Abstract
BACKGROUND
1,5-anhydroglucitol (1,5-AG), fructosamine, and glycated albumin are of increasing interest as alternative measures of hyperglycemia. We characterize the associations of these nontraditional glycemic markers with hemoglobin A1c (HbA1c) and fasting glucose and assess their ability to identify people with diabetes.
METHODS
We conducted a cross-sectional comparison of 1,5-AG, fructosamine, and glycated albumin with HbA1c and fasting glucose measurements in 1,719 participants from the Atherosclerosis Risk in Communities (ARIC) Study. Non-linear relationships were evaluated using R2 and F-statistics. Performance for identifying cases of diabetes was determined using the Area Under the Curve (AUC). Diabetes was defined by HbA1c ≥6.5%, fasting glucose ≥126 mg/dL (≥6.99 mmol/L), and/or a self-reported history of diagnosed diabetes.
RESULTS
Median values of HbA1c and fasting glucose were 5.8% and 109 mg/dL (6.05 mmol/L), respectively; 17.3% of the study population had diagnosed diabetes. Glycated albumin, fructosamine, and 1,5-AG were more strongly correlated with HbA1c compared with fasting glucose (all P-values <0.05). Nonlinear models provided the best fit for describing the relationships of the alternative markers to HbA1c. When diabetes was defined by an HbA1c≥6.5%, fructosamine (AUC 0.83; 95% CI: 0.79–0.87) and glycated albumin (AUC 0.87; 95% CI: 0.83–0.90) performed comparably to fasting glucose (AUC 0.83; 95% CI: 0.79–0.87), while 1,5-AG performed worse (AUC 0.74; 95% CI: 0.69–0.78) for identifying cases of undiagnosed diabetes.
CONCLUSIONS
Fructosamine and glycated albumin may be useful adjuncts to HbA1c and fasting glucose. Future studies should examine these markers in situations where fasting glucose or HbA1c measurements are invalid or not available.
Keywords: 1,5-anhydroglucitol; fructosamine; glycated albumin; hemoglobin A1c; fasting glucose; diabetes
Glucose and hemoglobin A1c (HbA1c) measurements are central to the diagnosis and management of diabetes. Fasting blood glucose provides an acute assessment of glycemia, while HbA1c reflects average glycemic exposure over the preceding 2–3 months (1). 1,5-anhydroglucitol (1,5-AG), fructosamine, and glycated albumin are alternative markers of hyperglycemia that can be readily measured in serum, and are of increasing interest in research and clinical practice. 1,5-AG is freely filtered by glomeruli, and in the setting of hyperglycemia there is accelerated urinary excretion of 1,5-AG with a corresponding drop in serum concentration (2). Fructosamine is formed via a nonenzymatic mechanism that involves the binding of blood glucose to serum proteins to form ketoamines (3). Glycated albumin, formed in a similar reaction as fructosamine, is specific to albumin (4). Both fructosamine and glycated albumin increase in the presence of increased blood glucose (3,4).
There is growing interest in these serum markers as alternatives to fasting glucose or HbA1c when these standard measurements are not available, when intermediate glycemic control (1 week to 1 month) is of interest, or in situations that reduce the validity of measurements of fasting glucose (e.g. nonfasting status, diurnal variation, glycolysis, or sample deterioration) or HbA1c (e.g. hemoglobinopathies, hemolysis, or altered red cell turnover) (5). However, little is known about the relationships between alternative serum markers of hyperglycemia to HbA1c and fasting glucose in the general adult population. Furthermore, recent publications have assumed a linear relationship between glycemic markers, which may not be a valid assumption (6,7). The comparative performance of 1,5-AG, fructosamine, and glycated albumin for the identification of persons with diagnosed and undiagnosed diabetes is also unclear.
We undertook this study to characterize the relationships of 1,5-AG, fructosamine, and glycated albumin to HbA1c and fasting glucose in persons with and without diabetes and to assess the performance of these serum glycemic markers in identifying cases of diabetes in a community-based population. We hypothesized that these alternative markers would more closely reflect HbA1c than fasting glucose, and that nonlinear models may more accurately capture the relationships between established and alternative markers of glycemia as compared to linear models. To investigate whether the associations between the glycemic markers might differ in population subgroups, we examined the correlations within strata of age, sex, coronary heart disease history, hypertension, and chronic kidney disease status.
