establish a baseline level of B8 excitability. B8 membrane potential was not maintained in these experiments. Increasing concentrations of either GABA or baclofen were then applied, and the same levels of DC were injected into B8. For the GABA experiments, GABA was always applied in the presence of 100 μM picrotoxin to avoid the fast inhibitory effects of GABA (Jing et al. 2003). Thus, picrotoxin was applied before GABA, and the effects of picrotoxin alone on B8 excitability were tested. B8 excitability was then tested for each concentration of GABA from 1 μM to 1 mM.
To measure the effect of the B40 or B34 slow EPSP on B8 excitability, B8 was injected five times with a 1-min interstimulus interval with sufficient DC to trigger ∼15 spikes over 4 s to establish a baseline level of excitability. Subsequently, B40 or B34 was stimulated at 10 Hz for the 3 s immediately preceding the DC injection to B8. Finally, DC was injected five times into B8 as a “recovery” measure to gauge if stimulating B40 or B34 for 3 s induced any persistent effects on B8 excitability. For experiments in which baclofen or GABA were used to occlude the effects of the slow EPSP on B8 excitability, the amount of current injected into B8 was reduced in the presence of baclofen or GABA to elicit 15 spikes over 4 s. Because GABA and baclofen increase B8 excitability, injecting the same amount of current as done for the control HiDi condition could have caused B8 to spike at a frequency that could not be further enhanced by B40. Thus, an inability of B40 to enhance B8 excitability could be due to a “ceiling effect.” Decreasing the amount of current injected into B8 to elicit 15 spikes over 4 s under all conditions, therefore, eliminated this potential confounding issue.
To induce the persistent changes in B8 excitability by B40, B40 was stimulated 10 times at 10 Hz, for 20 s with a 30-s intertrain interval. To trigger CBI-2 programs, CBI-2 was stimulated at 10 Hz with 15-ms DC pulses for the duration of the protraction phase (Dacks et al. 2012). For experiments in which the baseline activity of B8 in CBI-2 programs was determined, CBI-2 programs were triggered 3 min apart to prevent the induction of CBI-2 priming (Friedman and Weiss 2010), which induces an increase in B8 excitability (Dacks et al. 2012).
RESULTS
The slow excitatory PSP from B40 to B8 is mediated by GABA.
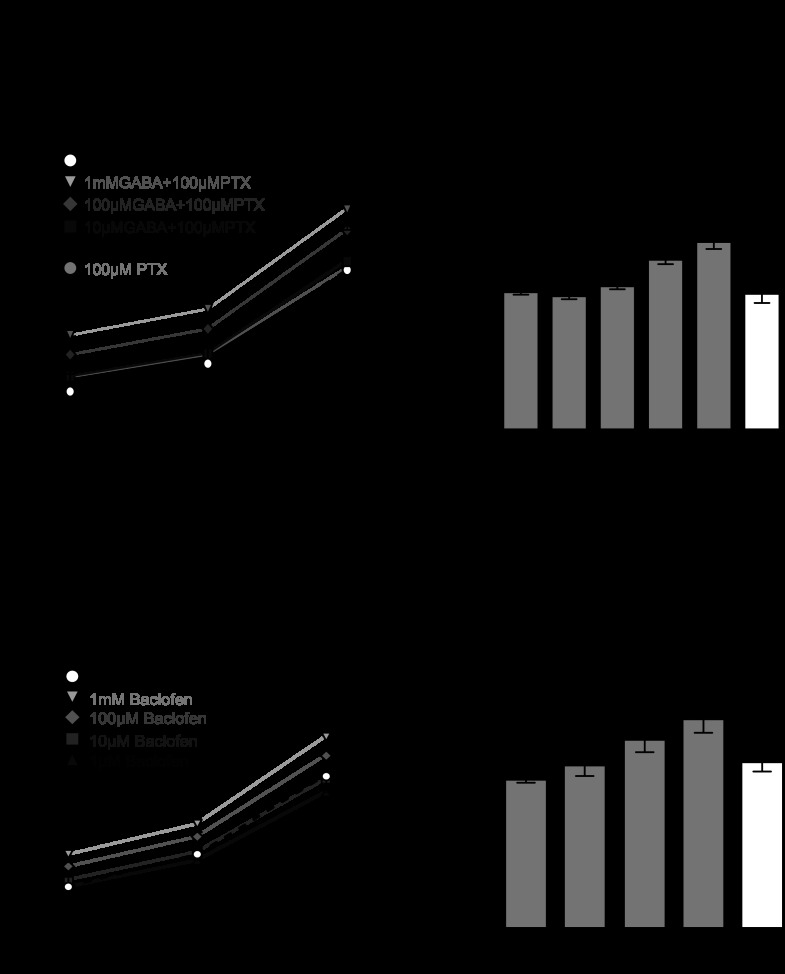
B40 exerts multiple synaptic actions on B8. These manifest themselves on different time scales (Fig. 1). B40 elicits a fast picrotoxin-sensitive IPSP (Jing et al. 2003) and a slow EPSP that is shunted by the fast IPSPs from B40 (Jing and Weiss 2002) and therefore is not expressed until after B40 ceases firing. B40 is GABAergic (Jing et al. 2003), and both GABA and baclofen (a GABA B receptor agonist) depolarize B8 (Diaz-Rios and Miller 2005), making GABA a potential candidate transmitter underlying the slow EPSP from B40 to B8. We therefore began by investigating whether GABA could increase B8 excitability as measured by the number of spikes elicited from B8 in response to injections of constant current pulses. Three levels of current were injected. To avoid the fast inhibitory effects of GABA on B8 (Jing et al. 2003), GABA was applied in the presence of 100 μM picrotoxin, which on its own did not affect B8 excitability (Fig. 2, A and B) relative to baseline. GABA increased the number of spikes elicited from B8 for all levels of current injection tested (Fig. 2, A and B). This is consistent with the finding that GABA produces a conductance decrease in B8 (Diaz-Rios and Miller 2005). A repeated-measures ANOVA revealed that GABA began to significantly increase the number of spikes elicited from B8 at 10 μM [Fig. 2C; F(6,30) = 28.44; P < 0.01; n = 6], and the effects of GABA on B8 excitability washed out. We then tested the effects of baclofen on B8 excitability. Because baclofen does not affect GABA A-receptors, picrotoxin was not included in the superfusate. Similar to GABA, baclofen increased the number of spikes elicited by B8 at all levels of current injection (Fig. 2, D and E). A repeated-measures ANOVA revealed that baclofen began to significantly increase the number of spikes elicited from B8 at 10 μM [Fig. 2F; F(5,25) = 17.6; P < 0.01; n = 6], but took much longer to wash out compared with GABA. Thus, both GABA and baclofen increased B8 excitability.
