Abstract
Nuclear factor E2-related factor 2 (Nrf2)/ antioxidant response element (ARE) pathway is an important cellular stress response pathway involved in neuroprotection. We previously screened several natural phytochemicals and identified plumbagin as a novel activator of the Nrf2/ARE pathway that can protect neurons against ischemic injury. Here we extended our studies to natural and synthetic derivatives of plumbagin. We found that 5,8-dimethoxy-1,4-naphthoquinone (naphthazarin) is a potent activator of the Nrf2/ARE pathway, up-regulates the expression of Nrf2-driven genes in primary neuronal and glial cultures, and protects neurons against glutamate-induced excitotoxicity.
Keywords: Nrf2, glutamate, naphthazarin, antioxidant response element
1. Introduction
Extracellular accumulation of glutamate has been linked to neuronal death associated with acute damage (i.e. stroke, spinal cord and head trauma), as well chronic neuropathologies such as Alzheimer’s, Parkinson’s and Huntington’s diseases [1]. Glutamate excitotoxicity results from the over-stimulation of postsynaptic glutamate receptors with massive calcium influx leading to rapid mitochondrial calcium overload and mitochondrial generation of reactive oxygen species (ROS) [1,2]. The increased ROS levels cause oxidative damage within the postsynaptic neurons, and can be released from them propagating the damage to surrounding cells [1,2].
The nuclear factor E2-related factor 2 (Nrf2)/ antioxidant response element (ARE) pathway is a critical signaling pathway regulating antioxidants and phase II detoxification enzymes, such as heme oxygenase 1 (HO1), NAD(P)H quinone oxidoreductase 1 (NQO1), and thioredoxin reductase 1 (TrxR1). The importance of Nrf2 in protecting cells against toxins and in pathological conditions characterized by enhanced oxidative stress has been clearly demonstrated using siRNA methodologies and Nrf2 knockout mice [3]. Furthermore, activation of the Nrf2/ARE pathway has beneficial roles in cell culture and animal models of neurodegenerative diseases, including Alzheimer’s and Parkinson’s diseases [3–5].
We previously screened natural botanical pesticides for their ability to activate adaptive stress response pathways, and identified plumbagin as a novel Nrf2/ARE activator and anti-ischemic compound [6]. In the present study we further our findings testing natural and synthetic plumbagin analogues with the aim to identify stronger Nrf2 activators and potential neuroprotective agents. Among the various analogues examined, naphthazarin showed higher levels of Nrf2/ARE activation than plumbagin in stable ARE reporter HepG2 cells. Naphthazarin induced expression of endogenous ARE-mediated target genes in primary neuronal and astrocytes cultures. Furthermore, naphthazarin protected neurons against glutamate-induced cell death and reduced α-fodrin cleavage in primary neurons.
2. Materials and Methods
2.1 Cell culture
The Nrf2 reporter cell line ARE-bla HepG2 was purchased from Invitrogen (Carlsbad, CA, USA) and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% dialyzed fetal bovine serum, 2 mM GlutaMAX, 0.1 mM non essential amino acids, 25 mM HEPES, 1% penicillin/streptomycin, and 5 μg/ml of blasticidin (Invitrogen) at 37°C in a humidified 5% CO2 atmosphere. Blasticidin was removed from the medium during experimental treatments. Neuronal cultures were generated as previously described [6] and plated at 0.1 × 106 cells/cm2 in Neurobasal medium supplemented with B27 (Invitrogen). Experiments were performed using 9- to 10-day-old cultures. Primary astrocytes cultures were generated from postnatal Sprague-Dawley rats as described [7]. Cultures were maintained in DMEM with 10% serum for 2–3 weeks, at which time microglia were removed by shaking and adherent astrocytes were passaged.
2.2. Cell viability and Nrf2/ARE reporter assay
ARE-bla HepG2 cells (5000 cells in 100 μl of medium) were seeded in 96-well plates 24 hours before treatment with the indicated concentration of 5,8-dihydroxy-1,4-naphthoquinone (naphthazarin) (Sigma, St. Louis. MO). Cell viability was measured 24 hours post-treatment using Celltiter 96® AQUEOUS One Solution reagent (Promega, Madison, WI, USA). β-lactamase activity was measured according to the manufacturer’s instructions (Invitrogen).