Materials and Methods
Study Population
The Atherosclerosis Risk in Communities (ARIC) Study is a community-based prospective cohort of 15,792 adults who have been followed for more than two decades. The original ARIC participants were enrolled between 1987–1989 from 4 U.S. communities (Forsyth County, North Carolina; Jackson, Mississippi; suburban Minneapolis, Minnesota; and Washington County, Maryland) (8–10). In 2004–2005, 2,045 ARIC participants were enrolled in the Carotid Magnetic Resonance Imaging (CARMRI) sub-study using a stratified sampling plan. Two-thirds of CARMRI participants were selected because they had a high value of maximum carotid artery intimal medial thickness on ultrasound (IMT), while the other third was randomly selected from among ARIC participants with a low intimal medial thickness (11). Magnetic resonance imaging (MRI), physical examinations, laboratory tests, and medical interviews were conducted as part of the CARMRI examination. The present study was limited to the 1,719 individuals who participated in the CARMRI examination, who were fasting 8 or more hours, and who had valid measurements of HbA1c, fasting glucose, 1,5-AG, fructosamine, and glycated albumin.
The study protocol was approved by institutional review boards at each clinical site. Written informed consent was obtained from all participants.
Glycemic Markers
HbA1c and glucose were measured in 2004–2005 as part of the original CARMRI protocol on a Roche Hitachi 911 Analyzer. Glucose was measured in serum using hexokinase (Roche Diagnostics). HbA1c was measured in whole blood using a Tina-quant II immunoassay method (Roche Diagnostics) and calibrated to the Diabetes Control and Complications Trial assay.
In 2009, we measured 1,5-AG (GlycoMark), fructosamine (Roche Diagnostics), and glycated albumin (Asahi Kasei Lucica GA-L; Asahi Kasei Pharma Corporation, Tokyo, Japan) in stored serum specimens using a Roche Modular P800 system. Glycated albumin was expressed as a percentage of total serum albumin, i.e., 100 × [glycated albumin]/[total albumin]. The interassay coefficients of variation were: 4.8% (1,5-AG, mean 19.4 μg/mL), 3.7% (fructosamine, mean 254.9 μmol/L), and 2.7% (glycated albumin, mean 12.8%). Previous studies have demonstrated high stability of these measurements in stored serum samples (12–14).
Diabetes
We used four definitions of diabetes: (1) HbA1c ≥ 6.5%; (2) fasting glucose ≥ 126 mg/dL (≥6.99 mmol/L); (3) a self-reported history of diabetes based on physician-diagnosis or glucose-lowering medication use in the last 4 weeks; or (4) a composite definition based on meeting any of the preceding three criteria. Medication use was based on a response to a question regarding use of diabetes medications in the four weeks prior to the examination.
Other Variables of Interest
All data were collected by trained study personnel using standardized protocols with extensive quality assurance measures, as described previously (11). Age, sex, and race were self-reported. Coronary heart disease was determined using a combination of self-reported history of coronary heart disease and adjudicated coronary heart disease event during active surveillance prior to the CARMRI visit (10,15). Hypertension was defined as use of blood pressure-lowering medication and/or an average of three systolic blood pressure measurements ≥140 mmHg (yes or no). We used the Chronic Kidney Disease Epidemiology Collaboration equation (eGFR) (16) to estimate glomerular filtration rate (eGFR) from using serum creatinine concentration, age, sex, and race. Chronic kidney disease was defined as an eGFR < 60 mL/min per 1.73m2 or albumin-to-creatinine ratio ≥ 30 mg/g (17).
Statistical Analyses
Characteristics of the study population were calculated overall and by self-reported history of diabetes. The population quantile distributions of 1,5-AG, fructosamine, and glycated albumin were calculated and compared across age, gender, and race groups. Spearman’s correlation coefficients (r) were calculated to describe the relationships between 1,5-AG, fructosamine, or glycated albumin and HbA1c or glucose. We compared the correlations overall and stratified by race, history of diabetes, history of coronary heart disease, chronic kidney disease and/or hypertension. We tested for differences among correlation coefficients with P-values obtained by bias corrected bootstrapping with 1,000 replications.
We compared the percentiles across the different glycemic markers and fit regression models to generate corresponding values between the different glycemic markers overall and stratified by diabetes status. We identified the best fitting models using multi-ordered, nested polynomials, logarithmic transformations, and inverse transformations. We utilized the F-statistic to evaluate whether additional terms added useful information to the models. Coefficients of determination were used to evaluate model performance. We visually displayed the associations between markers using scatterplots with overlaid lowess curves.
