Abstract
Slow afterhyperpolarizations (sAHPs) play an important role in establishing the firing pattern of neurons that in turn influence network activity. sAHPs are mediated by calcium-activated potassium channels. However, the molecular identity of these channels and the mechanism linking calcium entry to their activation are still unknown. Here we present several lines of evidence suggesting that the sAHPs in developing starburst amacrine cells (SACs) are mediated by two-pore potassium channels. First, we use whole cell and perforated patch voltage clamp recordings to characterize the sAHP conductance under different pharmacological conditions. We find that this conductance was calcium dependent, reversed at EK, blocked by barium, insensitive to apamin and TEA, and activated by arachidonic acid. In addition, pharmacological inhibition of calcium-activated phosphodiesterase reduced the sAHP. Second, we performed gene profiling on isolated SACs and found that they showed strong preferential expression of the two-pore channel gene kcnk2 that encodes TREK1. Third, we demonstrated that TREK1 knockout animals exhibited an altered frequency of retinal waves, a frequency that is set by the sAHPs in SACs. With these results, we propose a model in which depolarization-induced decreases in cAMP lead to disinhibition of the two-pore potassium channels and in which the kinetics of this biochemical pathway dictate the slow activation and deactivation of the sAHP conductance. Our model offers a novel pathway for the activation of a conductance that is physiologically important.
Keywords: retina, retinal waves, calcium imaging, multielectrode array recording, perforated patch, gene profiling
several types of neurons throughout the central and peripheral nervous system exhibit prolonged hyperpolarizations following bursts of action potentials (Hirst et al. 1985; Lancaster and Nicoll 1987; Schwindt et al. 1988). These slow afterhyperpolarizations (sAHPs) underlie oscillatory burst firing in cholinergic striatal neurons and gonadotropin-releasing hormone neurons (Goldberg et al. 2009; Lee et al. 2010), and they give rise to spike frequency adaptation in the principal cells of the cortex, hippocampus, and amygdala (Faber and Sah 2002; Lancaster and Adams 1986; Lorenzon and Foehring 1992). During development, retinal interneurons called starburst amacrine cells (SACs) exhibit sAHPs (Ford et al. 2012; Zheng et al. 2006). These sAHPs play an important functional role within the developing retina by setting the frequency of spontaneous retinal waves, which play an instructive role in the eye-specific and retinotopic organization of retinofugal projections (Ford et al. 2012; Godfrey and Swindale 2007; Hennig et al. 2009; Zheng et al. 2006).
Despite the prevalence and functional importance of sAHPs, the channels that give rise to them are unknown. It is generally accepted that sAHPs are mediated by potassium channels that are activated upon calcium influx through a variety of voltage-gated calcium channels (Sah and Faber 2002). No specific antagonists of sAHPs have been found, but several intracellular signaling pathways modulate the sAHP, including PKA, PKC, and PIP2 pathways (Lancaster et al. 2006; Lancaster and Nicoll 1987; Sah and Isaacson 1995; Villalobos et al. 2011; Vogalis et al. 2002). The slow kinetics of the sAHP have led to speculation that sAHP channels are not directly activated by calcium entry, but rather are indirectly opened by a signaling cascade, possibly involving phosphorylation of the channel (Abel et al. 2004). Implicated in this activation are the calcium-activated phosphatase calcineurin (Vogalis et al. 2004) and the calcium sensors hippocalcin (Tzingounis et al. 2007) and neurocalcin (Villalobos and Andrade 2010). However, the pathway leading from calcium entry to the channel's activation is still unknown.
Two-pore potassium (K2P) channels produce the hyperpolarized resting membrane potential in most neurons (Enyedi and Czirjak 2010), and they exhibit properties that are similar to those of channels underlying sAHPs. K2P channels are insensitive to most potassium channel antagonists but are modulated by second messenger cascades (Mathie 2007). Several K2P channel family members can be distinguished by their current rectification, modulation by protein kinases, and activation by heat, stretch, and lipids (Enyedi and Czirjak 2010; Lesage and Lazdunski 2000). While no family member is directly activated by calcium, their channel openings are gated by activation of calcium-dependent signaling cascades (Czirjak et al. 2004).
Here we combine perforated patch recordings with pharmacology in order to implicate a specific K2P channel, TREK1, in the generation of sAHPs in developing SACs. Second, we perform calcium imaging to investigate how modulating sAHP conductance affects circuit function. Last, we use multielectrode array recordings from mice lacking TREK1 to implicate this channel in the normal patterning of retinal waves.
METHODS
All animal procedures were approved by the University of California, Berkeley, and the Baylor School of Medicine, and they conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals, the Public Health Service Policy, and the Society for Neuroscience Policy on the Use of Animals in Neuroscience Research.
Animals.
All experiments were performed on acutely isolated mouse retinas. Male and female C57Bl/6 mice obtained from Harlan were used for all wild-type (WT) recordings. mGluR2-GFP mice contained a transgene insertion of interleukin-2 receptor-fused GFP under control of the mGluR2 promoter (Watanabe et al. 1998). ChAT-Cre/TdTom mice were generated by crossing a mouse in which an IRES-Cre recombinase was knocked in downstream of the endogenous choline acetyl transferase gene (Ivanova et al. 2010) with a separate tdTomato driver line [B6.129S6-ChATtm1(cre)lowl/J × B6.129S6-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J, Jackson Labs]. TREK1−/− mice (Namiranian et al. 2010) were used for assessing the role of TREK1 (gene name KCNK2) in retinal waves. Age-matched non-littermate C57BL/6 WT mice were used as controls. Genotypes were determined by genomic PCR using primer sequences described previously (Namiranian et al. 2010).
Whole mount retinal preparation.
P4-P7 mice were anesthetized with isoflurane and decapitated. Retinas were isolated in cold artificial cerebrospinal fluid (ACSF) (in mM: 119 NaCl, 26.2 NaHCO3, 11 glucose, 2.5 KCl, 1 K2HPO4, 2.5 CaCl2, 1.3 MgCl2) and mounted ganglion cell side up on filter paper. Retinas were incubated at room temperature in oxygenated ACSF until transfer to the recording chamber, where they were continuously superfused with oxygenated ACSF at 30–34°C.
Electrophysiology.
Perforated patch and whole cell recordings were performed on whole mount retinas from mice aged P4-P7. The inner limiting membrane was removed using a glass recording pipette, and SACs were identified using fluorescence and targeted using a Sutter micromanipulator. Voltage clamp recordings were sampled at 2.5 kHz and filtered at 1 kHz. Current clamp recordings were sampled at 5 kHz and filtered at 2 kHz. Analysis was performed using custom MATLAB (Mathworks) scripts. All reported voltages were corrected for liquid junction potential. Statistical significance was accessed using paired t-tests.
