Abstract
BACKGROUND
The BRAFV600E mutation is present in 62% of radioactive iodine resistant (RAIR) thyroid tumors and is associated with down-regulation of the sodium-iodide-symporter (NIS) and TSH-receptor (TSHr). We sought to evaluate the combined effect of BRAF inhibition and TSH supplementation on 131I uptake of BRAFV600E-mutant human thyroid cancer cells.
MATERIALS AND METHODS
WRO cells (a BRAFV600E-mutant follicular-derived papillary thyroid carcinoma cell line) were transfected with siRNA targeting BRAF for 72 hours in a physiologic TSH environment. NIS and TSHr expression were then evaluated at three levels: gene expression, protein levels, and 131I uptake. These three main outcomes were then reassessed in TSH-depleted media and media supplemented with supratherapeutic concentrations of TSH.
RESULTS
NIS gene expression increased 5.5-fold 36 hours after transfection (p=0.01) and TSHr expression increased 2.8-fold at 24 hours (p=0.02). NIS and TSHr protein levels were similarly increased 48 and 24 hours post-transfection, respectively. Seventy two hours after BRAF inhibition, 131I uptake showed was unchanged in TSH-depleted media, increased by 7.5-fold (p<0.01) in physiologic TSH media, and increased by 9.1-fold (p<0.01) in supratherapeutic TSH media.
CONCLUSIONS
The combined strategy of BRAF inhibition and TSH supplementation results in greater 131I uptake than when either technique is utilized alone. This represents a simple and feasible approach that may improve outcomes in patients with RAIR thyroid carcinomas for which current treatment algorithms are ineffective.
Keywords: Papillary thyroid cancer, BRAF(V600E) mutation, radioactive iodine, radioactive iodine resistance, thyroid stimulating hormone, sodium-iodine symptorer
INTRODUCTION
The loss of radioactive iodine (RAI) uptake in differentiated thyroid carcinomas accounts for significant morbidity and mortality of this increasingly common disease [1, 2]. Roughly 25% of primary well-differentiated thyroid tumors and 50% of metastases are RAI resistant (RAIR) and effective adjuvant medical therapies following thyroidectomy are limited [3].
The efficacy of RAI ablation following thyroidectomy is dependent on active intracellular transport and trapping of iodine by the sodium-iodide symporter (NIS) [4, 5]. NIS is a basolateral membrane protein that is regulated by a host of iodine metabolizing proteins such as the TSH receptor (TSHr), which controls transcription and posttranslational modification of NIS [6, 7]
A rapidly growing body of literature has implicated members of the MAPK signaling pathway, of which BRAF is the strongest activator, with tumorigenesis of aggressive thyroid carcinomas [8]. The BRAFV600E mutation is present in 40–50% of all papillary thyroid cancers (PTC) and is associated with aggressive features such as extrathyroidal extension, lymph node metastases, advanced tumor stage, and radioactive iodine resistance [9–11]. It is thought that the loss of responsiveness to 131I is due to loss of function of iodine metabolizing proteins such as NIS and TSHr [3, 12, 13]. Tumor cells harboring this mutation have decreased NIS and TSHr gene expression compared to similar cells without the mutation [12, 14–17]. Several recent in vitro and in vivo mouse studies have demonstrated that BRAF inhibition with small-molecule MAPK pathway inhibitors restores expression of iodine metabolizing proteins and increases susceptibility to RAI [18, 19].
It has also been shown that increasing circulating TSH to supratherapeutic levels prior to treatment with RAI increases its potency [20–22]. It is believed that TSH enhances the function of the sodium-iodide symporter and results in increased 131I uptake [7]. For this reason, many centers routinely administer human recombinant TSH or withhold thyroid hormone prior to 131I therapy in order to achieve supratherapeutic TSH levels at the time of treatment [22–24].
Thus, it has been independently demonstrated that both BRAF inhibition and TSH supplementation can increase RAI uptake in RAIR tumors, however the additive potential of implementing both strategies simultaneously has not been reported. We hypothesized that supplementing BRAFV600E -mutant human thyroid cancer cells with supratherapeutic concentrations of TSH while transiently inhibiting BRAF would result in greater 131I uptake compared to BRAF inhibition in lower TSH environments.
MATERIALS AND METHODS
Patient selection and analysis
First, to evaluate the prevalence of the BRAFV600E mutation and its effect on tumor behavior and levels of NIS and TSHr, a representative cohort of patients who underwent surgery for papillary thyroid carcinoma between 2003 and 2010 was selected. After obtaining approval from our Institutional Review Board and written informed consent from each patient, tumor and representative normal tissue samples from the contralateral lobe were collected. All samples were snap-frozen in liquid nitrogen and stored at −80°C until analysis. A retrospective review of a prospectively maintained patient database was performed for age, sex, and histopathologic features. All surgical specimens were reviewed by an endocrine pathologist.
