Abstract
Context
While the delivery of cell therapy following ST segment myocardial infarction (STEMI) has been evaluated in previous clinical trials, the influence of the timing of cell delivery on the effect on left ventricular (LV) function has not been analyzed in a trial that randomly designated the time of delivery.
Objective
To determine 1) the effect of intracoronary autologous bone marrow mononuclear cell (BMC) delivery following STEMI on recovery of global and regional LV function and 2) if timing of BMC delivery (3 versus 7 days following reperfusion) influences this effect.
Design, Setting, and Patients
Between July 17, 2008 and November 15, 2011, 120 patients were enrolled in a randomized, 2×2 factorial, double-blind, placebo-controlled trial of the National Heart, Lung, and Blood Institute (NHLBI)-sponsored Cardiovascular Cell Therapy Research Network (CCTRN) of patients with LV dysfunction (LV Ejection Fraction (LVEF) ≤45%) following successful primary percutaneous coronary intervention (PCI) of anterior STEMI.
Interventions
Intracoronary infusion of 150 × 106 BMCs or placebo (randomized 2:1 BMC:placebo) within 12 hours of aspiration and processing administered at Day 3 or Day 7 (randomized 1:1) post-PCI.
Main Outcome Measures
Co-primary endpoints were: 1) Change in global (LVEF) and regional (wall motion) LV function in infarct and border zones at 6 months measured by cardiac magnetic resonance imaging and 2) Change in LV function as affected by timing of treatment on Day 3 versus Day 7. Secondary endpoints included major adverse cardiovascular events as well as changes in LV volumes and infarct size.
Results
Patient mean age was 56.9±10.9 years with 87.5% male. At 6 months, LVEF increased similarly in both BMC (45.2±10.6 to 48.3±13.3 %) and placebo groups (44.5±10.8 to 47.8±13.6 %). No detectable treatment effect on regional LV function was observed in either infarct or border zones. Differences between therapy groups in the change in global LV function over time when treated at Day 3 (−0.9±2.9%, 95% CI 6.6 to 4.9%, p=0.763) or Day 7 (1.1±2.9%, 95% CI −4.7 to 6.9, p=0.702) were not significant, nor were they different from each other. Also, timing of treatment had no detectable effect on recovery of regional LV function. Major adverse events were rare with no difference between groups.
Conclusions
Patients with STEMI, who underwent successful primary PCI and administration of intra-coronary BMCs at either 3 or 7 days following the event, had recovery of global and regional LV function similar to placebo
Trial Registration
ClinicalTrials.gov Number, NCT00684021
INTRODUCTION
Cell therapy may eventually become a therapeutic option for patients following acute myocardial infarction (AMI), potentially preventing the transition to end-stage heart failure where cardiac transplantation is currently the only curative procedure available. Recent meta-analyses of bone marrow mononuclear cell (BMC) delivery to the infarct zone following AMI have shown small improvements in left-ventricular (LV) function after successful reperfusion1. However, despite a growing number of trials, many fundamental questions such as optimal timing of BMC delivery remain unanswered. Myocardium and bone marrow undergo important changes days to weeks following AMI that may affect stem/progenitor (S/P) cell engraftment and survival2. This notion has support from the REPAIR-AMI trial3 that determined in a prospectively specified analysis that delivery of BMCs 5-7 days post-AMI resulted in greater improvement in LV ejection fraction (LVEF) compared with earlier delivery. However, this important variable has never been evaluated in a prospective trial that randomly selects the day of cell delivery.
The National Heart, Lung, and Blood Institute (NHLBI) established the Cardiovascular Cell Therapy Research Network (CCTRN) to address mechanistic questions in cardiovascular cell therapy. The recently completed LateTIME4 trial found BMC administration did not influence the ongoing post-reperfusion recovery of either global or regional LV function when delivered 2-3 weeks following AMI. Here we present results of a companion trial investigating influences of timing of cell delivery within the first week following AMI on the course of improving global and regional LV function following reperfusion.
METHODS
Study Design
TIME (Timing In Myocardial infarction Evaluation) was a randomized, double-blinded, placebo-controlled trial investigating the timing of intracoronary autologous BMCs within the first week following reperfusion in a high-risk STEMI cohort5. Between July 17, 2008 and November 15, 2011, 120 patients with LVEF ≤45% by echocardiography following primary percutaneous coronary intervention (PCI) with stenting were enrolled. Exclusions included previous bypass surgery or prior STEMI with residual LV dysfunction (EF <55%).
Study Protocol
Each clinical center and the data coordinating center have independent institutional review board approvals and oversight. Briefly, all qualifying participants provided written informed consent and were randomized (1:1) to receive therapy on either Day 3 or 7 following primary PCI with stenting. Race/ethnicity was documented as self-described by participants. Demographic and clinical variables were determined by interview and the patient’s medical record. All patients had cardiac magnetic resonance imaging (MRI) at Day 3 (baseline) and those randomized to delivery on Day 7 had another MRI on Day 7 (baseline). Patients underwent bone marrow aspiration on the morning of their treatment day and BMCs were isolated using a closed, automated Ficoll cell processing system, (Sepax, Biosafe, Eysins, Switzerland)6 to ensure a uniform cellular product across centers. After the cell product passed stipulated lot release criteria, a second randomization to either cells (2:1) or cell-free placebo occurred. Patients randomized to BMCs received a product containing 150 × 106 total nucleated cells (TNCs) (70-80% BMCs). Patients randomized to placebo received a cell-free product of 5% albumin in normal saline, with 100 microliters of autologous blood added to ensure color and consistency matched the BMC group.
Within 12 hours of aspiration, the BMCs or placebo was infused in the infarct-related artery (Maverick balloon catheter, Boston Scientific, Natick, MA) in six aliquots of five ml each using stop-flow technique.5 All patients were heparinized during the procedure to activated clotting time (ACT) > 200 sec. and treated with aspirin and 75 mg of clopidogrel in addition to other guideline-recommended post-MI medications.
Study Outcomes
Wall Motion Imaging
Cardiac MRI of global and regional LV function has been previously described4; 5 Imaging using protocols developed by the MRI core laboratory (University of Florida) were performed using 1.5T scanners that had been certified before study initiation.
Statistical Analyses
Outcome Measures of Interest
Co-primary endpoints were: 1) change of global (LVEF) and regional LV function (infarct and border zone) by MRI between baseline and 6 months when administered within the first 7 days following PCI and 2) whether these changes were dependent on day of administration (Day 3 versus 7). Secondary endpoints included major adverse cardiovascular events as well as effects on LV volumes and infarct size. Subgroup analysis for age, gender, race, hypertension, diabetes, statins, drug-eluting stent (DES) versus bare metal stent (BMS) and LVEF was pre-specified. The distribution of participants across therapy groups precluded diabetes and statin analyses.