Fig. 2.
GABA and baclofen increase B8 excitability. A: example recording of GABA increasing the number of spikes elicited by a 4-s constant current pulse to a B8 motor neuron. B: GABA increases the number of spikes elicited from B8 by multiple levels of DC injection (sufficient to elicit 4, 7, and 15 spikes in the initial HiDi control phase) in a dose-dependent manner. 100 μM picrotoxin (PTX) is present in all GABA applications to eliminate any potential effects of GABA A-like receptor activation on B8 excitability. C: GABA causes a significant percent increase in B8 spikes at 10 μM. Percent increase was calculated for the spikes elicited by DC current injection sufficient to elicit 15 spikes from B8 during initial control conditions. D: example recording of baclofen increasing the number of spikes elicited by a 4-s constant current pulse to B8. E: baclofen increases the number of spikes elicited from B8 by multiple levels of DC injection (sufficient to elicit 4, 7, and 15 spikes in the initial HiDi control phase) in a dose-dependent manner. F: baclofen causes a significant percent increase in B8 spikes at 10 μM. Percent increase was calculated for the spikes elicited by DC current injection sufficient to elicit 15 spikes from B8 during initial control conditions. **P < 0.01, ***P < 0.001.
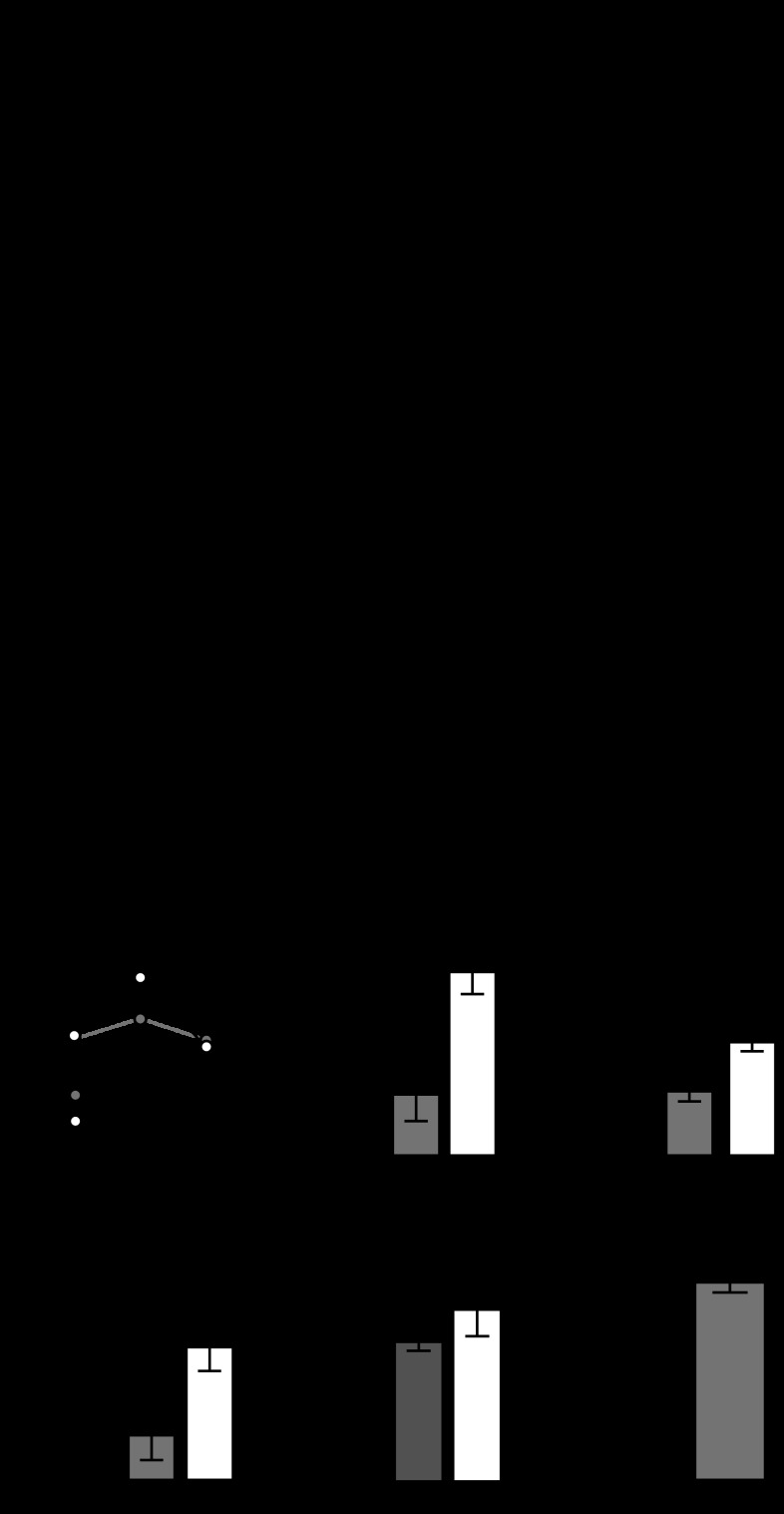
We then investigated whether the excitatory effects of the slow EPSP from B40 to B8 (Fig. 3A) could be mediated by GABA. The slow EPSP from B40 induces a conductance decrease in B8, increasing B8 excitability immediately after the motor program switches from protraction to retraction (Jing and Weiss 2002). We therefore tested if GABA could mediate the increased B8 excitability induced by B40 stimulation. Because we (see materials and methods) and others (Diaz-Rios and Miller 2005) were unable to identify any antagonists capable of blocking the excitatory effects of GABA on B8, we performed occlusion experiments to determine if either GABA or baclofen could prevent further enhancement of B8 excitability by B40. To quantify the excitatory effects of B40 on B8, we injected DC into B8 to establish a baseline level of its excitability in HiDi. We then stimulated B40 immediately before injecting DC into B8 (see materials and methods and Fig. 3B), and, as previously reported (Jing and Weiss 2002), prestimulation of B40 increased the number of B8 spikes elicited by current injections [Fig. 3C; F(8,40) = 19.53; P < 0.001; n = 6]. 1 mM GABA was then superfused onto the preparation, and the amount of current injected into B8 was reduced to elicit the same baseline firing frequency elicited during the initial HiDi superfusion (see materials and methods). The excitatory effect of B40 prestimulation on B8 was eliminated in the presence of 1 mM GABA and 100 μM picrotoxin (Fig. 3, B and C), and a repeated-measures ANOVA revealed that GABA significantly decreased the percent change in spikes elicited after B40 stimulation [Fig. 3D; F(2,10) = 8.705; P < 0.05; n = 6]. 