Cell culture
The BRAFV600E-mutant human follicular thyroid carcinoma-derived cell line WRO was used in this study. Of note, both BRAFV600E-mutant and wild-type WRO cells have been reported and it is accepted that two distinct cell lines have been distributed [25]. The WRO cells used in this report were confirmed to be the BRAFV600E variety. These cells were a generous gift from Dr. James A. Fagin (Memorial Sloan-Kettering, NY, USA) and were validated using short tandem repeat and single nucleotide polymorphism array analysis in 2008 [25].
Cell culture media preparation
In order to investigate the role of TSH in iodine uptake, cells were maintained in three different types of media: H6 (physiologic TSH concentration), H5 (no TSH), and supratherapeutic TSH media. H6 medium was made using HamsF12:DMEM supplemented with insulin (10μg/ml), human transferrin (5μg/ml), somatostatin (1mg/ml), glycyl-L histidyl-l-lysine acetate (2ng/ml), hydrocortisone (0.36 ng/ml), TSH (10 mU/ml), penicillin G (100 IU/ml), streptomycin sulfate (100 μg/ml), and amphotericin B (0.25 μg/ml). H5 medium contained the same components as H6 except without TSH. Supratherapeutic TSH media also contained the same components as H6 except for supplementation with 500 mU/ml of TSH. This concentration is similar to that which is achieved after administration of recombinant TSH prior to RAI ablation.
Small interfering RNA plasmids and transfection
Two oligonucleotides targeting an area of the BRAF gene outside of the V600E mutation site consisting of ribonucleosides with the presence of 2′-deoxyribonucleosides at the 3′ end, 5′-(CGAGACCGAUCCUCAUCAG)d(TT)-3′ and 5′-r(CUGAUGAGGAUCGGUCUCG)d(TT)-3 were synthesized and annealed as previously described (Qiagen, Valencia, CA, USA) [26]. Cells were transfected using Lipofectamine (HiPerFect Transfection Reagent, Qiagen, Valencia, CA, USA). Transfection experiments were performed using Fast-Forward Transfection protocol according to the manufacturer. 3.5 ×105 cells were seeded into a 6-well plate. Cells were exposed to 10 nmol, 20 nmol and 40 nmol of siRNA for up to 96 hours. Positive controls and transfection efficiency were measured with AllStars Hs Cell Death Control siRNA (Qiagen, Valencia, CA, USA). Negative control siRNA transfection was performed using a nonsilencing siRNA (AllStars Negative Control siRNA, Qiagen, Valencia, CA, USA).
RNA extraction, reverse transcription and real-time PCR
RNA was extracted from cells and frozen tissue by homogenization in RNeasy Lysis Buffer (Buffer RLT, Qiagen, Valencia, CA, USA) using the manufacturer’s instructions (RNeasy Mini Kit; Qiagen, Valencia, CA, USA). RNA purity was confirmed by spectrophotometry.
First-strand cDNA synthesis was performed using one microgram of each RNA sample primed with SuperScript™ First-Strand Synthesis system, Oligo (dT)12–18 primer, random hexamers and superscript II reverse transcriptase (Invitrogen, Carlsbad, CA). A reaction mixture containing 2.5 μl of cDNA template, 12.5 μl TaqMan Universal PCR master mix (Applied Biosystems, Foster city, CA, USA), and 1.25 μl primer probe mixture was amplified using the following thermal cycler parameters: incubation at 50°C for 2 minutes and denaturing at 95°C for 10 minutes, then 40 cycles of the amplification step (denaturation at 95°C for 15 seconds and annealing/extension at 60°C for 1 minute). BRAF, NIS, and TSHr gene expression were measured in triplicate and normalized relative to the house-keeping gene β-glucoronidase (Applied Biosystems, Foster city, CA, USA). Themean of the reference normalized expression measurements (ΔCt)in triplicate was used for statistical analysis. Gene expressionvalues were calculated according to the ΔΔCT method.
BRAFV600E mutation analysis
gDNA was extracted from cells and tumor samples using the QIAamp DNA Mini Kit (Qiagen, Valencia, CA, USA) according the manufacturer’s instructions. The following primers adapted from those previously reported [27] were used as a template for a standard PCR reaction: forward 5′-TGCTTGCTCTGATAGGAAAATG-3′, reverse 5′-GACTTTCTAGTAACTCAGCAGC-3′. The PCR product was purified using the QIAQuick PCR Purification Kit (Qiagen, Valencia, CA, USA) and visualized on a 2% agarose gel, which confirmed the presence of a single band of approximately 238 base pairs. The PCR product was then direct-sequenced using an Applied Biosystems Automated 3730×l DNA analyzer (Biotechnology Resource Center of Cornell University, Ithaca, NY. USA).