Perforated patch voltage and current clamp recordings were performed in the presence of DHβE (4 μM) and gabazine (5 μM) to block nAChR and GABA-A receptor-mediated synaptic conductances, and in the presence of tolbutamide (100 μM) to block an ATP-sensitive potassium conductance that develops during prolonged recordings (see Fig. 2). During recordings of conductance (Fig. 1E) and current-voltage relationships (Fig. 1, C and D), TTX (200 nM), 4-aminopyridine (4-AP) (1 mM), tetraethylammonium chloride (TEA) (1 mM), and cesium chloride (2 mM) were included in the bath solution to block voltage-activated conductances. A gluconate-based perforated patch internal solution (in mM: 122 K gluconate, 20 HEPES, 0.5 EGTA, 2 NaCl, pH 7.2, liquid junction potential: 14 mV) was frontfilled into electrodes and then backfilled with internal solution containing 750 μg/ml amphotericin B made fresh hourly. Seals were formed and then access resistance was monitored continuously. Recordings were performed when access resistance was stable and less than 5% of the input resistance of the cell (typically, Ra = 30–80 MΩ, Rin = 1–2 GΩ). While access resistance prevented efficient voltage clamp of voltage-gated calcium and potassium currents during depolarizing steps, the small current (∼5 pA) underlying the sAHP is unlikely to induce errors in voltage clamp during measurement of the reversal potential. Furthermore, maximum amplitudes and kinetics of the slow AHP were not correlated with the access resistance.
Fig. 2.

Whole cell recordings from SACs reveal a Katp conductance. A: whole cell voltage clamp recording from SAC. Cell is voltage clamped at −64 mV. Arrows indicate 500-ms voltage step to −14 mV followed by return to −64 mV. Dashed lines indicate changes in holding current at −64 mV in control, tolbutamide (100 μM), and rinse. B: peak amplitude of transient outward current following 500-ms depolarizing voltage step in whole cell configuration in the absence and presence of tolbutamide application (n = 5). C, left: example current clamp perforated patch recordings of sAHPs evoked by 500-ms, 100-pA current injection before and during tolbutamide bath application. C, middle: example voltage clamp perforated patch recording of IsAHP evoked by 500-ms step from −64 mV to −14 mV, before and during tolbutamide bath application. C, right: peak amplitude of IsAHP following 500-ms depolarizing voltage step in perforated patch configuration before and during tolbutamide application (n = 5).
Fig. 1.
Slow afterhyperpolarization (sAHP) in starburst amacrine cells (SACs) is mediated by a two-pore potassium channel. A: current clamp perforated patch recording of SAC shows sAHP evoked by 500-ms 100-pA current injection in presence of 5 µM gabazine and 8 μM dihydro-β-erthryoidine. Inset shows expanded version of initial spike (Scale: 10 mV, 100 ms). B: voltage clamp perforated patch recording of SAC shows IsAHP evoked by 500-ms voltage step to −14 mV from a holding potential of −64 mV. Trace shows average from n = 32 cells with gray indicating ±SD. C, left: voltage protocol used to determine current voltage relationship of IsAHP. Average IsAHP is shown above for reference. A series of 50-ms voltage steps to different holding potentials was given before and at the peak of IsAHP. Scale: 2 pA, 10 s. C, right, inset: example current traces from voltage steps before (1) and after (2) depolarizing step to activate IsAHP. Scale: 10 pA, 10 ms. Graph shows average current voltage relationship at peak of IsAHP. N = 4, means ± SD. D: reversal potential as a function of external potassium concentration. Dots represent individual cells; boxes indicate mean. Line is fit to prediction from Nernst equation for a potassium conductance. E: example conductance (top) and current (bottom) measurements taken from a baseline of −64 mV following a 500-ms voltage step to −14 mV. Conductance was determined from trains of 50-ms, 10-mV hyperpolarizing steps. Inset: voltage protocol used to determine conductance. F, top: average current evoked by 500-ms voltage step to −14 mV, as in B–D, in the presence (black) and absence (gray) of external calcium (n = 4), apamin (1 μM, n = 5), and TEA (1 mM, n = 5). F, bottom: peak amplitude of evoked currents for each cell before (Pre) and after (Post) application. Boxes represent the mean; error bars indicate SE. Scale: 2 pA, 10 s.
Whole cell recordings from SACs were made using potassium phosphate based internal solution (in mM: 110 KH2PO4, 6 MgCl2, 1 EGTA, 4 adenosine 5′-triphosphate magnesium salt, 0.3 guanosine 5′-triphosphate trisodium salt, 10 HEPES, and 10 phosphocreatine disodium salt, pH 7.2, liquid junction potential: 14 mV).
Calcium imaging.
Retinas from mice aged postnatal day (P)2-P6 were bulk loaded with the calcium indicator Oregon Green Bapta-1 AM (OGB-1 AM) using the multicell bolus loading technique (Blankenship et al. 2009; Stosiek et al. 2003). Epifluorescence imaging and analysis were performed as described earlier (Blankenship et al. 2009). Significance was assessed using paired t-tests.
Multi-electrode array recordings.
TREK1−/− and WT mice were dissected and placed ganglion cell side down onto a 60-electrode array (Multi-Channel Systems). The array electrodes were 10 μm in diameter and were arranged in an 8 × 8 grid (minus 4 corners) with 100-μm interelectrode spacing. The retina was held in place on the array with a weighted piece of dialysis membrane and was superfused continuously with oxygenated ACSF. The voltage trace on each electrode was sampled at 20 kHz and stored for offline analysis. The traces were then pass filtered between 120 and 2,000 Hz. Spikes that crossed a threshold of three times the root mean square of the noise on each electrode were sorted according to the two principal components of their voltage waveforms. A valley seeking algorithm was then used to sort spike clusters into individual units. To verify that each unit identified by this algorithm corresponded to a single cell, we inspected units manually. Furthermore, units that lacked a refractory period in their autocorrelation function were considered contaminated by other neurons and were excluded from the analysis. The mean spike rate, r, was calculated by dividing the total number of spikes for each unit by the recording duration. Units whose mean spike rate was <1/10 of the mean firing rate of all cells were excluded from additional analysis to reduce contamination from low spiking cells. After this cut, 20–45 units remained. Using the sorted spikes, waves were defined as events where the average firing rate increased over a selected threshold (values varied from 0.3 to 2.1 SD above the mean) and were separated by more than 10 s. The threshold was adjusted for each retina such that the number of defined waves was consistent with the number of waves identified by eye in the raster plots. Interwave intervals were pooled, and TREK−/− and WT waves were compared using a two-tailed t-test with a 99% confidence interval.