2.3. Western blot analysis
Cell lysates were processed for Western blot analysis as previously described [6]. Equal amounts of protein were separated by SDS-PAGE, transferred onto nitrocellulose membranes, and probed with antibodies against heme oxygenase 1 and thioredoxin reductase 1 (Santa Cruz Biotechnology, Santa Cruz, CA), glial fibrillary acid protein (GFAP) (Abcam, Cambridge, MA), α-fodrin (Millipore, Billerica, MA), and GAPDH (clone 6C5; Advanced Immunochemical Inc, Long Beach, CA). Immunoreactive bands were detected using HRP-conjugated secondary antibody (Amersham Bioscience, Piscataway, NJ) and chemiluminescence (Pierce). Autoradiograms were quantified using image J software.
2.4. Statistical analysis
Statistical analysis was performed by Student’s t-test or analysis of variance (ANOVA) as appropriate. The results are expressed as mean + S.E.M. Values of p<0.05 were considered statistically significant.
3. Results
3.1. Naphthazarin activates the Nrf2/ARE pathway in stable ARE –bla Hep G2 cell line
Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) (Figure 1A structure 1) is a natural quinoid with several therapeutic properties [6,8,9]. We investigated the ability of various structural analogs of plumbagin (Fig. 1A) for their ability to induce ARE-mediated transcription using the stable ARE reporter cell line ARE–bla Hep G2. None of the compounds tested showed toxicity at the concentration utilized for the reporter assay (Fig. 1B). Compounds 7-Hydroxy-1-Tetralone, 5-Methoxy-1-methyl-2-Tetralone, 4,7-Dihydroxy-3-methyl-indan-1-one, 1,4-Dihydroxy-7,8-dihydro-6H-benzocycloheptene-5,9-dione and 2-Hydroxy-2-(2-methyl-2,3-dihydro-1H-indol-5-yl)-1H-indene-1,3(2H)-dione showed similar or lower ARE-inducing activity compared to plumbagin (Fig. 1C). However 5,8-dihydroxy-1,4-naphthoquinone (naphthazarin) displayed significantly higher activity than plumbagin (Fig. 1C).
Figure 1. Naphthazarin activates the Nrf2/ARE pathway in ARE-bla Hep G2 cells.
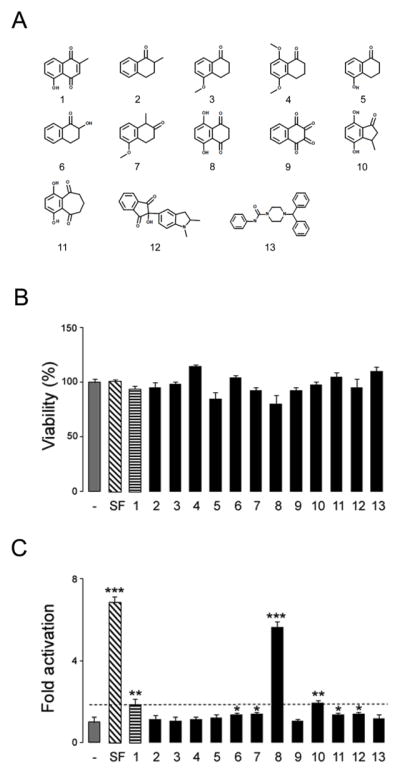
A. Chemical structure of plumbagin and its analogs tested in the study. 1: Plumbagin, 2: 2-Methyl-1-Tetralone, 3: 5-Methoxy-1-Tetralone, 4: 5,8-Dimethoxy-1-Tetralone, 5: 5-Hydroxy-1-Tetralone 6: 7-Hydroxy-1-Tetralone, 7: 5-Methoxy-1-methyl-2-Tetralone, 8: 5,8-Dihydroxy-1,4-naphthoquinone, 9: 1,2,3,4-Tetraoxo-1,2,3,4-tetrahydronaphthalene Dihydrate, 10: 4,7-Dihydroxy-3-methyl-indan-1-one, 11: 1,4-Dihydroxy-7,8-dihydro-6H-benzocycloheptene-5,9-dione,12: 2-Hydroxy-2-(2-methyl-2,3-dihydro-1H-indol-5-yl)-1H-indene-1,3(2H)-dione, 13:. 4-Benzhydryl-N-phenyl-1-piperazinecarboxamide, B and C. ARE-bla Hep G2 cells were treated with 5 μM sulforaphane (SF) or 1 μM of the various compounds. After 24 hours, cell viability (B) and ARE reporter activity (C) were assessed using MTS and β–lactamase activity assays respectively. Data are mean and S.E.M. *p<0.05, **p<0.01 and ***p<0.001 vs. control.
