There is growing interest in understanding quality of life (QOL) domains among non-Hodgkin lymphoma (NHL) survivors, the sixth most common cancer in the United States (NCI, 2012). Treatment modalities such as chemotherapy, biologic therapy, and stem cell transplantation have improved survival of patients with NHL to an overall 5 year survival rate of 68% (Horner, Reis, & Krapcho, 2011). Though the survival rate has increased, NHL remains an illness that elicits concerns related to late and long-term effects that affect QOL. A review describing QOL domains of older NHL survivors and the impact cancer has on health found that most studies lacked a conceptual or theoretical framework and representation of socio-demographic diversity, specifically age (Leak, Mayer & Smith, 2011). NHL research has focused primarily on examining the impact of NHL and treatment on survivor’s QOL. Oerlemans et al (2011) systematically reviewed lymphoma studies and found that having higher education, being married or living with a partner, and being male are associated with higher QOL in various cancer populations including NHL.
QOL is a widely accepted outcome measure in cancer research, but little is known about the moderating effect of age on QOL in NHL survivors. The aging population in the US will increase in numbers while NHL incidence rates are expected to rise. We need to better understand the interface of age on overall QOL and its determinants. Studies have focused on the moderator effects on QOL in lymphoma survivors including aerobic exercise training,, gender, general health, and body mass index (BMI) (Courneya, 2011). It is likely that age affects (moderates) the nature of relationships between other demographic and disease characteristics and QOL.
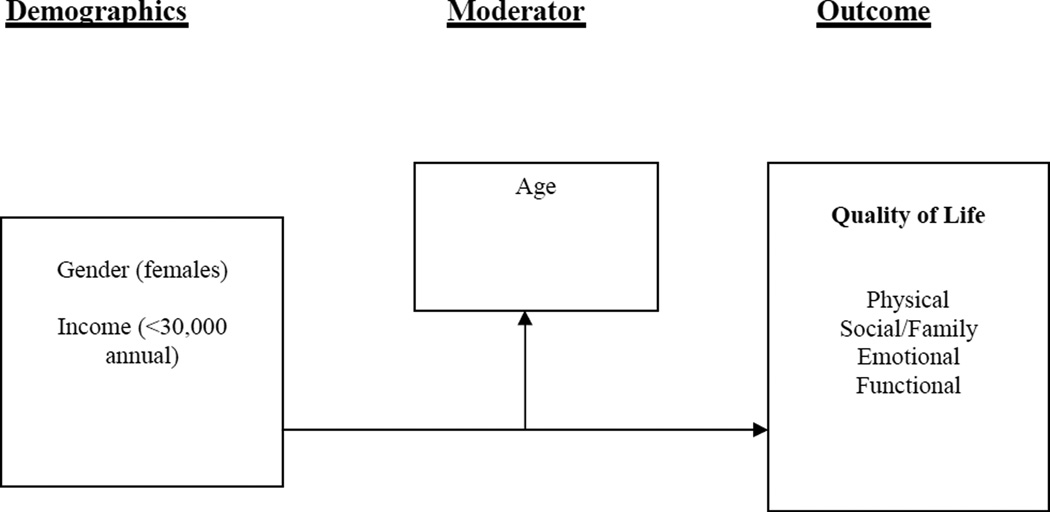
Moderators are independent variables that affect the strength and/or direction of the association between another independent variable and the outcome variable and help to determine when the relationship occurs (Bennett, 2000). Studies have evaluated the impact of sociodemographic and disease characteristics on QOL, however there are limited published reports in the NHL literature to provide insight for clinicians and researchers on the moderated effect of age and its association with QOL. Addressing the age association with personal characteristics could assist clinicians in identifying patients in where individualization of age appropriate cancer care is recommended. The role of age in the association between determinants and QOL has not been investigated in this population, therefore, nursing research can be focused on age sensitive assessments. Without keen assessments, it is difficult to offer interventions to make appropriate resources available without ongoing systematic assessments. The purpose of this study was to explore the relationship of demographic and disease characteristics to QOL and to examine the moderating effect of age among NHL survivors.