Microarray analysis.
A database of gene expression in 13 retinal neuron subtypes was generated using Affymetrix Mouse Genome 430 2.0 microarrays as described (Kay et al. 2011, 2012). The data was collected at P6, a time of strong retinal wave activity. To determine the expression profile of K2P family channels, we began by identifying Affymetrix probesets corresponding to each of the 13 genes in the mouse Kcnk gene family, which encode the K2P channels (Talley et al. 2003). This was done by searching the Affymetrix NetAffx online database and curating probesets by hand, using the Ensembl mouse genome viewer. To ensure completeness, we identified all probesets present on the 430 2.0 arrays that represent Kcnk family genes; some genes were represented on the array as multiple independent sequences, whereas others were represented by only one sequence. Next we queried our microarray database to determine the expression values of each of the Kcnk gene sequences across the 13 cell types. Probesets that were not expressed in any of the cell types were excluded from further analysis, which left 16 probesets (corresponding to the 13 Kcnk genes). Finally, we used a hierarchical clustering algorithm (dChip microarray data analysis software) to generate a heat map showing expression of these 16 sequences (see Fig. 3B). Sequences that have similar expression patterns are clustered together. Because preliminary analysis suggested that Kcnk2, which encodes TREK1, was specifically expressed in SACs, we included in the clustering analysis two known SAC-specific genes, encoding the vesicular acetylcholine transporter and choline acetyltransferase.
Fig. 3.
IsAHP has the pharmacological characteristics of K2P channels. A, top: peak amplitude of IsAHP before (Pre) and during (Post) bath application of barium (2 mM as BaCl2, n = 7), sanshool extract (0.02%, n = 4), arachidonic acid (10 μM, n = 7), or MMPX (100 μM, n = 8). A, bottom: average current evoked by 500-ms voltage step from −64 mV to −14 mV, in control (black) and during application of each drug (gray). Scale: 2 pA, 10 s. B: expression of the Kcnk gene family, which encodes K2P channels, was assessed in mouse retinal neurons using microarrays. Heatmap shows expression of the Kcnk genes across 13 different retinal neuron subtypes, which include subtypes of retinal ganglion cells (RGCs), amacrine cells (ACs), and bipolar cells (BCs). Color (red, high; blue, low; see bottom for scale) indicates gene expression level in each cell type relative to mean level for that gene. Unsupervised hierarchical clustering was used to determine the x-axis order in which the genes are listed, such that genes with similar expression patterns across the 13 cell types are found next to each other. All 13 members of the Kcnk family are represented at least once (some are represented twice because they appeared twice on the microarray). Expression of Kcnk2, the gene encoding TREK1, is specific to SACs, as shown both by the heatmap and by the fact that it clustered with two known SAC-specific genes, Chat and Vacht.
The Affymetrix probesets used for clustering (and the genes to which they correspond) were: 1455896_a_at (Kcnk1); 1448690_at (Kcnk1); 1449158_at (Kcnk2); 1445929_at (Kcnk2); 1425341_at (Kcnk3); 1421419_at (Kcnk4); 1421852_at (Kcnk5); 1435342_at (Kcnk6); 1425437_a_at (Kcnk7); 1445309_at (Kcnk9); 1431613_a_at (Kcnk10); 1441280_at (Kcnk12); 1447645_x_at (Kcnk13); 1424125_at (Kcnk13); 1447972_at (Kcnk15); 1429913_at (Kcnk16); 1440070_at (Chat, encoding choline acetyltransferase); 1422203_at (Slc18a3, encoding vesicular acetylcholine transporter). Cell type abbreviations in Fig. 3B are described in Kay et al. 2012.
RESULTS
Slow AHPs in SACs exhibit properties similar to those of K2P channels.
Following spontaneous depolarization during waves or evoked depolarization via current injection, SACs in developing mouse retina exhibit sAHPs (Fig. 1A) (Ford et al. 2012; Zheng et al. 2006). To determine the current underlying these sAHPs, we performed perforated patch voltage clamp recordings of SACs. We blocked synaptic input with cholinergic and GABAergic antagonists (4 μM dihydro-β-erthryoidine + 5 μM gabazine) to isolate the cell-intrinsic conductances. At this stage of development, glutamatergic inputs are immature and do not shape spontaneous activity patterns (Bansal et al. 2000). We found that, as previously described, depolarizing steps (of 500-ms duration from −64 mV to −14 mV) evoked a slow outward current at −64 mV (Fig. 1B) (Ford et al. 2012). This current has a slow rise and decay similar to sAHPs measured in current clamp (Fig. 1B). Thus, henceforth we refer to this current as IsAHP.
Several lines of evidence indicate that IsAHP is mediated by potassium channels and that it requires calcium entry for activation. First, channel activation was associated with an increase in conductance (Fig. 1E, n = 3). This implies the opening of channels rather than the activation of a transporter, a potential alternative source of the slow outward current (Pulver and Griffith 2010). Second, IsAHP reversed at the reversal potential for potassium (EK, Fig. 1C). At physiological external potassium concentration (4.5 mM), the current exhibited outward rectification. Third, as external potassium increased, the reversal potential for IsAHP shifted, consistent with the Nernst equation prediction for a potassium conductance (Fig. 1D). Fourth, IsAHP was reversibly blocked when calcium was removed from the bath solution (Fig. 1F, n = 4). Thus, our data indicate that IsAHP is mediated by a calcium-activated potassium channel.
To further characterize this channel, we conducted pharmacological experiments. Since calcium-activated potassium channels include the BK and SK families (Sah 1996), we investigated their involvement. BK channels mediate rapid repolarization during action potentials, while SK channels underlie the medium length afterhyperpolarization that follows individual action potentials. We blocked BK channels (Yellen 1984), as well as other voltage-gated channels, with 1 mM TEA and found that the amplitude of IsAHP was unaffected (Fig. 1F, n = 5). We blocked SK channels with its specific antagonist, apamin (Sah and Faber 2002), and found that the amplitude of IsAHP was again unaffected (Fig. 1F, n = 5). Hence, neither BK nor SK channels contribute to the generation of IsAHP.