1 mM baclofen also significantly decreased the enhancement of B8 excitability by B40 prestimulation [Fig. 3E; F(8,48) = 44.71; P < 0.001; n = 7]. The GABAergic interneuron B34 also elicits a fast IPSP and slow EPSP in B8, and 1 mM baclofen also occluded the effects of B34 prestimulation on B8 excitability [Fig. 3F; F(2,6) = 208.0; P < 0.001; n = 4]. Picrotoxin did not block the effects of B40 prestimulation on B8 excitability [Fig. 3G; F(2,6) = 0.9499; P = 0.4381; n = 4]. Both GABA and baclofen depolarize B8, causing it to spike spontaneously at ∼1 Hz. Depolarizing B8 to spike at 1 Hz by injecting DC actually increased the excitatory effects of B40 prestimulation (Fig. 3H; paired t-test; t = 3.361; df = 4; P < 0.05; n = 5), excluding the possibility that a depolarization of B8 could prevent the enhancement by B40 prestimulation. These results suggest that GABA mediates both the fast inhibition and slow excitation of B8 by B40.
Fig. 3.
GABA and baclofen occlude the slow excitatory actions of B40 on B8. A: stimulating B40 induces fast IPSPs and a slow EPSP that is unmasked once B40 spiking ceases. B: to quantify the excitatory action of B40 on B8, sufficient DC to elicit 15 spikes in 4 s is injected into B8. B40 is then stimulated at 10 Hz for 3 s immediately before DC injection to B8, which, in HiDi (Control), consistently resulted in a 20% increase in the number of spikes elicited. When this protocol is repeated in the presence of 1 mM GABA and 100 μM picrotoxin (GABA + PTX), B40 prestimulation does not increase the number of spikes elicited by B8. C: GABA with 100 μM picrotoxin occluded the increase in the number of spikes elicited from B8 with B40 prestimulation. D: GABA significantly decreased the percent increase in B8 spikes elicited by B40. Baclofen (Bac.) occludes the percent increase in B8 spikes induced by E B40 and F B34 prestimulation. G: 100 μM picrotoxin did not affect the percent increase in B8 spikes induced by B40 prestimulation. H: depolarizing B8 to spike at 1 Hz increased the percent enhancement of B8 spiking induced by B40 prestimulation. *P < 0.05, ***P < 0.001.
B40 induces a persistent increase of B8 excitability.
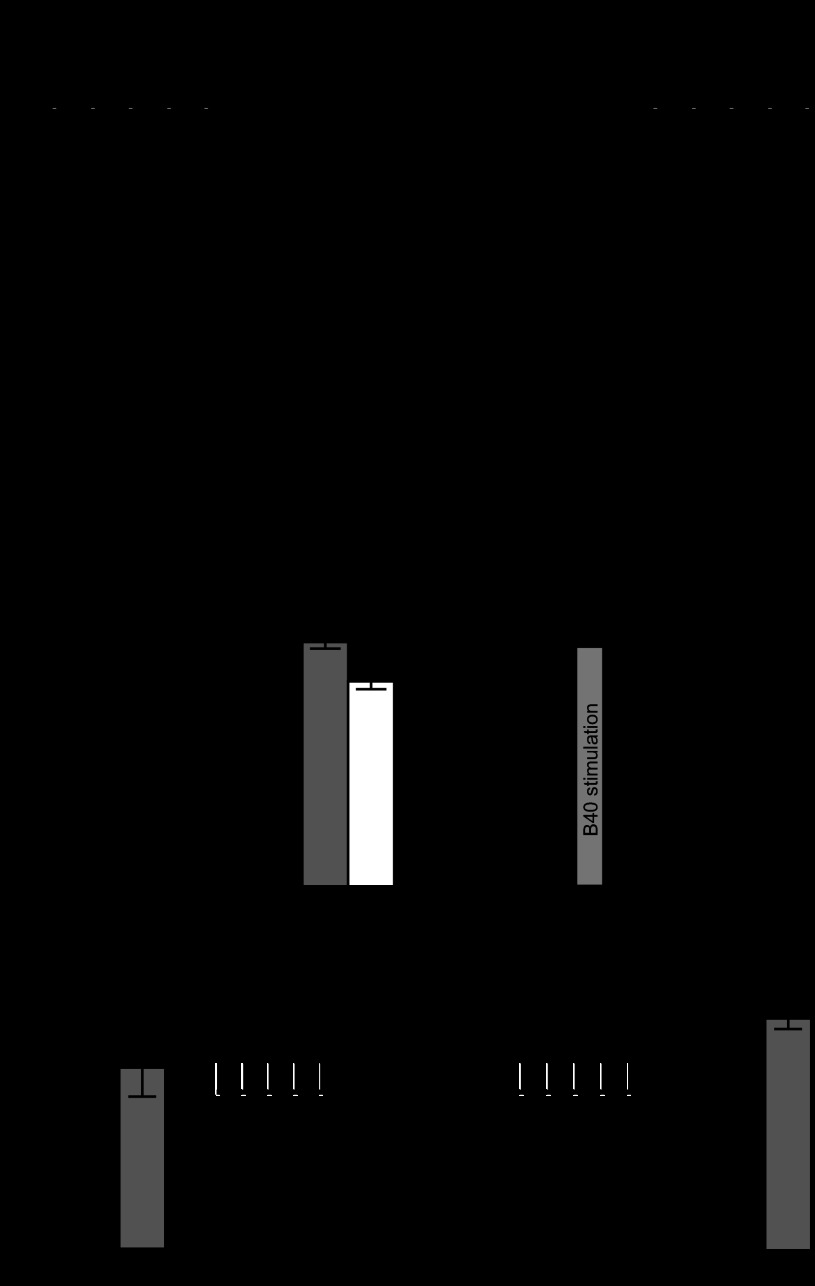
During CBI-2 motor programs, protraction duration (and therefore the duration of B40 activity) is ∼20 s (Dacks et al. 2012; Jing et al. 2003, 2004, 2007; Jing and Weiss 2002; Proekt et al. 2007). Because the slow excitatory effect of B40 develops over a relatively long time scale, we investigated whether repeated, long-duration B40 stimulation could have any persistent effects on B8 excitability. We elicited B40 action potentials in HiDi at frequencies and for durations (Fig. 4A; see materials and methods) similar to those occurring during buccal motor programs elicited by CBI-2 (Dacks et al. 2012; Jing and Weiss 2002). Figure 4B depicts the spiking activity of B8 after each successive B40 spike train which elicited an increasing number of B8 spikes. After 10 trains of B40 stimulation, B8 excitability increased significantly [Fig. 4, C and D; 1-way ANOVA; F(2,26) = 49.77; P < 0.001; n = 14], and this enhancement persisted for ∼5–10 min (Fig. 4E). B40 stimulation also induced a depolarization of B8 resting membrane potential (Fig. 4F; Wilcoxon signed rank test; P < 0.001; n = 23). Ten 3-s trains of B40 spiking elicited with the same intertrain interval as above (Fig. 4G) had no effect on B8 excitability (Fig. 4H; paired t-test; t = 1.662; df = 9; P = 0.1309), suggesting that the persistent effects on B8 excitability require more B40 activity compared with either the fast IPSP induced by B40 (which requires only a single spike) or the slow EPSP induced by B40 (which can be induced by a 3-s train of B40 spiking).
Fig. 4.