Finally, we evaluated the ability of 1,5-AG, fructosamine, and glycated albumin to identify cases of diabetes using four different definitions: HbA1c ≥ 6.5%, fasting glucose ≥ 126 mg/dL (≥6.99 mmol/L), self-reported history of diabetes, or any of the preceding three definitions. The performance of 1,5-AG, fructosamine, and glycated albumin were compared to the performance of HbA1c and fasting glucose where relevant. The weighted area under the curve (AUC) was calculated for each marker and diabetes definition. Confidence intervals for the AUC were calculated using bias corrected bootstrapping with 1,000 replications. To examine performance for identifying cases of undiagnosed diabetes, we conducted additional analyses excluding persons currently taking diabetes medications or with a self-reported history of diagnosed diabetes.
All analyses were conducted in Stata 11.1 (StataCorp LP) and were weighted to account for the CARMRI sampling design (11).
Results
Characteristics of the study population overall and by history of diabetes are shown in Table 1. The mean age of study participants was 70 years. Compared with participants without a history of diabetes, a higher percentage of participants with diabetes were male (48.7% versus 42.1%) or black (33.3% versus 17.5%). Hypertension, a history of coronary heart disease, and chronic kidney disease were more prevalent among participants with diabetes compared with those without a history of diabetes. In this study population, the median eGFR overall was 77.5 ml/min per 1.73 m2 (IQR: 64.6, 88.5) and the median ACR was 8.5 mg/g (IQR: 5.2, 16.7). As expected, mean levels of fasting glucose, HbA1c, fructosamine, and glycated albumin were substantially higher in persons with a history of diabetes compared to those without a history of diabetes, while 1,5-AG was lower among those with diabetes versus those without a history of diabetes.
Table 1.
Weighted participant characteristics overall and by history of diagnosed diabetes*
| Overall, n = 1,719 (SE) | History of diabetes, n= 343 (SE) | No history of diabetes, n= 1,376 (SE) | |
|---|---|---|---|
| Age, y | 70.27 (0.16) | 70.26 (0.35) | 70.27 (0.18) |
| Male, % | 43.21 (1.49) | 48.70 (3.46) | 42.06 (1.64) |
| Black, % | 20.21 (0.00) | 33.26 (0.01) | 17.47 (0.00) |
| History of hypertension, % | 70.81 (1.40) | 87.33 (2.48) | 67.34 (1.60) |
| Coronary heart disease, % | 9.67 (0.82) | 16.33 (2.35) | 8.27 (0.85) |
| Chronic kidney disease, %* | 27.89 (1.14) | 39.87 (3.01) | 67.34 (1.60) |
| Fasting glucose (mg/dL)*** | 108.85 (0.74) | 137.46 (2.77) | 102.84 (0.54) |
| Hemoglobin A1c (%) | 5.82 (0.02) | 6.72 (0.09) | 5.63 (0.02) |
| 1,5-Anhydroglucitol (μg/mL) | 17.03 (0.21) | 13.09 (0.51) | 17.86 (0.22) |
| Fructosamine (μmol/L) | 237.65 (0.94) | 273.07 (3.51) | 230.22 (0.71) |
| Glycated Albumin (%) | 14.31 (0.08) | 17.70 (0.34) | 13.60 (0.05) |
Estimates are means or proportions (SE)
Diabetes here is based on self-reported history of a physician diagnosis or glucose lowering medication use.
Chronic kidney disease was defined as an estimated glomerular filtration rate < 60 mL/min per 1.73m2 or an albumin-to-creatinine ratio of ≥ 30 mg/g. Among participants with chronic kidney disease, the median estimated glomerular filtration rate was 56.4 ml/min per 1.73 m2 (IQR: 48.3, 74.2) and the median albumin-to-creatinine ratio was 27.6 (IQR: 7.7, 61.1).
To convert glucose concentrations from mg/dL to mmol/L, multiply by 0.05551
We examined the population values of the alternative markers by age, gender, and race (Supplemental Table S1). Fructosamine values were higher in male participants, but neither 1,5-AG nor glycated albumin values differed by gender. Compared with whites, black participants had lower serum values of 1,5-AG and higher serum values of fructosamine and glycated albumin across all percentiles, as reported previously (18). The different glycemic markers were each strongly associated with age. At older ages, 1,5-AG was lower and fructosamine and glycated albumin were higher.
