Abstract
This chapter provides an overview of the polyamine field and introduces the 32 other chapters that make up this volume. These chapters provide a wide range of methods, advice, and background relevant to studies of the function of polyamines, the regulation of their content, their role in disease, and the therapeutic potential of drugs targeting polyamine content and function. The methodology provided in this new volume will enable laboratories already working in this area to expand their experimental techniques and facilitate the entry of additional workers into this rapidly expanding field.
Keywords: Putrescine, Spermidine, Spermine, Hypusine, Antizyme, Polyamine transport
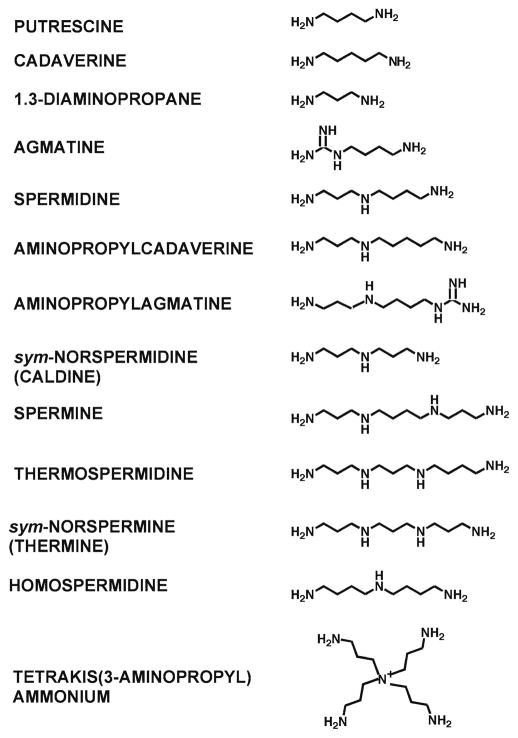
1. Structure of Physiological Polyamines
Mammalian tissues contain putrescine (1,4-diaminobutane), which is strictly speaking a diamine, spermidine, and spermine (1), while a wider spectrum of natural polyamines exists in other organisms (Fig. 1). Plants have these three polyamines and also thermospermine (2, 3) and agmatine. Some fungi and some, but not all, bacteria contain only putrescine and spermidine since they lack a spermine synthase. Specific organisms also contain other diamines such as 1,3-diaminopropane and cadaverine (1,5-diaminopentane) and sym-norspermidine (caldine). Thermophilic bacteria and archea are a rich source of polyamines including these compounds and a wide variety of other polyamines. Since thermophiles were the first source for isolation of polyamines such as thermospermine, caldine, and thermine (Fig. 1) this explains their trivial names, but these polyamines are not unique to organisms living at high temperature. Similarly, spermidine and spermine are so named because the first report of crystals in seminal plasma by Leeuwenhoek was subsequently shown to be due to the precipitation of insoluble spermine phosphate, but these polyamines are present in all tissues and many extracellular fluids of mammals (4).
Fig 1.
Structures of some naturally occurring polyamines. It should be noted that at physiological pH values the nitrogen atoms in these structures (and in subsequent figures) would be predominantly protonated (see Chapter 32). Additional polyamines found in thermophiles are described in Chapter 5.
2. Synthesis of Polyamines
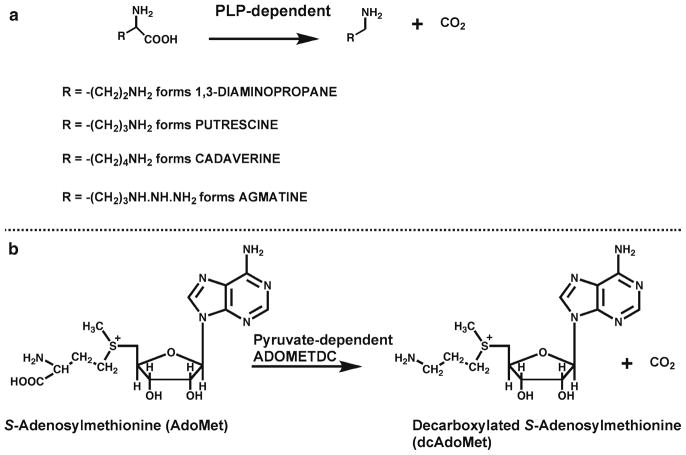
Diamines such as putrescine, cadaverine, and 1,3-diaminopropane are formed by the decarboxylation of the relevant amino acid (L-ornithine, L-lysine and L-1,4-diaminobutyric acid respectively); reactions catalyzed by pyridoxal phosphate-dependent decarboxylases (Fig. 2a) (5). An alternative pathway to putrescine occurs in plants and microorganisms in which L-arginine is decarboxylated forming agmatine (6). This can then be converted to putrescine either by the reaction of agmatinase releasing urea, as in E. coli, or by the combined actions of agmatine deiminase releasing ammonia forming N-carbamoylputrescine and N-carbamoylputrescine amidase as in plants.
Fig 2.
Decarboxylases involved in polyamine synthesis. (a) Pyridoxal phosphate (PLP)-dependent decarboxylases [L-1,4-diaminobutyric acid decarboxylase, L-ornithine decarboxylase (ODC), L-lysine decarboxylase and L-arginine decarboxylase]. (b) S-adenosylmethionine decarboxylase, which contains a covalently bound pyruvate group essential for activity.
There are two pathways for the addition of aminopropyl groups to form the higher polyamines. By far the most extensively studied is that first demonstrated by the Tabors (7), which occurs in mammals, plants, fungi, and many bacteria including E. coli, where decarboxylated S-adenosylmethionine (dcAdoMet) is used as the aminopropyl donor. This nucleoside is produced by the action of the pyruvoyl-dependent S-adenosylmethionine decarboxylase (AdoMetDC) (8, 9). Enzymes termed aminopropyl-transferases (10–12) use dcAdoMet and an amine acceptor to form the higher polyamines (Fig. 3). Thus, putrescine is used as a substrate by the aminopropyltransferase, termed spermidine synthase, to form spermidine. A distinct aminopropyltransferase, spermine synthase, then uses a second dcAdoMet molecule to add the aminopropyl group to the N8 of spermidine forming spermine. In addition to a spermine synthase, plants contain a thermospermine synthase that attacks the N1 end of spermidine producing thermospermine. The aminopropyltransferases mentioned above in mammals and plants, and in some bacteria and fungi, are quite specific for the amine acceptor (13). Other organisms including the thermophiles have less specific aminopropyltransferases that can make a variety of polyamines including thermine. Some contain an enzyme that uses agmatine to produce aminopropylagmatine (Fig. 3), which is then converted into spermidine and urea by an agmatinase-like enzyme allowing for the production of spermidine without a putrescine intermediate. It is likely that some thermophiles and some other organisms such as diatoms where very long chain polyamines are involved in biomineralization of the shell contain additional aminopropyltransferases supporting their synthesis but these enzymes have not been characterized.
Fig 3.
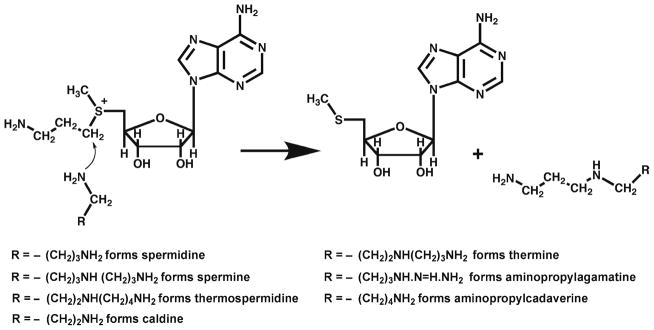
Aminopropyltransferases involved in polyamine synthesis. The reaction catalyzed by these enzymes involves the attack by the unprotonated terminal N of the amine substrate on the methylene C atom adjacent to the sulfonium center of the dcAdoMet aminopropyl donor. The deprotonation of the attacking N, which is facilitated by residues in the enzyme, and the positive charge on the sulfonium S of dcAdoMet allow the reaction to proceed forming the polyamine product. The reactions shown have all been demonstrated by purified aminopropyltransferases. Other members of this family of enzymes may occur to bring about the synthesis of longer chain polyamines.
The polyamine biosynthetic pathway dependent on dc-AdoMet described above is very well understood and methods for the assay of these enzymes including L-ornithine decarboxylase (ODC), AdoMetDC, and aminopropyltransferases were provided in Volume 79 of this series published in 1998.
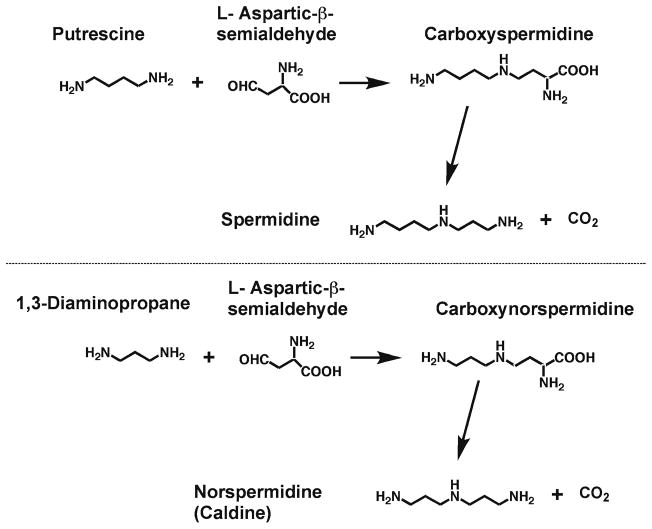
There is, however, a second pathway for the synthesis of polyamines containing aminopropyl moieties in which L-aspartic-β-semialdehyde is used to provide the aminopropyl group (14, 15) (Fig. 4). The condensation of this molecule with putrescine forms carboxyspermidine, which is then decarboxylated to form spermidine. Similar reactions using 1,3-diamino-propane form sym-norspermidine and the same enzymes (termed carboxynorspermidine synthase and carboxynorspermidine decarboxylase) are able to bring about both syntheses (Fig. 4). This pathway is used in many microbes.
Fig 4.
Condensation reactions forming polyamines from L-aspartic-β-semialdehyde. Reaction of L-aspartic–β-semialdehyde with putrescine or 1,3-diaminopropane by carboxynorspermidine synthase forms carboxyspermidine or carboxynorspermidine, which are decarboxylated by carboxynorspermidine decarboxylase to form spermidine or sym-norspermidine, respectively.
Finally, there is also a pathway for the synthesis of sym-homospermidine, which involves the use of NAD and putrescine with either a second molecule of putrescine or spermidine to generate the product via condensation with 4-aminobutyralehyde intermediate (16, 17) (Fig. 5a). In plants, the homospermidine synthase, which is responsible for the latter reaction, is derived from deoxyhypusine synthase (18), a critical enzyme for a posttranslational modification, which involves polyamines and is described below.
Fig 5.
Synthesis of sym-homospermine and of hypusine. sym-Homospermine can be formed from two molecules of putrescine or from putrescine and spermidine as shown in (a). In both cases, NAD is needed for hydrogen extraction, but is regenerated in the second half of the reaction. The reaction using spermidine and putrescine, which also generates 1,3-diaminopropane, is similar to that used for the hypusine modification of eIF5A shown in (b). In this case, a lysine residue in eIF5A is used instead of putrescine forming deoxyhypusine in eIF5A and free 1,3-diaminopropane. A second enzyme hydroxylates the deoxyhypusine to form the complete hypusine modification (see Chapters 12 and 13 for more details of the reactions forming hypusine).
The second chapter of this volume describes genomic methods for identifying key polyamine biosynthetic genes and thus determination of the pathways used in particular organisms. This is a very important area since polyamines are essential for normal growth and their biosynthetic pathways provide targets for drug development. Organisms such as Vibrio cholerae that use the L-aspartic–β-semialdehyde condensation pathway may be sensitive to potential therapeutics that are innocuous to host organisms that do not depend on these reactions.
Polyamines with quaternary ammonium centers such as tetrakis(3-aminopropyl)ammonium (Fig. 1) or tertiary N atoms such as mitsubishine (see Chapter 5) respectively are found in acute thermophiles and are needed for growth at extreme temperatures (19, 20). The biosynthetic reactions leading to these polyamines have not yet been elucidated.
3. Functions of Polyamines
Polyamines have a multitude of functions affecting growth and development, and these pleiotropic effects complicate efforts to understand the physiological and pathophysiological effects of perturbing polyamine content. Recent studies have identified a number of key areas in which polyamine effects are initiated (21–23). These include regulation of gene transcription, multiple effects on posttranscriptional regulation, control of the activity of ion channels as well as modulation of protein kinase activities, the cell cycle, membrane structure/function, and nucleic acid structure and stability.
A critical new concept increasing understanding of the role of polyamines in maintaining optimal growth rates and cell viability has been provided by studies showing that there is a bacterial “polyamine modulon” that consists of a set of genes whose expression is increased by polyamines as a result of increased translation (24, 25). Polyamines stimulate translation in a number of ways including alteration of mRNA structure allowing initiation of protein synthesis encoded by genes that lack Shine–Dalgarno sequences or have them placed at nonoptimal positions. The proteins whose synthesis is directly stimulated by polyamines also include transcription factors and kinases that can in turn enhance gene expression of other proteins. The polyamine modulon concept has been extended to yeast (26) and is likely to also apply to mammalian cells (27). Methods for identifying members of the polyamine modulon are described in Chapter 3.
In eukaryotes, another factor is also involved in the role of polyamines in stimulating gene expression. This is the protein eIF5A, which is only active after a posttranslational modification to form hypusine (Fig. 5b). This reaction has an absolute requirement for spermidine as a precursor. The functions of eIF5A have been the subject of considerable debate and it may have multiple functions, but recent studies indicate that it is a translation elongation factor (28–30). Many other sites of posttranscriptional gene regulation, such as mRNA transport, and turnover are also influenced by polyamines either via eIF-5A or other proteins. These include RNA-binding proteins such as the HuR family(31–33). The HuR proteins are highly regulated by polyamines and methods for their study are described in Chapter 4.
The novel polyamines present in thermophiles are essential for growth at higher temperatures and have effects on nucleic acid stability and structure, and in protein synthesis (19, 20). Methods for the synthesis and the study of the function of these polyamines are described in Chapter 5.
A new and critically important area in polyamine research was revealed when it was observed that the steep voltage-dependence of the inwardly rectifying potassium (Kir) channels is caused by the binding of polyamines (34) and that polyamines profoundly affect the activities of NMDA receptors (35). Subsequent studies have shown that a wide variety of ion channels are affected by polyamines including the Kir potassium channels, which control membrane potential and potassium homeostasis in many cell types, glutamate receptors that mediate excitatory synaptic transmission in the mammalian brain, as well as other channels affecting intracellular calcium signaling, Na+ transport, and some connexin-linked gap junctions (22, 23). Chapter 6 describes methodology characterizing polyamine interactions with both prokaryotic and eukaryotic Kir channels.
4. Use of Transgenics to Investigate Polyamine Function
The ability to generate transgenic rodents that have reductions or increases in polyamine content due to alteration in the activities of key enzymes has now provided important tools to evaluate polyamine function in mammals (36–38). Various aspects of these studies are covered in Chapters 7–9.
There is much evidence linking elevated polyamine content to tumor growth and development. Consequently a number of animal models have been developed in which polyamine content is disturbed and studies are carried out on their susceptibility to tumor induction by chemicals, oncogene activation, UV radiation, and other stimuli. Remarkably, even the small reduction in ODC activity brought about by the Odc heterozygosity in ODC (+/−) transgenic mice reduced lymphoma and skin tumor development (39, 40). Similarly, moderately increased levels of antizyme (AZ), which as described below is a negative regulator of ODC, dramatically lowered skin carcinogenesis in response to two-stage carcinogenesis protocols (37, 41). The use of these and other models for studying the role of polyamines in neoplasia is described in Chapter 7.