Conceptual Model
A cancer survivor adaptation (CSA) model of QOL guided this study (Naus, Ishler, Parrott & Kovacs, 2009). The three-component (personal characteristics, a moderator [age], and outcomes) model proposes that personal characteristics (demographic and disease factors) have a relationship with QOL and there is the potential that age may affect that relationship or its strength. The outcome variable for this model is QOL. The CSA provided a theoretical argument to move the NHL research beyond examining associations to explaining the components of the model.
Methods
The sample was recruited through mailed surveys to individuals who were treated for NHL from 2005 – 2006 at one of two comprehensive cancer centers in the southeastern United States: Duke University and the University of North Carolina at Chapel Hill (UNC). Eligible individuals were 18 years of age or older, at least two years post-diagnosis, and with or without active disease. The response rate was 74% (Smith, Zimmerman, Williams, Preisser & Clipp, 2008). This study was approved by the Duke and UNC Institutional Review Boards. The current study is a secondary analysis of a cross-sectional study of NHL survivors. The primary aim of the parent study was to estimate the prevalence of post-traumatic stress disorder (PTSD) symptoms in survivors of adult NHL who were at least two years post-diagnosis and to identify the risk factors associated with PTSD symptoms (Smith, Zimmerman, Williams, Preisser & Clipp, 2008).
Instruments
Demographic and disease characteristics
Socio-demographic and disease-related characteristics were self-reported obtained from the Tumor Registry. NHL type and stage was provided from the Tumor Registry and the other demographics were self-reported. Comorbidity was measured with the self-administered Comorbidity Questionnaire (SCQ) (Sangha, Stucki, Liang, Fossel, & Katz, 2003). This questionnaire was used to assess past and current health conditions including heart disease, high blood pressure, lung disease, diabetes, ulcer or stomach disease, kidney disease, liver disease, anemia or other blood disease, cancer other than lymphoma or non-melanoma skin cancer, depression, osteoarthritis, degenerative arthritis, back pain, and rheumatoid arthritis. A binary answer (yes-1 or no-0) was supplied for each question. The total comorbidity score ranged from 0 to 30 with higher scores indicating a greater comorbidity burden and lower scores indicating a lower burden.
Quality of life outcome
The 27-item Functional Assessment of Cancer Therapy-General (FACT-G, version 4) (Cella, Tulsky, Gray, et al, 1993; Cella, Webster & Cashy, 2005), was used to measure cancer-related QOL. A higher score indicates a higher perception of QOL. The FACT-G scores ranges from 0–108. There are 4 well-being domains that total a sum for FACT-G including FACT-Physical (FACT-PWB), FACT-Social/Family (FACT-SFWB), FACT-Emotional (FACT-EWB), and FACT-Functional (FACT-FWB). Evidence of satisfactory reliability and validity for FACT-G has been reported in psychometric studies (Cella, Tulsky, Gray, et al, 1993; Cella, Webster & Cashy, 2005), and in the parent study, the reliability of the FACT-G total score was 0.93 (Smith, Zimmerman, Williams, Preisser & Clipp, 2008).
Data Analysis
All analyses were conducted using SPSS 18.0. Multiple regression was used to analyze relationships between demographic and disease characteristics, age, and QOL. The regression model included demographic and disease characteristics that were statistically significant (all at p<.05) in bivariate analyses with QOL. Variables that were significant were jointly entered into the model, and the final model only retained those predictors that were significant at p<.05 (Table 3, Model 1). To test for moderation by age, the interaction between each demographic and disease characteristic and age was added to the model separately, and the significant interactions were jointly added to a single model (Table 3, Model 2). All variables had <5% missing data except for cancer stage (13%) which was therefore excluded from the analyses (Figure 1).