Western Blots
Protein was extracted with Radioimmunoprecipitation assay (RIPA) Lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA, USA) containing protease inhibitors. Protein concentration was determined by the Pierce BCA assay method according to the manufacturers protocol (Thermo Scientific, Waltham, MA, USA). Fifty μg of protein per lane was loaded on a 12% SDS-PAGE gel. After a semi-dry transfer onto nitrocellulose membrane, blots were incubated in a 5% milk in 0.05% Tris buffer saline solution with tween (TSBT) blocking solution for 1 hour. Membranes were probed overnight at 4°C with primary antibodies targeting NIS (1:200), TSH (1:1000), or phospho-ERK (1:200) in 5% milk in 0.05% TBST (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Blots were then incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) as appropriate for 1 hour at room temperature. Immunoreactive bands were visualized using Amersham ECL Enhanced Chemiluminescence System (GE Healthcare, Piscataway, NJ, USA). Blots were then stripped with 10 mL of Restore PLUS Western Blot Stripping Buffer (Thermo Scientific, Rockford, IL, USA) and re-probed for either GAPDH (Abcam, Cambridge, MA, USA, 1:10000) or -β-actin (Cell Signaling, 1:1000) as loading controls.
Radionucleotide Uptake in vitro
Radioactive iodine uptake was measured according to the protocol used by Haddad et al with slight modifications [28]. 131I was measured at 24, 48, 72 and 96 hours after siRNA transfection. Cells were trypsonized and 5 ×105 cells were transferred into each well of a 12-well plate in 1 ml of medium. One μCi of 131I was added to each well and incubated for 30 minutes. After completion of incubation time, 0.5 ml of fresh medium was added into each well. Cells were then centrifuged and the supernatant was transferred into a second test tube at which point the cells were washed with PBS three times. Tubes were then weighed (#1 containing the pellet of cells, #2 containing media, #3 containing wash 1, and #4 containing wash #2) and radiation was recorded by a Wizard scintillation well counter. A Pierce BCA protein assay was performed in parallel in order to measure protein concentration. Results were normalized to protein milligrams and expressed as cpm/mg.
Statistical analysis
Data was presented as mean ± standard deviation (normally distributed continuous variables), median and range (non-normally distributed continuous variables), % change, or fold-change of gene expression. Significance was assessed using Fisher’s exact test, student t- test, or Wilcoxon rank sum test as appropriate. Statistical significance was set at p=0.05. Statistical analysis was performed using SPSS 18.0 statistical software (Cornell University, NY).
RESULTS
BRAFV600E MUTATION AND THYROID IODINE METABOLIZING GENE EXPRESSION IN A PATIENT POPULATION
A representative cohort of 47 patients who underwent a thyroidectomy for cancer was selected (Table 1). The BRAFV600E mutation was detected in 24 of the 47 (51%) of the malignant tumors. The presence of extra-thyroidal extension was significantly higher (68% vs. 32%, p=0.020) and the prevalence of the follicular variant of papillary thyroid carcinoma (fvPTC) was significantly lower (18.2% vs. 81.8%, p=0.017) among tumors carrying the BRAFV600E mutation. There was no association between the presence of the BRAFV600E mutation and age, sex, tumor size, lymph node metastases, angiolympathic invasion, and multifocality.
Table 1.
Patient Demographics, Histopathologic Features, and Iodine Metabolizing Gene Expression.
| Total (N=47) | BRAF V600E (N=24) | BRAFwt (N=23) | P-value* | |
|---|---|---|---|---|
|
| ||||
| PATIENT DEMOGRAPHICS | ||||
|
| ||||
| Age (mean±SD) | 44.2±13.9 | 46.0±15.2 | 42.2±12.5 | 0.35 |
| Gender | ||||
| Male (N=12) | 12 (25.5%) | 7 (58.3%) | 5 (41.7%) | 0.74 |
| Female (N=35) | 35 (74.5%) | 17 (48.6%) | 18 (53.3%) | |
|
| ||||
| HISTOPATHOLOGIC FEATURES | ||||
|
| ||||
| Tumor size in cm [median (range)] | 1.5 (0.7–8.0) | 1.9 (0.8–8.0) | 1.2 (0.7–4.5) | 0.12 |
| Histologic Subtypes | ||||
| Papillary carcinoma (PTC) | 24 (51.1%) | 13 (54.2%) | 11 (45.8%) | 0.77 |
| Follicular variant of PTC | 11 (23.4%) | 2 (18.2%) | 9 (81.8%) | 0.02 |
| Tall cell variant of PTC | 6 (12.8%) | 5 (83.3%) | 1 (16.7%) | 0.19 |
| Follicular carcinoma | 2 (4.3%) | 0 (0%) | 2 (100%) | 0.23 |
| Poorly differentiated | 4 (8.5%) | 4 (100%) | 0 (0%) | 0.11 |
| Extrathyroidal extension | 25 (53.2%) | 17 (68%) | 8 (32%) | 0.02 |
| Lymphovascular invasion | 11 (23.4%) | 7 (63.4%) | 4 (36.4%) | 0.49 |
| Multifocality | 18 (38.3%) | 9 (50.0%) | 9 (50.0%) | 1.00 |
| Lymph node metastases | 20 (42.6%) | 11 (55.0%) | 9 (45.0%) | 0.77 |
|
| ||||
| IODINE METABOLIZING GENE EXPRESSION** | ||||
|
| ||||
| NIS | 0.53 ± 0.35 | 0.27 ± 0.03 | 0.78 ± 0.09 | <0.01 |
| TSHr | 0.62 ± 0.05 | 0.42 ± 0.09 | 0.71 ± 0.02 | <0.01 |
• p-values were derived using student’s t-test (age and gene expression), Wilcoxon rank-sum test (tumor size), and Fisher’s exact chi-squared (all other variables).