Sample Size Consideration
Statistical methods utilized have been detailed previously5. Briefly, global LV function was assessed by MRI derived LVEF, for which we assumed an effect size or placebo-adjusted change (difference in the change over time in the BMC group minus the change in the placebo group) of δ =5 % and a common group standard deviation of the difference of LVEF over time as σLVEF (Δ) = 7 as derived from the Wollert7, Lunde8, Schachinger9, and Janssens10 publications.
Regional LV function measure was defined as the change in wall motion over time in 1) the infarct zone and 2) the infarct border zone. The infarct zone was defined as the segments with the largest two late signal intensity enhancement (SIE) measures with gadolinium (using a 17 segment model). The border zone was defined as those regions adjacent to the infarct zone in which the SIE were in the 10 to 75% range of transmurality. For each of these measures of regional LV function, we assumed an effect size of δ =6.7 mm and a common group standard deviation of σ LVEF (Δ) = 9.57. Sixty patients each were required in an assessment of the 3- and 7-Day effects of therapy. This yield of 120 patients produced over 90% power for an overall assessment of therapy combining the Day 3 and 7 groups, as well as for comparing the Day 3 to the Day 7 effect.
Exact testing for categorical variables and Student’s t-testing for continuous variables assessed the compatibility of baseline variables between groups. All hypothesis testing was two-sided, and all effect sizes and their 95% confidence intervals (CIs), were evaluated using the general linear model adjusting for center and demographics. No adjustments for multiple comparisons were made in this Phase II study, and a p –value of 0.05 was used to assess statistical significance.
RESULTS
Screening and Enrollment
Between July 2008 and November 2011, a total of 3347 patients were screened with almost half excluded for LVEF >45% (Figure 1). There were no statistically significant differences between BMC and placebo groups in baseline characteristics except for higher peak creatine kinase (CK) and troponin levels among BMC patients randomized to Day 7 and lack of diabetes among Day 7 placebo group (Table 1). The qualifying LVEF (protocol-specified by echocardiography) within 48 hours of PCI ranged from 36.1 to 37.8%.
Figure 1.
CONSORT Diagram
Abbreviations: MI, myocardial infarction; PCI, percutaneous coronary intervention; EF, ejection fraction; AE, adverse event; SAE, serious adverse event; BMC, bone marrow mononuclear cell; MRI, magnetic resonance imaging
*A clinical hold is an order issued by FDA to the sponsor to suspend an ongoing investigation; this hold was issued to ensure proper screening and monitoring of subjects during the investigation by excluding subjects with LV thrombus or atrial fibrillation who required anticoagulation therapy
±All MRIs contraindicated because of ICD placement
Table 1.
Baseline Characteristics of BMC and Placebo Groupsa
| 3 Day | 7 Day | |||
|---|---|---|---|---|
| BMC N=43 |
Placebo N=24 |
BMC N=36 |
Placebo N=17 |
|
| Patient Characteristics: | ||||
| Age, mean (SD) | 55.6 (10.8) | 57.0 (12.4) | 58.2 (11.3) | 57.0 (8.0) |
| Female | 5 (11.6) | 3 (12.5) | 5 (13.9) | 2 (11.7) |
| Height in inches, mean (SD) | 69.7 (3.4) | 68.3 (3.1) | 68.9 (4.2) | 69.3 (4.5) |
| Weight in pounds, mean (SD) | 211.6 (41.4) | 197.6 (50.3) | 202.9 (45.1) | 214.7 (35.9) |
| BMI, mean (SD) | 30.5 (5.4) | 29.6 (6.8) | 29.9 (5.5) | 31.3 (3.3) |
| Race: | ||||
| White | 38 (88.4) | 20 (83.3) | 31 (86.1) | 15 (88.2) |
| Nonwhite | 5 (11.63) | 4 (16.67) | 5 (13.89) | 2 (11.76) |
| Qualifying LVEF % (echocardiogram), mean (SD) | 36.1 (6.1) | 37.8 (6.6) | 36.5 (6.3) | 36.6 (4.1) |
| History of: | ||||
| Diabetes | 10 (23.3) | 8 (33.3) | 4 (11.1) | 0 (0.0) |
| High Blood Pressure | 19 (44.2) | 15 (62.5) | 23 (63.9) | 13 (76.5) |
| Hyperlipidemia | 28 (65.1) | 15 (62.5) | 25 (69.4) | 13 (76.5) |
| Angina | 7 (16.3) | 2 (8.3) | 6 (16.7) | 5 (29.4) |
| Smoking | 28 (65.1) | 17 (70.8) | 19 (52.8) | 11 (64.7) |
| Initial Presentation at ED: | ||||
| Heart Rate | ||||
| Initial at ED, mean (SD) | 81.5 (14.2) | 78.7 (13.6) | 74.2 (15.3) | 82.3 (17.7) |
| median (range) | 82.0 (70.0-91.0) | 74.5 (70.0-90.5) | 75.5 (65.0-82.5) | 82.0 (75.0-89.0) |
| At discharge, mean (SD) (N=42 BMC 3 day) | 76.8 (12.1) | 79.5 (14.9) | 75.8 (10.4) | 78.1 (9.2) |
| median (range) | 74.5 (68.0-85.0) | 76.5 (70.0-88.5) | 77.5 (68.5-83.5) | 78.0 (72.0-82.0) |
| BP in mm Hg, mean (SD), (N=42 BMC 3 day) | ||||
| Systolic | 115.2 (14.0) | 115.4 (11.0) | 111.5 (16.4) | 112.0 (16.4) |
| Diastolic | 70.2 (10.7) | 68.3 (7.7) | 68.7 (11.2) | 69.5 (7.4) |
| Preinfarction Angina | 10 (23.3) | 7 (29.2) | 11 (30.6) | 7 (41.2) |
| Labs: | ||||
| Hemoglobin in gm/dL, mean (SD) (N=38 BMC 3 day, N=17 Placebo 3 day; N=29 BMC 7 day, N=15 Placebo 7 day) |
14.1 (1.7) | 12.8 (1.5) | 14.1 (1.5) | 13.9 (2.0) |
| hsCRP in mg/L, median (IQR) (N=39 BMC 3 day, N=21 Placebo 3 day; N=33 BMC 7 day, N=16 Placebo 7 day) |
20.8 (8.9-52.2) | 38.8 (10.8-49.4) | 28.1 (9.5-48.6)) | 34.0 (16.1-48.0) |