These studies correlate well with a number of investigations using human patients that show that interference with polyamine synthesis by drugs such as DFMO (an irreversible inactivator of ODC) has a profound cancer preventative effect (42). Thus, clinical trials examining the reoccurrence of colonic polyps have shown that prolonged treatment with this drug combined with sulindac caused a 70% reduction of recurrence of all adenomas, and over a 90% reduction in advanced and/or multiple adenomas (43). Treatment with DFMO also reduced the development of non-melanoma skin cancer in subjects with a previous history of this disease (44). Some encouraging results in a Phase IIb clinical trial suggest that DFMO may be useful for chemoprevention of prostate cancer (45). At present, all of the clinical data supporting the use of the polyamine pathway for cancer chemoprevention are derived from studies with DFMO, but animal studies suggest that polyamine analogs described below and the targeting of drugs to other enzymes in the pathway, such as spermidine synthase (which like ODC is regulated by the oncogene c-myc), may also be successful (46).
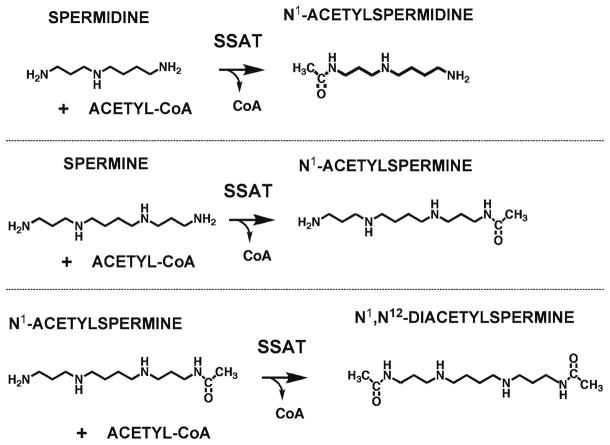
Remarkable phenotypic changes occur in rodents in which the activity of the catabolic enzyme spermidine/spermine-N1-acetyltransferase (SSAT) (Fig. 6) is increased. This contrasts to the relatively minimal effects in untreated mice with inactivation of the Sat1 gene. The lack of significant changes in these mice is consistent with the concept that normally SSAT levels are very low and that physiological and pathophysiological effects of SSAT occur in response to its induction (47, 48). Multiple lines of transgenic mice and transgenic rats overexpressing SSAT have been described (47, 49). These mice also show a wide variety of other defects including hair loss, female infertility, weight loss, CNS effects, altered carbohydrate and lipid metabolism, a tendency to develop pancreatitis and altered responses to chemical carcinogens. These effects are related not only to the alterations in polyamine content, but also to the effects on levels of acetyl-CoA, ATP, and oxidative damage associated with the increased activity of the SSAT/APAO pathway (Fig. 4) described below, and the increased metabolic flux through the polyamine pathway due to the futile cycle set up by compensatory increases in polyamine synthesis in response to constitutively elevated SSAT levels (48). Chapter 7 describes methods to study pancreatitis and altered lipid and carbohydrate metabolism in rodents with elevated expression of SSAT.
Fig 6.
Reactions catalyzed by SSAT.
The critical importance of polyamines for mammalian development is shown by studies with mouse knockouts of the ODC or AdoMetDC genes, which are lethal at very early embryonic stages. Spermidine synthase knockouts would almost certainly also be lethal since spermidine is essential for viability in yeast (50). In contrast, mice lacking spermine synthase do survive on an appropriate strain background and can be used to evaluate the specific functions of spermine (51). Chapter 9 describes the phenotypic changes in these mice and their use to evaluate the role of spermine in growth, behavior, fertility, hearing, and cardiac function.
Transgenic approaches have also been used to evaluate the functions of polyamines in plants. It is noteworthy that although many plants contain both ODC and arginine decarboxylase, the former is not required in all species since there is no gene for ODC in Arabidopsis thaliana (52). Genes for AdoMetDC and arginine decarboxylase are essential for normal plant development as is the gene for thermospermine synthase (2, 3, 13, 53, 54).
5. Polyamine Catabolism
The enzymatic reactions forming polyamines are effectively irreversible. Polyamines are interconverted and removed by a combination of acetylation, oxidation, and excretion to vacuoles or extracellular fluids. Putrescine can be attacked by diamine oxidase and other copper-dependent enzymes that oxidize the terminal amino groups of spermidine and spermine are well known (55–57). These enzymes are present extracellularly in mammals. The products of their reaction, which can rearrange to form acrolein, are highly toxic (58, 59) and this reaction is the basis of many publications that erroneously claim that polyamines exert direct toxic effects on the basis of observations that addition of polyamines to cell cultures causes cell death. The observed toxicity in such systems is due to the formation of toxic metabolites by enzymes in the serum used to support cell culture. Although elevated levels of intracellular polyamines can lead to apoptosis or increased formation of reactive oxygen species, the polyamine transport systems are highly regulated to avoid uptake of excess polyamines.
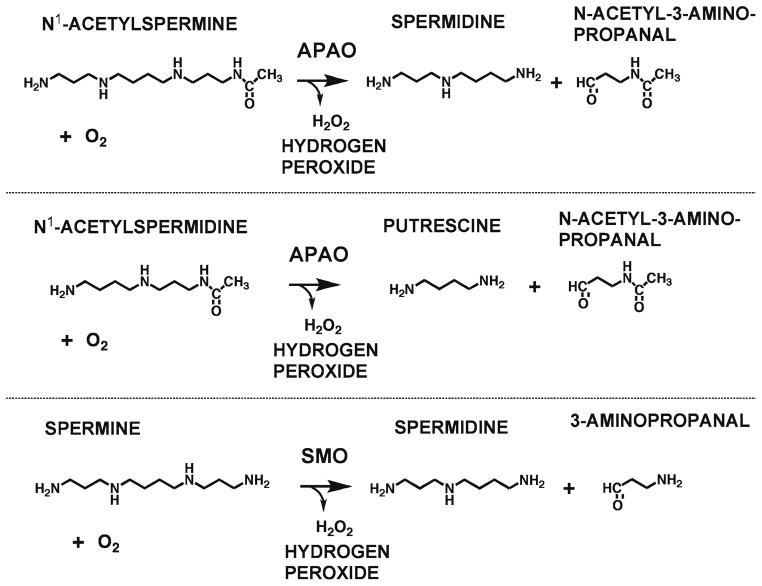
Interconversion of polyamines in mammals is brought about by two FAD-dependent oxidases (Fig. 7). Spermine oxidase (SMO), first described in 2001, is specific for spermine acting on it to produce spermine, H2O2, and 3-aminopropanal (48, 60). It exists as multiple forms due to splicing variations and is highly inducible by polyamine analogs and other stimuli. The other oxidase, N1-acetylpolyamine oxidase (APAO), is generally a constitutively expressed enzyme that has minimal activity on free polyamines, but is highly active on N1-acetylspermidine (forming putrescine) and N1-acetylspermine (forming spermidine). With both substrates, H2O2 and N-acetyl-3-aminopropanal are produced (48, 61). The substrates for APAO are produced by SSAT (Fig. 6), which is very highly regulated and inducible by polyamines (48). Therefore, the SSAT/APAO pathway acts to prevent the overaccumulation of polyamines. This occurs at the expense of the generation of reactive oxygen species (ROS) and potentially toxic aldehydes. Such damage may be limited by the subcellular localization of APAO, which is a peroxisomal enzyme, whereas similar production of ROS by SMO may be more damaging due to its nuclear and cytoplasmic localization. Assay of SMO is described in Chapter 10.
Fig 7.
Reactions catalyzed by APAO and SMO. APAO has a strong preference for N1-acetylated polyamines formed by SSAT releasing N-acetyl-3-aminopropanal and SMO is specific for spermine releasing 3-aminopropanal. Both reactions also generate hydrogen peroxide.
Plants also use polyamine oxidation to interconvert polyamines H2O2 that may and regulate polyamine content and also to form act as a signaling molecule (54, 62–64). Methods for the investigation of these enzymes are described in Chapter 11.
6. Formation and Function of Hypusine
One essential function of polyamines in eukaryotes, as stated above, is to serve as a precursor of hypusine [Nε-(4-amino-2-hydroxybutyl)lysine] (Fig. 5b). Hypusine production is a posttranslational modification of the protein eIF5A. This modification is essential for the activity of eIF5A, which as described above is needed for protein synthesis at the elongation step and probably also for other functions essential for growth and for viral replication (29, 50, 65–67). Drugs preventing the hypusination of eIF5A inhibit the expression of HIV-1 genes (68). The ability to serve as the source of hypusine can only be fulfilled by spermidine, which is used as a substrate by the enzyme deoxyhypusine synthase (66). This enzyme attaches the aminobutyl group of spermidine to a specific lysine residue in the protein eIF5A forming deoxyhypusine (Fig. 5b). This protein is then further modified by deoxyhypusine hydroxylase (69) to produce the complete hypusinated eIF5A. Assays for these enzymes are described in Chapters 12 and 13.
7. Regulation of Polyamine Content
In mammals, polyamine content is controlled primarily by alterations in the activity of the key enzymes ODC, AdoMetDC, and SSAT, along with alterations in efflux and uptake (22, 70, 71). Changes in ODC and SSAT activity are brought about by alterations in the content of enzyme protein and this level is controlled at the levels of transcription, mRNA stability, translation, and protein turnover (47, 48, 71–73). AdoMetDC is formed as a proenzyme that undergoes an autocatalytic cleavage to form its pyruvoyl cofactor and its two subunits (8, 22, 71). AdoMetDC activity is also varied by alterations in the level of the processed protein and, additionally, by activation of the protein by putrescine in mammals, yeast, and some other organisms (8, 22, 74) and by binding of an activating protein (75–77) in trypanosomes. This protein, which was discovered in 2007, has been named prozyme. Methods to identify and assay this important regulatory molecule in these parasites that are responsible for major human diseases including African sleeping sickness, Chagas disease, and Leishmaniasis are described in Chapter 14. DFMO is highly effective as a therapeutic agent for some forms of African sleeping sickness, but other forms and other parasitic diseases are less susceptible to DFMO and AdoMetDC may be a critical target for their treatment (78, 79).
It has been known for 40 years that ODC protein has a very rapid rate of turnover (80). More recent studies have shown that a key part of the regulation of ODC content is due to two proteins that influence the proteasomal degradation of ODC termed antizyme (AZ) and antizyme inhibitor (AZIn) (71, 72, 81, 82). AZ was first described and named in 1976 as an inducible protein inhibitor of ODC (83). It does act as an inhibitor by binding to the ODC monomer. ODC functions as a homodimer with two active sites formed at the dimer interface. Association between the two subunits is quite weak and the dimers are in rapid equilibrium with inactive monomers. Therefore, binding of AZ causes loss of ODC activity, but more importantly it targets the protein for degradation by the 26S proteasome. AZ acts in a catalytic manner in this reaction since most of the AZ is released and can cause further ODC degradation (72, 84). AZ is not restricted to mammals having been found in yeast and many other species (85, 86).
AZ synthesis is increased in response to high polyamine levels predominantly via increasing a +1 frameshifting mechanism, which is needed to allow read-through of a stop codon that prevents AZ synthesis (87, 88). Polyamines stimulate this frame-shifting, hence increasing the level of AZ and providing a regulatory feedback to control polyamine levels. Procedures for quantifying, assay, and characterization of AZ, which has at least four isoforms, some of which may be phosphorylated (89), are described in Chapter 15. AZ-3 is expressed specifically in germ line cells during spermatogenesis and is needed for haploid germ cell differentiation (90).
A second component of the system regulating ODC protein stability is AZIn (22, 73, 91). This protein, which has a structure similar to that of ODC itself, but does not dimerize or have any activity (92), binds to AZ more tightly than ODC. It can therefore displace ODC from the ODC:AZ complex or prevent this complex from forming, thus preventing ODC degradation. There are two AZIn genes (91, 93) and inactivation of the AZIn-1 gene is lethal (94). Methods for the study of AZIn are covered in Chapter 16.
The ODC gene promoter region contains sequences including E-boxes, a cAMP response element, CAAT and LSF motifs, AP-1 and AP-2 sites, GC-rich Sp1 binding sites, and a TATA box. These binding sites allow for regulation of the synthesis of ODC mRNA by many factors including oncogenes such as c-Myc, hormones, tumor promoters, and other growth factors (21, 22, 36, 65, 95, 96). There are also many studies showing that translational regulation of ODC synthesis via the long 5′UTR of its mRNA affects ODC production. Both cap-dependent and internal ribosome entry site (IRES)-mediated translation have been reported with the initiation factor eIF4E playing an important regulatory role (97, 98). Early studies also suggested that the 3′UTR of the ODC mRNA might exert regulatory activity (99) and this possibility has now been confirmed with studies showing that mRNA content is also regulated at the level of mRNA stability via interactions of key proteins with this region. Chapter 17 describes methods for studying the posttranscriptional regulation of ODC.
8. Polyamine Transport
The polyamines exist predominantly in a charged form at physiological pH and are excluded by cell membranes. The existence of transport systems underlying both uptake and efflux has been known for many years and several chapters outlining methods to determine polyamine transport into and out of the cell were included in Volume 79 of this series. At that time, almost nothing was known about the mechanism of this transport and the components of the transporters. Recently, great progress has been made in the characterization and study of these systems. This work has been led by a series of elegant studies in which the components of the carrier-mediated systems for both uptake and efflux of polyamines in bacteria have been fully characterized using proteins fromE. coli (100, 101). Detailed studies of these transport systems in yeast have also been published (101–103). Chapter 18 describes methods for investigating these transporters in detail.
Specific polyamine transporters have also been isolated from protozoan parasites (104, 105) and methods for investigation of these proteins are covered in Chapter 19. Some protozoa such as T. cruzi, which causes Chagas disease, lack enzymes capable of putrescine synthesis and uptake is therefore essential for their ability to possess adequate polyamines to maintain viability. Even in those parasites possessing a complete polyamine synthesis system, a full understanding of the uptake systems and methods for their inhibition or down-regulation may be needed to maintain the therapeutic activity of drugs targeting the polyamine pathway. Some progress has been made on the synthesis of inhibitors of polyamine transport using amino acid-spermidine conjugates (106–108). Lipophilic polyamine analogs where a C16-lipophilic substituent is attached to a Lys-spermine conjugate have been shown to have considerable promise, but reports so far have been limited to studies of inhibition of transport into cancer cells (108).
Many attempts to isolate similar carrier-mediated systems from higher organisms have not yet been successful, although recently a transporter protein CATP-5, a P5B-type ATPase associated with the plasma membrane that is involved in polyamine transport, has been identified in C. elegans (109). It should be noted that mammalian polyamine transport system can transport a number of related compounds including paraquat (110, 111), antiproliferative agents such as MGBG (112–114) and mepacrine (115), and synthetic drugs conjugated with a polyamine (115–118). The latter property may be valuable to deliver drugs to tumor cells, although obvious problems such as maintaining the tumor-killing properties of the drug or making a version cleavable inside the cell, as well as imparting tumor selectivity, since the uptake systems are not unique to tumor cells, must be overcome. An additional difficulty when reactive drugs are attached to polyamines is that these drugs may inactivate the carrier and thus rapidly inactivate the uptake process.
The polyamine transport system in mammals is also responsible for the uptake of many polyamine analogs, which, as described below, are under investigation and in some cases detailed clinical trials as antitumor agents. AZ (Chapter 15) has a dual function in maintaining polyamine homeostasis (72). It not only reduces putrescine synthesis by its effects reducing ODC activity, but also inhibits polyamine transport. The molecular mechanism for this inhibition is not yet understood. Many of the polyamine analogs also induce AZ synthesis and thus limit their own accumulation (119, 120). Finding an analog that lacks this property but is still actively transported is an important goal, although providing selectivity towards tumors is also a key limiting issue.
Many attempts to isolate mammalian carrier-mediated uptake systems similar to those characterized from bacteria, fungi, and protozoal parasites have not yet been successful. However, endocytic pathways for polyamine transport have been described and fluorescent polyamine derivatives have been localized in discrete vesicles (121). Polyamine transport can follow a dynamin-dependent and clathrin-independent endocytic uptake pathway.