Gene expression given as ΔΔ CT±SD.
Mean gene expression of NIS and TSHr was then measured from 10 of the tumors that carried the BRAFV600E mutation and 10 BRAFWT tumors. Tumors carrying the BRAFV600E mutation had significantly lower gene expression of NIS (ΔCT±SD 0.27±0.03 vs. 0.78±0.09, p <0.01) and TSHr (ΔCT±SD 0.42±0.09 vs. 0.71±0.02, p <0.01) compared to BRAFWT tumors (Table 1).
EFFECT OF BRAF KNOCKDOWN IN HUMAN THYROID CANCER CELLS IN A PHYSIOLOGIC (CONTROL) TSH ENVIRONMENT
Transient knockdown of BRAF in human thyroid cancer cells
First, to establish the effectiveness of siRNA transfection for inhibiting BRAF and restoring NIS and TSHr expression, transfections were carried out in a physiologic (control) TSH environment using three different siRNA doses (10 nmol, 20 nmol, and 40 nmol). Significant differences in BRAF, NIS, and TSHr expression were noted with all three concentrations compared to negative controls, but there were no significant differences among the different concentrations. For simplicity, only the results of the 20 nmol transfections are presented (results of the 10 nmol and 40 nmol transfections are available upon request).
BRAF gene expression was decreased by an average of 51% (p<0.01) 24 hours post-transfection (Figure 1A). Inhibition was first detected at 12 hours and returned to near-baseline levels at 36 hours. To measure BRAF activity at the protein level, Western blots for P-ERK (the main downstream actuator of the MAP/MEK/ERK cascade) were performed at baseline, 24, 48, and 72 hours after siRNA transfection. A transient 27% decrease in P-ERK protein expression occurred at 24 hours and returned to slightly above baseline by 72 hours (Figure 1B).
Figure 1.
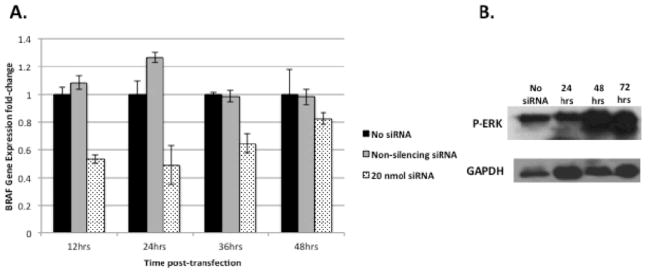
Knockdown of BRAF expression following transfection of siRNAin WRO human thyroid cancer cells. (A) Total RNA was quantified by qPCR at four different time points following transfection with 20 nmol of siRNA and compared to non-transfected cells and transfection with non-silencing siRNA. WRO cells exhibited a 51% (p<0.01) decrease in BRAF gene expression 24 hours post-transfection. This inhibitory effect was first observed at 12 hours and returned to near-baseline by 48 hours. (B) Western blot of P-ERK (a downstream actuator of the RAF/MEK/ERK signal pathway) showing a 27% reduction of protein levels when normalized to GAPDH 24 hours post-transfection and recovery of pre-transfection levels by 48 hours.
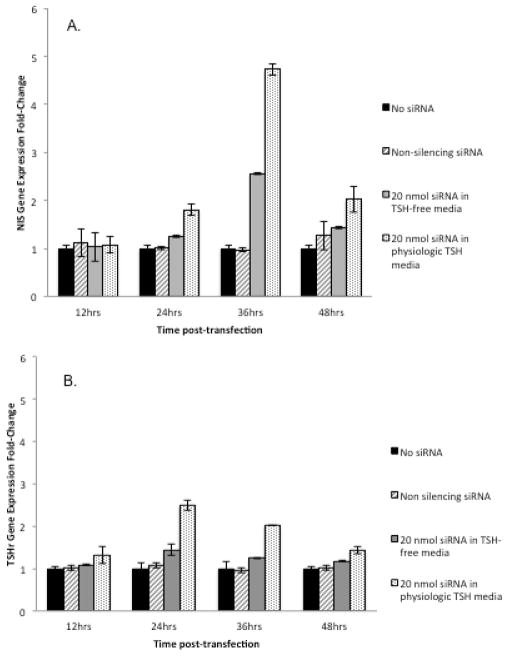
Restoration of NIS and TSHr gene expression and protein levels following siRNA transfection
To evaluate the effect of BRAF inhibition on iodine metabolizing proteins, NIS and TSHr gene expression and protein levels were measured pre- and post-transfection. NIS gene expression increased by an average of 3.9 fold (p=0.01) 36 hours after siRNA transfection relative to non-transfected cells and returned to near-baseline by 48 hours (Figure 2A). NIS Western blots performed at 24, 48, and 72 hours post-transfection revealed a band at 50 kDa (non-glycosylated NIS) and an additional wide band of approximately 87–150 kDa (glycosylated NIS), as has been described previously [29]. NIS protein levels increased by 50% 48 hours and returned to baseline by 72 hours (Figure 2B)
Figure 2.