Table 3.
Age as a Moderator of the Relationship of Demographic and Disease Characteristics to Quality of Life (FACT-G Score)
| Characteristics | Model 1: no moderation | Model 2: moderation by age | ||
|---|---|---|---|---|
| B (SE) | P value | B (SE) | P value | |
| Demographic | ||||
| Age | 0.29 | <.0001 | 0.25 | 0.0004 |
| Gender: Female | 1.88 | 0.08 | 10.34 | 0.04 |
| Income: < $30,000 | −6.03 | <.0001 | −24.11 | <.0001 |
| College Graduate | 2.48 | 0.056 | 2.71 | 0.04 |
| Employed | 2.78 | 0.04 | 1.99 | 0.13 |
| Disease | ||||
| Comorbidity Score | −1.36 | <.0001 | −1.36 | <.0001 |
| Currently Receiving | −4.84 | 0.003 | −4.92 | 0.002 |
| Treatment | ||||
| Cancer Treatments: | −4.58 | 0.003 | −4.87 | 0.002 |
| Bone Marrow | ||||
| Transplant | ||||
| Cancer Treatment: | −2.68 | 0.04 | −2.83 | 0.03 |
| Biologic | ||||
| Years Since Diagnosis | 0.16 | 0.048 | 0.13 | 0.11 |
| Female* Age | - | −0.14 | 0.08 | |
| Income *Age | - | 0.28 | 0.001 | |
Figure 1.
Results
Sample
The study sample included 741 NHL survivors who were, on average, 62.3 (SD 13.4) years of age at the time of study. More than 40% were 65 years of age or older (n = 322), and 86% were Caucasian. Over half (58%) were either retired or unemployed, and 27% earned less than $30,000 annually. The mean time since diagnosis for the sample was 10.2 years (SD 7.1). (Table 1). Disease characteristics for the sample included 28% of survivors in Stage 1 and 24% in Stage 4. A majority (80%) reported having had chemotherapy, and about half (48%) reported having had radiation as part of their treatment. The average total comorbidity score was 5.6 (SD 4.8; scale ranged from 0–30). Most commonly reported comorbidities were heart disease (19%), high blood pressure (18%), lung disease (17%), and diabetes (14%). (Table 2).
Table 1.
Demographic Characteristics of the Sample
| Variable | All Survivors (N = 741) |
Total Percent |
< 65 years of age (n = 419) |
Percent of Totsal |
≥ 65 years of age (n = 322) |
Percent of Total |
p value |
|---|---|---|---|---|---|---|---|
| Gender | |||||||
| Male | 377 | 50.9 | 220 | 52.5 | 157 | 48.8 | .312 |
| Female | 364 | 49.1 | 199 | 47.5 | 165 | 51.2 | |
| Race | |||||||
| White | 640 | 86.4 | 347 | 82.8 | 293 | 91.0 | .013 |
| Minority | 101 | 13.6 | 72 | 17.2 | 29 | 9.0 | |
| Ethnicity | |||||||
| Hispanic | 12 | 1.6 | 10 | 2.4 | 2 | 0.6 | .059 |
| Non-Hispanic | 729 | 98.4 | 409 | 97.6 | 320 | 99.4 | |
| Education | |||||||
| College Graduate | 309 | 41.7 | 197 | 47.0 | 112 | 34.8 | .000 |
| Not a College Graduate | 432 | 58.3 | 222 | 53.0 | 210 | 65.2 | |
| Marital Status | |||||||
| Married/Living with Partner | 568 | 76.7 | 328 | 78.3 | 240 | 74.5 | .000 |
| Not Married/Living with Partner | 172 | 23.2 | 91 | 21.7 | 81 | 25.2 | |
| Missing | 1 | 0.1 | |||||
| Employment Status | |||||||
| Retired/Unemployed | 427 | 57.6 | 150 | 35.8 | 277 | 86.0 | .000 |
| Employed | 314 | 42.4 | 269 | 64.2 | 45 | 14.0 | |
| Annual Income (US $) | |||||||
| < 30,000 | 202 | 27.3 | 93 | 22.2 | 109 | 33.9 | .000 |
| 30,000–59,999 | 228 | 30.8 | 111 | 26.5 | 117 | 36.3 | |
| 60,000–89,999 | 131 | 17.7 | 89 | 21.2 | 42 | 13.0 | |
| ≥ 90,000 | 180 | 24.3 | 126 | 30.1 | 54 | 16.8 |
Table 2.