| BNP reg in pg/ml, median (IQR) (N=34 BMC 3 day, N=20 Placebo 3 day; N=30 BMC 7 day, N=15 Placebo 7 day) |
189.0 (90.0- 394.0) |
205.5 (118.0- 394.5) |
177.5 (139.0- 238.0) |
150.0 (125.0- 370.0) |
| Peak CKMB in ng/ml, median (IQR) (N=29 BMC 3 day, N=19 Placebo 3 day; N=31 BMC 7 day, N=15 Placebo 7 day) |
180.9 (42.1- 1302.0) |
133.0 (62.0- 432.7) |
402.0 (234.0- 466.0) |
227.0 (76.0- 442.0) |
| Peak Troponin in ng/ml, mean (SD) | ||||
| T (N=18 BMC 3 day, N=12 Placebo 3 day; N=21 BMC 7 day, N=14 Placebo 7 day) |
9.0 (7.8) | 6.2 (4.7) | 12.3 (8.1) | 10.9 (8.3) |
| I, mean (SD) (N=14 BMC 3 day, N=4 Placebo 3 day; N=5 BMC 7 day, N=1 Placebo 7 day) |
58.6 (82.6) | 46.1 (38.5) | 189.4 (140.6) | 128.9 (−) |
| MI Treatment: | ||||
| Ischemic Time in hours, median (IQR) | 3.4 (2.4-7.6) | 3.6 (2.2-8.6) | 4.0 (2.1-6.5) | 3.5 (2.2-11.8) |
| Door to Balloon in hours, median (IQR) (N=42 BMC 3 day) |
1.2 (0.7-1.7) | 1.3 (0.6-2.4) | 1.5 (1.0-1.9) | 1.2 (0.6-2.2) |
| Transferred from outside hosp. after PCI | 5 (11.6) | 2 (8.3) | 2 (5.6) | 0 (0) |
| Time from bone marrow aspiration to infusion in hours, median (IQR) |
8.4 (7.9-9.2) | 8.8 (8.0-9.5) | 7.9 (7.5-8.9) | 8.6 (7.8-9.0) |
| Time from PCI to infusion in days, median (IQR) (N=42 BMC 3 day) |
3.3 (2.8-3.8) | 3.2 (2.5-4.1) | 7.4 (7.0-7.9) | 7.6 (7.0-8.3) |
| Drug Eluting Stent | 33 (76.74) | 21 (87.50) | 29 (80.56) | 14 (82.35) |
| Stent Region: | ||||
| LAD | 37 (86.0) | 23 (95.8) | 35 (97.2) | 17 (100) |
| LAD only | 35 (81.4) | 22 (91.7) | 33 (91.7) | 17 (100) |
| LAD + LCX | 0 (0.0) | 1 (4.2) | 1 (2.8) | 0 (0.0) |
| LAD + RCA | 2 (4.7) | 0 (0.0) | 1 (2.8) | 0 (0.0) |
| LCX only | 1 (2.3) | 1 (4.7) | 1 (2.8) | 0 (0.0) |
| RCA only | 5 (11.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Medications at Time of Randomization: | ||||
| ACE inhibitor | 35 (81.4) | 19 (79.2) | 33 (91.7) | 13 (76.5) |
| Plavix/Plasugrel/Clopidogel | 42 (97.7) | 23 (95.8) | 33 (91.7) | 17 (100) |
| Aspirin | 42 (97.7) | 24 (100) | 34 (94.4) | 17 (100) |
| BetaBlockers | 42 (97.7) | 24 (100) | 35 (97.2) | 16 (94.1) |
| Statins | 39 (90.7) | 22 (91.7) | 34 (94.4) | 17 (100) |
| Diuretics | 7 (16.3) | 5 (20.8) | 11 (30.6) | 2 (11.8) |
| Coumadin/Warfarin/Lovenox | 4 (9.3) | 3 (12.5) | 11 (30.6) | 3 (17.7) |
Abbreviations: BMC, bone marrow mononuclear cell; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); LVEF, left ventricular ejection fraction; ED, emergency department; hsCRP, highsensitivity C-reactive protein; BNP, brain natriuretic peptide; BP, blood pressure; IQR, interquartile range; PCI, percutaneous coronary intervention; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; RCA, right coronary artery
Data are presented as No. (%) unless otherwise specified.
Cell Harvesting and Processing
Mean time from PCI to bone marrow aspiration and cell processing was 3.3 days in the Day 3 group and 7.5 days in the Day 7 group. All BMC aspirates underwent automated cell processing at each center using Sepax. No patients suffered complications associated with the bone marrow harvesting.
Intracoronary Infusion
Median time from bone marrow aspiration to infusion was 8.3 hours in the BMC group (Table 1) and all patients received approximately 150 million TNCs. The mean viability of the final BMC product was 98.2% and contained 2.2% CD34+ cells and 1.1% CD34+/CD133+ cells (Table 2). The cell product was devoid of significant red blood cell contamination, contained only minuscule amounts of heparin (0.1 U/ml) and most participants were infused within an hour of completion of cell processing11 thereby avoiding concerns recently expressed in the literature. 12, 13 In vitro and in vivo studies comparing the delivery of Sepax-derived BMCs with that of open Ficoll-selected BMCs demonstrated phenotypic equivalence and equal efficacy on hind limb recovery in a murine model of hind limb perfusion. All patients received systemic heparin during treatment infusion, as in REPAIR-AMI and other trials using “stop-flow”. No complications were associated with intracoronary infusion.
Table 2.
Cell Characteristics of BMC and Placebo Groupsa
| 3 Day | 7 Day | |||
|---|---|---|---|---|
| BMC N=43 |
Placebo N=24 |
BMC N=36 |
Placebo N=17 |
|
| Total nucleated cells/product (x106), mean (SD) | 146.6 (22.3) | 149.5 (1.7) | 146.2 (12.0) | 145.4 (14.7) |
|
| ||||
| % Viability by Trypan Blue Exclusion, mean (SD) | 98.1 (1.7) | 98.7 (1.0) | 98.1 (1.4) | 97.9 (1.6) |
|
| ||||
| % CD34 cells/product, mean (SD)b (N=41 BMC 3 day, N=23 Placebo 3 day; N=28 BMC 7 day, N=16 Placebo 7 day) |
2.4 (1.3) | 2.2 (1.0) | 1.6 (0.8) | 2.4 (0.9) |
| % CD34+/CD133+ cells/product, mean (SD)b (N=41 BMC 3 day, N=23 Placebo 3 day; N=28 BMC 7 day, N=16 Placebo 7 day) | 1.1 (0.7) | 1.2 (0.8) | 0.9 (0.6) | 1.2 (0.6) |
|
| ||||
| Colony-forming units-Hill/product, median (IQR)b (N=30 BMC 3 day, N=17 Placebo 3 day; N=25 BMC 7 day, N=11 Placebo 7 day) |
120 (0-330) | 120 (0-180) | 165 (0-390) | 330 (60-750) |
|
| ||||
| Endothelial colony-forming cells/product, median (IQR)b (N=29 BMC 3 day, N=16 Placebo 3 day; N=26 BMC 7 day, N=10 Placebo 7 day) |
0 (0-480) | 0 (0-265) | 0 (0-300) | 120 (0-420) |
Abbreviations: BMC, bone marrow mononuclear cell.