Repeated B40 stimulation induces a persistent increase of B8 excitability. A: B40 stimulation protocol sufficient to induce the persistent increase in B8 excitability. B8 excitability is probed with five 4-s DC constant current pulses (depicted as square wave pulses) to B8 separated by 1 min. B40 is spiked at 10 Hz for 20 s for a total of 10 trains (represented as black squares) each separated by 30 s. B8 excitability is then probed with the same level of DC injection. B: example trace showing the number of spikes elicited from B8 by the slow EPSP evoked by B40 increases over the course of the B40 stimulation protocol. C: example recording of the spiking activity of B8 elicited by a 4-s constant current pulse before (Pre) and after (Post) B40 stimulation. D: the number of spikes elicited by a DC constant current pulse to B8 increases post-B40 stimulation and recovers. E: a timeline of the number of spikes elicited from B8 after B40 stimulation shows that the increase in B8 excitability recovers within 5–10 min. F: the resting membrane potential (RMP) of B8 becomes depolarized after the induction of the increase in B8 excitability by B40. G: B40 stimulation protocol sufficient to trigger the slow EPSP in B8 but insufficient to induce the persistent increase in B8 excitability. B8 excitability is probed with five 4-s DC constant current pulses (depicted as square wave pulses) to B8 separated by 1 min. B40 is spiked at 10 Hz for 3 s for a total of 10 trains each separated by 30 s. H: repeated 3-s B40 stimulation trains are not sufficient to induce a persistent increase in B8 excitability. ***P < 0.001.
We next investigated whether the persistent increase in B8 excitability induced by B40 could be occluded by baclofen. Because of the difficulty in holding B40 for a sufficient period of time to wash out baclofen, we twice induced the persistent increase in B8 excitability in HiDi (and allowed the preparation to recover back to baseline) to demonstrate that the persistent increase can be repeatedly induced (Fig. 5A). The first [1-way ANOVA; F(2,9) = 20.35; P < 0.001; n = 4] and second [1-way ANOVA; F(2,9) = 24.05; P < 0.001; n = 4] rounds of B40 stimulation both induced a persistent increase in B8 excitability (Fig. 5B), and the levels of increase were not significantly different from each other. However, B40 stimulation in the presence of baclofen did not increase B8 excitability [Fig. 5B; 1-way ANOVA; F(2,9) = 0.211; P = 0.82; n = 4] suggesting that in addition to the fast IPSP and the slow EPSP, the persistent effects of B40 on B8 excitability are also mediated by GABA.
Fig. 5.
The persistent increase in B8 excitability induced by B40 is occluded in baclofen. A: experimental protocol for the occlusion experiment. B8 excitability was probed (Pre bracket); then B40 was spiked as described in Fig. 4A, and B8 excitability was measured immediately afterwards (Post bracket) until it recovered to baseline (Rcv bracket). Because B40 recordings were typically too unstable to allow for the washout of baclofen, the persistent increase in B8 excitability was induced twice by B40 under control conditions and a third time in the presence of 1 mM baclofen. B: baclofen occludes the persistent effects of B40 on B8 excitability. Bar graph depicts number of spikes elicited before (Pre), immediately after (Post), and 45 min after B40 stimulation (Rcv) for first B40-induced increase in B8 excitability (1st Control; black), the second induction (2nd Control; gray), and in 1 mM baclofen (white). ***P < 0.001.