Cell surface heparin sulfate proteoglycans have been implicated in polyamine transport (122–124), and uptake of polyamines was blocked by a single chain variable fragment antiheparin sulfate antibody (125). Such uptake mechanisms are covered in Chapter 20. A caveolin-regulated system that transports polyamines in colon cancer cells has been described (126). Phosphorylation of caveolin-1 at Tyr14 is stimulated by the oncogene k-Ras and increases the activity of this system (126).
There is also compelling evidence for a polyamine efflux system which excretes excess putrescine and the acetylpolyamines formed by SSAT (127–129). Important recent studies have identified SLC3A2, previously known as a glycosylated heavy chain of a cationic amino acid transporter and its partner y+ LAT light chain, as a part of a putrescine and acetylpolyamine efflux system. This complex supports coupled arginine uptake and putrescine efflux, indicating that there is a putrescine/arginine exchange reaction (130). Methods for the study of these mammalian transport systems are included in Chapter 21. Interestingly, SSAT was colocalized with SLC3A2, so this efflux system may be closely linked to polyamine acetylation. Expression of SLC3A2 was negatively regulated by k-Ras (130). Thus, this oncogene can increase polyamine content by affecting both influx and efflux.
Since polyamines are ubiquitous components of plant and animal cells and are produced by intestinal microorganisms, there is substantial dietary exposure to polyamines (131). Uptake from this source is a significant potential problem leading to resistance to drugs that block endogenous polyamine synthesis which are used for cancer chemotherapy or chemoprevention (124, 132). Diets low in polyamines and supplemented with antibiotics to reduce synthesis by intestinal organisms have been designed to overcome this problem (133–135). On the other hand, enhanced use of dietary polyamines may be helpful in some conditions in which pathology is associated with depleted polyamines (136, 137). Detailed descriptions of the polyamine contents of various foodstuffs are available to aid in the design of diets with appropriate polyamine content (131, 138). Chapter 22 details simple and reliable methods for precisely measuring dietary polyamines from a number of food sources. Understanding the variety of dietary sources and their actual content of polyamines will be critical as chemotherapeutic and chemopreventive strategies using polyamine-like molecules move forward.
9. Deranged Polyamine Metabolism and Human Disease
There is an increasing realization that polyamines and/or their metabolites may have critical roles in human disease. Conditions causing reduced polyamine content, increased polyamine oxidation, or overproduction of certain polyamines are associated causatively in a number of conditions. In some cases, it is not entirely clear how the changes seen are produced or how they are linked to the conditions, but they provide an important diagnostic test. For example, urinary excretion of N1, N12-diacetylspermine has now been shown to be a valuable marker for a variety of cancers (139). The possible value of polyamine measurements in cancer diagnosis and monitoring of therapy was first proposed by Russell and colleagues (140, 141) who showed that increased extracellular polyamines were associated with neoplasia, but was not generally useful. The rationale/explanation for such increases was that the rapid turnover of cancerous cells led to release of increased polyamines that were found in blood and urine. However, many other conditions where there was increased cell proliferation/turnover, tissue damage, pregnancy, and dietary considerations can all influence such polyamine levels leading to a far more variation than is acceptable for such tests. More recent studies in which the focus has been on N1, N12-diacetylspermine have provided a more reliable assay (139). This product can be formed by SSAT as shown in Fig. 6 and is readily excreted from cells (142), but it is not clear how the N1, N12-diacetylspermine found in urine of cancer patients originates. Another advantage of N1, N12-diacetyl-spermine is that antibodies to this polyamine can be produced that have adequate specificity for analytical use (143), whereas it is difficult to produce antibodies for a free polyamines, such as spermine, which have sufficient selectivity over closely related molecules such as spermidine. Chapter 23 gives full details of this method and its diagnostic applicability.
Polyamine oxidation and the subsequent production of H2O2 (Fig. 7) leading to cell death by apoptosis may be an important tissue-remodeling event during development (144, 145). However, inadequately regulated oxidative damage caused by the production of H2O2 and/or aldehydes produced by the APAO and the SMO reactions may be involved in the initiation of cancer and the etiology of several other pathologies (48, 60). High levels of SMO may be particularly damaging since this enzyme is not located in peroxisomes as is APAO.
Recent work clearly implicates a significant role for ROS generated via the SSAT/APAO, and possibly SMO, pathway in ischemia reperfusion injury (IRI) (48, 146–148). SSAT was one of the genes that was significantly up-regulated in a mouse model of IRI (149) and plasmid-derived induction of SSAT in kidney epithelial cells (which also leads to SMO induction) showed ROS production leading to injury that could be prevented by catalase. H2O2-mediated DNA damage leads to decreased proliferation and repair capability in injured kidneys (148). Confirmation that SSAT plays an important role in kidney IRI has been provided by studies using the SSAT knockout mouse, which was less sensitive to both liver and kidney IRI (150). These results clearly have important implications in the treatment and prevention of IRI, and methods to study the role of ROS in IRI, which have more general potential for study of tissue damage related to increased polyamine catabolism, are described in Chapter 24.
Further studies in human renal failure (151, 152) and cerebral stroke (153, 154) have also focused on the role of the polyamine oxidation. These studies have shown that the metabolite, acrolein, may be of particular importance (154–157). The acute toxicity of acrolein, which can be formed from spermine by extracellular Cu-dependent oxidases present in serum, has been known for many years (58, 59, 158), but only recently this metabolite been demonstrated to be an important potential cause and biomarker for ischemic disease. Methods for these assays are given in Chapter 25.
SMO is likely to provide an important mechanistic link between infection, inflammation, and carcinogenesis (48). It is induced in ulcerative colitis leading to increased oxidative stress and altered immune responses (159). Infection of gut macrophages with Helicobacter pylori, which causes gastric ulcers and stomach cancer, induced arginase II and ODC increasing polyamine content and also induced SMO producing ROS including H2O2 leading to DNA damage and apoptosis (160–163). Thus, H. pylori infection may escape the immune system by killing the immune cells responsible for eradicating the infection. Furthermore, the increased SMO expression in the gastric epithelial cells could result in ROS production leading to mutagenic DNA damage and initiation of neoplastic transformation (163). Chapter 26 describes procedures for these investigations.
As mentioned above, animal models with increased SSAT activity have suggested that this enzyme may be involved in a wide variety of other conditions including pancreatitis, blockage of regenerative tissue growth, obesity, diabetes, and carcinogenesis. Such SSAT overexpression leads to a variety of biochemical alterations that may cause pathogenesis. These include: the loss of polyamines and the reduction in acetyl-CoA and ATP due to the futile cycle set up by increased degradation causing increased synthesis of polyamines and the formation of ROS (47, 49, 164–168). Human genetic studies also suggest that the SSAT/APAO and SMO pathways may be involved in heritable disease. One form of keratosis follicularis spinulosa decalvans (KFSD), a rare X-linked inherited disease affecting primarily the skin and eyes, may be associated with an increased gene copy of the Sat1 gene encoding SSAT (169). KFSD is characterized by keratosis and hair loss and elevated SSAT in mouse models does cause such changes (49, 170). Cells from patients with the neurodegenerative lysosomal storage disease Niemann–Pick type C are more sensitive to the toxic effects of aldehydes formed by the polyamine catabolic pathways (171). Low SSAT activity may be related to behavioral changes particularly a propensity to suicide (172–174). Decreased Sat1 gene expression due to reduced levels of SSAT mRNA that may be imparted by the C allele of the Sat1 342A/C gene polymorphism has been correlated to depression and a propensity to suicide. Microarray analyses indicating a link between decreased SSAT expression and suicide were supported by studies with reverse transcription-polymerase chain reaction; immunohistochemistry and Western blotting for SSAT have been used to show that SSAT content was lower in the brains of persons committing suicide than in normal controls. Alterations in polyamine levels in such depressive patients have also been reported (175) and methods to examine this and relate it to disease are described in Chapter 27.
The mechanism by which SSAT is linked to behavioral effects is not understood, but may be linked to polyamine-mediated alterations in ion channels including NMDA receptors, AMPA receptors, K+ channels, and Ca2 in the brain. Kainate-induced seizures increase SSAT activity (176) and transgenic overexpression of SSAT protects from kainate (177), and epilepsy-like seizure activity induction by pentylenetetrazol (177). These SSAT transgenic mice also showed a reduced activity and spatial learning impairment (36, 178). The critical role of polyamines in cognitive function is shown most clearly by the finding that an inherited defect in spermine synthase causes Snyder–Robinson syndrome (SRS) (179). SRS is an X-linked recessive disease that causes mild-to-moderate mental retardation as well as hypotonia, cerebellar circuitry dysfunction, thin habitus and kyphoscoliosis. The first gene alteration shown to cause this condition was a splice variant of the SMS gene that resulted in an inactive truncated protein. Subsequent studies showed that similar phenotypes were associated with point mutations in the coding region that produce virtually complete loss of spermine synthase activity (13, 180, 181). The analysis of human samples to determine SRS is covered in Chapter 28.
10. Polyamine Analogs and Derivatives as Research Tools and Therapeutics
A wide variety of polyamines have been produced by synthetic chemists and these have been used for a variety of experimental and therapeutic experiments. One interesting series of compounds first described by the laboratories of Coward and of Ganem has methyl substituents on the α carbon atom or both the terminal carbon atoms of spermidine or spermine (182–186). Such substitution does not interfere with the uptake or many physiological functions of the polyamines, but renders them resistant to some oxidative catabolism and as substrates for acetylation and the generation of ROS (187–189). They can therefore be used to investigate polyamine function and to replace polyamines in conditions of polyamine deficiency (188, 190). More recent studies, in which (S)- and (R)- isomers of α-methylspermidine and (S,S)-, (R,S)-, and (R,R)-diastereomers of α,α′-(dimethyl)spermine have been used, have confirmed and extended these important findings (67, 191–196). Such studies have shown the maintenance of nucleic acid structure (196, 197), the stereospecificity of oxidation reactions (192, 194), the ability of derivatives that can support hypusine synthesis to support growth (67, 188, 190), and the role of polyamines in other growth processes including hair synthesis (198), liver regeneration, and adipocyte differentiation (199). Of particular interest is the ability to overcome acute liver damage and pancreatitis related to polyamine depletion by provision of α-methylspermidine or α,α′-(dimethyl)spermine (168, 193, 195, 200, 201). The synthesis of such derivatives is covered by Chapter 29. Other active polyamine analogs have also been produced that contain unsaturated moieties leading to structural changes that can be used to investigate polyamine function (185, 186, 190, 202). N-(3-aminopropyl)-1,4-diamino-cis-but-2-ene [the cis isomer of the alkene analog of spermidine] was a good substrate for spermine synthase, but that the trans isomer and the alkyne analog [N-(3-aminopropyl)-1,4-diaminobut-2-yne] were not substrates. Only the cis isomer was able to act as a precursor of hypusine and support sustained growth in cells treated with an inhibitor of polyamine synthesis (190).
Other very useful polyamine derivatives involve substitution at N-groups. Such derivatives of both the terminal and internal N atoms have been used to provide analogs that are transported by the polyamine transport system and thus can be used to study this system (121, 203–206). Fluorescent derivatives have been particularly useful in this regard (121, 204, 206). They have been used to study transport and to provide useful assay substrates for polyamine metabolic enzymes as described in Chapter 30, and also to study the binding of polyamines to receptors and ion channels. Polyamines with N-substitutions are also potentially useful as delivery vehicles for drugs. They can incorporate additions that are known antitumor agents (118, 204, 207–209).
Many synthetic polyamine analogs are profoundly antiproliferative and/or cause apoptosis in cancer cells and some are at various stages of development as antitumor agents (210–216). Addition of terminal alkyl groups renders the analogs more resistant to metabolism and large numbers of analogs with both symmetrical terminal substituents and unsymmetrical terminal substituents have been made by the laboratories of Samejima, Bergeron, Woster, Frydman, and others (211, 217–223). Other internal modifications leading to conformational restriction of bis(ethyl) polyamines have provided particularly promising derivatives that are currently in clinical trials (224, 225).
The original hypothesis underlying the production of these N-alkyl substituted analogs was related to the homeostatic regulation of polyamine content and the importance of polyamines for neoplastic growth. Thus, it was hypothesized that if analogs could be developed, which did not themselves act in the roles of polyamine essential for cell growth, but were able to down-regulate polyamine synthesis and upregulate degradation/excretion, these would prevent tumor growth. Remarkably, many polyamine analogs such as N1, N11-bis(ethyl)norspermine and N1, N12-bis(ethyl)spermine do indeed seem to have these properties being strong inducers of antizyme and of SSAT, thus leading to a drastic fall in polyamine content and apoptosis (119, 226–228). Induction of high levels of SSAT also may exert antitumor effects via ROS production through the activated SSAT/APAO pathways (60, 229, 230) or a reduction in acetyl-CoA and malonyl-CoA, which would decrease fatty acid synthesis (231, 232).
However, many analogs that are also effective against human tumor cells in xenografts and cell cultures do not cause as large an increase in SSAT (213, 214, 224, 233–236). They may act by directly inducing SMO, which is highly responsive to many analogs, and thus reduce spermine and increase ROS (48, 60, 229), by metabolism generating toxic metabolites via SMO or the SSAT/APAO pathways (60, 237) or by additional mechanisms such as blocking binding of polyamines to key sites for normal effects on growth, interfering with polyamine-mediated cell signaling (238) or by acting on the cytoskeleton (230). It should also be noted that the potential of polyamine analogs for therapeutic use is not limited to cancer therapy. A variety of analogs have been shown to have antiparasitic activity (216, 239–242).
Recently, a novel use of polyamine analogs for therapy has been developed based on the observations that some of these compounds are potent and specific inactivators of enzymes that play key roles in chromatin modification (215, 238, 243). Alteration of chromatin structure to reverse epigenetic changes leading to neoplastic growth is a major current focus of the development of novel therapeutics. The synthesis of polyaminohydroxamic acid and polyaminobenzamide derivatives that selectively inactivate histone deacetylase isoforms is described in Chapter 31.
11. Chemical Properties and Analysis of Polyamines
It is important to realize that the properties of polyamines are not simply due to their total charge but that the spatial distribution of this charge and the abilities of cellular macromolecules to interact, not only via electrostatic interactions, but also by hydrophobic interactions with the aliphatic moieties, are critical to understanding their effects. The many crystal structures of proteins and nucleic acids complexed with polyamines that are now available show clearly the importance of both types of interaction.
Early studies with polyamine analogs containing fluorine substituents also demonstrate the importance of the charged nitrogen atoms in the ability of these analogs to fulfill polyamine functions (244–247), but with some exceptions (196) this aspect of the potential effects of synthetic substitutions of the polyamine backbone is not often explored. Other considerations of the charge distribution in the naturally occurring polyamines are of great importance and Chapter 32 provides a comprehensive discussion of this topic and the measurement of the pKa values of individual polyamines.
It is a critical part of experiments on polyamine metabolism and function to measure the content of polyamines. Many methods are available for this assay and, depending on the polyamines to be assayed and the equipment available, an appropriate method can be selected. Acid extraction followed by deproteinization provides a suitable starting material. Very early methods involved separation by thin layer chromatography of either the amines followed by detection with reagents such as ninhydrin (248) or of fluorescent derivatives formed by dansylation (249, 250) or use of an amino acid analyzer with modified elution to release the more basic polyamines (251). These have largely been replaced by HPLC separation, which can be carried out with either postcolumn derivatization using o-phthalaldehyde (252) or by precolumn derivatization to form dansyl or benzoyl- or other readily quantifiable derivatives (253, 254). These methods are covered in Volume 79 of this series. One advantage of the precolumn dansylation procedure is that polyamine analogs with terminal alkyl or acetyl substituents can still be detected. Extension of HPLC analysis to separation of the wider variety of polyamines first characterized in thermophiles is covered in Chapter 5 of the present volume.