1,5-AG, fructosamine, and glycated albumin were more strongly correlated with HbA1c as compared to fasting glucose overall (P-values <0.05) and in subgroups of interest (Supplemental Tables S2). Glycated albumin was more strongly correlated with either HbA1c or fasting glucose compared with fructosamine (both P-values < 0.05) or 1,5-AG (both P-values < 0.05). Similarly, fructosamine was more strongly correlated with either HbA1c or fasting glucose than 1,5-anhydroglucitol (both P-values < 0.001). Glycated albumin and fructosamine demonstrated similar correlations with fasting glucose of 0.38 and 0.33 respectively, while 1,5-AG showed a weaker relationship (r = −0.14). Correlations of the serum glycemic markers with HbA1c and fasting glucose did not differ substantially across age and race groups; although, 1,5-AG, fructosamine, and glycated albumin were all more highly correlated with HbA1c in men than in women. Stronger correlations were observed among persons with a history of diabetes. Correlations were largely similar among subgroups of persons with coronary heart disease, chronic kidney disease, or hypertension. The presence of chronic kidney disease as compared with no chronic kidney disease was associated with a higher Spearman correlation between 1,5-AG and fasting glucose (P = 0.02), but not the other markers. There were higher correlations between glycated albumin and both HbA1c and fasting glucose among persons with a history of hypertension, but not 1,5-AG or fructosamine. The overall Spearman correlation between HbA1c and fasting glucose was 0.48.
We developed equations to establish the relationship between each serum glycemic marker and HbA1c or fasting glucose overall and among persons with and without a history of diabetes (Table 2). Models had better fit (higher R2) among persons with a history of diabetes. Among persons with a history of diabetes, 1,5-AG, fructosamine, and glycated albumin were more strongly associated with fasting glucose as compared to HbA1c. Overall, adopting a nonlinear model improved the R2 from 0.10 to 0.21 for 1,5-AG and HbA1c and from 0.09 to 0.17 for 1,5-AG and fasting glucose, whereas linear and nonlinear models performed comparably for glycated albumin. Similarly, a nonlinear model improved the R2 for fructosamine and HbA1c from 0.39 to 0.46. This is consistent with evidence of non-linearity seen on the scatter plots and the fitted lowess curves (Figure 1).
Table 2.
Best fit models overall and by history of diagnosed diabetes
| 1,5-Anhydroglucitol
|
Fructosamine
|
Glycated Albumin
|
||||
|---|---|---|---|---|---|---|
| Model# | R2 | Model | R2 | Model | R2 | |
| Overall (N = 1719) | ||||||
| Best Fit | ||||||
| A1c | 5.3903 + 5.5048/x | 0.21 | 247.480 − 90.745ln(x) + 8.509(ln(x))2 | 0.46 | 26.275 − 17.612ln(x) + 3.720(ln(x))2 | 0.54 |
| FG | 212.182 − 66.594ln(x) + 10.153(ln(x))2 | 0.17 | 553.548 − 1.898e+05/x + 1.98e+07/x2 | 0.39 | 7.8278 + 7.3595x − 0.001262x3 | 0.41 |
| Linear | ||||||
| A1c | 6.4828 − 0.0388x | 0.10 | 2.0958 + 0.0157x | 0.39 | 2.9425 + 0.2011x | 0.52 |
| FG | 128.8306 − 1.1732x | 0.09 | −2.3199 + 0.4678x | 0.35 | 29.4777 + 5.5447x | 0.39 |
| History of Diabetes (N =343)* | ||||||
| Best Fit | ||||||
| A1c | 5.7696 + 8.8587/x − 5.1029/x2 | 0.33 | 250.611 − 90.873ln(x) + 8.443(ln(x))2 | 0.61 | 4.163 + 0.1287x + 3.82e+05x3 | 0.71 |
| FG | 208.73 − 32.56ln(x) + 0.9807(ln(x))2 | 0.23 | 426.93 − 1.2077e+05/x + 1.14e+07/x2 | 0.32 | 130.8 − 8.837x + 0.712x2 − 0.0108x3 | 0.37 |
| Linear | ||||||
| A1c | 7.7246 − 0.0770x | 0.20 | 1.8763 + 0.0177x | 0.56 | 3.1198 + 0.2032x | 0.69 |
| FG | 170.7132 − 2.5411x | 0.20 | 14.4649 + 0.4504x | 0.32 | 57.0838 + 4.5410x | 0.30 |
| No History of Diabetes (N = 1376)* | ||||||
| Best Fit | ||||||
| A1c | 5.5935 + 0.1047/x + 4.5559/x2 | 0.04 | 9.5088 − 0.03831x + 9.25e+5x2 | 0.12 | 14.8959 + 0.5714x − 6.5423ln(x) | 0.16 |
| FG | 101.7556 + 148.9338/x2 | 0.05 | 298.1406 − 1.8538x + 0.004328x2 | 0.24 | 603.933 + 27.0403x − 333.704ln(x) | 0.28 |
| Linear | ||||||
| A1c | 5.8152 − 0.0101x | 0.01 | 4.0437 + 0.0069x | 0.07 | 3.9098 + 0.1267x | 0.13 |
| FG | 106.6068 − 0.2107x | 0.01 | 42.2954 + 0.2630x | 0.12 | 43.5650 + 4.3574x | 0.18 |
Abbreviations: A1c, hemoglobin A1c; FG, fasting glucose.