BMC vs placebo group comparisons are not statistically significant.
A separate consent was used for the biorepository and four patients declined participation. Additionally four patients had insufficient product for the Biorepository analysis, and some analyzed data was unreportable.
Safety
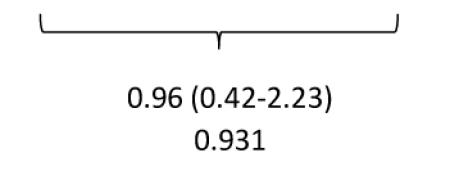
Despite a perceived high-risk cohort of patients with moderate to severe LV dysfunction following large STEMIs, there were very few clinical events (Table 3). One death occurred (due to subarachnoid hemorrhage) following randomization to the BMC group but before cell delivery was performed. Eleven patients underwent repeat revascularization and six received implantable cardiac defibrillators (ICDs). There was no significant difference between the relative incidences of events comparing BMC and placebo groups.
Table 3.
Clinical/Safety Outcomes at 6-Month End Point Window
| BMC (n=79) |
Placebo (n=41) |
Total Overall | |||
|---|---|---|---|---|---|
| Deaths | 1 | 1 | |||
| Reinfarctions | 1 | 2 | 3 | ||
| Repeat Revascularizations | 7 | 4 | 11 | ||
| Target Vessel | 2 | 3 | |||
| Non-Target Vessel | 5 | 1 | |||
| Hospitalization Heart Failure | 4 | 1 | 5 | ||
| ICD Placements | 3 | 3 | 6 | ||
|
| |||||
| Total Events | 16 | 10 | 26 | ||
| Patients | 13 (16%) | 7 (17%) | 20 (17%) | ||
| Crude Incidence Rate | 0.165 | 0.171 | 0.167 | ||
| Relative Risk (95% CI) p-value |
|
||||
Abbreviations: BMC, bone marrow mononuclear cell; ICD, implantable cardiac defibrillator
LV Function Assessment
Follow-up MRIs were not performed in 8 patients as 1 had died, 3 had ICD placements and 4 declined for miscellaneous reasons (discomfort, anxiety, scheduling/travel issues) (Figure 1).
Overall Effects of BMC versus Placebo
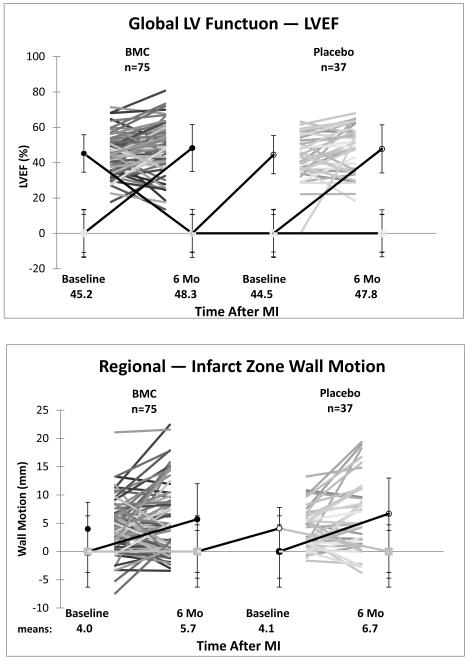
When both BMC groups (n=75) were combined and compared to a combined placebo group (n=37), LVEF in the BMC group increased from 45.2±10.6% at baseline to 48.3±13.3% at 6 months while the combined placebo group increased from 44.5±10.8% to 47.8±13.6%. Overall, there was no significant change in the difference between the two groups (−0.1±2.0%, 95% CI = −4.1 to 3.9, p=0.959). A similar failure for BMCs to improve regional wall motion in the infarct zone (−0.9 ± 1.1 mm, 95% CI = −3.0 to 1.2, p = 0.409) and border zone (−0.5 ± 1.7 mm, CI = −3.9 to 2.9, p=0.777) was observed (Table 4, Figure 2).
Table 4.
End Point Analyses of Global and Regional LV Function Between Baseline and 6 Months
| BMC | Placebo | Analysis Between- Group Difference in 6- Month Change (95% CI) |
||||||
|---|---|---|---|---|---|---|---|---|
| No. | Mean (SD) |
95% CI | No. | Mean (SD) |
95% CI |
P Value |
||
| Overall Effect of Therapy | ||||||||
| Global LV Function | ||||||||
| LVEF, % | ||||||||
| Baseline | 75 | 45.2(10.6) | 37 | 44.5(10.8) | ||||
| Follow-up | 75 | 48.3(13.3) | 37 | 47.8(13.6) | ||||
| Within-group change | 75 | 3.2(10.3) | (0.9 to 5.5) | 37 | 3.3(9.7) | (0.2 to 6.4) | −0.1 (−4.1 to 3.9) | 0.959 |
|
| ||||||||
| Regional LV Function | ||||||||
| Infarct zone wall motion, mm | ||||||||
| Baseline | 75 | 4.0(4.7) | 37 | 4.1(3.7) | ||||
| Follow-up | 75 | 5.7(6.3) | 37 | 6.7(6.3) | ||||
| Within-group change | 75 | 1.7(5.5) | (0.5 to 2.9) | 37 | 2.6(4.9) | (1.0 to 4.2) | −0.9 (−3.0 to 1.2) | 0.409 |
| Border zone wall motion, mm | ||||||||
| Border zone wall motion, mm | ||||||||
| Baseline | 75 | 15.2(9.9) | 37 | 13.1(10.4) | ||||
| Follow-up | 75 | 19.1(11.8) | 37 | 17.4(13.0) | ||||
| Within-group change | 75 | 3.8(8.8) | (1.8 to 5.8) | 37 | 4.3(8.0) | (1.7 to 6.9) | −0.5 (−3.9 to 2.9) | 0.777 |
|
| ||||||||
| Day 3 | ||||||||
| Global LV Function | ||||||||
| LVEF, % | ||||||||
| Baseline | 41 | 46.1 (11.1) |
22 | 41.6 (10.0) |
||||
| Follow-up | 41 | 49.6 (14.2) |
22 | 45.9 (13.8) |
||||
| Within-group change | 41 | 3.5 (11.0) | (0.1 to 6.9) |
22 | 4.4 (10.6) | (0.0 to 8.8) | −0.9 (−6.6 to 4.9) | 0.763 |
|
| ||||||||
| Regional LV Function | ||||||||
| Infarct zone wall motion, mm | ||||||||
| Baseline | 41 | 4.2 (5.2) | 22 | 3.7 (4.3) | ||||
| Follow-up | 41 | 6.3 (6.9) | 22 | 6.1 (6.7) | ||||
| Within-group change | 41 | 2.1 (5.9) | (0.3 to 3.9) |
22 | 2.4 (5.3) | (0.2 to 4.6) | −0.3 (−3.3 to 2.7) | 0.824 |
| Border zone wall motion, mm | ||||||||
| Baseline | 41 | 16.7 (11.2) | 22 | 12.6 (11.0) | ||||
| Follow-up | 41 | 20.2 (12.9) |
22 | 16.9 (12.9) |
||||
| Within-group change | 41 | 3.5 (9.3) | (0.7 to 6.3) |
22 | 4.3 (8.7) | (0.7 to 7.9) | −0.8 (−5.6 to 4.0) | 0.745 |
|
| ||||||||
| Day 7 | ||||||||