More recent analytical methods have employed gas chromatography/mass spectrometry (255–257). These assays, detailed procedures for which are described in Chapter 33 and for human samples in Chapter 27, are the method of choice with respect to specificity and sensitivity as long as the required equipment, marker compounds, and expertise are available and the extensive development of these assays has been a major advance.
In summary, it is hoped that the detailed methods provided in this new volume will facilitate the work in the many laboratories currently interested in polyamine function and metabolism and will foster a new generation of researchers interested in this exciting and challenging field. As we have entered a new era of polyamine research with our better understanding of their molecular functions, many challenges lie ahead. Our goal is to make meeting those challenges somewhat easier.
Acknowledgments
Research in the authors’ laboratories on polyamines has been supported by NIH (CA-018138 and GM-26290 to AEP; CA-51085 and CA-98454 to RAC) and by grants from Komen for the Cure KG08923, and the Samuel Waxman Cancer Research Foundation (to RAC).
References
- 1.Cohen SS. A guide to the polyamines. Oxford University Press; New York: 1998. [Google Scholar]
- 2.Naka Y, Watanabe K, Sagor GH, Niitsu M, Pillai MA, Kusano T, Takahashi Y. Quantitative analysis of plant polyamines including thermospermine during growth and salinity stress. Plant Physiol Biochem. 2010;48:527–533. doi: 10.1016/j.plaphy.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi T, Kakehi JI. Polyamines: ubiquitous polycations with unique roles in growth and stress responses. Ann Bot (Lond) 2010;105:1–6. doi: 10.1093/aob/mcp259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams-Ashman HG. NICOLAS LOUIS VAUQUELIN (1763-1829) Invest Urol. 1965;2:605–613. [PubMed] [Google Scholar]
- 5.Lee J, Michael AJ, Martynowski D, Goldsmith EJ, Phillips MA. Phylogenetic diversity and the structural basis of substrate specificity in the beta/alpha-barrel fold basic amino acid decarboxylases. J Biol Chem. 2007;282:27115–27125. doi: 10.1074/jbc.M704066200. [DOI] [PubMed] [Google Scholar]
- 6.Morris DR, Pardee AB. Multiple pathways of putrescine biosynthesis in Escherichia coli. J Biol Chem. 1966;241:3129–3135. [PubMed] [Google Scholar]
- 7.Tabor H, Rosenthal SM, Tabor CW. The biosynthesis of spermidine and spermine from putrescine and methionine. J Biol Chem. 1958;233:907–914. [PubMed] [Google Scholar]
- 8.Pegg AE. S-adenosylmethionine decarboxylase. Vol. 46. Portland, London: 2009. [Google Scholar]
- 9.Bale S, Ealick SE. Structural biology of S-adenosylmethionine decarboxylase. Amino Acids. 2010;38:451–460. doi: 10.1007/s00726-009-0404-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikeguchi Y, Bewley M, Pegg AE. Aminopropyltransferases: function, structure and genetics. J Biochem. 2006;139:1–9. doi: 10.1093/jb/mvj019. [DOI] [PubMed] [Google Scholar]
- 11.Wu H, Min J, Ikeguchi Y, Zeng H, Dong A, Loppnau P, Pegg AE, Plotnikov AN. Structure and mechanism of spermidine synthases. Biochemistry. 2007;46:8331–8339. doi: 10.1021/bi602498k. [DOI] [PubMed] [Google Scholar]
- 12.Wu H, Min J, Zeng H, McCloskey DE, Ikeguchi Y, Loppnau P, Michael AJ, Pegg AE, Plotnikov AN. Crystal structure of human spermine synthase: implications of substrate binding and catalytic mechanism. J Biol Chem. 2008;283:16135–16146. doi: 10.1074/jbc.M710323200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pegg AE, Michael AJ. Spermine synthase. Cell Mol Life Sci. 2010;67:113–121. doi: 10.1007/s00018-009-0165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J, Sperandio V, Frantz DE, Longgood J, Camilli A, Phillips MA, Michael AJ. An alternative polyamine biosynthetic pathway is widespread in bacteria and essential for biofilm formation in Vibrio cholerae. J Biol Chem. 2009;284:9899–9907. doi: 10.1074/jbc.M900110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tait GH. A new pathway for the biosynthesis of spermidine. Biochem Soc Trans. 1976;4:610–612. doi: 10.1042/bst0040610. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto S, Nagata S, Kusaba K. Purification and characterization of homospermidine synthase in Acinetobacter tartarogenes ATCC 31105. J Biochem. 1993;114:45–49. doi: 10.1093/oxfordjournals.jbchem.a124137. [DOI] [PubMed] [Google Scholar]
- 17.Ober D, Harms R, Witte L, Hartmann T. Molecular evolution by change of function. alkaloid-specific homospermidine synthase retained all properties of deoxyhypusine synthase except binding the eIF5A precursor protein. J Biol Chem. 2003;278:12805–12812. doi: 10.1074/jbc.M207112200. [DOI] [PubMed] [Google Scholar]
- 18.Shaw FL, Elliott KA, Kinch LN, Fuell C, Phillips MA, Michael AJ. Evolution and multifarious horizontal transfer of an alternative biosynthetic pathway for the alternative polyamine sym-homospermidine. J Biol Chem. 2010;285:14711–14723. doi: 10.1074/jbc.M110.107219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oshima T. Unique polyamines produced by an extreme thermophile Thermus thermophilus. Amino Acids. 2007;33:367–372. doi: 10.1007/s00726-007-0526-z. [DOI] [PubMed] [Google Scholar]
- 20.Terui Y, Ohnuma M, Hiraga K, Kawashima E, Oshima T. Stabilization of nucleic acids by unusual polyamines produced by an extreme thermophile. Biochem J. 2005;388:427–433. doi: 10.1042/BJ20041778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerner EW, Meyskens FL., Jr Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer. 2004;4:781–792. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- 22.Pegg AE. Mammalian polyamine metabolism and function. IUBMB Life. 2009;61:880–894. doi: 10.1002/iub.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Igarashi K, Kashiwagi K. Modulation of cellular function by polyamines. Int J Biochem Cell Biol. 2010;42:39–51. doi: 10.1016/j.biocel.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida M, Kashiwagi K, Shigemasa A, Taniguchi S, Yamamoto K, Makinoshima H, Ishihama A, Igarashi K. A unifying model for the role of polyamines in bacterial cell growth, the polyamine modulon. J Biol Chem. 2004;279:46008–46013. doi: 10.1074/jbc.M404393200. [DOI] [PubMed] [Google Scholar]
- 25.Igarashi K, Kashiwagi K. Polyamine modulon in Escherichia coli: genes involved in the stimulation of cell growth by polyamines. J Biochem (Tokyo) 2006;139:11–16. doi: 10.1093/jb/mvj020. [DOI] [PubMed] [Google Scholar]
- 26.Uemura T, Higashi K, Takigawa M, Toida T, Kashiwagi K, Igarashi K. Polyamine modulon in yeast-stimulation of COX4 synthesis by spermidine at the level of translation. Int J Biochem Cell Biol. 2009;41:2538–2545. doi: 10.1016/j.biocel.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura K, Okudaira H, Ochiai E, Higashi K, Kaneko M, Ishii I, Nishimura T, Dohmae N, Kashiwagi K, Igarashi K. Identification of proteins whose synthesis is preferentially enhanced by polyamines at the level of translation in mammalian cells. Int J Biochem Cell Biol. 2009;41:2251–2261. doi: 10.1016/j.biocel.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 28.Saini P, Eyler DE, Green R, Dever TE. Hypusine-containing protein eIF5A promotes translation elongation. Nature. 2009;459:118–121. doi: 10.1038/nature08034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park MH, Nishimura K, Zanelli CF, Valentini SR. Functional significance of eIF5A and its hypusine modification in eukaryotes. Amino Acids. 2010;41:2538–2545. doi: 10.1007/s00726-009-0408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landau G, Bercovich Z, Park MH, Kahana C. The role of polyamines in supporting growth of mammalian cells is mediated through their requirement for translation initiation and elongation. J Biol Chem. 2010;285:12474–12481. doi: 10.1074/jbc.M110.106419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zou T, Mazan-Mamczarz K, Rao JN, Liu L, Marasa BS, Zhang AH, Xiao L, Pullmann R, Gorospe M, Wang JY. Polyamine depletion increases cytoplasmic levels of RNA-binding protein HuR leading to stabilization of nucleophosmin and p53 mRNAs. J Biol Chem. 2006;281:19387–19394. doi: 10.1074/jbc.M602344200. [DOI] [PubMed] [Google Scholar]
- 32.Zou T, Liu L, Rao JN, Marasa BS, Chen J, Xiao L, Zhou H, Gorospe M, Wang JY. Polyamines modulate the subcellular localization of RNA-binding protein HuR through AMP-activated protein kinase-regulated phosphorylation and acetylation of importin alpha1. Biochem J. 2008;409:389–398. doi: 10.1042/BJ20070860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L, Rao JN, Zou T, Xiao L, Wang PY, Turner DJ, Gorospe M, Wang JY. Polyamines regulate c-Myc translation through Chk2-dependent HuR phosphorylation. Mol Biol Cell. 2009;20:4885–4898. doi: 10.1091/mbc.E09-07-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopatin AN, Makhina EN, Nichols CG. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994;372:366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- 35.Williams K. Modulation and block of ion channels: a new biology of polyamines. Cell Signal. 1997;9:1–13. doi: 10.1016/s0898-6568(96)00089-7. [DOI] [PubMed] [Google Scholar]
- 36.Jänne J, Alhonen L, Pietilä M, Keinänen T. Genetic approaches to the cellular functions of polyamines in mammals. Eur J Biochem. 2004;271:877–894. doi: 10.1111/j.1432-1033.2004.04009.x. [DOI] [PubMed] [Google Scholar]
- 37.Pegg AE, Feith DJ, Fong LYY, Coleman CS, O’Brien TG, Shantz LM. Transgenic mouse models for studies of the role of polyamines in normal, hypertrophic and neoplastic growth. Biochem Soc Trans. 2003;31:356–360. doi: 10.1042/bst0310356. [DOI] [PubMed] [Google Scholar]
- 38.Alhonen L, Uimari A, Pietila M, Hyvonen MT, Pirinen E, Keinanen TA. Transgenic animals modelling polyamine metabolism-related diseases. Essays Biochem. 2009;46:125–144. doi: 10.1042/bse0460009. [DOI] [PubMed] [Google Scholar]
- 39.Nilsson JA, Keller UB, Baudino TA, Yang C, Norton S, Old JA, Nilsson LM, Neale G, Kramer DL, Porter CW, Cleveland JL. Targeting ornithine decarboxylase in Myc-induced lymphomagenesis prevents tumor formation. Cancer Cell. 2005;7:433–444. doi: 10.1016/j.ccr.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 40.Guo Y, Cleveland JL, O’Brien TG. Haploinsufficiency for ODC modifies mouse skin tumor susceptibility. Cancer Res. 2005;65:1146–1149. doi: 10.1158/0008-5472.CAN-04-3244. [DOI] [PubMed] [Google Scholar]
- 41.Feith DJ, Fong LYY, Pegg AE. Antizyme inhibits N-nitrosomethylbenzylamine-induced mouse forestomach carcinogenesis in a p53-independent manner. Proc Am Assoc Cancer Res. 2005;46:A3887. [Google Scholar]
- 42.Rial NS, Meyskens FL, Gerner EW. Polyamines as mediators of APC-dependent intestinal carcinogenesis and cancer chemo-prevention. Essays Biochem. 2009;46:111–124. doi: 10.1042/bse0460008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerner EW, Meyskens FL., Jr Combination chemoprevention for colon cancer targeting polyamine synthesis and inflammation. Clin Cancer Res. 2009;15:758–761. doi: 10.1158/1078-0432.CCR-08-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bailey HH, Kim K, Verma AK, Sielaff K, Larson PO, Snow S, Lenaghan T, Viner JL, Douglas J, Dreckschmidt NE, Hamielec M, Pomplun M, Sharata HH, Puchalsky D, Berg ER, Havighurst TC, Carbone PP. A randomized, double-blind, placebo-controlled phase 3 skin cancer prevention study of α-difluoromethylornithine in subjects with previous history of skin cancer. Cancer Prev Res. 2010;3:35–47. doi: 10.1158/1940-6207.CAPR-09-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simoneau AR, Gerner EW, Nagle R, Ziogas A, Fujikawa-Brooks S, Yerushalmi H, Ahlering TE, Lieberman R, McLaren CE, Anton-Culver H, Meyskens FL., Jr The effect of difluoromethylornithine on decreasing prostate size and polyamines in men: results of a year-long phase IIb randomized placebo-controlled chemoprevention trial. Cancer Epidemiol Biomarkers Prev. 2008;17:292–299. doi: 10.1158/1055-9965.EPI-07-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forshell TP, Rimpi S, Nilsson JA. Chemoprevention of B-cell lymphomas by inhibition of the Myc target spermidine synthase. Cancer Prev Res. 2010;3:140–147. doi: 10.1158/1940-6207.CAPR-09-0166. [DOI] [PubMed] [Google Scholar]
- 47.Pegg AE. Spermidine/spermine N1-acetyltransferase: a key metabolic regulator. Am J Physiol Endocrinol Metab. 2008;294:E995–E1010. doi: 10.1152/ajpendo.90217.2008. [DOI] [PubMed] [Google Scholar]
- 48.Casero RA, Jr, Pegg AE. Polyamine catabolism and disease. Biochem J. 2009;421:323–338. doi: 10.1042/BJ20090598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jänne J, Alhonen L, Pietila M, Keinanen TA, Uimari A, Hyvonen MT, Pirinen E, Jarvinen A. Genetic manipulation of polyamine catabolism in rodents. J Biochem (Tokyo) 2006;139:155–160. doi: 10.1093/jb/mvj035. [DOI] [PubMed] [Google Scholar]
- 50.Chattopadhyay MK, Park MH, Tabor H. Hypusine modification for growth is the major function of spermidine in Saccharomyces cerevisiae polyamine auxotrophs grown in limiting spermidine. Proc Natl Acad Sci USA. 2008;105:6554–6559. doi: 10.1073/pnas.0710970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pegg AE, Wang X. Mouse models to investigate the function of spermine. Commun Integr Biol. 2009;2:271–274. doi: 10.4161/cib.2.3.8225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanfrey C, Sommer S, Mayer MJ, Burtin D, Michael AJ. Arabidopsis polyamine biosynthese: absence of ornithine decarboxylase and the mechanism of arginine decarboxylase activity. Plant J. 2001;27:551–560. doi: 10.1046/j.1365-313x.2001.01100.x. [DOI] [PubMed] [Google Scholar]