We next tested the involvement of ATP-dependent potassium channels (Katp). These channels are activated by depolarization, contribute to a slow afterhyperpolarization in hippocampal pyramidal cells (Tanner et al. 2011), and are thought to be activated by a decrease in the cell's ATP levels. We found these channels in the SACs. After gaining whole cell access to a SAC, we observed a rapidly developing conductance that produced a dramatic drop in input resistance (Fig. 2A). This conductance reversed at EK (data not shown), indicating the opening of potassium channels. Subsequent depolarizing steps activated a transient outward tail current (Fig. 2, A and B). Both this transient tail current and the whole cell-activated conductance were blocked by the Katp channel antagonist tolbutamide (100 μM; Rin for control = 0.33 ± 0.15 GΩ and for tolbutamide = 1.74 ± 0.22 GΩ; P = 0.0004, n = 5, Fig. 2B), indicating that these currents are mediated by Katp channels. To determine if these channels mediate IsAHP, we blocked with tolbutamide while using the perforated patch configuration and found that IsAHP did not change (Fig. 2C, n = 5). Thus, Katp channels in SACs are activated by the reduction of intracellular ATP caused by intracellular dialysis during whole cell recordings. However, when the intracellular milieu is left intact, these channels do not contribute to the sAHPs.
To determine if K2P channels mediate IsAHP, we first approached the family as a whole. The K2P family consists of 13 members, including the TWIK, TASK, TREK, TALK, THIK, and TRESK subfamilies (Talley et al. 2003). These subfamilies are insensitive to several potassium channel antagonists, including TEA, 4-AP, and cesium (Lesage 2003). Consistent with this, a combination of 1 mM TEA, 1 mM 4-AP, and 2 mM cesium did not block the IsAHP in SACs (n = 4, data not shown, see methods). However, IsAHP was blocked by barium (2 mM, n = 7, Fig. 3A), a blocker of the K2P channel subfamilies TASK, TREK, TWIK, and TRESK (Deng et al. 2009). These data, along with our finding that IsAHP is outwardly rectifying (Fig. 1C), suggest that IsAHP in SACs is mediated by one of the K2P subfamilies, which include TREK, TASK, and TRESK channels.
To distinguish between these subfamilies, we first conducted a microarray analysis of mRNA isolated from SACs (Kay et al. 2011, 2012). We found that kcnk2, the mRNA that encodes the K2P channel TREK1, is highly expressed in SACs during the first postnatal week (Fig. 3B). In fact, within the subset of cell types analyzed, it is specifically expressed only in SACs. Moreover, it is expressed with a degree of specificity that is similar to that seen for other markers of SACs, such as choline acetyl transferase and Megf10 (Kay et al. 2012). These results suggest that TREK1 channels likely mediate the slow potassium conductance underlying sAHPs.
We next conducted pharmacological experiments to ask whether other K2P subfamilies might also contribute to the sAHP. TRESK channels as well as TASK-3 and TASK-9 channels are inhibited by extract derived from sanshool chili peppers (Bautista et al. 2008). Bath application of 0.02% sanshool extract did not block IsAHP or increase the input resistance (Fig. 3A, n = 4), indicating that SACs do not express these K2P channel types. TREK and TRAAK channels exhibit potentiated currents with arachidonic acid (AA) (Lesage and Lazdunski 2000). Bath application of 10 μM AA led to a significant increase in the holding current at −60 mV and a corresponding decrease in the input resistance (in control, −1.0 ± 2.5 pA, 1.52 ± 0.24 GΩ; in AA, 10.0 ± 3.6 pA, 1.12 ± 0.23 GΩ; P = 0.0059 for holding current at −64 mV, P = 0.0117 for input resistance, n = 7). Moreover, IsAHP was increased in amplitude (Fig. 3A, n = 7). Thus, our results support the hypothesis that IsAHP in SACs is mediated by TREK, but not TASK or TRESK channels.
However, IsAHP requires calcium influx for activation, while TREK channels are not activated by changes in intracellular calcium (Fink et al. 1996). How might these channels be activated to generate the sAHP in SACs? Previously, we along with others showed that IsAHP is inhibited by the elevation of cAMP with forskolin (Ford et al. 2012; Zheng et al. 2006). Recently, TREK1 was shown to be activated by decreases in cAMP following the activation of metabotropic GABA receptors (Sandoz et al. 2012). Thus, we hypothesize that calcium influx into SACs activates calcium-activated phosphodiesterases (PDEs) leading to a decrease in cAMP level that then activates the TREK1 channels. We tested if PDE-1C, the calcium-activated phosphodiesterase that is expressed in SACs (Santone et al. 2006), plays a role in generating IsAHP. We applied 8-methoxymethyl-3-isobutyl-1-methyl xanthine (MMPX; 100 μM), a specific PDE-1C inhibitor, and found that it inhibited IsAHP (Fig. 3A, n = 8). Although at this concentration, MMPX may inhibit multiple PDEs, it exhibited specificity in pancreatic β-cells (Tian et al. 2012). Also, in a previous study based on imaging of PKA activity, we found that high concentrations of MMPX did not lead to a tonic increase in basal cAMP levels in retinal neurons as observed in the presence of the broad spectrum inhibitor IBMX (Dunn et al. 2009). In addition, these data are consistent with previous studies showing forskolin, an adenylate cyclase activator, decreases IsAHP (Ford et al. 2012; Zheng et al. 2006). Together, our findings indicate that IsAHP may be generated by activation of TREK channels via a calcium-dependent decrease in cAMP.
Changing the kinetics of sAHPs alters the frequency of retinal waves.
To determine the effect of sAHPs on network function in the developing retina, we used calcium imaging to investigate how changing sAHPs altered retinal waves (Ford et al. 2012). The timing of spontaneous retinal waves is critical for normal visual system development. We know that SACs control wave timing, but the mechanism is unclear. The sAHPs are well positioned to regulate the frequency of spontaneous retinal waves by generating a refractory period following the depolarization caused by a wave. If sAHPs really do set wave frequency, then blocking them should increase the frequency of waves. To test this hypothesis, we manipulated sAHPs via the cAMP-PDE pathway described above. We used MMPX to block calcium-dependent PDE activity, increasing cAMP levels and thereby inhibiting sAHPs. As predicted, this increased the frequency of waves, as we have previously shown using the broad spectrum PDE blocker IBMX and direct elevation of cAMP levels using forskolin (Ford et al. 2012) (Fig. 4, A and B, P = 0.001). By contrast, potassium channel antagonists, cesium and tolbutamide, that had no effect on IsAHP, also failed to influence the interwave interval (Fig. 4B, P = 0.42 and P = 0.29 for cesium and tolbutamide, respectively). These results support the notion that sAHP currents in SACs, and the calcium-regulated cAMP pathway that regulates them, are critical for controlling the timing of retinal waves. Thus, sAHPs appear to play an important role in the control of circuit function in the retina.