| Global LV Function | ||||||||
| LVEF, % | ||||||||
| Baseline | 34 | 44.0 (9.9) | 15 | 48.8 (10.9) |
||||
| Follow-up | 34 | 46.8 (12.3) |
15 | 50.4 (13.3) |
||||
| Within-group change | 34 | 2.8 (9.7) | (−0.5 to 6.1) |
15 | 1.7 (8.2) | (−2.4 to 5.8) | 1.1 (−4.7 to 6.9) | 0.702 |
|
| ||||||||
| Regional LV Function | ||||||||
| Infarct zone wall motion, mm | ||||||||
| Baseline | 34 | 3.8 (3.9) | 15 | 4.7 (2.7) | ||||
| Follow-up | 34 | 5.0 (5.5) | 15 | 7.4 (5.9) | ||||
| Within-group change | 34 | 1.2 (4.9) | (−0.4 to 2.8) | 15 | 2.8 (4.4) | (0.6 to 5.0) | −1.6 (−4.5 to 1.4) | 0.297 |
| Border zone wall motion, mm | ||||||||
| Baseline | 34 | 13.5 (7.7) | 15 | 13.8 (9.7) | ||||
| Follow-up | 34 | 17.7 (10.3) |
15 | 18.2 (13.6) |
||||
| Within-group change | 34 | 4.2 (8.3) | (1.4 to 7.0) |
15 | 4.4 (7.2) | (0.8 to 8.0) | −0.1 (−5.1 to 4.8) | 0.955 |
Figure 2.
Global Left Ventricular Function and Regional Infarct and Border Zone Wall Motion
BMC=bone marrow mononuclear cell; MI=myocardial infarction
Analysis of Global and Regional LV Function – Day 3 Group
A total of 41 BMC group patients and 22 placebo patients had paired MRI data at baseline and 6 months available for analysis of global and regional LV function in the Day 3 group. The LVEF in the BMC group on the day of treatment was 46.1±11.1% and increased to 49.6±14.2% at 6 months, while the placebo group increased from 41.6±10% to 45.9±13.8% at 6 months. There was no significant difference between the change in LVEF of the BMC group compared to the change in LVEF of the placebo group (−0.9±2.9%, 95% CI = −6.6 to 4.9%, p=0.763).
Similarly, infarct zone wall motion in the BMC group on day of treatment was 4.2±5.2mm compared to 3.7±4.3mm in the placebo group. The difference in the changes in infarct zone wall motion between the two groups was not significant (−0.3±1.5mm, 95% CI = −3.3 to 2.7, p=0.824). In the border zone, wall motion in the BMC group on day of treatment was 16.7±11.2mm versus 12.6±11.0mm in the placebo group. The difference between the 6-month changes in both groups was not significant (−0.8±2.4mm; 95% CI= −5.6 to 4.0, p=0.745).
Analysis of Global and Regional LV Function – Day 7 Group
A total of 34 patients in the BMC group and 15 patients in the placebo group had paired MRI data at baseline and 6 months available for analysis of global and regional LV function in the Day 7 group. Baseline LVEF measured on treatment day (Day 7) was 44.0±9.9% in the BMC group and increased to 46.8±12.3% at 6 months as placebo increased from 48.8±10.9% to 50.4±13.3% with no overall change in differences between groups (1.1±2.9%, 95%CI −4.7 to 6.9, p=0.702).
Regional wall motion in the infarct zone was 3.8±3.9 in the BMC group and 4.7±2.7mm with placebo. Overall, there was no significant difference in changes in infarct wall motion when comparing the groups (−1.6±1.5mm; 95%CI −4.5 to 1.4, p=0.297). Baseline border zone wall motion was 13.5±7.7mm in the BMC group and 13.8±9.7mm in the placebo group with no overall change in differences between the two groups (−0.1±2.5mm, 95%CI −5.1 to 4.8, p=0.955).
Comparison of the Day 3 to the Day 7 Effect
For LVEF, the placebo-adjusted effect of BMC at Day 3 was −0.9±2.9%, and Day 7 was 1.1±2.9%. The difference between the two was not significant (2.0±4.2, 95%CI - 6.3 to 10.2, p=0.635). For infarct zone wall motion, the placebo adjusted effect of BMC at Day 3 was −0.3±1.5 mm, and at Day 7 was −1.6±1.5mm. This difference was also not significant (−1.2±2.2; 95%CI −5.5 to 3.1; p=0.571). For border zone wall motion, placebo adjusted effect of BMC on Day 3 was −0.8±2.4mm, and Day 7 was −0.1±2.5mm. The difference between these was not significant (0.6±3.5, 95%CI −6.3 to 7.6; p=0.855).
Secondary Endpoints and Subgroup Analyses
LV end diastolic volume index (LVEDVI) increased by 11.7 ± 18.9 ml/m2 in the BMC group and 10.9 ±18.1 ml/m2 in the placebo group, which was not significantly different (change = 0.8 ml/m2 ± 3.7; 95% CI −6.6 to 8.2; p = 0.831). Likewise LV end systolic volume index (LVESVI) increased by 5.0 ± 16.0 ml/m2 in the BMC group and 4.3 ±14.9 ml/m2 in the placebo group (change = 0.7 ml/m2 ± 3.2; 95% CI –5.5 to 7.0; p = 0.817. Infarct volumes uniformly decreased in both groups at both times but again, the differences between BMC and placebo were not significant. Day of treatment did not influence secondary endpoints. Models adjusting for center, age, diabetes, hypertension, hyperlipidemia, weight, infarct location, infarct size (peak CK) and percent CD34+ cells did not change unadjusted results.