Diabetic status determined by self-reported history.
Modeling was performed using glucose concentrations expressed in mg/dL, 1,5-Anhydroglucitol concentrations in μg/mL, fructosamine concentrations in μmol/L and glycated albumin in %.
Figure 1.
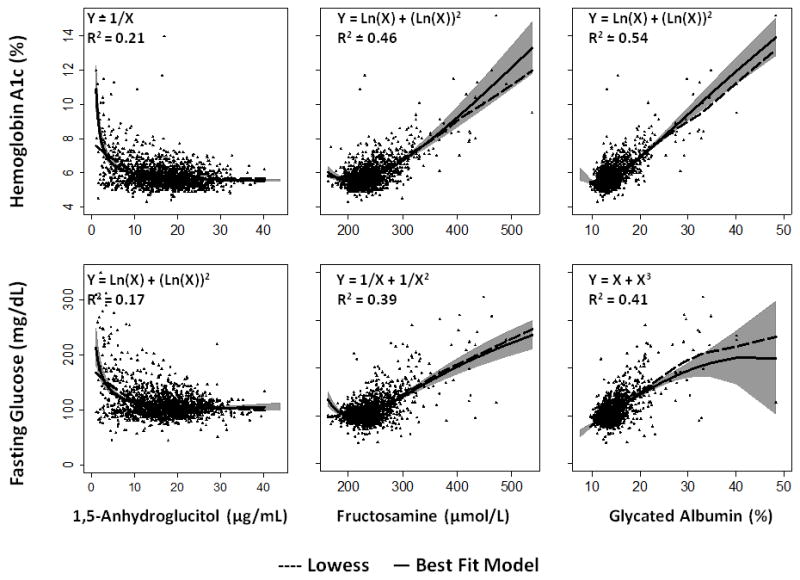
Scatterplots of 1,5-anhydroglucitol (μg/mL), fructosamine (μmol/L), and glycated albumin (%) versus hemoglobin A1c (%) or fasting glucose (mg/dL) overlaid with a lowess curve (dashed line) and best fit model (solid line). Best fit model equations (from Table 2) as well as the coefficient of determinations (R2) are displayed in the upper left hand corner of each panel. The gray shaded areas represent the 95% confidence intervals for the best fit model. To convert glucose concentrations from mg/dL to mmol/L, multiply by 0.05551.
We provide here corresponding values of HbA1c and fasting glucose from the best fit (nonlinear) models, ordinary least squares linear models, and corresponding weighted percentiles for 1,5-AG, fructosamine, and glycated albumin (Supplemental Table S3). Based on these models, an HbA1c value of 6.5% corresponded to 1,5-AG values ranging from 5.0 to 15.3 μg/mL, fructosamine values ranging from 254.7 to 289.5 μmol/L, and glycated albumin values of 16.1 to 18.3%. A fasting glucose of 126 mg/dL (6.99 mmol/L) corresponded to a 1,5-AG of 5.9 to 15.7 μg/mL, a fructosamine of 250.5 to 276.4 μmol/L, and a glycated albumin of 15.5 to 16.9%.
For all four different definitions of diabetes, glycated albumin and fructosamine identified cases of diabetes comparably to HbA1c or fasting glucose (Table 3). Fructosamine had non-significantly smaller AUCs than glycated albumin, while 1,5-AG was significantly worse than either fructosamine or glycated albumin. Excluding participants currently using glucose-lowering medication or participants with a history of diabetes each attenuated the diagnostic performance of all the markers.
Table 3.