Fig. 4.
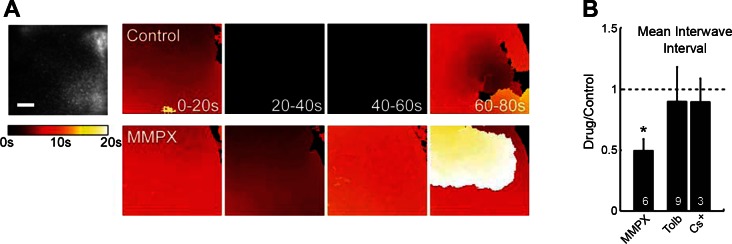
Modulation of sAHP alters the frequency of spontaneous retinal waves. A: calcium imaging of retinal waves. A, left: fluorescence image of a retina that is bolus loaded with OGB-1AM. Scale: 100 μm. A, right: pseudo-colored images show time progression of wave fronts. Each panel represents the activity during 4 consecutive 20-s time periods. Wave fronts are colored according to their location during the 20-s period, with darker colors occurring earlier in the period (see color scale). Black indicates the absence of a wave. Top shows a control retina, and bottom shows the same retina during application of 100 μM MMPX. B: mean interwave interval (relative to control) measured by calcium imaging in the presence of MMPX (100 μM), tolbutamide (100 μM), and cesium (2 mM as CsCl). Number of retinas per manipulation is shown on each bar. Means ± SD. *P = 0.001.
TREK1 knockout mice exhibit altered retinal waves.
Our molecular data suggest that the key channel mediating sAHPs in SACs is TREK1. If so, then loss of TREK1 function should also increase the frequency of waves. We were not able to import mice to use calcium imaging, so to investigate the functional role of TREK1 in retinal waves, we conducted multi-electrode array (MEA) recordings in P1-P3 WT and TREK1−/− mice (Namiranian et al. 2010). MEA recordings and calcium imaging led to the same results in terms of assaying the frequency of waves (Torborg and Feller 2005). Compared with WT animals, TREK1−/− mice exhibited interwave intervals that were approximately one-half the length (WT = 85.23 ± 15.96 s, TREK1−/− = 45.41 ± 15.51 s, t-test P < 0.001, Fig. 5), similar in scale to the effect of inhibiting the sAHP with MMPX (Fig. 4). Thus, TREK1 plays an important role in setting the pace for retinal waves. These data are consistent with the idea that TREK1 is a major contributor to the sAHP in SACs.
Fig. 5.
TREK1−/− mice exhibit more frequent cholinergic retinal waves. A: raster plots of single-unit spike trains over a 20-min period recorded from retinas of P1 WT (top) and TREK1−/− (bottom) mice. B: summary histograms show distribution of interwave intervals from WT (top, n = 4) and TREK1−/− (bottom, n = 4) retinas. C: average interwave intervals for WT (n = 52 waves from 4 retinas) and TREK1−/− (n = 83 waves from 4 retinas) mice. Error bars indicate SD; *P < 0.001.
DISCUSSION
We have demonstrated that sAHPs in developing SACs are mediated by a potassium channel that shows pharmacological and rectification properties consistent with the K2P channel, TREK1. TREK1 is highly and specifically expressed in SACs. The channel underlying sAHPs is inhibited by tonically elevating cAMP and is blocked by preventing the calcium-dependent degradation of cAMP by PDEs, consistent with TREK1. In addition, we have shown that altering sAHPs pharmacologically and in mice lacking TREK1 changes the frequency of retinal waves, indicating a functional role for TREK1 in circuit function. Below, we discuss the role of the K2P channel TREK1 in generating IsAHP, and we propose a model for its activation by the regulation of cAMP levels.
TREK1 as a candidate IsAHP channel.
It has become increasingly evident that K2P channels play a role in generating slow currents. Metabotropic GABAB receptors use cAMP-dependent disinhibition of TREK2 channels to generate second-long hyperpolarizations (Deng et al. 2009; Sandoz et al. 2012). Similarly, serotonin activates TWIK-1 via a decrease in cAMP (Deng et al. 2007) or inhibits TASK-1 channels via a G alpha q pathway (Talley et al. 2000) to alter neuronal excitability. Similarly, metabotropic glutamate receptors activate phospholipase C and inhibit TREK and TASK channels, generating slow depolarizations in neurons (Chemin et al. 2003). Thus, the slow modulation of second messengers, including cAMP and IP3, seems to be read out by K2P channels so they can generate slow hyperpolarizations and depolarizations.
In agreement, our results implicate the K2P channel TREK1 in the generation of IsAHP in retinal SACs using several lines of evidence. First, during development, SACs are enriched for kcnk2 transcripts, the mRNA that encodes the TREK1 channel (Fig. 3B). Second, IsAHP was insensitive to a variety of K-channel antagonists that are also known not to block K2P channels. In particular, TEA (1 mM), 4-AP (1 mM), cesium (2 mM), apamin (1 μM), and tolbutamide (100 μM) do not inhibit IsAHP.(Figs. 1 and 2). Thus, we can rule out the involvement of voltage-gated, A-type, SK, BK, or ATP-dependent potassium channels and Ih. Third, the IsAHP was blocked by 2 mM barium, a blocker of the K2P channel subfamilies TASK, TREK, TWIK, and TRESK (Fig. 3A). Fourth, application of the fatty acid AA increased IsAHP (Fig. 3A), consistent with the observation that some K2P channels are activated by manipulating pressure, pH, and lipids (Enyedi and Czirjak 2010; Lesage and Lazdunski 2000). Fifth, at physiological potassium concentrations, IsAHP was outwardly rectified (Fig. 1), consistent with the outward rectification of K2P channels (Goldstein et al. 2005). Furthermore, this outward rectification excludes the possibility that IsAHP is mediated by inwardly rectified channels that are sensitive to barium, such as Katp. Sixth, IsAHP is inhibited by blockade of the calcium-dependent phosphodiesterase PDE1C (Fig. 3A), consistent with the known inhibition of TREK channels by PKA phosphorylation. Seventh, mice lacking TREK1 display an increase in wave frequency (Fig. 5), consistent with the frequency increase seen with decreased IsAHP (Fig. 4).
A cAMP model for the activation of IsAHP.