Several predetermined subgroup analyses were performed in the BMC group. In contrast to previous studies14, 15 there was no improvement in the recovery of LV function among patients with more depressed LVEF at baseline (LVEF < 45 via MRI). No difference was observed in global or regional function in patients stratified by ischemic time.
DISCUSSION
TIME is the first cardiovascular cell therapy trial which was specifically designed to determine whether the timing of BMC administration following primary PCI influences LV functional recovery. There was no overall effect of BMC treatment on this ongoing improvement at 6 months vs. placebo in spite of previous supportive clinical data.1; 3 Additionally, the day of cell delivery did not demonstrate an effect on the recovery of LV function nor on LV volumes or infarct size.
The design of TIME was based on previous data that the timing of cell delivery may be critical.1, 3, 5 During the initial days to weeks following STEMI there are significant temporal changes in the release of cytokines such as stromal-derived factor (SDF)-116, and growth factors such as vascular endothelial growth factor (VEGF) and insulin-like growth factor (IGF)-1 that may support stem cell homing and angiogenesis leading to improved cell survival and engraftment. Conversely, reactive oxygen species and inflammatory cytokines such as Interleukin (IL)-6 and tumor necrosis factor (TNF)-α released by myocardium and circulating inflammatory cells may adversely affect the bone marrow and stem cell function and/or survival. These inflammatory mediators may impair the quality of cells harvested from the bone marrow as observed in a recent pre-clinical study demonstrating that BMCs are more potent several weeks following STEMI compared to those harvested a few days post-STEMI as a result of inflammatory changes in the bone marrow mediated by IL-117. The relative role of these potential positive and negative influences on cell therapy was uncertain.
TIME was developed shortly after early randomized trials suggested that autologous, intracoronary BMCs may improve LV function after AMI7, 9. Although several subsequent trials did not observe improved LV function8, 10, 18, 19, a recent Cochrane meta-analysis suggests very small improvement in LVEF (mean change 1.8%; 95% CI 0.3 to 3.3) when measured by MRI as used in TIME1. Currently, a study to detect such a difference in LV ejection fraction, would require 875 patients and would imply that this difference is biologically important. While these findings do not exclude this suggested effect size (95% CI for overall effect −4.1 to 3.9; for Day 3 effect −6.6 to 4.9, for Day 7 effect −4.7 to 6.9), it is reasonable to critically examine some possible contributing aspects so that future studies in this area may proceed from an enlightened position.
Going forward it is crucial to understand how well this cohort did with contemporary management. In the age of aggressive primary prevention and rapid and successful primary PCI, identifying patients with significant LV dysfunction following a first MI is challenging. The centers screened 3347 patients (of which about half did not have moderate or severe LV dysfunction) to identify 132 patients who were randomized.22. Among those qualifying with moderate or severe LV dysfunction ischemic time was remarkably brief (median 3-4 hours), all received PCI with stenting, and guideline-based medications were highly utilized. This management was associated with recovery of LV function yielding an aggregate EF at 6 months exceeding 48%. As has been reported elsewhere, existing data indicate that EF would be expected to continue to increase at 18 and 36 months in half of the cohort and links with mortality are no longer apparent when EF exceeds 45%.20 Indeed since initiation of TIME and LateTIME, the Network has observed only a single cardiovascular-related death (sub-arachnoid hemorrhage prior to receiving-study product) among 207 patients with moderate to large anterior STEMIs.
However, there is likely considerable heterogeneity among the cohort and it would be of interest to identify a population at greatest risk that might benefit (e.g. those at risk for EF <45% at 6 months). If prospective cohorts cannot be identified, then an alternative approach is to recruit patients who have already demonstrated incomplete recovery at later time points and/or to consider novel cell types.21 The development of novel and sensitive measures of LV function to serve as surrogate endpoints continues to be a requirement in this field.
Finally, the phenotype and functionality of the BMC product in this population may be an issue. BMCs from ischemic cardiomyopathy patients have reduced colony-forming unit capacities and impaired migration to SDF-1 and VEGF that translate into reduced blood flow in the ischemic hind limb model.22 Endothelial progenitor cells from coronary artery disease (CAD) patients also have impaired CXCR4 signaling with diminished neovascularization.23 Cytokine production from BMCs is reduced compared to other bone marrow and adipose-derived cell types.24 These considerations suggest that an autologous cell product derived from CAD patients, as in TIME, may have less regenerative capacity vs. allogeneic products obtained from younger healthy donors.25
Although the field of cell therapy in cardiovascular disease holds great promise, our study is consistent with the possibility that BMCs are not effective at improving LV function when delivered into the immediate post-STEMI myocardial environment. However, long-term follow-up of these patients and the development of new composite endpoints may still reveal a role for this cell type following AMI. Recent and ongoing studies continue to assess the role of BMCs in other areas such as heart failure and critical limb ischemia.26
Conclusions
Overall, the improvement in LV function observed 6 months after reperfusion of STEMI did not appear to be influenced by BMCs, regardless of the timing of delivery, within the initial week following reperfusion. These data should inform the future development of cell therapies for STEMI.
Acknowledgements
Funding/Support: Funding for this trial was provided by the National Heart, Lung, and Blood Institute under cooperative agreement 5 U01 HL087318-04.
Additional Acknowledgments: The Cardiovascular Cell Therapy Research Network would like to also acknowledge its industry partners: Biosafe (Geneva, Switzerland) and Boston Scientific Corporation (Natick, Massachusetts) for contributions of equipment and technical support during conduct of the trial.
We also thank the NHLBI Gene and Cell Therapies Data and Safety Monitoring Board and the NHLBI Protocol Review Committee for their review and guidance of the LateTIME trial.