Disease Characteristics
| Variable | All Survivors (N = 741) |
Total Percent |
< 65 years of age (n = 419) |
Percent of Total |
≥ 65 years of age (n = 322) |
Percent of Total |
p value |
|---|---|---|---|---|---|---|---|
| NHL Histology | |||||||
| Indolent | 365 | 49 | 202 | 48 | 163 | 51 | .244 |
| Aggressive | 335 | 45 | 200 | 48 | 135 | 42 | |
| Missing | 41 | 6 | 17 | 4 | 24 | 8 | |
| Stage at Diagnosis | |||||||
| I | 204 | 28 | 108 | 26 | 96 | 30 | .249 |
| II | 137 | 18 | 78 | 19 | 59 | 18 | |
| III | 125 | 17 | 79 | 19 | 46 | 14 | |
| IV | 178 | 24 | 108 | 26 | 70 | 22 | |
| Missing | 97 | 13 | 46 | 11 | 51 | 16 | |
| Currently in Remission | |||||||
| Yes | 579 | 78 | 321 | 77 | 258 | 80 | .415 |
| No | 97 | 13 | 60 | 14 | 37 | 12 | |
| Don’t Know | 63 | 9 | 38 | 8 | 25 | 8 | |
| Missing | 2 | <1 | 5 | 1 | 2 | <1 | |
| Currently receiving treatment | |||||||
| Yes | 105 | 1 | 69 | 17 | 36 | 11 | .041 |
| No | 636 | 86 | 350 | 84 | 286 | 89 | |
| Treatments Received | |||||||
| Chemotherapy | 592 | 80 | 340 | 81 | 252 | 78 | .331 |
| Radiation therapy | 358 | 48 | 214 | 51 | 144 | 45 | .086 |
| Surgery | 223 | 30 | 133 | 32 | 90 | 28 | .245 |
| Biologic therapy | 223 | 30 | 150 | 36 | 73 | 23 | .000 |
| Bone marrow/stem cell transplantation | 116 | 16 | 95 | 23 | 21 | 7 | .000 |
| Other therapy | 87 | 12 | 54 | 13 | 33 | 10 | .269 |
| Missing surgery variable | 18 | 2 | 11 | 3 | 7 | 2 | |
| Number of Comorbidities | |||||||
| 0 | 84 | 11 | 71 | 17 | 13 | 4 | .001 |
| 1 | 138 | 19 | 95 | 23 | 43 | 14 | |
| 2 | 134 | 18 | 78 | 19 | 56 | 17 | |
| ≥ 3 | 385 | 52 | 175 | 41 | 210 | 65 | |
|
Total Comorbidity Score(range 0–30) |
5.6 (4.8) |
4.6 (4.5) |
6.8 (5.1) |
||||
|
Mean age at time of study (range 21–90) |
62.3 (13.4) |
53.1 (9.3) |
74.4 (6.4) |
||||
|
Years since diagnosis (range 2–44) |
10.2 (7.1) |
9.6 (6.3) |
11.2 (7.9) |
Note. Numbers in parenthesis are standard deviations.
As shown in Table 1, younger survivors (<65 years) were more likely than older survivors to be of a minority race, college-educated, married or living with a partner, and employed. Younger survivors also had higher annual income than older survivors. There were no age differences for chemotherapy or radiation therapy, however younger survivors were more likely to have received biologic therapy or a hematopoietic stem cell transplant than older survivors. Comorbidities were more common in older survivors, with 28.3% having three or more, compared to 23.3% in younger survivors.