Changes in NIS and TSHr gene expression and protein levels following transfection of 20 nmol of siRNA in WRO cells compared to non-transfected cells and transfection with non-silencing siRNA in a physiologic (control) TSH environment. (A) NIS gene expression increased by 3.9 fold (p=0.01) 36 hours after transfection with 20 nmol of siRNA. (B) Western blot of NIS showing a 50% increase of protein levels 48 hours post-transfection that returned to baseline by 72 hours. (C) TSHr gene expression increased by 2.5 fold (p= 0.02) 24 hours post-transfection with 20nmol of siRNA. (D) Western blot of TSHr showing increased protein levels at 24 hours post-transfection which returned to baseline by 48 hours.
TSHr gene expression increased by an average of 2.5 fold (p= 0.02) 24 hours post-transfection (Figure 2C). TSHr protein levels similarly increased at 24 hours compared to pre-transfection levels and returned to baseline by 48 hours (Figure 2D).
EFFECT OF TSH WITHDRAWAL AND SUPPLEMENTATION ON IODINE UPTAKE FOLLOWING BRAF INHIBITION
Effect of TSH withdrawal on iodine metabolizing genes
WRO cells grown in H5 media (no TSH) were found to have significantly lower NIS gene expression 36 hours after transfection than cells grown in H6 media (physiologic TSH concentration) (ΔCT±SD 2.6±0.02 vs. 4.7±0.07, p=0.02) (Figure 3A). Similarly, TSHr gene expression was significantly lower in cells grown in H5 media compared to cells grown in H6 media 24 hours after transfection in WRO (ΔCT±SD 1.4±0.01 vs. 2.2±0.07, p =0.03) (Figure 3B).
Figure 3.
The effect of ambient TSH on NIS and TSHr gene expression following BRAF silencing. A) WRO cells grown in H6 media (physiologic TSH concentration) were found to have significantly increased NIS recovery 36 hours after transfection with siRNA than cells grown in H5 (TSH-free) media (4.7±0.07 vs. 2.6±0.02, p=0.02). B) TSHr gene expression recovery was also significantly higher in cells grown in H6 media compared to cells grown in H5 media 24 hours after transfection in WRO cells lp(2.2±0.07 vs. 1.4±0.01, p =0.03).
Effect of TSH concentration on 131I uptake after BRAF inhibition
TSH concentration was found to have a significant dose-dependent effect on 131I uptake following siRNA transfection (Figure 4). WRO cells grown in H5 media (no TSH) showed no increase in 131Iuptake 72 hours after siRNA transfection compared to non-transfected cells, while WRO cells grown in H6 media (physiologic TSH) showed a 7.5-fold (p<0.01) increase at the same time-point. Furthermore, supplementing the media with supratherapeutic TSH resulted in an even greater 9.1-fold increase of 131I uptake (p<0.01) at 72 hours compared to non-transfected cells. The maximal 131I uptake that was achieved in the supratherapeutic TSH environment was 1.7-fold higher than the maximum uptake achieved in physiologic TSH conditions (p<0.01). 131I uptake was not significantly changed when cells grown in any of the three conditions were not transfected or were transfected with non-silencing siRNA (Figure 4).
Figure 4.
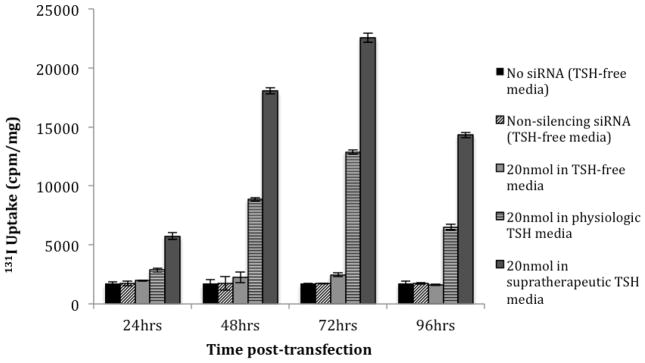
131I uptake of WRO cells following transfection of 20 nmol of siRNABRAF when grown in three different TSH environments: no TSH (H5), physiologic TSH (H6), and media supplemented with 50x the physiologic concentration of TSH (supratherapeutic). 131I uptake did not increase in TSH-free media, increased by 7.5-fold (p<0.01) in physiologic TSH media, and increased by 9.1 fold (p<0.01) in supratherapeutic TSH media 72 hours after siRNA transfection. Non-transfected WRO cells and WRO cells transfected with non-silencing siRNA grown in all three media conditions are shown as negative controls.