The persistent effects of B40 on B8 excitability can alter motor output.
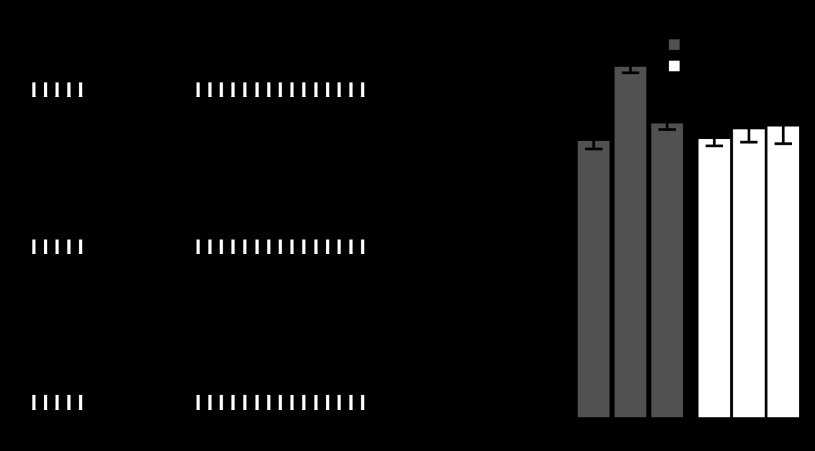
We next tested if the persistent effects of B40 on B8 excitability could have any consequences for activity of B8 during motor programs. We therefore tested whether the B40-induced persistent increase in B8 excitability altered the activity of B8 in CBI-2-triggered motor programs (Fig. 6A). In six experiments, B8 retraction firing rate (averaged across 3 CBI-2 programs separated by 3 min) was initially low, making programs intermediate in nature. After repeated B40 stimulation, B8 retraction firing rate increased, making the programs more ingestive (Fig. 6B). A paired t-test of the average B8 retraction firing rate showed a significant difference between CBI-2 programs before and after B40 stimulation (Fig. 6C; t = 6.037; df = 5; P < 0.01). We next tested if baclofen could bias the nature of CBI-2 programs. We therefore elicited three CBI-2 programs before, three during, and three after superfusion of 10 μM baclofen in ASW with each CBI-2 program separated by 3 min (Fig. 6D). Baclofen caused B8 retraction firing rate to increase significantly from ∼4 Hz to 6 Hz [Fig. 6E; 1-way ANOVA; F(2,18) = 9.796; P < 0.01; n = 7 B8s from 4 animals] with no effect on B8 protraction firing rate [F(2,18) = 1.385; P = 0.27; n = 7 B8s from 4 animals]. A study on the contribution of different motor neurons to the opening and closing of the radula found that a B8 firing rate of 6 Hz was sufficient to achieve functional closure in 67% of animals tested (Friedman et al. 2009). Furthermore, based on this study, the protraction firing rate achieved by B8 both before and after B40 stimulation (∼3 Hz) would not have been sufficient to close the radula. It should be noted, however, that our experiments only indirectly suggest that the B40-induced increase in B8 excitability increases B8 retraction firing rate during CBI-2 programs. Unfortunately, attempts to occlude the effects of B40 on B8 activity in CBI-2 programs using baclofen were unsuccessful. The concentration of baclofen required to occlude the effects of B40 in HiDi (1 mM; Figs. 3 and 5) was such that in ASW it induced a large amount of spontaneous activity from the buccal network and large amounts of movement from the sheath, making the intracellular recordings highly unstable. Despite this, our data suggest that B40 is capable of biasing B8 motor output towards ingestiveness on three different time scales using a single neurotransmitter.
Fig. 6.
B40 and baclofen can bias B8 output in motor programs. A: experimental protocol for testing the persistent effects of B40 on B8 activity in CBI-2 motor programs. Three CBI-2 programs are triggered with a 3-min interprogram interval (Pre B40). B40 is stimulated as described in Fig. 4A, and 3 CBI-2 programs are triggered with a 3-min interprogram interval (Post B40). B: example recording from B8 and B40 during CBI-2 programs before (Pre B40) and after (Post B40) repeated B40 stimulation. Protraction and retraction phases are indicated by the white and black bars, respectively. C: repeated B40 stimulation significantly increases B8 retraction firing rate in CBI-2 programs without affecting B8 protraction firing rate. D: example traces of B8 activity in CBI-2 motor programs triggered before (Control), during (Baclofen), and after (Wash) superfusion of 10 μM baclofen. E: B8 retraction firing rate in CBI-2 motor programs was significantly higher during 10 μM baclofen superfusion (gray circle) relative to control (black circle) and wash (white circle) treatments. **P < 0.01.
DISCUSSION
In this study we took advantage of the synaptic connections between two identified neurons (the interneuron B40 and the motor neuron B8) in the buccal ganglion of Aplysia to study the influence of the distinct components of neurotransmitter action for motor output. Interneuron B40 plays an important role in generating ingestive motor programs. Specifically, B40 activity in protraction controls the firing of motor neuron B8 so that it fires weakly during protraction and strongly during retraction. These effects of B40 on B8 are implemented through fast phasic IPSPs that are elicited during protraction and through a slow EPSP that manifests itself during retraction (Jing and Weiss 2002). In this study we describe yet another effect of B40 on B8: a persistent increase of B8 excitability that is induced by repeated trains of B40 spikes. This increase also biased B8 activity in motor programs to become more ingestive. Previous work provided evidence that B40 contains GABA and that the fast IPSPs are likely GABAergic (Jing et al. 2003). In this study, we obtained evidence consistent with the idea that the other two slower components are also mediated by GABA. Together with previous work, our data suggest that a single transmitter released from one neuron may produce a multi-component response with different signs and time courses, all of which can act together to promote generation of ingestive motor programs.