Relationships of demographic and disease characteristics to QOL (Model 1)
The associations between QOL and demographic/disease characteristics in the un-moderated multiple regression model (Model 1) are provided in Table 2. The significant demographic characteristics in this model were age, gender, annual income (<$30,000 vs. $30,000 +), education (college graduate vs. not a college graduate), and employment status (employed vs. not employed/retired). Significant disease characteristics were total comorbidity scores, some treatment types (BMT/SCT or biologic therapy), and years since diagnosis. QOL was worse for survivors who were males, younger at time of study, had a greater comorbidity burden, had received a transplant or biologic therapy, or had been diagnosed more recently (all at p <.05).
The FACT-G total mean score was 85.3 (16.9) with a range of 10–108 for this sample. The QOL subscales mean scores were FACT_PWB 22.7 (range was 0–28), FACT_SFWB 22.2 (range 4–28), FACT_EWB 19.6 (range 0–24), and FACT_FWB 20.7 (range 0–28). Compared to the overall cancer sample, NHL survivors had higher QOL mean scores on all domains.(Table 4). Higher QOL was observed for females (1.87 points), college graduates (2.47 points), and those who were employed (2.78 points), while earning less than $30,000 was associated with a 6.03 lower QOL score. In terms of disease characteristics, for every point increase in comorbidity score, the FACT-G score was 1.35 points lower. Lower QOL was also observed for those who were currently receiving treatment (4.84 points), and those who had received a transplant (4.58 points) or biologic therapy (2.68 points). On the other hand, longer time since diagnosis was associated with a higher score (.16 point increase for every year since diagnosis).
Table 4.
Quality of Life Scores
| QOL | Range | Mean Score | Normative Data on Cancer Samplea |
|---|---|---|---|
| FACT_G | 10–108 | 85.3 | 80.9 |
| FACT_PWB | 0–28 | 22.7 | 21.3 |
| FACT_SFWB | 4–28 | 22.2 | 22.1 |
| FACT_EWB | 0–24 | 19.6 | 18.7 |
| FACT_FWB | 0–28 | 20.7 | 18.9 |
=FACT-G Normative data on cancer sample. David Cella, PhD, 2004
Moderating Effect of Age on QOL
Moderation of the relationships by age was examined by adding the interaction between each disease/demographic characteristic and age to Model 1, one at a time. There were two demographic characteristics/disease factors whose relationships with QOL were moderated by age: income and gender (Figure 1). The corresponding interaction terms were both included in the final model (Table 3, Model 2). Moderation of the relationship between income and QOL remained significant (p<.01). Although lower income was associated with lower QOL scores, this difference was not as large in older survivors. The relationship between gender and QOL was only marginally moderated by age in the final model (p=.08). Females had higher QOL scores than men, but the difference was not as large in older survivors.
Discussion
The goals of this study were to explore the relationship of demographic and disease characteristics to QOL and to determine the moderating effect of age on QOL among NHL survivors. QOL was measured using FACT-G. Our study results are not consistent with the Jerkeman et al. prospective study of lymphoma survivors who found non-significant associations (2001), whereas we found significant associations between: 1) younger age and decreased QOL; and 2) lower income and decreased QOL. The sample was evenly distributed with numbers of males and females. While NHL is more common in older adults than younger adults with an unpredictable illness trajectory, we found that younger survivors had a more difficult time with their cancer diagnosis than older survivors. Breast cancer survivorship literature has explored the impact of cancer in younger survivors more than any other cancer and age has been shown as a moderator (Ganz et al, 2003; Kornblith et al, 2007); this is the first NHL study to our knowledge to explore age as a moderator on QOL.