A hallmark of both TREK channels and the channels underlying the sAHPs in other neurons is their regulation by intracellular signaling cascades. PKA phosphorylation inhibits TREK channels (Honore et al. 2002) and inhibits the channels underlying the sAHPs in hippocampal pyramidal neurons (Lancaster et al. 2006; Lancaster and Nicoll 1987; Sah and Isaacson 1995). In developing retinal SACs, forskolin, which elevates cAMP levels, inhibits IsAHP (Ford et al. 2012), indicating that IsAHP is sensitive to cAMP levels.
This finding could explain how TREK channels generate sAHPs in SACs even though IsAHP requires calcium influx for activation while TREK channels are not activated by changes in intracellular calcium (Fink et al. 1996). The activation of IsAHP occurs over several seconds, suggesting that channel opening following depolarization is mediated by a signaling cascade, rather than the direct activation of calcium. Calcium influx can activate adenylyl cyclases (ACs) and PDEs and thus can regulate levels of cAMP. Decreases in cAMP have been shown to activate TREK channels with the activation of metabotropic GABA (Deng et al. 2009; Sandoz et al. 2012) and glutamate receptors (Chemin et al. 2003; Lesage et al. 2000). Thus, calcium entry could regulate cAMP levels that would then control the TREK channel that produces IsAHP.
We propose a model for this mechanism, although it is important to note that this study does not specifically address the mechanism underlying calcium activation of the conductance. During depolarization, calcium influx through voltage-gated calcium channels (Zheng et al. 2006) may activate calcium-dependent PDE1C and cause a decrease in cAMP. We note that other pathways may also contribute to this calcium-dependent decrease in cAMP. Some adenylate cyclases, including AC5, AC6, and AC9, are inhibited by intracellular calcium signaling (Halls and Cooper 2011). Of these, AC5 and AC9 are expressed within the retina early in development (Nicol et al. 2006), although their specific localization is unknown. The decrease in cAMP caused by these potential pathways and by calcium influx may lead to activation of the channel underlying the sAHP. The corresponding decrease in PKA activity would then produce a decrease in phosphorylation that disinhibits the channel. In support of this idea, TREK1 channels in neurons associate with PKA anchoring proteins (AKAPs) that allow for rapid regulation by PKA (Sandoz et al. 2006). Furthermore, IsAHP in hippocampal CA1 neurons is modulated by both PKA and phosphatases (Pedarzani et al. 1998), suggesting that a balance between kinase and phosphatase activity actively regulates the channel phosphorylation state.
The role of IsAHP in retinal waves.
Retinal waves during the first postnatal week in mice critically depend on cholinergic transmission from SACs but not on other neurotransmitters (Bansal et al. 2000; Stacy et al. 2005; Wang et al. 2007). SACs are spontaneously active (Ford et al. 2012; Zheng et al. 2006), and stimulation of a single SAC is sufficient to initiate a wave (Ford et al. 2012). Thus, a network of SACs is both necessary and sufficient for generating retinal waves.
The intrinsic conductances in SACs dictate the spatio-temporal properties of retinal waves. During development of the visual system, the distinct spatio-temporal features convey information to target regions (Ackman et al. 2012; Bansal et al. 2000; Penn et al. 1994; Xu et al. 2011). Several computational models have been developed to investigate how the intrinsic properties of SACs give rise to the frequency, propagation speed, and spatial coverage of waves (Ford et al. 2012; Godfrey and Swindale 2007; Hennig et al. 2009). Each of these models relies on the spontaneous depolarization of SACs to initiate waves, the local excitatory connections to allow propagation, and a slow afterhyperpolarization to limit propagation of waves into recently active regions. Recent experimental evidence has indicated that decreasing the sAHP by elevating cAMP levels increases wave frequency (Ford et al. 2012; Zheng et al. 2006). In accordance, here we have shown that inhibiting calcium-activated PDE1C decreases IsAHP and wave frequency. This is consistent with predictions from modeling studies (Ford et al. 2012; Godfrey and Swindale 2007; Hennig et al. 2009). Furthermore, we have shown that animals lacking TREK1 exhibit waves with double the frequency of wild-type waves. Thus, our data support a role for TREK1 in generating the sAHP in SACs that limits wave frequency.
Why are waves not more frequent than 2–3 waves per minute when the sAHP is blocked or reduced? The sAHP prevents the propagation of waves into recently active regions but is not the only mechanism that sets wave frequency. The frequency of retinal waves is determined by the spontaneous depolarization rate of SACs. In mice, SACs are spontaneously active only about once every 10 min when neurotransmitters are blocked (but see in rabbit Zheng et al. 2006; Ford et al. 2012). This slow intrinsic depolarization rate may set an upper limit to the wave frequency when the sAHP is reduced. Alternatively, a reduction in sAHP might be compensated for with additional cellular mechanisms. During initial whole cell recordings, we found a Katp-mediated conductance following depolarizing voltage steps (Fig. 2). While this conductance was not activated during less invasive perforated patch recordings, a Katp-mediated sAHP may occur when sAHP is inhibited and causes excessive depolarization that depletes energy levels.
GRANTS
Support was contributed by National Institutes of Health (NIH) Grant RO1-EY-013528 (M. B. Feller), NIH Grant F31-NS-614663 (K. J. Ford), NSF Predoctoral Fellowship (D. Arroyo), Life Sciences Research Foundation Postdoctoral Fellowship (J. Kay), and NIH Grants R01-EY-022073R01 (J. Sanes), NS-046666 (R. M. Bryan), and R21-HL-098921 (R. M. Bryan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.J.F., D.A., J.K., E.E.L., R.M.B., J.S., and M.B.F. conception and design of research; K.J.F., D.A., J.K., and E.E.L. performed experiments; K.J.F., D.A., J.K., and M.B.F. analyzed data; K.J.F., D.A., J.K., J.S., and M.B.F. interpreted results of experiments; K.J.F., D.A., J.K., and M.B.F. prepared figures; K.J.F., D.A., J.K., and M.B.F. drafted manuscript; K.J.F., D.A., J.K., R.M.B., J.S., and M.B.F. edited and revised manuscript; D.A., J.K., E.E.L., R.M.B., J.S., and M.B.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Prof. Diana Bautista for sanshool extract and many useful discussions. We thank Prof. Sam Wu and Cameron Cowan at Baylor Univ. for the generous use of multielectrode array. Present addresses: K. J. Ford: Dept. of Biochemistry and Biophysics, Univ. of California, San Francisco, San Francisco, CA; J. Kay: Depts. of Neurobiology and Ophthalmology, Duke Univ., Durham, NC.