- 53.Hanfrey C, Franceschetti M, Mayer MJ, Illingworth C, Michael AJ. Abrogation of upstream open reading frame-mediated translational control of a plant S-adenosylmethionine decarboxylase results in polyamine disruption and growth perturbations. J Biol Chem. 2002;277:44131–44139. doi: 10.1074/jbc.M206161200. [DOI] [PubMed] [Google Scholar]
- 54.Vera-Sirera F, Minguet EG, Singh SK, Ljung K, Tuominen H, Blazquez MA, Carbonell J. Role of polyamines in plant vascular development. Plant Physiol Biochem. 2010;48:534–539. doi: 10.1016/j.plaphy.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 55.Seiler N, Bolkenius FN, Knodgen B. The influence of catabolic reactions on polyamine excretion. Biochem J. 1985;225:219–226. doi: 10.1042/bj2250219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morgan DM. Polyamine oxidases–enzymes of unknown function? Biochem Soc Trans. 1998;26:586–591. doi: 10.1042/bst0260586. [DOI] [PubMed] [Google Scholar]
- 57.Seiler N. Catabolism of polyamines. Amino Acids. 2004;26:217–233. doi: 10.1007/s00726-004-0070-z. [DOI] [PubMed] [Google Scholar]
- 58.Kimes BW, Morris DR. Inhibition of nucleic acid and protein synthesis in Escherichia coli by oxidized polyamines and acrolein. Biochim Biophys Acta. 1971;228:235–244. doi: 10.1016/0005-2787(71)90563-6. [DOI] [PubMed] [Google Scholar]
- 59.Kimes BW, Morris DR. Preparation and stability of oxidized polyamines. Biochim Biophys Acta. 1971;228:223–234. doi: 10.1016/0005-2787(71)90562-4. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y, Casero RA., Jr Mammalian polyamine catabolism: a therapeutic target, a pathological problem, or both? J Biochem (Tokyo) 2006;139:17–25. doi: 10.1093/jb/mvj021. [DOI] [PubMed] [Google Scholar]
- 61.Adachi MS, Juarez PR, Fitzpatrick PF. Mechanistic studies of human spermine oxidase: kinetic mechanism and pH effects. Biochemistry. 2010;49:386–392. doi: 10.1021/bi9017945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moschou PN, Sanmartin M, Andriopoulou AH, Rojo E, Sanchez-Serrano JJ, Roubelakis-Angelakis KA. Bridging the gap between plant and mammalian polyamine catabolism: a novel peroxisomal polyamine oxidase responsible for a full back-conversion pathway in Arabidopsis. Plant Physiol. 2008;147:1845–1857. doi: 10.1104/pp.108.123802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cona A, Rea G, Angelini R, Federico R, Tavladoraki P. Functions of amine oxidases in plant development and defence. Trends Plant Sci. 2006;11:80–88. doi: 10.1016/j.tplants.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 64.Rodriguez AA, Maiale SJ, Menendez AB, Ruiz OA. Polyamine oxidase activity contributes to sustain maize leaf elongation under saline stress. J Exp Bot. 2009;60:4249–4262. doi: 10.1093/jxb/erp256. [DOI] [PubMed] [Google Scholar]
- 65.Childs AC, Mehta DJ, Gerner EW. Polyamine-dependent gene expression. Cell Mol Life Sci. 2003;60:1394–1406. doi: 10.1007/s00018-003-2332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park MH. The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A) J Biochem (Tokyo) 2006;139:161–169. doi: 10.1093/jb/mvj034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hyvönen MT, Keinänen TA, Cerrada-Gimenez M, Sinervirta R, Grigorenko N, Khomutov AR, Vepsäläinen J, Alhonen L, Jänne J. Role of hypusinated eukaryotic translation initiation factor 5A in polyamine depletion-induced cytostasis. J Biol Chem. 2007;282:34700–34706. doi: 10.1074/jbc.M704282200. [DOI] [PubMed] [Google Scholar]
- 68.Hoque M, Hanauske-Abel HM, Palumbo P, Saxena D, D’Alliessi-Gandolfi D, Park MH, Pe’ery T, Mathews MB. Inhibition of HIV-1 gene expression by Ciclopirox and Deferiprone, drugs that prevent hypusination of eukaryotic initiation factor 5A. Retrovirology. 2009;6:90. doi: 10.1186/1742-4690-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vu VV, Emerson JP, Martinho M, Kim YS, Munck E, Park MH, Que L., Jr Human deoxyhypusine hydroxylase, an enzyme involved in regulating cell growth, activates O2 with a nonheme diiron center. Proc Natl Acad Sci USA. 2009;106:14814–14819. doi: 10.1073/pnas.0904553106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pegg AE. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986;234:249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Persson L. Polyamine homoeostasis. Essays Biochem. 2009;46:11–24. doi: 10.1042/bse0460002. [DOI] [PubMed] [Google Scholar]
- 72.Pegg AE. Regulation of ornithine decarboxylase. J Biol Chem. 2006;281:14529–14532. doi: 10.1074/jbc.R500031200. [DOI] [PubMed] [Google Scholar]
- 73.Kahana C. Regulation of cellular polyamine levels and cellular proliferation by antizyme and antizyme inhibitor. Essays Biochem. 2009;46:47–61. doi: 10.1042/bse0460004. [DOI] [PubMed] [Google Scholar]
- 74.Bale S, Lopez MM, Makhatadze GI, Fang Q, Pegg AE, Ealick SE. Structural basis for putrescine activation of human S-adenosylmethionine decarboxylase. Biochemistry. 2008;47:13404–13417. doi: 10.1021/bi801732m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Willert EK, Fitzpatrick R, Phillips MA. Allosteric regulation of an essential trypanosome polyamine biosynthetic enzyme by a catalytically dead homolog. Proc Natl Acad Sci USA. 2007;104:8275–8280. doi: 10.1073/pnas.0701111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Willert EK, Phillips MA. Regulated expression of an essential allosteric activator of polyamine biosynthesis in African trypanosomes. PLoS Pathog. 2008;4:e1000183. doi: 10.1371/journal.ppat.1000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Willert EK, Phillips MA. Cross-species activation of trypanosome S- adenosylmethionine decarboxylase by the regulatory subunit prozyme. Mol Biochem Parasitol. 2009;168:1–6. doi: 10.1016/j.molbiopara.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bacchi CJ. Chemotherapy of human African trypanosomiasis. Interdiscip Perspect Infect Dis. 2009;2009:195040. doi: 10.1155/2009/195040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barker RH, Jr, Liu H, Hirth B, Celatka CA, Fitzpatrick R, Xiang Y, Willert EK, Phillips MA, Kaiser M, Bacchi CJ, Rodriguez A, Yarlett N, Klinger JD, Sybertz E. Novel S-adenosylmethionine decarboxylase inhibitors for the treatment of human African trypanosomiasis. Antimicrob Agents Chemother. 2009;53:2052–2058. doi: 10.1128/AAC.01674-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Russell DH, Snyder SH. Amine synthesis in regenerating rat liver: extremely rapid turnover of ornithine decarboxylase. Mol Pharmacol. 1969;5:253–262. [PubMed] [Google Scholar]
- 81.Coffino P. Regulation of cellular polyamines by antizyme. Nat Rev Mol Cell Biol. 2001;2:188–194. doi: 10.1038/35056508. [DOI] [PubMed] [Google Scholar]
- 82.Kahana C. Antizyme and antizyme inhibitor, a regulatory tango. Cell Mol Life Sci. 2009;66:2479–2488. doi: 10.1007/s00018-009-0033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heller JS, Fong WF, Canellakis ES. Induction of a protein inhibitor to ornithine decarboxylase by the end products of its reaction. Proc Natl Acad Sci USA. 1976;73:1858–1862. doi: 10.1073/pnas.73.6.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hayashi S, Murakami Y, Matsufuji S. Ornithine decarboxylase antizyme: a novel type of regulatory protein. TIBS. 1996;21:27–30. [PubMed] [Google Scholar]
- 85.Yamaguchi Y, Takatsuka Y, Matsufuji S, Murakami Y, Kamio Y. Characterization of a counterpart to mammalian ornithine decarboxylase antizyme in prokaryotes. J Biol Chem. 2006;281:3995–4001. doi: 10.1074/jbc.M507545200. [DOI] [PubMed] [Google Scholar]
- 86.Ivanov IP, Matsufuji S, Murakami Y, Gesteland RF, Atkins JF. Conservation of polyamine regulation by translational frameshifting from yeast to mammals. EMBO J. 2000;19:1907–1917. doi: 10.1093/emboj/19.8.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Howard MT, Shirts BH, Zhou J, Carlson CL, Matsufuji S, Gesteland RF, Weeks RS, Atkins JF. Cell culture analysis of the regulatory frameshift event required for the expression of mammalian antizymes. Genes Cells. 2001;6:931–941. doi: 10.1046/j.1365-2443.2001.00477.x. [DOI] [PubMed] [Google Scholar]
- 88.Ivanov IP, Loughran G, Atkins JF. uORFs with unusual translational start codons autoregulate expression of eukaryotic ornithine decarboxylase homologs. Proc Natl Acad Sci USA. 2008;105:10079–10084. doi: 10.1073/pnas.0801590105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murai N, Shimizu A, Murakami Y, Matsufuji S. Subcellular localization and phosphorylation of antizyme 2. J Cell Biochem. 2009;108:1012–1021. doi: 10.1002/jcb.22334. [DOI] [PubMed] [Google Scholar]
- 90.Tokuhiro K, Isotani A, Yokota S, Yano Y, Oshio S, Hirose M, Wada M, Fujita K, Ogawa Y, Okabe M, Nishimune Y, Tanaka H. OAZ-t/OAZ3 is essential for rigid connection of sperm tails to heads in mouse. PLoS Genet. 2009;5:e1000712. doi: 10.1371/journal.pgen.1000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lopez-Contreras AJ, Ramos-Molina B, Cremades A, Penafiel R. Antizyme inhibitor 2: molecular, cellular and physiological aspects. Amino Acids. 2009;38:603–611. doi: 10.1007/s00726-009-0419-4. [DOI] [PubMed] [Google Scholar]
- 92.Su KL, Liao YF, Hung HC, Liu GY. Critical factors determining dimerization of human antizyme inhibitor. J Biol Chem. 2009;284:26768–26777. doi: 10.1074/jbc.M109.007807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Snapir Z, Keren-Paz A, Bercovich Z, Kahana C. ODCp, a brain- and testis-specific ornithine decarboxylase paralogue, functions as an antizyme inhibitor, although less efficiently than AzI1. Biochem J. 2008;410:613–619. doi: 10.1042/BJ20071423. [DOI] [PubMed] [Google Scholar]
- 94.Tang H, Ariki K, Ohkido M, Murakami Y, Matsufuji S, Li Z, Yamamura K. Role of ornithine decarboxylase antizyme inhibitor in vivo. Genes Cells. 2009;14:79–87. doi: 10.1111/j.1365-2443.2008.01249.x. [DOI] [PubMed] [Google Scholar]
- 95.Nilsson JA, Maclean KH, Keller UB, Pendeville H, Baudino TA, Cleveland JL. Mnt loss triggers Myc transcription targets, proliferation, apoptosis, and transformation. Mol Cell Biol. 2004;24:1560–1569. doi: 10.1128/MCB.24.4.1560-1569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shantz LM, Levin VA. Regulation of ornithine decarboxylase during oncogenic transformation: mechanisms and therapeutic potential. Amino Acids. 2007;33:213–223. doi: 10.1007/s00726-007-0531-2. [DOI] [PubMed] [Google Scholar]
- 97.Pyronnet S, Pradayrol L, Sonenberg N. Alternative splicing facilitates internal ribosome entry on the ornithine decarboxylase mRNA. Cell Mol Life Sci. 2005;62:1267–1274. doi: 10.1007/s00018-005-5020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Origanti S, Shantz LM. Ras transformation of RIE-1 cells activates cap-independent translation of ornithine decarboxylase: regulation by the Raf/MEK/ERK and phosphatidylinositol 3-kinase pathways. Cancer Res. 2007;67:4834–4842. doi: 10.1158/0008-5472.CAN-06-4627. [DOI] [PubMed] [Google Scholar]
- 99.Grens A, Scheffler IE. The 5′- and 3′-untranslated regions of ornithine decarboxylase mRNA affect the translational efficiency. J Biol Chem. 1990;265:11810–11816. [PubMed] [Google Scholar]
- 100.Igarashi K, Ito K, Kashiwagi K. Polyamine uptake systems in Escherichi coli. Res Microbiol. 2001;152:271–278. doi: 10.1016/s0923-2508(01)01198-6. [DOI] [PubMed] [Google Scholar]
- 101.Igarashi K, Kashiwagi K. Polyamine transport in bacteria and yeast. Biochem J. 1999;344:633–642. [PMC free article] [PubMed] [Google Scholar]
- 102.Aouida M, Leduc A, Poulin R, Ramotar D. AGP2 encodes the major permease for high affinity polyamine import in Saccharomyces cerevisiae. J Biol Chem. 2005;280:24267–24276. doi: 10.1074/jbc.M503071200. [DOI] [PubMed] [Google Scholar]
- 103.Uemura T, Kashiwagi K, Igarashi K. Polyamine uptake by DUR3 and SAM3 in Saccharomyces cerevisiae. J Biol Chem. 2007;282:7733–7741. doi: 10.1074/jbc.M611105200. [DOI] [PubMed] [Google Scholar]
- 104.Hasne MP, Ullman B. Identification and characterization of a polyamine permease from the protozoan parasite Leishmania major. J Biol Chem. 2005;280:15188–15194. doi: 10.1074/jbc.M411331200. [DOI] [PubMed] [Google Scholar]
- 105.Hasne MP, Coppens I, Soysa R, Ullman B. A high-affinity putrescine-cadaverine transporter from Trypanosoma cruzi. Mol Microbiol. 2010;76:78–91. doi: 10.1111/j.1365-2958.2010.07081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Burns MR, Carlson CL, Vanderwerf SM, Ziemer JR, Weeks RS, Cai F, Webb HW, Graminski GF. Amino acid/spermine conjugates: polyamine amides as potent spermidine uptake inhibitors. J Med Chem. 2001;44:3632–3644. doi: 10.1021/jm0101040. [DOI] [PubMed] [Google Scholar]
- 107.Covassin L, Desjardins M, Soulet D, Charest-Gaudreault R, Audette M, Poulin R. Xylylated dimers of putrescine and polyamines: influence of the polyamine backbone on spermidine transport inhibition. Bioorg Med Chem Lett. 2003;13:3267–3271. doi: 10.1016/s0960-894x(03)00668-1. [DOI] [PubMed] [Google Scholar]
- 108.Burns MR, Graminski GF, Weeks RS, Chen Y, O’Brien TG. Lipophilic lysine-spermine conjugates are potent polyamine transport Inhibitors for use in combination with a polyamine biosynthesis inhibitor. J Med Chem. 2009;52:1983–1993. doi: 10.1021/jm801580w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Heinick A, Urban K, Roth S, Spies D, Nunes F, Phanstiel O, IV, Liebau E, Luersen K. Caenorhabditis elegans P5B-type ATPase CATP-5 operates in polyamine transport and is crucial for norspermidine-mediated suppression of RNA interference. FASEB J. 2010;24:206–217. doi: 10.1096/fj.09-135889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rannels DE, Pegg AE, Clark RS, Addison JL. Interaction of paraquat and amine uptake by rat lungs perfused in situ. Am J Physiol. 1985;249:E506–E513. doi: 10.1152/ajpendo.1985.249.5.E506. [DOI] [PubMed] [Google Scholar]