Weighted diagnostic performance of 1,5-anhydroglucitol, fructosamine, and glycated albumin to identify cases of diabetes comparing four definitions of diabetes
| Total study population (N= 1,719)
|
Excluding persons taking diabetes medications (N=1,421)
|
Excluding persons with a history of diabetes* (N = 1376)
|
|
|---|---|---|---|
| AUC (95% CI)** | AUC (95% CI)** | AUC (95% CI)** | |
| Diabetes defined by hemoglobin A1c ≥ 6.5% (N = 252) | |||
| 1,5-Anhydroglucitol | 0.74 (0.69 to 0.78) | 0.66 (0.58 to 0.74) | 0.65 (0.56 to 0.73) |
| Fructosamine | 0.83 (0.79 to 0.87) | 0.71 (0.63 to 0.78) | 0.69 (0.61 to 0.77) |
| Glycated Albumin | 0.87 (0.83 to 0.90) | 0.73 (0.64 to 0.81) | 0.71 (0.62 to 0.78) |
| Fasting Glucose | 0.83 (0.79 to 0.87) | 0.76 (0.69 to 0.83) | 0.74 (0.66 to 0.81) |
| Diabetes defined by fasting glucose ≥ 126 mg/dL (≥6.99 mmol/L) (N = 297) | |||
| 1,5-Anhydroglucitol | 0.70 (0.65 to 0.75) | 0.60 (0.53 to 0.67) | 0.59 (0.51 to 0.66) |
| Fructosamine | 0.83 (0.79 to 0.87) | 0.78 (0.72 to 0.83) | 0.77 (0.71 to 0.83) |
| Glycated Albumin | 0.86 (0.82 to 0.89) | 0.80 (0.74 to 0.85) | 0.80 (0.74 to 0.85) |
| Hemoglobin A1c | 0.87 (0.85 to 0.90) | 0.82 (0.76 to 0.86) | 0.81 (0.75 to 0.86) |
| Diabetes defined by history of diabetes* (N = 343) | |||
| 1,5-Anhydroglucitol | 0.69 (0.65 to 0.74) | 0.60 (0.54 to 0.67) | - |
| Fructosamine | 0.81 (0.77 to 0.85) | 0.74 (0.66 to 0.82) | - |
| Glycated Albumin | 0.85 (0.81 to 0.88) | 0.74 (0.66 to 0.83) | - |
| Hemoglobin A1c | 0.87 (0.84 to 0.89) | 0.73 (0.64 to 0.82) | - |
| Fasting Glucose | 0.80 (0.76 to 0.84) | 0.73 (0.64 to 0.82) | - |
| Diabetes defined by hemoglobin A1c ≥ 6.5%, fasting glucose ≥ 126 mg/dL (≥6.99 mmol/L), or history of diabetes* (N = 491) | |||
| 1,5-Anhydroglucitol | 0.66 (0.63 to 0.70) | 0.59 (0.54 to 0.64) | 0.58 (0.52 to 0.64) |
| Fructosamine | 0.80 (0.76 to 0.83) | 0.73 (0.69 to 0.78) | 0.72 (0.67 to 0.78) |
| Glycated Albumin | 0.82 (0.79 to 0.85) | 0.75 (0.70 to 0.80) | 0.74 (0.68 to 0.79) |
Self-reported history of diabetes or diabetes medication use.
AUC represents the area under the receiver operating characteristic curve. Confidence intervals were calculated using bias corrected bootstrapping with 1,000 replications.
Discussion
This study described the relationships between alternative glycemic markers (1,5-AG, glycated albumin, and fructosamine) and established glycemic markers (HbA1c and fasting glucose) in a community-based population. We observed high correlations for glycated albumin and fructosamine with HbA1c and fasting glucose. Glycated albumin was more strongly correlated with HbA1c and fasting glucose as compared to fructosamine or 1,5-AG. Of the alternative glycemic markers, glycated albumin and fructosamine performed comparably to HbA1c and fasting glucose for identifying cases of diabetes in this population, regardless of definition of diabetes used.
1,5-AG is a monosaccharide that is filtered via the glomeruli at a rate of 5–10 mg/mL each day and has a half-life shorter than fructosamine or glycated albumin (2). In healthy persons, most of the filtered 1,5-AG is reabsorbed by the proximal tubule, however, in states of severe hyperglycemia and glycosuria, glucose in the urine competitively inhibits re-absorption of 1,5-AG, causing increased urinary excretion and a reduction in serum concentrations (2). 1,5-AG values are thought to reflect hyperglycemic exposure over approximately a 1-week period (19). This may explain our observation that 1,5-AG was virtually uncorrelated with HbA1c (r = −0.07) or fasting glucose (r = 0.03) in people without diabetes. Furthermore, the poorer performance of 1,5-AG in identifying diabetes cases is consistent with the fact that 1,5-AG concentrations are substantially lowered only when circulating glucose concentrations are very high(5).