REFERENCES
- Abel HJ, Lee JC, Callaway JC, Foehring RC. Relationships between intracellular calcium and afterhyperpolarizations in neocortical pyramidal neurons. J Neurophysiol 91: 324–335, 2004 [DOI] [PubMed] [Google Scholar]
- Ackman JB, Burbridge TJ, Crair MC. Retinal waves coordinate patterned activity throughout the developing visual system. Nature 490: 219–225, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J Neurosci 20: 7672–7681, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Sigal YM, Milstein AD, Garrison JL, Zorn JA, Tsuruda PR, Nicoll RA, Julius D. Pungent agents from Szechuan peppers excite sensory neurons by inhibiting two-pore potassium channels. Nat Neurosci 11: 772–779, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship AG, Ford KJ, Johnson J, Seal RP, Edwards RH, Copenhagen DR, Feller MB. Synaptic and extrasynaptic factors governing glutamatergic retinal waves. Neuron 62: 230–241, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemin J, Girard C, Duprat F, Lesage F, Romey G, Lazdunski M. Mechanisms underlying excitatory effects of group I metabotropic glutamate receptors via inhibition of 2P domain K+ channels. EMBO J 22: 5403–5411, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czirjak G, Toth ZE, Enyedi P. The two-pore domain K+ channel, TRESK, is activated by the cytoplasmic calcium signal through calcineurin. J Biol Chem 279: 18550–18558, 2004 [DOI] [PubMed] [Google Scholar]
- Deng PY, Poudel SK, Rojanathammanee L, Porter JE, Lei S. Serotonin inhibits neuronal excitability by activating two-pore domain k+ channels in the entorhinal cortex. Mol Pharmacol 72: 208–218, 2007 [DOI] [PubMed] [Google Scholar]
- Deng PY, Xiao Z, Yang C, Rojanathammanee L, Grisanti L, Watt J, Geiger JD, Liu R, Porter JE, Lei S. GABA(B) receptor activation inhibits neuronal excitability and spatial learning in the entorhinal cortex by activating TREK-2 K+ channels. Neuron 63: 230–243, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn TA, Storm DR, Feller MB. Calcium-dependent increases in protein kinase-A activity in mouse retinal ganglion cells are mediated by multiple adenylate cyclases. PLoS One 4: e7877, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev 90: 559–605, 2010 [DOI] [PubMed] [Google Scholar]
- Faber ES, Sah P. Physiological role of calcium-activated potassium currents in the rat lateral amygdala. J Neurosci 22: 1618–1628, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M, Duprat F, Lesage F, Reyes R, Romey G, Heurteaux C, Lazdunski M. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J 15: 6854–6862, 1996 [PMC free article] [PubMed] [Google Scholar]
- Ford KJ, Felix AL, Feller MB. Cellular mechanisms underlying spatiotemporal features of cholinergic retinal waves. J Neurosci 32: 850–863, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey KB, Swindale NV. Retinal wave behavior through activity-dependent refractory periods. PLoS Comput Biol 3: e245, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JA, Teagarden MA, Foehring RC, Wilson CJ. Nonequilibrium calcium dynamics regulate the autonomous firing pattern of rat striatal cholinergic interneurons. J Neurosci 29: 8396–8407, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein SA, Bayliss DA, Kim D, Lesage F, Plant LD, Rajan S. International union of pharmacology. LV. Nomenclature and molecular relationships of two-P potassium channels. Pharmacol Rev 57: 527–540, 2005 [DOI] [PubMed] [Google Scholar]
- Halls ML, Cooper DM. Regulation by Ca2+-signaling pathways of adenylyl cyclases. Cold Spring Harb Perspect Biol 3: a004143, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig MH, Adams C, Willshaw D, Sernagor E. Early-stage waves in the retinal network emerge close to a critical state transition between local and global functional connectivity. J Neurosci 29: 1077–1086, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GD, Johnson SM, van Helden DF. The slow calcium-dependent potassium current in a myenteric neurone of the guinea-pig ileum. J Physiol 361: 315–337, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore E, Maingret F, Lazdunski M, Patel AJ. An intracellular proton sensor commands lipid- and mechano-gating of the K(+) channel TREK-1. EMBO J 21: 2968–2976, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova E, Hwang GS, Pan ZH. Characterization of transgenic mouse lines expressing Cre recombinase in the retina. Neuroscience 165: 233–243, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay JN, Chu MW, Sanes JR. MEGF10 and MEGF11 mediate homotypic interactions required for mosaic spacing of retinal neurons. Nature 483: 465–469, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay JN, De la Huerta I, Kim IJ, Zhang Y, Yamagata M, Chu MW, Meister M, Sanes JR. Retinal ganglion cells with distinct directional preferences differ in molecular identity, structure, and central projections. J Neurosci 31: 7753–7762, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B, Adams PR. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol 55: 1268–1282, 1986 [DOI] [PubMed] [Google Scholar]
- Lancaster B, Hu H, Gibb B, Storm JF. Kinetics of ion channel modulation by cAMP in rat hippocampal neurones. J Physiol 576: 403–417, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B, Nicoll RA. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol 389: 187–203, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Duan W, Sneyd J, Herbison AE. Two slow calcium-activated afterhyperpolarization currents control burst firing dynamics in gonadotropin-releasing hormone neurons. J Neurosci 30: 6214–6224, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage F. Pharmacology of neuronal background potassium channels. Neuropharmacology 44: 1–7, 2003 [DOI] [PubMed] [Google Scholar]
- Lesage F, Lazdunski M. Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol Renal Physiol 279: F793–F801, 2000 [DOI] [PubMed] [Google Scholar]
- Lesage F, Terrenoire C, Romey G, Lazdunski M. Human TREK2, a 2P domain mechano-sensitive K+ channel with multiple regulations by polyunsaturated fatty acids, lysophospholipids, and Gs, Gi, and Gq protein-coupled receptors. J Biol Chem 275: 28398–28405, 2000 [DOI] [PubMed] [Google Scholar]
- Lorenzon NM, Foehring RC. Relationship between repetitive firing and afterhyperpolarizations in human neocortical neurons. J Neurophysiol 67: 350–363, 1992 [DOI] [PubMed] [Google Scholar]