3.2. Naphthazarin induces endogenous ARE-mediated target genes in primary neuronal cultures
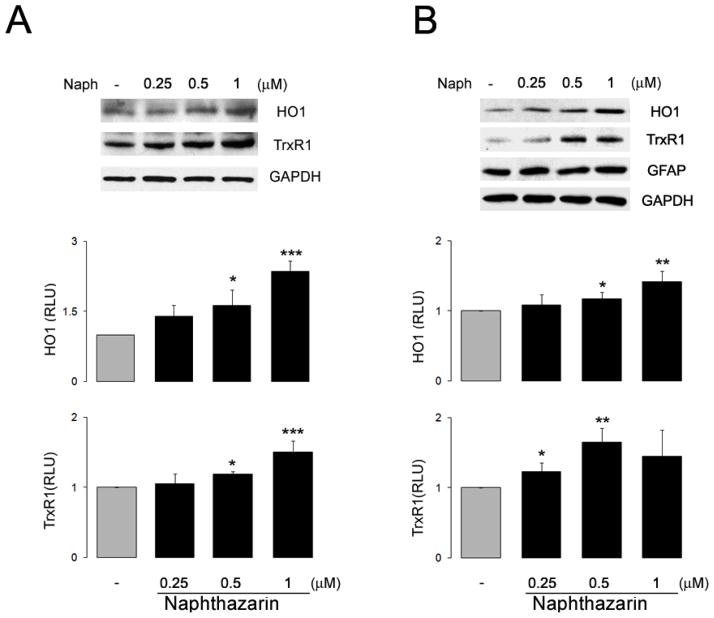
We then tested the ability of naphthazarin to induce the expression of endogenous ARE-mediated target genes in primary neuronal cultures. Neurons and astrocytes were treated with different concentrations of naphthazarin for 6 and 24 hours and levels of HO1 and TrxR1, respectively, were determined by immunoblotting. In both cell types naphthazarin increased the expression levels of the proteins in a concentration-dependent fashion (Fig. 2B) validating its use as an Nrf2/ARE activator.
Figure 2. Naphthazarin induces ARE-mediated target genes in rat primary cultures.
Representative immunoblots and quantification data of HO1 and TrxR1 expression in neurons (A) and astrocytes (B) following treatment with the indicated concentrations of naphthazarin. Data are mean and S.E.M. (n=3). *p<0.05, **p<0.01 and ***p<0.001 vs. control.
3.3. Naphthazarin protects against neuronal cell death and reduces α-fodrin cleavage induced by glutamate in rat primary neurons
In order to test the neuroprotective potential of naphtahzarin we used an acute excitotoxic cell culture model. Neuronal cultures were treated with the indicated concentrations of naphthazarin for 6 hours and were then subjected to glutamate challenge for 16 hours. A significant decrease in cell survival (Fig. 3A) paralleled by enhanced α-fodrin cleavage (Fig. 3B) was observed in glutamate-treated cells. However, naphthazarin pretreated cells displayed significantly higher resistance to glutamate excitotoxicity (Fig. 3A), as well decreased α-fodrin cleavage (Fig. 3B).
Figure 3. Naphthazarin protects neurons against glutamate excitotoxicity.
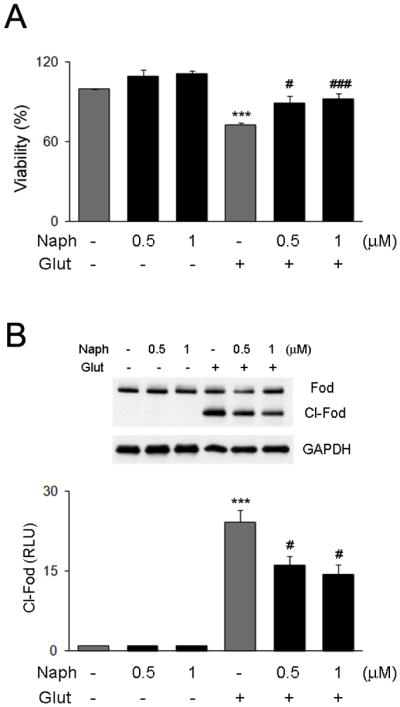
Primary rat neuronal cultures were pretreated with the indicated concentration of naphthazarin for 6 hours and exposed to 200 μM of glutamate for 16 hours. Cell viability was determined by MTS assay (A), and α-fodrin cleavage by western blot (B). GAPDH was used as control for equal loading. Data are mean and S.E.M. (n=3). ***p<0.001 vs. control; # p<0.05 and ## p< 001 vs. glutamate treated cells.