Fructosamine and glycated albumin are formed by the covalent binding of glucose to serum proteins via a nonezymatic glycation reaction (3,4). While fructosamine is a measure of serum ketoamines (3), glycated albumin is specific to albumin (4). Both of these markers have a half-life of about 17–21 days, reflecting exposure to glucose over the preceding 2–3 weeks (3,4,20–22). In our study we observed very low inter-assay coefficients (3.7% and 2.7% respectively), suggesting excellent laboratory performance of the assays implemented in this study. With regard to fructosamine, we observed a stronger correlation with HbA1c as compared to fasting glucose (r = 0.40 versus 0.33; P = 0.01). Furthermore, fructosamine performed well for the identification of diabetes cases with AUCs of approximately 0.8 regardless of the diabetes definition used. Our linear model relating fructosamine to HbA1c was similar to a previous report in the literature (23). However, our data suggest a nonlinear model may better describe the relationship of fructosamine to HbA1c, contrary to previous assumptions (6,7). Despite the similarities between fructosamine and glycated albumin, glycated albumin was more highly correlated with HbA1c and fasting glucose as compared to fructosamine. Glycated albumin also outperformed fructosamine and 1,5-AG for the identification of diabetes, performing comparably to both HbA1c and fasting glucose.
There are a number of limitations to our study that should be considered in the interpretation of our results. First, the mean age of participants in the CARMRI study was 70 years (range: 60–84 years) and it is unclear if the same results would be obtained in younger populations. Second, there were only 343 persons with diagnosed diabetes in this community-based population. As a result, the distribution of glycemic values available for evaluation of the relationships between markers was limited. In particular, our precision to characterize the interrelationships of these markers at high levels of hyperglycemia was correspondingly low. Additional studies are needed to evaluate the performance in persons with diabetes and across a wide range of glycemic control. Third, we only had single measurements of each glycemic marker and no information on 2-hour glucose concentrations (oral glucose tolerance tests were not conducted in the CARMRI Study). Fourth, we had limited power to conduct subgroup analyses. Indeed, our definition of chronic kidney disease was based on single measurements of serum creatinine and albuminuria, and there were few participants in the advanced stages of kidney disease.
This study also has several strengths, including its biracial, community-based population and rigorous assessment of multiple glycemic markers. To our knowledge, this is the largest study comparing fructosamine, glycated albumin, and 1,5-AG to HbA1c and fasting glucose in a community-based setting. We have previously shown in this same population that fructosamine, glycated albumin, and 1,5-AG are strongly associated with future diabetes risk, even after adjustment for HbA1c or fasting glucose (24).
In conclusion, our results suggest that glycated albumin and fructosamine may be useful as adjunct measures of hyperglycemia to HbA1c or in settings where HbA1c (or whole blood samples for its measurement) are not available. 1,5-AG may be useful in the setting of overt hyperglycemia. It remains unclear whether these serum glycemic markers may have clinical utility in settings where the performance of HbA1c is questionable (e.g. hemoglobinopathies, anemia, or end stage renal disease). Additional studies are needed to understand the possible roles of all three serum markers in diabetes care. In particular, future investigations should evaluate the associations of 1,5-AG, glycated albumin, and fructosamine with clinical complications of diabetes (e.g., microvascular and macrovascular outcomes).
Supplementary Material
Acknowledgments
This work was supported by NIH/NIDDK grants K01 DK076595 and R01 DK089174 to ES. SPJ was supported by NIH/NHLBI T32HL007024 Cardiovascular Epidemiology Training Grant. The Asahi Kasei Corporation provided materials for the glycated albumin assay.
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C) with the ARIC carotid MRI examination funded by U01HL075572-01. The authors thank the staff and participants of the ARIC study for their important contributions.
The authors thank the staff and participants of the ARIC Study for their important contributions.
Footnotes
No potential conflicts of interest relevant to this article were reported.
S.P.J. analyzed the data and wrote the article. M.W.S. collected the data and reviewed the article. E.S. collected the data, edited the article, and provided significant intellectual content to the study.