- 111.Minton KW, Tabor H, Tabor CW. Paraquat toxicity is increased in Escherichia coli defective in the synthesis of polyamines. Proc Natl Acad Sci USA. 1990;87:2851–2855. doi: 10.1073/pnas.87.7.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mandel J, Flintoff WF. Isolation of mutant mammalian cells altered in polyamine transport. J Cell Physiol. 1978;97:335–344. doi: 10.1002/jcp.1040970308. [DOI] [PubMed] [Google Scholar]
- 113.Heaton MA, Flintoff WF. Methylglyoxal bis (guanylhydrazone)- resistant Chinese hamster ovary cells: genetic evidence that more than a single locus controls uptake. J Cell Physiol. 1988;136:133–139. doi: 10.1002/jcp.1041360117. [DOI] [PubMed] [Google Scholar]
- 114.Byers TL, Kameji R, Rannels DE, Pegg AE. Multiple pathways for uptake of paraquat, methylglyoxal bis(guanylhydrazone), and polyamines. Am J Physiol. 1987;252:C663–C669. doi: 10.1152/ajpcell.1987.252.6.C663. [DOI] [PubMed] [Google Scholar]
- 115.Rossi T, Coppi A, Bruni E, Ruberto A, Giudice S, Baggio G. Mepacrine antagonises tumour cell growth induced by natural polyamines. Anticancer Res. 2008;28:2765–2768. [PubMed] [Google Scholar]
- 116.Holley JL, Mather A, Wheelhouse RT, Cullis PM, Hartley JA, Bingham JP, Cohen GM. Targeting of tumor cells and DNA by a chlorambucil-spermidine conjugate. Cancer Res. 1992;52:4190–4195. [PubMed] [Google Scholar]
- 117.Kaur N, Delcros JG, Archer J, Weagraff NZ, Martin B, Phanstiel O., IV Designing the polyamine pharmacophore: influence of N-substituents on the transport behavior of polyamine conjugates. J Med Chem. 2008;51:2551–2560. doi: 10.1021/jm701341k. [DOI] [PubMed] [Google Scholar]
- 118.Palmer AJ, Ghani RA, Kaur N, Phanstiel O, Wallace HM. A putrescine-anthracence conjugate: a paradigm for selective drug delivery. Biochem J. 2009;424:431–438. doi: 10.1042/BJ20090815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mitchell JL, Leyser A, Holtorff MS, Bates JS, Frydman B, Valasinas A, Reddy VK, Marton LJ. Antizyme induction by polyamine analogues as a factor of cell growth inhibition. Biochem J. 2002;366:663–671. doi: 10.1042/BJ20011612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mitchell JL, Simkus CL, Thane TK, Tokarz P, Bonar MM, Frydman B, Valasinas AL, Reddy VK, Marton LJ. Antizyme induction mediates feedback limitation of the incorporation of specific polyamine analogues in tissue culture. Biochem J. 2004;384:271–279. doi: 10.1042/BJ20040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Soulet D, Gagnon B, Rivest S, Audette M, Poulin R. A fluorescent probe of polyamine transport accumulates into intracellular acidic vesicles via a two-step mechanism. J Biol Chem. 2004;279:49355–49366. doi: 10.1074/jbc.M401287200. [DOI] [PubMed] [Google Scholar]
- 122.Belting M, Persson S, Fransson L-Å. Proteoglycan involvement in polyamine uptake. Biochem J. 1999;338:317–323. [PMC free article] [PubMed] [Google Scholar]
- 123.Belting M, Mani K, Jonsson M, Cheng F, Sandgren S, Jonsson S, Ding K, Delcros JG, Fransson LA. Glypican-1 is a vehicle for polyamine uptake in mammalian cells: a pivital role for nitrosothiol-derived nitric oxide. J Biol Chem. 2003;278:47181–47189. doi: 10.1074/jbc.M308325200. [DOI] [PubMed] [Google Scholar]
- 124.Belting M, Borsig L, Fuster MM, Brown JR, Persson L, Fransson LA, Esko JD. Tumor attenuation by combined heparan sulfate and polyamine depletion. Proc Natl Acad Sci USA. 2002;99:371–376. doi: 10.1073/pnas.012346499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Welch JE, Bengtson P, Svensson K, Wittrup A, Jenniskens GJ, Ten Dam GB, Van Kuppevelt TH, Belting M. Single chain fragment anti-heparan sulfate antibody targets the polyamine transport system and attenuates polyamine-dependent cell proliferation. Int J Oncol. 2008;32:749–756. [PubMed] [Google Scholar]
- 126.Roy UK, Rial NS, Kachel KL, Gerner EW. Activated K-RAS increases polyamine uptake in human colon cancer cells through modulation of caveolar endocytosis. Mol Carcinog. 2008;47:538–553. doi: 10.1002/mc.20414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hawell L, Tjandrawinata RR, Byus CV. Selective putrescine export is regulated by insulin and ornithine in Reuber H35 hepatoma cells. Biochim Biopyhs Acta. 1994;1222:15–26. doi: 10.1016/0167-4889(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 128.Hawel L, III, Byus CV. A streamlined method for the isolation and quantitation of nanomole levels of exported polyamines in cell culture media. Anal Biochem. 2002;311:127–132. doi: 10.1016/s0003-2697(02)00423-2. [DOI] [PubMed] [Google Scholar]
- 129.Pastorian KE, Byus CV. Tolerance to putrescine toxicity in Chinese hamster ovary cells is associated with altered uptake and export. Exp Cell Res. 1997;231:284–295. doi: 10.1006/excr.1996.3467. [DOI] [PubMed] [Google Scholar]
- 130.Uemura T, Yerushalmi HF, Tsaprailis G, Stringer DE, Pastorian KE, Hawel L, III, Byus CV, Gerner EW. Identification and characterization of a diamine exporter in colon epithelial cells. J Biol Chem. 2008;283:26428–26435. doi: 10.1074/jbc.M804714200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bardocz S, Duguid TJ, Brown DS, Grant G, Pusztai A, White A, Ralph A. The importance of dietary polyamines in cell regeneration and growth. Br J Nutr. 1995;73:819–828. doi: 10.1079/bjn19950087. [DOI] [PubMed] [Google Scholar]
- 132.Quemener V, Moulinoux JP, Havouis R, Seiler N. Polyamine deprivation enhances antitumoral efficacy of chemotherapy. Anticancer Res. 1992;12:1447–1454. [PubMed] [Google Scholar]
- 133.Quemener V, Blancard Y, Chamaillard L, Havouis R, Cipolla B, Moulinoux J. Polyamine deprivation: a new tool in cancer treatment. Anticancer Res. 1994;14:443–448. [PubMed] [Google Scholar]
- 134.Cipolla B, Guilli F, Moulinoux JP. Polyamine-reduced diet in metastatic hormone-refractory prostate cancer (HRPC) patients. Biochem Soc Trans. 2003;31:384–387. doi: 10.1042/bst0310384. [DOI] [PubMed] [Google Scholar]
- 135.Estebe JP, Legay F, Gentili M, Wodey E, Leduc C, Ecoffey C, Moulinoux JP. An evaluation of a polyamine-deficient diet for the treatment of inflammatory pain. Anesth Analg. 2006;102:1781–1788. doi: 10.1213/01.ane.0000205755.43562.2b. [DOI] [PubMed] [Google Scholar]
- 136.Soda K, Kano Y, Sakuragi M, Takao K, Lefor A, Konishi F. Long-term oral polyamine intake increases blood polyamine concentrations. J Nutr Sci Vitaminol (Tokyo) 2009;55:361–366. doi: 10.3177/jnsv.55.361. [DOI] [PubMed] [Google Scholar]
- 137.Sabater-Molina M, Larque E, Torrella F, Plaza J, Lozano T, Munoz A, Zamora S. Effects of dietary polyamines at physiologic doses in early-weaned piglets. Nutrition. 2009;25:940–946. doi: 10.1016/j.nut.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 138.Zoumas-Morse C, Rock CL, Quintana EL, Neuhouser ML, Gerner EW, Meyskens FL., Jr Development of a polyamine database for assessing dietary intake. J Am Diet Assoc. 2007;107:1024–1027. doi: 10.1016/j.jada.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kawakita M, Hiramatsu K. Diacetylated derivatives of spermine and spermidine as novel promising tumor markers. J Biochem (Tokyo) 2006;139:315–322. doi: 10.1093/jb/mvj068. [DOI] [PubMed] [Google Scholar]
- 140.Russell DH. Clinical relevance of polyamines as biochemical markers of tumor kinetics. Clin Chem. 1977;23:22–27. [PubMed] [Google Scholar]
- 141.Durie BG, Salmon SE, Russell DH. Polyamines as markers of response and disease activity in cancer chemotherapy. Cancer Res. 1977;37:214–221. [PubMed] [Google Scholar]
- 142.Miki T, Hiramatsu K, Kawakita M. Interaction of N1, N12-diacetylspermine with polyamine transport systems of polarized porcine renal cell line LLC-PK1. J Biochem (Tokyo) 2005;138:479–484. doi: 10.1093/jb/mvi141. [DOI] [PubMed] [Google Scholar]
- 143.Hamaoki M, Hiramatsu K, Suzuki S, Nagata A, Kawakita M. Two enzyme-linked immunosorbent assay (ELISA) systems for N1, N8-diacetylspermidine and N1, N12-diacetylspermine using monoclonal antibodies. J Biochem. 2002;132:783–788. doi: 10.1093/oxfordjournals.jbchem.a003287. [DOI] [PubMed] [Google Scholar]
- 144.Parchment RE. The implications of a unified theory of programmed cell death, polyamines, oxyradicals and histogenesis in the embryo. Int J Dev Biol. 1993;37:75–83. [PubMed] [Google Scholar]
- 145.Parchment RE, Pierce GB. Polyamine oxidation programmed cell death, and regulation of melanoma in the murine embryonic limb. Cancer Res. 1989;49:6680–6686. [PubMed] [Google Scholar]
- 146.Zahedi K, Wang Z, Barone S, Prada AE, Kelly CN, Casero RA, Yokota N, Porter CW, Rabb H, Soleimani M. Expression of SSAT, a novel biomarker of tubular cell damage, increases in kidney ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2003;284:F1046–F1055. doi: 10.1152/ajprenal.00318.2002. [DOI] [PubMed] [Google Scholar]
- 147.Zhao YJ, Xu CQ, Zhang WH, Zhang L, Bian SL, Huang Q, Sun HL, Li QF, Zhang YQ, Tian Y, Wang R, Yang BF, Li WM. Role of polyamines in myocardial ischemia/reperfusion injury and their interactions with nitric oxide. Eur J Pharmacol. 2007;562:236–246. doi: 10.1016/j.ejphar.2007.01.096. [DOI] [PubMed] [Google Scholar]
- 148.Zahedi K, Bissler JJ, Wang Z, Josyula A, Lu L, Diegelman P, Kisiel N, Porter CW, Soleimani M. Spermidine/spermine N1-acetyltransferase overexpression in kidney epithelial cells disrupts polyamine homeostasis, leads to DNA damage, and causes G2 arrest. Am J Physiol Cell Physiol. 2007;292:C1204–C1215. doi: 10.1152/ajpcell.00451.2006. [DOI] [PubMed] [Google Scholar]
- 149.Barone S, Okaya T, Rudich S, Petrovic S, Tenrani K, Wang Z, Zahedi K, Casero RA, Lentsch AB, Soleimani M. Distinct and sequential upregulation of genes regulating cell growth and cell cycle progression during hepatic ischemia-reperfusion injury. Am J Physiol Cell Physiol. 2005;289:C826–C835. doi: 10.1152/ajpcell.00629.2004. [DOI] [PubMed] [Google Scholar]
- 150.Zahedi K, Lentsch AB, Okaya T, Barone SL, Sakai N, Witte DP, Arend LJ, Alhonen L, Jell J, Jänne J, Porter CW, Soleimani M. Spermidine/spermine-N1-acetyltransferase ablation protects against liver and kidney ischemia reperfusion injury in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G899–G909. doi: 10.1152/ajpgi.90507.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sakata K, Kashiwagi K, Sharmin S, Ueda S, Igarashi K. Acrolein produced from polyamines as one of the uraemic toxins. Biochem Soc Trans. 2003;31:371–374. doi: 10.1042/bst0310371. [DOI] [PubMed] [Google Scholar]
- 152.Igarashi K, Ueda S, Yoshida K, Kashiwagi K. Polyamines in renal failure. Amino Acids. 2006;31:477–483. doi: 10.1007/s00726-006-0264-7. [DOI] [PubMed] [Google Scholar]
- 153.Tomitori H, Usui T, Saeki N, Ueda S, Kase H, Nishimura K, Kashiwagi K, Igarashi K. Polyamine oxidase and acrolein as novel biochemical markers for diagnosis of cerebral stroke. Stroke. 2005;36:2609–2613. doi: 10.1161/01.STR.0000190004.36793.2d. [DOI] [PubMed] [Google Scholar]
- 154.Yoshida M, Higashi K, Jin L, Machi Y, Suzuki T, Masuda A, Dohmae N, Suganami A, Tamura Y, Nishimura K, Toida T, Tomitori H, Kashiwagi K, Igarashi K. Identification of acrolein-conjugated protein in plasma of patients with brain infarction. Biochem Biophys Res Commun. 2010;391:1234–1239. doi: 10.1016/j.bbrc.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 155.Saiki R, Nishimura K, Ishii I, Omura T, Okuyama S, Kashiwagi K, Igarashi K. Intense correlation between brain infarction and protein-conjugated acrolein. Stroke. 2009;40:3356–3361. doi: 10.1161/STROKEAHA.109.553248. [DOI] [PubMed] [Google Scholar]
- 156.Yoshida M, Tomitori H, Machi Y, Hagihara M, Higashi K, Goda H, Ohya T, Niitsu M, Kashiwagi K, Igarashi K. Acrolein toxicity: comparison with reactive oxygen species. Biochem Biophys Res Commun. 2009;378:313–318. doi: 10.1016/j.bbrc.2008.11.054. [DOI] [PubMed] [Google Scholar]
- 157.Yoshida M, Tomitori H, Machi Y, Katagiri D, Ueda S, Horiguchi K, Kobayashi E, Saeki N, Nishimura K, Ishii I, Kashiwagi K, Igarashi K. Acrolein, IL-6 and CRP as markers of silent brain infarction. Atherosclerosis. 2009;203:557–562. doi: 10.1016/j.atherosclerosis.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 158.Seiler N. Polyamine metabolism. Digestion. 1990;46:319–330. doi: 10.1159/000200405. [DOI] [PubMed] [Google Scholar]
- 159.Hong SK, Chaturvedi R, Piazuelo MB, Coburn LA, Williams CS, Delgado AG, Casero RA, Jr, Schwartz DA, Wilson KT. Increased expression and cellular localization of spermine oxidase in ulcerative colitis and relationship to disease activity. Inflamm Bowel Dis. 2010;16:1557–1566. doi: 10.1002/ibd.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gobert AP, Cheng Y, Wang JY, Boucher JL, Iyer RK, Cederbaum SD, Casero RA, Jr, Newton JC, Wilson KT. Heliobacter pylori induces macrophage apoptosis by activation of arginase II. J Immunol. 2002;168:4692–4700. doi: 10.4049/jimmunol.168.9.4692. [DOI] [PubMed] [Google Scholar]
- 161.Cheng Y, Chaturvedi R, Asim M, Bussiere FI, Scholz A, Xu H, Casero RA, Jr, Wilson KT. Helicobacter pylori-induced macrophage apoptosis requires activation of ornithine decarboxylase by c-Myc. J Biol Chem. 2005;280:22492–22496. doi: 10.1074/jbc.C500122200. [DOI] [PubMed] [Google Scholar]
- 162.Chaturvedi R, Cheng Y, Asim M, Bussiere FI, Xu H, Gobert AP, Hacker A, Casero RA, Jr, Wilson KT. Induction of polyamine oxidase 1 by Helicobacter pylori causes macrophage apoptosis by hydrogen peroxide release and mitochondrial membrane depolarization. J Biol Chem. 2004;279:40161–40173. doi: 10.1074/jbc.M401370200. [DOI] [PubMed] [Google Scholar]