- Mathie A. Neuronal two-pore-domain potassium channels and their regulation by G protein-coupled receptors. J Physiol 578: 377–385, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namiranian K, Lloyd EE, Crossland RF, Marrelli SP, Taffet GE, Reddy AK, Hartley CJ, Bryan RM., Jr Cerebrovascular responses in mice deficient in the potassium channel, TREK-1. Am J Physiol Regul Integr Comp Physiol 299: R461–R469, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol X, Bennis M, Ishikawa Y, Chan GC, Reperant J, Storm DR, Gaspar P. Role of the calcium modulated cyclases in the development of the retinal projections. Eur J Neurosci 24: 3401–3414, 2006 [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Krause M, Haug T, Storm JF, Stuhmer W. Modulation of the Ca2+-activated K+ current sIAHP by a phosphatase-kinase balance under basal conditions in rat CA1 pyramidal neurons. J Neurophysiol 79: 3252–3256, 1998 [DOI] [PubMed] [Google Scholar]
- Penn AA, Wong RO, Shatz CJ. Neuronal coupling in the developing mammalian retina. J Neurosci 14: 3805–3815, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulver SR, Griffith LC. Spike integration and cellular memory in a rhythmic network from Na+/K+ pump current dynamics. Nat Neurosci 13: 53–59, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P. Ca2+-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci 19: 150–154, 1996 [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES. Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol 66: 345–353, 2002 [DOI] [PubMed] [Google Scholar]
- Sah P, Isaacson JS. Channels underlying the slow afterhyperpolarization in hippocampal pyramidal neurons: neurotransmitters modulate the open probability. Neuron 15: 435–441, 1995 [DOI] [PubMed] [Google Scholar]
- Sandoz G, Levitz J, Kramer RH, Isacoff EY. Optical control of endogenous proteins with a photoswitchable conditional subunit reveals a role for TREK1 in GABA(B) signaling. Neuron 74: 1005–1014, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoz G, Thummler S, Duprat F, Feliciangeli S, Vinh J, Escoubas P, Guy N, Lazdunski M, Lesage F. AKAP150, a switch to convert mechano-, pH- and arachidonic acid-sensitive TREK K(+) channels into open leak channels. EMBO J 25: 5864–5872, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santone R, Giorgi M, Maccarone R, Basso M, Deplano S, Bisti S. Gene expression and protein localization of calmodulin-dependent phosphodiesterase in adult rat retina. J Neurosci Res 84: 1020–1026, 2006 [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Spain WJ, Foehring RC, Chubb MC, Crill WE. Slow conductances in neurons from cat sensorimotor cortex in vitro and their role in slow excitability changes. J Neurophysiol 59: 450–467, 1988 [DOI] [PubMed] [Google Scholar]
- Stacy RC, Demas J, Burgess RW, Sanes JR, Wong ROL. Disruption and recovery of patterned retinal activity in the absence of acetylcholine. J Neurosci 25: 9347–9357, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stosiek C, Garaschuk O, Holthoff K, Konnerth A. In vivo two-photon calcium imaging of neuronal networks. Proc Natl Acad Sci USA 100: 7319–7324, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron 25: 399–410, 2000 [DOI] [PubMed] [Google Scholar]
- Talley EM, Sirois JE, Lei Q, Bayliss DA. Two-pore-domain (KCNK) potassium channels: dynamic roles in neuronal function. Neuroscientist 9: 46–56, 2003 [DOI] [PubMed] [Google Scholar]
- Tanner GR, Lutas A, Martinez-Francois JR, Yellen G. Single KATP channel opening in response to action potential firing in mouse dentate granule neurons. J Neurosci 31: 8689–8696, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian G, Sågetorp J, Xu Y, Shuai H, Degerman E, Tengholm A. Role of phosphodiesterases in the shaping of sub-plasma membrane cAMP oscillations and pulsatile insulin secretion. J Cell Sci 125: 5084–5095, 2012 [DOI] [PubMed] [Google Scholar]
- Torborg CL, Feller MB. Spontaneous patterned retinal activity and the refinement of retinal projections. Prog Neurobiol 76: 213–235, 2005 [DOI] [PubMed] [Google Scholar]
- Tzingounis AV, Kobayashi M, Takamatsu K, Nicoll RA. Hippocalcin gates the calcium activation of the slow afterhyperpolarization in hippocampal pyramidal cells. Neuron 53: 487–493, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos C, Andrade R. Visinin-like neuronal calcium sensor proteins regulate the slow calcium-activated afterhyperpolarizing current in the rat cerebral cortex. J Neurosci 30: 14361–14365, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos C, Foehring RC, Lee JC, Andrade R. Essential role for phosphatidylinositol 4,5-bisphosphate in the expression, regulation, and gating of the slow afterhyperpolarization current in the cerebral cortex. J Neurosci 31: 18303–18312, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogalis F, Harvey JR, Furness JB. Suppression of a slow post-spike afterhyperpolarization by calcineurin inhibitors. Eur J Neurosci 19: 2650–2658, 2004 [DOI] [PubMed] [Google Scholar]
- Vogalis F, Harvey JR, Neylon CB, Furness JB. Regulation of K+ channels underlying the slow afterhyperpolarization in enteric afterhyperpolarization-generating myenteric neurons: role of calcium and phosphorylation. Clin Exp Pharmacol Physiol 29: 935–943, 2002 [DOI] [PubMed] [Google Scholar]
- Wang CT, Blankenship AG, Anishchenko A, Elstrott J, Fikhman M, Nakanishi S, Feller MB. GABA(A) receptor-mediated signaling alters the structure of spontaneous activity in the developing retina. J Neurosci 27: 9130–9140, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe D, Inokawa H, Hashimoto K, Suzuki N, Kano M, Shigemoto R, Hirano T, Toyama K, Kaneko S, Yokoi M, Moriyoshi K, Suzuki M, Kobayashi K, Nagatsu T, Kreitman RJ, Pastan I, Nakanishi S. Ablation of cerebellar Golgi cells disrupts synaptic integration involving GABA inhibition and NMDA receptor activation in motor coordination. Cell 95: 17–27, 1998 [DOI] [PubMed] [Google Scholar]
- Xu HP, Furman M, Mineur YS, Chen H, King SL, Zenisek D, Zhou ZJ, Butts DA, Tian N, Picciotto MR, Crair MC. An instructive role for patterned spontaneous retinal activity in mouse visual map development. Neuron 70: 1115–1127, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Ionic permeation and blockade in Ca2+-activated K+ channels of bovine chromaffin cells. J Gen Physiol 84: 157–186, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Lee S, Zhou ZJ. A transient network of intrinsically bursting starburst cells underlies the generation of retinal waves. Nat Neurosci 9: 363–371, 2006 [DOI] [PubMed] [Google Scholar]