We examined the moderating effect of age in these relationships and found age differences in relationships between income and QOL. Younger NHLsurvivors (<65 years of age) with lower income have poorer QOL compared to older NHL survivors. Females have higher QOL than males.Time since diagnosis and the treatment period (e.g. active, surveillance) may be contributing factors for either improved or decreased QOL in younger survivors and female survivors. Younger NHL survivors are more likely to be of a minority race, with a college degree, married or living with a partner, and having an annual income greater than $30,000 than older NHL survivors (Bellizzi, Miller, Arora, & Rowland, 2001; Jerkeman, Kassa, Hjermstad & Kvaloy, 2001; Smith, Zimmerman, Williams, Preisser & Clipp, 2008). The association or influence of age on QOL was not fully described in these studies, but sociodemographic characteristics were highly correlated. Leak et al (2011) review found that older age was associated with worse physical QOL but better mental health compared to younger adults with NHL. Additionally, older survivors who received chemotherapy had poorer social and psychological wellbeing compared to those who did not receive chemotherapy. The overall FACT-G score for this population was 85.3 (range 10–108), which was higher than the normative data of the cancer sample. Emotional (19.6) and functional well-being (20.7) were the lowest scored among the remaining 2 QOL domains (physical and social/family).
Being female, a college graduate, employed, and having a longer time since diagnosis, were all independently, positively associated with QOL. Whereas earning less than $30,000 annually, having higher comorbidities, currently receiving treatment, having had a hematopoietic stem cell transplant or biologic therapy all had negative associations with QOL. Often times, providers focus on the number of comorbidities, and less attention is focused on type, severity, and duration of the comorbidity. There are challenges posed by multiple comorbidities and loss of function for patients at the time of diagnosis. Future work is needed to explore the impact of comorbidities on QOL, with special attention to type of comorbidity.
It has been well documented in the literature that lower socioeconomic status (SES) is associated with lower QOL and poorer survival (Byers et al, 2008; Du, Fang & Meyer, 2008; Newman, Griffith, Jatoi, Simon, Crowe & Colditz, 2006). Younger survivors earning less than $30,000 were associated with a lower QOL. Younger survivors often are diagnosed at a time when they have multiple responsibilities (e.g., primary caregiver for their spouse, parent, or child), working in or outside of the home, reproductive issues, and/or maintaining their career aspirations. Additionally, younger survivors usually have fewer coping skills to manage their diagnosis than older survivors and the thought of an early death may contribute to greater distress and poorer QOL (Stanton, 2006; Thewes, Butow, Girgis & Pendlebury, 2004). Younger breast cancer survivors felt their diagnosis had limited their family, career, and lifestyle priorities (Stewart et al, 2001; Thewes, Butow, Girgis, & Pendlebury, 2004).
Female survivors had higher QOL than male survivors with a marginal moderation by age. These data are mixed in the literature, however most cancer studies focus on increased coping skills (ie. cognitive restructuring) and support services for females with little attention focused on males (Mattson, Demshar & Daly, 2012; Thewes, Butow, Girgis & Pendlebury, 2004). Increasing social support systems and coping skills for males may increase QOL to favorably impact overall survival that are both age and gender specific.
Limitations
This secondary analysis is cross sectional in nature so we cannot establish a cause-effect relationship between demographic and disease characteristics and QOL, however we are able to assess the strength of the associations. This population had a high education level with 42% having a college degree and might have biased the findings. The strength of this study is the use of an existing dataset of NHL survivors to answer questions about an understudied cancer population. Future studies using other moderating effects on QOL such as type of comorbidity and sociodemographic variables can provide additional information about their associations with QOL. In addition, exploring the explanation for income variances between younger and older survivors is warranted. Perhaps there is a need for a more standardized approach to collecting demographic information. A longitudinal study would provide a more definitive answer as to whether demographic and disease characteristics differentially impact QOL changes with increasing age Future research is needed to further explore male’s QOL and the impact of socioeconomic factors on survivorship.