The diverse effects of neurotransmitters can have important consequences for the generation of a behavior. For instance, in the nudibranch Tritonia, the C2 interneuron is critical for the generation of rhythmic activity produced by the swim CPG, yet expresses significant spike frequency adaptation (Katz and Frost 1997). Because serotonin released by the dorsal swim interneuron has both fast synaptic and slow modulatory effects on C2, as well as other downstream neurons (Katz and Frost 1995a, b; Katz et al. 1994), the ability of the nervous system to repeatedly generate functional motor output is preserved (Katz and Frost 1997). These differences in time scale can also contribute to the specification of the phasing of neural activity. For instance, the MCN1 neuron in the stomatogastric nervous system of the crab generates gastric mill rhythms in part by exciting neurons active in both phases of the rhythm by eliciting fast GABA-mediated excitation of retraction phase neurons and a slow neuropeptide-mediated excitation of protraction phase neurons (Coleman et al. 1995; Stein et al. 2007; Wood et al. 2000). Thus, even though MCN1 is only active during retraction phase, cotransmission of a fast transmitter and a neuropeptide allows the consequences of MCN1 activity to be distributed over multiple phases. Our data suggest that in Aplysia, B40 controls the phasing of B8 motor output via the different components of the postsynaptic response elicited by GABA. During protraction, B40 shunts excitatory drive to B8 by eliciting increased conductance, one-for-one fast IPSPs (Jing and Weiss 2002; Sasaki et al. 2009). Concurrently, B40 elicits a slow EPSP that is not manifested until after B40 spiking ceases at the end of protraction (Jing and Weiss 2002). Thus, despite only being active in one phase of the motor program, B40 uses GABA to bias B8 spiking activity towards the subsequent phase by simultaneously inhibiting and enhancing B8 activity on different time scales.
Under specific conditions, the effects of a given transmitter can have relatively long lasting effects. These residual alterations of the biophysical properties of a neuron can influence the output of that neuron in responses subsequent to those that initially induced the persistent change. An obvious such example is the immediate excitatory effect of glutamate on hippocampal neurons via AMPA receptors and the persistent effects of glutamate on neuronal excitability via NMDA receptors after the induction of LTP (Malenka and Nicoll 1999). Furthermore, the persistence of the distinct effects of a single transmitter often increase in a concentration-dependent manner, such as with exogenous serotonin in Aplysia (Mauelshagen et al. 1998; Muller and Carew 1998; Philips et al. 2011), or glutamate during the induction of LTP (Bear and Malenka 1994). In this study, we observed that B40 could persistently increase B8 excitability if B40 is driven to spike at frequencies and durations observed during repeated CBI-2 motor programs (Dacks et al. 2012; Jing et al. 2004; Jing and Weiss 2002; Proekt et al. 2007), although B40 does not mediate the increase in B8 excitability associated with CBI-2 priming (Dacks et al. 2012). Similar to the slow EPSP elicited by B40 (Jing and Weiss 2002), the persistent increase in B8 excitability may also be due to a decrease in B8 conductance, as has been demonstrated for the increase in B8 excitability that is induced by CBI-2 (Dacks and Weiss 2013), which is cholinergic (Hurwitz et al. 2003). B40 is activated by other inputs and could in principle influence B8 excitability in these contexts. This persistent increase in B8 excitability was occluded by baclofen, suggesting that B40 relies on the different time scales of GABA actions to bias the nature of motor output in an activity-dependent manner. While B8 retraction firing rate increased in CBI-2 motor programs post-B40 stimulation, B8 protraction firing rate did not change. The lack of effect of B40 stimulation of B8 protraction firing rate was likely due to the potent shunting inhibition exerted by B40 during protraction (Jing et al. 2003; Jing and Weiss 2002). This shunting inhibition induces a high conductance state (Sasaki et al. 2009), and thus an increase in B8 excitability would likely have negligible effect on B8 protraction firing rate. The distinct effects of B40 on B8 all combine to coherently bias B8 motor output towards ingestiveness and do so both within individual programs, by increasing the “contrast” between B8 firing rates in protraction and retraction, and across programs, by increasing B8 excitability.
GRANTS
This research was funded by National Institute of Neurological Disorders and Stroke Grants NS-066587 and NS-070583 to K. R. Weiss.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.M.D. and K.R.W. conception and design of research; A.M.D. performed experiments; A.M.D. analyzed data; A.M.D. and K.R.W. interpreted results of experiments; A.M.D. prepared figures; A.M.D. and K.R.W. drafted manuscript; A.M.D. and K.R.W. edited and revised manuscript; A.M.D. and K.R.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. E. Cropper, Dr. J. Jing, Dr. B. Ludwar, M. Perkins, and M. Siniscalchi for technical assistance and helpful discussions.