DISCUSSION
RAIR thyroid cancers present a deadly dilemma for patients and clinicians, as currently alternate medical treatments for local recurrences and metastases are limited [24]. Since the BRAFV600E mutation is present in the majority of RAIR tumors, it is an attractive candidate for the development of targeted molecular therapies.
In the first part of this study, we demonstrated in a small representative patient population that the BRAFV600E mutation is associated with aggressive clinical features and decreased expression of NIS and TSHr. Both of these observations have been reported previously[30–34] and are included to provide a clinical context for the subsequent in vitro data.
The main aim of this study was to determine if the combination of BRAF inhibition and TSH supplementation results in greater cellular iodine uptake than implementation of each strategy independently. This multimodal approach utilizes two strategies that already have been established or are currently under clinical investigation independently and thus offers a simple and feasible strategy to restore susceptibility of RAIR tumors to 131I. To inhibit BRAF, we elected to use siRNA against the BRAF gene because of the highly specific inhibition of BRAF that is achieved. The same specificity is not achieved with similar strategies that use tyrosine kinase inhibitors or small molecules MAPK pathway inhibitors such as PLX4720.
To quantify activity of the RAF/MEK/ERK pathway at the protein level following BRAF inhibition, we elected to measure P-ERK levels rather than BRAF. P-ERK lies downstream of BRAF and activates hundreds of other transcription factors during activation of the cascade [35, 36]. Therefore, measuring this step of the pathway provides the most direct assessment of its overall activity. In addition, Western blots for P-ERK are more reliable and more reproducible than Western blots for BRAF and therefore, P-ERK is more suitable for use in this study. Although a profound inhibitory effect on BRAF and P-ERK was not observed with siRNA transfection, the inefficiency of knockdown of this pathway has previously been reported. Galabova-Kovacs and Matzen et al demonstrated that knocking out BRAF in mouse embryos resulted in virtual absence of P-ERK in the placenta but only a 60% reduction in the embryo [37]. Pritcherd et al similarly observed an incomplete reduction of P-ERK levels in fibroblasts of BRAF-knockout mice [38]. These findings have led to the conclusion that a complex array of cell signaling and kinase activity occurs along multiple steps of the RAF/MEK/ERK pathway that can circumvent BRAF-dependent kinase activity [39]. Thus, targeting a single step along this pathway in an attempt to abrogate downstream activity has proven to be a difficult and often inefficient endeavor.
Increasing expression of iodine metabolizing genes by knocking down BRAF is not, by itself, a novel concept. In 2007, Liu et al observed restoration of TSHr and NIS expression in BRAFV600E-mutant human papillary thyroid cancer cells after transfection with U0126, a direct MEK-pathway inhibitor [40]. More recently, Chakravarty et al demonstrated in mice engineered with doxycycline-inducible BRAFV600E expression that activation of the BRAF mutation results in near-complete abolition of several iodine metabolizing genes including NIS and TSHr (23). Expression of these genes as well as 124I thyroid uptake was partially recovered by switching off the BRAFV600E mutation and also by treating the mice with PLX4720, a small molecule BRAF inhibitor. Interestingly, dox-induction of BRAFV600E also rendered the mice hypothyroid and significantly increased plasma TSH concentrations at the time of the iodine-uptake assays. Although the mice returned to a euthyroid state two weeks after doxycycline withdrawal, iodine uptake was not reassessed at that time and, therefore, the influence of the elevated TSH is unclear from this study.
There are two main limitations to this paper. First, the siRNA utilized in this study targets a region of the BRAF gene outside of the V600E mutation site and thus silences BRAF wild-type and mutant cells equally. It is therefore unclear if these results are unique to BRAFV600E mutants or could be observed in BRAF wild-type cells as well. Second, the effect of the siRNA transfection on BRAF gene expression was modest, and the observed changes at the protein level were even smaller. As noted above, the intricacies of the RAF/MEK/ERK pathway are not yet completely understood and it is believed that multiple escape pathways exist involving other members of the RAF family of kinases to continue MEK and ERK phosphorylation despite BRAF inhibition.
In conclusion, we have demonstrated that the simultaneous implementation of two independent treatment strategies for RAIR thyroid tumors (BRAF inhibition and TSH supplementation) increases 131I uptake more effectively than when either modality is implemented alone. This promising approach represent a substantial advancement in the treatment of RAIR thyroid cancers and may offer a new option for patients who have failed our current treatment algorithms.
Acknowledgments
This investigation was supported in part by grant TL1RR024998 of the Clinical and Translational Science Center at Weill Cornell Medical College, and by the Dancers Care Foundation.