Implications for Nursing Practice
This study contributes to an important gap in the NHL literature about younger survivors and the association between demographic characteristics and QOL. Despite limitations, this study highlights the importance of considering age when trying to understand demographic and disease characteristics and its relationship on QOL. Increasing age does not necessarily mean that QOL will be lessened, and future studies are needed to look at age differences across cancer populations with other sociodemographic and disease variables. Increasing social support systems is one way to increase QOL to favorably impact overall survival in younger survivors (Chou, Stewart, Bloom, & Koo, 2003). It alone, social support systems, may not make a difference but nurses can effectively assess and refer patients to the most appropriate resources. Understanding the buffering effect of age on QOL expands new avenues for targeting younger survivors. Further research is needed on optimizing resources and services that could improve QOL for these survivors.
Acknowledgments
Supported by:
John A. Hartford Building Academic Geriatric Nursing Capacity (BAGNC) Program, Gordon H. DeFriese Career Development in Aging Research Award, and Cancer Care Quality Training Post-Doctoral Fellowship (5R25CA116339).
References
- Bellizzi KM, Miller MF, Arora NK, Rowland JH. Positive and negative life changes experienced by survivors of non-Hodgkin's lymphoma. Annals of Behavioral Medicine. 2007;34:188–199. doi: 10.1007/BF02872673. [DOI] [PubMed] [Google Scholar]
- Bennett J. Mediator and moderator variables in nursing research: Conceptual and statistical differences. Nursing Research. 2000;23:415–420. doi: 10.1002/1098-240x(200010)23:5<415::aid-nur8>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Byers T, Wolf H, Bauer K, Bolick-Aldrich S, Chen V, Finch J, et al. The impact of socioeconomic status on survival after cancer in the United States: findings from the National Program of Cancer Registries Patterns of Care Study. Cancer. 2008;113:582–591. doi: 10.1002/cncr.23567. [DOI] [PubMed] [Google Scholar]
- Cella D, Tulsky D, Gray G, et al. The Functional Assessment of Cancer Therapy Scale: development and validation of the general measure. Journal of Clinical Oncology. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- Cella D, Webster K, Cashy J. Development of a measure of health-related quality of life for non-Hodgkin’s lymphoma clinical research: The Functional Assessment of Cancer Therapy-Lymphoma (FACT-Lym) [Abstract] Blood. 2005;106:222A. [Google Scholar]
- Cella D. Manual of the Functional Assessment of Chronic Illness Therapy (FACIT Scales) (Version 4.1) Evanston, IL: Center on Outcomes Research and Education (CORE) Evanston Northwestern Healthcare; 2004. [Google Scholar]
- Chou, Stewart, Bloom, Koo Then and now: Quality of life of young breast cancer survivors. PsychoOncology. 2003;13:147–160. doi: 10.1002/pon.794. [DOI] [PubMed] [Google Scholar]
- Courneya K, Sellar C, Stevinson C, McNeely M, Friedenreich C, Peddle C, et al. Moderator Effects in a Randomized Controlled Trial of Exercise Training in Lymphoma Patients. Cancer Epidemiology, Biomarkers, Prevention. 2009;18:2600–2607. doi: 10.1158/1055-9965.EPI-09-0504. [DOI] [PubMed] [Google Scholar]
- Du X, Fang S, Meyer T. Impact of treatment and socioeconomic status on racial disparities in survival among older women with breast cancer. Journal of Clinical Oncology. 2008;31:125–132. doi: 10.1097/COC.0b013e3181587890. [DOI] [PubMed] [Google Scholar]
- Ganz P, Greendale GA, Petersen L, Kahn B, Bower JE. Breast Cancer in Younger Women: Reproductive and Late Health Effects of Treatment. Journal of Clinical Oncology. 2003;22:4184–4193. doi: 10.1200/JCO.2003.04.196. [DOI] [PubMed] [Google Scholar]
- Horner M, Ries L, Krapcho M, Neyman N, Aminou R, Howlader N, et al., editors. SEER Cancer Statistics Review, 1975–2006. 2009 Retrieved from National Cancer Institute Web site: http://seer.cancer.gov/csr/1975_2006.