REFERENCES
- Araneda R, Andrade R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine 1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience 40: 399–412, 1991 [DOI] [PubMed] [Google Scholar]
- Barreiro-Iglesias A, Cornide-Petronio ME, Anadon R, Rodicio MC. Serotonin and GABA are colocalized in restricted groups of neurons in the larval sea lamprey brain: insights into the early evolution of neurotransmitter colocalization in vertebrates. J Anat 215: 435–443, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol 4: 389–399, 1994 [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Somogyi P. Enrichment of cholinergic synaptic terminals on GABAergic neurons and coexistence of immunoreactive GABA and choline acetyltransferase in the same synaptic terminals in the striate cortex of the cat. J Comp Neurol 304: 666–680, 1991 [DOI] [PubMed] [Google Scholar]
- Beique JC, Campbell B, Perring P, Hamblin MW, Walker P, Mladenovic L, Andrade R. Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors. J Neurosci 24: 4807–4817, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beique JC, Imad M, Mladenovic L, Gingrich JA, Andrade R. Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proc Natl Acad Sci USA 104: 9870–9875, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic cotransmission. Exp Physiol 94: 20–24, 2009 [DOI] [PubMed] [Google Scholar]
- Coleman MJ, Meyrand P, Nusbaum MP. A switch between two modes of synaptic transmission mediated by presynaptic inhibition. Nature 378: 502–505, 1995 [DOI] [PubMed] [Google Scholar]
- Dacks AM, Siniscalchi MJ, Weiss K. Removal of default-state associated inhibition during repetition priming improves response articulation. J Neurosci 32: 17740–17752, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacks AM, Weiss K. Latent modulation: a basis for non-disruptive promotion of two incompatible behaviors by a single network state. J Neurosci 33: 3786–3798, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Rios M, Miller MW. Rapid dopaminergic signaling by interneurons that contain markers for catecholamines and GABA in the feeding circuitry of Aplysia. J Neurophysiol 93: 2142–2156, 2005 [DOI] [PubMed] [Google Scholar]
- Diaz-Rios M, Oyola E, Miller MW. Colocalization of gamma-aminobutyric acid-like immunoreactivity and catecholamines in the feeding network of Aplysia californica. J Comp Neurol 445: 29–46, 2002 [DOI] [PubMed] [Google Scholar]
- Friedman AK, Weiss KR. Repetition priming of motoneuronal activity in a small motor network: intercellular and intracellular signaling. J Neurosci 30: 8906–8919, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman AK, Zhurov Y, Ludwar B, Weiss KR. Motor outputs in a multitasking network: relative contributions of inputs and experience-dependent network states. J Neurophysiol 102: 3711–3727, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner D, Kandel ER. Physiological and kinetic properties of cholinergic receptors activated by multiaction interneurons in buccal ganglia of Aplysia. J Neurophysiol 40: 333–348, 1977 [DOI] [PubMed] [Google Scholar]
- Gorczyca MG, Budnik V, White K, Wu CF. Dual muscarinic and nicotinic action on a motor program in Drosophila. J Neurobiol 22: 391–404, 1991 [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Megias M, Emri Z, Freund TF. Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J Neurosci 19: 10082–10097, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G, Mills H, Wragg R, Hapiak V, Castelletto M, Korchnak A, Komuniecki RW. The monoaminergic modulation of sensory-mediated aversive responses in Caenorhabditis elegans requires glutamatergic/peptidergic cotransmission. J Neurosci 30: 7889–7899, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz I, Kupfermann I, Susswein AJ. Different roles of neurons B63 and B34 that are active during the protraction phase of buccal motor programs in Aplysia californica. J Neurophysiol 78: 1305–1319, 1997 [DOI] [PubMed] [Google Scholar]
- Hurwitz I, Kupfermann I, Weiss KR. Fast synaptic connections from CBIs to pattern-generating neurons in Aplysia: initiation and modification of motor programs. J Neurophysiol 89: 2120–2136, 2003 [DOI] [PubMed] [Google Scholar]
- Hurwitz I, Neustadter D, Morton DW, Chiel HJ, Susswein AJ. Activity patterns of the B31/B32 pattern initiators innervating the I2 muscle of the buccal mass during normal feeding movements in Aplysia californica. J Neurophysiol 75: 1309–1326, 1996 [DOI] [PubMed] [Google Scholar]
- Hurwitz I, Perrins R, Xin Y, Weiss KR, Kupfermann I. C-PR neuron of Aplysia has differential effects on “Feeding” cerebral interneurons, including myomodulin-positive CBI-12. J Neurophysiol 81: 521–534, 1999 [DOI] [PubMed] [Google Scholar]
- Hurwitz I, Susswein AJ. B64, a newly identified central pattern generator element producing a phase switch from protraction to retraction in buccal motor programs of Aplysia californica. J Neurophysiol 75: 1327–1344, 1996 [DOI] [PubMed] [Google Scholar]
- Jing J, Cropper EC, Hurwitz I, Weiss KR. The construction of movement with behavior-specific and behavior-independent modules. J Neurosci 24: 6315–6325, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Vilim FS, Horn CC, Alexeeva V, Hatcher NG, Sasaki K, Yashina I, Zhurov Y, Kupfermann I, Sweedler JV, Weiss KR. From hunger to satiety: reconfiguration of a feeding network by Aplysia neuropeptide Y. J Neurosci 27: 3490–3502, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Vilim FS, Wu JS, Park JH, Weiss KR. Concerted GABAergic actions of Aplysia feeding interneurons in motor program specification. J Neurosci 23: 5283–5294, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Weiss KR. Generation of variants of a motor act in a modular and hierarchical motor network. Curr Biol 15: 1712–1721, 2005 [DOI] [PubMed] [Google Scholar]
- Jing J, Weiss KR. Interneuronal basis of the generation of related but distinct motor programs in Aplysia: implications for current neuronal models of vertebrate intralimb coordination. J Neurosci 22: 6228–6238, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Weiss KR. Neural mechanisms of motor program switching in Aplysia. J Neurosci 21: 7349–7362, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz PS, Frost WN. Intrinsic neuromodulation in the Tritonia swim CPG: serotonin mediates both neuromodulation and neurotransmission by the dorsal swim interneurons. J Neurophysiol 74: 2281–2294, 1995a [DOI] [PubMed] [Google Scholar]