4. Discussion
Activation of the Nrf2/ARE pathway has been shown to counteract neurotoxicity resulting from glutathione depletion, intracellular calcium overload, mitochondria dysfunction, increased ROS and lipid peroxidation in different experimental models [10–11]. Given its multiple roles in regulating cell homeostasis the Nrf2/ARE pathway is considered a promising therapeutic target for neurodegenerative disorders [3,4]. Over the past decades several phytochemicals have been identified as Nrf2 inducers and neuroprotective agents. For example, sulforaphane protects against hydrogen peroxide-, glutamate-, and 6-hydroxydopamine-induced toxicity in cultured neurons and in vivo [12–13]. The naphthoquinones plumbagin and curcumin reduce brain damage and ameliorate the associated neurological deficits in a model of focal ischemia through Nrf2-dependent mechanisms [6, 14]. Licochalcone has anti-inflammatory and cytoprotective effects that are attenuated by siRNA-mediated Nrf2 silencing [15].
In most cases the study of potential neuroprotective agents is restricted to pure neuronal cultures. However astrocytes play a critical role in maintaining normal brain physiology and responding to injury or diseases. In addition to structural and metabolic support, astrocytes are important regulators of extracellular neurotransmitters and ion concentrations [16]. Furthermore, Nrf2/ARE responsive genes appear to be preferentially activated in astrocytes, which consequently may have stronger detoxification and antioxidant potential than neurons [17–19]. It is reasonable that agents able to modulate the Nrf2/ARE pathway both in neurons and astrocytes may provide a better protective spectrum. Here we show that, contrary to many known Nrf2 activators, naphthazarin can induce the expression of endogenous HO-1 and TrxR1 proteins both in neurons and astrocytes. Furthermore, we found that naphthazarin protects neurons against glutamate-induced cell death. Glutamatergic intracellular calcium mobilization in neuronal cells is crucial for neurotoxicity-induced death. In addition to impairment of mitochondrial functions and induction of oxidative stress, the elevation of intracellular calcium levels leads to the overstimulation of proteolytic enzymes such as calpains and caspases [1, 20]. We found that in our experimental conditions glutamate induced the accumulation of the calpain-specific 150 kDa α-fodrin cleavage product (Fig. 3B). Notably, we found that napthazarin attenuates the generation of the α-fodrin cleavage product suggesting that naphthazarin may reduce intracellular calcium levels. Although it is not known whether naphthazarin regulates neuronal calcium homeostasis via Nrf2/ARE-responsive genes, previous observations revealed an important role for Nrf2 in the maintenance of calcium homeostasis by showing that Nrf2−/ − neurons are more sensitive to increased intracellular calcium concentrations induced by ionomycin [21].
In conclusion, our findings identify naphthazarin as a novel potent Nrf2/ARE activator in neurons and astrocytes. In addition to inducing detoxification and antioxidant proteins, naphthazarin attenuates intracellular calcium-dependent protease activity and protects cortical neurons against glutamate-mediated excitotoxicity. These findings imply that naphthazarin may have therapeutic potential for various neurological diseases.
Highlights.
Naphthazarin activates the Nrf2/ ARE pathway
Naphthazarin induces Nrf2-driven genes in neurons and astrocytes
Naphthazarin protects neurons against excitotoxicity
Acknowledgments
This work was supported in part by the Intramural Research Program of the National Institute on Aging.