- Jerkeman M, Kassa S, Hjermstad M, Kvaloy S, Cavallin-Stahl E. Health-related quality of life and its potential prognostic implications in patients with aggressive lymphoma: A Nordic Lymphoma Group Trial. Medical Oncology. 2001;18:85–94. doi: 10.1385/MO:18:1:85. [DOI] [PubMed] [Google Scholar]
- Kornblith AB, Powell M, Regan MM, Bennett S, Krasner C, Moy B, et al. Long-term psychosocial adjustment of older vs youngersurvivors of breast and endometrial cancer. Psycho-Oncology. 2007;16:895–903. doi: 10.1002/pon.1146. [DOI] [PubMed] [Google Scholar]
- Leak A, Mayer DK, Smith SK. Quality of life domains among non-Hodgkin lymphoma survivors: An integrative literature review. Leukemia and Lymphoma. 2011;52:972–985. doi: 10.3109/10428194.2011.563884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MR, Demshar RK, Daly BJ. Quality of Life of Young Adult Survivors of Hematologic Malignancies. Cancer Nursing. 2012 doi: 10.1097/NCC.0b013e31824242dd. PMID: 22293158. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. US cancer survivors grows to nearly 20 million. 2011 Retrieved from http://www.cancer.gov/newscenter/pressreleases/2011/survivorshipMMWR2011.
- Naus M, Ishler M, Parrott C, Kovacs S. Cancer survivor adaptation model: Conceptualizing cancer as a chronic illness. Journal of Clinical Psychology. 2009;65:1350–1359. doi: 10.1002/jclp.20622. [DOI] [PubMed] [Google Scholar]
- Newman L, Griffith K, Jatoi I, Simon M, Crowe J, Colditz G. Meta-analysis of survival in African American and white American patients with breast cancer: ethnicity compared with socioeconomic status. Journal of Clinical Oncology. 2006;24:1342–1349. doi: 10.1200/JCO.2005.03.3472. [DOI] [PubMed] [Google Scholar]
- Oerlemanns S, Mols F, Nijziel M, Lybeert M, van de Poll-Franse L. The impact of treatment, socio-demographic and clinical characteristics on health-related quality of life among Hodgkin’s and non-Hodgkin’s lymphoma survivors: A systematic review. Annals of Hematology. 2011;9:993–1004. doi: 10.1007/s00277-011-1274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangha O, Stucki G, Liang M, Fossel A, Katz J. The self-administered comorbidity questionnaire: A new method to assess comorbidity clinical and health services research. Arthritis Rheumatology. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- Stewart DE, Cheung AM, Duff S, Wong F, McQuestion M, Cheng T, et al. Attributions of cause and recurrence in long-term breast cancer survivors. Psychooncology. 2001;10:179–183. doi: 10.1002/pon.497. [DOI] [PubMed] [Google Scholar]
- Smith S, Zimmerman S, Williams C, Preisser J, Clipp E. Post-traumatic stress outcomes in non-Hodgkin’s lymphoma survivors. Journal of Clinical Oncology. 2008;26:934–941. doi: 10.1200/JCO.2007.12.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton A. Psychosocial Concerns and Interventions for Cancer Survivors. Journal of Clinical Oncology. 2006;24:5132–5137. doi: 10.1200/JCO.2006.06.8775. [DOI] [PubMed] [Google Scholar]
- Thewes B, Butow P, Girgis A, Pendlebury S. The psychosocial needs of breast cancer survivors: A qualitative study of the shared and unique needs of younger versus older survivors. Psycho-Oncology. 2004;1:177–189. doi: 10.1002/pon.710. [DOI] [PubMed] [Google Scholar]