References
- 1.Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan DM, Peterson CM. Tests of glycemia in diabetes. Diabetes Care. 2003;26 (Suppl 1):S106–108. doi: 10.2337/diacare.26.2007.s106. [DOI] [PubMed] [Google Scholar]
- 2.Buse JB, Freeman JLR, Edelman SV, Jovanovic L, McGill JB. Serum 1,5-anhydroglucitol (GlycoMark ): a short-term glycemic marker. Diabetes Technol Ther. 2003;5:355–63. doi: 10.1089/152091503765691839. [DOI] [PubMed] [Google Scholar]
- 3.Armbruster DA. Fructosamine: structure, analysis, and clinical usefulness. Clin Chem. 1987;33:2153–63. [PubMed] [Google Scholar]
- 4.Rondeau P, Bourdon E. The glycation of albumin: structural and functional impacts. Biochimie. 2011;93:645–58. doi: 10.1016/j.biochi.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Saudek CD, Derr RL, Kalyani RR. Assessing glycemia in diabetes using self-monitoring blood glucose and hemoglobin A1c. JAMA. 2006;295:1688–97. doi: 10.1001/jama.295.14.1688. [DOI] [PubMed] [Google Scholar]
- 6.Rodríguez-Segade S, Rodríguez J, Cabezas-Agricola JM, Casanueva FF, Camiña F. Progression of nephropathy in type 2 diabetes: the glycation gap is a significant predictor after adjustment for glycohemoglobin (Hb A1c) Clin Chem. 2011;57:264–71. doi: 10.1373/clinchem.2010.144949. [DOI] [PubMed] [Google Scholar]
- 7.Nayak AU, Holland MR, Macdonald DR, Nevill A, Singh BM. Evidence for consistency of the glycation gap in diabetes. Diabetes Care. 2011;34:1712–6. doi: 10.2337/dc10-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 9.Jackson R, Chambless LE, Yang K, Byrne T, Watson R, Folsom A, et al. Differences between respondents and nonrespondents in a multicenter community-based study vary by gender ethnicity. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. J Clin Epidemiol. 1996;49:1441–6. doi: 10.1016/0895-4356(95)00047-x. [DOI] [PubMed] [Google Scholar]
- 10.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–33. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 11.Wagenknecht L, Wasserman B, Chambless L, Coresh J, Folsom A, Mosley T, et al. Correlates of carotid plaque presence and composition as measured by MRI: the Atherosclerosis Risk in Communities Study. Circ Cardiovasc Imaging. 2009;2:314–22. doi: 10.1161/CIRCIMAGING.108.823922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koskinen P, Irjala K. Stability of serum fructosamine during storage. Clin Chem. 1988;34:2545–6. [PubMed] [Google Scholar]
- 13.Nathan DM, Steffes MW, Sun W, Rynders GP, Lachin JM. Determining stability of stored samples retrospectively: the validation of glycated albumin. Clin Chem. 2011;57:286–90. doi: 10.1373/clinchem.2010.150250. [DOI] [PubMed] [Google Scholar]
- 14.Selvin E, Rynders GP, Steffes MW. Comparison of two assays for serum 1,5-anhydroglucitol. Clin Chim Acta. 2011;412:793–5. doi: 10.1016/j.cca.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selvin E, Francis LMA, Ballantyne CM, Hoogeveen RC, Coresh J, Brancati FL, et al. Nontraditional markers of glycemia: associations with microvascular conditions. Diabetes Care. 2011;34:960–7. doi: 10.2337/dc10-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Eckardt K-U, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 18.Selvin E, Steffes MW, Ballantyne CM, Hoogeveen RC, Coresh J, Brancati FL. Racial differences in glycemic markers: a cross-sectional analysis of community-based data. Ann Intern Med. 2011;154:303–9. doi: 10.1059/0003-4819-154-5-201103010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stettler C, Stahl M, Allemann S, Diem P, Schmidlin K, Zwahlen M, et al. Association of 1,5-anhydroglucitol and 2-h postprandial blood glucose in type 2 diabetic patients. Diabetes Care. 2008;31:1534–5. doi: 10.2337/dc08-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allgrove J, Cockrill BL. Fructosamine or glycated haemoglobin as a measure of diabetic control? Arch Dis Child. 1988;63:418–22. doi: 10.1136/adc.63.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones IR, Owens DR, Williams S, Ryder RE, Birtwell AJ, Jones MK, et al. Glycosylated serum albumin: an intermediate index of diabetic control. Diabetes Care. 1983;6:501–3. doi: 10.2337/diacare.6.5.501. [DOI] [PubMed] [Google Scholar]
- 22.Howey JE, Bennet WM, Browning MC, Jung RT, Fraser CG. Clinical utility of assays of glycosylated haemoglobin and serum fructosamine compared: use of data on biological variation. Diabet Med. 1989;6:793–6. doi: 10.1111/j.1464-5491.1989.tb01281.x. [DOI] [PubMed] [Google Scholar]
- 23.Narbonne H, Renacco E, Pradel V, Portugal H, Vialettes B. Can fructosamine be a surrogate for HbA(1c) in evaluating the achievement of therapeutic goals in diabetes? Diabetes Metab. 2001;27:598–603. [PubMed] [Google Scholar]
- 24.Juraschek SP, Steffes MW, Miller ER, 3rd, Selvin E. Alternative Markers of Hyperglycemia and Risk of Diabetes. Diabetes Care. 2012 doi: 10.2337/dc12-0787. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22875225. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