Abbreviations
- ARE
antioxidant response element
- HO1
heme oxygenase 1
- Nrf2
Nuclear factor E2-related factor 2
- NQO1
NAD(P)H quinine oxidoreductase 1
- ROS
reactive oxygen species
- TrxR1
thioredoxin reductase 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch. 2010;460:525–542. doi: 10.1007/s00424-010-0809-1. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Qin ZH. Molecular and cellular mechanisms of excitotoxic neuronal death. Apoptosis. 2010;15:1382–1402. doi: 10.1007/s10495-010-0481-0. [DOI] [PubMed] [Google Scholar]
- 3.Calkins MJ, Johnson DA, Townsend JA, et al. The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid Redox Signal. 2009;11:497–508. doi: 10.1089/ars.2008.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Mulswinkel FL, Kuiperij HB. The Nrf2-ARE signaling pathway: promising drug target to combat oxidative stress in neurodegenerative disorders. Curr Drug Targets CNS Neurol Disord. 2005;4:267–81. doi: 10.2174/1568007054038238. [DOI] [PubMed] [Google Scholar]
- 5.Son TG, Camandola S, Mattson MP. Hormetic dietary phytochemicals. Neuromolecular Med. 2008;10:236–46. doi: 10.1007/s12017-008-8037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Son TG, Camandola S, Arumugam TV, et al. Plumbagin, a novel Nrf2/ARE activator, protects against cerebral ischemia. J Neurochem. 2010;112:1316–26. doi: 10.1111/j.1471-4159.2009.06552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keller JN, Furukawa K, Barger SW, et al. Lysophosphatidic acid decreases glutamate and glucose uptake by astrocytes. J Neurochem. 1996;67:2300–5. doi: 10.1046/j.1471-4159.1996.67062300.x. [DOI] [PubMed] [Google Scholar]
- 8.Gupta SC, Kim JH, Prasad S, et al. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutracueticals. Cancer Metastasis Rev. 2010;29:405–434. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nambiar D, Rajamani P, Singh PP. Effects of phytochemicals on ionization radiation-mediated carcinogenesis and cancer therapy. Mutat Res. 2011;728:139–57. doi: 10.1016/j.mrrev.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 11.Yang YC, Lii CK, Lin AH, et al. Induction of glutathione synthesis and heme oxygenase 1 by the flavonoids butein and phloretin is mediated through the ERK/Nrf2 pathway and protects against oxidative stress. Free Radic Biol Med. 2011;51:2073–2081. doi: 10.1016/j.freeradbiomed.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Kraft AD, Johnson DA, Johnson JA. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J Neurosci. 2004;24:1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siebert A, Desai V, Chandrasekaran K, et al. Nrf2 activators provides neuroprotection against 6-hydroxydopamine toxicity in rat organotypic nigrostriatal cocultures. J Neurosci Res. 2009;15:1659–1669. doi: 10.1002/jnr.21975. [DOI] [PubMed] [Google Scholar]
- 14.Yang C, Zhang X, Fan H, et al. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009;1282:133–41. doi: 10.1016/j.brainres.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Kim SS, Lim J, Bang Y, et al. Licochalcone activates Nrf2/antioxidant response element signaling pathway in both neuronal and microglial cells: therapeutic relevance to neurodegenerative disease. J Nutr Biochem. 2012;23:1314–23. doi: 10.1016/j.jnutbio.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Barreto GE, Gonzales J, Torres Y, et al. Astrocytic-neuronal crosstalk: implications for neuroprotection from brain injury. Neurosci Res. 2011;71:107–13. doi: 10.1016/j.neures.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Shih AY, Johnson DA, Wong G, et al. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci. 2003;23:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson JA, Jonhson DA, Kraft AD, et al. The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann NY Acad Sci. 2008;1147:61–9. doi: 10.1196/annals.1427.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vargas MR, Johnson JA. The Nrf2-ARE cytoprotective pathway in astrocytes. Expert Rev Mol Med. 2009;11:e17. doi: 10.1017/S1462399409001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orrenius S, Nicotera P, Zhivotovsky B. Cell death mechanisms and their implication in toxicology. Toxicol Sci. 2011;119:3–19. doi: 10.1093/toxsci/kfq268. [DOI] [PubMed] [Google Scholar]
- 21.Lee JM, Shih AY, Murphy TH, et al. NF-E2-related factor 2 mediates neuroprotection against mitochondrial complex 1 inhibitors and increased concentrations of intracellular calcium in primary cortical neurons. J Biol Chem. 2003;278:37948–56. doi: 10.1074/jbc.M305204200. [DOI] [PubMed] [Google Scholar]