- 163.Xu H, Chaturvedi R, Cheng Y, Bussiere FI, Asim M, Yao MD, Potosky D, Meltzer SJ, Rhee JG, Kim SS, Moss SF, Hacker A, Wang Y, Casero RA, Jr, Wilson KT. Spermine oxidation induced by Helicobacter pylori results in apoptosis and DNA damage: implications for gastric carcinogenesis. Cancer Res. 2004;64:8521–8525. doi: 10.1158/0008-5472.CAN-04-3511. [DOI] [PubMed] [Google Scholar]
- 164.Kee K, Foster BA, Merali S, Kramer DL, Hensen ML, Diegelman P, Kisiel N, Vujcic S, Mazurchuk RV, Porter CW. Activated polyamine catabolism depletes acetyl-CoA pools and suppresses prostate tumor growth in TRAMP mice. J Biol Chem. 2004;279:40076–40083. doi: 10.1074/jbc.M406002200. [DOI] [PubMed] [Google Scholar]
- 165.Tucker JM, Murphy JT, Kisiel N, Diegelman P, Barbour KW, Davis C, Medda M, Alhonen L, Janne J, Kramer DL, Porter CW, Berger FG. Potent modulation of intestinal tumorigenesis in Apcmin/+ mice by the polyamine catabolic enzyme spermidine/spermine N1-acetyltransferase. Cancer Res. 2005;65:5390–5398. doi: 10.1158/0008-5472.CAN-05-0229. [DOI] [PubMed] [Google Scholar]
- 166.Hyvonen MT, Merentie M, Uimari A, Keinanen TA, Janne J, Alhonen L. Mechanisms of polyamine catabolism-induced acute pancreatitis. Biochem Soc Trans. 2007;35:326–330. doi: 10.1042/BST0350326. [DOI] [PubMed] [Google Scholar]
- 167.Jell J, Merali S, Hensen ML, Mazurchuk R, Spernyak JA, Diegelman P, Kisiel ND, Barrero C, Deeb KK, Alhonen L, Patel MS, Porter CW. Genetically altered expression of spermidine/spermine N1-acetyltransferase affects fat metabolism in mice via acetyl-CoA. J Biol Chem. 2007;282:8404–8413. doi: 10.1074/jbc.M610265200. [DOI] [PubMed] [Google Scholar]
- 168.Merentie M, Uimari A, Pietila M, Sinervirta R, Keinanen TA, Vepsalainen J, Khomutov A, Grigorenko N, Herzig KH, Janne J, Alhonen L. Oxidative stress and inflammation in the pathogenesis of activated polyamine catabolism-induced acute pancreatitis. Amino Acids. 2007;33:323–330. doi: 10.1007/s00726-007-0522-3. [DOI] [PubMed] [Google Scholar]
- 169.Gimelli G, Giglio S, Zuffardi O, Alhonen L, Suppola S, Cusano R, Lo Nigro C, Gatti R, Ravazzolo R, Seri M. Gene dosage of the spermidine/spermine N1-acetyltransferase (SSAT) gene with putrescine accumulation in a patient with a Xp21.1p22.12 duplication and keratosis follicularis spinulosa decalvans (KFSD) Hum Genet. 2002;111:235–241. doi: 10.1007/s00439-002-0791-6. [DOI] [PubMed] [Google Scholar]
- 170.Pietila M, Pirinen E, Keskitalo S, Juutinen S, Pasonen-Seppanen S, Keinanen T, Alhonen L, Jänne J. Disturbed keratinocyte differentiation in transgenic mice and organotypic keratinocyte cultures as a result of spermidine/spermine N1-acetyltransferase overexpression. J Invest Dermatol. 2005;124:596–601. doi: 10.1111/j.0022-202X.2005.23636.x. [DOI] [PubMed] [Google Scholar]
- 171.Kaufmann AM, Krise JP. Niemann-Pick C1 functions in regulating lysosomal amine content. J Biol Chem. 2008;283:24584–24593. doi: 10.1074/jbc.M803715200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sequeira A, Gwadry FG, Ffrench-Mullen JM, Canetti L, Gingras Y, Casero RA, Jr, Rouleau G, Benkelfat C, Turecki G. Implication of SSAT by gene expression and genetic variation in suicide and major depression. Arch Gen Psychiatry. 2006;63:35–48. doi: 10.1001/archpsyc.63.1.35. [DOI] [PubMed] [Google Scholar]
- 173.Guipponi M, Deutsch S, Kohler K, Perroud N, Le Gal F, Vessaz M, Laforge T, Petit B, Jollant F, Guillaume S, Baud P, Courtet P, La Harpe R, Malafosse A. Genetic and epigenetic analysis of SSAT gene dysregulation in suicidal behavior. Am J Med Genet B. 2009;50B:799–807. doi: 10.1002/ajmg.b.30901. [DOI] [PubMed] [Google Scholar]
- 174.Klempan TA, Rujescu D, Merette C, Himmelman C, Sequeira A, Canetti L, Fiori LM, Schneider B, Bureau A, Turecki G. Profiling brain expression of the spermidine/spermine N1-acetyltransferase 1 (SAT1) gene in suicide. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:934–943. doi: 10.1002/ajmg.b.30920. [DOI] [PubMed] [Google Scholar]
- 175.Chen GG, Fiori LM, Moquin L, Gratton A, Mamer O, Mechawar N, Turecki G. Evidence of altered polyamine concentrations in cerebral cortex of suicide completers. Neuropsychopharmacology. 2010;35:1477–1484. doi: 10.1038/npp.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ingi T, Worley PF, Lanahan AA. Regulation of SSAT expression by synaptic activity. Eur J Neurosci. 2001;13:1459–1463. doi: 10.1046/j.0953-816x.2001.01529.x. [DOI] [PubMed] [Google Scholar]
- 177.Kaasinen SK, Grohn OH, Keinanen TA, Alhonen L, Jänne J. Overexpression of spermidine/spermine N1-acetyltransferase elevates the threshold to pentylenetetrazol-induced seizure activity in transgenic mice. Exp Neurol. 2003;183:645–652. doi: 10.1016/s0014-4886(03)00186-9. [DOI] [PubMed] [Google Scholar]
- 178.Kaasinen SK, Oksman M, Alhonen L, Tanila H, Jänne J. Spermidine/spermine N1-acetyltransferase overexpression in mice induces hypoactivity and spatial learning impairment. Pharmacol Biochem Behav. 2004;78:35–45. doi: 10.1016/j.pbb.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 179.Cason AL, Ikeguchi Y, Skinner C, Wood TC, Lubs HA, Martinez F, Simensen RJ, Stevenson RE, Pegg AE, Schwartz CE. X-Linked spermine synthase gene (SMS) defect: the first polyamine deficiency syndrome. Eur J Human Genet. 2003;11:937–944. doi: 10.1038/sj.ejhg.5201072. [DOI] [PubMed] [Google Scholar]
- 180.de Alencastro G, McCloskey DE, Kliemann SE, Maranduba CM, Pegg AE, Wang X, Bertola DR, Schwartz CE, Passos-Bueno MR, Sertie AL. New SMS mutation leads to a striking reduction in spermine synthase protein function and a severe form of Snyder-Robinson X-linked recessive mental retardation syndrome. J Med Genet. 2008;45:539–543. doi: 10.1136/jmg.2007.056713. [DOI] [PubMed] [Google Scholar]
- 181.Becerra-Solano LE, Butler J, Castañeda-Cisneros G, McCloskey DE, Wang X, Pegg AE, Schwartz CE, Sánchez-Corona J, Garcia-Ortiz JE. A missense mutation, p.V132G, in the X-linked spermine synthase gene (SMS) causes Snyder-Robinson syndrome. Am J Med Genet A. 2009;149A:328–335. doi: 10.1002/ajmg.a.32641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lakanen JR, Coward JK, Pegg AE. α-Methylpolyamines: metabolically stable spermidine and spermine mimics capable of supporting growth in cells depleted of polyamines. J Med Chem. 1992;35:724–734. doi: 10.1021/jm00082a013. [DOI] [PubMed] [Google Scholar]
- 183.Varnado BL, Voci CJ, Meyer LM, Coward JK. Circular dichroism and NMR studies of metabolically stable α-methylpolyamines: special comparison with naturally occurring polyamines. Bioorg Chem. 2000;28:395–408. doi: 10.1006/bioo.2000.1195. [DOI] [PubMed] [Google Scholar]
- 184.Pegg AE, Poulin R, Coward JK. Use of aminopropyltransferase inhibitors and of non-metabolizable analogues to study polyamine regulation and function. Int J Biochem. 1995;27:425–442. doi: 10.1016/1357-2725(95)00007-c. [DOI] [PubMed] [Google Scholar]
- 185.Nagarajan S, Ganem B. Chemistry of naturally occurring polyamines. II. Unsaturated spermidine and spermine derivatives. J Org Chem. 1987;52:5044–5046. [Google Scholar]
- 186.Nagarajan S, Ganem B, Pegg AE. Studies of non-metabolizable polyamines that support growth of SV-3T3 cells depleted of natural polyamines by exposure to α-difluoromethylornithine. Biochem J. 1988;254:373–378. doi: 10.1042/bj2540373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yang J, Xiao L, Berkey KA, Tamez PA, Coward JK, Casero RA., Jr Significant induction of spermidine/spermine N1-acetyltransferase without cytotoxicity by the growth-supporting polyamine analogue 1, 12-dimethylspermine. J Cell Physiol. 1995;165:71–76. doi: 10.1002/jcp.1041650109. [DOI] [PubMed] [Google Scholar]
- 188.Byers TL, Lakanen JR, Coward JK, Pegg AE. The role of hypusine depletion in cytostasis induced by S-adenosyl-L-methionine decarboxylase inhibition: new evidence provided by 1-methylspermidine and 1, 12-dimethylspermine. Biochem J. 1994;303:363–368. doi: 10.1042/bj3030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Byers TL, Wechter R, Hu R, Pegg AE. Effects of the S-adenosylmethionine decarboxylase inhibitor, 5′-{[(Z)-4-amino-2-butenyl] methylamino}-5′-deoxyadenosine, on cell growth and polyamine polyamine metabolism and transport in Chinese hamster ovary cell cultures. Biochem J. 1994;303:89–96. doi: 10.1042/bj3030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Byers TL, Ganem B, Pegg AE. Cytostasis induced in L1210 murine leukemia cells by the S-adenosyl-L-methionine decarboxylase inhibitor 5′-{[(Z)-4-amino-2-butenyl]methylamino}-5′-deoxyadenosine may be due to the hypusine depletion. Biochem J. 1992;287:717–724. doi: 10.1042/bj2870717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jarvinen A, Grigorenko N, Khomutov AR, Hyvonen MT, Uimari A, Vepsalainen J, Sinervirta R, Keinanen TA, Vujcic S, Alhonen L, Porter CW, Janne J. Metabolic stability of α-methylated polyamine derivatives and their use as substitutes for the natural polyamines. J Biol Chem. 2005;280:6595–6601. doi: 10.1074/jbc.M412788200. [DOI] [PubMed] [Google Scholar]
- 192.Jarvinen AJ, Cerrada-Gimenez M, Grigorenko NA, Khomutov AR, Vepsalainen JJ, Sinervirta RM, Keinanen TA, Alhonen LI, Janne JE. α-methyl polyamines: efficient synthesis and tolerance studies in vivo and in vitro. First evidence for dormant stereospecificity of polyamine oxidase. J Med Chem. 2006;49:399–406. doi: 10.1021/jm050872h. [DOI] [PubMed] [Google Scholar]
- 193.Hyvonen MT, Herzig KH, Sinervirta R, Albrecht E, Nordback I, Sand J, Keinanen TA, Vepsalainen J, Grigorenko N, Khomutov AR, Kruger B, Janne J, Alhonen L. activated polyamine catabolism in acute pancreatitis: α-methylated polyamine analogues prevent trypsinogen activation and pancreatitis-associated mortality. Am J Pathol. 2006;168:115–122. doi: 10.2353/ajpath.2006.050518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jarvinen A, Keinanen TA, Grigorenko NA, Khomutov AR, Uimari A, Vepsalainen J, Narvanen A, Alhonen L, Janne J. Guide molecule-driven stereospecific degradation of α-methylpolyamines by polyamine oxidase. J Biol Chem. 2006;281:4589–4595. doi: 10.1074/jbc.M509959200. [DOI] [PubMed] [Google Scholar]
- 195.Jin HT, Lamsa T, Hyvonen MT, Sand J, Raty S, Grigorenko N, Khomutov AR, Herzig KH, Alhonen L, Nordback I. A polyamine analog bismethylspermine ameliorates severe pancreatitis induced by intraductal infusion of taurodeoxycholate. Surgery. 2008;144:49–56. doi: 10.1016/j.surg.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 196.Weisell J, Hyvonen MT, Vepsalainen J, Alhonen L, Keinanen TA, Khomutov AR, Soininen P. Novel isosteric charge-deficient spermine analogue-1, 12-diamino-3, 6, 9-triazadodecane: synthesis, pKa measurement and biological activity. Amino Acids. 2010;38:501–507. doi: 10.1007/s00726-009-0409-6. [DOI] [PubMed] [Google Scholar]
- 197.Nayvelt I, Hyvonen MT, Alhonen L, Pandya I, Thomas T, Khomutov AR, Vepsalainen J, Patel R, Keinanen TA, Thomas TJ. DNA condensation by chiral alpha-methylated polyamine analogues and protection of cellular DNA from oxidative damage. Biomacromolecules. 2010;11:97–105. doi: 10.1021/bm900958c. [DOI] [PubMed] [Google Scholar]
- 198.Fashe TM, Keinanen TA, Grigorenko NA, Khomutov AR, Janne J, Alhonen L, Pietila M. Cutaneous application of alpha-methylspermidine activates the growth of resting hair follicles in mice. Amino Acids. 2010;38:583–590. doi: 10.1007/s00726-009-0421-x. [DOI] [PubMed] [Google Scholar]
- 199.Vuohelainen S, Pirinen E, Cerrada-Gimenez M, Keinanen TA, Uimari A, Pietila M, Khomutov AR, Janne J, Alhonen L. Spermidine is indispensable in differentiation of 3T3-L1 fibroblasts to adipocytes. J Cell Mol Med. 2010;14:1683–1692. doi: 10.1111/j.1582-4934.2009.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Räsänen T-L, Alhonen L, Sinervirta R, Keinänen T, Herzig K-H, Suppola S, Khomutov AR, Vepsäläinen J, Jänne J. A polyamine analogue prevents acute pancreatitis and restores early liver regeneration in transgenic rats with activated polyamine catabolism. J Biol Chem. 2002;277:39867–39872. doi: 10.1074/jbc.M205967200. [DOI] [PubMed] [Google Scholar]
- 201.Hyvonen MT, Sinervirta R, Grigorenko N, Khomutov AR, Vepsalainen J, Keinanen TA, Alhonen L. alpha-Methylspermidine protects against carbon tetrachloride-induced hepatic and pancreatic damage. Amino Acids. 2010;38:575–581. doi: 10.1007/s00726-009-0418-5. [DOI] [PubMed] [Google Scholar]
- 202.Pegg AE, Nagarajan S, Naficy S, Ganem B. Role of unsaturated derivatives of spermidine as substrates for spermine synthase and in supporting growth of SV-3T3 cells. Biochem J. 1991;274:167–171. doi: 10.1042/bj2740167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Holley J, Mather A, Cullis P, Symons MR, Wardman P, Watt RA, Cohen GM. Uptake and cytotoxicity of novel nitroimidazole-polyamine conjugates in Ehrlich ascites tumour cells. Biochem Pharmacol. 1992;43:763–769. doi: 10.1016/0006-2952(92)90241-a. [DOI] [PubMed] [Google Scholar]
- 204.Cullis PM, Green RE, Merson-Davies L, Travis N. Probing the mechanism of transport and compartmentalisation of polyamines in mammalian cells. Chem Biol. 1999;6:717–729. doi: 10.1016/s1074-5521(00)80019-8. [DOI] [PubMed] [Google Scholar]
- 205.Wang C, Delcros JG, Biggerstaff J, Phanstiel O., IV Molecular requirements for targeting the polyamine transport system. Synthesis and biological evaluation of polyamine-anthracene conjugates. J Med Chem. 2003;46:2672–2682. doi: 10.1021/jm020598g. [DOI] [PubMed] [Google Scholar]
- 206.Soulet D, Covassin L, Kaouass M, Charest-Gaudreault R, Audette M, Poulin R. Role of endocytosis in the internalization of spermidine-C(2)-BODIPY, a highly fluorescent probe of polyamine transport. Biochem J. 2002;367:347–357. doi: 10.1042/BJ20020764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang C, Delcros JG, Biggerstaff J, Phanstiel OT. Synthesis and biological evaluation of N1-(anthracen-9-ylmethyl)triamines as molecular recognition elements for the polyamine transporter. J Med Chem. 2003;46:2663–2671. doi: 10.1021/jm030028w. [DOI] [PubMed] [Google Scholar]