References
- 1.Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. The Journal of clinical endocrinology and metabolism. 2006;91:2892–2899. doi: 10.1210/jc.2005-2838. [DOI] [PubMed] [Google Scholar]
- 2.Antonelli A, Fallahi P, Ferrari SM, et al. Dedifferentiated thyroid cancer: a therapeutic challenge. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2008;62:559–563. doi: 10.1016/j.biopha.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 3.Zarnegar R, Brunaud L, Kanauchi H, et al. Increasing the effectiveness of radioactive iodine therapy in the treatment of thyroid cancer using Trichostatin A, a histone deacetylase inhibitor. Surgery. 2002;132:984–990. doi: 10.1067/msy.2002.128690. discussion 990. [DOI] [PubMed] [Google Scholar]
- 4.Riesco-Eizaguirre G, Santisteban P. A perspective view of sodium iodide symporter research and its clinical implications. European journal of endocrinology/European Federation of Endocrine Societies. 2006;155:495–512. doi: 10.1530/eje.1.02257. [DOI] [PubMed] [Google Scholar]
- 5.Nilsson M. Iodide handling by the thyroid epithelial cell. Experimental and clinical endocrinology & diabetes: official journal, German Society of Endocrinology [and] German Diabetes Association. 2001;109:13–17. doi: 10.1055/s-2001-11014. [DOI] [PubMed] [Google Scholar]
- 6.Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature. 1996;379:458–460. doi: 10.1038/379458a0. [DOI] [PubMed] [Google Scholar]
- 7.Saito T, Endo T, Kawaguchi A, et al. Increased expression of the Na+/I- symporter in cultured human thyroid cells exposed to thyrotropin and in Graves’ thyroid tissue. The Journal of clinical endocrinology and metabolism. 1997;82:3331–3336. doi: 10.1210/jcem.82.10.4269. [DOI] [PubMed] [Google Scholar]
- 8.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 9.Lee JH, Lee ES, Kim YS. Clinicopathologic significance of BRAF V600E mutation in papillary carcinomas of the thyroid: a meta-analysis. Cancer. 2007;110:38–46. doi: 10.1002/cncr.22754. [DOI] [PubMed] [Google Scholar]
- 10.Michaloglou C, Vredeveld LC, Mooi WJ, Peeper DS. BRAF(E600) in benign and malignant human tumours. Oncogene. 2008;27:877–895. doi: 10.1038/sj.onc.1210704. [DOI] [PubMed] [Google Scholar]
- 11.Kim TH, Park YJ, Lim JA, et al. The association of the BRAF(V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: A Meta-Analysis. Cancer. 2011 doi: 10.1002/cncr.26500. [DOI] [PubMed] [Google Scholar]
- 12.Riesco-Eizaguirre G, Rodriguez I, De la Vieja A, et al. The BRAFV600E oncogene induces transforming growth factor beta secretion leading to sodium iodide symporter repression and increased malignancy in thyroid cancer. Cancer research. 2009;69:8317–8325. doi: 10.1158/0008-5472.CAN-09-1248. [DOI] [PubMed] [Google Scholar]
- 13.Ricarte-Filho JC, Ryder M, Chitale DA, et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer research. 2009;69:4885–4893. doi: 10.1158/0008-5472.CAN-09-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durante C, Puxeddu E, Ferretti E, et al. BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. The Journal of clinical endocrinology and metabolism. 2007;92:2840–2843. doi: 10.1210/jc.2006-2707. [DOI] [PubMed] [Google Scholar]
- 15.Espadinha C, Santos JR, Sobrinho LG, Bugalho MJ. Expression of iodine metabolism genes in human thyroid tissues: evidence for age and BRAFV600E mutation dependency. Clinical endocrinology. 2009;70:629–635. doi: 10.1111/j.1365-2265.2008.03376.x. [DOI] [PubMed] [Google Scholar]
- 16.Oler G, Cerutti JM. High prevalence of BRAF mutation in a Brazilian cohort of patients with sporadic papillary thyroid carcinomas: correlation with more aggressive phenotype and decreased expression of iodide-metabolizing genes. Cancer. 2009;115:972–980. doi: 10.1002/cncr.24118. [DOI] [PubMed] [Google Scholar]
- 17.Romei C, Ciampi R, Faviana P, et al. BRAFV600E mutation, but not RET/PTC rearrangements, is correlated with a lower expression of both thyroperoxidase and sodium iodide symporter genes in papillary thyroid cancer. Endocrine-related cancer. 2008;15:511–520. doi: 10.1677/ERC-07-0130. [DOI] [PubMed] [Google Scholar]
- 18.Chakravarty D, Santos E, Ryder M, et al. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. The Journal of clinical investigation. 2011;121:4700–4711. doi: 10.1172/JCI46382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nucera C, Nehs MA, Nagarkatti SS, et al. Targeting BRAFV600E with PLX4720 displays potent antimigratory and anti-invasive activity in preclinical models of human thyroid cancer. The oncologist. 2011;16:296–309. doi: 10.1634/theoncologist.2010-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pacini F, Ladenson PW, Schlumberger M, et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled study. The Journal of clinical endocrinology and metabolism. 2006;91:926–932. doi: 10.1210/jc.2005-1651. [DOI] [PubMed] [Google Scholar]