Author Contributions: Dr. Moye had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The following authors have made substantial contributions to the intellectual content of the paper in these respective areas:
Study Concept and Design: Aguilar, Baran, Bettencourt, Ellis, Forder, Gordon, Hatzopoulos, Henry, Kwak, Lai, Loghin, Moyé, Penn, Piller, Raveendran, Simari, Simpson, Skarlatos, Traverse, Vaughan, Willerson
Acquisition of Data: Anderson, Bowman, Chambers, Cogle, Ellis, Forder, Francescon, Gee, Geither, Handberg, Henry, Kappenman, Lambert, Lerman, Moyé, Olson, Penn, Pepine, Perin, Piller, Raveendran, Sayre, Simon, Simpson, Taylor, Traverse, Vojvodic, Westbrook, Willerson, Zhao, Zierold
Analysis and Interpretation of Data: Anderson, Baraniuk, Bettencourt, Chambers, Ebert, Ellis, Forder, Gee, Gordon, Henry, Kwak, Lai, Loghin, Moyé, Penn, Raveendran, Richman, Simari, Taylor, Thomas, Traverse, Willerson, Zhao, Zierold
Drafting of the Manuscript: Gee, Geither, Hatzopoulos, Henry, Kappenman, Moyé, Penn, Perin, Simari, Simpson, Traverse, Willerson
Critical Revision of the Manuscript for Important Intellectual Content: Aguilar, Anderson, Baran, Baraniuk, Bettencourt, Bowman, Chambers, Cogle, Ebert, Ellis, Forder, Francescon, Gordon, Handberg, Henry, Kwak. Lai, Lambert, Lerman, Loghin, Moyé, Olson, Penn, Pepine, Perin, Piller, Raveendran, Richman, Sayre, Simari, Simon, Skarlatos, Taylor, Thomas, Traverse, Vaughan, Vojvodic, Westbrook, Willerson, Zhao, Zierold
Statistical Analysis: Baraniuk, Gee, Kwak, Moyé, Traverse
Obtaining Funding: Hatzopoulos, Penn, Piller, Simari, Simpson, Traverse, Vaughan, Willerson
Administrative, Technical, or Material Support: Aguilar, Anderson, Bettencourt, Bowman, Ebert, Forder, Francescon, Gee, Geither, Gordon, Handberg, Lambert, Lerman, Loghin, Olson, Penn, Pepine, Piller, Raveendran, Sayre, Simari, Simpson, Skarlatos, Taylor, Thomas, Traverse, Vojvodic, Westbrook, Willerson
Supervision: Chambers, Ellis, Forder, Kwak, Lambert, Lerman, Penn, Perin, Raveendran, Simari, Simon, Simpson, Thomas, Traverse
Other: Richman (quality assurance), Taylor (data analysis)
NHLBI Project Office Team: Sonia Skarlatos, PhD, David Gordon, MD, PhD, Ray Ebert, PhD, Wendy Taddei-Peters, PhD, Min Jung Kwak, PhD, and Beckie Chamberlin
CCTRN Steering Committee Chair: Robert Simari, MD
TIME Trial Investigators and Clinical Teams: Minneapolis Heart Institute Foundation: Timothy Henry, MD, Jay Traverse, MD, David McKenna, MD, Beth Jorgenson, RN, and Rachel Olson, RN, MS
Cleveland Clinic Foundation: Stephen Ellis, MD, Marc Penn, MD, PhD, Saif Anwaruddin, MD, James Harvey, MD, Jane Reese Koc, MT, Carrie Geither, RN, Mark Jarosz, RN, and Cindy Oblak
Texas Heart Institute: James Willerson, MD, Emerson Perin, MD, PhD, Guilherme Silva, MD, James Chen, RN, Casey Kappenman, Deirdre Smith, RN, and Lynette Westbrook, RN, MS
University of Florida Department of Medicine: Carl Pepine, MD, Barry Byrne, MD, David Anderson, PhD, MD, John Wingard, MD, Eileen Handberg, PhD, Tempa Curry, RN, and Diann Fisk, MT
Vanderbilt University School of Medicine: David Zhao, MD, Antonis Hatzopoulos, PhD, Allen Naftilan, MD, Sherry Bowman, RN, Judy Francescon, RN, and Karen Prater
St. Paul Heart Clinic, United Hospital: Ken Baran, MD and Jody LaRock, RN
Metro Cardiology, Mercy Hospital: Jeffrey Chambers, MD and Betty Hargan, RN
University of Minnesota: Ganesh Raveendran, MD, Emily Caldwell, RN, and Barb Bruhn-Ding, RN
University Hospitals Case Medical Center: Daniel Simon, MD, Marco Costa, MD, and Stacey Mazzurco, RN
Florida Hospital Pepin Heart Institute: Charles Lambert, MD, PhD and Elizabeth Szymanski, RN
Mayo Clinic: Amir Lerman, MD and Kelly Noonan, RN
Michael E. DeBakey Medical Center: Biswajit Kar, MD
Data Coordinating Center and Laboratory Teams: University of Texas School of Public Health: Lemuel Moye, MD, PhD, Dejian Lai, PhD, Linda Piller, MD, MPH, Lara Simpson, PhD, Sarah Baraniuk, PhD, Shreela Sharma, PhD, Judy Bettencourt, MPH, Shelly Sayre, MPH, Rachel Vojvodic, MPH, Larry Cormier, Robert Brown, PhD, Diane Eady, Kristen Lucas, MS, Sibi Mathew, and Michelle Cohen, MPH
Baylor College of Medicine: Adrian Gee, PhD, Sara Richman, David Aguilar, MD
University of Texas Medical School: Catalin Loghin, MD
University of Florida Department of Medicine: John Forder, PhD (MRI Core Laboratory) and Christopher R. Cogle, MD and Elizabeth Wise, (Biorepository Core-Florida)
Cleveland Clinic C5 Research Imaging Core: James Thomas, MD, Allen Borowski, Annitta Flinn, and Cathy McDowell (Echo Core Laboratory)
Center for Cardiovascular Repair: Doris Taylor, PhD, Claudia Zierold, PhD, and Marjorie Carlson, (Biorepository Core-Minnesota)
Role of the Sponsor: The funding sponsor had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation of this manuscript. The funding sponsor reviewed and approved this manuscript.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. All authors reported receiving research grant funding and support for travel expenses to meetings for the Cardiovascular Cell Therapy Research Network (CCTRN) from the National, Heart, Lung and Blood Institute (NHLBI). Dr. Ellis reported receiving travel support from Abbott Vascular/Boston Scientific; Dr. Hanberg reported receiving grant funding from Amorcyte, Baxter and BDI; Dr. Hatzopoulos reported receiving funding from NHLBI for writing and review manuscripts, as well as participation in review activities; Dr. Henry reported serving as a consultant for Capricor and receiving grant funding from Capricor and Osiris; Dr. Penn reported serving as a consultant for Aastrom and Juventas and receiving grant funding from Athersys, as well as having patents, royalties, stock/stock options and travel expenses from Juventas Therapeutics; Dr. Pepine reported receiving funding for writing assistance, medicines, equipment or administrative support from NHLBI and Biosafe; Dr. Perin reported serving as a consultant for Cytori, Celgene, Biosense Webster, and Cephalon, as well as grant funding from NIH; Dr. Simon reported serving as a consultant for Cordis/Johnson & Johnson, Medtronic Vascular, Merck, Medicine Company, and Portola, as well as serving as an expert witness for Cordis/Johnson& Johnson and receiving payment for lectures from Abbott Vascular; Dr. Taylor reported receiving payment for lectures from AHA; Dr. Thomas reported that he served as president of the American Society of Echocardiography in 2011-12 and received grant funding from NSBRI/NASA; Ms. Westbrook reported receiving travel funding from Barnett International; Dr. Willerson reported receiving funding from NHLBI for participation in review activities;
REFERENCES