- Katz PS, Frost WN. Intrinsic neuromodulation in the Tritonia swim CPG: the serotonergic dorsal swim interneurons act presynaptically to enhance transmitter release from interneuron C2. J Neurosci 15: 6035–6045, 1995b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz PS, Frost WN. Removal of spike frequency adaptation via neuromodulation intrinsic to the Tritonia escape swim central pattern generator. J Neurosci 17: 7703–7713, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz PS, Getting PA, Frost WN. Dynamic neuromodulation of synaptic strength intrinsic to a central pattern generator circuit. Nature 367: 729–731, 1994 [DOI] [PubMed] [Google Scholar]
- Katz PS, Harris-Warrick RM. Serotonergic/cholinergic muscle receptor cells in the crab stomatogastric nervous system. II. Rapid nicotinic and prolonged modulatory effects on neurons in the stomatogastric ganglion. J Neurophysiol 62: 571–581, 1989 [DOI] [PubMed] [Google Scholar]
- Kupfermann I. Feeding behavior in Aplysia: a simple system for the study of motivation. Behav Biol 10: 1–26, 1974 [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation–a decade of progress? Science 285: 1870–1874, 1999 [DOI] [PubMed] [Google Scholar]
- Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci 7: 563–574, 2006 [DOI] [PubMed] [Google Scholar]
- Mauelshagen J, Sherff CM, Carew TJ. Differential induction of long-term synaptic facilitation by spaced and massed applications of serotonin at sensory neuron synapses of Aplysia californica. Learn Mem 5: 246–256, 1998 [PMC free article] [PubMed] [Google Scholar]
- Megias M, Emri Z, Freund TF, Gulyas AI. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience 102: 527–540, 2001 [DOI] [PubMed] [Google Scholar]
- Morgan PT, Perrins R, Lloyd PE, Weiss KR. Intrinsic and extrinsic modulation of a single central pattern generating circuit. J Neurophysiol 84: 1186–1193, 2000 [DOI] [PubMed] [Google Scholar]
- Morton DW, Chiel HJ. In vivo buccal nerve activity that distinguishes ingestion from rejection can be used to predict behavioral transitions in Aplysia. J Comp Physiol A 172: 17–32, 1993a [DOI] [PubMed] [Google Scholar]
- Morton DW, Chiel HJ. The timing of activity in motor neurons that produce radula movements distinguishes ingestion from rejection in Aplysia. J Comp Physiol A 173: 519–536, 1993b [DOI] [PubMed] [Google Scholar]
- Muller U, Carew TJ. Serotonin induces temporally and mechanistically distinct phases of persistent PKA activity in Aplysia sensory neurons. Neuron 21: 1423–1434, 1998 [DOI] [PubMed] [Google Scholar]
- Nargeot R, Baxter DA, Byrne JH. In vitro analog of operant conditioning in aplysia. I. Contingent reinforcement modifies the functional dynamics of an identified neuron. J Neurosci 19: 2247–2260, 1999a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargeot R, Baxter DA, Byrne JH. In vitro analog of operant conditioning in aplysia. II. Modifications of the functional dynamics of an identified neuron contribute to motor pattern selection. J Neurosci 19: 2261–2272, 1999b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum MP, Blitz DM, Swensen AM, Wood D, Marder E. The roles of co-transmission in neural network modulation. Trends Neurosci 24: 146–154, 2001 [DOI] [PubMed] [Google Scholar]
- Philips GT, Sherff CM, Menges SA, Carew TJ. The tail-elicited tail withdrawal reflex of Aplysia is mediated centrally at tail sensory-motor synapses and exhibits sensitization across multiple temporal domains. Learn Mem 18: 272–282, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proekt A, Brezina V, Weiss KR. Dynamical basis of intentions and expectations in a simple neuronal network. Proc Natl Acad Sci USA 101: 9447–9452, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proekt A, Jing J, Weiss KR. Multiple contributions of an input-representing neuron to the dynamics of the aplysia feeding network. J Neurophysiol 97: 3046–3056, 2007 [DOI] [PubMed] [Google Scholar]
- Rosen SC, Teyke T, Miller MW, Weiss KR, Kupfermann I. Identification and characterization of cerebral-to-buccal interneurons implicated in the control of motor programs associated with feeding in Aplysia. J Neurosci 11: 3630–3655, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Brezina V, Weiss KR, Jing J. Distinct inhibitory neurons exert temporally specific control over activity of a motoneuron receiving concurrent excitation and inhibition. J Neurosci 29: 11732–11744, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein W, DeLong ND, Wood DE, Nusbaum MP. Divergent co-transmitter actions underlie motor pattern activation by a modulatory projection neuron. Eur J Neurosci 26: 1148–1165, 2007 [DOI] [PubMed] [Google Scholar]
- Stevens CF. Neuronal diversity: too many cell types for comfort? Curr Biol 8: R708–R710, 1998 [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell 135: 422–435, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilim FS, Cropper EC, Price DA, Kupfermann I, Weiss KR. Peptide cotransmitter release from motorneuron B16 in aplysia californica: costorage, corelease, and functional implications. J Neurosci 20: 2036–2042, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilim FS, Cropper EC, Price DA, Kupfermann I, Weiss KR. Release of peptide cotransmitters in Aplysia: regulation and functional implications. J Neurosci 16: 8105–8114, 1996a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilim FS, Price DA, Lesser W, Kupfermann I, Weiss KR. Costorage and corelease of modulatory peptide cotransmitters with partially antagonistic actions on the accessory radula closer muscle of Aplysia californica. J Neurosci 16: 8092–8104, 1996b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos C, Beique JC, Gingrich JA, Andrade R. Serotonergic regulation of calcium-activated potassium currents in rodent prefrontal cortex. Eur J Neurosci 22: 1120–1126, 2005 [DOI] [PubMed] [Google Scholar]
- Wachtel H, Kandel ER. A direct synaptic connection mediating both excitation and inhibition. Science 158: 1206–1208, 1967 [DOI] [PubMed] [Google Scholar]
- Weiss KR, Brezina V, Cropper EC, Hooper SL, Miller MW, Probst WC, Vilim FS, Kupfermann I. Peptidergic co-transmission in Aplysia: functional implications for rhythmic behaviors. Experientia 48: 456–463, 1992 [DOI] [PubMed] [Google Scholar]
- Wood DE, Stein W, Nusbaum MP. Projection neurons with shared cotransmitters elicit different motor patterns from the same neural circuit. J Neurosci 20: 8943–8953, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]