- 208.Gardner RA, Delcros JG, Konate F, Breitbeil F, III, Martin B, Sigman M, Huang M, Phanstiel O., IV N1-substituent effects in the selective delivery of polyamine conjugates into cells containing active polyamine transporters. J Med Chem. 2004;47:6055–6069. doi: 10.1021/jm0497040. [DOI] [PubMed] [Google Scholar]
- 209.Kaur N, Delcros JG, Imran J, Khaled A, Chehtane M, Tschammer N, Martin B, Phanstiel O., IV A comparison of chloroambucil- and xylene-containing polyamines leads to improved ligands for accessing the polyamine transport system. J Med Chem. 2008;51:1393–1401. doi: 10.1021/jm070794t. [DOI] [PubMed] [Google Scholar]
- 210.Porter CW, McManis J, Casero RA, Jr, Bergeron RJ. Relative abilities of bis(ethyl) derivatives of putrescine spermidine and spermine to regulate polyamine biosynthesis and inhibit cell growth. Cancer Res. 1987;47:2821–2825. [PubMed] [Google Scholar]
- 211.Bergeron RJ, Neims AH, McManis JS, Hawthorne TR, Vinson JRT, Bortell R, Ingeno MJ. Synthetic polyamine analogues as antineoplastics. J Med Chem. 1988;31:1183–1190. doi: 10.1021/jm00401a019. [DOI] [PubMed] [Google Scholar]
- 212.Bergeron RJ, McManis JS, Liu CZ, Feng Y, Weimar WR, Luchetta GR, Wu Q, Ortiz-Ocasio J, Vinson JRT, Kramer D, Porter C. Antiproliferative properties of polyamine anlogues: a structure-activity study. J Med Chem. 1994;37:3464–3476. doi: 10.1021/jm00047a004. [DOI] [PubMed] [Google Scholar]
- 213.Casero RA, Jr, Frydman B, Stewart TM, Woster PM. Significance of targeting polyamine metabolism as an antineoplastic strategy: unique targets for polyamine analogues. Proc West Pharmacol Soc. 2005;48:24–30. [PubMed] [Google Scholar]
- 214.Casero RA, Jr, Marton LJ. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov. 2007;6:373–390. doi: 10.1038/nrd2243. [DOI] [PubMed] [Google Scholar]
- 215.Casero RA, Jr, Woster PM. Recent advances in the development of polyamine analogues as antitumor agents. J Med Chem. 2009;52:4551–4573. doi: 10.1021/jm900187v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Senanayake MD, Amunugama H, Boncher TD, Casero RA, Woster PM. Design of polyamine-based therapeutic agents: new targets and new directions. Essays Biochem. 2009;46:77–94. doi: 10.1042/bse0460006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Basu HS, Feuerstein BG, Deen DF, Lubich WP, Bergeron RJ, Samejima K, Marton LJ. Correlation between the effects of polyamine analogues on DNA conformation and cell growth. Cancer Res. 1989;49:5591–5597. [PubMed] [Google Scholar]
- 218.Chang BK, Bergeron RJ, Porter CW, Vinson JRT, Liang V. Regulatory and anti-proliferative effects of N-alkylated polyamine analogues in human and hamster pancreatic adenocarcinoma cell lines. Cancer Chemother Pharmacol. 1992;30:183–188. doi: 10.1007/BF00686309. [DOI] [PubMed] [Google Scholar]
- 219.Saab NH, West EE, Bieszk NC, Preuss CV, Mank AR, Casero RA, Jr, Woster PM. Synthesis and evaluation of unsymmetrically substituted polyamine analogues as modulators of human spermidine/spermine-N1-acetyl-transferase (SSAT) and as potential antitumor agents. J Med Chem. 1993;36:2998–3004. doi: 10.1021/jm00072a020. [DOI] [PubMed] [Google Scholar]
- 220.Reddy VK, Valasinas A, Sarkar A, Basu HS, Marton LJ, Frydman B. Conforma tionally restricted analogues of lN, 12N-bisethylspermine: synthesis and growth inhibitory effects on human tumor cell lines. J Med Chem. 1998;41:4723–4732. doi: 10.1021/jm980172v. [DOI] [PubMed] [Google Scholar]
- 221.Casero RA, Jr, Woster PM. Terminally alkylated polyamine analogues as chemotherapeutic agents. J Med Chem. 2001;44:1–26. doi: 10.1021/jm000084m. [DOI] [PubMed] [Google Scholar]
- 222.Valasinas A, Reddy VK, Blokhin AV, Basu HS, Bhattacharya S, Sarkar A, Marton LJ, Frydman B. Long-chain polyamines (oligoamines) exhibit strong cytotoxicities against human prostate cancer cells. Bioorg Med Chem. 2003;11:4121–4131. doi: 10.1016/s0968-0896(03)00453-x. [DOI] [PubMed] [Google Scholar]
- 223.Frydman B, Blokhin AV, Brummel S, Wilding G, Maxuitenko Y, Sarkar A, Bhattacharya S, Church D, Reddy VK, Kink JA, Marton LJ, Valasinas A, Basu HS. Cyclopropane-containing polyamine analogues are efficient growth inhibitors of a human prostate tumor xenograft in nude mice. J Med Chem. 2003;46:4586–4600. doi: 10.1021/jm030175u. [DOI] [PubMed] [Google Scholar]
- 224.Carew JS, Nawrocki ST, Reddy VK, Bush D, Rehg JE, Goodwin A, Houghton JA, Casero RA, Jr, Marton LJ, Cleveland JL. The novel polyamine analogue CGC-11093 enhances the antimyeloma activity of bortezomib. Cancer Res. 2008;68:4783–4790. doi: 10.1158/0008-5472.CAN-07-6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Frydman B, Porter CW, Maxuitenko Y, Sarkar A, Bhattacharya S, Valasinas A, Reddy VK, Kisiel N, Marton LJ, Basu HS. A novel polyamine analog (SL-11093) inhibits growth of human prostate tumor xenografts in nude mice. Cancer Chemother Pharmacol. 2003;51:488–492. doi: 10.1007/s00280-003-0598-8. [DOI] [PubMed] [Google Scholar]
- 226.Casero RA, Jr, Celano P, Ervin SJ, Porter CW, Bergeron RJ, Libby P. Differential induction of spermidine/spermine N1-acetyltransferase in human lung cancer cells by the bis(ethyl)polyamine analogues. Cancer Res. 1989;49:3829–3833. [PubMed] [Google Scholar]
- 227.McCloskey DE, Pegg AE. Properties of the spermidine/spermine N1-acetyltrans-ferase mutant L156F that decreases cellular sensitivity to the polyamine analogue N1, N11-bis(ethyl)norspermine. J Biol Chem. 2003;278:13881–13887. doi: 10.1074/jbc.M205689200. [DOI] [PubMed] [Google Scholar]
- 228.McCloskey DE, Pegg AE. Altered spermidine/spermine N1-acetyltransferase activity as a mechanism of cellular resistance to bis(ethyl)polyamine analogues. J Biol Chem. 2000;275:28708–28714. doi: 10.1074/jbc.M004120200. [DOI] [PubMed] [Google Scholar]
- 229.Pledgie A, Huang Y, Hacker A, Zhang Z, Woster PM, Davidson NE, Casero RA., Jr Spermine oxidase SMO(PAOh1), not N1-acetylpolyamine oxidase PAO, is the primary source of cytotoxic H2O2 in polyamine analogue-treated human breast cancer cell lines. J Biol Chem. 2005;280:39843–39851. doi: 10.1074/jbc.M508177200. [DOI] [PubMed] [Google Scholar]
- 230.Jiang R, Choi W, Hu L, Gerner EW, Hamilton SR, Zhang W. Activation of polyamine catabolism by N1, N11-diethylnorspermine alters the cellular localization of mTOR and downregulates mTOR protein level in glioblastoma cells. Cancer Biol Ther. 2007;6:1644–1648. doi: 10.4161/cbt.6.10.4800. [DOI] [PubMed] [Google Scholar]
- 231.Beckers A, Organe S, Timmermans L, Scheys K, Peeters A, Brusselmans K, Verhoeven G, Swinnen JV. Chemical inhibition of acetyl-CoA carboxylase induces growth arrest and cytotoxicity selectively in cancer cells. Cancer Res. 2007;67:8180–8187. doi: 10.1158/0008-5472.CAN-07-0389. [DOI] [PubMed] [Google Scholar]
- 232.Celik A, Kano Y, Tsujinaka S, Okada S, Takao K, Takagi M, Chohnan S, Soda K, Kawakami M, Konishi F. Decrease in malonyl-CoA and its background metabolic alterations in murine model of cancer cachexia. Oncol Rep. 2009;21:1105–1111. doi: 10.3892/or_00000330. [DOI] [PubMed] [Google Scholar]
- 233.Boncher T, Bi X, Varghese S, Casero RA, Jr, Woster PM. Polyamine-based analogues as biochemical probes and potential therapeutics. Biochem Soc Trans. 2007;35:356–363. doi: 10.1042/BST0350356. [DOI] [PubMed] [Google Scholar]
- 234.Huang Y, Pledgie A, Casero RA, Jr, Davidson NE. Molecular mechanisms of polyamine analogs in cancer cells. Anticancer Drugs. 2005;16:229–241. doi: 10.1097/00001813-200503000-00002. [DOI] [PubMed] [Google Scholar]
- 235.Wallace HM, Niiranen K. Polyamine analogues – an update. Amino Acids. 2007;33:261–265. doi: 10.1007/s00726-007-0534-z. [DOI] [PubMed] [Google Scholar]
- 236.Hacker A, Marton LJ, Sobolewski M, Casero RA., Jr In vitro and in vivo effects of the conformationally restricted polyamine analogue CGC-11047 on small cell and non-small cell lung cancer cells. Cancer Chemother Pharmacol. 2008;63:45–53. doi: 10.1007/s00280-008-0706-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hakkinen MR, Hyvonen MT, Auriola S, Casero RA, Jr, Vepsalainen J, Khomutov AR, Alhonen L, Keinanen TA. Metabolism of N-alkylated spermine analogues by polyamine and spermine oxidases. Amino Acids. 2010;38:369–381. doi: 10.1007/s00726-009-0429-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Huang Y, Greene E, Murray Stewart T, Goodwin AC, Baylin SB, Woster PM, Casero RA., Jr Inhibition of lysine-specific demethylase 1 by polyamine analogues results in reexpression of aberrantly silenced genes. Proc Natl Acad Sci USA. 2007;104:8023–8028. doi: 10.1073/pnas.0700720104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Bitonti AJ, Dumont JA, Bush TL, Edwards ML, Stemerick DM, McCann PP, Sjoerdsma A. Bis(benzyl)polyamine analogs inhibit the growth of chloroquine-resistant human malaria parasites (Plasmodium falciparum) in vitro and in combination with α-difluoromethylornithine cure murine malaria. Proc Natl Acad Sci USA. 1989;86:651–655. doi: 10.1073/pnas.86.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Edwards ML, Stemerick DM, Bitonti AJ, Dumont JA, McCann PP, Bey P, Sjoerdsma A. Antimalarial polyamine analogues. J Med Chem. 1991;34:569–574. doi: 10.1021/jm00106a015. [DOI] [PubMed] [Google Scholar]
- 241.Zou Y, Sirisoma N, Woster PM, Casero RA, Jr, Weiss LM, Rattendi D, Lane S, Bacchi CJ. Novel alkylpolyamine analogues that possess both antitrypanosomal and antimicrosporidial activity. Bioorg Med Chem Lett. 2001;11:1613–1617. doi: 10.1016/s0960-894x(01)00315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Woster PM. New therapies for parasitic Infection. Annu Rep Med Chem. 2001;36:99–108. [Google Scholar]
- 243.Huang Y, Marton LJ, Woster PM, Casero RA. Polyamine analogues targeting epigenetic gene regulation. Essays Biochem. 2009;46:95–110. doi: 10.1042/bse0460007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Seiler N, Sarhan S, Knödgen B, Gerhart F. Chain-fluorinated polyamines as tumor markers. II. Metabolic aspects in normal tissues. J Cancer Res Clin Oncol. 1988;114:71–80. doi: 10.1007/BF00390488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Hull WE, Kunz W, Port RE, Seiler N. Chain-fluorinated polyamines as tumor markers-III. Determination of geminal difluoropolyamines and their precursor 2, 2-difluoroputrescine in normal tissues and experimental tumors by in vitro and in vivo 19F NMR spectroscopy. NMR Biomed. 1988;1:11–19. doi: 10.1002/nbm.1940010104. [DOI] [PubMed] [Google Scholar]
- 246.Dezeure F, Sarhan S, Seiler N. Chain-fluorinated polyamines as tumor markers. IV. Comparison of 2-fluoroputrescine and 2, 2-difluoroputrescine as substrates of spermidine synthase in vitro and in vivo. Int J Biochem. 1988;20:1299–1312. doi: 10.1016/0020-711x(88)90235-2. [DOI] [PubMed] [Google Scholar]
- 247.Sarhan S, Knödgen B, Gerhart F, Seiler N. Chain-fluorinated polyamines as tumor markers-I. In vivo transformation of 2, 2-difluoroputrescine into 6, 6-difluorospermidine and 6, 6-difluorospermine. Int J Biochem. 1987;19:843–852. doi: 10.1016/0020-711x(87)90244-8. [DOI] [PubMed] [Google Scholar]
- 248.Hammond JE, Herbst EJ. Analysis of polyamines by thin-layer chromatography. Anal Biochem. 1968;22:474–484. doi: 10.1016/0003-2697(68)90288-1. [DOI] [PubMed] [Google Scholar]
- 249.Seiler N, Wiechmann M. Determination of amines on the 10-10-mole scale. Separation of 1-dimethylamino-naphthalene-5-sulfonyl amides by thin-layer chromatography. Experientia. 1965;21:203–204. doi: 10.1007/BF02141885. [DOI] [PubMed] [Google Scholar]
- 250.Fleisher JH, Russell DH. Estimation of urinary diamines and polyamines by thin-layer chromatography. J Chromatogr. 1975;110:335–340. doi: 10.1016/0021-9673(75)85014-x. [DOI] [PubMed] [Google Scholar]
- 251.Marton LJ, Russell DH, Levy CC. Measurement of putrescine, spermidine, and spermine in physiological fluids by use of an amino acid analyzer. Clin Chem. 1973;19:923–926. [PubMed] [Google Scholar]
- 252.Seiler N, Knödgen B. Determination of polyamines and related compounds by reversed-phase high-perfomance liquid chromatography: improved separation systems. J Chromatogr. 1985;339:45–57. [Google Scholar]
- 253.Kabra PM, Lee HK, Lubich WP, Marton LW. Solid-phase extraction and determination of dansyl derivatives of unconjugated and acetylated polyamines by reversed-phase liquid chromatography; improved separation systems for polyamines in cerebrospinal fluid, urine and tissue. J Chromatogr Biomed Appl. 1986;380:19–32. doi: 10.1016/s0378-4347(00)83621-x. [DOI] [PubMed] [Google Scholar]
- 254.Morgan DM. Determination of polyamines as their benzoylated derivatives by HPLC. Methods Mol Biol. 1998;79:111–118. doi: 10.1385/0-89603-448-8:111. [DOI] [PubMed] [Google Scholar]
- 255.Håkkinen MR, Keinanen TA, Vepsalainen J, Khomutov AR, Alhonen L, Janne J, Auriola S. Analysis of underivatized polyamines by reversed phase liquid chromatography with electrospray tandem mass spectrometry. J Pharm Biomed Anal. 2007;45:625–634. doi: 10.1016/j.jpba.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 256.Håkkinen MR, Keinanen TA, Vepsalainen J, Khomutov AR, Alhonen L, Janne J, Auriola S. Quantitative determination of underivatized polyamines by using isotope dilution RP-LC-ESI-MS/MS. J Pharm Biomed Anal. 2008;48:414–421. doi: 10.1016/j.jpba.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 257.Chen GG, Turecki G, Mamer OA. A quantitative GC-MS method for three major polyamines in postmortem brain cortex. J Mass Spectrom. 2009;44:1203–1210. doi: 10.1002/jms.1597. [DOI] [PubMed] [Google Scholar]