- 1.Clifford DM, Fisher SA, Brunskill SJ, et al. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2012;2:CD006536. doi: 10.1002/14651858.CD006536.pub3. doi: 10.1002/14651858.CD006536.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008;58(2):88–111. doi: 10.1016/j.phrs.2008.06.007. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schachinger V, Erbs S, Elsasser A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1210–1221. doi: 10.1056/NEJMoa060186. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 4.Traverse JH, Henry TD, Ellis SG, et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: The LateTIME randomized trial. JAMA. 2011;306(19):2110–2119. doi: 10.1001/jama.2011.1670. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Traverse JH, Henry TD, Vaughan DE, et al. Rationale and design for TIME: A phase II, randomized, double-blind, placebo-controlled pilot trial evaluating the safety and effect of timing of administration of bone marrow mononuclear cells after acute myocardial infarction. Am Heart J. 2009;158(3):356–363. doi: 10.1016/j.ahj.2009.06.009. doi: 10.1016/j.ahj.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aktas M, Radke TF, Strauer BE, Wernet P, Kogler G. Separation of adult bone marrow mononuclear cells using the automated closed separation system sepax. Cytotherapy. 2008;10(2):203–211. doi: 10.1080/14653240701851324. doi: 10.1080/14653240701851324. [DOI] [PubMed] [Google Scholar]
- 7.Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: The BOOST randomised controlled clinical trial. Lancet. 2004;364(9429):141–148. doi: 10.1016/S0140-6736(04)16626-9. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 8.Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1199–1209. doi: 10.1056/NEJMoa055706. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 9.Schachinger V, Erbs S, Elsasser A, et al. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: Final 1-year results of the REPAIR-AMI trial. Eur Heart J. 2006;27(23):2775–2783. doi: 10.1093/eurheartj/ehl388. doi: 10.1093/eurheartj/ehl388. [DOI] [PubMed] [Google Scholar]
- 10.Janssens S, Dubois C, Bogaert J, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: Double-blind, randomised controlled trial. Lancet. 2006;367(9505):113–121. doi: 10.1016/S0140-6736(05)67861-0. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 11.Gee AP, Richman S, Durett A, et al. Multicenter cell processing for cardiovascular regenerative medicine applications: The cardiovascular cell therapy research network (CCTRN) experience. Cytotherapy. 2010;12(5):684–691. doi: 10.3109/14653249.2010.487900. doi: 10.3109/14653249.2010.487900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assmus B, Tonn T, Seeger FH, et al. Red blood cell contamination of the final cell product impairs the efficacy of autologous bone marrow mononuclear cell therapy. J Am Coll Cardiol. 2010;55(13):1385–1394. doi: 10.1016/j.jacc.2009.10.059. doi: 10.1016/j.jacc.2009.10.059. [DOI] [PubMed] [Google Scholar]
- 13.Seeger FH, Rasper T, Fischer A, et al. Heparin disrupts the CXCR4/SDF-1 axis and impairs the functional capacity of bone marrow-derived mononuclear cells used for cardiovascular repair. Circ Res. 2012;111(7):854–862. doi: 10.1161/CIRCRESAHA.112.265678. doi: 10.1161/CIRCRESAHA.112.265678. [DOI] [PubMed] [Google Scholar]
- 14.Tendera M, Wojakowski W, Ruzyllo W, et al. Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: Results of randomized, multicentre myocardial regeneration by intracoronary infusion of selected population of stem cells in acute myocardial infarction (REGENT) trial. Eur Heart J. 2009;30(11):1313–1321. doi: 10.1093/eurheartj/ehp073. doi: 10.1093/eurheartj/ehp073. [DOI] [PubMed] [Google Scholar]
- 15.Miettinen JA, Ylitalo K, Hedberg P, et al. Determinants of functional recovery after myocardial infarction of patients treated with bone marrow-derived stem cells after thrombolytic therapy. Heart. 2010;96(5):362–367. doi: 10.1136/hrt.2009.171694. doi: 10.1136/hrt.2009.171694. [DOI] [PubMed] [Google Scholar]
- 16.Askari AT, Unzek S, Popovic ZB, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362(9385):697–703. doi: 10.1016/S0140-6736(03)14232-8. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Takagawa J, Lam VC, et al. Donor myocardial infarction impairs the therapeutic potential of bone marrow cells by an interleukin-1-mediated inflammatory response. Sci Transl Med. 2011;3(100):100ra90. doi: 10.1126/scitranslmed.3002814. doi: 10.1126/scitranslmed.3002814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsch A, Nijveldt R, van der Vleuten PA, et al. Intracoronary infusion of mononuclear cells from bone marrow or peripheral blood compared with standard therapy in patients after acute myocardial infarction treated by primary percutaneous coronary intervention: Results of the randomized controlled HEBE trial. Eur Heart J. 2011;32(14):1736–1747. doi: 10.1093/eurheartj/ehq449. doi: 10.1093/eurheartj/ehq449. [DOI] [PubMed] [Google Scholar]
- 19.Traverse JH, Henry TD, Vaughan DE, et al. LateTIME: A phase-II, randomized, double-blinded, placebo-controlled, pilot trial evaluating the safety and effect of administration of bone marrow mononuclear cells 2 to 3 weeks after acute myocardial infarction. Tex Heart Inst J. 2010;37(4):412–420. [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen JW, Forder JR, Thomas JD, et al. Quantification of myocardial segmental function in acute and chronic ischemic heart disease and implications for cardiovascular cell therapy trials: A review from the NHLBI-cardiovascular cell therapy research network. JACC Cardiovasc Imaging. 2011;4(6):671–679. doi: 10.1016/j.jcmg.2011.02.015. doi: 10.1016/j.jcmg.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): A prospective, randomised phase 1 trial. The Lancet. 2012;379(9819):895–904. doi: 10.1016/S0140-6736(12)60195-0. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heeschen C, Lehmann R, Honold J, et al. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109(13):1615–1622. doi: 10.1161/01.CIR.0000124476.32871.E3. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 23.Walter DH, Haendeler J, Reinhold J, et al. Impaired CXCR4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circ Res. 2005;97(11):1142–1151. doi: 10.1161/01.RES.0000193596.94936.2c. doi: 10.1161/01.RES.0000193596.94936.2c. [DOI] [PubMed] [Google Scholar]
- 24.Li T, Cheng K, Malliaras K, et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol. 2012;59(10):942–953. doi: 10.1016/j.jacc.2011.11.029. doi: 10.1016/j.jacc.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54(24):2277–2286. doi: 10.1016/j.jacc.2009.06.055. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perin EC, Willerson JT, Pepine CJ, et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: The FOCUS-CCTRN trial. JAMA. 2012;307(16):1717–1726. doi: 10.1001/jama.2012.418. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]