- 21.Pilli T, Brianzoni E, Capoccetti F, et al. A comparison of 1850 (50 mCi) and 3700 MBq (100 mCi) 131-iodine administered doses for recombinant thyrotropin-stimulated postoperative thyroid remnant ablation in differentiated thyroid cancer. The Journal of clinical endocrinology and metabolism. 2007;92:3542–3546. doi: 10.1210/jc.2007-0225. [DOI] [PubMed] [Google Scholar]
- 22.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid: official journal of the American Thyroid Association. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 23.Ma C, Xie J, Liu W, et al. Recombinant human thyrotropin (rhTSH) aided radioiodine treatment for residual or metastatic differentiated thyroid cancer. Cochrane database of systematic reviews. 2010:CD008302. doi: 10.1002/14651858.CD008302.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Neill CJ, Oucharek J, Learoyd D, Sidhu SB. Standard and emerging therapies for metastatic differentiated thyroid cancer. The oncologist. 2010;15:146–156. doi: 10.1634/theoncologist.2009-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schweppe RE, Klopper JP, Korch C, et al. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. The Journal of clinical endocrinology and metabolism. 2008;93:4331–4341. doi: 10.1210/jc.2008-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitsiades CS, Negri J, McMullan C, et al. Targeting BRAFV600E in thyroid carcinoma: therapeutic implications. Mol Cancer Ther. 2007;6:1070–1078. doi: 10.1158/1535-7163.MCT-06-0449. [DOI] [PubMed] [Google Scholar]
- 27.Arora N, Scognamiglio T, Lubitz CC, et al. Identification of borderline thyroid tumors by gene expression array analysis. Cancer. 2009;115:5421–5431. doi: 10.1002/cncr.24616. [DOI] [PubMed] [Google Scholar]
- 28.Haddad D, Chen NG, Zhang Q, et al. Insertion of the human sodium iodide symporter to facilitate deep tissue imaging does not alter oncolytic or replication capability of a novel vaccinia virus. Journal of translational medicine. 2011;9:36. doi: 10.1186/1479-5876-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levy O, Dai G, Riedel C, et al. Characterization of the thyroid Na+/I- symporter with an anti-COOH terminus antibody. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:5568–5573. doi: 10.1073/pnas.94.11.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimura ET, Nikiforova MN, Zhu Z, et al. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer research. 2003;63:1454–1457. [PubMed] [Google Scholar]
- 31.Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocrine reviews. 2007;28:742–762. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 32.Jin L, Sebo TJ, Nakamura N, et al. BRAF mutation analysis in fine needle aspiration (FNA) cytology of the thyroid. Diagnostic molecular pathology: the American journal of surgical pathology, part B. 2006;15:136–143. doi: 10.1097/01.pdm.0000213461.53021.84. [DOI] [PubMed] [Google Scholar]
- 33.Kim J, Giuliano AE, Turner RR, et al. Lymphatic mapping establishes the role of BRAF gene mutation in papillary thyroid carcinoma. Annals of surgery. 2006;244:799–804. doi: 10.1097/01.sla.0000224751.80858.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim KH, Kang DW, Kim SH, et al. Mutations of the BRAF gene in papillary thyroid carcinoma in a Korean population. Yonsei medical journal. 2004;45:818–821. doi: 10.3349/ymj.2004.45.5.818. [DOI] [PubMed] [Google Scholar]
- 35.Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth factors. 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- 36.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 37.Galabova-Kovacs G, Matzen D, Piazzolla D, et al. Essential role of B-Raf in ERK activation during extraembryonic development. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1325–1330. doi: 10.1073/pnas.0507399103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pritchard CA, Hayes L, Wojnowski L, et al. B-Raf acts via the ROCKII/LIMK/cofilin pathway to maintain actin stress fibers in fibroblasts. Molecular and cellular biology. 2004;24:5937–5952. doi: 10.1128/MCB.24.13.5937-5952.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galabova-Kovacs G, Kolbus A, Matzen D, et al. ERK and beyond: insights from B-Raf and Raf-1 conditional knockouts. Cell cycle. 2006;5:1514–1518. doi: 10.4161/cc.5.14.2981. [DOI] [PubMed] [Google Scholar]
- 40.Liu D, Hu S, Hou P, et al. Suppression of BRAF/MEK/MAP kinase pathway restores expression of iodide-metabolizing genes in thyroid cells expressing the V600E BRAF mutant. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13:1341–1349. doi: 10.1158/1078-0432.CCR-06-1753. [DOI] [PubMed] [Google Scholar]